SUMMARY
Sex determination in Drosophila is commonly thought to be a cell-autonomous process, where each cell decides its own sexual fate based on its sex chromosome constitution (XX vs. XY). This is in contrast to sex determination in mammals, which largely acts non-autonomously through cell-cell signaling. Here we examine how sexual dimorphism is created in the Drosophila gonad. We have identified a novel male-specific cell type in the embryonic gonad, the pigment cell precursors. Surprisingly, using sexually mosaic embryos, we find that sex determination in both the pigment cell precursors and the male-specific somatic gonadal precursors is non-cell autonomous. Male-specific expression of Wnt2 in the embryonic gonad is necessary and sufficient for pigment cell precursor formation. Our results indicate that non-autonomous sex determination is important for creating sexual dimorphism in the Drosophila gonad, similar to the manner in which sex-specific gonad formation is controlled in mammals.
Keywords: sex determination, sexual dimorphism, gonad development, Wnt2, doublesex, empty spiracles, Sox100B, Sox9
INTRODUCTION
A critical decision during development is whether an individual should develop as male or female. Sex determination imparts a sexual identity to an embryo, which is then used by cells and tissues to create different forms in males and females (sexual dimorphism). Cells can determine their sex in an autonomous manner by directly interpreting the sex determination switch (e.g., whether they are XX or XY). Alternatively, cells can undergo non-autonomous sex determination, in which local or systemic signals determine whether they should develop as male or female.
A commonly held view is that sex determination in Drosophila is almost exclusively cell autonomous, and “every cell decides for itself” what its sexual phenotype should be (Gilbert, 2006; Wolpert et al., 2006). For example, when cells of male or female genotype are present in the same individual (gynandromorphs), cells follow a male or female developmental program according to their genotype, independent of the cells around them. This is thought to contrast strongly with sex determination in mammals, which is largely non-cell autonomous. In the mouse, only a subset of cells in the somatic gonad is thought to directly respond to their sex chromosome genotype (XX vs. XY). Local cell-cell interactions non-autonomously control the sexual fate of the rest of the gonad, and systemic hormones such as testosterone influence other tissues of the embryo (reviewed in (Ross and Capel, 2005)). However, previous work has shown that cell-cell interaction is also important for creating sexual dimorphism in Drosophila, including in the germline (Nothiger et al., 1989; Steinmann-Zwicky et al., 1989), muscle of Lawrence (Lawrence and Johnston, 1986), and the genital disc (Ahmad and Baker, 2002; Keisman et al., 2001), suggesting that non-autonomous sex determination may be more prevalent in Drosophila than is commonly thought.
During somatic sex determination in Drosophila, the presence of two X chromosomes promotes a female identity by inducing expression of Sex lethal, which is responsible for splicing transformer (tra) RNA so as to produce functional TRA protein. tra controls all known aspects of sexual dimorphism in the soma, except for the difference in body size between males and females (Cline and Meyer, 1996). Downstream of tra, doublesex (dsx) is the critical regulator of male vs. female appearance (Baker and Ridge, 1980; Hildreth, 1965), while additional genes, such as fruitless, control sex-specific behavior (Ryner et al., 1996). TRA acts in conjunction with Transformer-2 (TRA-2) to splice dsx pre-RNA into the female form encoding the DSX-F transcription factor. In males, dsx is spliced in the default, male-specific manner to encode for DSX-M (Burtis and Baker, 1989). The DSX transcription factors are thought to regulate the genes that control sexual dimorphism, although only one direct DSX target, the yolk protein locus (Burtis et al., 1991; Coschigano and Wensink, 1993), has so far been identified.
Sex-specific development of the gonads may be the most fundamental and conserved aspect of sexual dimorphism, as it is required for the generation of male and female gametes that form the basis for sexual reproduction. The Drosophila embryonic gonad arises from the association of germ cells with specific somatic cells (somatic gonadal precursors or SGPs) that form within the mesoderm (Van Doren, 2006). We have previously shown that the gonad is already sexually dimorphic at the time of its initial formation, as male-specific SGPs (msSGPs) only join the male gonad and die by apoptosis in females (DeFalco et al., 2003). msSGPs express SOX100B, a homolog of the mammalian transcription factor SOX9 required for sex determination in humans and mice (Barrionuevo et al., 2006; Chaboissier et al., 2004; Foster et al., 1994; Huang et al., 1999; Qin and Bishop, 2005; Wagner et al., 1994). In addition, sex-specific development of the male germline stem cell niche, or hub, also occurs in the embryonic gonad (Le Bras and Van Doren, 2006). Finally, sex-specific signaling from the soma to the germline helps determine sexual identity of the germ cells in the embryo (Staab et al., 1996; Wawersik et al., 2005).
Another aspect of sexual dimorphism in the gonad is the presence of pigment cells (PCs) around the testis, but not the ovary (Fuller, 1993), which are likely to be important for testis function. PCs are also found around portions of the male reproductive tract, which is derived from the genital imaginal disc and only attaches to the gonad during metamorphosis. A male genital disc can induce PC formation when transplanted into female larvae (Hadorn and Bertani, 1948). Furthermore, mutants for one of the Wnt family of secreted ligands, Wnt2, lack pigment cells, and Wnt2 expression in the male genital disc is likely to account for the PC-inducing activity of this tissue (Kozopas et al., 1998). However, PCs normally originate from the testis and do not require contact with the genital disc for their formation (Stern and Hadorn, 1939). How PCs are normally specified in a sex-specific manner in the male gonad has not been investigated.
Here we provide evidence that PC precursors are recruited from the fat body mesoderm to join the testis in late embryos. Interestingly, the male somatic gonad induces PC precursor formation in a manner that involves non-autonomous sex determination; both the male and female fat body are capable of forming PC precursors when associated with a male gonad. Wnt2 is expressed male-specifically in the embryonic gonad, and is necessary and sufficient for PC precursor formation in this tissue. In addition, we show that sex-specific development of the msSGPs is also controlled through a non-cell autonomous mechanism, but this is independent of Wnt2. This work demonstrates that non-cell autonomous sex determination, involving local cell-cell interactions, is an important aspect of sex-specific gonad development in Drosophila, and that regulation of sexual dimorphism in flies shares many features with mechanisms observed in mammals.
RESULTS
Two distinct male-specific cell types express SOX100B in the embryonic gonad
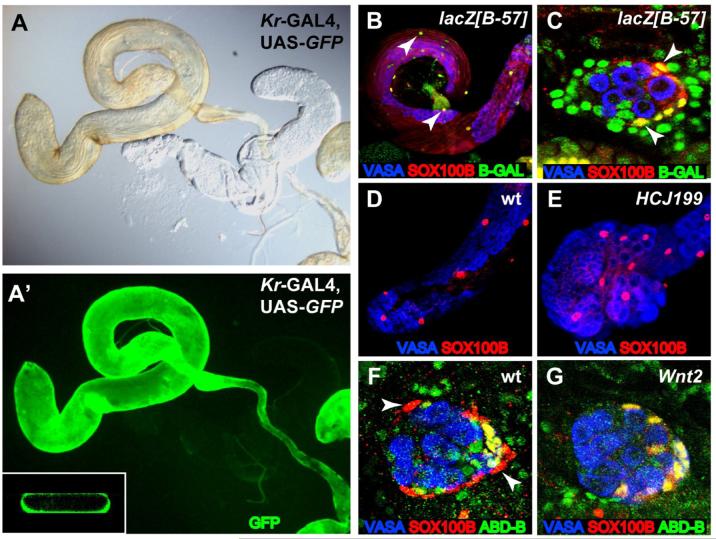
While examining the expression of SOX100B throughout gonad development (T.D., S. Nanda, M.V.D., and S. Russell, unpublished data, Figure 1), we observed two populations of SOX100B-positive cells in the late embryonic (stage 17) male gonad that were not present in the female gonad. The first was a tight cluster of SOX100B-positive cells in the posterior of the gonad, which appeared to be the msSGPs that we had observed at earlier stages (DeFalco et al., 2003). The second was a layer of SOX100B-positive cells surrounding the outside of the gonad (ensheathing cells), which we had not detected at earlier stages. Since these two cell types both express SOX100B, we investigated the relationship between the msSGPs and the ensheathing cells by first examining the expression of additional molecular markers. msSGPs express Abdominal-B (ABD-B) and Eyes Absent (EYA) (DeFalco et al., 2003), but we did not observe expression of either of these msSGP markers in the ensheathing cells (Figures 1A and 1B). In addition, we identified a GAL4 line (Kruppel(Kr)-GAL4) that drives UAS-GFP expression in the ensheathing cells but not in the msSGPs (Figures 1C and 1D). Thus, the msSGPs and ensheathing cells have different identities based on their pattern of gene expression.
Figure 1. SOX100B identifies two distinct sexually dimorphic cell types in the embryonic gonad.
