Abstract
An important facet of the Staphylococcus aureus host-pathogen interaction is the ability of the invading bacterium to evade host innate defenses, particularly the cocktail of host antimicrobial peptides. In this work, we showed that IsdA, a surface protein of S. aureus which is required for nasal colonization, binds to lactoferrin, the most abundant antistaphylococcal polypeptide in human nasal secretions. The presence of IsdA on the surface of S. aureus confers resistance to killing by lactoferrin. In addition, the bactericidal activity of lactoferrin was inhibited by addition of phenylmethylsulfonyl fluoride, implicating the serine protease activity of lactoferrin in the killing of S. aureus. Recombinant IsdA was a competitive inhibitor of lactoferrin protease activity. Reciprocally, antibody reactive to IsdA enhanced killing of S. aureus. Thus, IsdA can protect S. aureus against lactoferrin and acts as a protease inhibitor.
Staphylococcus aureus is an important gram-positive pathogen that is responsible for an extensive range of pathologies in humans (41, 65) and animals (37). Successful colonization and nasal carriage of S. aureus by patients and health care workers are linked to infection, which occurs when the organism spreads from its primary ecological niche, the anterior nares, to normally sterile parts of the body (64, 70, 73). In order to establish such an intimate association, an invading microorganism must be able to resist the actions of the host's innate defenses. Human nasal secretions form the first barrier against inhaled microorganisms and contain a cocktail of cationic proteins which are presumed to control bacterial growth and spread (21, 22). One such protein, lactoferrin (Lf), is the second most abundant antimicrobial polypeptide (21) (after lysozyme, to which S. aureus is insensitive [12]) in airway fluid, and significant quantities of this molecule are also found in other body secretions (49).
A globular 78-kDa glycoprotein, Lf is folded into homologous N and C lobes. Each lobe can bind one metal ion, usually Fe3+, to make hololactoferrin (hLf) (8). Apolactoferrin (aLf) carries no metal ions. The bactericidal activity of aLf, including activity against S. aureus, is well established (4, 14), but the mechanism(s) by which it kills S. aureus has not yet been elucidated. A stable peptide known as lactoferricin (Lfcin) results from proteolysis of Lf and is itself active against a range of bacteria, including S. aureus (11). However, its relevance to mucosa-colonizing bacteria in the absence of an inflammatory response to provide proteolytic conditions remains in question (66).
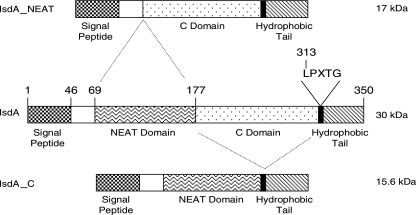
A study of S. aureus antigens expressed during human infection identified IsdA, an iron-regulated, covalently attached surface protein (18). Otherwise healthy individuals who were nasal carriers of S. aureus had lower serum concentrations of anti-IsdA antibodies than noncarriers, which led to the observation that vaccination with IsdA could reduce nasal carriage in the cotton rat model (18). Moreover, an isogenic isdA mutant was shown to be attenuated for nasal colonization in this model, and its ability to bind desquamated human nasal epithelial cells was reduced (18). IsdA is covalently bound to the cell wall peptidoglycan via the activity of sortase A (43). The mature IsdA protein has two domains with distinct functions. The N-terminal NEAT domain of IsdA binds a broad spectrum of human extracellular matrix and serum proteins, including transferrin (Tf), a protein with extensive homology to Lf (17, 44, 46, 47, 60, 63). The C-terminal domain of IsdA has recently been shown to decrease the cellular hydrophobicity of S. aureus and confer resistance to hydrophobic fatty acids and host antimicrobial peptides and thus aid survival on live human skin (20). IsdA is the first protein shown to have this function.
In this study, we analyzed Lf killing of S. aureus and found that it occurs via protease activity, which can be inhibited by binding to IsdA, leading to resistance to this bactericidal protein.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were grown on 2YT medium, using selection with the antibiotics ampicillin (100 μg/ml) and kanamycin (50 μg/ml) where appropriate. S. aureus strains were grown either on brain heart infusion medium (Oxoid) or on chemically defined CL medium (34). When included, antibiotics were added at the following concentrations: erythromycin, 5 μg/ml; lincomycin, 25 μg/ml; and chloramphenicol, 10 μg/ml. All bacterial cultures were grown at 37°C.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| Staphylococcus aureus strains | ||
| Newman | Wild type | 24 |
| SRC105 | isdA::Tn917 Emr in Newman | 18 |
| SRC108 | isdAΔNEAT in Newman | 20 |
| SRC109 | isdAΔC in Newman | 20 |
| SRC150 | ΔsrtA Emr in Newman | 20 |
| Escherichia coli BL21 (DE3) | F−ompT gal [dcm] [lon] hsdSB(rB− mB−) | Novagen |
| Plasmids | ||
| pIsdA | IsdA overexpression vector | 17 |
| pIsdAC | IsdA C domain overexpression vector | 17 |
| pMK4 | E. coli-S. aureus shuttle vector | 59 |
| pSRC001 | rIsdA complementation vector | 17 |
Overexpression of recombinant IsdA.
His6-tagged recombinant IsdA was purified using the Hi-Trap system (Amersham Biosciences) as described previously (17).
Ligand binding ELISAs.
An enzyme-linked immunosorbent assay (ELISA) was used to analyze the ability of recombinant IsdA to bind ligands, both immobilized and soluble, as described previously. Briefly, 100 μl of an appropriate ligand, either aLf, hLf, apotransferrin (aTf), or holotransferrin (hTf) (all purchased from Sigma), was added to the wells of a 96-well microtiter plate (Nunc), incubated overnight at 4°C, and probed as described previously (16).
Inhibition ELISAs were carried out by mixing recombinant IsdA (rIsdA) with an appropriate ligand or mouse anti-IsdA antibodies prepared as described previously (17). The reactions were carried out as described previously (16).
Western blot ligand binding assays.
Ligand binding was assayed by Western blotting, using biotinylated human serum proteins as described previously (16).
Bacterial killing assays.
