Abstract
Rationale: Abnormal inflammation and accelerated decline in lung function occur in patients with chronic obstructive pulmonary disease (COPD). Human sirtuin (SIRT1), an antiaging and antiinflammatory protein, is a metabolic NAD+-dependent protein/histone deacetylase that regulates proinflammatory mediators by deacetylating histone and nonhistone proteins.
Objectives: To determine the expression of SIRT1 in lungs of smokers and patients with COPD, and to elucidate the regulation of SIRT1 in response to cigarette smoke in macrophages, and its impact on nuclear factor (NF)-κB regulation.
Methods: SIRT1 and NF-κB levels were assessed in lung samples of nonsmokers, smokers, and patients with COPD. Human monocyte–macrophage cells (MonoMac6) were treated with cigarette smoke extract (CSE) to determine the mechanism of CSE-mediated regulation of SIRT1 and its involvement in RelA/p65 regulation and IL-8 release.
Measurements and Main Results: Peripheral lungs of smokers and patients with COPD showed decreased levels of nuclear SIRT1, as compared with nonsmokers, associated with its post-translational modifications (formation of nitrotyrosine and aldehyde carbonyl adducts). Treatment of MonoMac6 cells with CSE showed decreased levels of SIRT1 associated with increased acetylation of RelA/p65 NF-κB. Mutation or knockdown of SIRT1 resulted in increased acetylation of nuclear RelA/p65 and IL-8 release, whereas overexpression of SIRT1 decreased IL-8 release in response to CSE treatment in MonoMac6 cells.
Conclusions: SIRT1 levels were reduced in macrophages and lungs of smokers and patients with COPD due to its post-translational modifications by cigarette smoke–derived reactive components, leading to increased acetylation of RelA/p65. Thus, SIRT1 plays a pivotal role in regulation of NF-κB–dependent proinflammatory mediators in lungs of smokers and patients with COPD.
Keywords: reactive oxygen species, acetylation, nuclear factor-κB, inflammation, deacetylases
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Sirtuin (SIRT1) deacetylase is an antiaging and antiinflammatory protein. Cigarette smoke reduced SIRT1 levels by post-translational modifications, leading to acetylation of the RelA/p65 subunit of nuclear factor-κB.
What This Study Adds to the Field
SIRT1 deacetylase is decreased in macrophages, and in lungs of smokers and patients with chronic obstructive pulmonary disease.
Chronic obstructive pulmonary disease (COPD) is a major cause of disability, morbidity, and mortality in smokers. It is characterized by progressive and largely irreversible airflow limitation, which is associated with an abnormal inflammatory response in the lung (1–3). Cigarette smoking is the primary cause of COPD characterized by accelerated decline in lung function and alveolar destruction of the lung (3, 4). The inflammatory changes that occur in COPD are also seen in cigarette smokers without COPD, but to a lesser extent (5, 6). COPD is linked with the aging process of the lungs due to its direct encounter with inhaled cigarette smoke (CS)–derived oxidants and free radicals and other organic constituents (3, 4, 7). It is well known that abnormal inflammatory responses to smoking in patients with COPD are due to chronic inflammatory effects incited by CS (6, 8). However, due to the complex nature of the mechanism(s) of the inflammatory processes in COPD (6, 8), the precise molecular mechanisms whereby CS triggers abnormal and sustained lung inflammation and aging process still remain unclear.
The metabolic nicotinamide adenine dinucleotide (NAD+)–dependent protein deacetylases have emerged as important regulators of chronic inflammatory diseases, cancer, and aging (9). These proteins, which belong to class III histone/protein deacetylases (HDACs), are referred to as sirtuins. The founding member, ySir2 (yeast silent information regulator 2), homologous to human sirtuin 1 (SIRT1), is essential for maintaining silent chromatin via the deacetylation of histones. Activation or overexpression of SIRT1 has been shown to increase the lifespan of fly (Drosophila), yeast (Saccharomyces cerevisiae), worm (Caenorhabditis elegans) (up to 70%), and mouse (strain C57BL/6J) (10–15). Recently, it has been shown that SIRT1 plays an important role in a wide variety of processes, including stress resistance, metabolism, apoptosis, senescence, differentiation, and aging (9).
SIRT1 negatively regulates transcription factors, such as nuclear factor (NF)-κB, in the nucleus by the deacetylation of modified lysine residues on histones, transcription factors, and other nonhistone proteins (16–18). Recently, it has been shown that SIRT1 regulates NF-κB–dependent transcription and cell survival in response to tumor necrosis factor (TNF)-α (19). It has been suggested that SIRT1 deacetylase may directly bind to one or more constituents in the chromatin complex, resulting in structural reorganization, and therefore has the ability to establish silent chromatin domains (17). Evidence indicates that sirtuins have evolved to mediate signaling initiated by stress conditions such as metabolic alterations/nutritional deprivation and calorie restriction (20). However, environmental stress, such as CS exposure, has been shown to decrease the levels of SIRT1 dramatically both in macrophages in vitro and in rat lungs in vivo. This reduction has also been found to be associated with increased proinflammatory cytokine release (21). However, the regulation of SIRT1 in the lungs of smokers and patients with COPD, and its role in regulation of NF-κB–dependent proinflammatory cytokines is unknown.
The pathophysiology of COPD is multifactorial, with an inflammatory cell profile that includes macrophages, neutrophils, and T lymphocytes (6, 8, 22, 23). Macrophages are one of the most predominant inflammatory cell types involved in chronic inflammatory states, such as COPD, since they secrete both neutrophil and macrophage chemotactic factors, and related chemokines, and matrix metalloproteases (6, 24). The influx of macrophages in the lungs by cigarette smoking leads to increased expression of proinflammatory cytokines (24), which is now recognized to be an outcome of chromatin remodeling due to altered acetylation/deacetylation of histone proteins (25, 26). Therefore, the involvement of sirtuin in the regulation of proinflammatory cytokine gene expression by deacetylation of NF-κB subunit(s) in human macrophages appears to be highly possible. In light of this, we therefore hypothesized that SIRT1 regulates NF-κB–dependent proinflammatory cytokines in the lungs of smokers and patients with COPD, and that its function is altered by post-translational modifications by reactive components of CS. We therefore studied the levels and expression of SIRT1 in peripheral lungs of smokers with and without COPD, and determined the molecular regulation of SIRT1 by CS exposure in a monocyte–macrophage cell line (MonoMac6 cells). In addition, the role of SIRT1 in the regulation of proinflammatory cytokine release was also investigated.
