Abstract
Infection by vector-borne protozoa of the genus Leishmania occurs by the deposition of parasites within the skin of the mammalian host, where they eventually bind to and are phagocytized by Mφs. Our previous work supported the idea that parasites can interact with extracellular matrix and basement membrane proteins, such as fibronectin (FN), within the skin, leading to enhanced invasion. In this report, we extend these findings and show that both promastigotes and amastigotes of Leishmania species can bind directly to soluble FN and laminin (LM) and that promastigotes express a distinct surface protein of ∼60 kDa that binds both FN and LM. Promastigotes of multiple Leishmania species can rapidly degrade FN by using surface-localized and secreted metalloprotease (leishmanolysin). FN degradation at the surfaces of amastigotes is leishmanolysin dependent, whereas both secreted leishmanolysin and cysteine protease B contribute to extracellular FN degradation. Leishmania-degraded FN decreased the production of reactive oxygen intermediates by parasite-infected macrophages and affected the accumulation of intracellular parasites. These findings show that both parasite stages of Leishmania species bind to and proteolytically degrade FN at the parasite surface and distantly through secreted proteases and that degraded forms of FN can influence the activation state of parasite-infected macrophages.
Leishmania species are vector-borne pathogenic protozoa which cause considerable worldwide morbidity and mortality. Infection is initiated by the deposition of infective flagellated promastigotes within the skin of the mammalian host during the feeding of infected sand flies. Within host macrophages (Mφs), parasites differentiate into and replicate as intracellular, aflagellate amastigotes, eventually escaping extracellularly, where they are phagocytized by uninfected Mφs (7). Reactive-oxygen intermediates (ROI) and reactive-nitrogen intermediates are important for intraphagolysosomal parasite killing at early and late phases of infection, respectively (21).
The cell surface metalloproteases (also known as leishmanolysin, gp63, and msp) of Leishmania spp. are broad-spectrum, zinc-dependent proteases that aid parasite evasion of innate host immune factors, such as complement, and act as adhesins of host Mφs (5, 6, 16). Leishmanolysin also protects amastigotes from nonoxidative degradation within Mφs (8, 16). Cysteine proteases (CPs) are expressed by and important for the growth and differentiation of multiple Leishmania spp. (20). Of the three classes of CPs (CP-A, -B, and -C), CP-B is the most well studied in Leishmania mexicana and is crucial for the intracellular growth of amastigotes; released CP-B may be important for the cleavage of host proteins within phagolysosomes (2, 9, 29).
We have previously shown that the migration of Leishmania spp. through the extracellular matrix (ECM) in vitro is due to the leishmanolysin-mediated proteolysis of fibronectin (FN) and collagen type IV (18). FN is a large, multifunctional protein, the sequence of which is well conserved across species, that has multiple domains for interaction with ECM components such as heparin, collagen, and fibrin (12). FN is important in the structural framework of the ECM, where it plays a role in modulating cell behavior. Mφs can interact with different FN domains via different receptors. Peptide fragments produced by the proteolytic degradation of FN can have a dramatic and varied influence on Mφ activation and function. The binding of Mφs to peptide fragments containing the FN interconnecting segment (ICS) domain can decrease Mφ expression of gamma interferon, interleukin 12, monocyte chemoattractant protein 1, and transforming growth factor β (14). In contrast, the interaction of Mφs with intact FN (FNi) can lead to an increase in tumor necrosis factor alpha secretion (4). Mφs incubated with chymotrypsin-degraded FN (FNd) secrete more tumor necrosis factor alpha, fibroblast growth factor 1, insulinlike growth factor 1, and leukemia inhibitory factor than those incubated with FNi (4). This stimulatory capacity has been localized to a 110- to 120-kDa fragment of the cell-binding domain containing the RGD sequence (4, 26).
Here we show that a distinct parasite cell surface protein facilitates binding to FN and that both promastigotes and amastigotes of Leishmania species degrade FN. Leishmanolysin-dependent FN degradation by promastigotes of multiple Leishmania species occurs both at the parasite surface and distantly by secreted leishmanolysin. Amastigotes use both cell surface and secreted leishmanolysin and secreted CP to degrade FN. The degradation of FN by leishmanolysin leads to the production of multiple distinct fragments which have the capacity to down-regulate the ROI production of parasite-infected host Mφs. Together, these results suggest that Leishmania species interact with FN through a specific receptor protein and that parasites extensively degrade FN using two protease systems. This promotes the local ECM invasion and production of FN degradation products that can influence the activation state of host Mφs.
MATERIALS AND METHODS
Parasites and ECM proteins.
The Leishmania species used for these studies were (i) promastigotes and axenic amastigotes of a virulent strain of Leishmania amazonensis (LV7B) (16, 17), (ii) Leishmania major (MHOM/SN/74/Seidman) and the gp63 knockout (KO) mutant of this line (13), and (iii) Leishmania donovani (MHOM/N/1983/AG83) (11). Strains were cultivated as promastigotes at room temperature in M199 plus 10% heat-inactivated fetal bovine serum. Axenic amastigotes of L. amazonensis were routinely passaged in Grace's insect medium at pH 5.5 and 33°C. Thrice-washed cells were fixed in 0.5% glutaraldehyde (Glu) in phosphate-buffered saline (PBS) for 30 min on ice and then washed and resuspended at 108 per ml. It has been previously shown that the Glu fixation of cells does not significantly diminish the surface proteolytic activity of leishmanolysin (16-18). Purified bovine FN and laminin (LM) were purchased from a commercial source (Sigma-Aldrich), divided into aliquots, and stored at −20°C prior to use. Aliquots were used once to minimize freeze-thaw degradation.
Parasite surface labeling, ECM-basement membrane protein blotting, and immunoprecipitation analyses.
