Abstract
Inflammation during placental malaria (PM) is associated with low birth weight (LBW), especially during the first pregnancy, but the relative contribution of maternal or fetal factors that mediate this effect remains unclear and the role of gamma interferon (IFN-γ) has been controversial. We examined the relationship of maternal and cord plasma levels of IFN-γ, tumor necrosis factor alpha, interleukin-10, ferritin, and leptin to birth weight for Tanzanian women delivering in an area where there is a high rate of malaria transmission. The placental levels of inflammatory cytokines, including IFN-γ, increased significantly during PM in primigravid and multigravid women but not in secundigravid women. PM also increased maternal peripheral levels of all inflammatory markers except IFN-γ but had strikingly little effect on cord levels of these proteins. In a multivariate analysis, placental IFN-γ was negatively associated (P = 0.01) and cord ferritin was positively associated (P < 0.0001) with birth weight in infected (PM-positive [PM+]) first-time mothers. This relationship was not observed in other mothers, consistent with the epidemiology of PM and disease. Cord leptin had a strong positive relationship with birth weight in offspring of PM-negative women (P = 0.02 to P < 0.0001) but not in offspring of PM+ women (all differences were not significant) in the three gravidity groups. The results confirmed that placental IFN-γ is related to LBW due to PM during first pregnancies and suggest that fetal ferritin plays a protective role. Because fetal cells are a source of placental IFN-γ and cord ferritin, the fetal response to PM may modify the risk of LBW.
Placental malaria (PM) due to Plasmodium falciparum causes low birth weight (LBW), and this effect is estimated to kill tens of thousands or hundreds of thousands of infants annually (5, 13). PM is caused by parasite-infected erythrocytes that bind placental receptors, such as chondroitin sulfate A, to sequester in intervillous spaces (10). Women become resistant to PM over successive pregnancies as they acquire antibodies against placental or chondroitin sulfate A-binding parasite-infected erythrocytes (11, 35). First-time mothers lack these antibodies and commonly develop chronic PM with inflammatory infiltrates rich in monocytes and macrophages (19, 28). These inflammatory infiltrates have been associated with LBW and other poor pregnancy outcomes (16, 19, 34), but these relationships are seen primarily during first pregnancies.
Placental levels of the inflammatory cytokine tumor necrosis factor alpha (TNF-α) have also been related to LBW caused by PM (12, 33, 37), while the roles of gamma interferon (IFN-γ) and interleukin-10 (IL-10) have been controversial (12, 33, 37). TNF-α and IL-10 are expressed by the maternal inflammatory cells that infiltrate the infected placenta, while IFN-γ is secreted by placental villi, suggesting that the source is fetal (37). Elevated cord blood levels of ferritin, a marker of inflammation, were also recently found to increase the risk of LBW in a small study in Malawi (2). Other recent studies found elevated maternal levels of C-reactive protein (CRP) (3) and urokinase-type plasminogen activator (29) during infection, and the latter marker was significantly related to decreased birth weight. Taken together, these data suggest that inflammatory responses in the mother or fetus may play roles in the development of LBW, but the relative contributions of maternal or fetal factors remain unclear. No study of PM has analyzed all these potential risk factors in maternal and fetal blood simultaneously.
In healthy neonates, cord blood levels of the hormone leptin are positively associated with birth weight (39), but the role of leptin in PM has not been examined. Leptin, which regulates appetite and metabolism, is produced by adipocytes, and leptin levels normally reflect body fat stores (21). In addition to its role in metabolism, leptin potentiates inflammation by enhancing macrophage phagocytosis, as well as TNF-α and type 1 cytokine production by T cells (22, 30). In animal models, the leptin level increases during malaria infection (32). During pregnancy, the placenta acts as an additional source of leptin. Maternal plasma leptin concentrations increase (24) and do not correlate with body fat stores, indicating that leptin has an alternative function during pregnancy and fetal development (21). Fetal plasma leptin is derived from the placenta starting in early gestation (14) and from fetal adipose tissue which appears and develops progressively from 14 weeks of gestation to term (31).
Understanding the relative contributions of maternal and fetal factors that cause LBW during PM could focus the effort to develop interventions. In the present study, we measured levels of IFN-γ, TNF-α, IL-10, ferritin, and leptin in maternal and fetal blood samples from Tanzania. We examined whether these levels were associated with LBW using multivariate analysis, and we assessed the effects of gravidity or PM on these relationships. Our results show that factors expressed by the fetus may modify the risk of LBW.
MATERIALS AND METHODS
Study subjects and clinical procedures.
The subjects included in the present study were individuals participating in the Mother-Offspring Malaria Studies (MOMS) Project in Muheza district, northeastern Tanzania, between September 2002 and October 2005. Mother-infant pairs were recruited during delivery hospitalization at the Muheza Designated District Hospital. Clinical information, including antimalarial agent use during pregnancy to prevent malaria, was obtained by history and physical examination, as well as review of medical records. Pregnant women were eligible for the study if they were between 18 and 45 years old and had no clinical evidence of chronic or debilitating illness, such as recent significant weight loss or chronic diarrhea. Cases of twin or triplet gestation, human immunodeficiency virus infection, or early neonatal death were not included in the analyses in this study. LBW was defined as weight at birth of <2,500 g.
Protocols for procedures used in this study were approved by the International Clinical Studies Review Committee of the Division of Microbiology and Infectious Diseases at the U.S. National Institutes of Health. Ethical clearance was obtained from the institutional review boards of Seattle Biomedical Research Institute and the National Institute for Medical Research in Tanzania. Written informed consent was obtained from each mother before she entered the study.
Peripheral blood was obtained by venipuncture from women at delivery and anticoagulated with citrate phosphate dextrose. Cord blood samples were obtained by clamping the cord and cannulating umbilical vessels immediately after delivery. After removal of the umbilical cord and fetal membranes, placental blood samples were obtained by manual compression of the placental tissue in a grinder. Placental and cord blood samples were anticoagulated with EDTA. All samples were collected by trained nurses. Plasma was obtained by centrifugation at 3,000 × g for 3 min and was stored frozen at −70°C until it was thawed on the day that cytokine assays were performed.