The rate of S. aureus death was determined as described previously (20). Briefly, bacteria grown in CL broth were centrifuged and washed in sterile distilled H2O (dH2O) twice. Cell suspensions (ca. 1 × 108 CFU/ml in dH2O) were incubated at 37°C with various amounts of Lf. Portions (10 μl) were harvested at different time points, and serial dilutions were prepared to determine the numbers of viable bacteria present. The following compounds were added to the killing assay when appropriate: mouse anti-IsdA antibodies prepared as described previously (17), human serum, anti-Lf antibodies (ICN), CaCl2, NaCl, FeSO4, and rIsdA. Lf treated with CaCl2 or FeSO4 was dialyzed overnight against phosphate-buffered saline (PBS) to remove unbound excess ligand.
When appropriate, the statistical significance of the time necessary to obtain 50% killing was determined by using Student's t test.
Treatment of Lf with protease inhibitor.
Phenylmethylsulfonyl fluoride (PMSF) (final concentration, 1 mM) was added to Lf (1 mg/ml), and the preparation was incubated at room temperature with continuous mixing for 2 h. Subsequent dialysis against PBS overnight at 4°C removed residual PMSF.
Protease activity measurement.
N-α-Benzyloxycarbonyl-Phe-Arg-7-amido-4-methylcoumarin (Z-Phe-Arg-AMC) (Sigma) was used as a substrate, as described previously (42), and Lf-catalyzed hydrolysis was determined in PBS at room temperature (21°C). Lf was used at a concentration of 10 μM, and the substrate concentration ranged from 1 to 200 μM. The cleavage of the substrate was followed fluorometrically at 460 nm with an excitation wavelength of 355 nm by monitoring the increase with a Victor microtiter plate reader (Wallac). Protease activity inhibition studies were carried out by addition of rIsdA to the reaction mixture.
RESULTS
IsdA is an Lf binding protein.
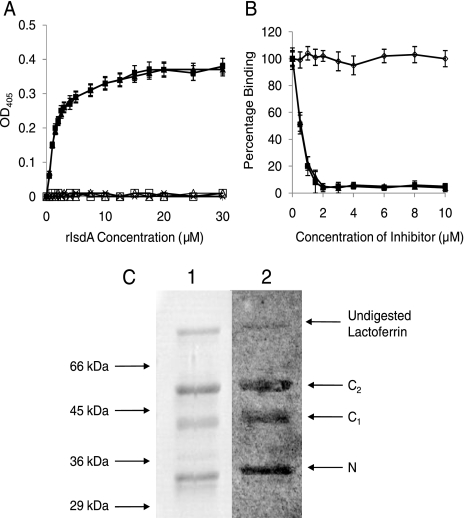
The Lf binding activity of rIsdA was tested by performing ELISAs. Binding to both aLf and hLf occurred in a dose-dependent and saturable manner. Saturation occurred at a concentration of ca. 15 μM (Fig. 1A). An apparent Kd (dissociation constant) of ca. 1.5 μM was calculated from the concentration giving half-maximum binding. This was an affinity similar to the affinity that we observed for IsdA binding to aTf (ca. 6.5 μM) (17). Addition of 1 M NaCl completely inhibited the interaction between IsdA and aLf or aTf, implicating ionic interactions in ligand binding. Furthermore, it was possible to inhibit binding of IsdA to immobilized aLf and aTf with excess quantities of both proteins but not with bovine serum albumin (BSA). Inhibition (≥95%) was observed for both proteins at a concentration of 2 μM (Fig. 1B). Additionally, the holo forms of Lf and Tf displayed IsdA binding properties similar to those of the respective apo forms (results not shown). Taken together, these data demonstrate that IsdA binds to Lf and Tf in similar ways, regardless of the iron status.
FIG. 1.
(A) Binding of rIsdA to microtiter plate wells coated with aLf (▪ and □), aTf (▴ and ▵), or BSA (×). In some cases 1 M NaCl was added to the reaction mixture (open symbols). Increasing concentrations of rIsdA were incubated in wells for 1 h at room temperature. Bound protein was detected with anti-IsdA antibodies and anti-mouse alkaline phosphatase-conjugated antibodies. The data for aLf and aTf without NaCl overlap extensively, as do the data for aLf and aTf with NaCl and the data for BSA. (B) Inhibition of rIsdA binding to immobilized aLf. rIsdA was preincubated for 1 h at room temperature with increasing concentrations of aLf (▪), aTf (▴), or BSA (⋄) before incubation in aLf protein-coated wells. Bound protein was detected with anti-IsdA antibodies and anti-mouse alkaline phosphatase-conjugated antibodies. The data for aLf and aTf overlap extensively. The values in panels A and B are the means for triplicate wells from three independent experiments. (C) Screening for binding of rIsdA to lobes of human aLf using the Western affinity blotting technique. Trypsin-digested aLf (20 μg) was separated by electrophoresis on a 12.5% (wt/vol) SDS-PAGE gel. rIsdA bound to aLf was detected using anti-rIsdA antibodies. The positions of the C lobe (bearing either one [C1]or two [C2] N-linked glycans) and the N lobe (N) are indicated on the right. Lane 1, Coomassie blue-stained aLf; lane 2, aLf probed with rIsdA.
To identify which domain of Lf contains the binding site for IsdA, a Western blot assay was used. Lf is globular and consists of homologous N and C lobes (3, 7, 8), which can be separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) after digestion with trypsin (62). This experiment revealed binding of rIsdA to both lobes of Lf (Fig. 1C).
Expression of IsdA inhibits killing of S. aureus by aLf.
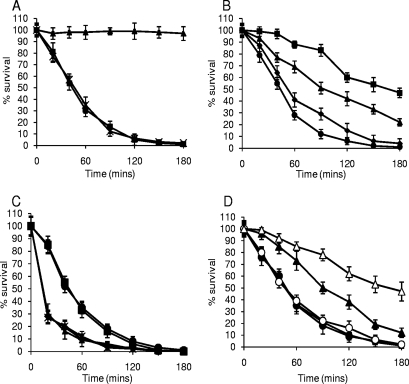
We hypothesized that expression of IsdA on the surface of S. aureus would alter the sensitivity of the organism to Lf, which is known to be bactericidal (1, 4, 14, 66). To test this, aLf and hLf isolated from human milk were used in killing assays. As observed in other studies, the bactericidal effect was seen only with aLf and not with hLf (results not shown). Indeed, addition of iron to aLf abrogated its bactericidal activity (Fig. 2A). Addition of anti-Lf antibodies to the killing assay mixture significantly reduced the bactericidal effect in a dose-dependent manner (P < 0.001 for all dilutions) (Fig. 2B). Taken together, these data confirm the findings of other workers, who showed that Lf is bactericidal only in its iron-free state (1, 5, 14) and that the bactericidal nature of the native Lf preparations is due to Lf rather than a contaminant.
