Abstract
Bacterial infections of the eye highlight a dilemma that is central to all immune-privileged sites. On the one hand, immune privilege limits inflammation to prevent bystander destruction of normal tissue and loss of vision. On the other hand, bacterial infections require a robust inflammatory response for rapid clearance of the pathogen. We demonstrate that the retina handles this dilemma, in part, by activation of a protective heat shock protein. During Staphylococcus aureus-induced endophthalmitis, the small heat shock protein αB-crystallin is upregulated in the retina and prevents apoptosis during immune clearance of the bacteria. In the absence of αB-crystallin, mice display increased retinal apoptosis and retinal damage. We found that S. aureus produces a protease capable of cleaving αB-crystallin to a form that coincides with increased retinal apoptosis and tissue destruction. We conclude that αB-crystallin is important in protecting sensitive retinal tissue during destructive inflammation that occurs during bacterial endophthalmitis.
Endophthalmitis is a vision-threatening bacterial infection that occurs when bacteria are introduced into the posterior of the eye following cataract surgery or a deep puncture wound. Staphylococcus aureus is the most common cause of destructive endophthalmitis following cataract surgery and accounts for 10% of all endophthalmitis cases (7, 12, 13, 16). Upon entering the posterior segment of the eye, S. aureus proliferates rapidly and produces exotoxins that directly damage retinal tissue (5, 7). In addition, the host mounts a massive neutrophil-mediated immune response in which degranulation of neutrophils releases cytotoxic mediators that further contribute to retinal-tissue damage (7, 8, 14). Typical treatment of endophthalmitis involves intravitreal injection of antibiotics, combined with vitrectomy in severe cases (7, 12). However, while intravitreal antibiotic treatment is effective in clearing the infection, in many cases it does not mitigate the tissue damage induced by the bacterial exotoxins and the by-products of the host immune response (7, 12).
The eye is an immune-privileged site where inflammation is inhibited to preserve vision by preventing damage to the delicate ocular tissues (34, 41). However, immune privilege is not absolute and can be breached, leaving the eye vulnerable to immune-mediated inflammation. Ocular infections, in particular, can overwhelm ocular immune privilege and trigger vigorous inflammation within the eye, raising a very important question (33): how does the eye protect the ocular tissues from nonspecific damage when immune privilege is overcome and inflammation is induced within the eye, as in the case of bacterial endophthalmitis? We propose that in addition to the multiple mechanisms that inhibit inflammation, an additional layer of immune privilege consists of special protective mechanisms that preserve the highly vulnerable ocular tissue when inflammation does occur. The present study examines one of these protective mechanisms and how it is compromised by pathogenic bacteria.
Using a murine model of S. aureus endophthalmitis, we identified a small heat shock protein, αB-crystallin, that plays a critical role in protecting the retina during endophthalmitis. αB-crystallin is a member of the small heat shock family of proteins and is expressed in long-lived tissues, such as muscle, brain, and lens (6, 9, 30, 38, 39, 42). Like other small heat shock proteins, αB-crystallin was first identified as a molecular chaperone that prevents aggregation of cellular proteins (21, 36, 37). More recently, αB-crystallin was shown to prevent apoptosis by inhibiting the activation of capase 3, a protease essential for apoptosis (1, 21, 22, 23, 26, 37). Furthermore, recent studies have demonstrated that αB-crystallin is expressed in the retina (in the ganglion cells, inner and outer nuclear layers, inner segments, and retinal pigment epithelium), where it is upregulated and prevents apoptosis in response to oxidative stress (43, 44). We hypothesized that one role for αB-crystallin is to protect the delicate retinal tissue from apoptosis induced by bacterial exotoxins, as well as bystander cell death induced by the host inflammatory response during S. aureus-induced endophthalmitis.
In the present study, we demonstrate that apoptosis is a critical component of the pathogenesis of S.aureus-induced endophthalmitis, leading to the loss of retinal function. In the early response to a pathogen, αB-crystallin is upregulated in the retina, which limits apoptosis. However, S. aureus produces a protease that cleaves and inactivates αB-crystallin, deranging the normal response of the host and increasing retinal-cell vulnerability to apoptosis. This study illuminates a novel protective mechanism used by the host to prevent retinal damage during endophthalmitis and identifies a new role for a protease of S. aureus in the pathogenesis of infection.
MATERIALS AND METHODS
Animals.
Female C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Male and female 129S6/SvEvTac control mice were purchased from Taconic Farms (Germantown, NY). The αB-crystallin knockout (KO) mice (129S6/SvEvTac) were obtained from the National Eye Institute (6). The genotypes of the αB-crystallin KO mice were confirmed by PCR according to a previously published protocol (6). All animals were treated according to the guidelines of the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Research. All procedures involving mice were approved by the Schepens IACUC.
Induction of endophthalmitis.
Mice received an intravitreal injection of 0.5 μl of sterile physiological saline (Hospira, Lake Forest, IL), in which 500 or 5,000 CFU S. aureus (strain RN6390) were suspended, into the left eye according to a previously described protocol (13). Briefly, the bacteria were grown for 4.5 h at 37°C with shaking to log phase and diluted to the appropriate concentrations. An intravitreal injection of S. aureus, just posterior to the limbus-parallel conjunctival vessels, was performed using a borosilicate microcapillary pipette pulled to a tip size of 50 μm. The right eye of each mouse was untreated and served as an internal control for electroretinography (ERG) studies. Before injection, S. aureus was grown in standard Bacto brain heart infusion (BHI) broth (BD Bioscience, Sparks, MD), harvested in log phase, and diluted in physiological saline to 500 CFU/0.5 μl or 5,000 CFU/0.5 μl.
Clinical scoring.
At 24, 48, 72, and 96 h following injection, clinical examinations were performed by slit lamp biomicroscopy. The mice were assigned a clinical score of 0 to 3, with a clinical score of 0 corresponding to a clear anterior chamber, clear vitreous, and clear view of the retina; 1 corresponded to mild aqueous flare, mild vitreal haze, and the view of the retina slightly obscured; 2 corresponded to moderate aqueous flare, dense vitreal haze, poor pupil dilation, and the view of the retina moderately obscured; 3 represented intense aqueous flare, opaque vitreous, and the view of the retina completely obscured.
Histological analysis.