The surface biotinylation and preparation of detergent lysates of L. amazonensis were done as described previously (18, 19). Briefly, the biotinylation of parasite surface proteins (using 107 washed, live cells) or 10 to 50 mg of FN or LM was done by incubation in PBS containing 1 mg/ml of EZ-Link N-hydroxysulfosuccinimide-long chain-biotin reagent (Pierce) on ice for 30 min. Thereafter, reaction mixtures were quenched by the addition of excess M199. Our two principal assays for the identification and purification of ECM-basement membrane binding proteins used nonreducing and nondenaturing conditions in order to preserve the native conformations of both parasite surface and ECM-basement membrane proteins to enhance their potential for interaction. For the bio-ECM blot assay, the denatured, reduced protein lysates of the parasites were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. Blots were blocked in Tris-buffered saline-Tween 20 (0.1%) containing 5% milk, washed, and then blotted with biotinylated FN or LM. After 1 h at 25°C, the blots were washed and probed with streptavidin-horseradish peroxidase (SA-HRP) and then washed thrice, followed by development by enhanced chemiluminescence (Amersham Biosciences, Inc.). For the bio-lysate ECM capture assay, the wells of a 96-well enzyme-linked immunosorbent assay plate were coated with either FN or LM proteins (1 or 5 mg) overnight at 4°C and then washed thrice with PBS. This was followed by the blocking of wells with 0.5% bovine serum albumin (BSA) in PBS for 1 h, followed by washing. Wells blocked with BSA alone were used as a negative control. Nondenatured lysates of surface-biotinylated parasites (∼107 cells) were added to the ECM-coated, blocked wells and controls. After a 1-h incubation, unbound proteins were washed away and the complexes were solubilized in SDS-PAGE buffer, fractionated by SDS-PAGE, and then immobilized on nitrocellulose paper and probed with SA-HRP to detect ECM-bound leishmanial proteins.
Parasite-ECM binding analysis.
The labeling of FN and LM with fluorescein isothiocyanate (FITC) was done using the protein labeling kit (Pierce) according to the protocol from the manufacturer. For flow cytometric analysis, stationary-phase promastigotes or axenically grown amastigotes (107) were incubated with FITC-labeled FN or LM for 30 min and then washed thrice with PBS, followed by flow cytometric analysis using a FACSCalibur flow cytometer and CellQuestPro software (Becton Dickinson, Mount View, CA). We tested increasing amounts (from 1 to 10 mg) of labeled FN or LM and found maximal binding at 5 mg and higher (not shown). A total of 5 mg was used for all further experiments. For competition assays, cells were preincubated in the same concentration of an unlabeled protein (FN, LM, or BSA) at 25°C for 30 min, washed and then incubated with labeled ECM for a further 30 min, and washed and then analyzed as described above. The preincubation of cells with anti-L. amazonensis gp63 rabbit polyclonal antiserum (1:200) was done under the same conditions as for the FN/LM competition assays. All experiments were performed at least three times, all of which yielded similar results.
Protein degradation and zymographic and Western blot analyses.
To test the capacity of parasites to digest components of FN, 10 mg each of FN was incubated at 37°C with 107 stationary-phase, Glu-fixed promastigotes or amastigotes for increasing lengths of time (from 5 min to 24 h). The Glu fixation of parasites has been shown to not affect gp63 proteolytic activity (17-19). Supernatants of the reaction mixtures were analyzed by SDS-PAGE and Coomassie blue staining of gels. Controls included protein incubated in buffer alone, cells that had been preincubated in 25 mM ortho-phenanthroline (OP), and/or cells incubated with 10 mM of the CP inhibitor E-64. The conditioned medium (CM) of promastigotes or amastigotes from that equivalent to 107 cells was also assayed for the ability to degrade ECM proteins under the same conditions. For the zymographic analysis of protease activity, 107 cells or the CM equivalent to this number of cells was fractionated under nondenaturing conditions in 10% SDS-PAGE gels containing 0.2% gelatin. After the gels were run, they were washed twice, 30 min each, in 0.5% Triton X-100 and then incubated at 37°C overnight in 40 mM Tris, pH 7.4, followed by staining in Coomassie blue and then destaining to visualize the proteolytic activity as negatively staining bands. Duplicate gels incubated in development buffer containing 25 mM OP and/or 10 mM E-64 served to differentiate between metallo- and cysteine-dependent proteolytic activities. Anti-CP-B-specific antisera were used for both immunoprecipitation (at 1:200 dilution) and Western blot (at 1:10,000 dilution) analyses of cells and CM.
Structural analysis of proteolytic fragments of FN.
FN degradation products were separated by preparative SDS-PAGE, and individual fragments were submitted for analysis by capillary liquid chromatography-tandem mass spectrometry on a hybrid quadrupole-time-of-flight Q-TOF II (Micromass, Wythenshawe, United Kingdom) mass spectrometer equipped with an orthogonal nanospray source (New Objective, Woburn, MA) operated in the positive-ion mode. Mass spectra were acquired using MassLynx 4.0. Sequence information from the tandem mass spectrometry data was processed using Mascot Distiller software, and database searches were performed using Mascot programs (Matrix Science, Boston, MA). Each fragment was identified and compared to the sequence of the entire FN protein in order to determine where within FN it was derived.
Analysis of Mφ ROI.
J774 mouse Mφ cultures (5 × 104) and mouse bone marrow-derived Mφs (BMDMs) were infected with stationary-phase L. amazonensis promastigotes (5 × 105) for 24 h, after which nonengulfed parasites were washed away. Primary BMDMs were harvested and prepared from the leg bones of 5- to 8-week-old BALB/c and B6 mice, as previously described (3, 24). Mφ cultures were then separately incubated at 37°C with differing amounts (1, 5, and 10 mg) of FNi or FNd produced by parasites. FNd was prepared by incubation with Glu-fixed L. amazonensis promastigotes, as described above. Cultures incubated with FNi or FNd were compared to controls containing PBS from Glu-fixed parasites alone under the same conditions. At the indicated times postinfection, the production of ROI was measured by a standardized assay using 2′,7′-dichlorodihydrofluorescein diacetate (DCF) (16), wherein infected-Mφs were incubated in RPMI medium containing 32 mM DCF for 30 min at 37°C. The fluorescence of DCF was measured every 2 min for a total of 90 min using a Fluostar 32 plate reader (BMG Lab Technologies, Durham, NC). Maximal effects were seen at 5 μg FNi and FNd, and these are shown below (see Fig. 1). Assays were performed in triplicate for three to five separate experiments. Results were analyzed for statistical significance using Student's t test. Endotoxin-free medium and serum were used for these experiments. All other buffers and reagents were assayed for the presence of lipopolysaccharide; those that were positive were treated with Detoxi-gel (Pierce Biochemicals) to remove lipopolysaccharide.