Parasitemia was defined as identification of any parasites in a placental blood smear or a cord blood smear by microscopy. Thick and thin smears were prepared for all samples; thin smears were fixed with methanol. Blood slides were stained for 10 min in 10% Giemsa stain, washed in tap water, air dried, and then examined using light microscopy at a magnification of ×100. Ten thousand red blood cells were examined in the thin smear before it was concluded that a placental blood slide was negative.
Plasma analyte assays.
Each plasma sample was analyzed using a multiplex, bead-based platform (BioPlex; Bio-Rad, Irvine, CA) and custom-made assay kits as previously described (8). For each serum sample, all analytes were assayed in a single day, thus eliminating freeze-thaw cycles. All pipetting and sample identification were performed with a bar code-enabled, high-speed pipetting robot (Megaflex; Tecan, Research Triangle Park, NC). The detection limits for the different soluble factors were as follows: IFN-γ, 0.04 pg/ml; TNF-α, 0.10 pg/ml; IL-10, 0.02 pg/ml; ferritin, 0.07 ng/ml; leptin, 1.28 pg/ml; and CRP, 0.01 μg/ml. The levels of soluble factors were adjusted to account for dilution in anticoagulant at the time of sample collection.
Statistical analyses.
Analyses were performed using Statview 5.0.1 (SAS Institute, Cary, NC). Differences in the proportions of placental blood samples with detectable IFN-γ from PM-positive (PM+) and PM-negative (PM−) women were analyzed by a chi-square test. Differences between groups in the levels of soluble factors or parasite densities were analyzed by a Mann-Whitney test or Kruskal-Wallis test, according to the number of groups. An unpaired t test was used to test for differences in maternal age and infant birth weight between the PM+ and PM− groups. Factorial analysis of variance was used to identify interactions between PM or gravidity on the relationship between soluble factors and birth weight. Univariate linear regression and multivariate linear regression were used to analyze the relationship of cytokines and other soluble factors to birth weight. Levels of IFN-γ, TNF-α, IL-10, leptin, and ferritin, as well as parasite density, were log transformed to obtain normal distributions for regression analyses.
RESULTS
Demographic characteristics of the study population.
After twins and triplets, cases of human immunodeficiency virus infection, and early neonatal deaths were excluded, 808 mother-infant pairs remained for analysis. Demographic characteristics of the cohort stratified by PM and gravidity are shown in Table 1. A total of 103 study participants had PM at delivery, while parasites were detected in cord blood smears of five participants. Primigravid and multigravid women with PM were significantly younger than their PM− counterparts. PM was associated with lower birth weight in all gravidity groups (P < 0.05 for all groups), but the smallest newborns were delivered by first-time mothers. Mean birth weight did not vary significantly based on the mother's history of taking antimalarials as preventive treatment during pregnancy (data not shown), possibly due to the known high rates of drug-resistant parasites in the study area. According to the Dubowitz score, the gestational age of LBW newborns was not significantly different from that of other newborns (data not shown), suggesting that intrauterine growth retardation was the primary cause of LBW in this cohort. Infected primigravid women had significantly higher parasitemia levels (median percentage of red blood cells infected, 2.9%; P = 0.0002) than infected secundigravid women (median, 0.6%) or multigravid women (median, 0.9%).
TABLE 1.
Characteristics of infants and mothers in the study population, stratified by maternal gravidity and PM statusa
| Group | PM status | n | Mean maternal age
|
Birth wt
|
Newborn gender
|
|||
|---|---|---|---|---|---|---|---|---|
| Age (yr) | P value | Wt (kg)b | P value | No. of males/no. of females | P value | |||
| Primigravidae | PM− | 185 | 20.8 | 0.07 | 3.101 (0.436) | 0.0007 | 98/86c | 0.3 |
| PM+ | 47 | 19.8 | 2.851 (0.444) | 21/26 | ||||
| Secundigravidae | PM− | 153 | 23.3 | 0.25 | 3.242 (0.436) | 0.001 | 82/71 | 0.1 |
| PM+ | 33 | 24 | 2.987 (0.303) | 14/19 | ||||
| Multigravidae | PM− | 367 | 30.1 | 0.01 | 3.280 (0.397) | 0.004 | 184/183 | 0.29 |
| PM+ | 23 | 28 | 3.047 (0.468) | 15/8 | ||||
Maternal age and newborn birth weight data for the PM+ and PM− groups were compared by using an unpaired t test, and newborn gender data were compared by using a chi-square test. P values for the differences are indicated.
The values are the means (standard deviations).
The gender of the newborn of one PM− primigravid mother was not recorded.
PM alters maternal but not fetal levels of inflammatory markers.
PM was associated with significant increases in the levels of inflammatory markers (including IFN-γ and TNF-α) in the placental blood of primigravid and multigravid women but not in the placental blood of secundigravid women (Table 2). Placental IFN-γ was detected more frequently in samples from PM+ primigravidae than in samples from PM− primigravidae (68.2% versus 48.4%; P = 0.02) or multigravidae (84.2% versus 48.9%; P = 0.003) but not secundigravidae (56.7% versus 54.8%; P > 0.999). Similarly, the levels of ferritin, a marker of inflammation, as well as iron stores, were significantly increased in placental blood of infected primigravid and multigravid women but not in placental blood of secundigravid women. The level of anti-inflammatory cytokine IL-10 was increased in placentas of infected women in all gravidity categories.
TABLE 2.