Mice were euthanized at 24, 48, 72, and 96 h following injection, and following euthanasia, the eyes were enucleated using Stevens curved sharp-tip scissors. The eyes were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE). Apoptotic cells were identified using a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) in situ cell death detection kit (TMR red; Roche Applied Science). The sections were mounted using DAPI (4′,6′-diamidino-2-phenylindole) pannuclear stain (Vectashield). Following staining, the ratio of TUNEL-positive to total DAPI-positive cells was calculated in six visual fields at ×100 magnification. These calculations were repeated in three sections per experimental eye, with at least four animals per group per time point. An anti-CD45 antibody was used to distinguish bone marrow-derived inflammatory cells from the resident cells of the retina. Some slides were double stained with TUNEL and anti-CD45 staining. CD45 staining was performed using a three-step staining procedure with rat anti-mouse CD45 antibody (BD Biosciences, San Jose, CA), biotin-conjugated anti-rat immunoglobulin G2b (BD Bioscience, Sparks, MD) as the secondary antibody, and streptavidin-horseradish peroxidase (BD Bioscience, Sparks, MD), together with the DAB detection system (Vector Laboratories, Burlingame, CA).
Intraocular bacterial quantification.
Mice were euthanized at 24, 48, 72, and 96 h following injection, and the eyes were enucleated as described above. The eyes were placed in 1.0 ml sterile phosphate-buffered saline on ice and homogenized by bead beating (FastPrep FP120; Thermo Fisher Scientific, Waltham, MA) with 1.0-mm glass beads for 90 seconds at maximum speed. The homogenates were serially diluted and plated on BHI agar plates.
ERG.
ERG was performed on the mice at 24, 48, 72, and 96 h postinoculation using a slightly modified protocol (13). Briefly, the mice were dark adapted for at least 4 h. The mice were then anesthetized, and the pupils were dilated using 1% tropicamide ophthalmic solution (Bausch & Lomb, Tampa, FL). Following anesthesia, the body temperature was maintained at 37°C using a microwave heat pad. Gold wire electrodes (0.25 mm; Alfa Aesar, Ward Hill, MA) were placed on the cornea after application of a hypromellose ophthalmic prism solution (Akorn, Inc.) and connected to a visual electrodiagnostic system (UTAS-E 3000; LKC Technologies, Gaithersburg, MD). Needle electrodes placed in the anterior scalp and the tail served as reference and ground leads, respectively. The b-wave amplitude (measured from the trough of the a-wave to the peak of the b-wave) in response to a bright flash in a Ganzfeld illumination sphere was recorded for the injected left eye and the contralateral (internal-control) right eye simultaneously. A total of 30 readings at 0.6 cd-s/m2 flash intensity with a 1-second interval between flashes were taken and averaged. The retinal function was defined as the ratio of the b-wave amplitude (from the trough of the a-wave to the peak of the b-wave) of the experimental treated eye divided by the value for the contralateral untreated eye.
Posterior-segment protein analysis.
Mice were euthanized at 24, 48, 72, and 96 h following injection, and following euthanasia, the eyes were enucleated and snap-frozen in liquid nitrogen; eyes that received no injections were enucleated as 0-h controls. The eyes were dissected frozen in a cold room on dry ice, and the posterior segment (vitreous, retina, retinal pigment epithelium, sclera, choroids, and optic nerve) of each eye was collected. Posterior segments were homogenized in 8 M urea/2% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} buffer with 1× Complete minicocktail protease inhibitor (Roche Applied Science, Indianapolis, IN). The protein concentration was quantified using a Bio-Rad DC protein assay (Bio-Rad Laboratories, Hercules, CA). Posterior-segment proteins were analyzed by Western blotting. The proteins were separated on 12% Tris-glycine gels (Invitrogen, Carlsbad, CA) and transferred onto Invitrolon polyvinylidene difluoride membranes (Invitrogen, Carlsbad, CA). The membranes were blocked with 5% milk and probed for αB-crystallin, caspase 3, and actin using monoclonal mouse anti-αB-crystallin (Assay Designs, Ann Arbor, MI), polyclonal rabbit anti-cleaved caspase 3 (Cell Signaling Technology, Danvers, MA), and polyclonal goat anti-actin (Santa Cruz Biotechnology, Santa Cruz, CA) with the appropriate secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA): goat anti-mouse, goat anti-rabbit, and bovine anti-goat, respectively.
In vitro αB-crystallin assay.
Recombinant αB-crystallin (50 ng) (Assay Designs, Ann Arbor, MI) was incubated overnight with 24 μl of either (i) sterile BHI broth (Becton, Dickinson and Company, Sparks, MD), (ii) nonsterile S. aureus supernatant, or (iii) nonsterile S. aureus supernatant with 1× Complete minicocktail protease inhibitor (Roche Applied Science, Indianapolis, IN). Samples were analyzed by Western blotting and probed using monoclonal mouse anti-αB-crystallin with goat anti-mouse secondary antibody, as described above.
Statistical analysis.
Where normally distributed, the data were analyzed with an unpaired t test with a P value of ≤0.05 as the basis for rejection of the null hypothesis; nonparametric data were analyzed using a Mann-Whitney U test. Where appropriate, the Q test was used to eliminate one outlier per experimental group. Statistical analysis and graphing were performed using Prism and Microsoft Excel.
RESULTS
Induction of bacterial endophthalmitis.
We used a murine model of S. aureus-induced endophthalmitis (13) to determine whether αB-crystallin plays a significant role in protecting ocular tissue from apoptosis in response to infection. Since the S. aureus endophthalmitis model was developed recently, the first series of experiments served as an independent confirmation that the laboratory model was reproducible.
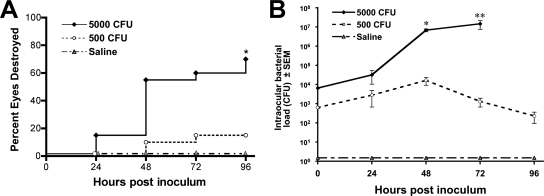
Similar to what was previously reported, eyes infected with 500 CFU of S. aureus developed a mild infection in which the intraocular bacterial load peaked at 48 h (maximum, 1.6 × 104 CFU/eye) but the infection was cleared by 96 h in 85% of the mice, resulting in destruction of the eyes in only 15% of the mice (Fig. 1). By contrast, eyes infected with 5,000 CFU of S. aureus developed a progressive infection that increased in severity over 96 h (maximum, 1.4 × 107 CFU), resulting in complete destruction of the eyes in 70% of the mice. As expected, no ocular damage was observed in the control mice that received an intravitreal injection of sterile saline.
FIG. 1.