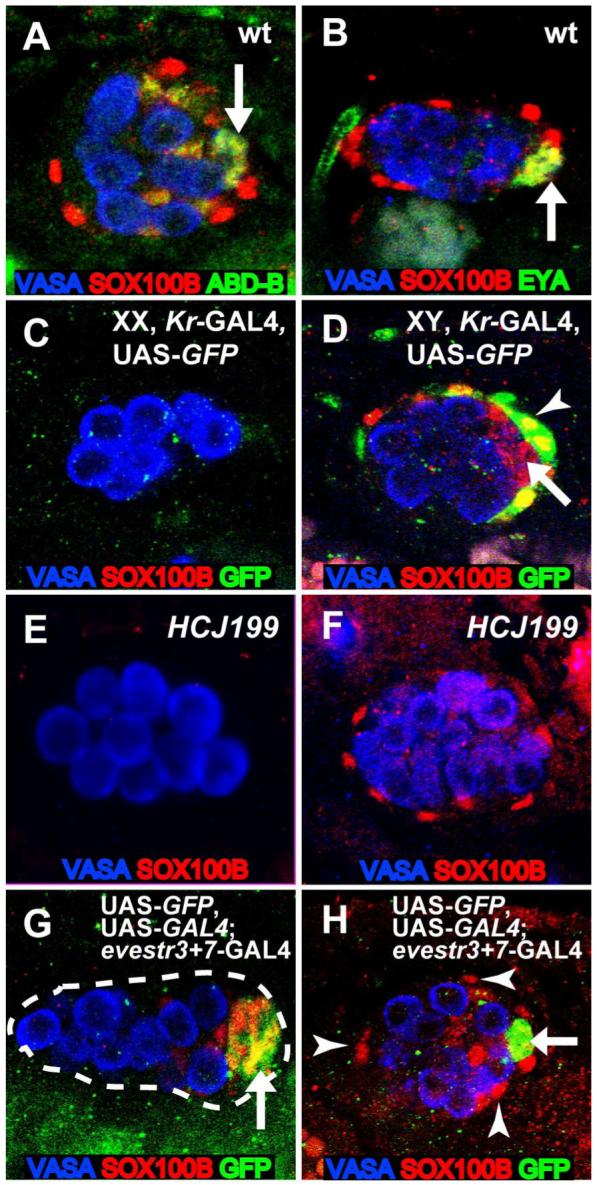
Embryonic gonads immunostained as indicated in the figure. Anterior is to the left in each panel. (A,B) Wild-type st. 17 male gonads. msSGPs express ABD-B (A) and EYA (B) (arrows), in addition to SOX100B, whereas the ensheathing cells express only SOX100B. In this genetic background, a few posterior SGPs also express both SOX100B and ABD-B, as has been observed previously (DeFalco et al., 2003). (C,D) St. 17 XX (C) and XY (D) gonads from embryos expressing Kr-GAL4, UAS-GFP constructs on a TM3 balancer chromosome. GFP is expressed in cells ensheathing male gonads (arrowhead), but is not expressed in msSGPs (arrow). (E,F) Embryos homozygous for the HCJ199 allele of Abd-B. Male gonads lack msSGPs at both st. 15 (E) and st. 17 (F), but still exhibit SOX100B-positive ensheathing cells at st. 17. (G,H) Embryos expressing evestr3+7-GAL4 in combination with UAS-GFP and UAS-GAL4 for permanent lineage tracing. msSGPs (arrows) express GFP at st. 13 (G) and st. 17 (H) but SOX100B-positive ensheathing cells do not (arrowheads).
We next investigated whether the ensheathing cells arise independently of the msSGPs by examining gonads that lack msSGPs. In Abd-B mutant gonads, msSGPs are absent (Figure 1E) (DeFalco et al., 2004), yet we still observed the SOX100B-positive ensheathing cells at stage 17 (Figure 1F). Furthermore, in shotgun/E-cadherin mutants the msSGPs sometimes fail to join the gonad (Jenkins et al., 2003), but SOX100B-positive cells were still observed around the gonad at stage 17 (data not shown). These data suggest that msSGPs are not required for the ensheathing cells to be present.
Finally, to verify that the ensheathing cells are independent of the msSGPs, we conducted lineage tracing of the msSGPs. We identified a GAL4 line that, within the gonad, is expressed only in the msSGPs (Figure 1G, even-skipped stripe 3+7-GAL4, a gift from S. Small). We combined this with UAS-GAL4 (Hassan et al., 2000) to permanently express UAS-GFP in the msSGPs and their descendents. We observed robust expression of GFP in the msSGPs at stage 13 (Figure 1G) and in the posterior cluster of SOX100B-expressing cells at stage 17 (Figure 1H, arrow). However, the SOX100B-positive ensheathing cells (Figure 1H, arrowheads) did not exhibit GFP expression, nor did any other cells in the embryonic gonad. This indicates that the msSGPs do not give rise to the ensheathing cells and that these two male-specific cell types are specified independently in the embryonic gonad.
SOX100B-positive ensheathing cells appear to give rise to testis PCs
In adult testes, SOX100B labels a population of cells with large nuclei that surround the outside of the testis, reminiscent of the PCs (T.D., S. Nanda, M.V.D., and S. Russell, unpublished data). To investigate whether the ensheathing cells in the stage 17 embryonic gonad give rise to the adult PCs, we first examined whether these two cell types express other molecular markers in common. We found that Kr-GAL4, which labels the ensheathing cells in the embryonic gonad, is also expressed in the adult PCs. When Kr-GAL4 was combined with UAS-GFP, we observed GFP expression along the entire length of the adult testis and seminal vesicle, coincident with the PC layer that gives a yellow color to the testis (Figures 2A and 2A’). GFP expression was only in the outer layer of the testis where the PCs reside (inset, Figure 2A’). We next examined two markers of the adult PCs, lacZ [B-57] and lacZ [857] (Gönczy, 1995). We found that SOX100B was co-expressed with these markers in the adult testis, demonstrating that SOX100B is expressed in adult PCs (Figure 2B and data not shown). Furthermore, lacZ [B-57] marker expression was also seen in stage 17 embryonic gonads, where it overlapped with SOX100B expression in the ensheathing cells (Figure 2C). Therefore, the ensheathing cells and PCs share common molecular marker expression, consistent with the ensheathing cells giving rise to the PCs.
Figure 2. SOX100B-expressing cells give rise to testis PCs.
St. 17 gonads and adult testes immunostained as indicated in the figure. Anterior is to the left in each panel. (A,A’) Kr-GAL4, UAS-GFP adult testis examined by either light microscopy (A) or immunofluorescence (B). Inset: Slice of three-dimensional reconstruction of testis. Note that GFP expression is coincident with the yellow pigmentation of the testis and is only in the outer layer of cells. (B,C) The B-57 enhancer trap labels PCs in the adult testis (B) and st. 17 embryonic male gonad (C), and co-localizes with SOX100B (arrowheads). (D,E) Adult testes showing SOX100B-positive PCs in both wild-type (D) and AbdB[HCJ199] mutants (E). The AbdB[HCJ199] mutant testes do not have the normal, elongated appearance of wild-type testes, likely due to defects in interaction with the genital disc. (F,G) St. 17 embryonic male gonads immunostained to reveal msSGPs (co-expressing ABD-B and SOX100B) and PCs (SOX100B only, arrowheads). In addition, a few posterior SGPs also co-express ABD-B and SOX100B (DeFalco et al., 2003). Note that PCs are observed in wild-type (F) but not Wnt2 mutants (G).
Since the msSGPs also express SOX100B, we wanted to verify that msSGPs were not contributing to the adult PCs. We examined viable Abd-B mutants in which msSGPs are absent in the embryo (Figure 1E and data not shown) and still observed a wild-type number of SOX100B-positive PCs around the adult testis (Figures 2D and 2E), indicating that msSGPs are not required for adult PC formation.
Wnt2 mutant larval and adult gonads specifically lack PCs (Kozopas et al., 1998), indicating that this factor is important in PC specification or maintenance. If the ensheathing cells around the embryonic gonad are PC precursors, we reasoned that they might also be missing in Wnt2 mutants. We found that the ensheathing cells (SOX100B-positive, ABD-B-negative) are indeed missing in Wnt2 mutant embryos (Figures 2F vs. 2G). In contrast, the msSGPs (SOX100B, ABD-B double-positive) were unaffected in Wnt2 mutants; they still joined the gonad in males (Figure 2G) and were not present in females (data not shown). Taken together, our results indicate that the ensheathing cells of the embryonic gonad are likely to give rise to the PCs of the adult testis, and that Wnt2 plays a role in the initial specification of these cells in the embryo (see below). Consequently, we will refer to the ensheathing cells as PC precursors.
PC precursors are recruited to the gonad from surrounding fat body
Since the PC precursors appear around the outside of the late embryonic gonad, we investigated whether they are derived from the fat body mesoderm that surrounds the gonad. Two genes that are required for fat body development, and are often used as markers for fat body identity, are seven up (svp) and serpent (srp) (Hoshizaki et al., 1994; Sam et al., 1996). We observed expression of both svp (as assayed with a svp-lacZ enhancer trap) and SRP (as determined by an anti-SRP antibody) in the PC precursors (Figures 3A and 3B), consistent with a fat body origin for these cells. In addition, we found that the PC precursors failed to form in srp mutant embryos, in which fat body development is severely defective. In contrast, the msSGPs (co-labeled with SOX100B and EYA, Figure 3C) and SGPs (Supplemental Figure S1A) (Moore et al., 1998, Riechmann, et al., 1998) were still present. These data indicate that a subset of cells from the fat body mesoderm are recruited to join the developing testis and form PC precursors.
Figure 3. PC precursors are specified from fat body cells surrounding the male gonad.