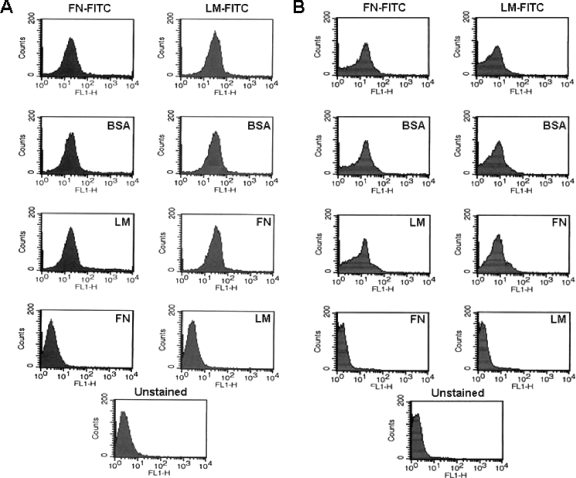
FIG. 1.
Flow cytometric analysis of FN and LM binding to stationary-phase promastigotes (A) and amastigotes (B) of L. amazonensis. Cells (107) were washed in PBS and incubated for 30 min at 25°C with 1, 5, or 10 μg of FN- or LM-FITC (as indicated at the top of the panels), washed thrice, and analyzed by flow cytometry. We observed maximal surface fluorescence with 5 μg or higher, and results using 5 μg are shown. This resulted in a definitive shift in the surface fluorescence of the entire population of cells (compare top panels with those of unlabeled cells in the bottom panels). The specificity of binding was tested by the preincubation of cells with unlabeled proteins (indicated within each group) under the same conditions prior to incubation with FN- or LM-FITC.
RESULTS
The surfaces of both life stages of Leishmania species bind to FN.
Leishmania species can degrade various ECM proteins in vitro in a leishmanolysin-dependent manner, leading to enhanced parasite migration through the ECM. We hypothesized that Leishmania species have the capacity for binding FN as a prerequisite to this process. In order to test this, we developed a flow cytometric approach using FITC-labeled FN and LM (Fig. 1 and 2B and C). FN-FITC readily labeled L. amazonensis promastigotes and amastigotes (Fig. 1A and B, respectively, top left panels). Surface FN binding was specific since the preincubation of cells with unlabeled FN prior to incubation with FN-FITC ablated binding, yet the preincubation of cells with the same concentration of LM or BSA did not. Assays using LM-FITC also showed that both forms of L. amazonensis can also readily bind this protein (Fig. 1A and B, top right panels). Unlabeled LM efficiently competed with LM-FITC, whereas the preincubation of cells with either unlabeled FN or BSA did not reduce LM-FITC binding.
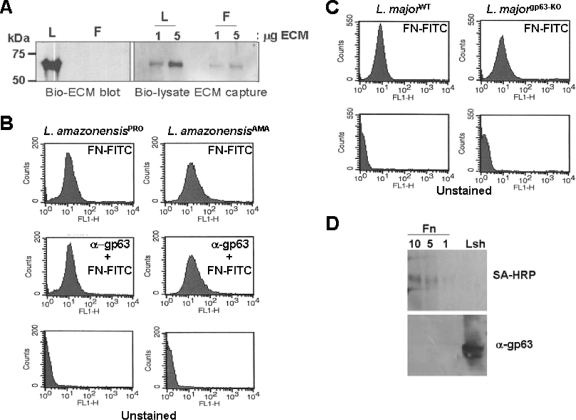
FIG. 2.
The cell surface binding of both life stages of Leishmania species is mediated by a protein distinct from leishmanolysin. (A) Analysis of the FN- or LM-binding protein of L. amazonensis was performed using a biotinylated ligand to probe denatured and reduced protein lysates of promastigotes (Bio-ECM blot). Nondenatured detergent lysates of surface biotinylated parasites were incubated with either 1 or 5 μg of plastic immobilized FN or LM (F and L, respectively), washed and solubilized in Laemmli buffer, fractionated by SDS-PAGE, blotted, and then probed with SA-HRP (Bio-lysate ECM capture). (B) Both promastigotes (PRO) and amastigotes (AMA) of L. amazonensis bind FN-FITC. The preincubation of cells with antileishmanolysin polyclonal antibody (1:200 for 30 min at 25°C) followed by incubation with FN-FITC (indicated within the graphs) did not diminish FN-FITC surface binding. (C) FN-FITC labels the surfaces of both wild-type (WT) and gp63KO L. major parasites, indicating both that FN can bind to multiple Leishmania species and that this is a leishmanolysin-independent process. (D) Lysates used in the bio-lysate ECM capture experiment were harvested from wells with increasing amounts of FN (as indicated in micrograms) and blotted, and the blots were probed with SA-HRP (upper panel) or with antileishmanolysin polyclonal antisera (lower panel). The lysates of parasites (Lsh lane) served as a positive control for antiserum reactivity.
Leishmania species express an FN-binding protein which is distinct from leishmanolysin.
Since our ECM binding assays suggested that Leishmania species readily bound FN and LM, we tested the hypothesis that parasites express a specific receptor(s) to recognize these proteins. Since proteins, glycans, and glycolipid conjugates are abundant on the surfaces of Leishmania spp. species and could serve to bind FN and LM, we tested trypsinized promastigotes and amastigotes (not shown) for their abilities to bind FN- and LM-FITC. The binding of FN and LM by both parasite stages was completely abolished by trypsin treatment, highly suggesting that binding to these ECM proteins was dependent on a parasite surface protein. We investigated this protein further by using two additional methods. First, we probed nitrocellulose-blotted, SDS-PAGE-fractionated parasite protein lysates separately with biotinylated FN or LM, followed by probing with SA-HRP (Fig. 2A, bio-ECM blot). This method allowed us to visualize an ∼60-kDa protein which reacted to LM but not FN. Second, we incubated detergent lysates of surface-biotinylated promastigotes with increasing amounts of LM or FN prebound to the plastic wells of an enzyme-linked immunosorbent assay plate. Following the washing of unbound material, bound material was solubilized in Laemmli buffer and fractionated by SDS-PAGE gels, blotted, and then probed with SA-HRP to visualize surface-labeled parasite proteins (Fig. 2A, bio-lysate ECM capture blot). This method allowed us to visualize an ∼60-kDa protein that bound to both FN and LM. In each case, we found higher levels of the ECM-binding protein in reaction mixtures containing more ECM than in mixtures containing less ECM. We found no binding of the labeled proteins of Leishmania species to wells containing BSA (not shown).
