Abstract
Gap junctions permit the direct passage of small molecules from the cytosol of one cell to that of its neighbor, and thus form a system of cell-cell communication that exists alongside familiar secretion/receptor signaling. Because of the rich potential for regulation of junctional conductance, and directional and molecular gating (specificity), gap junctional communication (GJC) plays a crucial role in many aspects of normal tissue physiology. However, the most exciting role for GJC is in the regulation of information flow that takes place during embryonic development, regeneration, and tumor progression. The molecular mechanisms by which GJC establishes local and long-range instructive morphogenetic cues are just beginning to be understood. This review summarizes the current knowledge of the involvement of GJC in the patterning of both vertebrate and invertebrate systems and discusses in detail several morphogenetic systems in which the properties of this signaling have been molecularly characterized. One model consistent with existing data in the fields of vertebrate left-right patterning and anterior-posterior polarity in flatworm regeneration postulates electrophoretically-guided movement of small molecule morphogens through long-range GJC paths. The discovery of mechanisms controlling embryonic and regenerative GJC-mediated signaling, and identification of the downstream targets of GJC-permeable molecules, represent exciting next areas of research in this fascinating field.
Keywords: gap junctional communication, embryonic patterning, asymmetry, model, electrophoresis
Introduction
The mechanisms that allow an embryo to reliably self-assemble the complex morphology, physiology, and behavior appropriate to its species represent one of the most fundamental and fascinating areas of research in modern science. Pattern is acquired simultaneously on three orthogonal axes, and on several logs of scale, from the molecular (e.g., cytoskeletal elements) to the organismic (graded antero-posterior (AP), dorso-ventral (DV), and left-right (LR) axial specification). Though some animals undergo a mosaic mode of development, most embryos show impressive abilities to regulate in the face of environmental or genetic perturbations. The events underlying this generation and maintenance of form require a complex web of information flow between cell and tissue subsystems during development. With the advent of multi-cellularity, the cell membrane has become a key nexus for regulatory information flow. Receptor-mediated signal exchange via secreted messenger molecules has been studied extensively. However, another important system of signaling exists: the direct cell-cell exchange of small molecules through gap junctions.
The cell biology of gap junctions has been described in a number of excellent recent reviews (Falk, 2000; Goodenough et al., 1996); the reader is also referred to the classic (Loewenstein, 1981) for a historical and detailed perspective of some of the definitive studies on gap junction properties. This extremely versatile system for communication allows for rapid synchronization among cells in a tissue and the passage of signals, both of which can be regulated at many levels. Indeed cells often up-regulate gap junctional communication when they need to share ions with their neighbors (Aslanidi et al., 1991; Larre et al., 2006). Thus, GJC is a perfect conduit for information flow during development, which depends on the ability of cells and tissues to communicate. The converse however is also paramount - embryos contain independent compartments (Rela and Szczupak, 2004; Sutor and Hagerty, 2005) that must remain isolated for proper morphology to result.
Gap junctions have been studied for years using biophysical methods (such as electron microscopy and X-ray diffraction), but some of the most exciting information on gap junctions has come more recently, from the use of molecular embryology techniques to probe the role of GJC in physiological processes (Goodenough and Musil, 1993; Lo, 1999). A number of human syndromes have been identified as mutations in gap junction genes (Maestrini et al., 1999; Richard et al., 2002), and transgenic mice are beginning to allow the molecular dissection of GJC function in these contexts (Krutovskikh and Yamasaki, 2000). For example, GJC is now known to be a general mechanism for achieving rapid syncitial communication within a tissue. Contexts include the spread of electric waves in cardiac tissue (Kimura et al., 1995; Severs, 1999) and the brain (Budd and Lipton, 1998; Momose-Sato et al., 2004; Momose-Sato et al., 2005), Ca++ transients controlling interkinetic nuclear movement in retinal cells (Pearson et al., 2005b), and the spread of signals through gland cells to synchronize hormonal action and secretion (Meda, 1996).
Molecularly, gap junctions can consist of proteins from a number of gene families. Vertebrate gap junctions are commonly assumed to consist of connexins (Cruciani and Mikalsen, 2006; Sohl and Willecke, 2003; Sohl and Willecke, 2004). In contrast, invertebrate gap junctions are made of innexins, a family formerly called OPUS (Barnes, 1994). The ability of innexins to form functional gap junction channels has recently been demonstrated directly for a number of innexins (Landesman et al., 1999; Phelan et al., 1998; Stebbings et al., 2000). Innexins show no sequence homology to connexins but have the same topology, including four transmembrane domains (Phelan, 2005; Stebbings et al., 2002). Interestingly, this simple division does not reflect the complexity that is now becoming uncovered. First, it is becoming increasingly clear that proteins from the innexin family exist in vertebrates, prompting their proposed renaming to “pannexins” (Bao et al., 2004; Baranova et al., 2004; Bruzzone et al., 2005; Bruzzone et al., 2003; Connors and Long, 2004; Ray et al., 2005; Sohl et al., 2005). Indeed, members of these genes have also been identified in viruses (Turnbull and Webb, 2002; Turnbull et al., 2005), as well as being expressed in tissues known to rely on GJC (Dvoriantchikova et al., 2006; Ray et al., 2005; Sohl et al., 2005). Second, although connexin genes have not been demonstrated in lower invertebrate genomes, connexins from invertebrate chordates have been described (Sasakura et al., 2003; White et al., 2004).
There have even been suggestions of connexin-like proteins in plants (Meiners et al., 1991; Yahalom et al., 1991), although this is quite controversial (Mushegian and Koonin, 1993). Moreover, in hydra, an antibody generated to a vertebrate connexin appears to modulate GJC-dependent morphogenesis (Fraser et al., 1987). Hydra is known by ultrastructural methods to contain gap junctions (Wood and Kuda, 1980), which have been proposed to serve as the conduit for positional information during regeneration (Wakeford, 1979) because they form between graft and host tissue during inhibition of head regeneration by grafted head tissue. The functional data in hydra using a connexin-based reagent suggests the possibility that connexin-like motifs remain to be discovered in other invertebrate systems. Finally, there is ductin - a VATPase H+ pump component that appears to form a hexamer trans-membrane channels on its own in both vertebrate and invertebrate systems (Bohrmann and Lammel, 1998; Finbow et al., 1992; Finbow et al., 1991; Finbow et al., 1995; Finbow and Pitts, 1998; Inoue et al., 1999; Saito et al., 1998; Skinner et al., 1999). Ductin (like connexins) has four transmembrane domains, exists in plants and most metazoan animals, and has approximately 85% identity between insects and human. The role of ductin in endogenous GJC is currently controversial and remains an important area for investigation (Bruzzone and Goodenough, 1995; Finbow et al., 1994; Finbow and Pitts, 1993).
What sorts of signals endogenously traverse gap junctions during patterning? In general, a variety of small molecules and metabolites are thought to permeate. cAMP (Bedner et al., 2003; Burnside and Collas, 2002; Webb et al., 2002), ATP (Bao et al., 2004; Pearson et al., 2005a), and Ca++ (Blomstrand et al., 1999; Paemeleire et al., 2000; Toyofuku et al., 1998) have been investigated in a number of physiological contexts, but endogenous morphogenetic roles for these signals have not yet been defined in the context of GJC. Interestingly, several recent reports have demonstrated that molecules much larger than the normal ≈1 kDa size cutoff can penetrate through gap junctions under some circumstances (Brooks and Woodruff, 2004; Valiunas et al., 2005b). These include siRNA’s and calmodulin; one possibility is that the crucial parameter is shape, not overall size, and that long, thin molecules may be able to traverse gap junction channels. The alignment of such molecules to facilitate GJC-mediated transfer has been suggested to be performed by an endogenous electric field (Woodruff, 2005). The issues of endogenous GJC-permeable signals and the roles of electric fields in regulating movement of signals through GJC in patterning systems will be examined in detail below. However, it is crucial that junctional selectivity can result in radically-different permeabilities of gap junctions to different types of molecules (Bevans et al., 1998; Goldberg et al., 1999; Goldberg et al., 2002; Nicholson et al., 2000). This may have important consequences for the morphogenetic system, and means that studies of this process in vivo may be strongly dependent upon the experimental probe used. For example, at 7.5 days, the mouse embryo was found to be subdivided into at least nine GJC compartments with respect to Lucifer Yellow (LY) transfer, but only two domains with respect to ionic coupling (Kalimi and Lo, 1989). In the loach (Misgurnus fossilis), LY, fluorescein, and DAPI showed consistent differences in their ability to transfer between tissues during mesoderm induction and patterning (Bozhkova and Rozanova, 2000). Moreover, the chemical selectivity of the gap junctions connecting early embryonic cells changes appreciably during loach development (Bozhkova, 1998), suggesting that embryonic patterning can utilize regulation of not only the amount of GJC but also of the various types of molecules being passed through the gap junctions. These results clearly underscore the need to explore GJC with a variety of different probes to truly understand what paths are available for endogenous small molecules and current in the embryo. Moreover, the determinants of GJC selectivity, including PKC-dependent phosphorylation and specific connexin make-up of the gap junctions (Ayad et al., 2006; Ek-Vitorin et al., 2006; Weber et al., 2004), represent important upstream regulatory factors that must be characterized during embryogenesis.
Before considering the role of GJC in complex pattern formation, it is important to be aware of gap junctional signaling’s role in two special cases: control of stem cell behavior and the misregulation of pattern that occurs during neoplastic growth (Pierce, 1983; Pierce et al., 1986; Ruiz i Altaba et al., 2004). One of the most tantalizing roles for GJC is in the processes that distinguish normal tissue from tumor cells (Krutovskikh and Yamasaki, 1997; Li and Herlyn, 2000; Loewenstein and Rose, 1992; Mesnil et al., 2005; Omori et al., 2001; Yamasaki et al., 1995). It has been shown that normal tissue generally possesses a much higher degree of GJC than tumor tissue, and a loss of GJC accompanies early steps in neoplastic transformation (Pitts et al., 1988). Moreover, a neoplastic phenotype can be induced in cell culture by ectopic closing of gap junctions using pharmacological agents or dominant-negative constructs (Omori and Yamasaki, 1998). Most interestingly, neoplastic characteristics can be suppressed by ectopic induction of GJC in tumor tissue (Hellmann et al., 1999; Mehta et al., 1991; Rose et al., 1993). More recently it has been shown that non-transformed cells can effectively normalize the conductance of gap junctions expressed by adjacent tumor cells (Valiunas et al., 2005a). These data are consistent with a role of GJC in mediating the information flow that is necessary for coordinated tissue activity and morphogenesis, which is lost in tumors (Pierce, 1983; Pierce et al., 1986; Rubin, 1985). However, in some cases, the epistasis of these events is still under investigation, since for example, data from cervical cancer progression suggests that loss of GJIC is perhaps a consequence of, rather than being causal to, epithelial dysplasia (Aasen et al., 2003).
The fine balance of proliferation and differentiation must also be maintained in stem cell populations. Gap junctions are beginning to be uncovered as an element of this process (Sheardown and Hooper, 1992; Tazuke et al., 2002; Trosko et al., 2000; Trosko, 2005; Wong et al., 2004). Interestingly, neural stem cells exhibit a unique signature based on GJC and ion transporters, and GJC based on C×43 and C×45 is essential for their survival and proliferation (Cai et al., 2004). For example, the gap junction protein Zero Population Growth (zpg) is required for germ cell differentiation in Drosophila ovary. In the absence of ZPG, the stem cell daughter destined to differentiate instead dies (Gilboa et al., 2003). Germ line stem cells differentiate upon losing contact with their niche, which likely involves GJC; as an example, it has been proposed that gap junction-mediated cAMP signaling between blastomeres and somatic cells results in changes in somatic cell gene expression (Burnside and Collas, 2002).
Overview of GJC in developmental and regenerative morphogenesis
The first step towards formulation of developmental models is knowledge of exactly what GJC paths exist among which cells in a given patterning system. Comprehensive expression analyses of gap junction genes now exist in several species (de Boer and van der Heyden, 2005; Katbamna et al., 2004; Nogi and Levin, 2005; Stebbings et al., 2002). Because of the many levels of regulation to which gap junctions are subject, the presence of connexin mRNA in a tissue is no guarantee of the existence of gap junction complexes or of functional cell-cell connectivity. Thus, some studies have addressed the localization of connexin protein by immunohistochemistry. For example, in the rabbit, C×43 and C×32 are expressed in the blastoderm at streak stages (Liptau and Viebahn, 1999). The rich regulation to which mature gap junctions are subject, and the potential for compensation among co-expressed GJC proteins, make it necessary to ascertain functional GJC in addition to mRNA and protein localization data. Direct dye coupling assays have demonstrated compartments of communication in a number of embryonic systems, including a very early observation in the squid embryo (Potter et al., 1966), the detection of ionic coupling among cells of the Triturus (Ito and Loewenstein, 1969), an investigation of electrotonic GJC in the chick blastoderm (Sheridan, 1966), and evidence for preferentially directional and pH-dependent GJC-mediated transfer in the early Xenopus embryo (Guthrie, 1984; Guthrie et al., 1988; Nagajski et al., 1989; Turin and Warner, 1980). It has also been shown using these techniques that the wing imaginal disk in Drosophila is subdivided into a number of communication compartments during differentiation (Weir and Lo, 1984).
The regulation of GJC paths by endogenous factors in embryos is key to formulating models of their role in patterning. For example, using the scrape-loading/dye transfer technique, it was shown that chick limbs exhibit a gradient of GJC along the AP axis (Coelho and Kosher, 1991). The highest GJC was observed in cells adjacent to the zone of polarizing activity, while no GJC was present at the opposite end of the limb bud. Mesenchymal tissues in the middle of the limb had an intermediate level of dye coupling and it has been hypothesized that polarizing region cells communicate to anterior mesenchyme cells via gap junctions (Allen et al., 1990). These patterns appear to be directed by FGF-4 (Makarenkova et al., 1997), while asymmetries in gap junctional coupling in the early frog embryo (Nagajski et al., 1989; Turin and Warner, 1980) appear to be controlled by members of the wnt pathway (Olson et al., 1991; Olson and Moon, 1992). Unidirectional junctions are thought to form between C×32 and C×43 (Robinson et al., 1993; Xin and Bloomfield, 1997), potentially allowing an embryo to establish one-way signaling paths. Together with pH, membrane voltage, and other asymmetries, it is clear that the potential for incredibly complex and specific signal transfer exists through GJC.