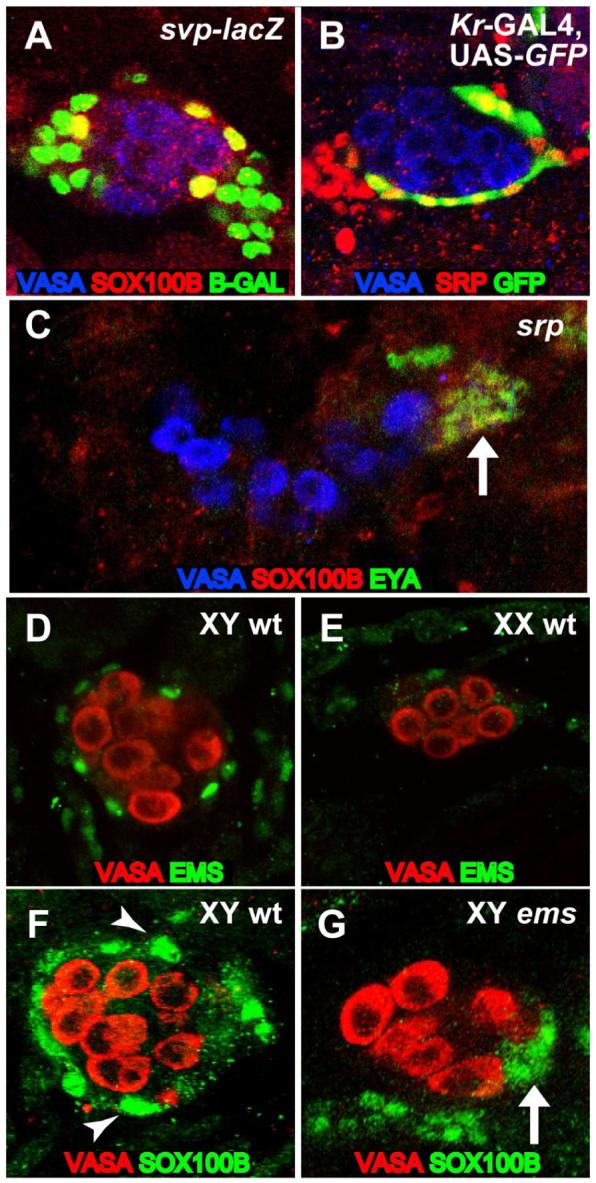
St. 17 gonads immunostained as indicated in the figure. Anterior is to the left in each panel. (A) Expression of the svp-lacZ fat body enhancer trap overlaps with SOX100B-positive PC precursors. (B) Cells that express the PC precursor marker Kr-GAL4, UAS-GFP also express SRP. (C) A srp mutant gonad that lacks PC precursors but has msSGPs (co-expressing SOX100B and EYA, arrow). (D,E) EMS is expressed in cells that ensheath the male (D), but not female (E) embryonic gonad. (F,G) SOX100B-positive PC precursors surround the wild-type male gonad (F, arrowhead) but are not observed in ems mutant gonads (G), which only exhibit SOX100B expression in msSGPs (arrow).
We have also found that PC precursor formation requires empty spiracles (ems), which encodes a homeobox transcription factor (Dalton et al., 1989). Immunostaining with an anti-EMS antibody revealed that EMS is expressed in the PC precursors (Figures 3D and 3E) in the same pattern as SOX100B and in the same cells that express Kr-GAL4 (data not shown). In addition, an analysis of ems mutants revealed a lack of SOX100B-positive PC precursors surrounding the gonads, while the posterior cluster of SOX100B-positive msSGPs was still observed (Figures 3F and 3G). Staining with anti-SRP antibody revealed that, apart from the lack of PC precursors, the fat body is otherwise properly specified and organized around the gonad in ems mutants (data not shown). Thus, ems is specifically required for the formation of this male-specific cell type in the Drosophila gonad.
Regulation of pigment cell precursor formation by tra and dsx
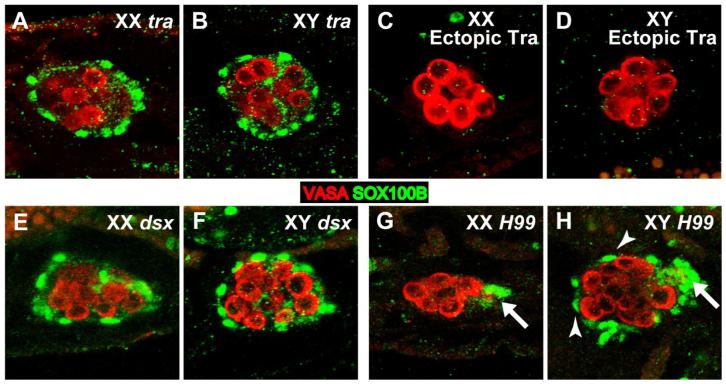
Since the PC precursors are a male-specific cell type, we examined how their specification is regulated by key genes in the sex determination pathway. transformer (tra) and doublesex (dsx) are required for sexual dimorphism of the soma, and we have previously shown these two genes are necessary for proper development of sexual dimorphism in the embryonic gonad (DeFalco et al., 2003; Le Bras and Van Doren, 2006). tra promotes female development, so we expect tra mutant gonads to be masculinized. Indeed, we observed SOX100B-positive PC precursors in both XX and XY gonads in tra mutants (Figures 4A and 4B). Conversely, when we expressed TRA ubiquitously in the soma (using tubulin-GAL4 and UAS-traF), both XX and XY gonads lacked PC precursors (Figures 4C and 4D). Interestingly, in dsx mutant embryos, PC precursors were observed in both XX and XY gonads (Figures 4E and 4F), and both appeared similar to wild-type males. This indicates that dsx is not required in males to specify PC precursors, but rather is only necessary in females to repress PC precursor specification (although dsx may still play a role later in PC development in males).
Figure 4. tra and dsx control sexual dimorphism in the PC precursors.
St. 17 gonads immunostained with anti-VASA (germ cells, red) and anti-SOX100B (green). Anterior is to the left in each panel. (A,B) SOX100B-positive PC precursors are observed in both XX (A) and XY (B) tra mutant embryos. (C,D) PC precursors are not observed in either XX (C) or XY (D) gonads when the female form of tra is ectopically expressed using UAS-traF; tubulin-GAL4. (E,F) PC precursors are observed in both XX (A) and XY (B) dsx mutant embryos. (G,H) Df (H99) mutants defective for apoptosis. Note that PC precursors (arrowheads) are still observed in XY (H) but not XX (G) embryos, similar to wild type. In contrast, msSGPs (arrows) are observed in both XY and XX embryos.
We also wanted to determine whether male-specific development of the PC precursors is regulated similarly to the msSGPs, which are initially specified in both sexes but undergo programmed cell death in females. To test this, we examined Df(H99) mutant embryos that are deficient for programmed cell death (White et al., 1994). msSGPs survive in both males and females in this background (DeFalco et al., 2003) (Figures 4G and 4H, arrows). However, we found that in Df(H99) mutants, sexual dimorphism of PC precursors was unaffected; SOX100B-positive cells were observed only around the gonads in XY embryos, and not in XX embryos, as in wild-type (Figures 4G and 4H). Thus, although msSGPs and PC precursors both depend on dsx for sex-specific development, the cellular mechanisms employed to ensure sexual dimorphism of the two cell types are different.
Non-cell autonomous control of sex determination in the Drosophila gonad
We next investigated whether the sex determination pathway acts cell-autonomously or non-cell autonomously to control sex-specific development of the Drosophila gonad. To do this, we used sexual mosaics in which some TRA-expressing, and therefore female, cells are present in an otherwise male embryo. We could then assess whether TRA expression was sufficient to dictate the sex-specific development of a particular cell type (cell-autonomously) or whether those cells developed according to the sex of the cells around them (non-autonomously). We studied three different male-specific cell types in the embryonic gonad: the hub cells that form the male germline stem cell niche (Le Bras and Van Doren, 2006), the msSGPs and the PC precursors. Ubiquitous expression of TRA in XY embryos (using tubulin-GAL4 and UAS-traF) is sufficient to feminize the gonad and block the formation of each of these male-specific cell types (DeFalco et al., 2003; Le Bras and Van Doren, 2006) (Figure 4D). To create sexual mosaics, we used paired-GAL4 (prd-GAL4) to express UAS-traF in subsets of cells in XY embryos (Figure 5). prd-GAL4 is expressed in alternating parasegments (PSs) of the embryo, including SGPs in the middle of the gonad (PS11), msSGPs (PS13), and subsets of cells in the fat body surrounding the gonad (Figure 5C). To verify where UAS-traF is expressed, we used UAS-GFP to label the prd-GAL4-expressing cells directly with GFP.
Figure 5. Non-cell autonomous control of sex determination in the Drosophila gonad.