Levels of soluble factors in cord, placental, and peripheral blood of primigravid women, secundigravid women, and multigravid women, stratified by PM status
| Location | Cytokine | PM− women
|
PM+ women
|
P valueb | ||
|---|---|---|---|---|---|---|
| No. of samples | Median concn (interquartile range)a | No. of samples | Median concn (interquartile range)a | |||
| Primigravid women | ||||||
| Cord | TNF-α | 168 | 105.4 (63.9-180.2) | 40 | 118.2 (70.5-155.7) | NS |
| IFN-γ | 168 | 0.0 (0.0-0.0) | 40 | 0.0 (0.0-0.0) | NS | |
| IL-10 | 168 | 3.5 (1.8-5.7) | 40 | 3.3 (1.9-8.0) | NS | |
| Ferritin | 168 | 101.3 (52.4-187.1) | 40 | 140.2 (69.8-194.4) | NS | |
| Leptin | 168 | 2,917.8 (1,091.5-6,811.4) | 40 | 2,000.2 (754.4-6,193.7) | NS | |
| Placenta | TNF-α | 165 | 282.8 (161.9-535.1) | 40 | 359.6 (287.7-668.6) | 0.005 |
| IFN-γ | 165 | 0.0 (0-75.5) | 40 | 55.7 (0-190.9) | 0.004 | |
| IL-10 | 165 | 10.0 (6.8-17.5) | 40 | 67.3 (29.2-180.0) | <0.0001 | |
| Ferritin | 165 | 776.6 (445.4-1,061.8) | 40 | 1,024.0 (651.5-1,739.1) | 0.0002 | |
| Leptin | 165 | 10,475.5 (4,774.0-20,984.2) | 40 | 12,083.1 (7,732.8-28,661.3) | NS | |
| Peripheral | TNF-α | 165 | 24.1 (9.8-49.0) | 41 | 70.6 (31.7-189.1) | <0.0001 |
| IFN-γ | 165 | 0.0 (0.0-0.0) | 41 | 0.0 (0.0-6.6) | NS | |
| IL-10 | 165 | 5.7 (3.5-11.4) | 41 | 22.7 (13.8-64.8) | <0.0001 | |
| Ferritin | 165 | 14.5 (8.3-33.0) | 41 | 74.6 (22.5-150.5) | <0.0001 | |
| Leptin | 165 | 2,448.7 (1,145.6-6,348.7) | 41 | 940.8 (634.1-10,269.8) | 0.0004 | |
| Secundigravid women | ||||||
| Cord | TNF-α | 141 | 118.8 (63.4-179.6) | 31 | 101.5 (50.9-178.2) | NS |
| IFN-γ | 141 | 0.0 (0.0-0.0) | 31 | 0.0 (0.0-0.0) | NS | |
| IL-10 | 141 | 2.3 (0.9-4.0) | 31 | 3.5 (1.5-5.6) | 0.03 | |
| Ferritin | 141 | 110.5 (68.4-171.9) | 31 | 74.7 (44.3-153.5) | NS | |
| Leptin | 141 | 2,568.9 (1,091.6-7,003.2) | 31 | 3,441.6 (1,212.5-5,356.8) | NS | |
| Placenta | TNF-α | 135 | 324.1 (169.1-506.9) | 31 | 263.2 (186.6-448.2) | NS |
| IFN-γ | 135 | 13.1 (0-68.1) | 31 | 15.9 (0.-0-55.6) | NS | |
| IL-10 | 135 | 10.4 (5.6-17.5) | 31 | 30.9 (14.5-57.3) | <0.0001 | |
| Ferritin | 135 | 639.5 (404.5-972.0) | 31 | 613.4 (441.5-1,030.1) | NS | |
| Leptin | 135 | 8,090.0 (4,279.9-16,420.0) | 31 | 11,917.3 (3,475.7-21,359.0) | NS | |
| Peripheral | TNF-α | 134 | 21.8 (11.0-40.1) | 31 | 55.0 (24.7-79.1) | 0.0003 |
| IFN-γ | 134 | 0.0 (0.0-0.0) | 31 | 0.0 (0.0-0.0) | NS | |
| IL-10 | 134 | 6.1 (3.1-10.0) | 31 | 22.6 (14.0-41.4) | <0.0001 | |
| Ferritin | 134 | 12.2 (7.4-20.5) | 31 | 38.2 (21.2-79.9) | <0.0001 | |
| Leptin | 134 | 2,318.6 (1,111.0-6,581.3) | 31 | 2,268.6 (446.8-4,679.7) | NS | |
| Multigravid women | ||||||
| Cord | TNF-α | 340 | 103.1 (58.6-149.9) | 23 | 149.3 (85.9-171.5) | NS |
| IFN-γ | 340 | 0.0 (0.0-0.0) | 23 | 0.0 (0.0-0.0) | NS | |
| IL-10 | 340 | 2.8 (0.9-5.2) | 23 | 2.7 (0.9-6.6) | NS | |
| Ferritin | 340 | 90.2 (47.8-164.6) | 23 | 105.7 (80.5-155.5) | NS | |
| Leptin | 340 | 3,577.6 (1,725.6-6,880.1) | 23 | 2,445.6 (881.8-3,892.9) | 0.05 | |
| Placenta | TNF-α | 328 | 263.6 (158.7-439.8) | 20 | 512.3 (301.2-746.4) | 0.005 |
| IFN-γ | 328 | 0.0 (0-55.7) | 20 | 49.3 (6.2-265.4) | 0.001 | |
| IL-10 | 328 | 9.8 (6.0-17.6) | 20 | 49.8 (30.4-204.7) | <0.0001 | |
| Ferritin | 328 | 640.1 (385.8-960.6) | 20 | 867.3 (746.4-1,196.4) | 0.0007 | |
| Leptin | 328 | 7,193.0 (3,049.9-14,887.0) | 20 | 9,495.4 (5,165.5-27,492.2) | NS | |
| Peripheral | TNF-α | 322 | 19.5 (9.0-44.4) | 18 | 67.4 (17.9-98-6) | 0.01 |
| IFN-γ | 322 | 0.0 (0.0-0.0) | 18 | 0.0 (0.0-0.0) | NS | |
| IL-10 | 322 | 6.2 (2.9-12.1) | 18 | 27.2 (14.0-54.5) | <0.0001 | |
| Ferritin | 322 | 11.9 (6.9-28.0) | 18 | 45.3 (12.0-97.9) | 0.004 | |
| Leptin | 322 | 2,130.4 (895.9-5,236.7) | 18 | 1,965.1 (1,108.0-5,776.6) | NS | |
The concentrations of TNF-α, IFN-γ, IL-10, and leptin are expressed in picograms per milliliter, and the concentrations of ferritin are expressed in nanograms per milliliter.