Clinical examination and bacterial quantification. C57BL/6J mice received intravitreal injections of either sterile saline or 500 CFU or 5,000 CFU S. aureus. (A) Eyes were examined by slit lamp and given a score of 0, 1, 2, or 3 (0, cleared, and 3, destroyed). At 96 h, 70% of eyes receiving 5,000 CFU and 15% of eyes receiving 500 CFU were destroyed (score, 3). Sterile saline, n = 9; 500 and 5,000 CFU, n = 30; * P < 0.001. (B) The bacterial load in eyes injected with 500 CFU peaked at 48 h (1.6 × 104 CFU/eye); eyes injected with 5,000 CFU did not clear the infection, and the bacterial load increased to 1.4 × 107 at 72 h. No bacteria grew in eyes injected with sterile saline at any time point. Saline, n = 3; 500 CFU, n ≥ 9; 5,000 CFU, n ≥ 9; *, P < 0.0001; **, P = 0.01.
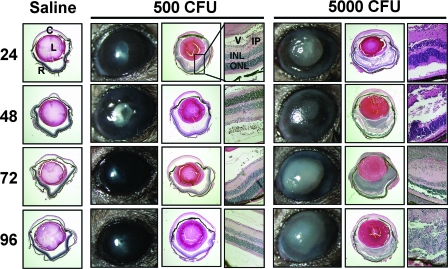
In the eyes of mice that cleared an infection of 500 CFU of S. aureus, slit lamp examinations revealed minimal corneal opacity and poor pupil dilation that peaked at 48 h and subsequently resolved (Fig. 2). Histological analysis revealed mild inflammation with a minimal vitreous infiltrate at 24 and 48 h that resolved and coincided with slight retinal folding in the inner and outer nuclear layers. By contrast, mice that received 5,000 CFU of S. aureus displayed severe inflammation, characterized by significant corneal opacity, corneal edema, and poor pupil dilation within 48 h. Histological analysis revealed a robust inflammatory infiltrate, retinal hemorrhaging, severe retinal folding, and visible bacterial growth in the vitreous at 24 to 48 h with complete destruction of the retina by 72 to 96 h (Fig. 2). Control mice receiving an injection of sterile saline did not exhibit any signs of inflammation at any time postinjection (Fig. 2).
FIG. 2.
Representative clinical pictures and histology. C57BL/6J mice received intravitreal injections of either sterile saline or 500 CFU or 5,000 CFU S. aureus. At 24, 48, 72, and 96 h, the eyes were photographed and then removed, sectioned, and stained for HE. Mild inflammation was observed in eyes that received 500 CFU, and severe retinal damage was observed in eyes that received 5,000 CFU. Retinal detachment in saline- and 500-CFU-injected eyes is an artifact of tissue sectioning. HE pictures were taken of whole eyes at ×2 magnification, and the retina at ×20 magnification. C, cornea; L, lens; R, retina; V, vitreous; IP, inner plexiform; INL, inner nuclear layer; ONL, outer nuclear layer.
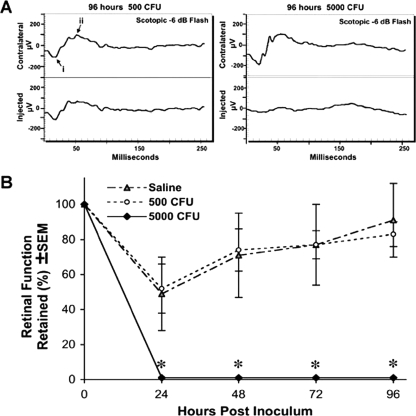
Retinal function, as determined by the b-wave amplitude measured by ERG, corresponded with the clinical and histological findings. Control mice that received sterile-saline intravitreal injections displayed a transient loss of retinal function at 24 h postinjection, returning to 91% of normal by 96 h (Fig. 3B). Mice that received 500 CFU of S. aureus displayed a loss in retinal function to 48% of normal at 24 h that returned to 83% of normal by 96 h as the bacterial infection was cleared. By contrast, mice that received 5,000 CFU of S. aureus displayed a complete loss of retinal function at 24 h that never recovered. Representative ERG recordings at 96 h revealed a total loss of the b-wave amplitude in eyes that received 5,000 CFU of S. aureus; by contrast, mouse eyes that received 500 CFU of S. aureus displayed 83% of the normal (contralateral-eye) b-wave amplitude at 96 h (Fig. 3A).
FIG. 3.
ERG assessment of retinal function. C57BL/6J mice received intravitreal injections of sterile saline or 500 CFU or 5,000 CFU S. aureus. (A) Representative ERG response to 0.6 cd-s/m2 flash intensity at 96 h following 500 CFU or 5,000 CFU S. aureus injection (right and left, respectively). The b-wave amplitude (from the trough of the a-wave [i] to the peak of the b-wave [ii]) was determined, and the ratio of injected eye to contralateral internal control eye was calculated. (B) The 5,000-CFU group lost all retinal function, while the 500-CFU and saline groups displayed only a transient loss of retinal function. The data are expressed as percent retinal function in the experimental left eye compared to the control right eye. Saline, n = 3; 500 CFU, n = 10; 5,000 CFU, n = 10. *, P < 0.001 for 5,000 CFU versus saline and 5,000 CFU versus 500 CFU.
Expression of αB-crystallin during endophthalmitis.
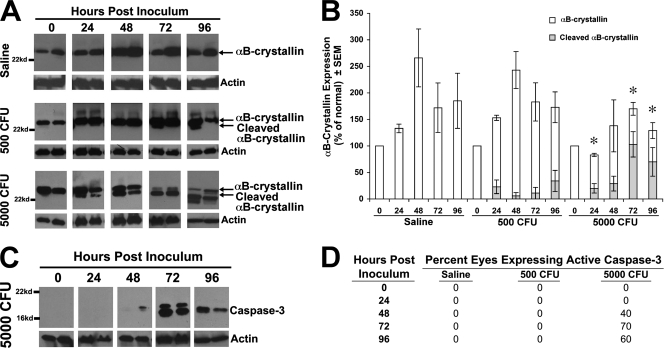
To determine whether αB-crystallin was upregulated during endophthalmitis, the posterior segment of each eye was isolated following an intravitreal injection of either saline or 500 or 5,000 CFU of S. aureus. Eyes that did not receive an intravitreal injection (0 h) yielded a 22-kDa αB-crystallin band (Fig. 4A). Interestingly, an intravitreal injection of sterile saline alone induced a significant increase in αB-crystallin expression that peaked at 48 h (more than 250% of the unperturbed normal eye) and remained elevated at 96 h. This up-regulation of αB-crystallin at 48 h coincided with the recovery of retinal function (Fig. 3B and 4B). Mice that received 500 CFU of S. aureus displayed a similar upregulation of αB-crystallin at 48 h postinfection, but a smaller, 18-kDa αB-crystallin band began to appear at 72 h (Fig. 4A and B). However, the 18-kDa band was always expressed at much lower levels than full-length (22-kDa) αB-crystallin. This smaller, 18-kDa band may represent a cleavage product of αB-crystallin. By contrast, in mice that received 5,000 CFU of S. aureus, the 22-kDa αB-crystallin band decreased below baseline expression at 72 and 96 h postinfection, and the smaller, 18-kDa band increased steadily from 24 to 96 h, so that the level of the 18-kDa αB-crystallin was significantly higher (P < 0.05) than the level of full-length (22-kDa) αB-crystallin. The increase in the 18-kDa band coincided with the increase in the bacterial load and retinal damage in these eyes. These data imply that the upregulation of full-length αB-crystallin is associated with retinal protection, while cleavage of αB-crystallin is associated with retinal destruction and loss of retinal function.