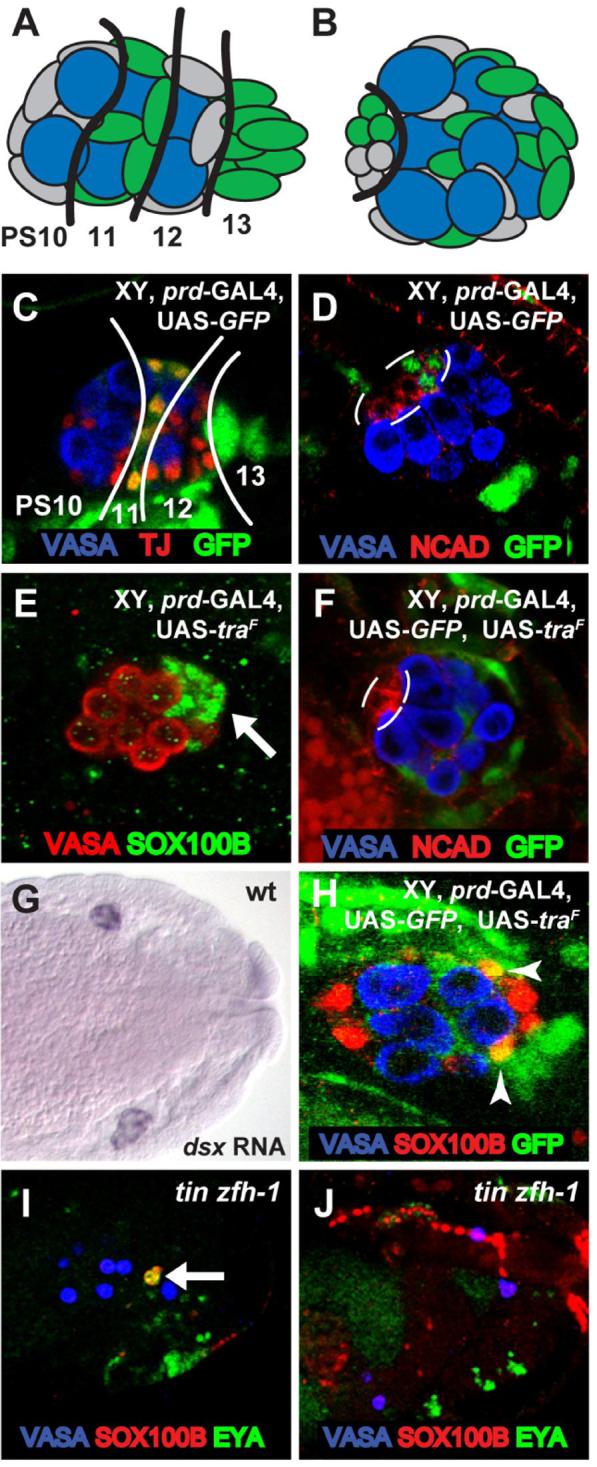
(A,B) Diagram of prd-GAL4 expression (green) in the embryonic gonad at st. 15 (A) and st. 17 (B). Anterior is to the left in each panel. Approximate PS numbers are indicated in (A) based on the known pattern of prd gene expression. The black line in (B) outlines the hub. (C-F, H-J) Immunostainings of embryonic gonads as indicated. (C) GFP expression from st. 15 male embryo expressing prd-GAL4 and UAS-GFP. Traffic Jam (TJ) staining labels PS10-12 SGP nuclei. Lines separate GFP-positive and GFP-negative regions of the gonad. Note that GFP is expressed in PS 11 SGPs and strongly in the msSGPs from PS 13. GFP is also expressed in subsets of cells surrounding the gonad. (D) GFP expression from st. 17 male embryo expressing prd-GAL4 and UAS-GFP. Note that the hub (outline, labeled with DN-cadherin) is normally composed of GFP-positive (PS11) and GFP-negative (PS10) cells. (E) A similar embryo as in (C) but expressing TRA instead of GFP. Note that the msSGPs are still present in the XY gonad even though they express TRA. (F) A similar embryo as in (D) but now expressing TRA in addition to GFP. Note that GFP/ TRA expressing cells do not contribute to the hub (outline), in contrast to GFP-alone expressing cells in (D). (G) St. 15 wild type embryo labeled by in situ hybridization for dsx mRNA. (H) St. 17 embryo expressing prd-GAL4, UAS-GFP and UAS-traF, immunostained for SOX100B and GFP. Note that GFP/ TRA expressing cells (arrowheads) can still become PC precursors based on their expression of SOX100B and their position and morphology. (I,J) tin zfh-1 double-mutant embryos. Note that msSGPs (arrows, co-labeled with EYA and SOX100B) are present at st. 12 (I), but not at st. 15 (J).
Previously, we found that the hub normally forms during late embryogenesis (stage 17) from two types of SGPs; those that express prd-GAL4 (PS11) and those that do not (PS10) (Le Bras and Van Doren, 2006) (Figure 5D). However, when TRA was expressed using prd-GAL4 and UAS-traF, we observed that cells expressing TRA were now excluded from forming part of the hub (Figure 5F), and the hub was formed entirely of cells that lacked prd-GAL4, and therefore TRA expression. Thus, feminizing an SGP through expression of TRA is sufficient to prevent it from taking on a male-specific hub cell identity, independent of the fate of surrounding cells. This indicates that sex determination in the hub acts through a cell-autonomous mechanism.
A very different result was obtained when the same experiment was conducted with the msSGPs. Even though prd-GAL4 is expressed strongly in the msSGPs compared to the SGPs (Figure 5C), we did not observe any effect on msSGP development when UAS-traF was expressed using this driver (Figure 5E). The msSGPs still survived and joined the posterior of the gonad as they do in wild-type males. This indicates that expression of TRA in the msSGPs themselves is unable to cause them to develop in the female mode, in contrast to what we observed when UAS-traF was expressed throughout the embryo using tubulin-GAL4 (DeFalco et al., 2003). We conclude that sex determination in the msSGPs acts through a non-cell autonomous mechanism; another cell type besides the msSGPs themselves must determine whether the msSGPs survive, as they normally would in males, or undergo apoptosis, as they normally do in females.
We next investigated which other cell type might be regulating the survival of the msSGPs. dsx is essential for proper sex-specific development of the msSGPs (DeFalco et al., 2003), and dsx expression in the embryo is limited to the somatic gonad (SGPs and msSGPs) (Hemple and Oliver, 2007, Berkeley Drosophila Genome Project Gene Expression Database, Figure 5G). This suggests that the SGPs might regulate the development of the msSGPs, either by producing a survival signal in males, or a pro-death signal in females. To test this hypothesis, we examined the development of the msSGPs in embryos that lack SGPs. We utilized two different genetic backgrounds that block SGP specification, but not msSGP specification: double mutants of tinman and zinc finger homeodomain 1 (tin zfh-1) (Broihier et al., 1998) and mutants of abdominal A (abd-A) (Brookman et al., 1992). In both of these genotypes, the initial specification of the msSGPs in both sexes at stage 12 is normal (DeFalco et al., 2003) (Figure 5I). However, we no longer observed normal msSGPs in either females or males at later stages (stage 15), indicating that the msSGPs now died in both sexes (Figure 5J and data not shown). In tin zfh-1 mutants, msSGPs were observed in 94% (n=34) of embryos at stage 12, but msSGPs were not observed in any embryos at stage 15 (n=21). In abd-A mutants, msSGPs were observed in 100% of embryos at stage 12 (n=26), while at stage 15 (n=28), 29% had no msSGPs and 71% exhibited greatly reduced numbers of msSGPs (only 1-3 remained); no embryos exhibited a normal msSGP cluster. These data indicate that the SGPs are required for survival of the msSGPs.
We next wanted to verify that the loss of msSGPs in these genetic backgrounds was due to apoptosis, as opposed to other explanations, such as improper msSGP specification. msSGPs normally undergo apoptosis in females in a manner that is dependent on the programmed cell death gene head involution defective (hid) (DeFalco et al., 2003). If the msSGPs are lost through a similar mechanism in abd-A mutants, we expect that this would also be dependent on hid. Indeed, we found that msSGPs were restored in abd-A hid double mutants (92% of double mutant embryos had >4 msSGPs at stage 15, n=37). We conclude that msSGPs undergo apoptosis in both sexes in embryos that lack SGPs. This suggests that the SGPs regulate sex-specific development of the msSGPs, and do so through a survival signal produced only in males. The nature of this survival signal has not yet been identified, but it does not appear to act via the JAK/STAT or Wnt pathways that we have shown are active in the male but not female gonad (below) (Wawersik et al., 2005).
Finally, we investigated whether sex determination in the PC precursors acts through a cell-autonomous or non-cell autonomous mechanism. Again, we expressed TRA using prd-GAL4 and UAS-traF, and expected that if TRA is acting cell autonomously to repress PC precursor identity, then TRA-expressing cells should be unable to take on a male identity and become PC precursors. However, we found that TRA-expressing cells did exhibit SOX100B expression indicative of becoming PC precursors (Figure 5H). In contrast, expression of TRA more generally (via tubulin-GAL4 and UAS-traF) was able to completely block PC precursor formation (Figure 4D). This indicates that fat body cells do not need to be male themselves to take on the PC identity and that, like the msSGPs, the PC precursors exhibit a non-cell autonomous mechanism of sex determination.
Male-specific expression of Wnt2 in the somatic gonad regulates PC precursor formation
We next wanted to determine the mechanism that regulates non-autonomous sex determination in the PC precursors of the embryonic gonad. Since Wnt2 mutant adults lack PCs (Kozopas et al., 1998), and Wnt2 is required for formation of PC precursors in the embryonic gonad (Figure 2G), we tested whether it represents the non-autonomous signal that directly controls sexually dimorphic development of these cells. We first examined the expression of Wnt2 at the time of PC precursor specification to ascertain if there is a difference between males and females. Earlier in embryogenesis, Wnt2 is expressed in the posterior of the embryonic gonad of both sexes (Kozopas et al., 1998; Russell et al., 1992), including the msSGPs (DeFalco et al., 2003). However, at the time that PC precursors first form (stage 17), Wnt2 was observed exclusively in male gonads and was not detectable in female gonads (Figures 6A and 6B). Expression appears to be primarily in the somatic gonad (SGPs and msSGPs), and little or no expression is detected in the germ cells. Co-labeling for Wnt2 RNA and a PC precursor marker (Kr-GAL4, UAS-GFP) showed that Wnt2 expression is only in SGPs and not in the PC precursors or surrounding fat body itself (Figure S1B). Given the role of dsx in PC specification, we looked at Wnt2 expression in dsx mutants and found that Wnt2 was expressed in 100% of dsx mutant embryos (i.e., in both XX and XY gonads, n=25) (Figure 6C). This is consistent with our observations that PC precursors are present in both XX and XY dsx mutant gonads (Figures 4E and 4F).