Since we have shown that leishmanolysin can degrade FN and that the size of the FN-binding protein is similar to that of leishmanolysin, we sought to test the hypothesis that the FN-binding protein is distinct from leishmanolysin. We used three assays to test this. First, we used antileishmanolysin polyclonal antibody in our competitive FN-FITC-parasite binding flow cytometric assay to test whether the binding of this antibody to leishmanolysin could reduce the FN-FITC association with the parasite surface (Fig. 2B). We tested both L. amazonensis promastigotes and amastigotes for this analysis and found that preincubation with antileishmanolysin antibody did not diminish the association of FN-FITC with either parasite form. Second, we compared both the wild type and the gp63KO mutant of L. major lines (13, 15) for their degree of FN-FITC binding (Fig. 2C). We found that both lines had similar levels of FN-FITC binding, indicating both that FN binding occurs with multiple Leishmania species and that FN binding is independent of leishmanolysin. Finally, we tested the reactivity of the FN-binding protein with antileishmanolysin antisera. For this, material from the bio-ECM lysate was fractionated by SDS-PAGE, blotted, and probed with either SA-HRP or antileishmanolysin antisera (Fig. 2D). Only blots probed with SA-HRP demonstrated the presence of the ∼60-kDa binding protein, whereas replicate blots probed with the antileishmanolysin antiserum reacted only with the Leishmania lysate control.
Rapid and extensive surface proteolytic degradation of FN by promastigotes of multiple Leishmania species.
We have previously shown that ECM proteins are degraded by surface-localized, zinc-dependent proteolysis at the parasite surface (18). In order to determine the kinetics of proteolytic FN degradation, Glu-fixed promastigotes from multiple Leishmania species were separately incubated with FN, and the products were analyzed at increasing time points of incubation by SDS-PAGE fractionation, followed by Coomassie blue staining (Fig. 3A). Both cutaneous (L. amazonensis and L. major) and visceral (L. donovani) species extensively degraded FN into multiple peptide fragments. FN degradation was evident as early as at 5 min for L. major, 1 h for L. amazonensis, and 2 h for L. donovani. Complete degradation occurred by 24 h for all parasite lines, and an analysis of FNd at later times of incubation did not change the abundance or sizes of FN fragments (not shown). The leishmanolysin degradation pattern of bovine and human FN by L. amazonensis led to the production of similarly sized proteins (not shown). FN was cleaved into 10 to 13 fragments that ranged in size from 240 to 25 kDa. At 24 h, abundant levels of larger degradation products were seen in reactions with L. amazonensis but were not as abundant in reactions with the two other species.
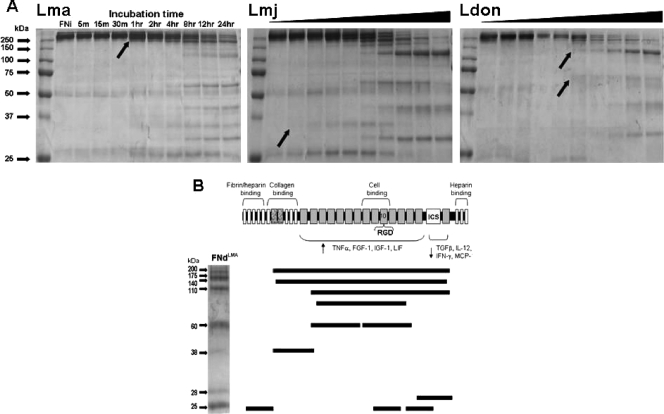
FIG. 3.
Time-dependent surface proteolytic degradation of FN by promastigotes of multiple Leishmania species (A) and mapping of the FN peptide fragments produced by L. amazonensis degradation (B). (A) Glu-fixed, stationary-phase promastigotes (107 in 100 ml PBS containing 10 μg FN at 25°C) of L. amazonensis (Lma), L. major (Lmj), and L. donovani (Ldon) were incubated for the indicated lengths of time (black triangles for Lmj and Ldon represent the same time course as shown for Lma), parasites were removed by microcentrifugation, and supernatants were processed for SDS-PAGE fractionation and Coomassie blue staining. L. major degraded FN the most rapidly (within 5 min), followed by L. amazonensis (1 h) and L. donovani (2 h), as indicated by the visualization of smaller FN proteins (black arrows). Each species produced a number of similarly sized protein fragments as well as some unique ones. (B) FNi was incubated with Glu-fixed L. amazonensis promastigotes for 24 h. FN fragments were separated by SDS-PAGE and stained with Coomassie blue. The visualized fragments shown were excised and submitted for mass spectroscopic analysis. The peptide sequences of each FN fragment were mapped according to their positions within the FNi. The functional domains of FN are indicated above the schematic, with the 10th repeat of the cell association domain containing the RGD sequence. The regions shown to activate (4) or deactivate (14) Mφs to produce different proteins are indicated below the schematic. TNF-α, tumor necrosis factor alpha; FGF-1, fibroblast growth factor 1; IGF-1, insulinlike growth factor 1; LIF, leukemia inhibitory factor; TGFβ, transforming growth factor β; IL-12, interleukin 12; IFN-γ, gamma interferon; MCP, monocyte chemoattractant protein.
In order to characterize FNd and identify domains that may be potentially important for altering the activity of Mφs, we fractionated FNd by SDS-PAGE and submitted individual fragments for mass spectroscopic analysis (data not shown). The sequence data indicated that FN was cleaved in multiple places (Fig. 3B). Interestingly, several fragments encompassed nearly the entire FN protein being degraded at the extreme N- and C-terminal ends. Smaller fragments of ∼60 and 25 kDa were each composed of two and three comigrating fragments of the same size, respectively. Two other singular fragments of 38 and 28 kDa were also identified. Of note, one of the 60-kDa fragments encompassed the region of FN containing the RGD domain, and both the 28-kDa and one of the 25-kDa fragments overlap and encompass the FN ICS domain which functions to link FN monomers (1).
