Abstract
The spatial arrangement and chemical reactivity of the activation-dependent thiol in the mitochondrial Complex I was studied using the membrane penetrating N-ethylmaleimide (NEM) and non-penetrating anionic 5,5′-dithiobis-(2-nitrobenzoate) (DTNB) as the specific inhibitors of the enzyme in mitochondria and inside-out submitochondrial particles (SMP). Both NEM and DTNB rapidly inhibited the de-activated Complex I in SMP. In mitochondria NEM caused rapid inhibition of Complex I, whereas the enzyme activity was insensitive to DTNB. In the presence of the channel-forming antibiotic alamethicin, mitochondrial Complex I became sensitive to DTNB. Neither active nor de-activated Complex I in SMP was inhibited by oxidized glutathione (10 mM, pH 8.0, 75 min). The data suggest that the active/de-active transition sulfhydryl group of Complex I which is sensitive to inhibition by NEM is located at the inner membrane–matrix interface. These data include the sidedness dependency of inhibition, effect of pH, ionic strength, and membrane bilayer modification on enzyme reactivity towards DTNB and its neutral analogue.
Keywords: NADH:ubiquinone oxidoreductase, Respiratory Complex I, Active/de-active transition, Sulfhydryl group, Mitochondria
1. Introduction
The mitochondrial NADH:ubiquinone oxidoreductase (respiratory Complex I) serves as the main entry point for reducing equivalents to the respiratory chain and as an energy-transducing device. Mammalian Complex I is composed of at least 46 different polypeptides [1]. In bacteria, structurally and functionally homologous enzymes (NDH-1) that bound to the plasma membrane comprises a minimal set of 13−14 subunits [2–4]. The number of subunits in Complex I in other eukaryotes different in evolutionary lineages has been recently determined: fungi, Yarrowia lipolytica (37) [5] and Neurospora crassa (39) [6], algae, Chlamydomonas reinhardtii (31) [7], and plant, Arabidopsis thaliana (30) [8]. The basic catalytic properties of the mammalian [9], fungal [10] and bacterial [11] enzymes are remarkably similar. These include turnover number, Km and Ki for substrates and inhibitors and also thermodynamic efficiency of energy transduction [12,13]. The functional role of the ∼16−32 supernumerary subunits in eukaryotic Complexes I thus remains to be established.
The central position of Complex I in the metabolic network implies that the enzymes activity must be finely regulated both in long-term scale at the level of its biogenesis and in shorter-term scale via covalent modification or alteration of its catalytic capacity by certain specific ligands. An obvious although somewhat general suggestion, for the function of the supernumerary subunits is that they are required for the enzymes regulation. Little is known, however, about the regulatory mechanisms of Complex I. Evidence has been reported that the nuclear encoded bovine heart 18 kDa subunit (AQDQ) containing phosphorylation consensus site (RVS) at 129−131 position is phosphorylated by a cAMP-dependent protein kinase [14]. Cholera toxin-induced phosphorylation of this subunit in mouse fibroblast culture was associated with an increase of respiration with NAD-linked substrates [15] and it has been proposed by Papa and his associates that the mammalian Complex I activity is under control of the cAMP-cascade [16]. Walker's group found two c-AMP-dependent phosphorylated subunits in bovine heart Complex I which were identified as 6 kDa, MWFE, and 18 kDa, ESSS [17]. Recently, the 42 kDa subunit phosphorylated at serine-59 have been identified in bovine heart Complex I isolated from mitochondria without prior protein kinase treatment [18,19].
Another possible regulatory mechanism that may operate under physiological conditions is the slow reversible interconversion between the catalytically active (A) and inactive (D) forms of Complex I [20]. Incubation of Complex I at elevated temperature under conditions where enzyme turnover is not permitted either because ubiquinone is fully reduced or because NADH is absent, results in a slow decline of enzyme activity measured as the initial rate of the NADH:quinone reductase or oxidase reactions, the process operationally called de-activation. The A to D transition for the mammalian enzyme is extremely sensitive to temperature [21] and it is easily detectable only at >30 °C. The D to A transition proceeds (also slowly) when enzyme turnover is permitted and a lag-phase in onset of the catalytic activity is seen when NADH oxidation is initiated by the D-form [21,22]. The duration of this lag is strongly pH-dependent and activation is inhibited by bivalent cations (Ca2+,Mg2+) [22] and by free fatty acids [23].