Mechanistic investigations of GJC in patterning have been fairly few (Levin, 2001; Lo, 1996; Sheardown and Hooper, 1992; Warner, 1999), but a number of early functional experiments indicated that endogenous patterns of embryonic GJC have important morphogenetic roles. Introduction of antibodies raised to specific portions of connexin proteins in mouse embryos resulted in developmental defects (Becker et al., 1995). In Xenopus, microinjection of antibodies has been reported to disrupt axial patterning (Warner et al., 1984); the treated embryos contained differentiated mesodermal derivatives such as notochord and muscle tissue and Warner et al. argued that GJC has a role in pattern formation per se, rather than in the induction of specific cell types. These are summarized in Table 1; the most data from mammalian systems are available for the limb, heart, gastrulation, and neurulation.
Table 1.
Summary of GJC involved in patterning events (only those not discussed in detail in the main text)
In invertebrate systems, C. elegans and Drosophila have been paramount in providing examples of GJC roles. Numerous defects were observed in a C. elegans innexin-3 mutant, including rupture of hypodermis, failure of elongation, and failure of pharynx to attach to anterior (Starich et al., 2003). Likewise, epithelial organization and polarity of the embryonic epidermis is dependent on heteromerization of innexins 2 and 3 in Drosophila (Bauer et al., 2004; Lehmann et al., 2006). Establishment of the proventriculus requires Innexin-2, which is a target of Wingless signaling (Bauer et al., 2002; Bauer et al., 2001). This work is particularly interesting because it links GJC to a canonical signaling pathway. Wnt signaling is likewise upstream of GJC patterns in vertebrate systems (Ai et al., 2000; Olson et al., 1991; Olson and Moon, 1992; van der Heyden et al., 1998), suggesting possible conservation of signaling modules involving GJC. Interestingly, overexpression of C×43 provides a partial recovery of the formation of the cerebellum in Wnt-1 knock-out mice (Melloy et al., 2005), strengthening the link to the Wnt pathway and suggesting that establishing ectopic domains of cell:cell communication may be a useful modality to control certain patterning defects.
Gene targeting studies of connexins in mice have provided evidence that gap junctions are important not only for the function of the mature heart (Hagendorff and Plum, 2000; Severs, 2001; Verheijck et al., 2001), but also for septation (Kirchhoff et al., 2000), and morphogenesis of the outflow tract (Gu et al., 2003a) and endocardial cushions (Kumai et al., 2000). Many of these effects appear mediated by effects on the behavior of neural crest (Ewart et al., 1997; Huang et al., 1998a; Huang et al., 1998b; Li et al., 2002; Lo et al., 1997; Lo et al., 1999; Sullivan et al., 1998; Waldo et al., 1999; Waller et al., 2000; Xu et al., 2001), although some have argued for a role of C×43 in suppressing cellular delamination from the neural tube (Wei et al., 2004). Because of the increasing data that connexins or ductin can have functions independent of their GJC activity (Huss et al., 2002; Jiang and Gu, 2005; Kalra et al., 2006), it is important to keep in mind that genetic loss-of-function studies do not guarantee that any particular phenotype is in fact due to reduction of GJC per se. For example, in contrast to models based on GJC-permeable morphogens, Wei et al. propose that in the case of cardiac cell effects, C×43 protein directly interacts with a number of structural membrane proteins (such as β-catenin, cytoskeletal proteins, and cadherins), leading to the activation of pathways that control adhesion, motility, and cell shape (Wei et al., 2004).
How might gap junction-dependent signals control pattern formation? One mechanism is through guidance of cell differentiation (Araya et al., 2005; Araya et al., 2003; Bani-Yaghoub et al., 1999; Gu et al., 2003b; Hirschi et al., 2003; Zhang et al., 2002). Another is through the regulation of proliferation (Paraguassu-Braga et al., 2003; Pearson et al., 2005a). This is likely to mediate the stunting effects of connexin loss-of-function on craniofacial morphogenesis in the chick (McGonnell et al., 2001). Importantly, the ability of connexins to regulate progression through the cell cycle may also function through direct roles of connexins in the nucleus not dependent on GJC (Dang et al., 2003; Doble et al., 2004) and this possibility needs to be considered in genetic work on gap junction proteins that does not assay GJC directly. Finally, GJC seems to control migration in some contexts (Huang et al., 1998a; Kjaer et al., 2004; Lecanda et al., 2000; Minkoff et al., 1997; Oviedo-Orta et al., 2002).
A final but very important mechanistic role for GJC is to establish patterns of isopotential, iso-pH, and homogenous [K+] cell fields (Contreras et al., 1995; Fitzharris and Baltz, 2006; Potapova et al., 1990; Sherman and Rinzel, 1991). This epigenetic “prepattern” overlaid upon the embryonic morphology, working in concert with the molecular fields defined by gene expression domains, is now recognized to be an important control element in embryonic patterning. Though the field is too broad to be reviewed here, the control of endogenous bioelectric signals by GJC paths allows gap junction-permeable molecules to ultimately direct cell migration, proliferation, and differentiation (Levin, 2003a; McCaig et al., 2005; Nuccitelli, 2003a; Nuccitelli, 2003b; Robinson and Messerli, 2003).
The phenomenon of regeneration involves a profound example of pattern formation, as the system must not only build a complex structure, but unlike the case of embryonic development, must also know which components have been damaged and must be restored; in this case, the normal time-line of morphogenesis is disrupted and the system cannot rely on a pre-programmed sequence of events but must instead exchange signals to inform the various cells and tissues of the current morphogenetic state. An ideal example is provided by planaria - flatworms with three tissue layers, bilateral symmetry, and a centralized brain, possess remarkable powers of regeneration (Sanchez Alvarado, 2003; Sanchez Alvarado, 2004; Sanchez Alvarado et al., 2003; Slack, 2003; Tessmar-Raible and Arendt, 2003). After bisection, one of the edge blastemas regenerates a head, while the other forms a tail. The ability of previously-adjacent cells to adopt radically different fates highlights the necessity of long-range signaling allowing determination of position relative to, and the identity of, remaining tissue.
Following the hypothesis that GJC may underlie this signaling, Nogi et al. (Nogi and Levin, 2005) cloned and characterized the expression of the Innexin gene family during planarian regeneration. Planarian innexins fall into 3 groups according to both sequence and expression, and the concordance between expression-based and phylogenetic grouping suggests diversification of 3 ancestral innexin genes into the large family of planarian innexins. Innexin expression was detected throughout the animal, as well as specifically in regeneration blastemas, consistent with a role in long-range signaling relevant to specification of blastema positional identity as well as signaling among cells local to the regenerating tissue. Exposure to a GJC-blocking reagent that does not distinguish among gap junctions composed of different Innexin proteins resulted in bipolar (2-headed) animals, demonstrating the alteration of a high-level morphogenetic signal (“build a head”, as opposed to “build a tail” in the posterior-facing blastema).
Taken together, the expression data and the re-specification of the posterior blastema to an anteriorized fate by GJC loss-of-function suggest that innexin-based GJC mediates instructive signaling during regeneration in planaria. Because of the availability of a simple assay in a clear-cut example of GJC-dependent patterning, and the existence of large amounts of classical as well as molecular and genomic data, this system is very likely to allow important progress on the mechanisms utilized in this spatial regulation. Although the pharmacological GJC blockers, not being subject to compensation or redundancy effects, allowed the rapid identification of an important phenotype that might have been missed if single innexin genetic loss-of-function experiments were pursued, it is now crucial to utilize RNAi-based gene abrogation to determine precisely which innexins are responsible for mediating the anterior-posterior polarity signals, and use their expression pattern and biophysical properties to formulate testable mechanistic models of how large-scale polarity is imposed on tissue.
GJC and left-right asymmetry
A considerable amount of data on the function of GJC in vertebrate patterning, including evolutionary conservation in two species, is now available in the field of left-right (LR) axial patterning. The vertebrate body-plan is based on a bilaterally-symmetrical structure; however, the visceral organs and brain display marked and consistent asymmetries in their location or geometry with respect to the embryonic midline. Left-right asymmetry is a fascinating example of large-scale embryonic patterning and consistent laterality of vertebrates raises many deep theoretical issues linking molecular stereo-chemistry with multi-cellular pattern control (Burdine and Schier, 2000; Levin, 2005). Because no macroscopic force distinguishes right from left, a powerful paradigm has been proposed to leverage large-scale asymmetry from the chirality of sub-cellular components (Brown et al., 1991; Brown and Wolpert, 1990). In this class of models, some molecule or organelle with a fixed chirality is oriented with respect to the antero-posterior and dorso-ventral axes, and its chiral nature is thus able to nucleate asymmetric processes. Thus, the first developmental event that distinguishes left from right is generally thought to take place on a subcellular scale. However, it is now known that a pathway of multi-cellular fields of asymmetric gene expression directs the laterality of asymmetric organs (Levin, 1998; Yost, 2001). A mechanism must then exist to transduce subcellular signals to cell fields, and thus convert information about LR direction to that of a cell’s position with respect to the midline.
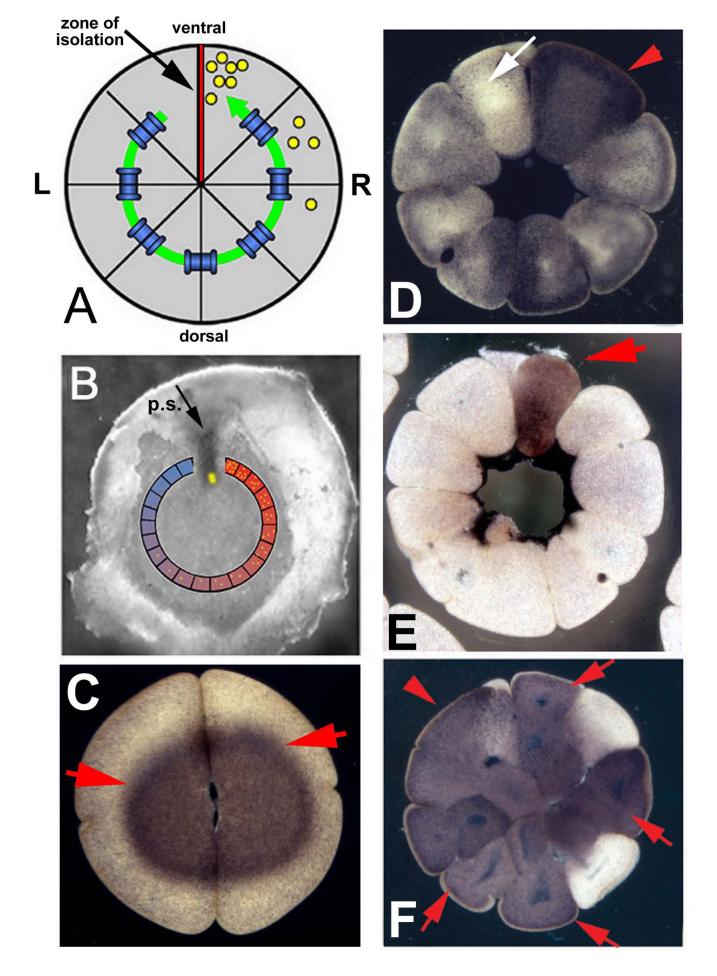
Levin and Mercola tested the hypothesis that GJC could play a role in the mechanism by which asymmetry at the level of a cell can be transduced into embryo-wide asymmetry of gene expression (Levin and Mercola, 1998a; Levin and Mercola, 1999). The initial hint of long-range signaling was provided by the observation that removing a distal lateral portion of the chick blastoderm causes ectopic gene induction in cells on the opposite side of the embryo (Fig. 1). Following initial observations on GJC in frog embryos (Guthrie et al., 1988; Warner et al., 1984), we pursued this question in embryos of Xenopus laevis. Using injections of a system of a small junctionally-permeable fluorescent dye together with a large molecule junctionally-impermeable dye (to mark the injected cell and rule out false positives due to cytoplasmic bridges and incomplete cell cleavage, Fig. 1G), we showed that there is indeed an asymmetry, with a zone of isolation across the ventral midline and good junctional coupling on the dorsal side (Fig. 2A). Misexpression of connexins lacking a regulatory region (C×26) can reveal induction of GJC in sectioned embryos (Fig. 1H), countering previous proposals that the changes in early frog embryo GJC, due to various treatments and previously reported by a number of labs (Brizuela et al., 2001; Guthrie, 1984; Guthrie and Gilula, 1989; Guthrie et al., 1988; Nagajski et al., 1989; Olson et al., 1991; Olson and Moon, 1992), were artifacts caused by lens flare effects from adjacent blastomeres and by pigmentation differences between dorsal and ventral Xenopus cells (Landesman et al., 2000).
Figure 1.

Evidence for long-range GJC in chick and frog embryos determining left-right asymmetry
Chick embryos cultured as whole blastoderms (A) exhibit the normal left-sided expression of the markers Nodal (B) and Sonic hedgehog in Hensen’s node (C), indicating normal asymmetry. In contrast, when the distal left side of the embryo is cut off before culture (D), cells on the far right side begin to express Nodal (E) and Sonic hedgehog expression in the node is bilateral (F).
Panels A and D are schematics of st. 2 chick embryos (primitive streak stage); the region encompassed by the square indicates the part that was cultured. The purple stain in panels B-F is signal, indicating expression of the relevant gene marker via in situ hybridization. Red arrowheads indicate expression; white arrowheads indicate absence of expression.