Figure 6. Wnt2 acts non-autonomously to regulate sex-specific PC precursor specification.
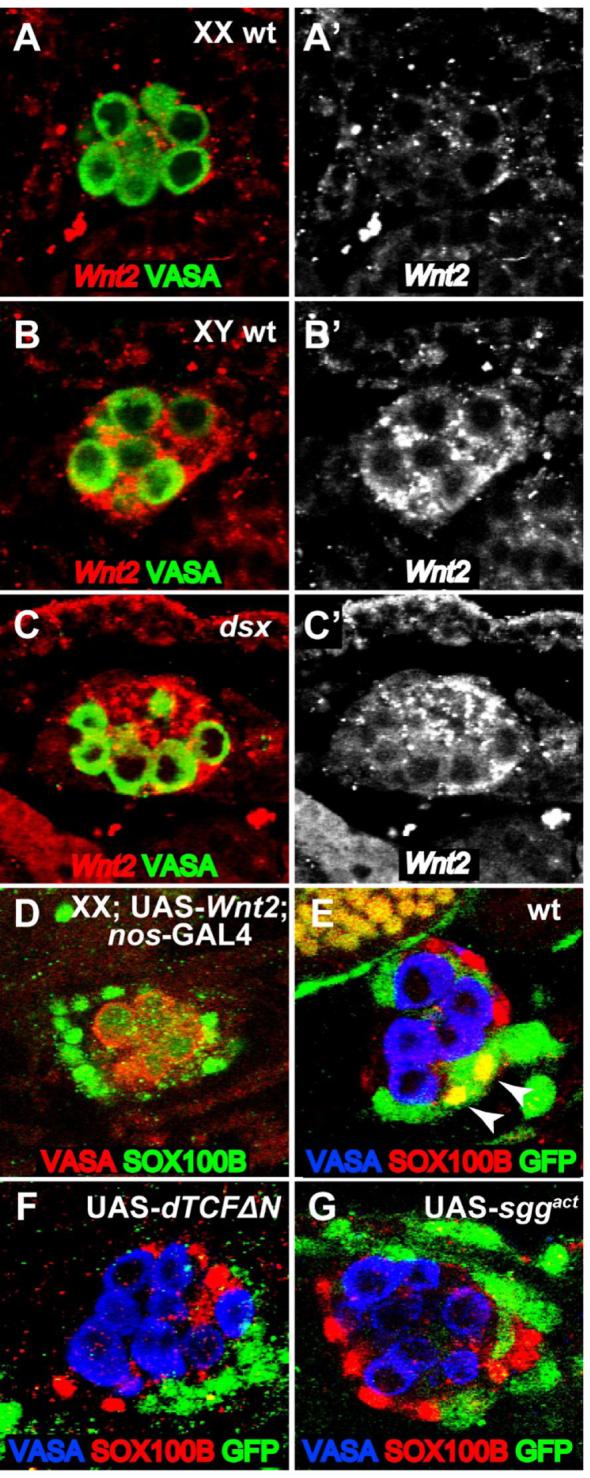
(A-C) St. 17 embryos labeled by fluorescent in situ hybridization for Wnt2 mRNA (red) and anti-VASA immunostaining (green). Anterior is to the left in each panel. (A’-C’) Wnt2 mRNA channel alone. (A,B) Wild type embryos. Wnt2 expression is robust in male embryonic gonads at st. 17 (B,B’) but is not detectable above background in females (A,A’) at this stage. (C) dsx1/dsx23 mutant gonad. Wnt2 expression is now observed in 100% of embryos. (D-G) St. 17 embryos immunostained as indicated in the figure. (D) Female embryo exhibiting SOX100B-positive PC precursors when Wnt2 is ectopically expressed in the germ cells (UAS-Wnt2, nos-GAL4). (E-G) Embryos expressing prd-GAL4 and UAS-GFP either alone (E), or in combination with UAS-dTCFΔN (F) or UAS-sggact (G). Note that some GFP-positive cells become SOX100B-positive PC precursors when expressing GFP alone (E, arrowheads), but not when expressing UAS-dTCFΔN (F) or UAS-sggact (G).
Previous work showed that Wnt2 expression is sufficient to either induce or maintain PCs in larval and adult females (Kozopas et al., 1998). To see if Wnt2 expression is sufficient to induce PC precursors at the time and place that they normally appear in the embryonic gonad, we expressed Wnt2 in gonads of both sexes. We used a germ cell-specific GAL4 driver to localize Wnt2 to the gonad and examined SOX100B expression in females. Strikingly, we found that Wnt2 expression was sufficient to induce the formation of SOX100B-expressing cells around an otherwise female gonad (Figure 6D). Expression of Wnt2 in females also induced EMS expression in these surrounding cells (Figure S1C), indicating that they are truly PC precursors. We conclude that male-specific expression of Wnt2 is necessary and sufficient to control the sexually dimorphic formation of PC precursors in the embryonic gonad.
Interestingly, SOX100B-expressing cells are only observed around the gonads at stage 17 in this experiment, even though Wnt2 expression in the gonad (germ cells) begins much earlier (Van Doren et al., 1998). This is consistent with the fact that Wnt2 is normally expressed in the somatic gonad in both males and females at earlier stages, yet PC precursors are not specified in females. Thus, the fat body must only be competent to form PC precursors at stage 17, when Wnt2 expression has normally become male-specific (Figures 6A and 6B). In addition, although Wnt2 was sufficient to induce a robust number of PC precursors around the female gonads, these cells did not exhibit the same morphology as in males and did not appear to attach as closely to the female gonads (Figure 6D). There may be other differences in the properties of the male vs. female gonads, besides Wnt2 expression, that regulate interactions with the PC precursors. We also expressed Wnt2 in other segments of the embryo (using prd-GAL4), and observed ectopic SOX100B-expressing cells in the fat body in other regions (data not shown). This suggests that the competence to form PC precursors is not limited to the fat body immediately surrounding the gonad. Lastly, the Wnt2-expressing females grow up to be fertile adults, indicating that the presence of ectopic PCs does not interfere with female gonad development.
We next wanted to address whether the Wnt2 signal is being received directly by the fat body to influence PC precursor formation. Signaling through the canonical Wnt pathway can be blocked in a cell-autonomous manner by expressing dominant negative pangolin/TCF (UAS-dTCF ΔN) (van de Wetering et al., 1997) or constitutively active shaggy (glycogen synthase kinase, UAS-sggact) (Hazelett et al., 1998). We again used the prd-GAL4 driver to express these reagents in a subset of cells in the gonad and fat body. If Wnt signaling is required in fat body cells for PC specification, we expect that cells that cannot transduce the Wnt signal will not become PC precursors and will not express SOX100B. In control embryos expressing solely prd-GAL4 and UAS-GFP, we observed that 100% of male gonads (n=27 gonads) contained some PC precursors that co-express SOX100B and GFP (Figure 6E). However, when prd-GAL4 was also driving expression of either UAS-dTCF ΔN or UAS-sggact, GFP-expressing cells near the gonad never exhibited SOX100B expression (n=26 gonads) (Figures 6F and 6G), indicating that they could no longer form PC precursors. Anti-SRP antibody staining revealed that the fat body was specified normally in these embryos, even in the domains expressing the Wnt pathway inhibitors (data not shown). Since PC precursors were still formed, but did not arise from the pool of fat body cells in which Wnt signaling was blocked, we conclude that the Wnt2 signal is received directly by the fat body to control PC specification and that it acts through the canonical Wnt pathway.
DISCUSSION
Here we have shown that two distinct male-specific cell types in the Drosophila gonad exhibit non-autonomous sex determination. For both the msSGPs and the PC precursors, the sex determination pathway does not act in these cells themselves, and both are dependent on sex-specific signaling from the SGPs in order to develop properly as male or female. These findings are in contrast to the commonly held view that sex determination in Drosophila is a cell-autonomous process, and demonstrate the similarity in sex-specific gonad development between flies and mammals.
The mechanism for creating sexual dimorphism in the PC precursors
We have identified a novel, sex-specific cell type in the Drosophila embryonic gonad, the PC precursors, and studied the mechanism by which the sex determination switch controls the sex-specific development of these cells (Figure 7). Our data indicate that male-specific expression of Wnt2 in the SGPs of the gonad signals non-autonomously to the fat body to form PC precursors. dsx ensures that PC formation is male-specific by repressing Wnt2 expression in female gonads in late-stage embryos (stage 17). The sex of the fat body itself does not affect PC precursor formation, as cells with a female identity can form PC precursors when associated with a male gonad or with a female gonad that expresses Wnt2. Furthermore, Wnt2 acts directly on the fat body, since blocking Wnt signaling in male fat body cells prevents them from forming PC precursors. PC precursor identity in the fat body is regulated by ems acting upstream of Sox100B in response to the Wnt2 signal. An interesting question is whether Wnt2 is a direct downstream target of DSX in controlling sexual dimorphism. The DNA binding specificity for DSX has been determined (Erdman et al., 1996), and there are a number of sites upstream of the Wnt2 start of transcription that either exactly match or closely match the DSX binding consensus sequence. Several of these sites are conserved between different Drosophila species (Figure S2). However, we have not yet identified a fragment of the Wnt2 promoter that allows us to test whether Wnt2 expression in the somatic gonad is directly regulated by DSX, since the upstream region that includes the putative DSX binding sites does not promote expression in the gonad (data not shown).