METHODS
Materials
Unless otherwise stated, all reagents used in this study were purchased from Sigma (St. Louis, MO). Penicillin, streptomycin, and RPMI (Roswell Park Memorial Institute) 1640 were obtained from Life Technologies (Gaithersburg, MD). Rabbit polyclonal anti-SIRT1 (cat. no. Ab7343) and mouse monoclonal anti-SIRT1 (cat. no. 05-707) antibodies were procured from Abcam (Cambridge, MA) and Upstate (Lake Placid, NY), respectively. Antibodies against NF-κB–RelA/p65 (rabbit polyclonal; cat. no. sc-372), lamin B (goat polyclonal; sc-6216), and α-tubulin (mouse monoclonal; cat. no. sc-5286) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibodies against 4-hydroxy-2-nonenal (cat. no. 24327), nitrotyrosine (cat. no. 05-233), and β-actin (cat. no. CP01) were obtained from Oxis International (Foster City, CA), Upstate, and Calbiochem (La Jolla, CA), respectively. Human SIRT1 siRNA (cat. no. L-003540-00), nontarget scrambled siRNA (cat. no. D-001810-01) and DharmaFECT2 transfection reagent (cat. no. T-2002-01) were purchased from Dharmacon (Lafayette, CO). The antiacetylated RelA/p65 (AcK310) antibody (33) was provided by Dr. Leonard Buckbinder at Pfizer Global R&D (Groton, CT). Overexpression SIRT1 plasmid and SIRT1 construct lacking deacetylase domain (SIRT1-H363Y) were obtained from Addgene (Cambridge, MA).
Collection of Human Lung Tissues
Lung tissue specimens from 37 subjects/patients including 10 lifelong nonsmokers, 10 current smokers with normal lung function, and 9 patients with COPD (3 former and 6 current smokers; 2 patients had been prescribed inhaled steroids) undergoing resection for suspected lung tumor (either malignant or nonmalignant–local carcinoma or hamartoma), and 8 patients (former smokers; all had been prescribed inhaled and/or low-dosage oral corticosteroids) with severe COPD undergoing lung transplantation were collected (both COPD groups were pooled; see Table 1) from the Departments of Medicine and Pathology, Helsinki University Hospital. COPD was defined according to GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria (FEV1 < 80% of predicted, FEV1/FVC < 70% and bronchodilatation effect < 12%) (1). None of the patients had suffered from acute exacerbation for 2 months. Tumor-free peripheral lung tissues were immediately stored at −80°C for Western blot analysis and preserved for immunohistochemistry as described by Dail and colleagues (27). The clinical characteristics of the patients are shown in Table 1.
TABLE 1.
SUMMARY OF THE CLINICAL CHARACTERISTICS OF SUBJECTS AND PATIENTS
| Nonsmokers | Smokers | COPD | |
|---|---|---|---|
| Number | 10 | 10 | 17 |
| M:F ratio | 6:4 | 8:2 | 9:8 |
| Age, yr | 59 ± 15 | 58 ± 4 | 60 ± 7 |
| Smoking, pack-years | 0 | 34 ± 18* | 42 ± 19* |
| FEV1% predicted | 98 ± 4 | 97 ± 13 | 39 ± 27† |
| FEV1/FVC, % | 81 ± 3 | 81 ± 2 | 51 ± 23‡ |
| DlCO, % predicted | 80 ± 12 | 90 ± 10 | 54 ± 23‡ |
| DlCO/Va, % predicted | 86 ± 14 | 103 ± 13 | 70 ± 24 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; DlCO = diffusing capacity of lung for carbon monoxide; M:F = male:female.
Data shown represents mean ± SEM unless otherwise indicated.
P < 0.001 compared with nonsmokers.
P < 0.001 compared with nonsmokers and smokers without COPD.
P < 0.05 compared with nonsmokers and smokers without COPD.
MonoMac6 Cell Culture
Monocyte–macrophage cell line (mature monocytes) MonoMac6, which was established from peripheral blood of a patient with monoblastic leukemia (28, 29), was grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 μg/ml penicillin, 100 U/ml streptomycin, 1% nonessential amino acids, 1 mM sodium pyruvate, 1 μg/ml human holo-transferrin, 8 μg/ml polymixin B, 9 μg/ml bovine insulin, and 1 mM oxaloacetic acid. The cells were cultured at 37°C in a humidified atmosphere containing 7.5% CO2.
Preparation of CS Extract
CS extract (CSE) (10%) was prepared by bubbling smoke from one research grade cigarette (1R3F; University of Kentucky, Lexington, KY) into 10 ml of RPMI 1640 medium with 1% FBS, as described previously (21, 30, 31). See the online supplement for further details.
In Vitro Treatments
MonoMac6 cells were seeded at a density of 1.5 × 106 cells/well in six-well plates containing 2 ml of culture medium supplemented with 1% FBS, starved overnight, and then treated with different concentrations of CSE (0.1, 0.5, and 1.0%) at 37°C. After 1, 4, or 24 hours of treatment, the cells and culture media were harvested.
Experimental Procedures
The detailed procedures for Western blotting, immunoprecipitation, immunohistochemistry, immunocytochemistry, mRNA expression, and siRNA or cDNA transfection are provided in the online supplement.
Statistical Analysis
Statistical analysis of significance was calculated by one-way analysis of variance followed by Tukey's post hoc test, using StatView software (SAS Institute Inc., Cary, NC). Results are shown as mean ± SEM of at least three independent experiments.
Ethical Considerations
The study was approved by the ethics committee of Helsinki University Hospital District. The volunteers gave their written, informed consent.
RESULTS
Decreased Levels of Nuclear SIRT1 in Peripheral Lung Tissues of Smokers and Patients with COPD Were Associated with Increased Post-translational Modifications by Reactive Aldehydes and Nitric Oxide Products
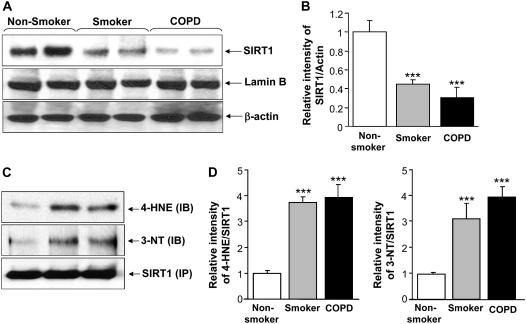
To determine the levels of SIRT1, the peripheral lung samples were collected from nonsmokers, smokers, and patients with COPD. Levels of nuclear SIRT1 were measured by Western blotting and normalized with the amount of β-actin (loading control). The levels were significantly lower (P < 0.001) in nuclear extracts of peripheral lung tissues of smokers and patients with COPD than in the lungs of nonsmokers (Figures 1A and 1B). The reduction in the levels of SIRT1 was more pronounced in patients with COPD compared with that of smokers. Similar reduction in SIRT1 activity was also observed in nuclear extracts (data not shown). In view of the presence of prooxidative and nitrosative components in CS and our observation of decreased SIRT1 levels in response to CSE (see above), we investigated whether oxidative/nitrosative protein modifications are involved in the observed reduction in SIRT1 levels. Post-translational/covalent modification of SIRT1 proteins was assessed by immunoprecipitation, followed by Western blot analysis using monoclonal antibodies for 4-hydroxy-2-nonenal (4-HNE) and 3-nitrotyrosine. There was a significant increase in tyrosine nitration and 4-HNE (carbonyl adducts) modification on SIRT1 in lungs of smokers and patients with COPD compared with nonsmokers (Figures 1C and 1D).