The A/D transition for mammalian Complex I has been demonstrated for preparations of different degrees of resolution: i.e., purified Complex I [24], submitochondrial particles [21], intact rat heart mitochondria [25] and, perhaps most importantly, in ex vivo experiments on perfused rat hearts during anoxia–oxygen reperfusion cycles [26]. The phenomenon with some variations in the particular kinetic parameters has also been documented for fungal mitochondrial Complex I (N. crassa [10,27], Y. lipolytica [27]) and for mitochondrial preparations obtained from a number of vertebrate and invertebrate species [27]. The “simpler” prokaryotic NADH:quinone oxidoreductases (NDH-1) composed of only 14 “core” subunits (Paracoccus denitrificans [10,28], Rhodobacter capsulatus [11]) do not show any sign of the A/D transition implicating the eukaryotic supernumerary subunits in the phenomenon. The most prominent difference between the A- and D-forms of Complex I besides their catalytic capacity is seen as their different sensitivity to SH-reagents: the D-form is rapidly and irreversibly inactivated by NEM whereas the A-form is insensitive to NEM if treated at a temperature which is low enough to be not permissive for the de-activation [22,29]. In fact, the sensitivity of NADH oxidation to prior treatment with NEM at 15 °C is a measure of the fractional de-activation of any particular preparation of Complex I. The differential labeling of A- and D-forms by fluoresceine-maleimide have shown that a small-peptide preliminary assigned as IP-15 (nomenclature used for bovine heart Complex I) became susceptible for alkylation when the enzyme is converted into its D-form [29].
Protein sulfhydryl groups are sensitive reactive sites for interaction with NO, oxygen free radicals and oxidized glutathione, the natural species which are thought to play important roles in a number of pathophysiological states. The present studies were undertaken to gain information on the topography and chemical reactivity of the A/D-transition-sensitive sulfhydryl group(s) (A/D-SH) in mitochondrial Complex I.
2. Materials and methods
Bovine heart submitochondrial particles (SMP) were prepared [21] and their NADH:ubiquinone oxidoreductase (Complex I) was activated [30] as described. The NADH oxidase activity was determined as a decrease of absorption at 340 nm with 100 μM NADH in the standard assay mixture comprising of 0.25 M sucrose, 1 mM EDTA, 50 mM Tris/Cl−(pH 8.0) and the enzyme preparation (∼10 μg protein/ml). Complex I was de-activated by incubation of SMP in 0.25 M sucrose, 1 mM EDTA, 50 mM Tris/Cl−(pH 8.0) for 15 min at 37 °C.
Rat heart mitochondria were isolated from trypsin-treated muscle essentially as described [31]. The mitochondria were suspended in 0.3 M sucrose, 10 mM HEPES, 0.2 mM EDTA (pH 7.4) and stored on ice.
As a compromise in order to obtain the highest quality preparations of mostly intact mitochondria rat hearts were used, whereas, to obtain inside-out SMP bovine heart material was used. This assumes that there is no significant difference in the spatial arrangements and structure of Complex I in the mitochondrial membranes from Bos taurus and Rattus rattus.
Protein content was determined with biuret reagent using BSA as the standard.
All fine chemicals were purchased from “Sigma”, USA. The experimental details are indicated in the legends to the figures and tables.
3. Results
3.1. Total content of DTNB-reactive thiols in mitochondria and SMP
NEM and its fluorescent derivative, F-NEM, are SH-reagents which are likely to be membrane-penetrating and have been used in our previous studies on the A/D transition-sensitive thiol(s) in Complex I [22,29]. Thus, no topographical assignments of the A/D-SH could be made. DTNB was chosen as the SH-reagent in these studies in order to gain insight into the topography of the A/D-SH because the reagent is a dianion over a wide range of pH [32] and thus is hardly expected to penetrate the inner mitochondrial membrane. It has been shown that alamethicin, the pore-forming antibiotic, makes the inner mitochondrial membrane permeable for NADH, succinate, and ATP with no loss of the matrix-located proteins or gross alterations of the mitochondrial structure [33]. Alamethicin was used in the present studies as a tool to establish the spatial location of the A/D-SH in the mitochondrial preparations. The total amount of DTNB-reactive SH-groups in SMP and intact mitochondria were measured before and after their permeabilization for the low molecular mass components (Table 1). As expected, alamethicin greatly increased the amount of DTNB-reactive thiols in mitochondria whereas no significant effect was seen in SMP. Solubilization of the membranes by Triton X-100 resulted in further increase of the DTNB-reactive groups both in mitochondria and SMP showing that the SH-reactivity depends not only on permeability of the membranes but also on steric constraints due to specific arrangements of the thiol-groups within the native structure of proteins. The content of Complex I in SMP and in mitochondria is within the range of 0.1−0.2 nmol per mg of protein [25], a value which amounts to at most 0.9% of the total DTNB-reactive SH-groups (40 and 16 nmol per mg of protein for alamethicin-treated mitochondria and SMP, respectively, Table 1). This was taken into account when kinetics of the inhibitory effects of DTNB and other SH-reagents on Complex I activity were studied in the experiments reported below.
Table 1.