Zinc-dependent FN degradation by Leishmania is due to surface and secreted leishmanolysin.
Leishmanolysin is abundant on the surfaces of all pathogenic Leishmania species, and proteolytically active leishmanolysin is also released from some species (19, 30). Both Glu-fixed L. amazonensis and L. donovani promastigotes cleaved FN in a Zn-dependent manner (Fig. 4A, lanes 1 and 2, respectively). The chelation of Zn by the preincubation of parasites with OP prior to their incubation with FN completely ablated FN degradation. We further confirmed this by comparing the abilities of the wild type and a leishmanolysin KO mutant of L. major to degrade FN (Fig. 4B). The wild-type but not the KO mutant of L. major degraded FN in the presence of Zn (Fig. 4B, left panel), whereas the chelation of Zn (right panel) ablated FN degradation. These data firmly establish that FN degradation by parasites observed here and in our previous work is due directly to leishmanolysin. Finally, we compared the abilities of surface and secreted leishmanolysin to degrade FN (Fig. 4C). Cell-free CM from cultures of Leishmania promastigotes (shown for L. amazonensis) degraded FN in a Zn-dependent manner. The ability of CM to degrade FN was abolished by the removal of leishmanolysin from CM with a leishmanolysin-specific antibody (not shown). The patterns of FN degradation produced by released and cell-associated leishmanolysin were nearly identical, with the exception of a minor fragment of ∼36 kDa (Fig. 4C) seen in reaction mixtures containing CM.
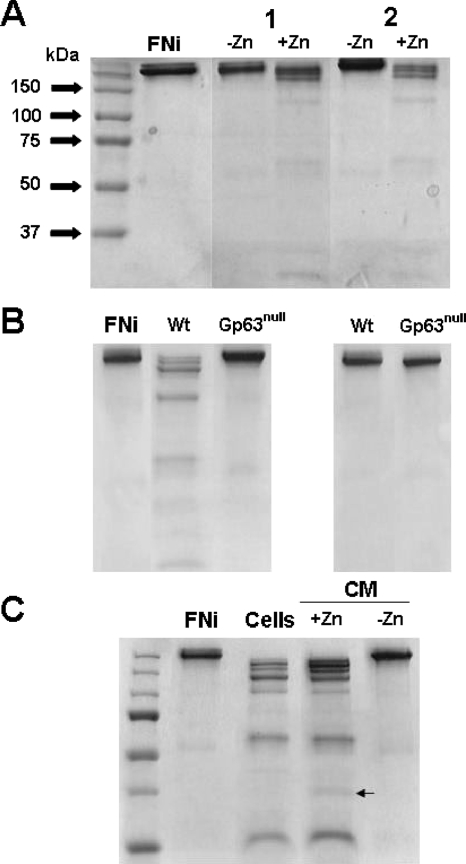
FIG. 4.
Surface and secreted leishmanolysin from Leishmania promastigotes proteolytically degrade FN. (A) Glu-fixed promastigotes of L. amazonensis (lanes 1) and L. donovani (lanes 2) were incubated with FN for 4 h in PBS with and without Zn (as indicated) and then processed for SDS-PAGE analysis and Coomassie blue staining. (B) Fixed wild-type (Wt) and a gp63KO mutant (Gp63null) of L. major were compared for their ability to degrade FN in the presence (left panel) and absence (right panel) of zinc. (C) Comparative SDS-PAGE analysis of the cell surface (Cells) and CM of L. amazonensis (CM) for FN proteolytic degradation. CM was tested in the presence and absence of Zn (as indicated). The arrow denotes a unique FN fragment in the CM reaction mixtures. FNi, FNi alone.
Both secreted leishmanolysin and CP-B of L. amazonensis amastigotes participate in the cleavage of FN.
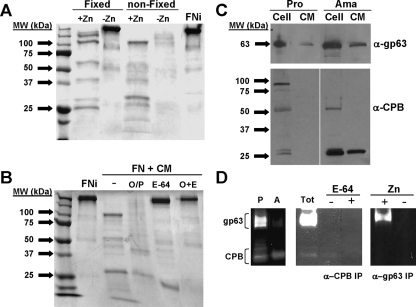
During mammalian infection, infected Mφs are disrupted, leading to the release of amastigotes which come into contact with ECM prior to their reengulfment by uninfected Mφs. We tested the hypothesis that amastigotes of L. amazonensis could also degrade FN. We compared the abilities of fixed and unfixed amastigotes of L. amazonensis to degrade FN in the presence and absence of Zn (Fig. 5A) and found that unfixed parasites degraded FN more extensively than fixed parasites. In the absence of Zn, unfixed parasites showed less-extensive degradation than they did in the presence of Zn, suggesting that FN degradation by live amastigotes was only partially due to leishmanolysin. FN degradation by fixed parasites, in contrast, was completely abolished by the removal of Zn, suggesting that live amastigotes release additional proteases that degrade FN. Amastigote CM readily degraded FN into multiple fragments, and this degradation was only partially inhibited by either preincubation with OP or the CP inhibitor E-64, suggesting that both released CPs and leishmanolysin from amastigotes contribute to FN degradation (Fig. 5B). Preincubation of CM with both E-64 and OP completely abolished FN degradation, confirming that these are the only proteases from amastigotes that degrade FN. Since CP-B is known to be expressed by both promastigotes and amastigotes of L. mexicana, but only amastigotes release CP-B, we tested CP-B expression and activity by our system using L. amazonensis (Fig. 5C). Western blot analysis of the cell lysates and CM of both promastigotes and amastigotes using antiserum specific for leishmanolysin and CP-B clearly showed that amastigotes released both leishmanolysin and CP-B, whereas we could detect only leishmanolysin released from promastigotes. Both enzymes were proteolytically active and readily detectable by gelatin zymographic analysis of total cell lysates and CM after immunoprecipitation with corresponding antisera (Fig. 5D).
FIG. 5.