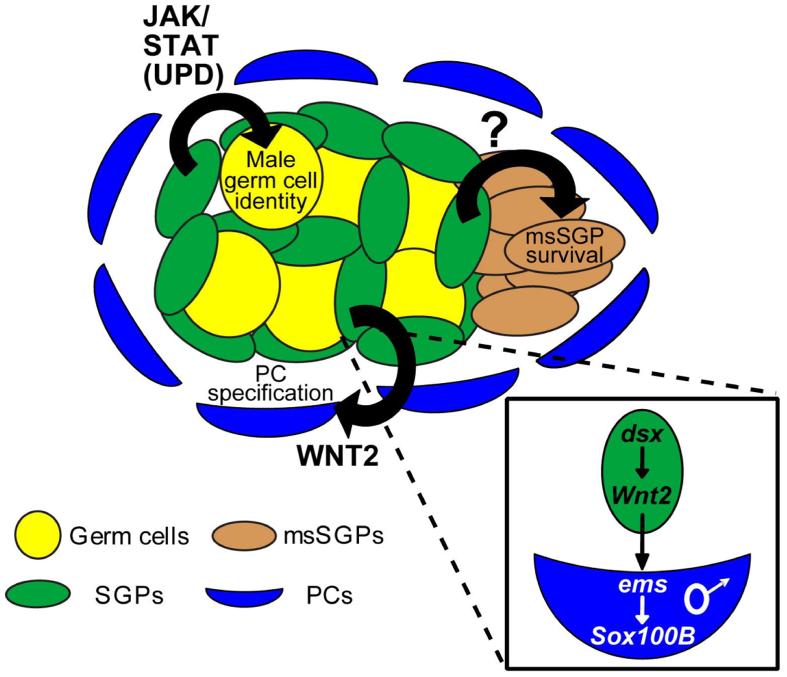
Figure 7. Model for non-autonomous control of sexual dimorphism in the Drosophila gonad.
Diagram of a male gonad. Anterior is to the left. Sexual dimorphism of three cell types is controlled non-autonomously via different types of male-specific cell-cell signaling from the SGPs: Wnt2 expression induces PC precursor formation, the JAK/STAT pathway regulates male germ cell development, and an unknown signal promotes msSGP survival in male gonads. Inset shows a model for PC specification, in which Wnt2 is regulated by dsx to induce male PC fate in surrounding fat body cells. Although Wnt2 is genetically downstream of dsx as indicated, our data indicate that dsx acts in the female gonad to directly or indirectly repress Wnt2 expression.
The creation of sexual dimorphism in the PC precursors differs from that of the msSGPs. While the PC precursors are apparently only specified in males and recruited to form part of the testis (this work), msSGPs are initially specified in both sexes, and are only present in the male gonad because they undergo programmed cell death specifically in females (DeFalco et al., 2003). Furthermore, the germline stem cell niche in the testis (the hub) is formed from a population of anterior SGPs that are present in the gonads of both sexes, but only form the hub in males and presumably form part of the ovary in females (Le Bras and Van Doren, 2006). These events are all regulated by dsx, and demonstrate the diverse cellular mechanisms that a sex determination gene can utilize to control sexual dimorphism. Interestingly, in dsx null mutant embryos each of these cell types develops as if it were male. Thus, the male mode of development can at least be initiated in these cell types in the absence of dsx function, and dsx primarily acts in females to repress male development. dsx is clearly required in males at some point for proper testis formation (Hildreth, 1965), therefore some cell types in the gonad may not be entirely masculinized in dsx mutants.
Non-autonomous sex determination in Drosophila
The non-autonomous nature of PC precursor specification contrasts with the commonly held view that sex determination in Drosophila is a cell-autonomous process, where “every cell decides for itself” whether it should develop as male or female based on its own intrinsic sex chromosome constitution. We have also shown that the msSGPs undergo non-autonomous sex determination; our data indicate that a male-specific survival signal coming from the SGPs allows the msSGPs to survive and join the male gonad, while they undergo apoptosis in females (Figure 7). Finally, we have previously shown that non-autonomous sex determination in the germ cells requires a male-specific signal from the SGPs that acts through the JAK/STAT pathway (Wawersik et al., 2005). Thus, not only does non-cell autonomous sex determination occur in the Drosophila gonad, it appears to be the predominant mechanism of sex determination. Of the cell types tested so far, only the hub cells, which form from a subset of SGPs (Le Bras and Van Doren, 2006), appear to decide their sexual fate in an autonomous manner. Our current model (Figure 7) is that the SGPs determine their sex in a cell autonomous manner, and then signal to other cell types in the gonad (PC precursors, msSGPs, and germ cells) to control the sex-specific development of these cells via non-autonomous sex determination.
Non-autonomous sex determination is not limited to the gonad; other tissues have been shown to decide their sex through cell-cell signaling. In the genital imaginal disc, the sexual identity of a signaling center, the A/P organizer, largely determines whether the disc will develop in the male or female mode. This is controlled non-cell autonomously through Wingless and Decapentaplegic signaling (Keisman et al., 2001). In addition, sex-specific migration of mesodermal cells into the male genital disc is regulated by male-specific expression of the Fibroblast Growth Factor Branchless in the genital disc (Ahmad and Baker, 2002). Finally, in the nervous system, male neurons can non-cell autonomously induce the formation of the male-specific muscle of Lawrence from female muscle precursors (Lawrence and Johnston, 1986). Given the large number of tissues and cell types that undergo non-autonomous sex determination, it seems that we can abandon the conventional view that sex determination in Drosophila is an obligatorily cell-autonomous process; while some cell types utilize a cell-autonomous mechanism, many cell types clearly do not.
One reason why sex determination has been traditionally thought of as a cell-autonomous process in Drosophila is due to its relationship with X chromosome dosage compensation. This is the process by which gene expression from the single X chromosome in males is regulated to match that from the two X chromosomes in females. Both sex determination and X chromosome dosage compensation are regulated by the number of X chromosomes, acting through the master control gene Sex lethal (Sxl) (Cline and Meyer, 1996). It is likely that most or all cells count their X chromosomes and use this information to control X chromosome dosage in a cell-autonomous manner. However, as discussed above, it is now clear that cells do not necessarily use this information to decide their sex. Consistent with this idea, the expression of dsx, a key regulator of sex determination downstream of Sxl, is surprisingly tissue specific. Within the embryo, dsx is only expressed in the SGPs and msSGPs of the gonad (Hemple and Oliver, 2007, BDGP GEP, Figure 5G). Thus, not all cells even express the machinery to translate their sex chromosome constitution into sexual identity, and it is therefore necessary that sex-specific development of many cell types be controlled non-autonomously.
Common mechanisms control gonad sexual dimorphism in diverse species
The non-autonomous cell-cell interactions that control gonad sexual dimorphism in Drosophila show great similarity to sex-specific gonad development in other species. In mammals, somatic sex determination is based on the presence or absence of the Y chromosome. The critical Y chromosome gene Sry is mainly expressed in a subset of cells in the somatic gonad in the mouse embryo (Albrecht and Eicher, 2001; Koopman et al., 1990), similar to dsx expression in the Drosophila embryonic gonad. Sry is only thought to be important for formation of Sertoli cells in males, and the sexually dimorphic development of all other cell types is thought to be regulated by local cell-cell interaction or hormonal cues (reviewed in (Ross and Capel, 2005)). An excellent example of non-autonomous sex determination in the mouse is the recruitment of cells from the neighboring mesoderm (mesonephros) to form specific cell types in the mouse testis (Buehr et al., 1993; Martineau et al., 1997). Recruitment of these cells is dependent on the sex of the gonad, not the sex of the mesonephros (Capel et al., 1999; Tilmann and Capel, 1999). In addition, sex-specific development of other somatic cell types in the mouse gonad is regulated non-autonomously by cell-cell interaction, as is sexual identity in the germline. Thus, the use of non-cell autonomous sex determination and sex-specific cell recruitment are common mechanisms for creating gonad sexual dimorphism in flies and mice.
Non-autonomous sex determination in the mouse also utilizes signaling through the Wnt pathway. Wnt4 acts as a “pro-female” gene that opposes Fibroblast growth factor 9 to regulate sex determination in the gonad (Kim et al., 2006). In early stages of gonad development, Wnt4 knockout females form a male-specific coelomic blood vessel and exhibit ectopic migratory steroidogenic cells, suggesting that Wnt4 acts to inhibit endothelial cell and steroid cell migration from the mesonephros into the female gonad (Jeays-Ward et al., 2003). Interestingly, Wnt4 also has been shown to have a role in the male gonad, as male knockout mice show defects in Sertoli cell differentiation, downstream of Sry but upstream of Sox9 (Jeays-Ward et al., 2004). Wnt7a also has been implicated in sexual dimorphism in the reproductive tract, as Wnt7a knockout mice fail to express Mullerian-inhibiting substance (MIS) type II receptor in the Mullerian duct mesenchyme, which is required for regression of the duct in male embryos (Parr and McMahon, 1998). In addition, a number of Wnt genes have been found to be expressed sex-specifically in the gonad through sex-specific gene profiling (Nef et al., 2005), indicating that other Wnt family members play a role in creating sexual dimorphism in the mammalian gonad.