Content of DTNB-reactive thiols in the mitochondrial preparations a
| DTNB-reactive groups (nmol per mg of protein) |
||
|---|---|---|
| Without alamethicin | Pretreated with alamethicin b | |
| Rat heart mitochondria | 16±2 | 40±2 |
| +Triton X-100 | 48±2 | — |
| Bovine heart SMP | 16±2 | 16±2 |
| +Triton X-100 | 35±2 | 35±2 |
Rat heart mitochondria or bovine heart submitochondrial particles (0.2 mg/ml) were suspended in 0.25 M sucrose, 1 mM EDTA, 50 mM Tris/Cl−, pH 8.0. Alamethicin (30 μg/ml) and Triton X-100 (1%) were added where indicated. DTNB (2 · 10−4 M, 1 μmol/mg of protein) was added and absorption increase at 412−510 nm was traced. The final absorption change reached after the reaction has been completed (∼30 min) was used for calculations of the total thiols content in the samples.
3.2. Location of A/D-SH in mitochondria
To establish the location of the A/D-SH in mitochondria we compared the effect of alamethicin on the inhibition of Complex I by the penetrating (NEM) and presumably non-penetrating (DTNB) SH-reagents. An excess of either inhibitor (over the total content of the reactive thiols, Table 1) was added to the samples of mitochondria where Complex I was de-activated by preincubation under strictly anaerobic conditions at elevated temperature (see Materials and methods and Ref. [25]). The results depicted in Fig. 1 show that permeabilization of the inner membrane greatly potentiated the inhibitory effect of DTNB whereas NEM inhibited the enzyme with equal efficiency in intact and permeabilized preparations. No substantial inhibition of the active Complex I by either reagent was seen in the presence or absence of alamethicin. These findings show that the A/D-SH is located in the mitochondrial matrix and no SH-groups essential for the catalytic activity of Complex I are exposed at the cytosolic (intermembraneous space) side of mitochondria.
Fig. 1.
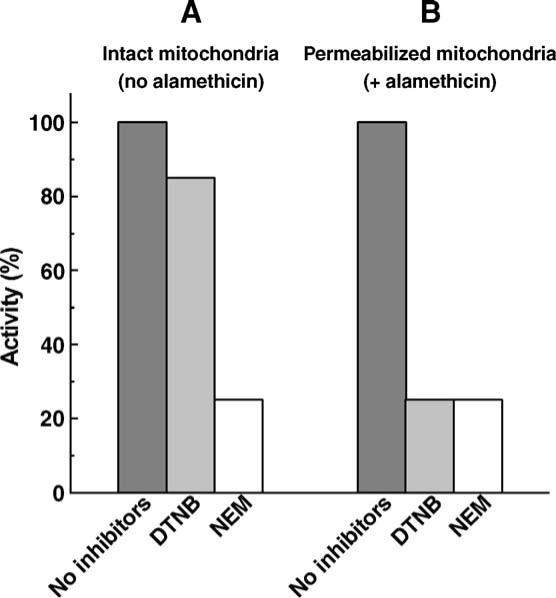
Effect of permeabilization on inhibition of the de-activated Complex I by SH-reagents in mitochondria. Rat heart mitochondria (1 mg protein/ml) were incubated in 0.25 M sucrose, 0.2 mM EDTA, 1.5 mM potassium succinate, 50 mM Tris/Cl−(pH 8.0) at 37 °C for 1 h in 2 ml closed vessels filled by the reaction medium up to the rubber stopper to prevent contact with air. The anaerobic samples were then placed in a thermostat at 15 °C and the inhibitors (0.5 mM NEM or DTNB) were injected by syringes through the rubber stoppers. The incubation was continued for 30 min. Alamethicin (20 μg/ml) and MgCl2 (0.6 mM) were injected into the samples (B) just before the inhibitors. The samples were diluted 5 times with the reaction medium and mitochondria were precipitated (1 h at 16,000 r.p.m., Beckman JA-20 rotor). The pellets were suspended in 0.25 M sucrose, 1 mM EDTA, 50 mM Tris/Cl−(pH 8.0) and the NADH oxidase activity was measured at 30 °C in a reaction mixture of 0.25 M sucrose, 0.2 mM EDTA, 0.6 mM MgCl2, 20 μg/ml alamethicin, 100 μM NADH, 50 mM Tris/Cl−(pH 8.0) and 20−30 μg of protein/ml. Only slight inhibition (about 15%, the data are not shown) of the NADH oxidase activity by NEM and DTNB was seen in the samples incubated aerobically (open well stirred vessels) and further treated as described above. One hundred percent corresponds to the specific NADH oxidase activity (0.6 μmol/min/mg of protein) of untreated anaerobic mitochondria.