Differential pattern FN degradation by amastigotes is due to secreted metallo- and cysteine proteases. (A) Glu-fixed and nonfixed axenic amastigotes of L. amazonensis were compared for their capacity to degrade FN in the presence and absence of Zn (as indicated). The conditions used were identical to those described in the legend to Fig. 4 except that the reactions were performed at 35°C. (B) The CM from axenic amastigotes was incubated with FN under various conditions inhibitory to cysteine (E-64) or metalloproteases (OP, O/P) or both (O+E). (C) Western blot analysis of the stationary-phase cells (Cell) and medium (CM) of promastigotes and amastigotes of L. amazonensis with antisera specific for leishmanolysin (α-gp63) and CP-B (α-CPB). (D) Gelatin zymographic analysis of total cell lysates of promastigotes (P) and amastigotes (A) (left panel) and from CM (Tot) and immunoprecipitated CP-B (α-CPB IP) and leishmanolysin (α-gp63 IP) from amastigotes in the presence and absence of E-64 and Zn, as indicated.
FNd produced by leishmanolysin decreases the production of ROI of parasite-infected Mφs and increases the accumulation of amastigotes.
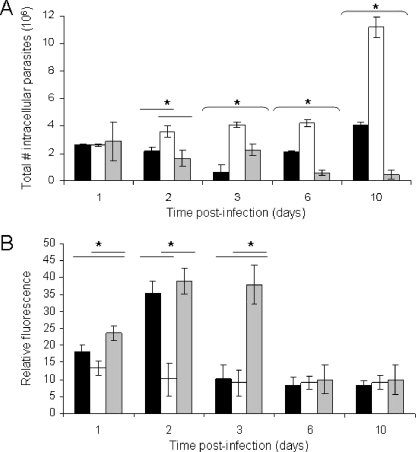
Since different FN subdomains can lead to the differential activation or deactivation of Mφs, we tested the hypothesis that FNd produced by leishmanolysin can influence the activation state of parasite-infected Mφs. We analyzed the ROI production of parasite-infected Mφs exposed to FNi and FNd for up 10 days (Fig. 6A). FNd-treated cultures had substantially diminished production of ROI compared to that of cultures treated with FNi or buffer alone. The production levels of ROI in the last two cultures did not differ significantly until day 3 postinfection, when the ROI production dropped to the low level equivalent to that of the FN-treated cultures. Thereafter, on days 6 and 10 postinfection, all cultures produced the same low levels of ROI. We have not detected any differences in reactive-nitrogen intermediates between treatment groups (data not shown).
FIG. 6.
FNd produced by leishmanolysin enhances the intracellular growth of L. amazonensis by diminishing the production of ROI. Mφs (5 × 105) bound to coverslips were incubated with 10 μg of FNi (gray bars), FNd (white bars), or buffer alone (black bars) for 30 min prior to the addition of late-stationary-phase promastigotes. (A) Growth of intracellular amastigotes within Mφs. (B) Measurement of ROI produced within infected Mφs. Means ± standard deviations are shown. The asterisks over the horizontal lines denote statistically significant differences (P ≤ 0.05) between the column under the asterisk and the spanned value(s).
We measured the accumulation of amastigotes over a 10-day course of infection and found a substantial rise in the accumulation of amastigotes in the FNd-treated cultures starting at 48 h postinfection and reaching a maximum at 10 days postinfection (Fig. 6B). In contrast, FNi-treated cultures showed a slow decline in the number of parasites, while untreated controls, initially showing a decrease by 72 h postinfection, slowly accumulated more parasites than the FNi-treated cultures at days 6 to 10 postinfection. We also determined the effect of FNi and FNd on the ROI production and the number of intracellular L. amazonensis parasites in BMDMs from BALB/c mice over 48 h. Parasite-infected BMDMs incubated with FNi over 48 h produced approximately twofold more ROI than those incubated with FNd (4,891 ± 195 versus 2,451 ± 80 arbitrary units [mean ± standard deviation]). We observed comparable differences in ROI in BMDMs harvested from B6 mice (not shown). The total parasite burdens of the cultures 48 h postinfection were approximately seven to eightfold larger (2,696 ± 361 versus 355 ± 96 parasites/high-power field). We observed no differences in ROI production levels or intracellular parasite numbers compared to those at 4 h postincubation.
DISCUSSION
For this study, we have characterized the interactions of Leishmania species with FN and have shown that both stages of Leishmania species can bind to and degrade FN. We have provided evidence that Leishmania promastigotes express a distinct cell surface receptor for FN that may serve to bind multiple ECM proteins. The results presented strongly indicate that the protein(s) which binds FN and LM is distinct from leishmanolysin. FN and LM bound to parasite surface proteins of nearly identical molecular weights, suggesting that they may bind the same surface receptor. The finding that FN bound to a protein(s) in the bio-lysate ECM capture assay but not in the bio-ECM blot assay but that LM bound to a protein(s) in both assays suggests that FN binding is more dependent on the intact conformation of the receptor and that LM binding may be less stringent in this regard, requiring only a linear peptide epitope(s). The results of the flow cytometric ECM-binding competition assay suggest that FN and LM may not directly compete for the same binding site of the receptor. A less likely possibility is that two or more ECM receptors of similar sizes are expressed and present on the cell surface. The binding of FN and LM via this receptor may increase the proximity of surface-localized leishmanolysin with FN, resulting in its enhanced degradation. The intensity of FN-FITC binding to the surfaces of wild-type parasites was maximal at 10 min in our assays, with a slow decline in fluorescent intensity during incubation for up to 60 min (not shown), suggesting that FN initially binds to the parasite surface and then may be released by leishmanolysin degradation. Such a decline was not seen with the leishmanolysin KO mutant (not shown). This, together with the fact that no differences in FN-FITC binding were seen in comparisons of the wild type and the KO mutant of L. major, suggests that FN binds to the surface FN receptor and then is released by leishmanolysin degradation. Whether FNi or FNd fragments remain bound to the surfaces of parasites is not yet known.
Various microbial pathogens also express cell surface proteins (microbial surface components recognizing adhesive matrix molecules) which promote binding to ECM and BM proteins (23, 27, 28). Thus, our findings support the idea that cutaneous Leishmania species express a receptor protein functionally analogous to microbial surface components recognizing adhesive matrix molecules. Leishmania donovani has a partially characterized 67-kDa surface LM binding protein that is postulated to promote visceralization of infection (10, 11). Whether the protein that we have identified is similar to this awaits further investigation. The binding of ECM proteins, such as FN, to the cell surface receptor may lead to signal transduction within parasites, resulting in changes in gene expression that facilitate further parasite invasion or stage transformation.