FIG. 2.
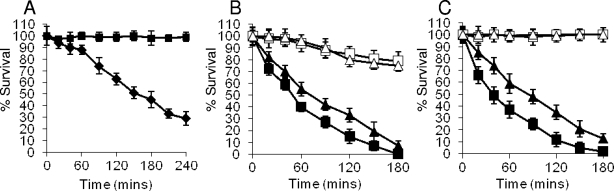
(A) aLf is bactericidal for S. aureus Newman. FeSO4 or CaCl2 was added to preparations of aLf used in killing assays prior to dialysis to remove excess salts. S. aureus Newman was treated with 0.5 μM aLf (▪), aLf plus 10 μM FeSO4 (▴), or aLf plus 10 μM CaCl2 (×). (B) Addition of rabbit anti-Lf antibodies inhibits Lf bactericidal activity. S. aureus Newman was treated with aLf and different dilutions of anti-Lf antibodies, including 1:10 (▪), 1:100 (▴), and 1:1,000 (⧫), as well as no anti-Lf antibody (•). (C) IsdA protects S. aureus Newman against killing by 0.5 μM aLf. The following strains were used: ▪, Newman (wild type); ▴, SRC105 (isdA); •, SRC105(pSRC001); ⧫, SRC105(pMK4); and ×, SRC150 (srtA). The data for strains Newman and SRC105(pSRC001) overlap extensively, as do the data for strains SRC105, SRC105(pMK4), and SRC150. (D) Addition of a molar excess of rIsdA, but not addition of a molar excess of rIsdAC, inhibits killing of S. aureus Newman by 0.5 μM aLf. Symbols: ▪, aLf; ▴, rIsdA plus aLf (5:1 molar excess); ▵, rIsdA plus aLf (10:1 molar excess); •, rIsdAC plus aLf (5:1 molar excess); ○, rIsdAC plus aLf (10:1 molar excess). In all panels, the values are the means of three independent experiments.
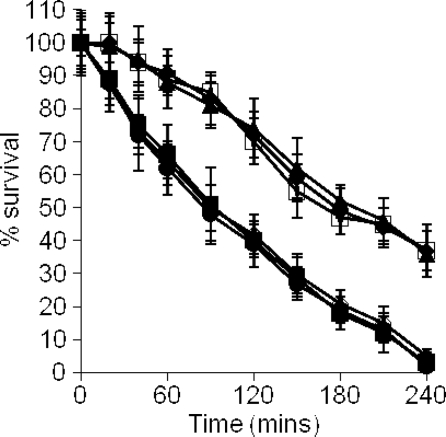
In killing assays, the isdA mutant was significantly more sensitive than the parental strain to aLf (P < 0.0002), and complementation of the isdA mutation fully restored the resistance phenotype (Fig. 2C). In order to determine if any other covalently attached surface proteins were involved in resistance to aLf, a sortase A mutant (S. aureus SRC150), which lacked nearly all such proteins, was used and in killing assays was found to be just as sensitive to aLf as strain SRC105 (isdA). This indicates that under these growth conditions, IsdA is the major covalently attached surface protein responsible for resistance to aLf (Fig. 2C). To confirm that binding of aLf by IsdA mediated resistance, molar excesses of rIsdA and its recombinant C domain (rIsdAC) were added to killing assay mixtures using the isdA mutant strain, and a concomitant reduction in the lethality of aLf was observed when rIsdA was added (P < 5 × 10−6) but not when rIsdAC (P > 0.5) was added (Fig. 2D).
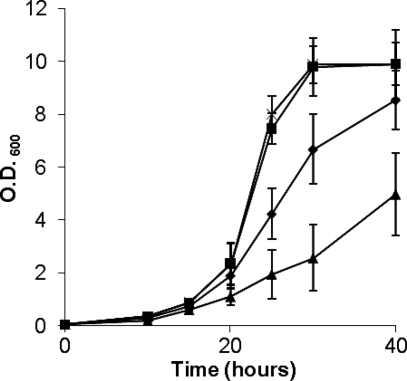
The effect which aLf had on the growth rate of S. aureus in CL broth (a chemically defined medium in which IsdA is expressed [17, 20]) at 37°C was measured. Addition of aLf to the culture resulted in a decrease in the growth rate (Fig. 3). IsdA is involved in resistance to the detrimental effects of aLf as SRC105 (isdA) grew more slowly in its presence than the parental strain.
FIG. 3.
IsdA protects against the growth-inhibiting effect of aLf. Symbols: ▪, Newman (wild type); ×, SRC105 (isdA); ⧫, Newman plus 1 μM aLf; ▴, SRC105 plus 1 μM aLf. The values are the means of three independent experiments. The data for strains Newman and SRC105 overlap extensively. O.D. 600, optical density at 600 nm.
These data make a strong case for the hypothesis that IsdA is able to reduce the bactericidal efficacy of aLf. The ability of IsdA to readily prevent killing by aLf, whether bound to the cell surface or in solution, suggests that IsdA may block aLf-mediated killing by neutralizing the site(s) on Lf which is responsible for the bactericidal activity.
Anti-IsdA antibodies inhibit Lf resistance.
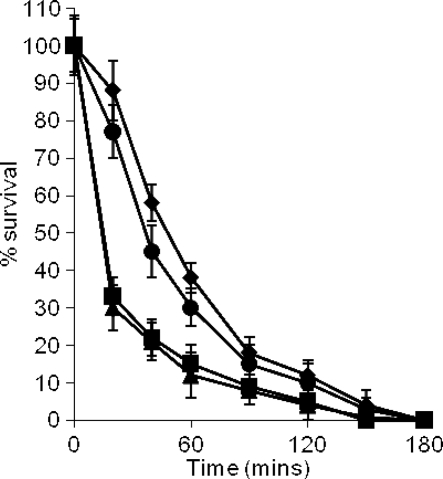
Given that IsdA is required for nasal colonization and that antibodies reactive to IsdA can prevent this colonization, it was hypothesized that anti-IsdA antibodies might prevent binding of aLf to IsdA and thus enhance the killing activity. When strain Newman was incubated with dilutions of mouse anti-IsdA, increased killing was observed, but no difference was observed when SRC105 (isdA) was used (Fig. 4A and B). Addition of mouse anti-IsdH to the killing assay mixtures had no effect on aLf killing efficacy for either strain (results not shown). Taken together, these data demonstrate that antibodies reactive to IsdA enhance the killing activity of aLf.