3.3. Chemical reactivity of the A/D-SH
The chemical reactivity of free thiol groups in the native proteins towards the specific reagents is quite variable being dependent on a number of factors such as the steric accessibility, hydrogen bond formation and the specific microenvironment of a given SH-group. To obtain insight into the reactivity of the A/D-SH we examined the inhibitory efficiencies of different SH-reagents on the D-form of Complex I in inside-out SMP. The pseudo-first order rate constants of the time-dependent irreversible inhibition of Complex I were determined for those reagents as exemplified by the experiments with DTNB (Fig. 2). Inhibition of the D-form followed simple first order kinetics both in terms of the time-dependency and the SH-reagent concentration if an excess of the latter (over the total SH-group content in SMP) was added to the thermally de-activated enzyme. Almost no inhibition was seen when pre-activated SMP were exposed to DTNB (Fig. 2A, upper curve). Slight inhibition (about 15%) corresponds to the ratio between A- and D-forms at the equilibrium reached after turnover-dependent activation of Complex I [34]. The inhibition of de-activated Complex I by DTNB was completely reversed by an excess of SH-containing dithiothreitol (DTT). Qualitatively the same inhibitory patterns were observed for other SH-reagents (NEM and DTNP, the uncharged analogue of DTNB) except that the NEM-induced inhibition was not reversed by dithiothreitol. The characteristic pseudo-first order rate constants for DTNB and DTNP are summarized in Table 2. We were unable to observe any inhibition of either A or D forms of the membrane-bound Complex I by oxidized glutathione, a natural disulfide which is potentially capable of participation in thiol-disulfide exchange reactions. The following Complex I specific ligands also had no effect on the reactivity of DTNB towards the D-form of Complex I: NAD+ (2 mM), ADP-ribose (0.25 mM), capsaicin (0.2 mM).
Fig. 2.
Kinetics of DTNB-induced inhibition of Complex I in inside-out SMP (A). Preactivated (A-form) or de-activated (D-form) SMP (1 mg/ml) were incubated in 0.25 M sucrose, 1 mM EDTA, 50 mM Tris/Cl−(pH 8.0) and 200 μM DTNB at 15 °C. The residual NADH oxidase activity was assayed at 30 °C in the same mixture without DTNB containing 100 μM NADH and uncoupler (gramicidin D, 0.05 μg/ml). 5 mM DTT was added where indicated. A lag-phase in onset of NADH oxidase activity was seen when de-activated samples were assayed. All the activities were estimated after the rate of NADH oxidation had reached a constant level (∼30 s after the reaction was initiated by the addition of SMP) (B) Semi-log plots for the data shown for D-form in (A) at different DTNB concentrations.
Table 2.
Reactivity of different SH-reagents towards A/D transition-sensitive SH-group of Complex I in SMP (25 °C)
| SH-reagent | Pseudo-first order rate constant (M−1·min−1·104) |
Relative reactivity (ki/kDTT) | |
|---|---|---|---|
| Inhibition of Complex I (ki) | Reaction with DTTa (kDTT) | ||
| DTNB (standard buffer)b | 0.30 | 6.0 | 0.05 |
| +KCl (100 mM)c | 0.70 | 7.0 | 0.10 |
| +SDS (200 μM) | 0.17 | 6.0 | 0.03 |
| DTNP (standard buffer)b | 50 | 69 | 0.72 |
| +KCl (100 mM)c | 100 | 80 | 1.25 |
| +SDS (200 μM) | 25 | 69 | 0.36 |
| Oxidized glutathione (10 mM, 75 min, pH 8.0)b | no inhibition | not determined | |
The reaction was carried out with 1 μM DTT and 10 μM DTNB or DTNP in the standard buffer (see Fig. 2) at pH 7.0. At this pH the reaction proceeded slow enough for reliable rate constant determination.
The rate constants were determined as described in Fig. 2. Concentrations of DTNB and DTNP were 200 μM and 1 μM and protein content in the samples was 1 mg/ml and 10 μg/ml, respectively.
Other salts (NaCl, RbCl, Sodium acetate) showed almost the same effect on the rate constants.
Prior treatment (inhibition) of de-activated Complex I by DTNB followed by removal of the inhibitor (see Fig. 2A) protected the enzyme against irreversible inactivation by NEM (Fig. 3), thus showing that both DTNB and NEM modify the same sulfhydryl group(s).
Fig. 3.

Protective effect of DTNB against on irreversible inhibition of Complex I by NEM. De-activated SMP (1 mg/ml) were incubated in 0.25 M sucrose, 1 mM EDTA, 50 mM Tris/Cl−(pH 8.0) in the presence or absence of 0.5 mM DTNB for 10 min at 25 °C, precipitated by centrifugation and suspended in the same buffer. The samples were then subjected to NEM treatment (0.5 mM, 5 min 25 °C), precipitated and suspended in the same buffer containing 5 mM DTT (where indicated as: +DTT) to remove DTNB. NADH oxidase activity was assayed at 30 °C as described in Fig. 2.