Figure 1.
Decreased levels of sirtuin (SIRT1) protein in lung tissue of smokers and patients with chronic obstructive pulmonary disease (COPD). (A) Western blot analysis of SIRT1 in soluble nuclear proteins (30 μg) extracted from the lung tissue of nonsmokers (n = 10), smokers (n = 10), and patients with COPD (n = 17). The proteins were electrophoresed on a 7.5% polyacrylamide gel electrophoresis and electroblotted onto a nitrocellulose membrane. The level of SIRT1 protein was determined using mouse monoclonal anti-SIRT1 antibody. The purity of nuclear extract was shown by the presence of lamin B (nuclear envelope protein) and the absence of the cytoskeletal protein α-tubulin (not shown). (B) After densitometric analysis, the values were normalized against the loading control, β-actin. The relative level (% of control) of SIRT1 showed decreased levels of nuclear SIRT1 protein in the lung tissues of smokers and patients with COPD. (C) SIRT1 protein was immunoprecipitated from the nuclear extract of lung homogenates. The levels of SIRT1 adducts with 4-hydroxy-2-nonenol (4-HNE) and nitration of tyrosine residues on SIRT1 were analyzed by immunoblotting with anti–4-HNE and anti–3-nitrotyrosine (3-NT) antibodies, respectively. Equal amount of immunoprecipitated SIRT1 protein (100 μg) was used for Western blotting. (D) Relative intensity of 4-HNE/SIRT1 and 3-NT/SIRT1 represents the increased post-translational modifications of SIRT1 protein in lungs of smokers and patients with COPD compared with nonsmokers. A representative blot is shown, which was obtained from several blotting experiments. Results are expressed as mean ± SEM. ***P < 0.001, significant compared with nonsmokers.
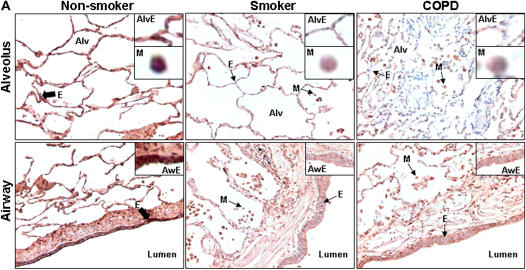
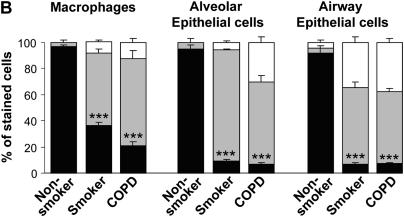
Immunohistochemical staining of fixed peripheral lung tissues confirmed the decrease in the levels of SIRT1 in macrophages and airway/alveolar epithelial cells both in the lungs of smokers and in patients with COPD when compared with nonsmokers (Figures 2A and 2B). These data suggest that the level of SIRT1 is decreased by oxidative and/or proinflammatory effects of CS both in smokers and in patients with COPD.
Figure 2.
Decreased staining of sirtuin (SIRT1) in lung macrophages and alveolar/airway epithelial cells of smokers with and without chronic obstructive pulmonary disease (COPD). (A) Representative photographs (original magnification, ×400) from immunostaining for SIRT1 in lung tissues from nonsmokers (n = 10) and smokers with (n = 17) and without (n = 10) COPD. The levels of SIRT1 were measured in the fixed lung sections (3-μm thick) by immunohistochemical staining using rabbit polyclonal anti-SIRT1 antibody (1:100 dilution) with avidin–biotin–peroxidase complex method followed by hematoxylin counter staining. Dark brown color represents the presence of SIRT1 (indicated with thick arrow), which was decreased in smokers' lung (indicated with thin arrow). Alv = alveoli; Aw = airway; E = epithelial cells; M = macrophage. (B) Immunostaining scores for SIRT1 per cell type in alveolar and airway regions of the lung. The assessment of immunostaining intensity was performed semiquantitatively and in a blinded fashion. Solid bars, intense staining; shaded bars, moderate/weak staining; open bars, no staining. Results are represented as mean ± SEM. ***P < 0.001, significant compared with nonsmokers.
Decreased Level of SIRT1 Was Associated with Increased Activation of RelA/p65 NF-κB in the Lungs of Smokers and Patients with COPD
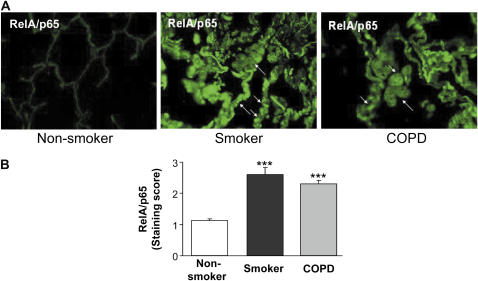
To determine whether decreased levels of SIRT1 are associated with the expression of NF-κB–dependent proinflammatory cytokines, the level of RelA/p65 subunit of NF-κB was assessed in the peripheral lung tissues of smokers and patients with COPD, and compared with nonsmokers. The expression of NF-κB was increased in lung macrophages and epithelial cells of smokers and patients with COPD as compared with nonsmokers (Figures 3A and 3B), suggesting that SIRT1 reduction is associated with NF-κB activation.
Figure 3.
Decreased levels of sirtuin (SIRT1) in smokers and patients with chronic obstructive pulmonary disease (COPD) were associated with increased levels of RelA/p65 nuclear factor (NF)-κB. The expression of NF-κB was measured in fixed lung sections (3-μm thick) of nonsmokers (n = 10), and smokers with (n = 17) and without (n = 10) COPD by immunohistochemical staining using anti-RelA/p65 NF-κB antibody (1:100 dilution) followed by incubations with fluorescein isothiocyanate conjugated anti-rabbit secondary antibody. Representative immunofluorescent images (original magnification, ×400) showed increased levels of NF-κB in the lungs (especially in macrophages and epithelial cells) of smokers with and without COPD as compared with nonsmokers. Arrows indicate the cells (airway/alveolar epithelial cells and macrophages) that express increased levels of RelA/p65 NF-κB proteins. (B) Immunostaining score for RelA/p65 was performed semiquantitatively and in a blinded fashion: 0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = intense staining. Results are expressed as mean ± SEM. ***P < 0.001, significant compared with nonsmokers.