Our findings indicate that multiple Leishmania species can extensively degrade FN in a rapid manner using surface leishmanolysin, which suggests that this is a functionally conserved process and may contribute to the pathogenesis of different forms of leishmaniasis. The kinetics of degradation are variable, however, depending on the species, becoming detectable between 5 min and 2 h. In our experience, the uptake of promastigotes by Mφs, tested in vitro, can take up to 24 h after binding is complete. While the kinetics of binding and uptake of parasites by Mφs in vivo have not been well studied, we envision that the complete internalization of parasites may take several hours. The initiation of FN degradation within 30 min occurs well within this time and is consistent with the idea that FN degradation contributes to the local invasion of parasites. While FN degradation using Glu-fixed cells is due to surface-localized leishmanolysin, Leishmania species express multiple differentially localized leishmanolysin isoforms that may be involved in different functions in the parasite life cycle (19, 22, 30). Structural differences between leishmanolysin isoforms expressed by different species probably contribute to their differential substrate specificities, accounting for the differing patterns of FN degradation. Leishmanolysin is released in two forms: that directly secreted from the flagellar pocket and that released from the cell membrane (19). FN degradation in vivo probably occurs by a combined action of the two forms of extracellular leishmanolysin. The difference in the patterns of FN degradation between extracellular and cell surface leishmanolysin may be due to the differential substrate specificities of these different protease isoforms.
These results clearly demonstrate that leishmanolysin is present on the surfaces of axenically grown amastigotes and that both leishmanolysin and CP-B are released from amastigotes, both of which can extensively degrade FN. We hypothesize that the degradation of FN, and probably other ECM proteins, by parasites may contribute to the disruption of ECM, facilitating the local spread of parasites, and that breakdown of the basement membrane may lead to the metastatic spread of parasites to other organs. ECM breakdown could also lead to the production of peptide fragments with chemokine activity, attracting additional host Mφs into inflammatory sites for the uptake of parasites. The leishmanolysin and cysteine group of proteases, which mediate FN degradation, probably have multiple synergistic functions within the parasite and at the host-pathogen interface. Leishmanolysin-deficient parasites have been shown to have diminished virulence in mice, which has been attributed to their enhanced susceptibility to complement-mediated lysis (13, 25). We hypothesize that multiple additional leishmanolysin-dependent events may account for these results, including the inability of these parasites to degrade FN and generate FNd for Mφ deactivation and the ability of these parasites to degrade and inactivate host antimicrobial peptides (15). CPs may also contribute to these processes. Finally, the degradation of FN by parasites leads to the generation of FN peptides that may facilitate the uptake of parasites by host Mφs and/or directly stimulate the intracellular growth of parasites.
Our results suggest not only that the degradation of FN within ECM may play a role in allowing parasites to spread locally but also that FN degradation products may affect Mφ function. Since FN peptides containing the ICS domain have been shown to deactivate Mφs, we hypothesize that the proteolytic degradation of FN may expose this region for interaction with Mφs in our assay and that the interaction of Mφs with this or other FN fragments may lead to their deactivation. The effect of FNd on infected J-line Mφs suppressed the rise in ROI production over 48 h compared to that of cultures treated with buffer alone, suggesting that FNd dampened parasite-induced Mφ activation. Furthermore, the presence of FNi served to enhance the activation of infected J-line Mφs over 24 h compared to that of the control and for 72 h longer than those treated with FNd, suggesting that FNi may enhance Mφ activation. The transient depression of ROI production by infected Mφs led to an increase in the number of amastigotes. We find similar changes in ROI production and intracellular parasite number in parasite-infected BMDMs, suggesting that the effect of parasite FN degradation may influence infection in vivo. During host infection, the intracellular growth and release of parasites from infected Mφs may allow the continuous exposure of parasites to extracellular FN. This may lead to the consumption of FNi due to proteolysis and the production of FNd. These two processes together may significantly diminish the activation of parasite-infected Mφs in lesions, allowing parasites to flourish intracellularly. These alterations in FN may also influence the binding and engulfment of parasites by Mφs. We hypothesize that the interactions of parasites with other ECM and BM proteins, such as collagen subtypes and LM, in addition to FN, may also have importance in the disease pathogenesis of different types of leishmaniasis.
Acknowledgments
This study was supported by internal funds and a Davis Bremer Medical Research grant from The Ohio State University to B.S.M. and a Canadian Institutes of Health grant (MOP 739) to W.R.M.
We thank J. Mottram and S. Rafati for the anti-CP-B antisera, K.-P. Chang for the axenic amastigotes of L. amazonensis, K. Green-Church from the OSU Mass Spectrometry and Proteomics facility for help with the FN structural studies, and Amal Amer for help with the isolation of BMDMs. We thank Chad Rappleye, John Gunn, and Uday Sandbhor for critical reading of the manuscript.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 22 January 2008.