The reactivity of the A/D-SH towards alkylating NEM or thiol–disulfide exchange reagents as such tells little about the local environment of that particular sulfhydryl. More information can be obtained if the relative reactivities towards negatively charged hydrophilic DTNB and its neutral more hydrophobic analogue, DTNP, a reagent interacting with SH-groups by the same thiol–disulfide exchange mechanism [35], are compared. The rate of inhibition, i.e., SH-reactivity depends on susceptibility of the inhibitor disulfide bridge sulfur to nucleophilic substitution by the mercaptide anion of the protein thiol. This parameter is different for DTNB and DTNP [32,35] and a comparison between their reactivities with the A/D-SH seems meaningful only if normalized to their relative reactivities with a “standard” thiol. Thus the pseudo-first order reaction rate constants for the inhibition of Complex I by DTNB and DTNP and those for the reactions with a “standard” neutral thiol, DTT, were compared. Table 2 shows that DTNP reacted with DTT about 170 times faster than DTNB did and the normalized reactivity of DTNP towards A/D-SH was still about 14 times higher than that of DTNB (0.72 versus 0.05, last column). This suggests that the A/D-SH is located in a relatively hydrophobic and negatively charged local environment. It has been shown that only the rotenone-sensitive ubiquinone reduction is affected by the A/D-transition, while the reactivity of Complex I towards artificial hydrophilic electron acceptors such as ferricyanide or hexaammineruthenium (III) is not sensitive to NEM in either state of the enzyme [9,22]. It seemed thus reasonable to propose that A/D-SH is located in close vicinity to the ubiquinone-reactive site(s), i.e. at the matrix–membrane interphase. If correct this proposal predicts that an increase of the membrane surface charge would decrease the reactivity of the A/D-SH because of the electrostatic effect on its deprotonation (shift of apparent pKa). Indeed, lauryl sulphate previously used to modify the mitochondrial membrane in SMP [36] decreased the inhibition rate while the effect on the reaction with DTT was negligible. Also, as expected, an increase of ionic strength resulted in a considerable increase of the A/D-SH-reactivity presumably due to the secondary salt effect. Another prediction of the model is that the apparent pKa for the A/D-SH should be abnormally high due to the effect of local negative charge on dissociation of the proton. The apparent pKa of a particular SH-group can be determined from the pH-dependency of its reactivity in the addition or substitution reactions because of much stronger nucleophilicity of the mercaptide anion than that of protonated thiol. The pH-profile of DTNB–Complex I interaction is shown in Fig. 4. We were unable to determine the apparent pKa of the A/D-SH because the inhibition rate was strongly increased and not saturated up to pH of 11, the highest value at which the inhibition time-course could be reliably measured. Significant spontaneous inactivation of the enzyme preparation and hydrolytic decomposition of DTNB were observed at higher pH. Nevertheless the data shown in Fig. 4 suggest that apparent pKa for the A/D-SH is higher than 10.
Fig. 4.
pH-Dependence of A/D-SH reactivity towards DTNB. De-activated SMP (1 mg/ml) were treated with 40 μM DTNB at 25 °C as described in Fig. 2 and the pseudo-first order inhibition rate constant were determined from semi-log plots.
4. Discussion
We have shown here that the A/D-SH is located at the matrix protruding part of mitochondrial Complex I. The pattern of its reactivities towards different SH-reagents and their alteration by pH, ionic strength, and the membranotropic modifier, lauryl sulphate, strongly suggest that this thiol group (s) is (are) located at the inner membrane–matrix interface. The structural rearrangements associated with the A/D transition can be visualized as follows. The protonated A/D-SH is located within the hydrophobic lipid bilayer when the active form of the enzyme resides in the inner mitochondrial membrane. At this state the A/D-SH is not reactive to either hydrophilic (DTNB) or more hydrophobic (DTNP, NEM) reagents because protonation drastically reduces the nucleophilicity of sulfur. De-activation of Complex I results in relocation of the sulfhydryl group to the aqueous interface where only a mercaptide anion which is in equilibrium with its protonated form is highly reactive in the nucleophilic addition (NEM) or thiol–disulfide exchange (DTNB, DTNP) reactions. This model is in accord with the observation of a strong decrease of the D-to-A transition at alkaline pH [22]. It should be noted that similar phenomenology of the A/D-SH reactivity would also be seen if it is located elsewhere than at the membrane–matrix interface, such as in a matrix exposed part of the protein in close vicinity to negatively charged amino acid residues. However, this seems unlikely because, as it is mentioned above, the A/D-transition deactivates the ubiquinone-reactive site leaving the reactions with the hydrophilic electron acceptors unaffected. The final conclusion on exact location of A/D-SH should wait until the peptide which contains this sulfhydryl will be identified and complete atomic structure of mammalian Complex I will be available.