In frog embryos, small molecule fluorescent probes are able to penetrate several cell diameters through gap junctions (G). Panel G shows four large blastomeres of the 16-cell embryo. The left-most cell (indicated by red arrow) was injected with a fluorescent small molecule tracer dye, which is seen spreading to all 4 of its neighbors in a clock-wise direction. Panel H shows, in section, induced GJC (via injection of C×26 mRNA) in early frog embryos. The transfer of Lucifer Yellow (< 1kD) but not rhodamine-labeled dextran (RLD, =10 kD) in section demonstrates true gap junctional communication and rules out cytoplasmic bridges and incomplete cleavages.
Figure 2.
Serotonin movement through gap junctions during LR patterning
(A) One model consistent with the data is that in Xenopus, the long-range GJC path around the ventral zone of isolation results in a LR-asymmetric distribution of a small molecule morphogen (yellow). (B) The same model can be superimposed on the chick, where connexin43 is expressed circumferentially around, but not in, the primitive streak. (C) Serotonin is initially homogenously distributed in the early frog embryo. Within the next few hours, serotonin appears as a circumferential gradient around the zone (D) and ultimately coalesces into a single cell on one side of the midline (E). If the gap junctions are blocked, serotonin is unable to move (F). The frog embryo stages shown are 16-32-cell stages in panels A,D,E,F, and 2-cell stage in panel C. The chick embryo in panel B is at the early primitive-streak stage.
The asymmetry in ability to transfer dye could be altered in predictable ways by chemical agents that are known to regulate junction permeability. Using dominant negative and constitutively-active connexin constructs, it was shown that interruption of the circumferential GJC path, as well as introduction of ectopic open gap junctions across the zone of isolation, both randomized early asymmetric gene expression and position of the asymmetric viscera in frog embryos (in the absence of other defects). Similar data were obtained in the chick - an embryo with very different gastrulation architecture - using antisense oligonucleotides and a variety of surgical manipulations to interrupt the GJC zone (Fig. 2B). Taken together, the results indicated that a zone of circumferential Connexin43 expression around the primitive streak (which does not express C×43) is required for normal laterality.
While much remains to be learned about the individual connexins functioning in the frog embryo and the permeabilities of the native gap junctions for different permeant molecules (Dicaprio et al., 1975; Landesman et al., 2000; Landesman et al., 2003), these experiments revealed a common system functioning in both species. Thus, a very similar picture emerged of the role of GJC in LR patterning in two embryonic systems. In both chicks and frogs, a circumferential large-scale pattern of GJC exists around a zone of isolation. The contiguity of this path is crucial for normal LR expression of asymmetric genes; interruption of this path by surgical, pharmacological, or molecular-biological methods specifically causes left-right randomization. Conversely, the zone of isolation is likewise crucial for LR patterning. Introducing GJC through this zone randomizes gene expression, probably because it can no longer serve as a barrier for accumulation of LR morphogens on either side of the midline.
The main features of this system appear to be the same in birds and amphibians; however, several differences have been found. Firstly, due to the different modes of gastrulation, the circumferential path is overlaid differently on the two embryos’ body-plans: it is perpendicular to the DV axis in chicks, but to the animal-vegetal axis in Xenopus. Also, the zone of isolation appears to be maintained at the mRNA level in chick (based on the exclusion of C×43 from the streak). In contrast, Xenopus appears to rely on a protein-level mechanism to exclude junctional transfer from the zone of isolation because drug agents that open existing gap junctions are effective in inducing GJC on the ventral side in Xenopus embryos.
The differences in GJC observed in chick are LR-symmetrical at the mRNA level. Likewise, the differences in GJC in Xenopus are along the dorso-ventral axis, downstream of the Wnt pathway (Olson et al., 1991; Olson and Moon, 1992). So, how may these asymmetries of GJC be translated into differential signaling to the left vs. right-sided cells? One model that is consistent with these data is a radial movement of small molecule morphogens through the GJC path, allowing them to accumulate on one side of the midline. If the net movement was biased (clockwise or counterclockwise), such sorting of a small LR morphogen through gap junctional paths would lead to the formation of a concentration gradient across the primitive streak, which could then induce asymmetric gene expression cascades in conventional ways (Levin and Mercola, 1998b).
The investigation of GJC in chick and frog asymmetry raised two main lines of inquiry. First, what controls the unidirectional (chiral) flow of LR information through the gap junctions? We have previously proposed (Levin, 2003b; Levin and Nascone, 1997) that asymmetries in electrical polarization among L vs. R cells (Levin et al., 2002) can result in an electrophoretic force that can drag certain small molecules through gap junctional paths in a preferentially-chiral direction.
Second, what is the molecular nature of the small-molecule LR signals which are exchanged between cells on the L and R sides? The ideal candidate would be smaller than the size cut-off of gap junctions (< 1 kDa), be water-soluble (signaling molecules such as retinoic acid do not need gap junctions to move between cells), and be charged (to enable regulation of movement via ion pump-dependent voltage gradients that have been shown to be required for normal asymmetry (Adams et al., 2006; Levin et al., 2002). Serotonin fits these criteria, has been demonstrated to go through gap junctions in some contexts (Wolszon et al., 1994), and offers the benefits of a well-developed pharmacological tool-set (Gaster and King, 1997).
Serotonin is thus an ideal candidate for an early LR signal. Thus, taking advantage of the large number of well-characterized reagents available to test and characterize its role in left-right asymmetry in chick and frog embryos, serotonin’s role in asymmetry, prior to the formation of neurons, was recently probed in chick and frog (Fukumoto et al., 2005a; Fukumoto et al., 2005b). Using analysis of endogenous localization of serotonin and its receptors, as well as gain- and loss-of-function experiments using pharmacological serotonergic blockers and dominant-negative and wild-type expression constructs, it was shown that serotonergic signaling through receptor subtypes R3 and R4 was crucial for normal asymmetry. In early Xenopus embryos, serotonin was distributed in a striking radial pattern, eventually forming a gradient and coalescing into a single blastomere on one side of the midline by the 32/64-cell stage (Fig. 2C-E). While direct movement of serotonin through gap junctions in this system has not been demonstrated in vivo (serotonin is too small to be modified by fluorescent tags without significantly changing its properties), it was crucially shown that the ability of serotonin to localize asymmetrically through the GJC-connected blastomeres was dependent upon open gap junctions (Fig. 2F) and upon the function of the H,K-ATPase and V-ATPase ion pumps (Adams et al., 2006; Fukumoto et al., 2005b). Moreover, evidence was presented for a novel intracellular locus of serotonin activity - this is a requirement of the model since serotonin arriving within the target cells’ cytosol has to be able to activate receptor mechanisms there. This is an important general feature of GJC-dependent signaling since intracellular receptor mechanisms must be characterized for gap junction-dependent morphogens.
Models of Morphogen Movement through GJC paths
Taken together, these data suggested a model (Fig. 3) that provides a possible answer to the chirality of the morphogen movement through gap junctions in left-right patterning (Esser et al., 2006): that serotonin moves asymmetrically through the field of GJC-connected cells under an electrophoretic force provided by differential membrane voltages in cells at opposite ends of the circumferential cell field (Fukumoto et al., 2005b; Levin, 2003b; Levin et al., 2006). One unique aspect of this dataset is that most of the important properties are known or can be estimated quantitatively. This allowed a mathematical model to be formulated (Esser et al., 2006), which tested and supported the hypothesis that the known voltage difference across the GJ-coupled cell field can actually generate a significant LR morphogen gradient in the developmentally-available time, using an electrophoretic mechanism. The computer simulation used realistic values for the physical constants related to serotonin, cytoplasm, etc. to demonstrate that electrophoresis of small molecules across GJ-coupled cell fields (Fig. 3A) can give rise to significant gradients of the small molecule (Fig. 3B) under physiological voltage differences. Moreover, analysis of the model revealed that not only are chemical gradients formed across the entire GJ-coupled cell field, but also are present in a complex pattern across each individual cell (Fig. 3C), providing a potential mechanism by which cells in large GJ-coupled fields can derive directional information.
Figure 3.
A model of electrophoretic movement of morphogens through gap-junctional paths
(A) Schematically, a number of patterning systems can be visualized as a field of gap junctions terminating on a cell group at one end that establishes a strong polarization by ion exchange with the outside world. Gray level within the cells illustrate voltage gradient produced by pumps at the edge. (B) This results in an electrophoretic force that can, given realistic estimates of the physical parameters of cytoplasm and small molecules, result in a significant gradient of a small molecule across the cell field (yellow dots in panel A; this is now thought to be serotonin in the case of frog left-right patterning). (C) Importantly, mathematical analysis of the model illustrates that local gradients of GJC-permeable morphogens are thus set up within cells as well as across cell fields, potentially providing local directional cues.
This class of models makes a number of specific predictions. First, the influx of the LR morphogens into cells (as distinct from the well-understood serotonin receptor activation on the cell surface) has to control cell fate. This appears to be borne out in other systems, as the function of the SERT importer is involved in tumor growth (Nordenberg et al., 1999; Serafeim et al., 2002), as is serotonin (Dizeyi et al., 2004). An important aspect is the identification of gene transcription modulated by the intracellular arrival of GJC-dependent morphogens into cells. While these studies are just beginning in our lab in the context of the asymmetric gene cascade, others have begun to characterize, using transcriptome analysis, the genetic targets of gap junction-mediated signaling (Iacobas et al., 2005; Iacobas et al., 2004; Iacobas et al., 2003). In some cases, GJC modulates transcription by altering Sp1 and Sp3 transcription factors (Stains and Civitelli, 2005; Stains et al., 2003).
It is now abundantly clear that large number of important gene networks are potentially controlled by GJC signaling and these represent excellent targets for future molecular dissection. For example, recent analysis links the expression of Syndecan-2 with connexin43 in mouse embryos (Iacobas et al., 2005), which is the first glimpse of conservation of regulatory circuits among the known roles of GJC and Syndecan-2 in lower vertebrates and mammals in establishment of left-right asymmetry (Fukumoto and Levin, 2005; Kramer et al., 2002; Kramer and Yost, 2002). Finally, the use of this mechanism in various patterning systems must include significant filtering and selectivity among the gap junctions, since tissues cannot afford all charged molecules to be moved in a particular direction - this force must be reasonably specific for the intended morphogens, and such developmental selectivity is supported by direct measurements (Bozhkova, 1998; Bozhkova and Rozanova, 2000; Goldberg et al., 2002; Suchyna et al., 1999).
An alternative set of models based on reaction-diffusion systems (Kondo, 2002; Takagi and Kaneko, 2005) are suggested by recent data on zebrafish pigmentation. The zebrafish leopard mutation gives rise to distinct pigmentation changes that are likely to reveal reaction-diffusion as the mechanism controlling pigment pattern (Asai et al., 1999). Interestingly, it has recently been discovered that the mutation is in connexin41.8 (Watanabe et al., 2006), suggesting that in this case of pigment system morphogenesis, the relevant signaling molecules may be moving through gap junctions. Theoretical discussion of this possibility has suggested the GJC-permeable molecules cAMP and ATP as the relevant Turing couple (Schiffmann, 1991).
The Future of GJC in Patterning
The characterization of GJC in embryonic patterning is currently at a very exciting stage; molecular tools now exist to probe every aspect of this type of intercellular information exchange in contexts where it is known to be important. It is expected that newly-developed confocal microscopy uncaging and FRET methodologies will greatly facilitate the study of endogenous GJC paths in embryos in vivo (Bedner et al., 2003; Braet et al., 2003; Cannell et al., 2004; Dakin et al., 2005; Di et al., 2005). Key future research areas include the characterization of factors which set up patterns of differential GJC in various embryonic tissues (at the transcriptional level, as well as at the level of controlling GJC states, such as by endogenous patterns of pH and voltage gradients (Calero et al., 1998; Ek-Vitorin et al., 1996; Francis et al., 1999; Gu et al., 2000; Morley et al., 1997), and the mapping of paths which exist in and between different tissues to molecules of various charges and sizes. It is already known that gap junctions are selectively permeable based on a complex function of molecular weight, size, net charge, shape, and interactions with specific connexins (Ek-Vitorin and Burt, 2005; Goldberg et al., 2004). Particularly interesting is the possible role of chemically-rectifying (unidirectional) gap junctions in embryogenesis (Robinson et al., 1993; Xin and Bloomfield, 1997; Zhang et al., 2004). A better understanding of endogenous GJC-permeable molecules will be crucial.
The zebrafish will likely emerge as an excellent embryonic system for studying GJC since the embryos are transparent, allowing easy dye-based analysis of GJC paths in vivo as well as use of more sophisticated pH and voltage-sensitive dyes for studying the spatial distribution of factors which control junctional gating. Expression of C×43.4 has already been followed in zebrafish using a GFP fusion construct (Essner et al., 1996). The amenability of the zebrafish to genetic screens may also be used to analyze mutants in junctional transfer, by designing lines containing fluorescent small molecules.
Experiments in mouse (and other systems) must, of necessity, be performed in the context of multiple connexin knock-out, since it is becoming increasingly clear that due to compensation and redundancy, single loss of gap junction genes can fail to reveal important phenotypes (Simon et al., 2004). Indeed, it is now known that ablation of Connexin43 can lead to up-regulation of a pannexin (Iacobas et al., 2006), suggesting that cell-cell communication can be restored in a regulative manner by alterations of transcription of GJC genes across families. Knock-in of dominant negative mutants with different specificities for endogenous connexin families is likely to reveal many important and novel roles (Beahm et al., 2006; Fiorini et al., 2004; Paul et al., 1995). Likewise, more sophisticated technologies allowing expression of dominant negative mutants restricted in space and time during development will allow the circumvention of embryonic lethal phenotypes and likely to lead to the discovery of novel patterning mechanisms (Bakirtzis et al., 2003; Becker et al., 1992). While we now have some understanding of downstream transcriptional changes that occur when gap junctional signaling is perturbed (Kim et al., 2005), microarray analysis of GJC-inhibited tissues may lead to the discovery of proximal early-response genes to GJC-permeable morphogens. Our lab is currently pursuing such efforts in the context of embryonic left-right patterning.