It is also interesting that several conserved transcription factors act during gonad development in diverse species. Sox100B is the fly homolog of SOX9/Sox9, a critical regulator of sex determination and male gonad development in humans and mice (Chaboissier et al., 2004; Foster et al., 1994; Huang et al., 1999; Wagner et al., 1994). Similarly, a mouse homolog of ems, Emx2, is expressed in the developing gonad and is required for development of the urogenital system (Miyamoto et al., 1997). Lastly, dsx homologs of the DMRT family have been implicated in sex-specific gonad development in diverse species (Hodgkin, 2002). Thus, not only are the cellular mechanisms, such as non-cell autonomous sex determination and cell-cell recruitment, common between flies and mice, but the specific genes that regulate sexually dimorphic gonad development may also be conserved. Since the formation of testes vs. ovaries, and sperm vs. egg, are critical features of sexual reproduction, they may represent processes that are highly conserved across the animal kingdom.
EXPERIMENTAL PROCEDURES
Fly Stocks
The following stocks were used: HCJ199(ry-) (W. Bender) (Bender and Hudson, 2000), Wnt2I, Wnt2O (R. Nusse), svp07842 (svp-lacZ), srp3, ems1, tra1, dsx1, dsx23, abd-AMX1 , tinGC14 zfh-175.26 (R. Lehmann), hidWR+X1 (A. Bergmann), Df(3L)H99, UAS-GAL412B, UAS-GFP.nls8, UAS-GFP.nls14, UAS-traF-20J7 (Ferveur et al., 1995), UAS-dTCF ΔN (van de Wetering et al., 1997) and UAS-sggS9A (also called UAS-sggact) (Hazelett et al., 1998) (J. Treisman), even-skipped stripe3+7-GAL4 (S. Small), paired-GAL4 (Brand and Perrimon, 1993), nanos 3′UTR::VP16-GAL4 (nos-GAL4) (Van Doren et al., 1998), tubulin-GAL4-LL7, lacZ[857] (E. Matunis; Gönczy, 1995), lacZ[B-57] (E. Matunis; Gönczy, 1995). Kr-GAL4, UAS-GFP used was present on both TM3 and CyO balancer chromosomes (TM3, P[GAL4-Kr.C]DC2, P[UAS-GFP.S65T]DC10 and CyO, P[GAL4-Kr.C]DC3, P[UAS-GFP.S65T]DC7) (Casso et al., 2000) and showed similar gonad expression with both chromosomes. Lineage tracing using the UAS-GAL4 construct has been successfully used in the nervous system (Hassan et al., 2000) and in the gonad (Le Bras and Van Doren, 2006). w1118 was used as a wild-type control. Information about unspecified stocks can be found on Flybase (http://flybase.bio.indiana.edu).
Antibody Stainings and In Situ Hybridization
Embryos were fixed and stained as described in (DeFalco et al., 2003). Stage 17 embryos were subjected to a single three-second pulse with a Branson Sonifier 250 in order to facilitate antibody penetration through cuticle, as in (Le Bras and Van Doren, 2006). Following staining, embryos were mounted in 2.5% DABCO (Sigma-Aldrich, St. Louis, MO, USA) and mounted on slides for imaging on a Zeiss 510 Meta confocal microscope. Pictures of embryos and embryonic gonads are always oriented so that anterior is to the left. Adult testes and ovaries were dissected in PBS, followed by a 30-minute room temperature fixation in 4.5% formaldehyde in PBS containing 0.1% Triton X-100 (PBTx). Staining was performed as described in (Gönczy et al., 1997), and samples were mounted on slides in 2.5% DABCO.
The following antibodies were used: chicken anti-VASA (K.Howard) at 1:5,000 or 1:10,000; rabbit anti-VASA (R. Lehmann) at 1:10,000; rabbit anti-SOX100B at 1:1,000 (S. Russell); mouse anti-ABD-B 1A2E9 (Development Studies Hybridoma Bank: DSHB, S. Celniker) at 1:50; mouse anti-EYA 10H6 (DSHB, S. Benzer/N.M. Bonini) at 1:25; rabbit anti-GFP (Torrey Pines) at 1:2,000; mouse anti-GFP (Santa Cruz) at 1:50; mouse anti-FAS3 7G10 (DSHB, C. Goodman) at 1:30; rabbit anti-β-GAL (Cappel) at 1:10,000; mouse anti-β-GAL (Promega) at 1:10,000; mouse anti-SXL M18 (DSHB, P. Schedl) at 1:50; rat anti-DN-cadherin Ex #8 (DSHB, T. Uemura) at 1:20, rabbit anti-EMS at 1:500 (U. Walldorf); guinea pig anti-TJ (D. Godt) at 1:3,000 and rabbit anti-SRP (R. Reuter) at 1:1,000. The following secondary antibodies were all used at 1:500: Cy5 goat anti-chicken (Rockland), Alexa 546 goat anti-chicken, Alexa 546 or 488 anti-rabbit, Alexa 546 or 488 goat anti-mouse and Alexa 488 goat anti-guinea pig. All Alexa antibodies are from Molecular Probes (Invitrogen, Carlsbad, CA, USA).
In situ hybridization was performed as previously described (DeFalco et al., 2003), either using Fast Red as a fluorescent substrate (Roche, Indianapolis, IN, USA) or a colorimetric (NBT/BCIP) substrate, except that antibody staining was performed after the in situ reaction was developed.
Genotyping and sexing of embryos
In these experiments, GFP or lacZ-expressing balancer chromosomes were used to distinguish homozygous mutant embryos from balancer-containing heterozygous siblings. Sexing of embryos was done via female-specific anti-SXL staining (Figures 1E-1F, 2F-2G, 3C, 3F-3G, 4A-4H, 5E, and 6D) or lacZ-expressing X chromosomes (Figures 1A-1D, 3B, 3D-3E, and 6A-6B) as described in (DeFalco et al., 2003). Additionally, male-specific anti-SOX100B staining (Figures 1G-1H, 2C, 3A, 5C, 5H, and 6E-6G) (DeFalco et al., 2003) or male-specific anti-NCAD staining (Figures 5D and 5F) (Le Bras and Van Doren, 2006) was also used to determine sex of embryos.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the numerous colleagues who have generously supplied us with fly stocks and reagents for this work, and we have specifically acknowledged them within the Experimental Procedures. We also acknowledge the Bloomington Stock Center (Indiana University) and the Developmental Studies Hybridoma Bank (University of Iowa) for providing reagents. We are grateful to Leonie Hemple and Brian Oliver, along with the Berkeley Drosophila Genome Project’s Gene Expression Patterns Project, for information regarding the doublesex expression pattern. We thank Michael McCaffery and the JHU Integrating Imaging Center for help with confocal microscopy. We thank members of the Van Doren Lab for helpful discussions and critical reading of the manuscript. This work was supported by the National Institutes of Health (HD46619).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmad SM, Baker BS. Sex-specific deployment of FGF signaling in Drosophila recruits mesodermal cells into the male genital imaginal disc. Cell. 2002;109:651–661. doi: 10.1016/s0092-8674(02)00744-4. [DOI] [PubMed] [Google Scholar]
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92–107. doi: 10.1006/dbio.2001.0438. [DOI] [PubMed] [Google Scholar]
- Baker BS, Ridge KA. Sex and the single cell. I. On the action of major loci affecting sex determination in Drosophila melanogaster. Genetics. 1980;94:383–423. doi: 10.1093/genetics/94.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod. 2006;74:195–201. doi: 10.1095/biolreprod.105.045930. [DOI] [PubMed] [Google Scholar]
- Bender W, Hudson A. P element homing to the Drosophila bithorax complex. Development. 2000;127:3981–3992. doi: 10.1242/dev.127.18.3981. [DOI] [PubMed] [Google Scholar]
- Boyle M, DiNardo S. Specification, migration and assembly of the somatic cells of the Drosophila gonad. Development. 1995;121:1815–1825. doi: 10.1242/dev.121.6.1815. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Broihier HT, Moore LA, Van Doren M, Newman S, Lehmann R. zfh-1 is required for germ cell migration and gonadal mesoderm development in Drosophila. Development. 1998;125:655–666. doi: 10.1242/dev.125.4.655. [DOI] [PubMed] [Google Scholar]
- Brookman JJ, Toosy AT, Shashidhara LS, White RA. The 412 retrotransposon and the development of gonadal mesoderm in Drosophila. Development. 1992;116:1185–1192. doi: 10.1242/dev.116.4.1185. [DOI] [PubMed] [Google Scholar]
- Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development. 1993;117:273–281. doi: 10.1242/dev.117.1.273. [DOI] [PubMed] [Google Scholar]
- Burtis KC, Baker BS. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989;56:997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- Burtis KC, Coschigano KT, Baker BS, Wensink PC. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J. 1991;10:2577–2582. doi: 10.1002/j.1460-2075.1991.tb07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel B, Albrecht KH, Washburn LL, Eicher EM. Migration of mesonephric cells into the mammalian gonad depends on Sry. Mech Dev. 1999;84:127–131. doi: 10.1016/s0925-4773(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Casso D, Ramirez-Weber F, Kornberg TB. GFP-tagged balancer chromosomes for Drosophila melanogaster. Mech Dev. 2000;91:451–454. doi: 10.1016/s0925-4773(00)00248-3. [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- Cline TW, Meyer BJ. Vive la difference: males vs females in flies vs worms. Annu Rev Genet. 1996;30:637–702. doi: 10.1146/annurev.genet.30.1.637. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Wensink PC. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev. 1993;7:42–54. doi: 10.1101/gad.7.1.42. [DOI] [PubMed] [Google Scholar]