We were unable to see any inhibition of either active or de-activated membrane-bound Complex I by oxidized glutathione. The reversible glutathionylation of 51 and 75 kDa subunits of Complex I correlated with inhibition of its rotenone-sensitive activity and increased superoxide production have been reported [37]. The apparent discrepancy between the results of Taylor et al. (Fig. 4a, Ref. [37]) and those reported here (Table 2) are not clear and may be explained by differences in enzyme preparations and experimental conditions employed by the two groups. The insensitivity of Complex I to oxidized glutathione as reported here does not exclude the possibility that the intramitochondrial GSH/GSSG ratio may affect the enzyme catalytic capacity. Clearly GSH would protect the A/D-SH in the de-activated Complex I against its modification (reversible or irreversible) by any natural SH-reactive species via a simple competition mechanism.
An exposure of highly reactive SH-group(s) in the D-form is unlikely to be the only difference between the active and de-activated Complex I. The exceptionally high activation energy for the A-to-D-form transition in mammalian mitochondria suggests by itself that gross structural rearrangements of the protein are involved. For example, it has been reported that the 29.9 kDa subunit of N. crassa Complex I is also involved in the A/D-transition [38]. It might be expected that the sensitivity of the enzyme to other than SH-targeted regulatory signals, such as multiple site-directed protein phosphorylation/dephosphorylation events [14–19], would also be A/D-transition sensitive. The structural heterogeneity of the mitochondrial Complex I due to its A/D-transition evident from in vitro [20–25] and ex vivo studies [26] seems to be an important factor which may determine accessibility of particular amino acid residue for post-translational regulatory modifications.
Acknowledgments
This work was supported by Russian Foundation for the Fundamental Studies (grant 05-04-48809 to A.D.V.), by the program Leading Schools in Russian Science (grant 596.2003.4 to A.D.V.) and by NIH Fogarty International Research grant 1R03TW006041 (to G.C. and A.D.V.) and the Department of Veterans Affairs (G.C.).
Abbreviations
- NEM
N-ethylmaleimide
- DTNB
5,5′-dithiobis-(2-nitrobenzoate)
- DTNP
2,2′-dithiobis-(5-nitropyridine)
- DTT
dithiothreitol
References
- 1.Carroll J, Fearnley IM, Shannon RJ, Hirst J, Walker JE. Analysis of the subunit composition of complex I from bovine heart mitochondria. Mol. Cell. Proteomics. 2003;2:117–126. doi: 10.1074/mcp.M300014-MCP200. [DOI] [PubMed] [Google Scholar]
- 2.Xu X, Matsuno-Yagi A, Yagi T. DNA sequencing of the seven remaining structural genes of the gene cluster encoding the energy-transducing NADH-quinone oxidoreductase of Paracoccus denitrificans. Biochemistry. 1993;32:968–981. doi: 10.1021/bi00054a030. [DOI] [PubMed] [Google Scholar]
- 3.Weidner U, Geier S, Ptock A, Friedrich T, Leif H, Weiss H. The gene locus of the proton-translocating NADH: ubiquinone oxidoreductase in Escherichia coli. Organization of the 14 genes and relationship between the derived proteins and subunits of mitochondrial complex I. J. Mol. Biol. 1993;233:109–122. doi: 10.1006/jmbi.1993.1488. [DOI] [PubMed] [Google Scholar]
- 4.Dupuis A, Chevallet M, Darrouzet E, Duborjal H, Lunardi J, Issartel JP. The complex I from Rhodobacter capsulatus. Biochim. Biophys. Acta. 1998;1364:147–165. doi: 10.1016/s0005-2728(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 5.Abdrakhmanova A, Zickermann V, Bostina M, Radermacher M, Schagger H, Kerscher S, Brandt U. Subunit composition of mitochondrial complex I from the yeast Yarrowia lipolytica. Biochim. Biophys. Acta. 2004;1658:148–156. doi: 10.1016/j.bbabio.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Videira A, Duarte M. From NADH to ubiquinone in Neurospora mitochondria. Biochim. Biophys. Acta. 2002;1555:187–191. doi: 10.1016/s0005-2728(02)00276-1. [DOI] [PubMed] [Google Scholar]