The ultimate proof of mechanistic understanding of a process is the ability to synthesize the elements, characterized quantitatively, back into a predictive computer model that can make and facilitate the testing of specific predictions. Theoretical analyses of GJC signaling have begun via mathematical models (Cooper, 1984; Cooper et al., 1989; Fortier and Bagna, 2006; Hofer et al., 2002; Nygren and Giles, 2000; Vogel and Weingart, 1998; Vogel and Weingart, 2002) and in future work it will be very important to apply these to the understanding of specific patterning processes in embryonic and regenerative morphogenesis (Esser et al., 2006). It is probable that the understanding of gap junctional signals will prove as important to basic developmental and evolutionary biology as has been the explosion in receptor-mediated signaling. The implications for the ability to control pattern formation are profound, and we are only beginning to glimpse the elusive, subtle, yet crucial molecular cross-talk between the interior machinery of cells.
Acknowledgements
I thank Jose F. Ek Vitorin, Edgar Spalding, Winslow Briggs, Malcolm Maden, Jiri Friml, Daniel Goodenough, Ken Robinson, and Richard Borgens for many useful discussions on these topics, and Dany S. Adams for the embryo shown in Fig. 1H. This work was supported in part by NIH grant 1-R01-GM-06227, NSF grant IBN-0234388, and NHTSA grant DTNH22-06-G-00001. This review was written in a Forsyth Institute facility renovated with support from Research Facilities Improvement Grant Number CO6RR11244 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aasen T, Hodgins MB, Edward M, Graham SV. The relationship between connexins, gap junctions, tissue architecture and tumour invasion, as studied in a novel in vitro model of HPV-16-associated cervical cancer progression. Oncogene. 2003;22:7969–80. doi: 10.1038/sj.onc.1206709. [DOI] [PubMed] [Google Scholar]
- Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, McSweeney M, Levin M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development. 2006;133:1657–1671. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. 2000;105:161–71. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen F, Tickle C, Warner A. The role of gap junctions in patterning of the chick limb bud. Development. 1990;108:623–34. doi: 10.1242/dev.108.4.623. [DOI] [PubMed] [Google Scholar]
- Araya R, Eckardt D, Maxeiner S, Kruger O, Theis M, Willecke K, Saez JC. Expression of connexins during differentiation and regeneration of skeletal muscle: functional relevance of connexin43. J Cell Sci. 2005;118:27–37. doi: 10.1242/jcs.01553. [DOI] [PubMed] [Google Scholar]
- Araya R, Eckardt D, Riquelme MA, Willecke K, Saez JC. Presence and importance of connexin43 during myogenesis. Cell Commun Adhes. 2003;10:451–6. doi: 10.1080/cac.10.4-6.451.456. [DOI] [PubMed] [Google Scholar]
- Arita K, Akiyama M, Tsuji Y, McMillan JR, Eady RA, Shimizu H. Gap junction development in the human fetal hair follicle and bulge region. Br J Dermatol. 2004;150:429–34. doi: 10.1046/j.1365-2133.2004.05775.x. [DOI] [PubMed] [Google Scholar]
- Asai R, Taguchi E, Kume Y, Saito M, Kondo S. Zebrafish leopard gene as a component of the putative reaction-diffusion system. Mech Dev. 1999;89:87–92. doi: 10.1016/s0925-4773(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Aslanidi KB, Boitsova L, Chailakhyan LM, Kublik LN, Marachova, Potapova TV, Vinogradova TA. Energetic cooperation via ion-permeable junctions in mixed animal cell cultures. FEBS Lett. 1991;283:295–7. doi: 10.1016/0014-5793(91)80612-7. [DOI] [PubMed] [Google Scholar]
- Ayad WA, Locke D, Koreen IV, Harris AL. Heteromeric, but not homomeric, connexin channels are selectively permeable to inositol phosphates. J Biol Chem. 2006 doi: 10.1074/jbc.M600136200. [DOI] [PubMed] [Google Scholar]
- Bakirtzis G, Jamieson S, Aasen T, Bryson S, Forrow S, Tetley L, Finbow M, Greenhalgh D, Hodgins M. The effects of a mutant connexin 26 on epidermal differentiation. Cell Commun Adhes. 2003;10:359–64. doi: 10.1080/cac.10.4-6.359.364. [DOI] [PubMed] [Google Scholar]
- Bani-Yaghoub M, Felker JM, Sans C, Naus CC. The effects of bone morphogenetic protein 2 and 4 (BMP2 and BMP4) on gap junctions during neurodevelopment. Exp Neurol. 2000;162:13–26. doi: 10.1006/exnr.2000.7294. [DOI] [PubMed] [Google Scholar]
- Bani-Yaghoub M, Underhill TM, Naus CC. Gap junction blockage interferes with neuronal and astroglial differentiation of mouse P19 embryonal carcinoma cells. Dev Genet. 1999;24:69–81. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<69::AID-DVG8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Bannerman P, Nichols W, Puhalla S, Oliver T, Berman M, Pleasure D. Early migratory rat neural crest cells express functional gap junctions: evidence that neuralcrest cell survival requires gap junction function. J Neurosci Res. 2000;61:605–15. doi: 10.1002/1097-4547(20000915)61:6<605::AID-JNR4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–8. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–16. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Barnes TM. OPUS: a growing family of gap junction proteins? Trends Genet. 1994;10:303–5. doi: 10.1016/0168-9525(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Bauer R, Lehmann C, Fuss B, Eckardt F, Hoch M. The Drosophila gap junction channel gene innexin 2 controls foregut development in response to Wingless signalling. J Cell Sci. 2002;115:1859–67. doi: 10.1242/jcs.115.9.1859. [DOI] [PubMed] [Google Scholar]
- Bauer R, Lehmann C, Hoch M. Gastrointestinal development in the Drosophila embryo requires the activity of innexin gap junction channel proteins. Cell Commun Adhes. 2001;8:307–10. doi: 10.3109/15419060109080743. [DOI] [PubMed] [Google Scholar]
- Bauer R, Lehmann C, Martini J, Eckardt F, Hoch M. Gap junction channel protein innexin 2 is essential for epithelial morphogenesis in the Drosophila embryo. Mol Biol Cell. 2004;15:2992–3004. doi: 10.1091/mbc.E04-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beahm DL, Oshima A, Gaietta GM, Hand GM, Smock AE, Zucker SN, Toloue MM, Chandrasekhar A, Nicholson BJ, Sosinsky GE. Mutation of a conserved threonine in the third transmembrane helix of alpha- and beta-connexins creates a dominant-negative closed gap junction channel. J Biol Chem. 2006;281:7994–8009. doi: 10.1074/jbc.M506533200. [DOI] [PubMed] [Google Scholar]
- Becker DL, Evans WH, Green CR, Warner A. Functional analysis of amino acid sequences in connexin43 involved in intercellular communication through gap junctions. J Cell Sci. 1995;108:1455–67. doi: 10.1242/jcs.108.4.1455. [DOI] [PubMed] [Google Scholar]
- Becker DL, Leclerc-David C, Warner A. The relationship of gap junctions and compaction in the preimplantation mouse embryo. Dev Suppl. 1992:113–8. [PubMed] [Google Scholar]
- Bedner P, Niessen H, Odermatt B, Willecke K, Harz H. A method to determine the relative cAMP permeability of connexin channels. Exp Cell Res. 2003;291:25–35. doi: 10.1016/s0014-4827(03)00323-9. [DOI] [PubMed] [Google Scholar]
- Berthoud VM, Singh R, Minogue PJ, Ragsdale CW, Beyer EC. Highly restricted pattern of connexin36 expression in chick somite development. Anat Embryol (Berl) 2004;209:11–8. doi: 10.1007/s00429-004-0416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevans CG, Kordel M, Rhee SK, Harris AL. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem. 1998;273:2808–16. doi: 10.1074/jbc.273.5.2808. [DOI] [PubMed] [Google Scholar]
- Blomstrand F, Aberg ND, Eriksson PS, Hansson E, Ronnback L. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience. 1999;92:255–65. doi: 10.1016/s0306-4522(98)00738-6. [DOI] [PubMed] [Google Scholar]
- Bohrmann J, Haas-Assenbaum A. Gap junctions in ovarian follicles of Drosophila melanogaster: inhibition and promotion of dye-coupling between oocyte and follicle cells. Cell Tissue Res. 1993;273:163–73. doi: 10.1007/BF00304623. [DOI] [PubMed] [Google Scholar]
- Bohrmann J, Lammel H. Microinjected antisera against ductin affect gastrulation in Drosophila melanogaster. Int J Dev Biol. 1998;42:709–21. [PubMed] [Google Scholar]
- Bozhkova VP. The specificity of gap junctional channels in early fish embryos and its significance for pattern formation in development. Membr Cell Biol. 1998;11:803–15. [PubMed] [Google Scholar]
- Bozhkova VP, Rozanova NV. Local changes in gap junctional permeability accompany regionalization of the mesoderm in early fish (loach, Misgurnus fossilis) embryos. Membr Cell Biol. 2000;14:189–98. [PubMed] [Google Scholar]
- Braet K, Vandamme W, Martin PE, Evans WH, Leybaert L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium. 2003;33:37–48. doi: 10.1016/s0143-4160(02)00180-x. [DOI] [PubMed] [Google Scholar]
- Brizuela BJ, Wessely O, De Robertis EM. Overexpression of the Xenopus tight-junction protein claudin causes randomization of the left-right body axis. Developmental Biology. 2001;230:217–229. doi: 10.1006/dbio.2000.0116. [DOI] [PubMed] [Google Scholar]
- Brooks RA, Woodruff RI. Calmodulin transmitted through gap junctions stimulates endocytic incorporation of yolk precursors in insect oocytes. Dev Biol. 2004;271:339–49. doi: 10.1016/j.ydbio.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Brown N, McCarthy A, Wolpert L. Development of handed body asymmetry in mammals. CIBA Found. Symp. 1991;162:182–196. doi: 10.1002/9780470514160.ch11. [DOI] [PubMed] [Google Scholar]
- Brown N, Wolpert L. The development of handedness in left/right asymmetry. Development. 1990;109:1–9. doi: 10.1242/dev.109.1.1. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–43. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Goodenough DA. Gap junctions: ductin or connexins--which component is the critical one? Bioessays. 1995;17:744–5. doi: 10.1002/bies.950170812. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–9. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd S, Lipton S. Calcium tsunamis. Nature Neuroscience. 1998;1:431–432. doi: 10.1038/2147. [DOI] [PubMed] [Google Scholar]
- Burdine R, Schier A. Conserved and divergent mechanisms in left-right axis formation. Genes Dev. 2000;14:763–776. [PubMed] [Google Scholar]
- Burnside AS, Collas P. Induction of Oct-3/4 expression in somatic cells by gap junction-mediated cAMP signaling from blastomeres. Eur J Cell Biol. 2002;81:585–91. doi: 10.1078/0171-9335-00286. [DOI] [PubMed] [Google Scholar]
- Cai J, Cheng A, Luo Y, Lu C, Mattson MP, Rao MS, Furukawa K. Membrane properties of rat embryonic multipotent neural stem cells. J Neurochem. 2004;88:212–26. doi: 10.1046/j.1471-4159.2003.02184.x. [DOI] [PubMed] [Google Scholar]
- Calero G, Kanemitsu M, Taffet SM, Lau AF, Delmar M. A 17mer peptide interferes with acidification-induced uncoupling of connexin43. Circ Res. 1998;82:929–35. doi: 10.1161/01.res.82.9.929. [DOI] [PubMed] [Google Scholar]
- Cannell MB, Jacobs MD, Donaldson PJ, Soeller C. Probing microscopic diffusion by 2-photon flash photolysis: measurement of isotropic and anisotropic diffusion in lens fiber cells. Microsc Res Tech. 2004;63:50–7. doi: 10.1002/jemt.10422. [DOI] [PubMed] [Google Scholar]
- Chatterjee B, Meyer RA, Loredo GA, Coleman CM, Tuan R, Lo CW. BMP regulation of the mouse connexin43 promoter in osteoblastic cells and embryos. Cell Commun Adhes. 2003;10:37–50. doi: 10.1080/15419060302064. [DOI] [PubMed] [Google Scholar]
- Chen JR, Chatterjee B, Meyer R, Yu JC, Borke JL, Isales CM, Kirby ML, Lo CW, Bollag RJ. Tbx2 represses expression of Connexin43 in osteoblastic-like cells. Calcif Tissue Int. 2004;74:561–73. doi: 10.1007/s00223-003-0106-5. [DOI] [PubMed] [Google Scholar]
- Coelho CN, Kosher RA. A gradient of gap junctional communication along the anterior-posterior axis of the developing chick limb bud. Dev Bio. 1991;148:529–35. doi: 10.1016/0012-1606(91)90271-4. [DOI] [PubMed] [Google Scholar]
- Coleman CM, Loredo GA, Lo CW, Tuan RS. Correlation of GDF5 and connexin 43 mRNA expression during embryonic development. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1117–21. doi: 10.1002/ar.a.10125. [DOI] [PubMed] [Google Scholar]
- Coleman CM, Tuan RS. Functional role of growth/differentiation factor 5 in chondrogenesis of limb mesenchymal cells. Mech Dev. 2003;120:823–36. doi: 10.1016/s0925-4773(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- Contreras RG, Lazaro A, Bolivar JJ, Flores-Maldonado C, Sanchez SH, Gonzalez-Mariscal L, Garcia-Villegas MR, Valdes J, Cereijido M. A novel type of cell-cell cooperation between epithelial cells. J Membr Biol. 1995;145:305–10. doi: 10.1007/BF00232722. [DOI] [PubMed] [Google Scholar]
- Cooper MS. Gap junctions increase the sensitivity of tissue cells to exogenous electric fields. J Theor Biol. 1984;111:123–30. doi: 10.1016/s0022-5193(84)80200-3. [DOI] [PubMed] [Google Scholar]