FIG. 4.
Mouse anti-IsdA serum enhances aLf killing in an IsdA-dependent manner. Different concentrations of sera were added to killing assay mixtures. (A) Symbols: •, Newman plus 0.5 μM aLf (P < 0.01); ▪, Newman plus 0.5 μM aLf plus anti-IsdA (1:100 dilution of antibody; P < 4 × 10−5); □, Newman plus 0.5 μM aLf plus anti-IsdA (1:1,000 dilution of antibody; P < 2 × 10−5). (B) Symbols: ×, SRC105 plus 0.5 μM aLf; ▴, SRC105 plus 0.5 μM aLf plus anti-IsdA (1:100 dilution of antibody); ▵, SRC105 plus 0.5 μM aLf plus anti-IsdA (1:1,000 dilution of antibody). The data for all three mixtures overlap extensively. The values are the means of three independent experiments.
Protease activity of human aLf is bactericidal.
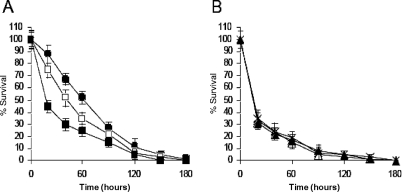
The mechanism by which aLf elicits killing of S. aureus remains uncertain. Previous reports have shown that Lfcin, like many other antimicrobial peptides, is inactive under saline conditions, such as those described above. Conversely, the antimicrobial activity of whole Lf has been shown to require such conditions (5). The killing efficacies of whole Lf and Lfcin were tested to confirm the previous findings, and the results showed that bovine Lfcin was not active against S. aureus when PBS was used; however, killing did occur when the experiment was carried out with dH2O (Fig. 5A). Both human aLf and bovine aLf were active in PBS, but the activity was poor in dH2O (Fig. 5b). Thus, in the assays conditions used in this work, the killing activity of Lf is not due to Lfcin.
FIG. 5.
Effect of assay conditions on Lfcin and aLf activity. (A) Lfcin B is active in dH2O but not in PBS. S. aureus Newman was incubated with Lfcin B in PBS (▪) and dH2O (⧫). (B) aLf (0.5 μM) is active in PBS but only poorly active in dH2O. S. aureus Newman was incubated with human (▪ and □) or bovine (▴ and ▵) aLf. Filled symbols, incubation in PBS; open symbols, incubation in dH2O. The data are the means of three independent experiments. (C) Serine protease activity of aLf is bactericidal. S. aureus Newman was incubated with 0.5 μM human aLf (▪ and □) and 0.5 μM bovine aLf (▴ and ▵). Some preparations were pretreated with PMSF (open symbols). The values are the means of three independent experiments.
The serine protease activity of aLf has been described extensively previously (33, 42, 52, 57, 68). To test whether this activity was responsible for S. aureus killing, aliquots of bovine aLf and human aLf were treated with PMSF, a potent inhibitor of serine proteases, and used in killing assays. Treatment of both human and bovine aLf completely inhibited killing of S. aureus (Fig. 5C). Similarly, aliquots of aLf which had been boiled for 2 min were completely inactive (results not shown). As a control, aLF samples which had not been treated with PMSF were subjected to parallel dialysis, and they maintained full protease and killing activity (results not shown). Thus, the anti-S. aureus activity of whole aLf is due to its serine protease activity. Experiments aimed at identifying components of S. aureus released by aLf proteolysis by SDS-PAGE, followed by Coomassie blue or silver staining of killing assay supernatants, failed to yield specific fragments (results not shown).
NEAT domain of IsdA is an inhibitor of aLf activity.
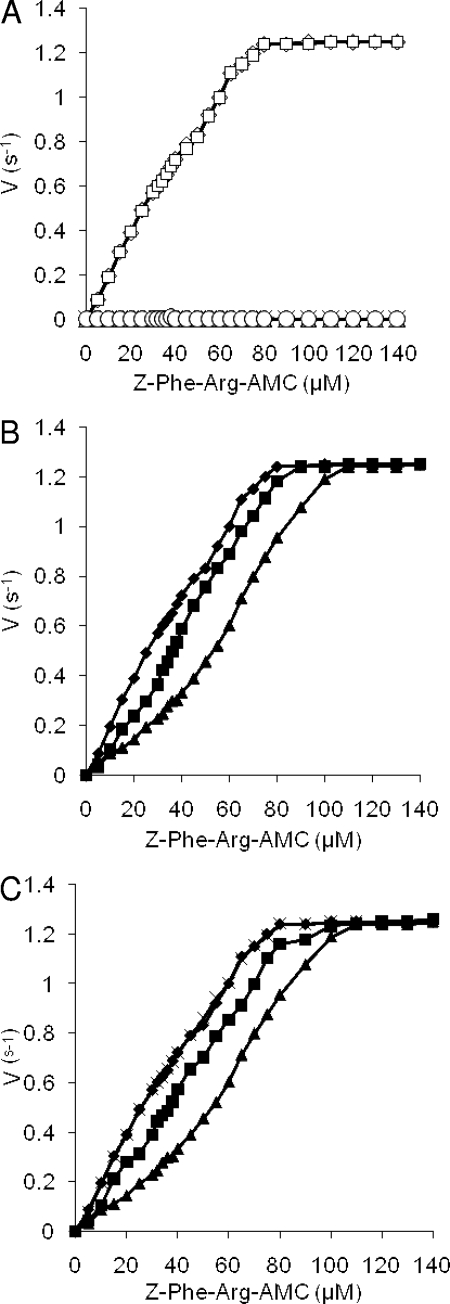
To investigate the possibility that IsdA directly inhibits the serine protease activity of aLf, proteolysis of Z-Phe-Arg-AMC, a fluorescent substrate of aLf (42), was measured. The proteolytic activity of aLf followed simple Michaelis-Menten kinetics (kcat = 0.8 s−1, Km = 34 μM) (Fig. 6A) and disappeared when the aLf was either denatured by heating or treated with PMSF or 10 μM FeSO4 (see above), but not when it was treated with 10 μM CaCl2, another Lf ligand (53) (Fig. 6A). Addition of rIsdA to the assay mixture decreased the serine protease activity of aLf in a competitive manner (Fig. 6B). Addition of anti-IsdA antiserum at 1:1,000 and 1:100 dilutions inhibited protease inhibitor activity (Fig. 6C). No inhibition was observed with rIsdAC (results not shown). Recombinant NEAT domain could not be used in these experiments due to its insolubility. Increasing the concentration of substrate added to the rIsdA-aLf reaction mixture did increase the reaction rate, indicating that IsdA is a classical competitive inhibitor of aLf. No proteolytic degradation of rIsdA was observed under any conditions used (results not shown).