FIG. 4.
Expression of αB-crystallin and active caspase 3. C57BL/6J mice received intravitreal injections of sterile saline or 500 CFU or 5,000 CFU S. aureus. (A) Representative Western blot results probing for αB-crystallin (22 kDa) and actin (43 kDa) loading control. Two samples per group per time point are shown. (B) Densitometry of Western blot results, probing for αB-crystallin. The data are shown as the mean [(αB-crystallin/actin) injected sample]/[(αB-crystallin/actin) normal control]. Saline, n = 3; 500 CFU, n = 4; 5,000 CFU, n ≥ 5. Standard error of the mean: *, P < 0.05 for 5,000 versus saline or 500 CFU full αB-crystallin. (C) Representative Western blot results using an active caspase 3-specific antibody in eyes that received 5,000 CFU S. aureus. (D) Percentages of eyes by Western blot analysis expressing active caspase 3 at 24, 48, 72, and 96 h in C57BL/6J mice following sterile-saline or 500- or 5,000-CFU S. aureus injection. Saline, n = 3; 500 CFU, n = 4; 5,000 CFU, n ≥ 5.
Expression of active caspase 3.
Recent studies demonstrated that αB-crystallin prevents apoptosis by binding to caspase 3 and directly blocking its activation (21, 22, 23). αB-crystallin can also indirectly block the activation of caspase 3 by (i) binding to the proapoptotic factors Bax and Bcl-X(S), (ii) preventing their translocation into the mitochondria, and (iii) restricting the release of cyochrome c (27). Both the direct and indirect antiapoptotic activities may be terminated if αB-crystallin is cleaved. The next series of experiments determined whether the loss of full-length αB-crystallin (the 22-kDa band) and the appearance of the smaller (18-kDa band) αB-crystallin protein coincided with the activation of caspase 3. Western blot analysis was performed on the posterior-segment protein lysates using a caspase 3-specific antibody that recognizes only the active form of caspase 3. As predicted, there was no detectable active caspase 3 at any time point in the eyes of mice receiving either sterile saline or 500 CFU of S. aureus (Fig. 4D). However, the percentage of eyes expressing active caspase 3 in mice receiving 5,000 CFU of S. aureus increased steadily following the intravitreal injections (Fig. 4C), so that by 96 h, 60% of the mice expressed active caspase 3 (Fig. 4D). The appearance of active caspase 3 beginning in eyes that received 5,000 CFU at 48 h coincided with the increased level of the smaller, 18-kDa αB-crystallin band and the decrease in the full-length 22-kDa band, suggesting that the antiapoptotic activity of αB-crystallin may be lost when it is cleaved.
In vitro cleavage of αB-crystallin.
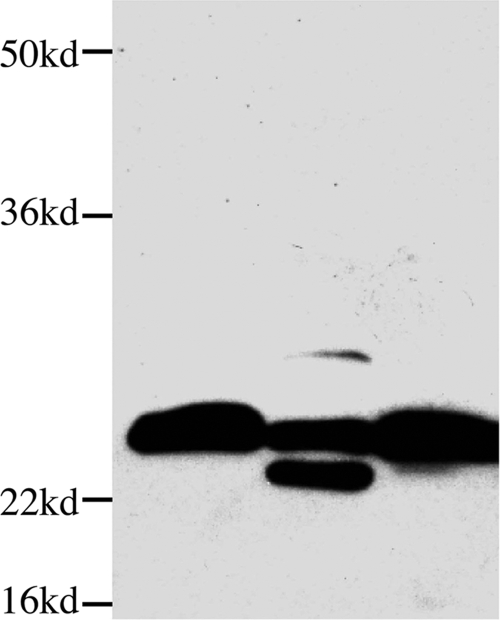
S. aureus also produces a variety of exotoxin proteases that contribute to virulence and are capable of breaking down both host and pathogen matrix proteins (5, 10, 24, 40). The best-studied S. aureus extracellular proteases include SspA, SspB, and aureolysin, which have been linked to virulence and are involved in activating other exotoxins (40). In order to determine whether S. aureus produces an exotoxin that cleaves αB-crystallin, recombinant αB-crystallin was incubated overnight with an S. aureus supernatant and analyzed by Western blotting. The data in Fig. 5 reveal that S. aureus produces a protease that cleaves recombinant αB-crystallin in vitro, and the cleavage band (∼18 kDa) was the same size as the band observed in the posterior segments of mice receiving 5,000 CFU of S. aureus. The addition of a protease inhibitor cocktail to the S. aureus supernatant blocked the cleavage and formation of the 18-kDa band (Fig. 5). These data indicate that an extracellular protease produced by S. aureus cleaves αB-crystallin in vitro and imply that a similar cleavage may occur in vivo during bacterial endophthalmitis.
FIG. 5.
Cleavage of recombinant αB-crystallin by S. aureus supernatant. Western blot analysis of recombinant αB-crystallin treated with (i) sterile BHI broth, (ii) S. aureus culture supernatant, or (iii) S. aureus culture supernatant plus protease inhibitor cocktail.
To determine the protease class responsible for cleaving αB-crystallin, recombinant αB-crystallin was incubated overnight with an S. aureus supernatant in the presence or absence of specific inhibitors of serine, cysteine, or metalloproteases (Pefabloc SC, E-64, and EDTA, respectively). Unexpectedly, all three protease inhibitors blocked the cleavage of αB-crystallin (data not shown), indicating that a complex network of proteases are involved in cleaving αB-crystallin.