- Dalton D, Chadwick R, McGinnis W. Expression and embryonic function of empty spiracles: a Drosophila homeo-box gene with two patterning functions on the anterior-posterior axis of the embryo. Genes Dev. 1989;3:1940–1956. doi: 10.1101/gad.3.12a.1940. [DOI] [PubMed] [Google Scholar]
- DeFalco T, Le Bras S, Van Doren M. Abdominal-B is essential for proper sexually dimorphic development of the Drosophila gonad. Mech Dev. 2004;121:1323–1333. doi: 10.1016/j.mod.2004.07.001. [DOI] [PubMed] [Google Scholar]
- DeFalco TJ, Verney G, Jenkins AB, McCaffery JM, Russell S, Van Doren M. Sex-specific apoptosis regulates sexual dimorphism in the Drosophila embryonic gonad. Dev Cell. 2003;5:205–216. doi: 10.1016/s1534-5807(03)00204-1. [DOI] [PubMed] [Google Scholar]
- Erdman SE, Chen HJ, Burtis KC. Functional and genetic characterization of the oligomerization and DNA binding properties of the Drosophila doublesex proteins. Genetics. 1996;144:1639–1652. doi: 10.1093/genetics/144.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur JF, Stortkuhl KF, Stocker RF, Greenspan RJ. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science. 1995;267:902–905. doi: 10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Fuller MT. Spermatogenesis. In: Bate M, Martinez-Arias M, editors. The Development of Drosophila. Cold Spring Harbor Press; Cold Spring Harbor, New York: 1993. pp. 71–147. [Google Scholar]
- Gilbert SF. Developmental Biology. 8 edn Sinauer Associates; Sunderland, Mass.: 2006. [Google Scholar]
- Gönczy P. Thesis: Towards a molecular genetic analysis of spermatogenesis in Drosophila. The Rockefeller University; New York: 1995. p. 184. [Google Scholar]
- Gönczy P, Matunis E, DiNardo S. bag-of-marbles and benign gonial cell neoplasm act in the germline to restrict proliferation during Drosophila spermatogenesis. Development. 1997;124:4361–4371. doi: 10.1242/dev.124.21.4361. [DOI] [PubMed] [Google Scholar]
- Hadorn E, Bertani G. Induktion mannlicher Pigmentierung in somatischen Zellen von Drosophila-Ovarien. Rev Suisse Zool. 1948;55:232–248. [Google Scholar]
- Hassan BA, Bermingham NA, He Y, Sun Y, Jan YN, Zoghbi HY, Bellen HJ. atonal regulates neurite arborization but does not act as a proneural gene in the Drosophila brain. Neuron. 2000;25:549–561. doi: 10.1016/s0896-6273(00)81059-4. [DOI] [PubMed] [Google Scholar]
- Hazelett DJ, Bourouis M, Walldorf U, Treisman JE. decapentaplegic and wingless are regulated by eyes absent and eyegone and interact to direct the pattern of retinal differentiation in the eye disc. Development. 1998;125:3741–3751. doi: 10.1242/dev.125.18.3741. [DOI] [PubMed] [Google Scholar]
- Hemple LU, Oliver B. Sex-specific Doublesex[M] expression in subsets of Drosophila somatic gonad cells. BMC Dev Biol. 2007;7:113. doi: 10.1186/1471-213X-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth PE. Doublesex, Recessive Gene That Transforms Both Males and Females of Drosophila into Intersexes. Genetics. 1965;51:659–678. doi: 10.1093/genetics/51.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J. The remarkable ubiquity of DM domain factors as regulators of sexual phenotype: ancestry or aptitude? Genes Dev. 2002;16:2322–2326. doi: 10.1101/gad.1025502. [DOI] [PubMed] [Google Scholar]
- Hoshizaki DK, Blackburn T, Price C, Ghosh M, Miles K, Ragucci M, Sweis R. Embryonic fat-cell lineage in Drosophila melanogaster. Development. 1994;120:2489–2499. doi: 10.1242/dev.120.9.2489. [DOI] [PubMed] [Google Scholar]
- Huang B, Wang S, Ning Y, Lamb AN, Bartley J. Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet. 1999;87:349–353. doi: 10.1002/(sici)1096-8628(19991203)87:4<349::aid-ajmg13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Jeays-Ward K, Dandonneau M, Swain A. Wnt4 is required for proper male as well as female sexual development. Dev Biol. 2004;276:431–440. doi: 10.1016/j.ydbio.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- Jenkins AB, McCaffery JM, Van Doren M. Drosophila E-cadherin is essential for proper germ cell-soma interaction during gonad morphogenesis. Development. 2003;130:4417–4426. doi: 10.1242/dev.00639. [DOI] [PubMed] [Google Scholar]
- Keisman EL, Christiansen AE, Baker BS. The sex determination gene doublesex regulates the A/P organizer to direct sex-specific patterns of growth in the Drosophila genital imaginal disc. Dev Cell. 2001;1:215–225. doi: 10.1016/s1534-5807(01)00027-2. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4:e187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P, Munsterberg A, Capel B, Vivian N, Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990;348:450–452. doi: 10.1038/348450a0. [DOI] [PubMed] [Google Scholar]
- Kozopas KM, Samos CH, Nusse R. DWnt-2, a Drosophila Wnt gene required for the development of the male reproductive tract, specifies a sexually dimorphic cell fate. Genes Dev. 1998;12:1155–1165. doi: 10.1101/gad.12.8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Johnston P. The muscle pattern of a segment of Drosophila may be determined by neurons and not by contributing myoblasts. Cell. 1986;45:505–513. doi: 10.1016/0092-8674(86)90282-5. [DOI] [PubMed] [Google Scholar]
- Le Bras S, Van Doren M. Development of the male germline stem cell niche in Drosophila. Developmental biology. 2006;294:92–103. doi: 10.1016/j.ydbio.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Curr Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development. 1997;124:1653–1664. doi: 10.1242/dev.124.9.1653. [DOI] [PubMed] [Google Scholar]
- Moore LA, Broihier HT, Van Doren M, Lehmann R. Gonadal mesoderm and fat body initially follow a common developmental path in Drosophila. Development. 1998;125:837–844. doi: 10.1242/dev.125.5.837. [DOI] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Nothiger R, Jonglez M, Leuthold M, Meier-Gerschwiler P, Weber T. Sex determination in the germ line of Drosophila depends on genetic signals and inductive somatic factors. Development. 1989;107:505–518. doi: 10.1242/dev.107.3.505. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395:707–710. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- Qin Y, Bishop CE. Sox9 is sufficient for functional testis development producing fertile male mice in the absence of Sry. Hum Mol Genet. 2005;14:1221–1229. doi: 10.1093/hmg/ddi133. [DOI] [PubMed] [Google Scholar]
- Riechmann V, Rehorn KP, Reuter R, Leptin M. The genetic control of the distinction between fat body and gonadal mesoderm in Drosophila. Development. 1998;125:713–723. doi: 10.1242/dev.125.4.713. [DOI] [PubMed] [Google Scholar]
- Ross AJ, Capel B. Signaling at the crossroads of gonad development. Trends in endocrinology and metabolism: TEM. 2005;16:19–25. doi: 10.1016/j.tem.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Russell J, Gennissen A, Nusse R. Isolation and expression of two novel Wnt/wingless gene homologues in Drosophila. Development. 1992;115:475–485. doi: 10.1242/dev.115.2.475. [DOI] [PubMed] [Google Scholar]
- Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/s0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- Sam S, Leise W, Hoshizaki DK. The serpent gene is necessary for progression through the early stages of fat-body development. Mech Dev. 1996;60:197–205. doi: 10.1016/s0925-4773(96)00615-6. [DOI] [PubMed] [Google Scholar]
- Staab S, Heller A, Steinmann-Zwicky M. Somatic sex-determining signals act on XX germ cells in Drosophila embryos. Development. 1996;122:4065–4071. doi: 10.1242/dev.122.12.4065. [DOI] [PubMed] [Google Scholar]
- Steinmann-Zwicky M, Schmid H, Nothiger R. Cell-autonomous and inductive signals can determine the sex of the germ line of drosophila by regulating the gene Sxl. Cell. 1989;57:157–166. doi: 10.1016/0092-8674(89)90181-5. [DOI] [PubMed] [Google Scholar]
- Stern C, Hadorn E. The Relation between the Color of Testes and Vasa Efferentia in Drosophila. Genetics. 1939;24:162–179. doi: 10.1093/genetics/24.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilmann C, Capel B. Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development. 1999;126:2883–2890. doi: 10.1242/dev.126.13.2883. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- Van Doren M. Development of the Somatic Gonad and Fat Bodies. In: Sink H, editor. Muscle Development in Drosophila. Springer; New York: 2006. pp. 51–61. [Google Scholar]
- Van Doren M, Williamson AL, Lehmann R. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol. 1998;8:243–246. doi: 10.1016/s0960-9822(98)70091-0. [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Wawersik M, Milutinovich A, Casper AL, Matunis E, Williams B, Van Doren M. Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature. 2005;436:563–567. doi: 10.1038/nature03849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- Wolpert L, Smith J, Jessell T, Lawrence P, Robertson E, Meyerowitz E. Principles of Development. 3 edn Oxford University Press; Oxford: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.