FIG. 6.
Protease activity of human aLf with a fluorescent substrate. (A) Effect of substrate concentration on the rate of 0.1 μM aLf-catalyzed hydrolysis of Z-Phe-Arg-AMC. Symbols: □, 0.1 μM aLf; ⋄, 0.1 μM aLf plus 10 μM CaCl2; ▵, 0.1 μM aLf plus 10 μM FeSO4; ○, PMSF-treated 0.1 μM aLf; ×, boiled 0.1 μM aLf. The data for PMSF-treated 0.1 μM aLf, 0.1 μM aLf plus 10 μM FeSO4, and boiled 0.1 μM aLf overlap extensively, as do the data for 0.1 μM aLf and 0.1 μM aLf plus 10 μM CaCl2. (B) rIsdA inhibits protease activity. Symbols: ⧫, 0.1 μM aLf; ▴, 0.1 μM aLf plus 1 μM rIsdA; ▪, 0.1 μM aLf plus 0.2 μM rIsdA. (C) Anti-IsdA antibodies inhibit IsdA protease inhibitor action. Symbols: ⧫, 0.1 μM aLf; ▴, 0.1 μM aLf plus 1 μM rIsdA; ▪, 0.1 μM aLf plus 0.2 μM rIsdA plus 1:1,000 dilution of anti-IsdA; ×, 0.1 μM aLf plus 0.2 μM rIsdA plus 1:100 dilution of anti-IsdA. The values are the means of three independent experiments.
The ligand binding activity of IsdA has been mapped to the NEAT domain. Using S. aureus strains carrying deletions of the NEAT and C domains of IsdA (Fig. 7) (20), the resistance to aLf killing was determined. When grown under iron starvation conditions, S. aureus SRC108 (isdAΔNEAT) was as sensitive to the action of aLf as strain SRC105 (isdA), exhibiting the same rate of death (P > 0.5) (Fig. 8). Conversely, S. aureus SRC109 (isdAΔC) was partially resistant to the action of aLf (P < 0.0004) but was significantly less resistant (P < 0.001) than the wild type (Fig. 8).
FIG. 7.
Domain structure of IsdA in S. aureus. The organization and predicted mature sizes of IsdA, IsdAΔC, and IsdAΔNEAT are shown. The numbers indicate the positions of the relevant amino acid residues.
FIG. 8.
NEAT domain of IsdA protects S. aureus against the killing activity of 0.5 μM aLf in PBS. The following strains were used: ⧫, Newman (wild type); ▪, SRC105 (isdA); ▴, SRC108 (isdAΔNEAT); and •, SRC109 (isdAΔC). The data for strain SRC105 and SRC108 overlap extensively. The values are the means of three independent experiments.
C domain of IsdA confers resistance to bovine Lf.
Recent work has shown that the C domain of IsdA confers resistance to various mammalian antimicrobial peptides, probably due to inhibition of hydrophobic interactions with the cell envelope (20). We tested the ability of IsdA and specifically the C domain to resist Lf. In killing assays, S. aureus SRC108 (isdAΔNEAT) and its parent were similarly resistant to killing by bovine Lfcin compared to strains SRC105 (isdA) and SRC109 (isdAΔC) (P < 5 × 10−6), which were equally more sensitive. Complementation of SRC105 (isdA) by pSRC001 (isdA+) specifically restored the resistance phenotype (Fig. 9). These data show that IsdA, specifically its C domain, is able to resist the action of bovine Lfcin, possibly by inhibiting the hydrophobic interactions which are required between host antimicrobial peptides and their target, the cell membrane.
FIG. 9.
C domain of IsdA protects S. aureus against 1 μM bovine Lfcin killing activity. The following strains were used: ⧫, Newman (wild type); ▪, SRC105 (isdA); ▴, SRC108 (isdAΔNEAT); •, SRC109 (isdAΔC); □, SRC105(pSRC001); and ⋄, SRC105(pMK4). The data for strains Newman, SRC108, and SRC105(pSRC001) overlap extensively, as do the data for strains SRC105, SRC109, and SRC105(pMK4). The data are the means of three independent experiments.
DISCUSSION
There is a complex interaction between S. aureus and its human host as the bacterium is able to colonize several host niches, both as an opportunist pathogen that has great medical significance and as a commensal in the nares (41, 63, 65, 70, 73). In order to defend against colonization by microorganisms, the host produces a barrage of antimicrobial peptides. In the human nose, the primary ecological niche of S. aureus, Lf is the predominant anti-S. aureus protein (21, 22). Indeed, transgenic mice producing human Lf were more able to clear S. aureus from infected mucosa than mice lacking the protein (30). Moreover, in vitro studies using bovine mammary tissue provided evidence that production of Lf is up-regulated in response to infection (71, 74). The antibiotic activity of Lf against a variety of bacteria has been recognized for a long time, and the antimicrobial activity of Lf was initially attributed to iron sequestration, until the irreversible inhibition of Streptococcus mutans in vitro was shown not to be attributable to iron deprivation (6). Indeed, even the role of Lf in host iron homeostasis is now being reconsidered. It is well established that the host uses Tf to sequester iron and deliver it to its cells (13, 36, 38, 61), but in Lf double-knockout mice the iron homeostasis in adults is no different from that in wild-type mice, and indeed there is a mild iron overload in neonates, suggesting that Lf may reduce iron uptake (67). However, numerous further functions have been ascribed to Lf (for reviews, see references 9, 15, 25, 39, 40, 66, 68, and 69), including serine protease activity, which has been shown to be responsible for the removal of colonization and virulence factors from the surface of Haemophilus influenzae (52) and Porphyromonas gingivalis (57), respectively. Furthermore, serine protease activity could also account for Lf inhibition of virulence, cellular invasion, type III secretion, and biofilms in a variety of pathogenic bacteria (2, 27, 50, 58). Binding of iron has been shown to significantly alter the structural conformation of Lf (29), which correlates with a loss of both protease and antibacterial activities against a number of organisms, including S. aureus (1, 4, 14, 66). In this study, we showed that inhibition of serine protease activity abrogates killing activity. Furthermore, expression of IsdA by S. aureus confers resistance to Lf killing, and rIsdA inhibits protease activity in vitro.