- 7.Cardol P, Vanrobaeys F, Devreese B, Van Beeumen J, Matagne RF, Remacle C. Higher plant-like subunit composition of mitochondrial complex I from Chlamydomonas reinhardtii: 31 conserved components among eukaryotes. Biochim. Biophys. Acta. 2004;1658:212–224. doi: 10.1016/j.bbabio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Heazlewood JL, Howell KA, Millar AH. Mitochondrial complex I from Arabidopsis and rice: orthologs of mammalian and fungal components coupled with plant-specific subunits. Biochim. Biophys. Acta. 2003;1604:159–169. doi: 10.1016/s0005-2728(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 9.Vinogradov AD. Catalytic properties of the mitochondrial NADH-ubiquinone oxidoreductase (complex I) and the pseudo-reversible active/inactive enzyme transition. Biochim. Biophys. Acta. 1998;1364:169–185. doi: 10.1016/s0005-2728(98)00026-7. [DOI] [PubMed] [Google Scholar]
- 10.Grivennikova VG, Serebryanaya DV, Isakova EP, Belozerskaya TA, Vinogradov AD. The transition between active and de-activated forms of NADH:ubiquinone oxidoreductase (Complex I) in the mitochondrial membrane of Neurospora crassa. Biochem. J. 2003;369:619–626. doi: 10.1042/BJ20021165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grivennikova VG, Roth R, Zakharova NV, Hagerhall C, Vinogradov AD. The mitochondrial and prokaryotic proton-translocating NADH:ubiquinone oxidoreductases: similarities and dissimilarities of the quinone-junction sites. Biochim. Biophys. Acta. 2003;1607:79–90. doi: 10.1016/j.bbabio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Galkin AS, Grivennikova VG, Vinogradov AD. H+/2e-stoichiometry in NADH-quinone reductase reactions catalyzed by bovine heart submitochondrial particles. FEBS Lett. 1999;451:157–161. doi: 10.1016/s0014-5793(99)00575-x. [DOI] [PubMed] [Google Scholar]
- 13.Grivennikova VG, Ushakova AV, Hagerhall C, Vinogradov AD. Proton translocation catalyzed by Paracoccus denitrificans NADH: quinone oxidoreductase (NDH-1) Biochim. Biophys. Acta: EBEC Short Reports. 2002;12:209. [Google Scholar]
- 14.Papa S, Sardanelli AM, Cocco T, Speranza F, Scacco SC, Technikova-Dobrova Z. The nuclear-encoded 18 kDa (IP) AQDQ subunit of bovine heart complex I is phosphorylated by the mitochondrial cAMP-dependent protein kinase. FEBS Lett. 1996;379:299–301. doi: 10.1016/0014-5793(95)01532-9. [DOI] [PubMed] [Google Scholar]
- 15.Scacco S, Vergari R, Scarpulla RC, Technikova-Dobrova Z, Sardanelli A, Lambo R, Lorusso V, Papa S. cAMP-dependent phosphorylation of the nuclear encoded 18-kDa (IP) subunit of respiratory complex I and activation of the complex in serum-starved mouse fibroblast cultures. J. Biol. Chem. 2000;275:17578–17582. doi: 10.1074/jbc.M001174200. [DOI] [PubMed] [Google Scholar]
- 16.Papa S, Sardanelli AM, Scacco S, Petruzzella V, Technikova-Dobrova Z, Vergari R, Signorile A. The NADH: ubiquinone oxidoreductase (complex I) of the mammalian respiratory chain and the cAMP cascade. J. Bioenerg. Biomembr. 2002;34:1–10. doi: 10.1023/a:1013863018115. [DOI] [PubMed] [Google Scholar]
- 17.Chen R, Fearnley IM, Peak-Chew SY, Walker JE. The phosphorylation of subunits of complex I from bovine heart mitochondria. J. Biol. Chem. 2004;279:26036–26045. doi: 10.1074/jbc.M402710200. [DOI] [PubMed] [Google Scholar]
- 18.Schulenberg B, Aggeler R, Beechem JM, Capaldi RA, Patton WF. Analysis of steady-state protein phosphorylation in mitochondria using a novel fluorescent phosphosensor dye. J. Biol. Chem. 2003;278:27251–27255. doi: 10.1074/jbc.C300189200. [DOI] [PubMed] [Google Scholar]
- 19.Schilling B, Aggeler R, Schulenberg B, Murray J, Row RH, Capaldi RA, Gibson BW. Mass spectrometric identification of a novel phosphorylation site in subunit NDUFA10 of bovine mitochondrial complex I. FEBS Lett. 2005;579:2485–2490. doi: 10.1016/j.febslet.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 20.Vinogradov AD, Grivennikova VG. The mitochondrial complex I: progress in understanding of catalytic properties. IUBMB Life. 2001;52:129–134. doi: 10.1080/15216540152845920. [DOI] [PubMed] [Google Scholar]
- 21.Kotlyar AB, Vinogradov AD. Slow active/inactive transition of the mitochondrial NADH-ubiquinone reductase. Biochim. Biophys. Acta. 1990;1019:151–158. doi: 10.1016/0005-2728(90)90137-s. [DOI] [PubMed] [Google Scholar]
- 22.Kotlyar AB, Sled VD, Vinogradov AD. Effect of Ca2+ ions on the slow active/inactive transition of the mitochondrial NADH-ubiquinone reductase. Biochim. Biophys. Acta. 1992;1098:144–150. doi: 10.1016/s0005-2728(05)80329-9. [DOI] [PubMed] [Google Scholar]