REFERENCES
- 1.Aguirre, K. M., R. J. McCormick, and J. E. Schwarzbauer. 1994. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. J. Biol. Chem. 26927863-27868. [PubMed] [Google Scholar]
- 2.Alexander, J., G. H. Coombs, and J. C. Mottram. 1998. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J. Immunol. 1616794-6801. [PubMed] [Google Scholar]
- 3.Amer, A. O., and M. S. Swanson. 2005. Autophagy is an immediate macrophage response to Legionella pneumophila. Cell. Microbiol. 7765-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beezhold, D. H., and C. Personius. 1992. Fibronectin fragments stimulate tumor necrosis factor secretion by human monocytes. J. Leukoc. Biol. 5159-64. [DOI] [PubMed] [Google Scholar]
- 5.Brittingham, A., C. J. Morrison, W. R. McMaster, B. S. McGwire, K. P. Chang, and D. M. Mosser. 1995. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J. Immunol. 1553102-3111. [PubMed] [Google Scholar]
- 6.Chang, C. S., and K. P. Chang. 1986. Monoclonal antibody affinity purification of a Leishmania membrane glycoprotein and its inhibition of leishmania-macrophage binding. Proc. Natl. Acad. Sci. USA 83100-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, K. P., and D. M. Dwyer. 1976. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science 193678-680. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhuri, G., M. Chaudhuri, A. Pan, and K. P. Chang. 1989. Surface acid proteinase (gp63) of Leishmania mexicana. A metalloenzyme capable of protecting liposome-encapsulated proteins from phagolysosomal degradation by macrophages. J. Biol. Chem. 2647483-7489. [PubMed] [Google Scholar]
- 9.Denise, H., K. McNeil, D. R. Brooks, J. Alexander, G. H. Coombs, and J. C. Mottram. 2003. Expression of multiple CPB genes encoding cysteine proteases is required for Leishmania mexicana virulence in vivo. Infect. Immun. 713190-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh, A., K. Bandyopadhyay, L. Kole, and P. K. Das. 1999. Isolation of a laminin-binding protein from the protozoan parasite Leishmania donovani that may mediate cell adhesion. Biochem. J. 337551-558. [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh, A., L. Kole, K. Bandyopadhyay, K. Sarkar, and P. K. Das. 1996. Evidence of a laminin binding protein on the surface of Leishmania donovani. Biochem. Biophys. Res. Commun. 226101-106. [DOI] [PubMed] [Google Scholar]
- 12.Hynes, R. 1985. Molecular biology of fibronectin. Annu. Rev. Cell. Biol. 167-90. [DOI] [PubMed] [Google Scholar]
- 13.Joshi, P. B., B. L. Kelly, S. Kamhawi, D. L. Sacks, and W. R. McMaster. 2002. Targeted gene deletion in Leishmania major identifies leishmanolysin (GP63) as a virulence factor. Mol. Biochem. Parasitol. 12033-40. [DOI] [PubMed] [Google Scholar]
- 14.Korom, S., W. W. Hancock, A. J. Coito, and J. W. Kupiec-Weglinski. 1998. Blockade of very late antigen-4 integrin binding to fibronectin in allograft recipients. II. Treatment with connecting segment-1 peptides prevents chronic rejection by attenuating arteriosclerotic development and suppressing intragraft T cell and macrophage activation. Transplantation 65854-859. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni, M. M., W. R. McMaster, E. Kamysz, W. Kamysz, D. M. Engman, and B. S. McGwire. 2006. The major surface-metalloprotease of the parasitic protozoan, Leishmania, protects against antimicrobial peptide-induced apoptotic killing. Mol. Microbiol. 621484-1497. [DOI] [PubMed] [Google Scholar]
- 16.McGwire, B., and K. P. Chang. 1994. Genetic rescue of surface metalloproteinase (gp63)-deficiency in Leishmania amazonensis variants increases their infection of macrophages at the early phase. Mol. Biochem. Parasitol. 66345-347. [DOI] [PubMed] [Google Scholar]
- 17.McGwire, B. S., and K. P. Chang. 1996. Posttranslational regulation of a Leishmania HEXXH metalloprotease (gp63). The effects of site-specific mutagenesis of catalytic, zinc binding, N-glycosylation, and glycosyl phosphatidylinositol addition sites on N-terminal end cleavage, intracellular stability, and extracellular exit. J. Biol. Chem. 2717903-7909. [DOI] [PubMed] [Google Scholar]
- 18.McGwire, B. S., K.-P. Chang, and D. M. Engman. 2003. Migration through the extracellular matrix by the parasitic protozoan Leishmania is enhanced by surface metalloprotease gp63. Infect. Immun. 711008-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGwire, B. S., W. A. O'Connell, K. P. Chang, and D. M. Engman. 2002. Extracellular release of the glycosylphosphatidylinositol (GPI)-linked Leishmania surface metalloprotease, gp63, is independent of GPI phospholipolysis: implications for parasite virulence. J. Biol. Chem. 2778802-8809. [DOI] [PubMed] [Google Scholar]
- 20.Mottram, J. C., G. H. Coombs, and J. Alexander. 2004. Cysteine peptidases as virulence factors of Leishmania. Curr. Opin. Microbiol. 7375-381. [DOI] [PubMed] [Google Scholar]
- 21.Murray, H. W., and C. F. Nathan. 1999. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 189741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramamoorthy, R., J. E. Donelson, K. E. Paetz, M. Maybodi, S. C. Roberts, and M. E. Wilson. 1992. Three distinct RNAs for the surface protease gp63 are differentially expressed during development of Leishmania donovani chagasi promastigotes to an infectious form. J. Biol. Chem. 2671888-1895. [PubMed] [Google Scholar]
- 23.Sillanpaa, J., Y. Xu, S. R. Nallapareddy, B. E. Murray, and M. Hook. 2004. A family of putative MSCRAMMs from Enterococcus faecalis. Microbiology 1502069-2078. [DOI] [PubMed] [Google Scholar]
- 24.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 633609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiakaki, M., B. Kolli, K. P. Chang, and K. Soteriadou. 2006. Down-regulation of gp63 level in Leishmania amazonensis promastigotes reduces their infectivity in BALB/c mice. Microbes Infect. 81455-1463. [DOI] [PubMed] [Google Scholar]
- 26.Trial, J., R. D. Rossen, J. Rubio, and A. A. Knowlton. 2004. Inflammation and ischemia: macrophages activated by fibronectin fragments enhance the survival of injured cardiac myocytes. Exp. Biol. Med. 229538-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Visai, L., Y. Xu, F. Casolini, S. Rindi, M. Hook, and P. Speziale. 2000. Monoclonal antibodies to CNA, a collagen-binding microbial surface component recognizing adhesive matrix molecules, detach Staphylococcus aureus from a collagen substrate. J. Biol. Chem. 27539837-39845. [DOI] [PubMed] [Google Scholar]
- 28.Wann, E. R., S. Gurusiddappa, and M. Hook. 2000. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J. Biol. Chem. 27513863-13871. [DOI] [PubMed] [Google Scholar]
- 29.Williams, R. A., L. Tetley, J. C. Mottram, and G. H. Coombs. 2006. Cysteine peptidases CPA and CPB are vital for autophagy and differentiation in Leishmania mexicana. Mol. Microbiol. 61655-674. [DOI] [PubMed] [Google Scholar]
- 30.Yao, C., K. G. Leidal, A. Brittingham, D. E. Tarr, J. E. Donelson, and M. E. Wilson. 2002. Biosynthesis of the major surface protease GP63 of Leishmania chagasi. Mol. Biochem. Parasitol. 121119-128. [DOI] [PubMed] [Google Scholar]