Differences between groups of women with and without PM were analyzed by using the Mann-Whitney U test. NS, not significant.
The levels of TNF-α, IL-10, and ferritin were also increased in the maternal peripheral blood of women with PM. Unlike the pattern seen in placental blood, the increases occurred in secundigravid women as well as in primigravid and multigravid women. The peripheral blood IFN-γ levels in PM+ women were not significantly different from those in PM− women.
The placental leptin levels in PM+ samples were not different from those in PM− samples for any of the gravidity groups. The peripheral leptin levels were significantly lower in PM+ primigravid women than in PM− primigravid women, but this relationship was not observed for other gravidity groups.
In general, the fetal levels of cytokines and other soluble factors were not altered during PM. PM was related to a small but significant increase in the level of cord blood IL-10 in secundigravid women. PM did not significantly alter cord blood levels of TNF-α, IFN-γ, leptin, or ferritin in any of the gravidity groups. The levels of the inflammatory marker CRP were elevated (>8.2 μg/ml) in only 17/457 (3.1%) cord blood samples tested, and elevated levels of CRP were seen in similar proportions of PM+ cases (4/62, 6.4%) and PM− cases (13/395, 3.3%) (P = 0.22).
Gravidity and PM modify relationships of soluble factors to LBW.
We examined the relationship between all measured analytes and birth weight by using univariate linear regression. Placental IL-10 had a significant inverse relationship with birth weight (β = −0.114, P = 0.002), while cord leptin had a significant positive relationship with birth weight (β = 0.277, P < 0.0001). Cord ferritin, placental IFN-γ, and placental TNF-α were not significantly related to birth weight in these aggregate analyses.
PM and gravidity are known to influence relationships between inflammatory cytokines and birth weight in malaria-exposed individuals, and the relationship is limited to first pregnancies (12). We performed factorial analysis of variance to examine whether gravidity and PM modified relationships between soluble factors and birth weight in this cohort. Interaction terms involving gravidity and/or PM significantly or nearly significantly modified relationships between birth weight and several soluble factors, including cord ferritin (significance of term PM * parity, P = 0.04) and placental TNF-α (significance of term placental TNF-α * PM, P = 0.07). We therefore stratified our analyses by gravidity (Fig. 1) and by PM status (Fig. 2).
FIG. 1.
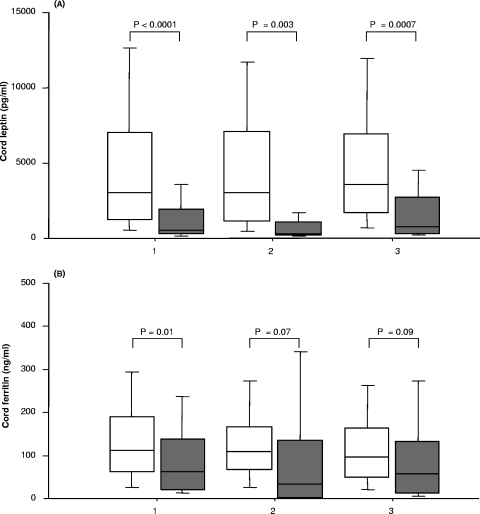
Cord blood levels of (A) leptin and (B) ferritin for normal-birth-weight (open boxes) and LBW (shaded boxes) neonates. 1, primigravidae (188 normal-birth-weight babies and 20 LBW babies); 2, secundigravidae (170 normal-birth-weight babies and 5 LBW babies); 3, multigravidae (345 normal-birth-weight babies and 12 LBW babies). Each box plot indicates the median (horizontal line) and interquartile range (box), whereas the whiskers indicate the 10th and 90th percentiles. The differences between two groups were analyzed by the Mann-Whitney U test. Associated P values are shown.
FIG. 2.
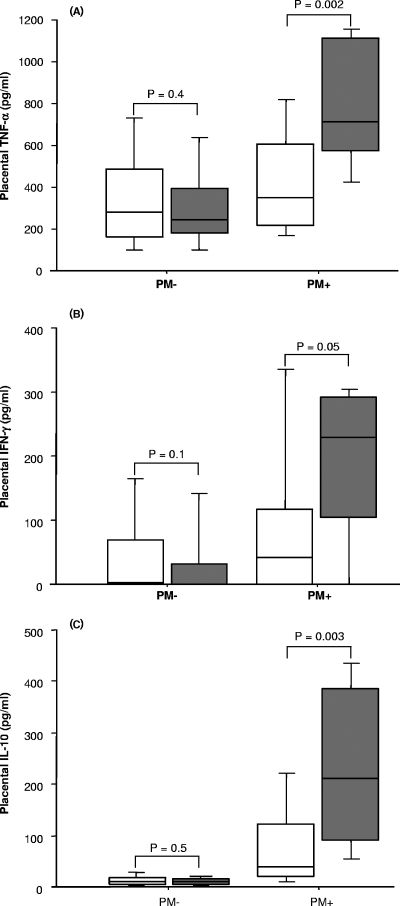
Placental blood concentrations of (A) TNF-α, (B) IFN-γ, and (C) IL-10 for normal-birth-weight (open boxes) and LBW (shaded boxes) neonates. For the PM+ group, there were 78 normal-birth-weight babies and 9 LBW babies; for the PM− group, there were 603 normal-birth-weight babies and 26 LBW babies. Each box plot indicates the median (horizontal line) and interquartile range (box), whereas the whiskers indicate the 10th and 90th percentiles. The differences between two groups were analyzed by the Mann-Whitney U test. Associated P values are shown.