Retinal apoptosis during endophthalmitis.
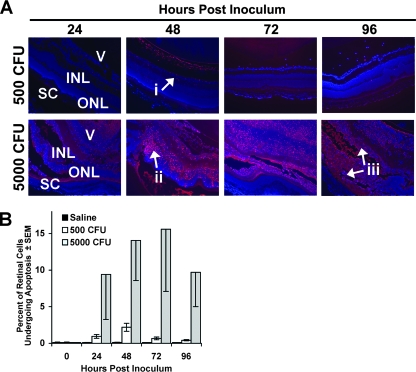
Our data indicate that cleavage of αB-crystallin coincides with increased activation of caspase 3. Therefore, we predicted that the loss of the full-length αB-crystallin and subsequent increase in the cleaved αB-crystallin would correspond with increased apoptosis in the retina. Retinal apoptosis following intravitreal infection was determined by TUNEL staining of retinal sections. TUNEL staining identified very few apoptotic cells in retinas of eyes that received either sterile saline or 500 CFU of S. aureus. The proportion of cells in the retina undergoing apoptosis never increased above 2 to 3% of total cells (Fig. 6A and B). However, the fraction of apoptotic cells in the retina increased steadily in mice that received 5,000 CFU, with 9% apoptotic retinal cells at 24 h, peaking at 16% at 72 h.
FIG. 6.
Retinal apoptosis during endophthalmitis. C57BL/6J mice received intravitreal injections of either sterile saline or 500 CFU or 5,000 CFU S. aureus. (A) Representative TUNEL staining (×10 magnification) in C57BL/6J mouse retinas (red fluorescence signifies TUNEL staining). No apoptosis was observed in mice receiving saline injections. Very little apoptosis (i) was observed at any of the time points in mice receiving 500 CFU. High levels of apoptosis were observed in the inner nuclear layer at 24 to 48 h (ii) and throughout the retina at 72 to 96 h (iii) in mice receiving 5,000 CFU S. aureus. V, vitreous; INL, inner nuclear layer; ONL, outer nuclear layer; SC, sclera/choroid. (B) Percentages of TUNEL-positive cells in the retina. Saline, n = 2; 500 CFU, n = 4; 5,000 CFU, n ≥ 5.
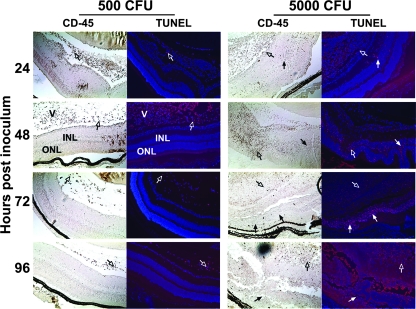
Double staining with an anti-CD45 antibody revealed that the cells undergoing apoptosis in the saline and 500-CFU groups were primarily CD45-positive (CD45+) infiltrating inflammatory cells (Fig. 7). By contrast, the cells undergoing apoptosis in the retinas of the mice that received 5,000 CFU were primarily CD45 negative. We conclude that during destructive bacterial endophthalmitis, cleavage of αB-crystallin coincides with increased retinal-cell apoptosis.
FIG. 7.
TUNEL and CD45 double staining. C57BL/6J mice received intravitreal injections of either sterile saline or 500 CFU or 5,000 CFU S. aureus. The open arrows indicate apoptosis of infiltrating CD45-positive immune cells; the closed arrows indicate apoptosis of CD45-negative retinal cells. Magnification, ×10. V, vitreous; INL, inner nuclear layer; ONL, outer nuclear layer; SC, sclera/choroid.
Endophthalmitis in αB-crystallin KO mice.
Our data demonstrate that increased expression of αB-crystallin coincides with reduced retinal apoptosis and reduced retinal damage, while cleavage of αB-crystallin coincides with increased retinal apoptosis and increased retinal damage. Based upon these data, we hypothesize that upregulation of full-length αB-crystallin is required for prevention of apoptosis in the retina and preservation of retinal function, even during infections that are successfully cleared. To test this hypothesis, we compared the development of bacterial endophthalmitis in αB-crystallin KO mice with that in wild-type mice that received intravitreal injections of 500 CFU of S. aureus.
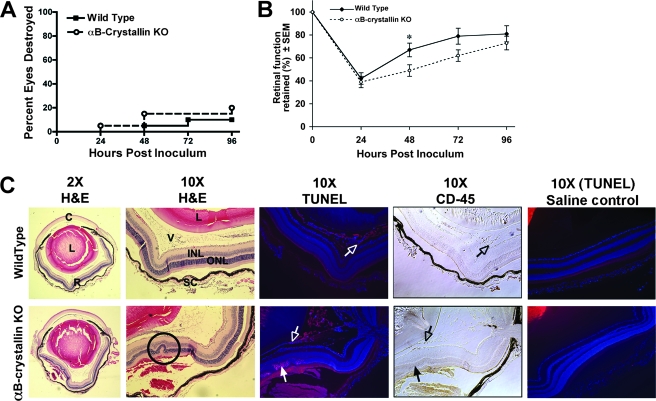
αB-crystallin KO mice and wild-type mice displayed no significant difference in their ability to clear an infection of 500 CFU of S. aureus. Seventy-seven percent of the eyes from αB-crystallin KO mice and 83% of the eyes from wild-type mice cleared the infection by 96 h (Fig. 8A). Clearance of the bacteria was confirmed by determining the bacterial load at 96 h (data not shown). Interestingly, although there was no difference in the ability to clear the bacteria in αB-crystallin KO mice, retinal function was significantly reduced (P < 0.05) at 48 h in αB-crystallin KO eyes (49% of normal) compared with wild-type eyes (69% of normal), and they were slower to recover retinal function (Fig. 8B). To determine whether the difference in retinal function at 48 h was due to increased retinal apoptosis in αB-crystallin KO mice, histological sections of eyes from wild-type and αB-crystallin KO mice were double stained for TUNEL and CD45 at 48 h postinfection. Increased levels of apoptosis of CD45-negative cells were observed in the retinas of αB-crystallin KO mice compared with the wild-type control mice (Fig. 8C). Apoptosis in the αB-crystallin KO eyes was concentrated in pockets and coincided with increased retinal damage, as demonstrated by retinal folds and hemorrhaging. However, at 48 h postinjection of saline alone, no apoptosis was observed in the retinas of wild-type or αB-crystallin KO mice. The results from the saline controls indicated that an intravitreal injection of saline alone did not induce apoptosis in either the wild-type or the αB-crystallin KO mice. Therefore, the increased apoptosis observed in the retinas of αB-crystallin KO mice was specifically induced by the bacterial infection and not by the intravitreal injection alone. Together, these data indicate that αB-crystallin prevents retinal apoptosis during a bacterial infection and that in the absence of αB-crystallin, there is increased apoptosis and increased retinal damage, which occur even when the bacterial infection is successfully cleared.