SIRT1 Protein Levels and mRNA Expression Were Reduced by CSE Treatment in MonoMac6 Cells
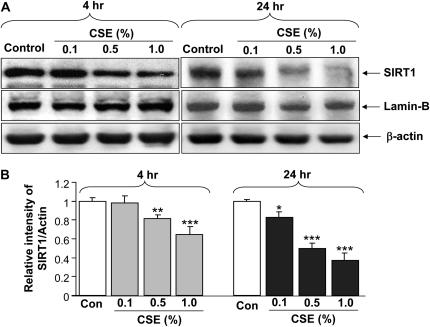
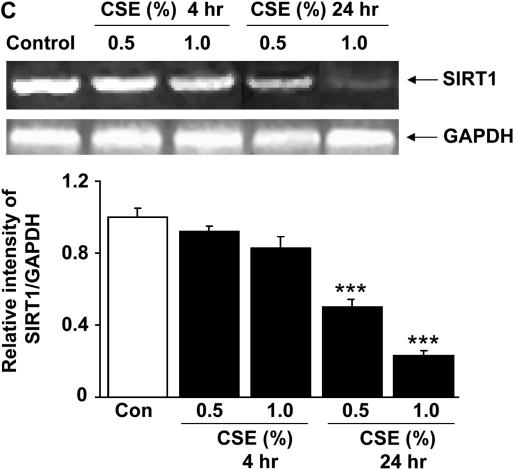
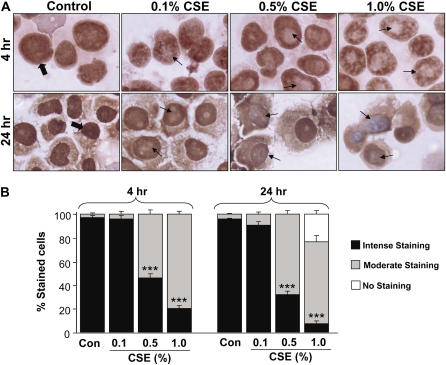
To determine the molecular regulation of decreased SIRT1 levels in lungs (predominantly in macrophages) of smokers and patients with COPD, the effect of CSE on SIRT1 levels was investigated in a human monocyte–macrophage cell line (MonoMac6). CSE (0.1, 0.5, and 1.0%) decreased nuclear SIRT1 protein levels at 4 and 24 hours of treatment (Figures 4A and 4B). Similar reduction in SIRT1 activity was also observed in response to CSE treatments in MonoMac6 cells (data not shown). To further characterize the mechanism of CS-mediated decrease in SIRT1 levels, we examined the level of SIRT1 mRNA in CSE-treated MonoMac6 cells using qualitative reverse transcriptase–polymerase chain reaction (RT-PCR). CSE produced marked decreases in SIRT1 mRNA levels in macrophages at 24 hours but not at 4 hours (Figure 4C). Immunocytochemical staining for SIRT1 confirmed the localization of SIRT1 in the nucleus of MonoMac6 cells, which were dramatically decreased in response to CSE treatments (Figures 5A and 5B). This finding suggests that CS-mediated modification of SIRT1 occurs at earlier time points (4 h), which leads to its degradation, whereas the reduction of mRNA level occurs at later time points (24 h). This finding corroborates with our results that CSE decreased SIRT1 protein levels in MonoMac6 cells at 4 hours. Thus, it appears that decrease in SIRT1 levels/enzyme activity due to CS (post-translational effect) might precede the transcriptional effects (see below).
Figure 4.
Decreased levels of sirtuin (SIRT1) protein and mRNA expression by cigarette smoke extract (CSE) treatment in monocyte–macrophage (MonoMac6) cells. (A) Western blots of soluble nuclear proteins (30 μg) extracted from CSE-exposed MonoMac6 cells. Expression of SIRT1 was determined using mouse monoclonal anti-SIRT1 antibody. The purity of nuclear extract was shown by the presence of lamin B (nuclear envelope protein) and the absence of the cytoskeletal protein α-tubulin (bands not shown). Gel pictures shown are representative of at least three separate experiments. (B) Densitometric values of SIRT1 were normalized against the loading control, β-actin. The relative level (% of control) of SIRT1 in MonoMac6 cells showed decreased levels of SIRT1 protein in response to CSE at 4 and 24 hours. (C) CSE decreased the levels of SIRT1 mRNA in MonoMac6 cells. After 4 and 24 hours of CSE treatment, total RNA was extracted from MonoMac6 cells using RNeasy kit (Qiagen, Germantown, MD). Reverse transcriptase–polymerase chain reaction was performed. Amplified products (SIRT1: 200 bp; GAPDH: 600 bp) were resolved by 1.5% agarose gel electrophoresis, stained with ethidium bromide. SIRT1 mRNA expression was decreased after 24-hour exposure to low concentrations of CSE (0.5 and 1%) compared with control. Data represent mean ± SEM of three experiments (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with control values.
Figure 5.
Decreased sirtuin (SIRT1) protein staining in response to cigarette smoke extract (CSE) treatment in monocyte–macrophage (MonoMac6) cells. (A) CSE decreased the levels of SIRT1 in MonoMac6 cells at 4 and 24 hours. MonoMac6 cells were treated with different concentrations of CSE (0.1–1.0%). Cells were harvested and the cytospin slides were prepared at 4 and 24 hours of treatments. Immunostaining was performed using a rabbit polyclonal antibody specific for SIRT1 followed by the avidin–biotin–peroxidase complex method, and counterstained with hematoxylin. The dark brown color represents the presence of SIRT1, which was decreased in response to CSE treatment. (B) Graph showing the percentage of SIRT1-positive cells from the total number of cells in CSE-treated MonoMac6 cells. The assessment of immunostaining intensity was performed semiquantitatively and in a blinded fashion. Results shown are means ± SEM of three separate experiments (n = 3). ***P < 0.001, significant compared with control values.
CSE Induced IL-8 Release in MonoMac6 Cells
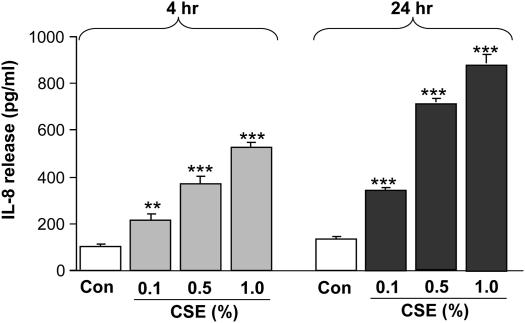
To determine the effect of CS on proinflammatory cytokine release in vitro, MonoMac6 cells were exposed to different concentrations of CSE. Culture media were collected to assay the IL-8 release by ELISA. CSE (0.1, 0.5, and 1.0%) significantly increased IL-8 release from these cells at 4 (P < 0.01 or P < 0.001) and 24 hours (P < 0.001) of treatment (Figure 6). Assay of lactate dehydrogenase leakage showed no cytotoxicity of CSE (0.1–1.0%) in MonoMac6 cells at 4 and 24 hours. These results confirmed the proinflammatory effect of CS, and the increased release of proinflammatory cytokines was associated with decreased levels of SIRT1 in MonoMac6 cells.
Figure 6.