In analyses stratified by gravidity, LBW was related to significant or nearly significant reductions in levels of cord leptin and cord ferritin in all gravidity groups (Fig. 1). Although the placental levels of TNF-α, IFN-γ, and IL-10 were elevated in primigravid and multigravid women delivering LBW babies compared to women delivering normal-birth-weight babies (data not shown), the differences were not significant.
In analyses stratified by PM status, LBW was strongly related to inflammatory cytokines when the mother was PM+. The placental levels of TNF-α, IFN-γ, and IL-10 were all significantly and substantially increased in PM+ mothers delivering LBW babies compared to women delivering normal-birth-weight babies (Fig. 2). Conversely, the cord levels of both leptin and ferritin were significantly decreased in LBW babies compared with normal-birth-weight babies, and this was observed for both PM− and PM+ groups (data not shown).
Placental IFN-γ is specifically related to decreased birth weight.
We performed multivariate regression analysis of the factors that were related to birth weight in the univariate analyses (Table 3). For multivariate analysis, we stratified by gravidity and PM status, since these variables interacted with several plasma factors and modified their relationship to birth weight. Because one-half of women had undetectable placental IFN-γ levels (which therefore could not be normalized by transformation), we analyzed the groups with detectable and undetectable IFN-γ levels separately.
TABLE 3.
Soluble factors related to birth weight in multivariate linear regression analysis stratified by PM and gravidity of women with detectable placental IFN-γ and without detectable placental IFN-γ
| Women | n | PM status | Factor | β | P valuea |
|---|---|---|---|---|---|
| Detectable placental IFN-γ | |||||
| Primigravid | 80 | PM− | Cord leptin | 0.393 | <0.0001 |
| Cord ferritin | −0.079 | NS | |||
| Placental TNF-α | −0.283 | NS | |||
| Placental IL-10 | 0.111 | NS | |||
| Placental IFN-γ | −0.009 | NS | |||
| Secundigravid | 74 | PM− | Cord leptin | 0.266 | 0.004 |
| Cord ferritin | −0.458 | 0.005 | |||
| Placental TNF-α | −0.261 | NS | |||
| Placental IL-10 | −0.06 | NS | |||
| Placental IFN-γ | 0.141 | NS | |||
| Multigravid | 161 | PM− | Cord leptin | 0.191 | 0.007 |
| Cord ferritin | −0.117 | NS | |||
| Placental TNF-α | 0.127 | NS | |||
| Placental IL-10 | −0.101 | NS | |||
| Placental IFN-γ | −0.01 | NS | |||
| Primigravid | 27 | PM+ | Cord leptin | 0.079 | NS |
| Cord ferritin | 0.8 | <0.0001 | |||
| Placental TNF-α | 0.065 | NS | |||
| Placental IL-10 | 0.208 | NS | |||
| Placental IFN-γ | −0.393 | 0.01 | |||
| Secundigravid | 18 | PM+ | Cord leptin | 0.252 | NS |
| Cord ferritin | −0.217 | NS | |||
| Placental TNF-α | 0.393 | NS | |||
| Placental IL-10 | −0.228 | NS | |||
| Placental IFN-γ | 0.018 | NS | |||
| Multigravid | 16 | PM+ | Cord leptin | 0.053 | NS |
| Cord ferritin | 0.168 | NS | |||
| Placental TNF-α | −0.591 | NS | |||
| Placental IL-10 | −0.242 | NS | |||
| Placental IFN-γ | 0.157 | NS | |||
| No detectable placental IFN-γb | |||||
| Primigravid | 85 | PM− | Cord leptin | 0.318 | 0.0002 |
| Cord ferritin | 0.192 | NS | |||
| Placental TNF-α | −0.079 | NS | |||
| Placental IL-10 | 0.16 | NS | |||
| Secundigravid | 61 | PM− | Cord leptin | 0.206 | 0.02 |
| Cord ferritin | 0.475 | 0.0001 | |||
| Placental TNF-α | −0.597 | 0.009 | |||
| Placental IL-10 | 0.289 | NS | |||
| Multigravid | 167 | PM− | Cord leptin | 0.277 | <0.0001 |
| Cord ferritin | −0.08 | NS | |||
| Placental TNF-α | 0.15 | NS | |||
| Placental IL-10 | 0.03 | NS | |||
| Primigravid | 13 | PM+ | Cord leptin | −0.511 | NS |
| Cord ferritin | 0.11 | NS | |||
| Placental TNF-α | −1.444 | NS | |||
| Placental IL-10 | −0.09 | NS | |||
| Secundigravid | 13 | PM+ | Cord leptin | 0.277 | NS |
| Cord ferritin | −0.459 | NS | |||
| Placental TNF-α | 0.169 | NS | |||
| Placental IL-10 | 0.009 | NS |
NS, not significant.
For multigravid PM+ women with no detectable IFN-γ there were not enough cases to perform an analysis (n = 4).
In PM+ primigravidae with detectable placental IFN-γ, cord blood ferritin was strongly related to increased birth weight (β = 0.80, P < 0.0001), while placental IFN-γ was related to decreased birth weight (β = −0.39, P < 0.01). These relationships were not seen in other PM+ women (Table 3). In multivariate analysis that excluded IFN-γ data but included all PM+ primigravidae, cord blood ferritin was still related to increased birth weight (β = 0.41, P = 0.01), while placental TNF-α was related to decreased birth weight (β = −0.50, P = 0.03). The effect of cord blood ferritin was different in different groups. When placental inflammatory cytokines were related to decreased birth weight (such as IFN-γ or TNF-α in PM+ primigravidae or TNF-α in PM− secundigravidae without detectable IFN-γ), ferritin had a positive effect on birth weight. Conversely, ferritin had an inverse relationship with birth weight in PM− secundigravidae with detectable IFN-γ, in whom none of the cytokines had an effect on the outcome (Table 3).
In PM− women, cord blood leptin levels were significantly associated with increased birth weight in all gravidity groups. Leptin levels were not related to birth weight in PM+ women.