Lf is globular and consists of N and C lobes connected by a three-turn α-helix (3, 7, 8). The two lobes are homologous and have very similar tertiary structures, consistent with the hypothesis that they arose as products of gene duplication. Each lobe is further subdivided into two domains with a single iron binding site situated between the inner faces of the interdomain cleft. IsdA binds both lobes, and it therefore seems likely that the binding site is situated in an area of homology. The serine protease activity resides in the N lobe, but the precise location is a matter of contention. One study proposed that it consists of a serine-lysine catalytic dyad located in the area adjacent to the interlobe cleft of human Lf (33), but in another study, pKa shift calculations indicated that several serine residues of bovine Lf display the high nucleophilicity required to potentially catalyze substrate cleavage (42). Definitive identification of the active site awaits further study.
Iron deprivation is a common host environmental stimulus, and in response to this stimulus S. aureus makes a cell wall-bound protein, IsdA (17, 18, 48, 72). Previously, IsdA has been shown to be an adhesin required for nasal colonization (17, 18). In this paper, we describe how IsdA binds Lf, a protein similar to Tf (46), which has also been described as a binding substrate for IsdA (17, 60). Previous studies have shown that IsdA binds a range of iron-containing ligands, including Tf, and have proposed that it has a role in iron uptake across the cell envelope; however, there is no phenotypic evidence for such uptake from Tf. Indeed, Park et al. (51) showed that IsdA does not have the ability to remove iron directly from Tf and that a siderophore-mediated iron acquisition system plays a dominant and, more importantly, essential role in this process. The IsdA NEAT domain has also been shown to bind heme, and it has been proposed that the bacterium uses this activity to acquire iron (28). Several S. aureus surface proteins which bind hemoglobin, hemin, and haptoglobin have been identified, which may substitute for the loss of IsdA (23, 44).
The interaction between IsdA and Lf appears to be very similar to the interaction of IsdA with Tf, which is not surprising given the homologous nature of the two proteins. The ligand binding activity of IsdA resides in its single-copy NEAT domain (17), whose structure has recently been determined and shown to consist of a beta-sheet (28). It was also speculated that the broad-spectrum ligand binding activity of IsdA is due to nonspecific ionic interactions (28). However, although we show here that ionic interactions are important in the binding of Lf by IsdA, these interactions are unlikely to be nonspecific, as the net charges at pH 7 of Lf and Tf are positive and negative, respectively (35, 47, 55). In this study, no inhibition of Lf activity was observed for the C domain of IsdA; only full-length rIsdA inhibited protease activity. We were unable to use recombinant NEAT domain due to insolubility of the polypeptide. Instead, S. aureus strains displaying mutated forms of IsdA, lacking either the NEAT or C domain, were used in killing assays. No resistance to killing was observed for the isdAΔNEAT strain, but isdAΔC cells were partially resistant compared to the wild type. It has been proposed that the C domain extends the NEAT domain into the extracellular milieu (63). Thus, in the IsdAΔC truncated version of the protein, the NEAT domain may be insufficiently exposed or oriented for full activity.
In addition to protease activity, a small peptide, Lfcin, is released by proteolysis from the N terminus of Lf (10, 11, 54). This cationic antimicrobial peptide has been compared with other molecules, such as defensins, suggesting that it has a similar mode of action. However, the significance of Lfcin to bacteria colonizing mucosal surfaces, such as the nares, is in doubt because this peptide is generated by proteolysis, such as the proteolysis produced by an inflammatory response, and not under normal conditions (66). In this study, we found that IsdA, specifically the C domain, inhibits Lfcin activity. However, this was not responsible for the IsdA-derived resistance to Lf. It is the Lf interaction with the IsdA NEAT domain that confers resistance.
The array of surface proteins possessed by S. aureus poses interesting questions concerning their functions. This organism inhabits a variety of environments even within the same host, and thus there may be a requirement for more than one function for a protein; indeed, the spectrum of ligands bound by some surface proteins continues to widen (for reviews, see references 19 and 26). IsdA is known to be expressed upon association with the host, most significantly in the nares. In Streptococcus pneumoniae, another upper-airway commensal also able to cause life-threatening disease, the capacity of surface protein PspA to bind Lf has been linked to its ability to inhibit killing by this protein (31, 32, 56). Thus, binding of Lf and subsequent inhibition of its proteolytic activity may represent a common mechanism in pathogenic bacteria. Interestingly, antibodies to both IsdA and PspA have been shown to abrogate their inhibition of Lf killing (56; this study). It is already known that increased titers of anti-IsdA and -PspA antibodies are associated with decreased rates of carriage of both organisms (18, 45). Thus, such acquired immune responses mounted to resistance factors like IsdA and PspA may represent a mechanism for control of bacterial carriage.
Acknowledgments
This work was funded by Biosynexus Inc. (S.R.C.).
We thank Olaf Schneewind, University of Chicago, for providing the srtA mutant.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 28 January 2008.