Cigarette smoke extract (CSE) induced IL-8 release from monocyte–macrophage (MonoMac6) cells. MonoMac6 cells were treated with freshly prepared CSE (0.1, 0.5, and 1.0%) for 4 and 24 hours. IL-8 release was measured in the culture media by sandwich ELISA duo-antibody kit (R&D Systems, Minneapolis, MN). CSE showed increase in the levels of IL-8 as compared with controls at 4 and 24 hours. Each histogram represents the mean ± SEM of three experiments (n = 3). **P < 0.01, ***P < 0.001, significant compared with controls.
SIRT1 Deacetylase Regulates IL-8 Release from MonoMac6 Cells in Response to CSE
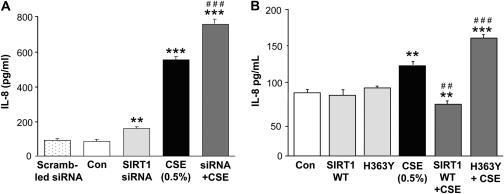
To characterize the role of SIRT1 in CS-mediated IL-8 release, we knocked down the endogenous SIRT1, overexpressed SIRT1 or its mutant lacking catalytic activity (SIRT1 H363Y lacking deacetylase domain) in MonoMac6 cells, which were then treated with CSE (0.5%) for 4 or 12 hours. SIRT1 levels were decreased by SIRT1 siRNA transfection (∼60%, data not shown; without any change in SIRT2 or SIRT3 levels) and/or CSE treatment. Knockdown of SIRT1 or CSE treatment resulted in significantly (P < 0.001) increased IL-8 release in MonoMac6 cells as compared with control and nonscrambled siRNA transfected groups. There was a further increase in the level of IL-8 release in CSE-treated SIRT1 siRNA-transfected cells as compared with CSE-treated cells alone (Figure 7A). Overexpression of SIRT1 or H363Y did not show any significant change in IL-8 release at 4 hours, but in response to CSE treatment, overexpression of SIRT1 decreased the IL-8 release, whereas H363Y increased the IL-8 release as compared with the CSE-treated group (Figure 7B). Taken together, these data show a negative trend in the levels/deacetylase activity of SIRT1 and IL-8 release, further confirming the involvement of SIRT1 in CS-mediated IL-8 release.
Figure 7.
Cigarette smoke extract (CSE)–mediated IL-8 release was modified by sirtuin (SIRT1) knockdown, mutation, and overexpression in monocyte–macrophage (MonoMac6) cells. (A) MonoMac6 cells were transfected with predesigned human SIRT1 siRNA or scrambled nontarget siRNA using DharmaFect2 siRNA transfection reagent. After 36–48 hours of transfection (cells > 85% viable), the cells were treated with CSE (0.5%) for 12 hours. At the end of the experiment, culture media was collected by centrifugation for IL-8 assay. SIRT1 knockdown led to significant increase in IL-8 release in response to CSE treatment in MonoMac6 cells as compared with nontarget scrambled siRNA. (B) MonoMac6 cells were transfected with SIRT1 overexpressing plasmid or SIRT1-H363Y (mutated in the deacetylase domain) using the calcium phosphate method. Overexpression of SIRT1 deceased IL-8 release, whereas SIRT1-H363Y lacking SIRT1 deacetylase domain increased IL-8 release in response to CSE treatment at 4 hours. Each value is the mean ± SEM of triplicate determinations (n = 3). **P < 0.001, ***P < 0.001, significant compared with control; ##P < 0.01, ###P < 0.001, significant compared with CSE-treated group.
SIRT1 Is Post-translationally Modified by CSE-derived Reactive Oxygen/Nitrogen Species and Reactive Aldehydes in MonoMac6 Cells
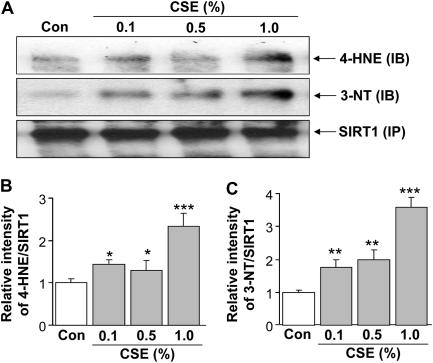
Based on above data of post-translational modifications of SIRT1 by reactive aldehydes and nitric oxide in lungs of smokers and patients with COPD, we determined whether CSE is directly involved in such modifications by its reactive components in MonoMac6 cells. Indeed, our data show post-translational modification of SIRT1 protein by 4-HNE and 3-nitrotyrosine in response to CSE in MonoMac6 cells, confirming the direct involvement of CS components, rather than inflammatory cell reactive oxygen species (ROS) production, in covalent modification of SIRT1 (Figures 8A and 8B).
Figure 8.
Cigarette smoke extract (CSE) caused post-translational modifications of sirtuin (SIRT1). (A) SIRT1 protein was immunoprecipitated from the nuclear extract of monocyte–macrophage (MonoMac6) cells treated with CSE (0.1, 0.5, and 1.0%) for 4 hours. The levels of SIRT1 adducts with 4-hydroxy-2-nonenol (4-HNE) and nitration of tyrosine residues on SIRT1 were analyzed by immunoblotting with anti–4-HNE and anti–3-nitrotyrosine (3-NT) antibodies, respectively. Equal amount of immunoprecipitated SIRT1 protein (100 μg) was used for Western blotting. Relative intensity of 4-HNE/SIRT1 (B) and 3-NT/SIRT1 (C) represents the increased post-translational modifications of SIRT1 protein in response to CSE treatment. Results are means ± SEM of three separate experiments (n = 3). Significant differences are shown as compared to controls: *P < 0.05, **P < 0.01, and ***P < 0.001.
CS-mediated Reduction of SIRT1 Was Associated with Increased Acetylation and Activation of RelA/p65 NF-κB
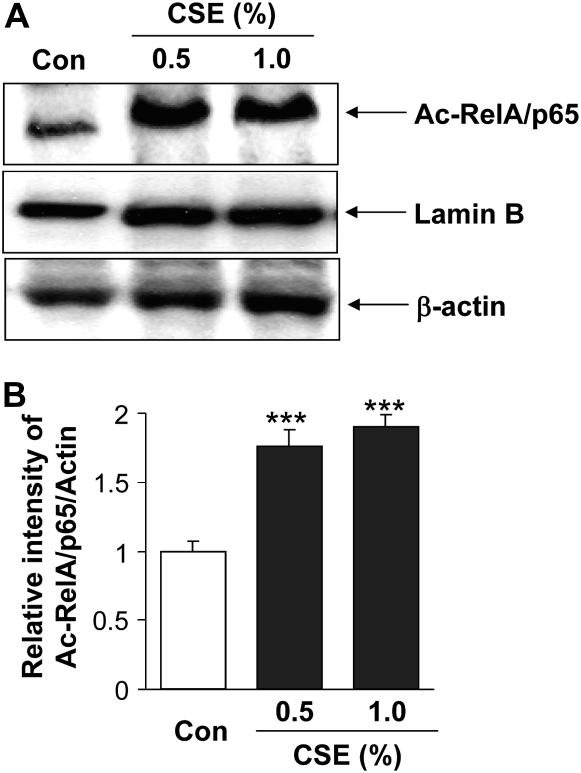
To examine the relationship between CSE-mediated acetylation/activation of NF-κB and IL-8 release, MonoMac6 cells were treated with CSE (0.5 and 1.0%) and analyzed for acetylated RelA/p65 using a specific acetylated lysine 310 residue (K310) antibody for RelA/p65. Acetylation of RelA/p65 at lysine 310 residue is critical for sustained proinflammatory cytokine release (32, 33). Western blot analysis revealed that CSE significantly increased the acetylation and thereby activation of RelA/p65 (Figures 9A and 9B). CSE treatment decreased the SIRT1 deacetylase level (Figures 4 and 5), which was associated with increased acetylation of RelA/p65 levels in the nucleus of MonoMac6 cells.