- Cooper MS, Miller JP, Fraser SE. Electrophoretic repatterning of charged cytoplasmic molecules within tissues coupled by gap junctions by externally applied electric fields. Dev Bio. 1989;132:179–88. doi: 10.1016/0012-1606(89)90216-9. [DOI] [PubMed] [Google Scholar]
- Cruciani V, Mikalsen SO. The vertebrate connexin family. Cell Mol Life Sci. 2006;63:1125–40. doi: 10.1007/s00018-005-5571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin K, Zhao Y, Li WH. LAMP, a new imaging assay of gap junctional communication unveils that Ca2+ influx inhibits cell coupling. Nat Methods. 2005;2:55–62. doi: 10.1038/nmeth730. [DOI] [PubMed] [Google Scholar]
- Dang X, Doble BW, Kardami E. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol Cell Biochem. 2003;242:35–8. [PubMed] [Google Scholar]
- Davy A, Bush JO, Soriano P. Inhibition of Gap Junction Communication at Ectopic Eph/ephrin Boundaries Underlies Craniofrontonasal Syndrome. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer TP, Kok B, Neuteboom KI, Spieker N, De Graaf J, Destree OH, Rook MB, Van Veen TA, Jongsma HJ, Vos MA, De Bakker JM, Van Der Heyden MA. Cloning and functional characterization of a novel connexin expressed in somites of Xenopus laevis. Dev Dyn. 2005;233:864–71. doi: 10.1002/dvdy.20420. [DOI] [PubMed] [Google Scholar]
- de Boer TP, van der Heyden MA. Xenopus connexins: how frogs bridge the gap. Differentiation. 2005;73:330–40. doi: 10.1111/j.1432-0436.2005.00026.x. [DOI] [PubMed] [Google Scholar]
- Di WL, Gu Y, Common JE, Aasen T, O′Toole EA, Kelsell DP, Zicha D. Connexin interaction patterns in keratinocytes revealed morphologically and by FRET analysis. J Cell Sci. 2005;118:1505–14. doi: 10.1242/jcs.01733. [DOI] [PubMed] [Google Scholar]
- Dicaprio RA, French AS, Sanders EJ. Intercellular connectivity in the eight-cell Xenopus embryo - correlation of electrical and morphological investigations. Biophys J. 1975;15:373–89. doi: 10.1016/S0006-3495(75)85824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizeyi N, Bjartell A, Nilsson E, Hansson J, Gadaleanu V, Cross N, Abrahamsson PA. Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. Prostate. 2004;59:328–36. doi: 10.1002/pros.10374. [DOI] [PubMed] [Google Scholar]
- Doble BW, Dang X, Ping P, Fandrich RR, Nickel BE, Jin Y, Cattini PA, Kardami E. Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocytes. J Cell Sci. 2004;117:507–14. doi: 10.1242/jcs.00889. [DOI] [PubMed] [Google Scholar]
- Dvoriantchikova G, Ivanov D, Panchin Y, Shestopalov VI. Expression of pannexin family of proteins in the retina. FEBS Lett. 2006;580:2178–82. doi: 10.1016/j.febslet.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Ek-Vitorin JF, Burt JM. Quantification of Gap Junction Selectivity. Am J Physiol Cell Physiol. 2005 doi: 10.1152/ajpcell.00182.2005. [DOI] [PubMed] [Google Scholar]
- Ek-Vitorin JF, Calero G, Morley GE, Coombs W, Taffet SM, Delmar M. pH regulation of connexin43: molecular analysis of the gating particle. Biophys J. 1996;71:1273–84. doi: 10.1016/S0006-3495(96)79328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res. 2006;98:1498–505. doi: 10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser AT, Smith KC, Weaver JC, Levin M. Mathematical model of morphogen electrophoresis through gap junctions. Dev Dyn. 2006;235:2144–59. doi: 10.1002/dvdy.20870. [DOI] [PubMed] [Google Scholar]
- Essner JJ, Laing JG, Beyer EC, Johnson RG, Hackett PB., Jr. Expression of zebrafish connexin43.4 in the notochord and tail bud of wild-type and mutant no tail embryos. Dev Bio. 1996;177:449–62. doi: 10.1006/dbio.1996.0177. [DOI] [PubMed] [Google Scholar]
- Ewart JL, Cohen MF, Meyer RA, Huang GY, Wessels A, Gourdie RG, Chin AJ, Park SM, Lazatin BO, Villabon S, Lo CW. Heart and neural tube defects in transgenic mice overexpressing the C×43 gap junction gene. Development. 1997;124:1281–84. doi: 10.1242/dev.124.7.1281. [DOI] [PubMed] [Google Scholar]
- Falk MM. Biosynthesis and structural composition of gap junction intercellular membrane channels. Eur J Cell Biol. 2000;79:564–74. doi: 10.1078/0171-9335-00080. [DOI] [PubMed] [Google Scholar]
- Finbow M, Eliopoulos E, Jackson P, Keen J, Meagher L, Thompson P, Jones P, Findlay J. Structure of a 16 kDa integral membrane protein that has indentity to the putative proton channel of the vacuolar H+-ATPase. Protein Eng. 1992;5:7–15. doi: 10.1093/protein/5.1.7. [DOI] [PubMed] [Google Scholar]
- Finbow M, Pitts J, Goldstein D, Schlegel R, Findlay J. The E5 oncoprotein target: a 16-kDa channel-forming protein with diverse functions. Mol Carcinog. 1991;4:441–444. doi: 10.1002/mc.2940040605. [DOI] [PubMed] [Google Scholar]
- Finbow ME, Goodwin SF, Meagher L, Lane NJ, Keen J, Findlay JB, Kaiser K. Evidence that the 16 kDa proteolipid (subunit c) of the vacuolar H(+)-ATPase and ductin from gap junctions are the same polypeptide in Drosophila and Manduca: molecular cloning of the Vha16k gene from Drosophila. J Cell Sci. 1994;107(Pt 7):1817–24. doi: 10.1242/jcs.107.7.1817. [DOI] [PubMed] [Google Scholar]
- Finbow ME, Harrison M, Jones P. Ductin--a proton pump component, a gap junction channel and a neurotransmitter release channel. Bioessays. 1995;17:247–55. doi: 10.1002/bies.950170311. [DOI] [PubMed] [Google Scholar]
- Finbow ME, Pitts JD. Is the gap junction channel--the connexon--made of connexin or ductin? J Cell Sci. 1993;106:463–71. doi: 10.1242/jcs.106.2.463. [DOI] [PubMed] [Google Scholar]
- Finbow ME, Pitts JD. Structure of the ductin channel. Biosci Rep. 1998;18:287–97. doi: 10.1023/a:1020205231507. [DOI] [PubMed] [Google Scholar]
- Fiorini C, Mograbi B, Cronier L, Bourget I, Decrouy X, Nebout M, Ferrua B, Malassine A, Samson M, Fenichel P, Segretain D, Pointis G. Dominant negative effect of connexin33 on gap junctional communication is mediated by connexin43 sequestration. J Cell Sci. 2004 doi: 10.1242/jcs.01335. Pt.
- Fitzharris G, Baltz JM. Granulosa cells regulate intracellular pH of the murine growing oocyte via gap junctions: development of independent homeostasis during oocyte growth. Development. 2006;133:591–9. doi: 10.1242/dev.02246. [DOI] [PubMed] [Google Scholar]
- Fortier PA, Bagna M. Estimating conductances of dual-recorded neurons within a network of coupled cells. J Theor Biol. 2006;240:501–10. doi: 10.1016/j.jtbi.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Francis D, Stergiopoulos K, Ek-Vitorin JF, Cao FL, Taffet SM, Delmar M. Connexin diversity and gap junction regulation by pHi. Dev Gen. 1999;24:123–36. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<123::AID-DVG12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Fraser S, Green C, Bode H, Gilula M. Selective disruption of gap junctional communication interferes with a patterning process in hydra. Science. 1987;237:49–55. doi: 10.1126/science.3037697. [DOI] [PubMed] [Google Scholar]
- Fujimoto E, Satoh H, Negishi E, Ueno K, Nagashima Y, Hagiwara K, Yamasaki H, Yano T. Negative growth control of renal cell carcinoma cell by connexin 32: possible involvement of Her-2. Mol Carcinog. 2004;40:135–42. doi: 10.1002/mc.20025. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Blakely R, Levin M. Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev Neurosci. 2005;27(a):349–63. doi: 10.1159/000088451. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol. 2005;15(b):794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Levin M. Asymmetric expression of Syndecan-2 in early chick embryogenesis. Gene Expr Patterns. 2005;5:525–8. doi: 10.1016/j.modgep.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Ganfornina MD, Sanchez D, Herrera M, Bastiani MJ. Developmental expression and molecular characterization of two gap junction channel proteins expressed during embryogenesis in the grasshopper Schistocerca americana. Dev Gen. 1999;24:137–50. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<137::AID-DVG13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gaster LM, King FD. Serotonin 5-HT3 and 5-HT4 receptor antagonists. Med Res Rev. 1997;17:163–214. doi: 10.1002/(sici)1098-1128(199703)17:2<163::aid-med2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Gilboa L, Forbes A, Tazuke SI, Fuller MT, Lehmann R. Germ line stem cell differentiation in Drosophila requires gap junctions and proceeds via an intermediate state. Development. 2003;130:6625–34. doi: 10.1242/dev.00853. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol. 1999;1:457–9. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Lampe PD. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem. 2002;277:36725–30. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Valiunas V, Brink PR. Selective permeability of gap junction channels. Biochim Biophys Acta. 2004;1662:96–101. doi: 10.1016/j.bbamem.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Goodenough D, Musil L. Gap junctions and tissue business: problems and strategies for developing specific functional reagents. J Cell Sci Suppl. 1993;17:133–8. doi: 10.1242/jcs.1993.supplement_17.19. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Simon AM, Paul DL. Gap junctional intercellular communication in the mouse ovarian follicle. Novartis Found Symp. 1999;219:226–35. doi: 10.1002/9780470515587.ch14. [DOI] [PubMed] [Google Scholar]
- Gu H, Ek-Vitorin JF, Taffet SM, Delmar M. Coexpression of connexins 40 and 43 enhances the pH sensitivity of gap junctions: a model for synergistic interactions among connexins. Circ Res (Online) 2000;86:E98–E103. [PubMed] [Google Scholar]
- Gu H, Smith FC, Taffet SM, Delmar M. High incidence of cardiac malformations in connexin40-deficient mice. Circ Res. 2003;93(a):201–6. doi: 10.1161/01.RES.0000084852.65396.70. [DOI] [PubMed] [Google Scholar]
- Gu S, Yu XS, Yin X, Jiang JX. Stimulation of lens cell differentiation by gap junction protein connexin 45.6. Invest. Ophthalmol. Vis. Sci. 2003;44(b):2103–11. doi: 10.1167/iovs.02-1045. [DOI] [PubMed] [Google Scholar]
- Guillotin B, Bourget C, Remy-Zolgadri M, Bareille R, Fernandez P, Conrad V, Amedee-Vilamitjana J. Human primary endothelial cells stimulate human osteoprogenitor cell differentiation. Cell Physiol Biochem. 2004;14:325–32. doi: 10.1159/000080342. [DOI] [PubMed] [Google Scholar]
- Guthrie S. Patterns of junctional communication in the early amphibian embryo. Nature. 1984;311:149–51. doi: 10.1038/311149a0. [DOI] [PubMed] [Google Scholar]
- Guthrie S, Gilula N. Gap junctional communication and development. Trends Neuroscis. 1989;12:12–6. doi: 10.1016/0166-2236(89)90150-1. [DOI] [PubMed] [Google Scholar]
- Guthrie S, Turin L, Warner A. Patterns of junctional communication during development of the early amphibian embryo. Development. 1988;103:769–83. doi: 10.1242/dev.103.4.769. [DOI] [PubMed] [Google Scholar]
- Hagendorff A, Plum A. [Cardiac arrhythmias in targeted connexin deficient mice: significance for the arrhythmia field] Z Kardiol. 2000;89:1108–18. doi: 10.1007/s003920070138. [DOI] [PubMed] [Google Scholar]
- Hellmann P, Grummer R, Schirrmacher K, Rook M, Traub O, Winterhager E. Transfection with different connexin genes alters growth and differentiation of human choriocarcinoma cells. Exp Cell Res. 1999;246:480–90. doi: 10.1006/excr.1998.4332. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, Burt JM, Hirschi KD, Dai C. Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ Res. 2003;93:429–37. doi: 10.1161/01.RES.0000091259.84556.D5. [DOI] [PubMed] [Google Scholar]
- Hofer T, Venance L, Giaume C. Control and plasticity of intercellular calcium waves in astrocytes: a modeling approach. J Neurosci. 2002;22:4850–9. doi: 10.1523/JNEUROSCI.22-12-04850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GY, Cooper ES, Waldo K, Kirby ML, Gilula NB, Lo CW. Gap junction-mediated cell-cell communication modulates mouse neural crest migration. J Cell Biol. 1998;143(a):1725–34. doi: 10.1083/jcb.143.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GY, Wessels A, Smith BR, Linask KK, Ewart JL, Lo CW. Alteration in connexin 43 gap junction gene dosage impairs conotruncal heart development. Dev Biol. 1998;198(b):32–44. doi: 10.1006/dbio.1998.8891. [DOI] [PubMed] [Google Scholar]
- Huss M, Ingenhorst G, Konig S, Gassel M, Drose S, Zeeck A, Altendorf K, Wieczorek H. Concanamycin A, the specific inhibitor of V-ATPases, binds to the Vo subunit c. J Biol Chem. 2002;277:40544–8. doi: 10.1074/jbc.M207345200. [DOI] [PubMed] [Google Scholar]