FIG. 8.
Clinical examination, bacterial clearance, and retinal apoptosis. αB-crystallin KO and wild-type mice received 500-CFU S. aureus intravitreal injections. (A) Eyes were examined by slit lamp and given a score of 0, 1, 2, or 3 (0, cleared, and 3, destroyed). At 96 h, there was no significant difference in percentages of destroyed (score, 3) eyes (23% of αB-crystallin KO; 17% of wild-type). (B) Retinal function, as determined by ERG, was significantly depressed at 48 h (49% of normal) in αB-crystallin KO mice compared to wild-type controls (69% of normal) (n = 10; *, P = 0.05). (C) HE and TUNEL staining in eyes enucleated at 48 h revealed increased retinal folding and bleeding (circled in ×10 HE image) and retinal apoptosis in αB-crystallin KO mice compared to the wild type (closed arrows, CD45 negative). Low levels of CD45-positive inflammatory-cell apoptosis were observed in both wild-type and KO eyes (open arrows, CD45 positive). No apoptosis was observed in wild-type or αB-crystallin KO mice that received saline only. C, cornea; L, lens; R, retina; V, vitreous; INL, inner nuclear layer; ONL, outer nuclear layer; SC, sclera/choroid.
DISCUSSION
Our studies identified upregulation of αB-crystallin as a defense mechanism of the immune-privileged eye that protects delicate retinal tissue from bacterial and host-mediated bystander damage, as occurs during S. aureus endophthalmitis. Furthermore, our studies revealed that S. aureus produces a protease capable of cleaving αB-crystallin into a form identified as being ineffective in preventing retinal apoptosis and destructive endophthalmitis.
A unique property of the eye is the ability to protect against pathogens while simultaneously protecting the delicate visual axis from the sight-destroying effects of immunogenic inflammation (for a review, see reference 41). This property is called immune privilege and was first described by Medawar in 1948. Medawar demonstrated that foreign tissue allografts survive for a prolonged period of time in immune-privileged sites such as the eye, whereas placement of identical allografts at conventional (nonprivileged) body sites led to immune rejection (28). Work over the last several decades has revealed that ocular immune privilege restricts both adaptive and innate immune effectors in a way that balances the challenge of pathogenic infection against the challenge of inflammation-induced vision loss (41). The present study shows that αB-crystallin contributes to the protection of the retina from the inflammatory effects of infection and that this protection is undermined by S. aureus, a leading cause of severe endophthalmitis.
A member of the small heat shock protein family, αB-crystallin was first identified as a lens protein involved in maintaining lens clarity (18). However, in 1989, Bhat and Nagineni and Dubin et al. demonstrated extralenticular expression of crystallin (4, 11). Over the last decade, several investigators demonstrated that αB-crystallin is an important molecular chaperone that is induced by a variety of stress stimuli and confers cellular protection by suppressing the nonspecific aggregation of denatured proteins (17, 25). More recently, studies have demonstrated that αB-crystallin also inhibits apoptosis by blocking the proteolytic activation of caspase 3 (21, 22). Furthermore, while αB-crystallin is expressed constitutively within the neural retina and retinal pigment epithelium, oxidative stress dramatically increases the level of expression (43, 44). In vitro studies have demonstrated that overexpression of αB-crystallin in retinal pigment epithelium cells coincides with reduced caspase 3 activity and apoptosis in response to oxidative stress (44). These studies imply that upregulation of αB-crystallin in the retina amplifies resistance to cell death, allowing the cells to adapt to changes in their environment and to survive otherwise-lethal conditions.
In this model of S. aureus-induced endophthalmitis, the antiapoptotic effect of αB-crystallin was most evident during a mild infection of the eye that was successfully cleared by the immune system. An intravitreal injection of 500 CFU of S. aureus induced a low level of inflammation, as well as upregulation of αB-crystallin in the retina. The inflammatory response successfully cleared the infection within 72 h with little apoptosis. Studies using the αB-crystallin KO mice further confirmed the protective effects of αB-crystallin. While αB-crystallin KO mice did not show impaired ability to clear infection, KO mice showed increased retinal damage and apoptosis compared to wild-type mice.
In addition to demonstrating the role of αB-crystallin in protecting the retina during infection, we also identified a novel mechanism by which S. aureus may impede this protection. The heat shock protein αB-crystallin is constitutively expressed in the retina and, as others have demonstrated, is upregulated in response to many forms of stress (43, 44). However, unique to our studies is the finding that S. aureus produces a protease that cleaves and inactivates αB-crystallin, thus eliminating its protective effect. When the host did not readily clear an S. aureus infection, we observed that retinal αB-crystallin was cleaved, and this cleavage coincided with increased retinal apoptosis, significant tissue damage, and loss of retinal function. The cleavage of αB-crystallin was observed only following infection with S. aureus, and no cleavage of αB-crystallin was observed in wild-type mice treated with saline alone. In addition, in vitro studies determined that S. aureus produced one or more proteases that cleave αB-crystallin. Although we have not elucidated the cleavage site, based on the molecular weight of the cleavage product, we predict that cleavage occurs in either the N-terminal or the C-terminal domain of αB-crystallin, which are involved in oligomerization and solubility of oligomers, respectively (23, 32). The data from Kamradt et al. indicates that, if the oligomerization of αB-crystallin is impaired (by N-terminal domain mutation), then this impaired form of αB-crystallin allows increased activation of caspase 3 and increased apoptosis (23). Therefore, if the cleavage of αB-crystallin observed in infected retinas interferes with N-terminal oligomerization, then this will coincide with increased caspase 3 activation and increased apoptosis. These data reveal another pathogenic mechanism by which S. aureus increases the vulnerability of retinal cells to apoptotic cell death. Conceivably, other endophthalmitis pathogens, such as Pseudomonas and Bacillus, produce proteases with similar activities.