REFERENCES
- 1.Aguila, A., A. G. Herrera, D. Morrison, B. Cosgrove, A. Perojo, I. Montesinos, J. Perez, G. Sierra, C. G. Gemmell, and J. H. Brock. 2001. Bacteriostatic activity of human lactoferrin against Staphylococcus aureus is a function of its iron-binding properties and is not influenced by antibiotic resistance. FEMS Immunol. Med. Microbiol. 31145-152. [DOI] [PubMed] [Google Scholar]
- 2.Ajello, M., R. Greco, F. Giansanti, M. T. Massucci, G. Antonini, and P. Valenti. 2002. Anti-invasive activity of bovine lactoferrin towards group A streptococci. Biochem. Cell Biol. 80119-124. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, B. Y., H. M. Baker, E. J. Dodson, G. E. Norris, S. V. Rumball, J. M. Waters, and E. N. Baker. 1987. Structure of human lactoferrin at 3.2-Å resolution. Proc. Natl. Acad. Sci. USA 841769-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold, R. R., M. Brewer, and J. J. Gauthier. 1980. Bactericidal activity of human lactoferrin: sensitivity to a variety of microorganisms. Infect. Immun. 28893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold, R. R., J. E. Russell, W. J. Champion, and J. J. Gauthier. 1981. Bactericidal activity of human lactoferrin: influence of physical conditions and metabolic state of the target microorganism. Infect. Immun. 32655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold, R. R., J. E. Russell, W. J. Champion, M. Brewer, and J. J. Gauthier. 1982. Bactericidal activity of human lactoferrin: differentiation from the stasis of iron deprivation. Infect. Immun. 35792-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker, E. N., B. F. Anderson, H. M. Baker, C. L. Day, M. Haridas, G. E. Norris, S. V. Rumball, C. A. Smith, and D. H. Thomas. 1994. Three-dimensional structure of lactoferrin in various functional states. Adv. Exp. Med. Biol. 3571-12. [DOI] [PubMed] [Google Scholar]
- 8.Baker, E. N., and H. M. Baker. 2005. Molecular structure, binding properties and dynamics of lactoferrin. Cell. Mol. Life Sci. 622531-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baveye, S., E. Elass, J. Mazurier, G. Spik, and D. Legrand. 1999. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin. Chem. Lab. Med. 37281-286. [DOI] [PubMed] [Google Scholar]
- 10.Bellamy, W., M. Takase, K. Yamauchi, H. Wakabayashi, K. Kawase, and M. Tomita. 1992. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1121130-136. [DOI] [PubMed] [Google Scholar]
- 11.Bellamy, W., M. Takase, H. Wakabayashi, K. Kawase, and M. Tomita. 1992. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 73472-479. [DOI] [PubMed] [Google Scholar]
- 12.Bera, A., S. Herbert, A. Jakob, W. Vollmer, and F. Götz. 2005. Why are pathogenic staphylococci so lysozyme resistant? The peptidoglycan O-acetyltransferase OatA is the major determinant for lysozyme resistance of Staphylococcus aureus. Mol. Microbiol. 55778-787. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein, S. E. 1987. Hereditary hypotransferrinemia with hemosiderosis, a murine disorder resembling human atransferrinemia. J. Lab. Clin. Med. 110281-286. [PubMed] [Google Scholar]
- 14.Bhimani, R. S., Y. Vendrov, and P. Furmanski. 1999. Influence of lactoferrin feeding and injection against systemic staphylococcal infections in mice. J. Appl. Microbiol. 86135-144. [DOI] [PubMed] [Google Scholar]
- 15.Brock, J. 1995. Lactoferrin: a multifunctional immunoregulatory protein? Immunol. Today 16417-419. [DOI] [PubMed] [Google Scholar]
- 16.Clarke, S. R., L. G. Harris, R. G. Richards, and S. J. Foster. 2002. Analysis of Ebh, a 1.1-megadalton cell wall-associated fibronectin-binding protein of Staphylococcus aureus. Infect. Immun. 706680-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke, S. R., M. D. Wiltshire, and S. J. Foster. 2004. IsdA of Staphylococcus aureus is a broad spectrum, iron-regulated adhesin. Mol. Microbiol. 511509-1519. [DOI] [PubMed] [Google Scholar]
- 18.Clarke, S. R., K. J. Brummell, M. J. Horsburgh, P. W. McDowell, S. A. Syed Mohamad, M. R. Stapleton, J. Acevedo, R. C. Read, N. P. J. Day, S. J. Peacock, J. J. Mond, J. F. Kokai-Kun, and S. J. Foster. 2006. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J. Infect. Dis. 1931098-1108. [DOI] [PubMed] [Google Scholar]
- 19.Clarke, S. R., and S. J. Foster. 2006. Surface adhesins of Staphylococcus aureus. Adv. Microb. Physiol. 51187-224. [DOI] [PubMed] [Google Scholar]
- 20.Clarke, S. R., R. Mohamed, L. Bian, A. F. Routh, J. F. Kokai-Kun, J. J. Mond, A. Tarkowski, and S. J. Foster. 2007. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe 1199-212. [DOI] [PubMed] [Google Scholar]
- 21.Cole, A. M., P. Dewan, and T. Ganz. 1999. Innate antimicrobial activity of nasal secretions. Infect. Immun. 673267-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole, A. M., H.-I. Liao, O. Stuchlik, J. Tilan, J. Pohl, and T. Ganz. 2002. Cationic polypeptides are required for antibacterial activity of human airway fluid. J. Immunol. 1696985-6991. [DOI] [PubMed] [Google Scholar]
- 23.Dryla, A., D. Gelbmann, A. von Gabain, and E. Nagy. 2003. Identification of a novel iron regulated staphylococcal surface protein with haptoglobin-haemoglobin binding activity. Mol. Microbiol. 4937-53. [DOI] [PubMed] [Google Scholar]
- 24.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 695-107. [DOI] [PubMed] [Google Scholar]
- 25.Farnaud, S., and R. W. Evans. 2003. Lactoferrin—a multifunctional protein with antimicrobial properties. Mol. Immunol. 40395-405. [DOI] [PubMed] [Google Scholar]
- 26.Foster, T. J. 2002. Surface protein adhesins of staphylococci, p. 3-26. In M. Wilson (ed.), Bacterial adhesion to host tissues: mechanisms and consequences. Cambridge University Press, Cambridge, United Kingdom.