- Iacobas DA, Iacobas S, Urban-Maldonado M, Spray DC. Sensitivity of the brain transcriptome to connexin ablation. Biochim Biophys Acta. 2005;1711:183–96. doi: 10.1016/j.bbamem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Iacobas DA, Scemes E, Spray DC. Gene expression alterations in connexin null mice extend beyond the gap junction. Neurochem Int. 2004;45:243–50. doi: 10.1016/j.neuint.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Iacobas DA, Urban-Maldonado M, Iacobas S, Scemes E, Spray DC. Array analysis of gene expression in connexin-43 null astrocytes. Physiol Genomics. 2003;15:177–90. doi: 10.1152/physiolgenomics.00062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobas DA, Iacobas S, Spray DC. Connexin43 and the brain transcriptome of newborn mice. Genomics. 2006 doi: 10.1016/j.ygeno.2006.09.007. in press
- Inoue H, Noumi T, Nagata M, Murakami H, Kanazawa H. Targeted disruption of the gene encoding the proteolipid subunit of mouse vacuolar H(+)-ATPase leads to early embryonic lethality. Biochim Biophys Acta. 1999;1413:130–8. doi: 10.1016/s0005-2728(99)00096-1. [DOI] [PubMed] [Google Scholar]
- Ito S, Loewenstein WR. Ionic communication between early embryonic cells. Dev Bio. 1969;19:228–43. doi: 10.1016/0012-1606(69)90062-1. [DOI] [PubMed] [Google Scholar]
- Jiang JX, Gu S. Gap junction- and hemichannel-independent actions of connexins. Biochim Biophys Acta. 2005;1711:208–14. doi: 10.1016/j.bbamem.2004.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneja SC, Barr KJ, Enders GC, Kidder GM. Defects in the germ line and gonads of mice lacking connexin43. Biol Reprod. 1999;60:1263–70. doi: 10.1095/biolreprod60.5.1263. [DOI] [PubMed] [Google Scholar]
- Jursnich VA, Fraser SE, Held LI, Jr., Ryerse J, Bryant PJ. Defective gap-junctional communication associated with imaginal disc overgrowth and degeneration caused by mutations of the dco gene in Drosophila. Dev Bio. 1990;140:413–29. doi: 10.1016/0012-1606(90)90090-6. [DOI] [PubMed] [Google Scholar]
- Kalimi GH, Lo CW. Gap junctional communication in the extraembryonic tissues of the gastrulating mouse embryo. J Cell Biol. 1989;109:3015–26. doi: 10.1083/jcb.109.6.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra J, Shao Q, Qin H, Thomas T, Alaoui-Jamali MA, Laird DW. C×26 inhibits breast MDA-MB-435 cell tumorigenic properties by a gap junctional intercellular communication-independent mechanism. Carcinogenesis. 2006 doi: 10.1093/carcin/bgl110. [DOI] [PubMed] [Google Scholar]
- Katbamna B, Jelaso AM, Ide CF. Connexin 43 expression in glial cells of developing rhombomeres of Xenopus laevis. Int J Dev Neurosci. 2004;22:47–55. doi: 10.1016/j.ijdevneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Kidder GM, Winterhager E. Intercellular communication in preimplantation development: the role of gap junctions. Front Biosci. 2001;6:D731–6. doi: 10.2741/kidder. [DOI] [PubMed] [Google Scholar]
- Kim JY, Cho SW, Lee MJ, Hwang HJ, Lee JM, Lee SI, Muramatsu T, Shimono M, Jung HS. Inhibition of connexin 43 alters Shh and Bmp-2 expression patterns in embryonic mouse tongue. Cell Tissue Res. 2005;320:409–15. doi: 10.1007/s00441-005-1091-y. [DOI] [PubMed] [Google Scholar]
- Kimura H, Oyamada Y, Ohshika H, Mori M, Oyamada M. Reversible inhibition of gap junctional intercellular communication, synchronous contraction, and synchronism of intracellular Ca2+ fluctuation in cultured neonatal rat cardiac myocytes by heptanol. Exp Cell Res. 1995;220:348–56. doi: 10.1006/excr.1995.1325. [DOI] [PubMed] [Google Scholar]
- Kirchhoff S, Kim JS, Hagendorff A, Thonnissen E, Kruger O, Lamers WH, Willecke K. Abnormal cardiac conduction and morphogenesis in connexin40 and connexin43 double-deficient mice. Circ Res. 2000;87:399–405. doi: 10.1161/01.res.87.5.399. [DOI] [PubMed] [Google Scholar]
- Kjaer KW, Hansen L, Eiberg H, Leicht P, Opitz JM, Tommerup N. Novel Connexin 43 (GJA1) mutation causes oculo-dento-digital dysplasia with curly hair. Am J Med Genet A. 2004;127:152–7. doi: 10.1002/ajmg.a.20614. [DOI] [PubMed] [Google Scholar]
- Kondo S. The reaction-diffusion system: a mechanism for autonomous pattern formation in the animal skin. Genes Cells. 2002;7:535–41. doi: 10.1046/j.1365-2443.2002.00543.x. [DOI] [PubMed] [Google Scholar]
- Kramer KL, Barnette JE, Yost HJ. PKCgamma regulates syndecan-2 inside-out signaling during xenopus left-right development. Cell. 2002;111:981–90. doi: 10.1016/s0092-8674(02)01200-x. [DOI] [PubMed] [Google Scholar]
- Kramer KL, Yost HJ. Ectodermal syndecan-2 mediates left-right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Dev Cell. 2002;2:115–24. doi: 10.1016/s1534-5807(01)00107-1. [DOI] [PubMed] [Google Scholar]
- Krutovskikh V, Yamasaki H. The role of gap junctional intercellular communication (GJIC) disorders in experimental and human carcinogenesis. Histol. Histopathol. 1997;12:761–768. [PubMed] [Google Scholar]
- Krutovskikh V, Yamasaki H. Connexin gene mutations in human genetic diseases. Mutat Res. 2000;462:197–207. doi: 10.1016/s1383-5742(00)00037-5. [DOI] [PubMed] [Google Scholar]
- Kumai M, Nishii K, Nakamura K, Takeda N, Suzuki M, Shibata Y. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development. 2000;127:3501–12. doi: 10.1242/dev.127.16.3501. [DOI] [PubMed] [Google Scholar]
- Laird DW, Yancey SB, Bugga L, Revel JP. Connexin expression and gap junction communication compartments in the developing mouse limb. Dev Dyn. 1992;195:153–61. doi: 10.1002/aja.1001950302. [DOI] [PubMed] [Google Scholar]
- Landesman Y, Goodenough DA, Paul DL. Gap junctional communication in the early Xenopus embryo. J Cell Biol. 2000;150:929–36. doi: 10.1083/jcb.150.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landesman Y, Postma FR, Goodenough DA, Paul DL. Multiple connexins contribute to intercellular communication in the Xenopus embryo. J Cell Sci. 2003;116:29–38. doi: 10.1242/jcs.00182. [DOI] [PubMed] [Google Scholar]
- Landesman Y, White TW, Starich TA, Shaw JE, Goodenough DA, Paul DL. Innexin-3 forms connexin-like intercellular channels. J Cell Sci. 1999;112:2391–6. doi: 10.1242/jcs.112.14.2391. [DOI] [PubMed] [Google Scholar]
- Larre I, Ponce A, Fiorentino R, Shoshani L, Contreras RG, Cereijido M. Contacts and cooperation between cells depend on the hormone ouabain. Proc Natl Acad Sci U S A. 2006;103:10911–6. doi: 10.1073/pnas.0604496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151:931–44. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Gilula NB, Warner AE. Gap junctional communication and compaction during preimplantation stages of mouse development. Cell. 1987;51:851–60. doi: 10.1016/0092-8674(87)90108-5. [DOI] [PubMed] [Google Scholar]
- Lehmann C, Lechner H, Loer B, Knieps M, Herrmann S, Famulok M, Bauer R, Hoch M. Heteromerization of Innexin Gap Junction Proteins Regulates Epithelial Tissue Organization in Drosophila. Mol Biol Cell. 2006 doi: 10.1091/mbc.E05-11-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Left-right asymmetry and the chick embryo. Semin Cell Dev Biol. 1998;9:67–76. doi: 10.1006/scdb.1997.0192. [DOI] [PubMed] [Google Scholar]
- Levin M. Isolation and community: the role of gap junctional communication in embryonic patterning. J Membr Biol. 2001;185:177–192. doi: 10.1007/s00232-001-0129-7. [DOI] [PubMed] [Google Scholar]
- Levin M. Bioelectromagnetic patterning fields: roles in embryonic development, regeneration, and neoplasm. Bioelectromagnetics. 2003;24(a):295–315. [Google Scholar]
- Levin M. Hypothesis: motor proteins and ion pumps, not ciliary motion, initiate LR asymmetry. BioEssays. 2003;25(b):1002–1010. doi: 10.1002/bies.10339. [DOI] [PubMed] [Google Scholar]
- Levin M. Left-right asymmetry in embryonic development: a comprehensive review. Mech Dev. 2005;122:3–25. doi: 10.1016/j.mod.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Levin M, Buznikov GA, Lauder JM. Of minds and embryos: left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci. 2006;28:171–85. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junctions are involved in the early generation of left right asymmetry. Dev Biol. 1998;203(a):90–105. doi: 10.1006/dbio.1998.9024. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. The compulsion of chirality: toward an understanding of left-right asymmetry. Genes Dev. 1998;12(b):763–769. doi: 10.1101/gad.12.6.763. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junction-mediated transfer of left-right patterning signals in the early chick blastoderm is upstream of Shh asymmetry in the node. Development. 1999;126:4703–4714. doi: 10.1242/dev.126.21.4703. [DOI] [PubMed] [Google Scholar]
- Levin M, Nascone N. Two molecular models of initial left-right asymmetry generation. Med. Hypotheses. 1997;49:429–435. doi: 10.1016/s0306-9877(97)90092-x. [DOI] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Li G, Herlyn M. Dynamics of intercellular communication during melanoma development. Mol Med Today. 2000;6:163–9. doi: 10.1016/s1357-4310(00)01692-0. [DOI] [PubMed] [Google Scholar]
- Li WE, Waldo K, Linask KL, Chen T, Wessels A, Parmacek MS, Kirby ML, Lo CW. An essential role for connexin43 gap junctions in mouse coronary artery development. Development. 2002;129:2031–42. doi: 10.1242/dev.129.8.2031. [DOI] [PubMed] [Google Scholar]
- Liptau H, Viebahn C. Expression patterns of gap junctional proteins connexin 32 and 43 suggest new communication compartments in the gastrulating rabbit embryo. Differentiation. 1999;65:209–19. doi: 10.1046/j.1432-0436.1999.6540209.x. [DOI] [PubMed] [Google Scholar]
- Lo CW. The role of gap junction membrane channels in development. J Bioenerg Biomembr. 1996;28:379–85. doi: 10.1007/BF02110114. [DOI] [PubMed] [Google Scholar]
- Lo CW. Genes, gene knockouts, and mutations in the analysis of gap junctions. Dev Gen. 1999;24:1–4. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<1::AID-DVG1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Lo CW, Cohen MF, Huang GY, Lazatin BO, Patel N, Sullivan R, Pauken C, Park SM. C×43 gap junction gene expression and gap junctional communication in mouse neural crest cells. Dev Gen. 1997;20:119–32. doi: 10.1002/(SICI)1520-6408(1997)20:2<119::AID-DVG5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Lo CW, Waldo KL, Kirby ML. Gap junction communication and the modulation of cardiac neural crest cells. Trends in Cardiovascular Medicine. 1999;9:63–9. doi: 10.1016/s1050-1738(99)00015-8. [DOI] [PubMed] [Google Scholar]
- Loewenstein WR. Junctional intercellular communication: the cell-to-cell membrane channel. Physiological Reviews. 1981;61:829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Loewenstein WR, Rose B. The cell-cell channel in the control of growth. Semin Cell Biol. 1992;3:59–79. doi: 10.1016/s1043-4682(10)80008-x. [DOI] [PubMed] [Google Scholar]
- Maestrini E, Korge BP, Ocana-Sierra J, Calzolari E, Cambiaghi S, Scudder PM, Hovnanian A, Monaco AP, Munro CS. A missense mutation in connexin26, D66H, causes mutilating keratoderma with sensorineural deafness (Vohwinkel′s syndrome) in three unrelated families. Hum Mol Genet. 1999;8:1237–43. doi: 10.1093/hmg/8.7.1237. [DOI] [PubMed] [Google Scholar]
- Makarenkova H, Becker DL, Tickle C, Warner AE. Fibroblast growth factor 4 directs gap junction expression in the mesenchyme of the vertebrate limb Bud. J Cell Biol. 1997;138:1125–37. doi: 10.1083/jcb.138.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenkova H, Patel K. Gap junction signalling mediated through Connexin-43 is required for chick limb development. Dev Bio. 1999;207:380–392. doi: 10.1006/dbio.1998.9171. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev. 2005;85:943–78. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- McGonnell IM, Green CR, Tickle C, Becker DL. Connexin43 gap junction protein plays an essential role in morphogenesis of the embryonic chick face. Dev Dyn. 2001;222:420–438. doi: 10.1002/dvdy.1208. [DOI] [PubMed] [Google Scholar]
- Meda P. The role of gap junction membrane channels in secretion and hormonal action. J. Bioenerg. Biomembr. 1996;28:369–377. doi: 10.1007/BF02110113. [DOI] [PubMed] [Google Scholar]
- Mege R, Goudou D, Giaume C, Nicolet M, Rieger F. Is intercellular communication via gap junctions required for myoblast fusion? Cell Adhes Commun. 1994;2:329–43. doi: 10.3109/15419069409014208. [DOI] [PubMed] [Google Scholar]
- Mehta PP, Hotz-Wagenblatt A, Rose B, Shalloway D, Loewenstein WR. Incorporation of the gene for a cell-cell channel protein into transformed cells leads to normalization of growth. J Membr Biol. 1991;124:207–25. doi: 10.1007/BF01994355. [DOI] [PubMed] [Google Scholar]
- Meiners S, Xu A, Schindler M. Gap junction protein homologue from Arabidopsis thaliana: evidence for connexins in plants. Proc Natl Acad Sci U S A. 1991;88:4119–22. doi: 10.1073/pnas.88.10.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloy PG, Kusnierczyk MK, Meyer RA, Lo CW, Desmond ME. Overexpression of connexin43 alters the mutant phenotype of midgestational wnt-1 null mice resulting in recovery of the midbrain and cerebellum. Anat Rec A Discov Mol Cell Evol Biol. 2005;283:224–38. doi: 10.1002/ar.a.20158. [DOI] [PubMed] [Google Scholar]