DISCUSSION
Malaria-related LBW is a major public health problem in tropical countries and is thought to kill tens of thousands or hundreds of thousands of infants each year. We report here a comprehensive assessment of cytokines and other soluble factors measured in maternal and fetal blood that may be involved in the pathogenesis of malaria-related LBW. Our results confirm the relationship between placental inflammation and LBW during PM of first-time mothers and not other mothers. Notably, only placental IFN-γ remained significantly associated with decreased birth weight in the multivariate analysis. The associations of placental IFN-γ and cord blood ferritin with LBW indicate that fetal responses may modify the risk of LBW.
Elevated placental levels of TNF-α during PM have been a consistent finding at different study sites (12, 33, 36), but the effect of PM on placental IFN-γ levels has been controversial (12, 33). Differences between studies may be related to the sensitivity of various assays used to measure IFN-γ or to biologic differences between study sites with different malaria transmission intensities. As observed in a previous study (12), the effect of PM that elicited inflammatory cytokines in the placenta was limited to primigravid and multigravid women and was not observed in secundigravid women in this cohort. The reasons that secundigravid women appear to be a distinct immunological group are unknown, but we speculate that secundigravidae comprise a transitional immune state between the full susceptibility of primigravidae and the significant immunity of multigravidae. This transitional state may involve distinct immune mediators in the response to infection, reflected by the distinct cytokine profile of secundigravidae.
Unlike the elevated levels measured in placental blood, the level of IFN-γ was not elevated in maternal peripheral blood during PM. This suggests that maternal or fetal cells in the placenta may be the primary source of IFN-γ during PM. In a previous study in Cameroon, IFN-γ was produced by fetal villi but not by maternal immune cells collected from infected placentas (37). The placenta secretes numerous cytokines and immunomodulatory molecules in addition to IFN-γ, and these molecules could play an important role in maternal and fetal outcomes. For example, a recent study in Tanzania found that fetal trophoblast cells express soluble vascular endothelial growth receptor 1 during PM, possibly as a strategy to modulate the maternal inflammatory response (26).
Our finding that PM did not substantially alter cytokines in fetal blood highlights the ability of the placental barrier to shield the fetus from inflammation. Proinflammatory cytokines like IL-1β, TNF-α, and IL-6 do not cross the placenta (1), which explains in part the unperturbed environment of the fetus. However, in utero sensitization to malaria antigens is not uncommon (9, 17, 23, 25, 38), indicating that the fetus is exposed to parasites or parasite antigens. The lack of fetal inflammation may suggest that fetal exposure to viable parasites does not occur or is limited or else that the fetus may actively suppress inflammation (for example, by increasing the number of regulatory T cells during PM) (6).
In the multivariate analysis of all the factors measured in the current study, only IFN-γ remained significantly associated with decreased birth weight, a relationship observed in PM+ primigravidae but not in other groups. In areas where the rate of malaria transmission is high, like Muheza, where LBW due to PM is mainly a problem of first-time mothers, this primigravid-specific association is consistent with the epidemiology of poor outcomes. The relationship between inflammation and LBW was also restricted to first-time mothers in an area of Kenya where the rate of transmission is high (12).
This is the first study to suggest that fetal ferritin levels may play a protective role in intrauterine growth during PM episodes. In a previous study in Malawi, increased cord blood ferritin levels were associated with lower birth weight during PM (2), but in that study there were important differences that may explain the discordant findings. The Malawi study involved a small cohort consisting of 32 PM+ mother-newborn pairs, and the analyses were not stratified by parity. In an unstratified analysis of our Tanzanian data, we did not observe a relationship between cord blood ferritin and birth weight.
In the United States, giving iron supplements to pregnant women has been shown to increase birth weight without an effect on maternal hemoglobin or maternal iron stores (7), suggesting that an increased amount of iron transferred to the fetus may have direct benefits for weight. The level of cord blood ferritin may reflect a similar process in our cohort, indicating either increased maternal-fetal transfer or increased fetal iron stores with attendant benefits for birth weight. However, ferritin levels were related to improved outcomes only in the groups in which placental IFN-γ or TNF-α had a negative effect on birth weight, suggesting that ferritin may specifically counteract the deleterious effects of placental inflammation.
Hepatocytes and immune cells are known to be sources of plasma ferritin, and plasma ferritin levels increase during inflammation and decrease during iron deficiency. In our cohort, the cord levels of ferritin and the inflammatory marker CRP were not related, suggesting that changes in ferritin levels were not due to systemic inflammation of the fetus. Ferritin is also actively expressed by the placenta. Immunolocalization studies suggested that there is abundant ferritin production in the placental stroma but that ferritin is also detectable in the endothelium of fetal vessels within the placenta (4). The abundance and localization of ferritin in placental tissue during PM have not been studied previously but are of interest because of the association of cord ferritin levels with increased birth weight in this study.
Cord blood leptin was positively associated with birth weight in PM− mothers but not in PM+ mothers. During pregnancy, the human placenta (40) and other fetal tissues, including adipose, heart, and liver tissues (15), produce leptin. Cord blood leptin levels correlate with fetal size (20, 27), and both placental and fetal leptin levels are decreased in pregnancies complicated by fetal growth retardation (18, 39). However, PM appears to disrupt the relationship between cord leptin and birth weight. This may indicate that leptin plays additional roles during PM, and further study is warranted.
In summary, PM increases inflammatory cytokine levels in placental and maternal peripheral blood but not in fetal blood. Second-time mothers are distinct because their placental levels of inflammatory cytokines do not increase during PM. Inflammatory cytokines in the placenta are associated with poor outcomes during PM, and this relationship is limited to first pregnancies, consistent with the epidemiology of PM and disease. In first-time mothers, placental IFN-γ is associated with decreased birth weight, while cord ferritin is associated with increased birth weight, suggesting that fetal responses to infection may play a key role in the pathogenesis of malaria-related LBW. Future studies should localize and quantify IFN-γ and ferritin expression by cell subsets in the placenta and relate the measurements to outcomes, including birth weight during PM episodes.