Figure 9.
Cigarette smoke extract (CSE)–mediated decrease in sirtuin (SIRT1) level was associated with increased acetylation of RelA/p65 nuclear factor (NF)-κB. (A) Monocyte–macrophage (MonoMac6) cells were treated with CSE (0.5 and 1.0%) for 4 hours. Acetylation of the lysine residue (K310) on RelA/p65 NF-κB protein was determined in soluble nuclear proteins (30 μg) by Western blotting using anti–acetyl RelA/p65 (K310) antibody. β-Actin was measured as a loading control. Lamin B (nuclear envelope protein) and the absence of the cytoskeletal protein α-tubulin (bands not shown) were measured to confirm the purity of nuclear extracts. (B) The relative density (% of control) of acetylated RelA/p65 NF-κB in nuclear fraction of MonoMac6 cells showed increased acetylation of RelA/p65 NF-κB in response to CSE treatment at 4 hours. Each histogram represents the means ± SEM (n = 3). ***P < 0.001, compared with control values.
Depletion of SIRT1 Leads to Augmentation of CSE-mediated Acetylation of RelA/p65 NF-κB
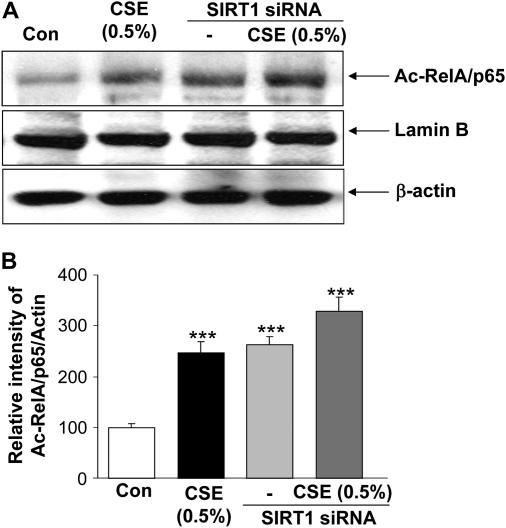
Further experiments were performed to determine whether SIRT1 regulates acetylation/activation of NF-κB, because it is known that SIRT1 interacts with RelA/p65 NF-κB (21), and the depletion of SIRT1 was associated with increased expression of RelA/p65 NF-κB in the peripheral lungs of smokers and patients with COPD (Figure 3). SIRT1 knockdown alone significantly (P < 0.001) increased the acetylation of RelA/p65 NF-κB in MonoMac6 cells. In combination with CSE treatment, SIRT1 knockdown augmented the acetylating effect of CSE on RelA/p65 (Figures 10A and 10B). This suggested that SIRT1 regulates the acetylation and activation of RelA/p65 NF-κB (lysine 310 residue) in the nucleus.
Figure 10.
siRNA silencing of sirtuin (SIRT1) augmented the cigarette smoke extract (CSE)–mediated acetylation of RelA/p65 nuclear factor (NF)-κB. Monocyte–macrophage (MonoMac6) cells were transfected with predesigned human SIRT1 siRNA duplex (100 nM) using DharmaFect2 transfection reagent for 36–48 hours and then treated with CSE (0.5%) for 12 hours. Nontargeting scrambled siRNA was used as a negative control. Actin was measured as a loading control. (A) Acetylation of RelA/p65 NF-κB was determined using rabbit anti-Ac-RelA/p65 (K310) antibody in the soluble nuclear extract. The purity of nuclear extract was shown by the presence of lamin B (nuclear envelope protein) and the absence of the cytoskeletal protein α-tubulin (bands not shown). (B) The relative level (% of control) of Ac-RelA/p65 showed increased acetylation of nuclear RelA/p65 in response to SIRT1 knockdown and/or CSE treatment. Each value is the mean ± SEM of triplicate determinations (n = 3). ***P < 0.001, significant compared with control.
DISCUSSION
Recent focus on COPD research is to understand the factors or molecular mechanisms involved in underlying pathogenesis of lung inflammation in smokers who are susceptible to COPD. We have previously shown that proinflammatory cytokine release was increased in a human monocytic–macrophage cell line (MonoMac6) in vitro and in rat lung in vivo in response to CS exposure (21, 34). This was associated with increased NF-κB activation and acetylation of histone proteins (25, 31). However, the molecular mechanisms of CS-mediated lung inflammation, particularly in patients with COPD, are not completely understood. This study was focused on the role of class III deacetylases, SIRT1, which is an important protein involved in deacetylation of proteins/histones, regulation of proinflammatory cytokine release, apoptosis, stress resistance, metabolism, senescence, differentiation, and aging (19, 21, 35, 36)—all of which are linked to the pathogenesis of COPD (4–6, 8, 24). The levels of SIRT1 were decreased in peripheral lungs, particularly in alveolar macrophages, airway epithelium, and in alveolar epithelium of smokers and patients with COPD as compared with nonsmokers. Because SIRT1 is involved in the regulation of NF-κB (19, 21), the decreased levels of SIRT1 may result in an NF-κB–mediated abnormal chronic inflammatory effect, which is observed in lungs of smokers and patients with COPD. Consistent with this notion, we observed the decreased levels of SIRT1 and increased activation of RelA/p65 in peripheral lungs of smokers and patients with COPD. The importance of SIRT1 further gains credence from the observation of McBurney and colleagues (37) that genetic ablation of SIRT1 leads to increased neutrophil infiltration in mouse lung, suggesting that knockdown of SIRT1 leads to exaggerated lung inflammation. Therefore, it is possible that CS-mediated reduction in SIRT1 may in part be responsible for increased neutrophil influx, NF-κB activation, and inflammatory response seen in lungs of smokers and patients with COPD.