- Mesnil M, Crespin S, Avanzo JL, Zaidan-Dagli ML. Defective gap junctional intercellular communication in the carcinogenic process. Biochim Biophys Acta. 2005;1719:125–45. doi: 10.1016/j.bbamem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Cohen MF, Recalde S, Zakany J, Bell SM, Scott WJ, Jr., Lo CW. Developmental regulation and asymmetric expression of the gene encoding C×43 gap junctions in the mouse limb bud. Dev Gen. 1997;21:290–300. doi: 10.1002/(SICI)1520-6408(1997)21:4<290::AID-DVG6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Minkoff R, Parker SB, Rundus VR, Hertzberg EL. Expression patterns of connexin43 protein during facial development in the chick embryo: associates with outgrowth, attachment, and closure of the midfacial primordia. Anat Rec. 1997;248:279–90. doi: 10.1002/(SICI)1097-0185(199706)248:2<279::AID-AR15>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Momose-Sato Y, Honda Y, Sasaki H, Sato K. Optical mapping of the functional organization of the rat trigeminal nucleus: initial expression and spatiotemporal dynamics of sensory information transfer during embryogenesis. J Neurosci. 2004;24:1366–76. doi: 10.1523/JNEUROSCI.4457-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose-Sato Y, Honda Y, Sasaki H, Sato K. Optical imaging of large-scale correlated wave activity in the developing rat CNS. J Neurophysiol. 2005;94:1606–22. doi: 10.1152/jn.00044.2005. [DOI] [PubMed] [Google Scholar]
- Morley GE, Ek-Vitorin JF, Taffet SM, Delmar M. Structure of connexin43 and its regulation by pHi. J Cardiovasc Electrophysiol. 1997;8:939–51. doi: 10.1111/j.1540-8167.1997.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Mushegian AR, Koonin EV. The proposed plant connexin is a protein kinase-like protein. Plant Cell. 1993;5:998–9. doi: 10.1105/tpc.5.9.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagajski D, Guthrie S, Ford C, Warner A. The correlation between patterns of dye transfer through gap junctions and future developmental fate in Xenopus. Development. 1989;105:747–752. [Google Scholar]
- Nicholson BJ, Weber PA, Cao F, Chang H, Lampe P, Goldberg G. The molecular basis of selective permeability of connexins is complex and includes both size and charge. Braz J Med Biol Res. 2000;33:369–78. doi: 10.1590/s0100-879x2000000400002. [DOI] [PubMed] [Google Scholar]
- Nogi T, Levin M. Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration. Dev Biol. 2005;287:314–35. doi: 10.1016/j.ydbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Nordenberg J, Fenig E, Landau M, Weizman R, Weizman A. Effects of psychotropic drugs on cell proliferation and differentiation. Biochem Pharmacol. 1999;58:1229–36. doi: 10.1016/s0006-2952(99)00156-2. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol. 2003;58(a):1–26. doi: 10.1016/s0070-2153(03)58001-2. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R. Endogenous electric fields in embryos during development, regeneration and wound healing. Radiat Prot Dosimetry. 2003;106(b):375–83. doi: 10.1093/oxfordjournals.rpd.a006375. [DOI] [PubMed] [Google Scholar]
- Nygren A, Giles WR. Mathematical simulation of slowing of cardiac conduction velocity by elevated extracellular. Ann Biomed Eng. 2000;28:951–7. doi: 10.1114/1.1308489. [DOI] [PubMed] [Google Scholar]
- Olson DJ, Christian JL, Moon RT. Effect of wnt-1 and related proteins on gap junctional communication in Xenopus embryos. Science. 1991;252:1173–6. doi: 10.1126/science.252.5009.1173. [DOI] [PubMed] [Google Scholar]
- Olson DJ, Moon RT. Distinct effects of ectopic expression of Wnt-1, activin B, and bFGF on gap junctional permeability in 32-cell Xenopus embryos. Dev Bio. 1992;151:204–212. doi: 10.1016/0012-1606(92)90227-8. [DOI] [PubMed] [Google Scholar]
- Omori Y, Yamasaki H. Mutated connexin43 proteins inhibit rat glioma cell growth suppression mediated by wild-type connexin43 in a dominant-negative manner. Int J Cancer. 1998;78:446–53. doi: 10.1002/(sici)1097-0215(19981109)78:4<446::aid-ijc10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Omori Y, Zaidan Dagli ML, Yamakage K, Yamasaki H. Involvement of gap junctions in tumor suppression: analysis of genetically-manipulated mice. Mutat Res. 2001;477:191–6. doi: 10.1016/s0027-5107(01)00120-8. [DOI] [PubMed] [Google Scholar]
- Oviedo-Orta E, Errington RJ, Evans WH. Gap junction intercellular communication during lymphocyte transendothelial migration. Cell Biol Int. 2002;26:4253–63. doi: 10.1006/cbir.2001.0840. [DOI] [PubMed] [Google Scholar]
- Paemeleire K, Martin PE, Coleman SL, Fogarty KE, Carrington WA, Leybaert L, Tuft RA, Evans WH, Sanderson MJ. Intercellular calcium waves in HeLa cells expressing GFP-labeled connexin 43, 32, or 26. Mol Biol Cell. 2000;11:1815–27. doi: 10.1091/mbc.11.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraguassu-Braga FH, Borojevic R, Bouzas LF, Barcinski MA, Bonomo A. Bone marrow stroma inhibits proliferation and apoptosis in leukemic cells through gap junction-mediated cell communication. Cell Death Differ. 2003;10:1101–8. doi: 10.1038/sj.cdd.4401279. [DOI] [PubMed] [Google Scholar]
- Paul D, Yu K, Bruzzone R, Gimlich R, Goodenough D. Expression of a dominant negative inhibitor of intercellular communication in the early Xenopus embryo causes delamination and extrusion of cells. Development. 1995;121:371–81. doi: 10.1242/dev.121.2.371. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46(a):731–44. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Luneborg NL, Becker DL, Mobbs P. Gap junctions modulate interkinetic nuclear movement in retinal progenitor cells. J Neurosci. 2005;25(b):10803–14. doi: 10.1523/JNEUROSCI.2312-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan P. Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta. 2005;1711:225–45. doi: 10.1016/j.bbamem.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Phelan P, Stebbings LA, Baines RA, Bacon JP, Davies JA, Ford C. Drosophila Shaking-B protein forms gap junctions in paired Xenopus oocytes. Nature. 1998;391:181–4. doi: 10.1038/34426. [DOI] [PubMed] [Google Scholar]
- Pierce GB. The cancer cell and its control by the embryo. Rous-Whipple Award lecture. Am J Pathol. 1983;113:117–24. [PMC free article] [PubMed] [Google Scholar]
- Pierce GB, Arechaga J, Wells RS. Embryonic control of cancer. Prog Clin Biol Res. 1986;226:67–77. [PubMed] [Google Scholar]
- Pitts JD, Finbow ME, Kam E. Junctional communication and cellular differentiation. Br J Cancer Suppl. 1988;9:52–7. [PMC free article] [PubMed] [Google Scholar]
- Pizard A, Burgon PG, Paul DL, Bruneau BG, Seidman CE, Seidman JG. Connexin 40, a target of transcription factor tbx5, patterns wrist, digits, and sternum. Mol Cell Biol. 2005;25:5073–83. doi: 10.1128/MCB.25.12.5073-5083.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova TV, Aslanidi KB, Boitzova L. Energy transfer via cell-to-cell junctions. Ouabain-resistant cells maintain a membrane potential in ouabain-sensitive cells. FEBS Lett. 1990;262:69–71. doi: 10.1016/0014-5793(90)80156-d. [DOI] [PubMed] [Google Scholar]
- Potter DD, Furshpan EJ, Lennox ES. Connections between cells of the developing squid as revealed by electrophysiological methods. Proceedings of the National Academy of Sciences of the United States of America. 1966;55:328–36. doi: 10.1073/pnas.55.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R. Site-specific and developmental expression of pannexin1 in the mouse nervous system. Eur J Neurosci. 2005;21:3277–90. doi: 10.1111/j.1460-9568.2005.04139.x. [DOI] [PubMed] [Google Scholar]
- Rela L, Szczupak L. Gap junctions: their importance for the dynamics of neural circuits. Mol Neurobiol. 2004;30:341–57. doi: 10.1385/MN:30:3:341. [DOI] [PubMed] [Google Scholar]
- Richard G. Connexin disorders of the skin. Adv Dermatol. 2001;17:243–77. [PubMed] [Google Scholar]
- Richard G, Rouan F, Willoughby CE, Brown N, Chung P, Ryynanen M, Jabs EW, Bale SJ, DiGiovanna JJ, Uitto J, Russell L. Missense mutations in GJB2 encoding connexin-26 cause the ectodermal dysplasia keratitis-ichthyosis-deafness syndrome. Am J Hum Genet. 2002;70:1341–8. doi: 10.1086/339986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risek B, Klier FG, Gilula NB. Multiple gap junction genes are utilized during rat skin and hair development. Development. 1992;116:639–51. doi: 10.1242/dev.116.3.639. [DOI] [PubMed] [Google Scholar]
- Robinson KR, Messerli MA. Left/right, up/down: the role of endogenous electrical fields as directional signals in development, repair and invasion. Bioessays. 2003;25:759–66. doi: 10.1002/bies.10307. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Hampson EC, Munro MN, Vaney DI. Unidirectional coupling of gap junctions between neuroglia. Science. 1993;262:1072–4. doi: 10.1126/science.8093125. [DOI] [PubMed] [Google Scholar]
- Roger C, Mograbi B, Chevallier D, Michiels JF, Tanaka H, Segretain D, Pointis G, Fenichel P. Disrupted traffic of connexin 43 in human testicular seminoma cells: overexpression of C×43 induces membrane location and cell proliferation decrease. J Pathol. 2004;202:241–6. doi: 10.1002/path.1509. [DOI] [PubMed] [Google Scholar]
- Rong P, Wang X, Niesman I, Wu Y, Benedetti LE, Dunia I, Levy E, Gong X. Disruption of Gja8 (alpha8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development. 2002;129:167–74. doi: 10.1242/dev.129.1.167. [DOI] [PubMed] [Google Scholar]
- Rose B, Mehta PP, Loewenstein WR. Gap-junction protein gene suppresses tumorigenicity. Carcinogenesis. 1993;14:1073–5. doi: 10.1093/carcin/14.5.1073. [DOI] [PubMed] [Google Scholar]
- Rubin H. Cancer as a dynamic developmental disorder. Cancer Res. 1985;45:2935–42. [PubMed] [Google Scholar]
- Ruiz i Altaba A, Stecca B, Sanchez P. Hedgehog--Gli signaling in brain tumors: stem cells and paradevelopmental programs in cancer. Cancer Lett. 2004;204:145–57. doi: 10.1016/S0304-3835(03)00451-8. [DOI] [PubMed] [Google Scholar]
- Saito T, Schlegel R, Andresson T, Yuge L, Yamamoto M, Yamasaki H. Induction of cell transformation by mutated 16K vacuolar H+-atpase (ductin) is accompanied by down-regulation of gap junctional intercellular communication and translocation of connexin 43 in NIH3T3 cells. Oncogene. 1998;17:1673–80. doi: 10.1038/sj.onc.1202092. [DOI] [PubMed] [Google Scholar]
- Sanchez Alvarado A. The freshwater planarian Schmidtea mediterranea: embryogenesis, stem cells and regeneration. Curr Opin Genet Dev. 2003;13:438–444. doi: 10.1016/s0959-437x(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Sanchez Alvarado A. Planarians. Curr Biol. 2004;14:R737–8. doi: 10.1016/j.cub.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Sanchez Alvarado A, Reddien PW, Bermange A, Oviedo N, Robb SM. Functional Studies of Regeneration in the Planarian Schmidtea mediterranea. Dev Bio. 2003;259:525. [Google Scholar]
- Sasakura Y, Shoguchi E, Takatori N, Wada S, Meinertzhagen IA, Satou Y, Satoh N. A genomewide survey of developmentally relevant genes in Ciona intestinalis. X. Genes for cell junctions and extracellular matrix. Dev Genes Evol. 2003;213:303–13. doi: 10.1007/s00427-003-0320-1. [DOI] [PubMed] [Google Scholar]
- Schiffmann Y. An hypothesis: phosphorylation fields as the source of positional information and cell differentiation--(cAMP, ATP) as the universal morphogenetic Turing couple. Prog Biophys Mol Biol. 1991;56:79–105. doi: 10.1016/0079-6107(91)90015-k. [DOI] [PubMed] [Google Scholar]
- Serafeim A, Grafton G, Chamba A, Gregory CD, Blakely RD, Bowery NG, Barnes NM, Gordon J. 5-Hydroxytryptamine drives apoptosis in biopsylike Burkitt lymphoma cells: reversal by selective serotonin reuptake inhibitors. Blood. 2002;99:2545–53. doi: 10.1182/blood.v99.7.2545. [DOI] [PubMed] [Google Scholar]
- Serras F, Fraser S, Chuong CM. Asymmetric patterns of gap junctional communication in developing chicken skin. Development. 1993;119:85–96. doi: 10.1242/dev.119.Supplement.85. [DOI] [PubMed] [Google Scholar]
- Severs NJ. Cardiovascular disease. Novartis Found Symp. 1999;219:188–206. doi: 10.1002/9780470515587.ch12. [DOI] [PubMed] [Google Scholar]
- Severs NJ. Gap junction remodeling and cardiac arrhythmogenesis: cause or coincidence? J Cell Mol Med. 2001;5:355–66. doi: 10.1111/j.1582-4934.2001.tb00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheardown SA, Hooper ML. A relationship between gap junction-mediated intercellular communication and the in vitro developmental capacity of murine embryonic stem cells. Exp Cell Res. 1992;198:276–82. doi: 10.1016/0014-4827(92)90380-q. [DOI] [PubMed] [Google Scholar]