- 23.Loskovich MV, Grivennikova VG, Cecchini G, Vinogradov AD. Inhibitory effect of palmitate on the mitochondrial NADH:ubiqinon oxidoreductase (Complex I) as related to the active–de-active enzyme transition. Biochem. J. 2005;387:677–683. doi: 10.1042/BJ20041703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maklashina EO, Sled' VD, Vinogradov AD. Hysteresis behavior of complex I from bovine heart mitochondria: kinetic and thermodynamic parameters of retarded reverse transition from the inactive to active state. Biokhimiia. 1994;59:946–957. [PubMed] [Google Scholar]
- 25.Grivennikova VG, Kapustin AN, Vinogradov AD. Catalytic activity of NADH-ubiquinone oxidoreductase (complex I) in intact mitochondria. Evidence for the slow active/inactive transition. J. Biol. Chem. 2001;276:9038–9044. doi: 10.1074/jbc.M009661200. [DOI] [PubMed] [Google Scholar]
- 26.Maklashina E, Sher Y, Zhou HZ, Gray MO, Karliner JS, Cecchini G. Effect of anoxia/reperfusion on the reversible active/de-active transition of NADH-ubiquinone oxidoreductase (complex I) in rat heart. Biochim. Biophys. Acta. 2002;1556:6–12. doi: 10.1016/s0005-2728(02)00280-3. [DOI] [PubMed] [Google Scholar]
- 27.Maklashina E, Kotlyar AB, Cecchini G. Active/de-active transition of respiratory complex I in bacteria, fungi, and animals. Biochim. Biophys. Acta. 2003;1606:95–103. doi: 10.1016/s0005-2728(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 28.Kotlyar AB, Albracht SP, van Spanning RJ. Comparison of energization of complex I in membrane particles from Paracoccus denitrificans and bovine heart mitochondria. Biochim. Biophys. Acta. 1998;1365:53–59. doi: 10.1016/s0005-2728(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 29.Gavrikova EV, Vinogradov AD. Active/de-active state transition of the mitochondrial complex I as revealed by specific sulfhydryl group labeling. FEBS Lett. 1999;455:36–40. doi: 10.1016/s0014-5793(99)00850-9. [DOI] [PubMed] [Google Scholar]
- 30.Burbaev D.Sh., Moroz IA, Kotlyar AB, Sled VD, Vinogradov AD. Ubisemiquinone in the NADH:ubiquinone reductase region of the mitochondrial respiratory chain. FEBS Lett. 1989;254:47–51. [Google Scholar]
- 31.Jacobus WE, Saks VA. Creatine kinase of heart mitochondria: changes in its kinetic properties induced by coupling to oxidative phosphorylation. Arch. Biochem. Biophys. 1982;219:167–178. doi: 10.1016/0003-9861(82)90146-1. [DOI] [PubMed] [Google Scholar]
- 32.Riddles PW, Blakeley RL, Zerner B. Ellman's reagent: 5,5′-dithiobis (2-nitrobenzoic acid)—A reexamination. Anal. Biochem. 1979;94:75–81. doi: 10.1016/0003-2697(79)90792-9. [DOI] [PubMed] [Google Scholar]
- 33.Gostimskaya IS, Grivennikova VG, Zharova TV, Bakeeva LE, Vinogradov AD. In situ assay of the intramitochondrial enzymes: use of alamethicin for permeabilization of mitochondria. Anal. Biochem. 2003;313:46–52. doi: 10.1016/s0003-2697(02)00534-1. [DOI] [PubMed] [Google Scholar]
- 34.Grivennikova VG, Maklashina EO, Gavrikova EV, Vinogradov AD. Interaction of the mitochondrial NADH-ubiquinone reductase with rotenone as related to the enzyme active/inactive transition. Biochim. Biophys. Acta. 1997;1319:223–232. doi: 10.1016/s0005-2728(96)00163-6. [DOI] [PubMed] [Google Scholar]
- 35.Swatditat A, Tsen CC. Determining simple sulfhydryl compounds (low molecular weight) and their contents in biological samples by using 2,2′-dithiobis-(5-nitropyridine) Anal. Biochem. 1972;45:349–356. doi: 10.1016/0003-2697(72)90198-4. [DOI] [PubMed] [Google Scholar]
- 36.Grivennikova VG, Ushakova AV, Cecchini G, Vinogradov AD. Unidirectional effect of lauryl sulfate on the reversible NADH:ubiquinone oxidoreductase (Complex I) FEBS Lett. 2003;549:39–42. doi: 10.1016/s0014-5793(03)00765-8. [DOI] [PubMed] [Google Scholar]
- 37.Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J. Biol. Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- 38.Ushakova AV, Duarte M, Vinogradov AD, Videira A. The 29.9 kDa subunit of mitochondrial complex I is involved in the enzyme active/de-active transitions. J. Mol. Biol. 2005;351:327–333. doi: 10.1016/j.jmb.2005.06.005. [DOI] [PubMed] [Google Scholar]