We further determined the molecular mechanism of SIRT1 reduction and its role in proinflammatory cytokine release in response to CS exposure in human macrophage-like cells (MonoMac6) in vitro. Alveolar macrophages are considered to be important cells in perpetuating the inflammatory response of CS (6, 24, 38). CSE treatment significantly increased the release of proinflammatory cytokine IL-8 concomitant with decreased levels of SIRT1 protein and mRNA expression in MonoMac6 cells. Recently, Yang and coworkers (21) reported that CS-mediated decrease in SIRT1 levels was correlated with decreased SIRT1 activity in macrophages and rat lungs. Because this decrease was correlated with increased release of IL-8, we speculated that CSE-mediated decrease in the levels of SIRT1 plays an important role in release of proinflammatory cytokines. To support this observation and to determine the specific effect of SIRT1 in CS-induced proinflammatory cytokine release, further experiments were performed on MonoMac6 cells by knocking down SIRT1 or overexpressing SIRT1 as well as using a SIRT1 defective mutant lacking deacetylase activity. Knockdown of endogenous SIRT1 and mutation of SIRT1 deacetylase domain augmented the CS-stimulated proinflammatory cytokine (IL-8) release, whereas SIRT1 overexpression resulted in decreased IL-8 release in response to CSE exposure. We have previously shown that pharmacologic activation of SIRT1 reduced the CSE-mediated IL-8 release in MonoMac6 cells (21) and the present findings further support these observations and emphasize the importance of SIRT1 in regulation of proinflammatory mediators, such as IL-8 and other NF-κB–dependent genes (matrix metalloproteinases, growth factors, and mucin genes). The mechanism whereby CS alters the levels of SIRT1 is not known, but it is possible that SIRT1 is regulated by post-translational modifications and/or by kinase signaling mechanisms. The other possible mechanism would be nucleocytoplasmic shuttling of SIRT1 by kinase signaling mechanism leading to proteasomal degradation of SIRT1 in the cytoplasm.
It is well known that CS-induced oxidative stress is responsible for proinflammatory cytokine release in the lung (39). Previously, we have shown that levels of lipid peroxidation products, such as 4-HNE, were increased in lungs of patients with COPD (40). Post-translational modifications of various proteins by oxidative/nitrosative stress have been shown to have influence on various cellular functions (25, 41, 42). To determine the CS-mediated reduction in SIRT1 levels and its post-translational modifications, SIRT1 modification was evaluated by measuring the SIRT1 adducts with 4-HNE (reactive aldehydes which form protein carbonyls), a highly reactive diffusible product of lipid peroxidation and a key mediator of oxidant-induced cell signaling and apoptosis (43). SIRT1 adducts with 4-HNE and 3-nitrotyrosine in lungs were increased in smokers and patients with COPD compared with nonsmokers. CSE induced the formation of SIRT1–4-HNE adducts in MonoMac6 cells. Cysteine, histidine, and lysine, the three nucleophilic amino acids, have been shown to be the target of modification by 4-HNE (43). The high affinity of 4-HNE toward lysine becomes important with respect to SIRT1, because lysine residues 1020/1024 present on the active site domain of SIRT1 may be the direct target of 4-HNE. Thus, the increased SIRT1–4-HNE adduct formation seen after smoke exposure may therefore form a part of the mechanism responsible for the reduction in SIRT1 activity/level. However, it may also be possible that other residues, such as cysteine(s), present on SIRT1 are involved in formation of SIRT1–4-HNE adducts. Both oxidation and nitration can damage proteins; nitration of protein tyrosine residues to form 3-nitrotyrosine is considered a hallmark of tissue injury caused by inflammation (41, 42). Post-translationally modified proteins can be a direct target of proteolytic degradation and removal (44). The increased SIRT1 protein tyrosine nitration seen after CSE exposure in MonoMac6 cells may trigger increased proteolytic degradation of this protein, resulting in decreased SIRT1 levels. Thus, the decreased SIRT1 levels in smokers and patients with COPD may be explained on the basis of the CS-mediated oxidative/nitrosative (which occurs in patients with COPD) alterations on the SIRT1 proteins. In view of the fact that SIRT1 is an antiaging and antiinflammatory molecule (9, 15), the CS-mediated SIRT1 modification/reduction may have a role in lung inflammation and aging seen in patients with COPD (3, 4, 7). However, it remains to be determined whether SIRT1 reduction is directly associated with the decline in lung function in smokers or disease progression/severity of COPD. Because some of the patients with COPD were ex-smokers and some of them were receiving inhaled steroids, it is likely that, once initiated, the alterations of SIRT1 may not be fully reversible (irreversible epigenetic events), which in turn might be one contributor to the persistence of many inflammatory changes observed even after cessation of smoking.
Reduction/inactivation of other deacetylases, such as HDACs, has been reported to cause transactivation of NF-κB and induction of proinflammatory cytokine release (25, 31, 34). Recently, Yeung and colleagues (19) demonstrated that SIRT1 physically interacts with the RelA/p65 subunit of NF-κB and inhibits gene transcription by deacetylating RelA/p65 at lysine 310, suggesting that acetylated lysine 310 might form a platform for the binding of a bromodomain-containing protein that is required for full transcriptional activity of RelA/p65 (45, 46). Because acetylation at lysine 310 is required for full transactivation function of RelA/p65 (32, 33), the levels of acetylated lysine 310 moiety of RelA/p65 subunit of NF-κB in CSE-treated MonoMac6 cells were determined. It was found that CS-mediated SIRT1 reduction was associated with increased acetylation of lysine 310 moiety on RelA/p65. SIRT1 knockdown also increased the acetylation of RelA/p65 (K310) acetylation and this effect was further augmented by the acetylation effect of CS on RelA/p65. These data indicate that CSE caused acetylation of RelA/p65 NF-κB by reduction of SIRT1 deacetylase level and/or post-translational modifications of its lysine residues. Therefore, increased post-translational modifications of SIRT1 lead to disruption of RelA/p65-SIRT1 complex, which would then culminate into NF-κB acetylation and persistent activation of NF-κB in response to CSE exposure in macrophages. Apart from NF-κB regulation, SIRT1 also regulates the stress/protective pathway via deacetylation of the forkhead box class (FOXO3) transcription factor. SIRT1 reduction leads to acetylation of FOXO3, which would then result in loss of its transcription activity for transcription of GADD45 (DNA repair) and MnSOD genes (9). Therefore, loss of SIRT1 by CS will lead to acetylation of FOXO3 and tumor suppressor p53, resulting in lung cell senescence and apoptosis.