- Sheridan JD. Electrophysiological study of special connections between cells in the early chick embryo. J Cell Biol. 1966;31:C1–5. doi: 10.1083/jcb.31.1.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman A, Rinzel J. Model for synchronization of pancreatic beta-cells by gap junction coupling. Biophys J. 1991;59:547–59. doi: 10.1016/S0006-3495(91)82271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR, Dones JA, Jackson CL, Chen H. Heart and head defects in mice lacking pairs of connexins. Dev Biol. 2004;265:369–83. doi: 10.1016/j.ydbio.2003.09.036. [DOI] [PubMed] [Google Scholar]
- Skinner MA, MacLaren LA, Wildeman AG. Stage-dependent redistribution of the V-ATPase during bovine implantation. J Histochem Cytochem. 1999;47:1247–54. doi: 10.1177/002215549904701004. [DOI] [PubMed] [Google Scholar]
- Slack JM. Regeneration research today. Dev Dyn. 2003;226:162–6. doi: 10.1002/dvdy.10232. [DOI] [PubMed] [Google Scholar]
- Sohl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes. 2003;10:173–80. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–32. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Stains JP, Civitelli R. Gap junctions regulate extracellular signal-regulated kinase signaling to affect gene transcription. Mol Biol Cell. 2005;16:64–72. doi: 10.1091/mbc.E04-04-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stains JP, Lecanda F, Screen J, Towler DA, Civitelli R. Gap junctional communication modulates gene transcription by altering the recruitment of Sp1 and Sp3 to connexin-response elements in osteoblast promoters. J Biol Chem. 2003;278:24377–87. doi: 10.1074/jbc.M212554200. [DOI] [PubMed] [Google Scholar]
- Starich TA, Lee RY, Panzarella C, Avery L, Shaw JE. eat-5 and unc-7 represent a multigene family in Caenorhabditis elegans involved in cell-cell coupling. J Cell Biol. 1996;134:537–48. doi: 10.1083/jcb.134.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich TA, Miller A, Nguyen RL, Hall DH, Shaw JE. The caenorhabditis elegans innexin INX-3 is localized to gap junctions and is essential for embryonic development. Dev Biol. 2003;256:403–17. doi: 10.1016/s0012-1606(02)00116-1. [DOI] [PubMed] [Google Scholar]
- Stebbings L, Todman M, Phillips R, Greer C, Tam J, Phelan P, Jacobs K, Bacon J, Davies J. Gap junctions in Drosophila: developmental expression of the entire innexin gene family. Mech Dev. 2002;113:197–205. doi: 10.1016/s0925-4773(02)00025-4. [DOI] [PubMed] [Google Scholar]
- Stebbings LA, Todman MG, Phelan P, Bacon JP, Davies JA. Two Drosophila innexins are expressed in overlapping domains and cooperate to form gap-junction channels. Mol Biol Cell. 2000;11:2459–70. doi: 10.1091/mbc.11.7.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchyna TM, Nitsche JM, Chilton M, Harris AL, Veenstra RD, Nicholson BJ. Different ionic selectivities for connexins 26 and 32 produce rectifying gap junction channels. Biophys J. 1999;77:2968–87. doi: 10.1016/S0006-3495(99)77129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Huang GY, Meyer RA, Wessels A, Linask KK, Lo CW. Heart malformations in transgenic mice exhibiting dominant negative inhibition of gap junctional communication in neural crest cells. Dev Bio. 1998;204:224–34. doi: 10.1006/dbio.1998.9089. [DOI] [PubMed] [Google Scholar]
- Sutor B, Hagerty T. Involvement of gap junctions in the development of the neocortex. Biochim Biophys Acta. 2005;1719:59–68. doi: 10.1016/j.bbamem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Takagi H, Kaneko K. Dynamical systems basis of metamorphosis: diversity and plasticity of cellular states in reaction diffusion network. J Theor Biol. 2005;234:173–86. doi: 10.1016/j.jtbi.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Grossman HB. Connexin 26 gene therapy of human bladder cancer: induction of growth suppression, apoptosis, and synergy with Cisplatin. Hum Gene Ther. 2001;12:2225–36. doi: 10.1089/10430340152710568. [DOI] [PubMed] [Google Scholar]
- Tazuke SI, Schulz C, Gilboa L, Fogarty M, Mahowald AP, Guichet A, Ephrussi A, Wood CG, Lehmann R, Fuller MT. A germline-specific gap junction protein required for survival of differentiating early germ cells. Development. 2002;129:2529–39. doi: 10.1242/dev.129.10.2529. [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Bio (Orlando) 1994;161:563–96. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Tessmar-Raible K, Arendt D. Emerging systems: between vertebrates and arthropods, the Lophotrochozoa. Curr Opin Genet Dev. 2003;13:331–40. doi: 10.1016/s0959-437x(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Todman M, Baines R, Stebbings L, Davies J, Bacon J. Gap-Junctional communication between developing Drosophila muscles is essential for their normal development. Dev Gen. 1999;24:57–68. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<57::AID-DVG7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Intercellular calcium signaling via gap junction in connexin-43-transfected cells. J Biol Chem. 1998;273:1519–28. doi: 10.1074/jbc.273.3.1519. [DOI] [PubMed] [Google Scholar]
- Trosko J, Chang C, Wilson M, Upham B, Hayashi T, Wade M. Gap junctions and the regulation of cellular functions of stem cells during development and differentiation. Methods. 2000;20:245–264. doi: 10.1006/meth.1999.0941. [DOI] [PubMed] [Google Scholar]
- Trosko JE. The role of stem cells and gap junctions as targets for cancer chemoprevention and chemotherapy. Biomed Pharmacother. 2005;59(Suppl 2):S326–31. doi: 10.1016/s0753-3322(05)80065-4. [DOI] [PubMed] [Google Scholar]
- Turin L, Warner AE. Intracellular pH in early Xenopus embryos: its effect on current flow between blastomeres. J Physiol. 1980;300:489–504. doi: 10.1113/jphysiol.1980.sp013174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull M, Webb B. Perspectives on polydnavirus origins and evolution. Adv Virus Res. 2002;58:203–54. doi: 10.1016/s0065-3527(02)58006-4. [DOI] [PubMed] [Google Scholar]
- Turnbull MW, Volkoff AN, Webb BA, Phelan P. Functional gap junction genes are encoded by insect viruses. Curr Biol. 2005;15:R491–2. doi: 10.1016/j.cub.2005.06.052. [DOI] [PubMed] [Google Scholar]
- Valiunas V, Bechberger JF, Naus CC, Brink PR, Goldberg GS. Nontransformed cells can normalize gap junctional communication with transformed cells. Biochem Biophys Res Commun. 2005;333(a):174–9. doi: 10.1016/j.bbrc.2005.05.104. [DOI] [PubMed] [Google Scholar]
- Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS, Brink PR. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568(b):459–68. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heyden MA, Rook MB, Hermans MM, Rijksen G, Boonstra J, Defize LH, Destree OH. Identification of connexin43 as a functional target for Wnt signalling. J Cell Sci. 1998;111:1741–9. doi: 10.1242/jcs.111.12.1741. [DOI] [PubMed] [Google Scholar]
- Verheijck EE, van Kempen MJ, Veereschild M, Lurvink J, Jongsma HJ, Bouman LN. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc Res. 2001;52:40–50. doi: 10.1016/s0008-6363(01)00364-9. [DOI] [PubMed] [Google Scholar]
- Vogel R, Weingart R. Mathematical model of vertebrate gap junctions derived from electrical measurements on homotypic and heterotypic channels. J Physiol. 1998;510(Pt 1):177–89. doi: 10.1111/j.1469-7793.1998.177bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R, Weingart R. The electrophysiology of gap junctions and gap junction channels and their mathematical modelling. Biol Cell. 2002;94:501–10. doi: 10.1016/s0248-4900(02)00022-9. [DOI] [PubMed] [Google Scholar]
- Wakeford RJ. Cell contact and positional communication in hydra. J Embryol Exp Morphol. 1979;54:171–83. [PubMed] [Google Scholar]
- Waldo KL, Lo CW, Kirby ML. Connexin 43 expression reflects neural crest patterns during cardiovascular development. Dev Bio. 1999;208:307–23. doi: 10.1006/dbio.1999.9219. [DOI] [PubMed] [Google Scholar]
- Waller BR, 3rd, McQuinn T, Phelps AL, Markwald RR, Lo CW, Thompson RP, Wessels A. Conotruncal anomalies in the trisomy 16 mouse: an immunohistochemical analysis with emphasis on the involvement of the neural crest. Anat Rec. 2000;260:279–93. doi: 10.1002/1097-0185(20001101)260:3<279::AID-AR65>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Wang SQ. [The appearance and changes of gap junctions during myogenesis of Cynops embryos] Shi Yan Sheng Wu Xue Bao. 1989;22:189–203. [PubMed] [Google Scholar]
- Warner A. Interactions between growth factors and gap junctional communication in developing systems. Novartis Found Symp. 1999;219:60–72. doi: 10.1002/9780470515587.ch5. [DOI] [PubMed] [Google Scholar]
- Warner AE, Guthrie SC, Gilula NB. Antibodies to gap-junctional protein selectively disrupt junctional communication in the early amphibian embryo. Nature. 1984;311:127–31. doi: 10.1038/311127a0. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Iwashita M, Ishii M, Kurachi Y, Kawakami A, Kondo S, Okada N. Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene. EMBO Rep. 2006 doi: 10.1038/sj.embor.7400757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb RJ, Marshall F, Swann K, Carroll J. Follicle-stimulating hormone induces a gap junction-dependent dynamic change in [cAMP] and protein kinase a in mammalian oocytes. Dev Biol. 2002;246:441–54. doi: 10.1006/dbio.2002.0630. [DOI] [PubMed] [Google Scholar]
- Weber PA, Chang HC, Spaeth KE, Nitsche JM, Nicholson BJ. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys J. 2004;87:958–73. doi: 10.1529/biophysj.103.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CJ, Xu X, Lo CW. Connexins and Cell Signaling in Development and Disease. Annu Rev Cell Dev Biol. 2004 doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- Weir MP, Lo CW. Gap-junctional communication compartments in the Drosophila wing imaginal disk. Dev Bio. 1984;102:130–46. doi: 10.1016/0012-1606(84)90181-7. [DOI] [PubMed] [Google Scholar]
- White TW, Wang H, Mui R, Litteral J, Brink PR. Cloning and functional expression of invertebrate connexins from Halocynthia pyriformis. FEBS Lett. 2004;577:42–8. doi: 10.1016/j.febslet.2004.09.071. [DOI] [PubMed] [Google Scholar]
- Wolszon LR, Gao WQ, Passani MB, Macagno ER. Growth cone ″collapse″ in vivo: are inhibitory interactions mediated by gap junctions? J Neurosci. 1994;14:999–1010. doi: 10.1523/JNEUROSCI.14-03-00999.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RC, Pebay A, Nguyen LT, Koh KL, Pera MF. Presence of functional gap junctions in human embryonic stem cells. Stem Cells. 2004;22:883–9. doi: 10.1634/stemcells.22-6-883. [DOI] [PubMed] [Google Scholar]
- Wood RL, Kuda AM. Formation of junctions in regenerating hydra: gap junctions. J Ultrastruct Res. 1980;73:350–60. doi: 10.1016/s0022-5320(80)90094-5. [DOI] [PubMed] [Google Scholar]
- Woodruff RI. Calmodulin transit via gap junctions is reduced in the absence of an electric field. J Insect Physiol. 2005;51:843–52. doi: 10.1016/j.jinsphys.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield S. Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina. J Comp Neurol. 1997;383:512–528. doi: 10.1002/(sici)1096-9861(19970714)383:4<512::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, Thomas MP, Radice GL, Lo CW. N-cadherin and C×43alpha1 gap junctions modulates mouse neural crest cell motility via distinct pathways. Cell Commun Adhes. 2001;8:321–4. doi: 10.3109/15419060109080746. [DOI] [PubMed] [Google Scholar]
- Ya J, Erdtsieck-Ernste EB, De Boer PA, van Kempen MJ, Jongsma H, Gros D, Moorman AF, Lamers WH. Heart defects in connexin43-deficient mice. Circ Res. 1998;82:360–6. doi: 10.1161/01.res.82.3.360. [DOI] [PubMed] [Google Scholar]
- Yahalom A, Warmbrodt RD, Laird DW, Traub O, Revel JP, Willecke K, Epel BL. Maize mesocotyl plasmodesmata proteins cross-react with connexin gap junction protein antibodies. Plant Cell. 1991;3:407–17. doi: 10.1105/tpc.3.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Mesnil M, Omori Y, Mironov N, Krutovskikh V. Intercellular communication and carcinogenesis. Mutat Res. 1995;333:181–8. doi: 10.1016/0027-5107(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Yancey SB, Biswal S, Revel JP. Spatial and temporal patterns of distribution of the gap junction protein connexin43 during mouse gastrulation and organogenesis. Development. 1992;114:203–12. doi: 10.1242/dev.114.1.203. [DOI] [PubMed] [Google Scholar]
- Yost HJ. Establishment of left-right asymmetry. Int Rev Cytol. 2001;203:357–81. doi: 10.1016/s0074-7696(01)03011-x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Green C, Stott NS. Bone morphogenetic protein-2 modulation of chondrogenic differentiation in vitro involves gap junction-mediated intercellular communication. J Cell Physiol. 2002;193:233–43. doi: 10.1002/jcp.10168. [DOI] [PubMed] [Google Scholar]
- Zhang ZQ, Hu Y, Wang BJ, Lin ZX, Naus CC, Nicholson BJ. Effective asymmetry in gap junctional intercellular communication between populations of human normal lung fibroblasts and lung carcinoma cells. Carcinogenesis. 2004;25:473–82. doi: 10.1093/carcin/bgh036. [DOI] [PubMed] [Google Scholar]