These data also raise important questions about the pathogenesis of S. aureus endophthalmitis. Specifically, how do bacteria induce apoptosis of cells in the retina? Bantel et al. reported that S. aureus induces apoptosis in Jurkat cells in vitro without bacterial internalization (2). This was accomplished via the release of the bacterial toxin alpha-toxin, which forms pores within the plasma membranes of target cells (2). Genestier and colleagues identified another pore-forming toxin, Panton-Valentine leukocidin (PVL), which induces apoptosis in neutrophils (15). Genestier proposed that PVL forms large pores in the membranes of neutrophils, resulting in necrosis. They hypothesized, however, that sublytic concentrations of PVL form a few small pores that allow the entry of S. aureus-derived toxins and proteases. This induced apoptosis via the intrinsic death pathway, in which cytochrome c was released within the mitochondria, triggering the pathway that leads to the activation of downstream caspases, such as caspase 3. Support for the prospect that S. aureus is capable of introducing protein factors that manipulate the biology of target cells stems from the observation that the S. aureus C3-like ADP-ribosyltransferase is capable of accessing its intracellular substrate in an otherwise-intact cell (31). However, αB-crystallin inhibits the activation of caspase 3, increasing the resistance of retinal cells to apoptosis. We propose that cleaved αB-crystallin is nonfunctional and unable to prevent activation of caspase 3. Therefore, entry of the protease into retinal cells through mechanisms such as membrane pores could lead to cleavage of αB-crystallin, making the retinal cells more susceptible to the apoptosis that is triggered by S. aureus toxins, such as alpha-toxin and PVL.
Alternatively, S. aureus may induce apoptosis following internalization into retinal cells. While S. aureus is not generally considered an intracellular pathogen, many host cells, including corneal epithelial cells, internalize S. aureus (19). Furthermore, internalization of S. aureus can induce apoptosis in many cell types, including epithelial cells, endothelial cells, and keratinoyctes (3, 20, 29, 35). If retinal cells internalize S. aureus, bacterial toxins and the proteases could be released through pores in a phagocytic vacuole, potentially making the cell more vulnerable to apoptosis. Studies are ongoing in our laboratory to (i) identify the S. aureus-derived protease that cleaves αB-crystallin and (ii) determine whether retinal cells internalize S. aureus during endophthalmitis and/or whether S. aureus secretes bacterial toxins that enter retinal cells through membrane pores.
Acknowledgments
We thank Marie Ortega and Santina Caruso for their excellent assistance with animal surgical procedures and animal breeding. We also thank Caroline Hackett for experimental assistance.
This work was supported by National Institutes of Health RO1-EY016145 (to M. S. Gregory), EY08289 (to M. S. Gilmore), and Department of Defense W81XWH-07-2-0038 (to M. S. Gregory).
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 28 January 2008.
REFERENCES
- 1.Alge, C. S., S. G. Priglinger, A. S. Neubauer, A. Kampik, M. Zillig, H. Bloemendal, and U. Welge-Lussen. 2002. Retinal pigment epithelium is protected against apoptosis by αB-crystallin. Investig. Ophthalmol. Vis. Sci. 433575-3582. [PubMed] [Google Scholar]
- 2.Bantel, H., B. Sinha, W. Domschke, G. Peters, K. Schulze-Osthoff, and R. U. Janicke. 2001. Alpha-toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 155637-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayles, K. W., C. A. Wesson, L. E. Liou, L. K. Fox, G. A. Bohach, and W. R. Trumble. 1998. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect. Immun. 66336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat, S. P., and C. N. Nagineni. 1989. Alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem. Biophys. Res. Comm. 158319-325. [DOI] [PubMed] [Google Scholar]
- 5.Booth, M. C., A. L. Cheung, K. L. Hatter, B. D. Jett, M. C. Callegan, and M. S. Gilmore. 1997. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect. Immun. 651550-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady, J. P., D. L. Garland, D. E. Green, E. R. Tamm, F. J. Giblin, and E. F. Wawrousek. 2001. αB-crystallin in lens development and muscle integrity: a gene knockout approach. Investig. Ophthalmol. Vis. Sci. 422924-2934. [PubMed] [Google Scholar]
- 7.Callegan, M. C., M. Engelbert, D. W. Parke, B. D. Jett, and M. S. Gilmore. 2002. Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin. Microbiol. Rev. 15111-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callegan, M. C., M. S. Gilmore, M. Gregory, R. T. Ramadan, B. J. Wiskur, A. L. Moyer, J. J. Hunt, and B. D. Novosad. 2007. Bacterial endophthalmitis: therapeutic challenges and host-pathogen interactions. Prog. Ret. Eye Res. 26189-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, J. I., and P. J. Muchowski. 2000. Small heat-shock proteins and their potential role in human disease. Curr. Opin. Struct. Biol. 1052-59. [DOI] [PubMed] [Google Scholar]
- 10.Dubin, G. 2002. Extracellular proteases of Staphylococcus spp. Biol. Chem. 3831075-1086. [DOI] [PubMed] [Google Scholar]
- 11.Dubin, R. A., E. F. Warwrousek, and J. Piatigorsky. 1989. Expression of the murine αB-crystallin gene is not restricted to the lens. Mol. Cell. Biol. 91083-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endophthalmitis Vitrectomy Study Group. 1995. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch. Ophthalmol. 1131479-1496. [PubMed] [Google Scholar]
- 13.Engelbert, M., and M. S. Gilmore. 2005. Fas ligand but not complement is critical for control of experimental Staphylococcus aureus endophthalmitis. Investig. Ophthalmol. Vis. Sci. 462479-2486. [DOI] [PubMed] [Google Scholar]
- 14.Engstrom, R. E., Jr., B. J. Mondino, B. J. Glasgow, H. Pitchekian-Halabi, and S. A. Adamu. 1991. Immune response to Staphylococcus aureus endophthalmitis in a rabbit model. Investig. Ophthalmol. Vis. Sci. 321523-1533. [PubMed] [Google Scholar]