Acknowledgments
We gratefully acknowledge the participation of mothers and their infants in the MOMS Project and the work of the MOMS Project staff, including assistant medical officers, nurses, village health workers, laboratory technicians, microscopists, and data entry personnel. Gretchen Langdon of the Institute of International Health, Brown University, organized the cytokine assays. Wil Dolmans of Kilimanjaro Christian Medical College, Tumaini University, Moshi, Tanzania, reviewed the manuscript and provided discussions.
This work was supported by Bill & Melinda Gates Foundation grant 29202 and by grants NIH R01 AI 52059 and NIH TW 05509 to P.E.D.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 22 January 2008.
REFERENCES
- 1.Aaltonen, R., T. Heikkinen, K. Hakala, K. Laine, and A. Alanen. 2005. Transfer of proinflammatory cytokines across term placenta. Obstet. Gynecol. 106802-807. [DOI] [PubMed] [Google Scholar]
- 2.Abrams, E. T., J. J. Kwiek, V. Mwapasa, D. D. Kamwendo, E. Tadesse, V. M Lema, M. E. Molyneux, S. J. Rogerson, and S. R. Meshnick. 2005. Malaria during pregnancy and foetal haematological status in Blantyre, Malawi. Malar. J. 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adegnika, A. A., J. J. Verweij, S. T. Agnandji, S. K. Chai, L. P. Breitling, M. Ramharter, M. Frolich, S. Issifou, P. G. Kremsner, and M. Yazdanbakhsh. 2006. Microscopic and sub-microscopic Plasmodium falciparum infection, but not inflammation caused by infection, is associated with low birth weight. Am. J. Trop. Med. Hyg. 75798-803. [PubMed] [Google Scholar]
- 4.Bastin, J., H. Drakesmith, M. Rees, I. Sargent, and A. Townsend. 2006. Localisation of proteins of iron metabolism in the human placenta and liver. Br. J. Haematol. 134532-543. [DOI] [PubMed] [Google Scholar]
- 5.Brabin, B., and S. Rogerson. 2001. The epidemiology and outcomes of maternal malaria, p. 27-52. In P. E. Duffy and M. Fried (ed.), Malaria in pregnancy. Deadly parasite susceptible host. 1st ed. Taylor and Francis, New York, NY.
- 6.Brustoski, K., U. Moller, M. Kramer, F. C. Hartgers, P. G. Kremsner, U. Krzych, and A. J. Luty. 2006. Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J. Infect. Dis. 193146-154. [DOI] [PubMed] [Google Scholar]
- 7.Cogswell, M. E., I. Parvanta, L. Ickes, R. Yip, and G. M. Brittenham. 2003. Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. Am. J. Clin. Nutr. 78773-781. [DOI] [PubMed] [Google Scholar]
- 8.Coutinho, H. M., S. T. McGarvey, L. P. Acosta, D. L. Manalo, G. C. Langdon, T. Leenstra, H. K. Kanzaria, J. Solomon, H. Wu, R. M. Olveda, J. D. Kurtis, and J. F. Friedman. 2005. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J. Infect. Dis. 192528-536. [DOI] [PubMed] [Google Scholar]
- 9.Fievet, N., P. Ringwald, K. Bickii, B. Dubois, B. Maubert, J. Y. Le Hesran, M. Cot, and P. Deloron. 1996. Malaria cellular immune responses in neonates from Cameroon. Parasite. Immunol. 18483-490. [DOI] [PubMed] [Google Scholar]
- 10.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 2721502-1504. [DOI] [PubMed] [Google Scholar]
- 11.Fried, M., F. Nosten, A. Brockman, B. J. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395851-852. [DOI] [PubMed] [Google Scholar]
- 12.Fried, M., R. O. Muga, A. O. Misore, and P. E. Duffy. 1998. Malaria elicits type 1 cytokines in the human placenta: IFN-γ and TNF-α associated with pregnancy outcomes. J. Immunol. 1602523-2530. [PubMed] [Google Scholar]
- 13.Guyatt, H. L., and R. W. Snow. 2004. Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin. Microbiol. Rev. 17760-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henson, M. C., K. F. Swan, and J. S. O'Neil. 1998. Expression of placental leptin and leptin receptor transcripts in early pregnancy and at term. Obstet. Gynecol. 921020-1028. [DOI] [PubMed] [Google Scholar]
- 15.Hoggard, N., L. Hunter, R. G. Lea, P. Trayhurn, and J. G. Mercer. 2000. Ontogeny of the expression of leptin and its receptor in the murine fetus and placenta. Br. J. Nutr. 83317-326. [DOI] [PubMed] [Google Scholar]
- 16.Jilly, P. 1969. Anaemia in parturient women, with special reference to malaria infection of the placenta. Ann. Trop. Med. Parasitol. 63109-116. [DOI] [PubMed] [Google Scholar]
- 17.King, C. L., I. Malhotra, A. Wamachi, J. Kioko, P. Mungai, S. A. Wahab, D. Koech, P. Zimmerman, J. Ouma, and J. W. Kazura. 2002. Acquired immune responses to Plasmodium falciparum merozoite surface protein-1 in the human fetus. J. Immunol. 168356-364. [DOI] [PubMed] [Google Scholar]
- 18.Lea, R. G., D. Howe, L. T. Hannah, O. Bonneau, L. Hunter, and N. Hoggard. 2000. Placental leptin in normal, diabetic and fetal growth-retarded pregnancies. Mol. Hum. Reprod. 6763-769. [DOI] [PubMed] [Google Scholar]
- 19.Leopardi, O., W. Naughten, L. Salvia, M. Colecchia, A. Matteelli, A. Zucchi, A. Shein A. Muchi, G. Carosi, and M. Ghione. 1996. Malaria placentas. A quantitative study and clinico-pathological correlations. Pathol. Res. Pract. 192892-898. [DOI] [PubMed] [Google Scholar]