In conclusion, we have shown for the first time that the level of nuclear SIRT1 protein was decreased in peripheral lung tissue of smokers and patients with COPD. SIRT1 proteins undergo post-translational covalent modifications in response to CS exposure, which may not be fully reversible. These changes render SIRT1 inactive, leading to acetylation/activation of RelA/p65, and thereby uncontrolled expression of proinflammatory mediators, which is seen in macrophages/lungs of smokers and patients with COPD. In view of the role of SIRT1 in regulation of proinflammatory mediators, apoptosis, senescence, cell survival, differentiation, and aging, it is tempting to propose that CS-mediated alterations in SIRT1 would have ramifications on these processes that are directly linked to the pathogenesis of COPD (4–6, 8, 24). Further studies are required to understand the mechanism of CS-mediated down-regulation of SIRT1 and its involvement in chronic inflammatory and injurious processes in the lung using genetic gain and loss of function, and whether up-regulation or genetic modifications of SIRT1 can attenuate such processes in animal models of COPD.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Leonard Buckbinder (Pfizer Global R&D, Groton, CT) for the kind gift of Ac RelA/p65 (K310) NF-κB antibody, and Dr. Kaisa Salmenkivi and Mrs. Tiina Marjomaa for collecting the tissue specimens.
Supported by the National Institute of Environmental Health Sciences, Environmental Health Sciences Center grant ES-01247; funding from the Helsinki University Hospital; and the Finnish Antituberculosis Association Foundation.
This article has an online supplement, which is accessible at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200708-1269OC on January 3, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med 2001;163:1256–1276. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997;349:1269–1276. [DOI] [PubMed] [Google Scholar]
- 3.Vogelmeier C, Bals R. Chronic obstructive pulmonary disease and premature aging. Am J Respir Crit Care Med 2007;175:1217–1218. [DOI] [PubMed] [Google Scholar]
- 4.Tuder RM. Aging and cigarette smoke: fueling the fire. Am J Respir Crit Care Med 2006;174:490–491. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med 2000;343:269–280. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003;22:672–688. [DOI] [PubMed] [Google Scholar]
- 7.Burchfiel CM, Marcus EB, Curb JD, Maclean CJ, Vollmer WM, Johnson LR, Fong KO, Rodriguez BL, Masaki KH, Buist AS. Effects of smoking and smoking cessation on longitudinal decline in pulmonary function. Am J Respir Crit Care Med 1995;151:1778–1785. [DOI] [PubMed] [Google Scholar]
- 8.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 9.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J 2007;404:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 2000;289:2126–2128. [DOI] [PubMed] [Google Scholar]
- 11.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001;410:227–230. [DOI] [PubMed] [Google Scholar]
- 12.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem 2002;277:45099–45107. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature 2003;423:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003;425:191–196. [DOI] [PubMed] [Google Scholar]
- 15.Trapp J, Jung M. The role of NAD+ dependent histone deacetylases (sirtuins) in ageing. Curr Drug Targets 2006;7:1553–1560. [DOI] [PubMed] [Google Scholar]
- 16.Sauve AA, Schramm VL. SIR2: the biochemical mechanism of NAD(+)-dependent protein deacetylation and ADP-ribosyl enzyme intermediates. Curr Med Chem 2004;11:807–826. [DOI] [PubMed] [Google Scholar]
- 17.Grubisha O, Smith BC, Denu JM. Small molecule regulation of Sir2 protein deacetylases. FEBS J 2005;272:4607–4616. [DOI] [PubMed] [Google Scholar]
- 18.Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem 2006;75:435–465. [DOI] [PubMed] [Google Scholar]
- 19.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004;23:2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guarente L, Picard F. Calorie restriction–the SIR2 connection. Cell 2005;120:473–482. [DOI] [PubMed] [Google Scholar]
- 21.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol 2007;292:L567–L576. [DOI] [PubMed] [Google Scholar]
- 22.Jeffery PK. Comparison of the structural and inflammatory features of COPD and asthma. Giles F. Filley Lecture. Chest 2000;117:251S–260S. [DOI] [PubMed] [Google Scholar]
- 23.Saetta M, Turato G, Baraldo S, Zanin A, Braccioni F, Mapp CE, Maestrelli P, Cavallesco G, Papi A, Fabbri LM. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med 2000;161:1016–1021. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro SD. The macrophage in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:S29–S32. [DOI] [PubMed] [Google Scholar]
- 25.Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, Donaldson K, Macnee W, Rahman I. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol 2004;31:633–642. [DOI] [PubMed] [Google Scholar]
- 26.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 2005;352:1967–1976. [DOI] [PubMed] [Google Scholar]
- 27.Dail DH, Liebow AA, Gmelich JT, Friedman PJ, Miyai K, Myer W, Patterson SD, Hammar SP. Intravascular, bronchiolar, and alveolar tumor of the lung (IVBAT): an analysis of twenty cases of a peculiar sclerosing endothelial tumor. Cancer 1983;51:452–464. [DOI] [PubMed] [Google Scholar]
- 28.Moesby L, Jensen S, Hansen EW, Christensen JD. A comparative study of Mono Mac 6 cells, isolated mononuclear cells and Limulus amoebocyte lysate assay in pyrogen testing. Int J Pharm 1999;191:141–149. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler-Heitbrock HW, Thiel E, Futterer A, Herzog V, Wirtz A, Riethmuller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer 1988;41:456–461. [DOI] [PubMed] [Google Scholar]
- 30.Carp H, Janoff A. Possible mechanisms of emphysema in smokers: in vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis 1978;118:617–621. [DOI] [PubMed] [Google Scholar]
- 31.Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J 2004;18:1897–1899. [DOI] [PubMed] [Google Scholar]
- 32.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J 2002;21:6539–6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol 2005;25:7966–7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol 2006;291:L46–L57. [DOI] [PubMed] [Google Scholar]
- 35.Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell 2004;16:93–105. [DOI] [PubMed] [Google Scholar]
- 36.Clayton AL, Hazzalin CA, Mahadevan LC. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell 2006;23:289–296. [DOI] [PubMed] [Google Scholar]
- 37.McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol 2003;23:38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes PJ. Alveolar macrophages as orchestrators of COPD. COPD 2004;1:59–70. [DOI] [PubMed] [Google Scholar]
- 39.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 2006;28:219–242. [DOI] [PubMed] [Google Scholar]
- 40.Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:490–495. [DOI] [PubMed] [Google Scholar]
- 41.Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM, Keller JN. Protein oxidation and cellular homeostasis: emphasis on metabolism. Biochim Biophys Acta 2007;1773:93–104. [DOI] [PubMed] [Google Scholar]
- 42.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys 1998;356:1–11. [DOI] [PubMed] [Google Scholar]
- 43.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 1991;11:81–128. [DOI] [PubMed] [Google Scholar]
- 44.Souza JM, Choi I, Chen Q, Weisse M, Daikhin E, Yudkoff M, Obin M, Ara J, Horwitz J, Ischiropoulos H. Proteolytic degradation of tyrosine nitrated proteins. Arch Biochem Biophys 2000;380:360–366. [DOI] [PubMed] [Google Scholar]
- 45.Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain. Nature 1999;399:491–496. [DOI] [PubMed] [Google Scholar]
- 46.Polesskaya A, Naguibneva I, Duquet A, Bengal E, Robin P, Harel-Bellan A. Interaction between acetylated MyoD and the bromodomain of CBP and/or p300. Mol Cell Biol 2001;21:5312–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.