- 15.Genestier, A. L., M. C. Michallet, G. Prevost, G. Bellot, L. Chalabreysse, S. Peyrol, F. Thivolet, J. Etienne, G. Lina, F. M. Vallette, F. Vandenesch, and L. Genestier. 2005. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Investig. 1153117-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanscom, T. A. 2004. Postoperative endophthalmitis. Clin. Infect. Dis. 38542-546. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz, J. 2000. The function of alpha-crystallin in vision. Semin. Cell Dev. Biol. 1153-60. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz, J., T. Emmons, and L. Takemoto. 1992. The ability of lens alpha crystallin to protect against heat-induced aggregation is age-dependent. Curr. Eye Res. 11817-822. [DOI] [PubMed] [Google Scholar]
- 19.Jett, B. D., and M. S. Gilmore. 2002. Internalization of Staphylococcus aureus by human corneal epithelial cells: role of bacterial fibronectin-binding protein and host cell factors. Infect. Immun. 704697-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahl, B. C., M. Goulian, W. van Wamel, M. Herrmann, S. M. Simon, G. Kaplan, G. Peters, and A. L. Cheung. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect. Immun. 685385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamradt, M. C., F. Chen, and V. L. Cryns. 2001. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J. Biol. Chem. 27616059-16063. [DOI] [PubMed] [Google Scholar]
- 22.Kamradt, M. C., F. Chen, S. Sam, and V. L. Cryns. 2002. The small heat shock protein alpha B-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J. Biol. Chem. 27738731-38736. [DOI] [PubMed] [Google Scholar]
- 23.Kamradt, M. C., M. Lu, M. E. Werner, T. Kwan, F. Chen, A. Strohecker, S. Oshita, J. C. Wilkinson, C. Yu, P. G. Oliver, C. S. Duckett, D. J. Buchsbaum, A. F. LoBuglio, V. C. Jordan, and V. L. Cryns. 2005. The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J. Biol. Chem. 28011059-11066. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson, A., P. Saravia-Otten, K. Tegmark, E. Morfeldt, and S. Arvidson. 2001. Decreased amounts of cell wall-associated protein A and fibronectin-binding proteins in Staphylococcus aureus sarA mutants due to up-regulation of extracellular proteases. Infect. Immun. 694742-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klemenz, R., E. Frohli, R. H. Steiger, R. Schafer, and A. Aoyama. 1991. Alpha B-crystallin is a small heat shock protein. Proc. Natl. Acad. Sci. USA 883652-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, D. W., J. P. Liu, Y. W. Mao, H. Xiang, J. Wang, W. Y. Ma, Z. Dong, H. M. Pike, R. E. Brown, and J. C. Reed. 2005. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Mol. Biol. Cell 164437-4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao, Y. W., J. P. Liu, H. Xiang, and D. W. Li. 2004. Human αA- and αB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during saturosporine-induced apoptosis. Cell Death Differ. 11512-526. [DOI] [PubMed] [Google Scholar]
- 28.Medawar, P. 1948. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 12958-69. [PMC free article] [PubMed] [Google Scholar]
- 29.Menzies, B. E., and I. Kourteva. 2000. Staphylococcus aureus alpha-toxin induces apoptosis in endothelial cells. FEMS Immunol. Med. Microbiol. 2939-45. [DOI] [PubMed] [Google Scholar]
- 30.Minami, M., T. Mizutani, R. Kawanishi, Y. Suzuki, and H. Mori. 2003. Neuronal expression of αB crystallin in cerebral infarction. Acta Neuropathol. 105549-554. [DOI] [PubMed] [Google Scholar]
- 31.Molinari, G., M. Rohde, C. Wilde, I. Just, K. Aktories, and G. S. Chhatwal. 2006. Localization of the C3-like ADP-ribosyltransferase from Staphylococcus aureus during bacterial invasion of mammalian cells. Infect. Immun. 743673-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moyano, J. V., J. R. Evans, F. Chen, M. Lu, M. E. Werner, F. Yehiely, L. K. Diaz, D. Turbin, G. Karaca, E. Wiley, T. O. Nielsen, C. M. Perou, and V. L. Cryns. 2006. αB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J. Clin. Investig. 116261-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niederkorn, J. Y. 2004. Immune privilege of the eye, p. 143-154. In L. S. Chan (ed.), Animal models of human inflammatory skin diseases. CRC Press, New York, NY.
- 34.Niederkorn, J. Y. 2006. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat. Immunol. 7354-359. [DOI] [PubMed] [Google Scholar]
- 35.Nuzzo, I., M. R. Sanges, A. Folgore, and C. R. Carratelli. 2000. Apoptosis of human keratinocytes after bacterial invasion. FEMS Immunol. Med. Microbiol. 27235-240. [DOI] [PubMed] [Google Scholar]
- 36.Organisciak, D., R. Darrow, X. Gu, L. Barsalou, and J. W. Crabb. 2006. Genetic, age and light mediated effects on crystallin protein expression in the retina. Photochem. Photobiol. 821088-1096. [DOI] [PubMed] [Google Scholar]
- 37.Parcellier, A., E. Schmitt, M. Brunet, A. Hammann, E. Solary, and C. Garrido. 2005. Small heat shock proteins HSP27 and αB-crystallin: cytoprotective and oncogenic functions. Antiox. Redox. Signaling 7404-413. [DOI] [PubMed] [Google Scholar]
- 38.Ray, P. S., J. L. Martin, E. A. Swanson, H. Otani, W. H. Dillmann, and D. K. Das. 2001. Transgene overexpression of αB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. FASEB J. 15393-402. [DOI] [PubMed] [Google Scholar]
- 39.Sax, C. M., and J. Piatigorsky. 1994. Expression of the alpha-crystallin/small heat-shock protein/molecular chaperone genes in the lens and other tissues. Adv. Enzymol. Mol. Biol. 69155-201. [DOI] [PubMed] [Google Scholar]
- 40.Shaw, L., E. Golonka, J. Potempa, and S. J. Foster. 2004. The role and regulation of the extracellular proteases of Staphylococcus aureus. Microbiology 150217-228. [DOI] [PubMed] [Google Scholar]
- 41.Streilein, J. W. 2003. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. 3879-889. [DOI] [PubMed] [Google Scholar]
- 42.Sugiyama, Y., A. Suzuki, M. Kishikawa, R. Akutsu, T. Hirose, M. M. Waye, S. K. Tsui, S. Yoshida, and S. Ohno. 2000. Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J. Biol. Chem. 2751095-1104. [DOI] [PubMed] [Google Scholar]
- 43.Xi, J., R. Farjo, S. Yoshida, T. S. Kern, A. Swaroop, and U. P. Andley. 2003. A comprehensive analysis of the expression of crystallins in mouse retina. Mol. Vis. 9410-419. [PubMed] [Google Scholar]
- 44.Yaung, J., M. Jin, E. Barron, C. Spee, E. F. Wawrousek, R. Kannan, and D. R. Hinton. 2007. Alpha-crystallin distribution in retinal pigment epithelium and effect of gene knockouts on sensitivity to oxidative stress. Mol. Vis. 13566-577. [PMC free article] [PubMed] [Google Scholar]