- 20.Lepercq, J., N. Lahlou, J. Timsit, J. Girard, and S. H. Mouzon. 1999. Macrosomia revisited: ponderal index and leptin delineate subtypes of fetal overgrowth. Am. J. Obstet. Gynecol. 181621-625. [DOI] [PubMed] [Google Scholar]
- 21.Linnemann, K., A. Malek, H. Schneider, and C. Fusch. 2001. Physiological and pathological regulation of feto/placento/maternal leptin expression. Biochem. Soc. Trans. 2986-90. [DOI] [PubMed] [Google Scholar]
- 22.Loffreda, S., S. Q. Yang, H. Z. Lin, C. L. Karp, M. L. Brengman, D. J. Wang, A. S. Klein, G. B. Bulkley, C. P. W. Noble, M. D. Lane, and A. M. Diehl. 1998. Leptin regulates proinflammatory immune responses. FASEB J. 1257-65. [PubMed] [Google Scholar]
- 23.Malhotra, I., P. Mungai, E. Muchiri, J. Ouma, S. Sharma, J. W. Kazura, and C. L. King. 2005. Distinct Th1- and Th2-type prenatal cytokine responses to Plasmodium falciparum erythrocyte invasion ligands. Infect. Immun. 733462-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy, J. F., D. N. Misra, and J. M. Roberts. 1999. Maternal plasma leptin is increased in preeclampsia and positively correlates with fetal cord concentration. Am. J. Obstet. Gynecol. 180731-736. [DOI] [PubMed] [Google Scholar]
- 25.Metenou, S., A. L. Suguitan, Jr., C. Long, R. G. Leke, and D. W. Taylor. 2007. Fetal immune responses to Plasmodium falciparum antigens in a malaria-endemic region of Cameroon. J. Immunol. 1782770-2777. [DOI] [PubMed] [Google Scholar]
- 26.Muehlenbachs, A., T. K. Mutabingwa, S. Edmonds, M. Fried, and P. E. Duffy. 2006. Hypertension and maternal-fetal conflict during placental malaria. PLoS Med. 3e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong, K. K., M. L. Ahmed, A. Sherriff, K. A. Woods, A. Watts, J. Golding, and D. B. Dunger. 1999. Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. J. Clin. Endocrinol. Metab. 841145-1148. [DOI] [PubMed] [Google Scholar]
- 28.Ordi, J., M. R. Ismail, P. J. Ventura, E. Kahigwa, R. Hirt, A. Cardesa, P. L. Alonso, and C. Menendez. 1998. Massive chronic intervillositis of the placenta associated with malaria infection. Am. J. Surg. Pathol. 221006-1011. [DOI] [PubMed] [Google Scholar]
- 29.Ostrowski, S. R., C. E. Shulman, N. Peshu, T. Staalsøe, G. Høyer-Hansen, B. K. Pedersen, K. Marsh, and H. Ullum. 2007. Elevated plasma urokinase receptor predicts low birth weight in maternal malaria. Parasite Immunol. 2937-46. [DOI] [PubMed] [Google Scholar]
- 30.Pacifico, L., L. Di Renzo, C. Anania, J. F. Osborn, F. Ippoliti, E. Schiavo, and C. Chiesa. 2006. Increased T-helper interferon-gamma-secreting cells in obese children. Eur. J. Endocrinol. 154691-697. [DOI] [PubMed] [Google Scholar]
- 31.Poissonnet, C. M., A. R. Burdi, and S. M. Garn. 1984. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum. Dev. 101-11. [DOI] [PubMed] [Google Scholar]
- 32.Polio-Mendez, M., J. De Sanctis, and A. Rodriguez-Acosta. 2002. Leptin and leptin receptors during malaria infection in mice. Folia Parasitol. (Prague) 49249-251. [DOI] [PubMed] [Google Scholar]
- 33.Rogerson, S. J., H. C. Brown, E. Pollina, E. T. Abrams, E. Tadesse, V. M. Lema, and M. E. Molyneux. 2003. Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infect. Immun. 71267-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogerson, S. J., E. Pollina, A. Getachew, E. Tadesse, V. M. Lema, and M. E. Molyneux. 2003. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am. J. Trop. Med. Hyg. 68115-119. [PubMed] [Google Scholar]
- 35.Staalsoe, T., C. E. Shulman, J. N. Bulmer, K. Kawuondo, K. Marsh, and L. Hviid. 2004. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 363283-289. [DOI] [PubMed] [Google Scholar]
- 36.Suguitan, A. L., R. G. F. Leke, G. Fouda, A. Zhou, L. Thuita, S. Metenou, J. Fogako, R. Megnekou, and D. W. Taylor. 2003. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J. Infect. Dis. 1881074-1082. [DOI] [PubMed] [Google Scholar]
- 37.Suguitan, A. L., Jr., T. J. Cadigan, T. A. Nguyen, A. Zhou, R. J. Leke, S. Metenou, L. Thuita, R. Megnekou, J. Fogako, R. G. Leke, and D. W. Taylor. 2003. Malaria-associated cytokine changes in the placenta of women with pre-term deliveries in Yaounde, Cameroon. Am. J. Trop. Med. Hyg. 69574-581. [PubMed] [Google Scholar]
- 38.Xi, G., R. G. Leke, L. W. Thuita, A. Zhou, R. J. Leke, R. Mbu, and D. W. Taylor. 2003. Congenital exposure to Plasmodium falciparum antigens: prevalence and antigenic specificity of in utero-produced antimalarial immunoglobulin M antibodies. Infect. Immun. 711242-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yildiz, L., B. Avci, and M. Ingec. 2002. Umbilical cord and maternal blood leptin concentrations in intrauterine growth retardation. Clin. Chem. Lab Med. 401114-1117. [DOI] [PubMed] [Google Scholar]
- 40.Yura, S., N. Sagawa, H. Mise, T. Mori, H. Masuzaki, Y. Ogawa, and K. Nakao. 1998. A positive umbilical venous-arterial difference of leptin level and its rapid decline after birth. Am. J. Obstet. Gynecol. 178926-930. [DOI] [PubMed] [Google Scholar]