- 27.Gomez, H. F., T. J. Ochoa, L. G. Carlin, and T. G. Cleary. 2003. Human lactoferrin impairs virulence of Shigella flexneri. J. Infect. Dis. 18787-95. [DOI] [PubMed] [Google Scholar]
- 28.Grigg, J. C., C. L. Vermeiren., D. E. Heinrichs, and M. E. P. Murphy. 2007. Haem recognition by a Staphylococcus aureus NEAT domain. Mol. Microbiol. 63139-149. [DOI] [PubMed] [Google Scholar]
- 29.Grossmann, J. G., M. Neu, E. Pantos, F. J. Schwab, R. W. Evans, E. Townes-Andrews, H. Appel, W. G. Theis, and S. S. Hasnain. 1992. X-ray solution scattering reveals conformational changes upon iron uptake in lactoferrin, serum and ovo-transferrins. J. Mol. Biol. 225811-819. [DOI] [PubMed] [Google Scholar]
- 30.Guillén, C., I. B. McInnes, D. M. Vaughan, S. Kommajosyula, P. H. C. van Berkel, B. P. Leung, A. Aguilla, and J. H. Brock. 2002. Enhanced Th1 response to Staphylococcus aureus infection in human lactoferrin-transgenic mice. J. Immunol. 1683950-3957. [DOI] [PubMed] [Google Scholar]
- 31.Håkansson, A., H. Roche, S. Mizra, L. S. McDaniel, A. Brooks-Walter, and D. E. Briles. 2001. Characterization of binding human lactoferrin to pneumococcal surface protein A. Infect. Immun. 693372-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammerschmidt, S., G. Bethe, P. H. Remane, and G. S. Chhatwal. 1999. Identification of pneumococcal surface protein A as a lactoferrin binding protein of Streptococcus pneumoniae. Infect. Immun. 671683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendrixson, D. R., J. Qiu, S. C. Shewry, D. L. Fink, S. Petty, E. N. Baker, A. G. Plaut, and J. W. St. Geme III. 2003. Human milk lactoferrin is a serine protease that cleaves Haemophilus surface proteins at arginine-rich sites. 2003. Mol. Microbiol. 47607-617. [DOI] [PubMed] [Google Scholar]
- 34.Horsburgh, M. J., E. Ingham, and S. J. Foster. 2001. In Staphylococcus aureus, Fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J. Bacteriol. 183468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hovanessian, A. G., and Z. L. Awdeh. 1976. Gel isoelectric focussing of human serum transferrin. Eur. J. Biochem. 68333-338. [DOI] [PubMed] [Google Scholar]
- 36.Iyer, S., and B. Lonnerdal. 1993. Lactoferrin, lactoferrin receptors and iron metabolism. J. Clin. Nutr. 47232-241. [PubMed] [Google Scholar]
- 37.Leonard, F. C., and B. K. Markey. 2008. Methicillin-resistant Staphylococcus aureus in animals: a review. Vet. J. 17527-36. [DOI] [PubMed] [Google Scholar]
- 38.Levy, J. E., O. Jin, Y. Fujikaway, F. Kuo, and C. Andrews. 1999. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat. Genet. 21396-399. [DOI] [PubMed] [Google Scholar]
- 39.Levy, P. F., and M. Viljoen. 1995. Lactoferrin: a general review. Haemotologica 80252-267. [PubMed] [Google Scholar]
- 40.Ling, J. M. L., and A. B. Schryvers. 2006. Perspectives on interactions between lactoferrin and bacteria. Biochem. Cell Biol. 84275-281. [DOI] [PubMed] [Google Scholar]
- 41.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339520-532. [DOI] [PubMed] [Google Scholar]
- 42.Massucci, M. T., F. Giansanti, G. Di Nino, M. Turacchio, M. F. Giardi, D. Botti, R. Ippoliti, B. De Giulio, R. Siciliano, G. Donnarumma, P. Valenti, A. Bocedi, F. Polticelli, P. Ascenzi, P. Ascenzi, and G. Antonini. 2004. Proteolytic activity of bovine lactoferrin. Biometals 17249-255. [DOI] [PubMed] [Google Scholar]
- 43.Mazmanian, S. K., H. Ton-That, K. Su, and O. Schneewind. 2002. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. USA 992293-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazmanian, S. K., E. P. Skaar, A. H. Gasper, M. Humayan, P. Gorniki, J. Jelenska, A. Joachmiak, D. M. Missiakas, and O. Schneewind. 2003. Passage of heme iron across the envelope of Staphylococcus aureus. Science 299906-909. [DOI] [PubMed] [Google Scholar]
- 45.McCool, T. L., T. R. Cate, G. Moy, and J. N. Weiser. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metz-Boutigue, M-H., J. Jollès, J. Mazurier, F. Schoentgen, D. Legrand, G. Spik, J. Montreuil, and P. Jollès. 1984. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur. J. Biochem. 145659-676. [DOI] [PubMed] [Google Scholar]
- 47.Moguilevsky, N., L. A. Retegui, and P. L. Masson. 1985. Comparison of human lactoferrins from milk and neutrophilic leucocytes. Relative molecular mass, isoelectric point, iron-binding properties and uptake by the liver. Biochem. J. 229353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrissey, J. A., A. Cockayne, J. Hammacott, K. Bishop, A. Denman-Johnson, P. J. Hill, and P. Williams. 2002. Conservation, surface exposure, and in vivo expression of the Frp family of iron-regulated cell wall proteins in Staphylococcus aureus. Infect. Immun. 732399-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nuijens, J. H., P. H. Van Berkel, and F. L. Schanbacher. 1996. Structure and biological actions of lactoferrin. J. Mammary Gland Biol. Neoplasia 1285-295. [DOI] [PubMed] [Google Scholar]
- 50.Ochoa, T. J., M. Noguera-Obanza, F. Ebel, C. A. Guzman, H. F. Gomez, and T. G. Cleary. 2003. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect. Immun. 715149-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park, R.-Y., H.-Y. Sun, M.-H. Choi, Y.-H. Bai, and S. H. Shin. 2005. Staphylococcus aureus siderophore-mediated iron-acquisition system plays a dominant and essential role in the utilization of transferrin-bound iron. J. Microbiol. 43183-190. [PubMed] [Google Scholar]
- 52.Qiu, J., D. R. Hendrixson, E. N. Baker, T. F. Murphy, J. W. St. Geme III, and A. G. Plaut. 1998. Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc. Natl. Acad. Sci. USA 9512641-12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossi, P., F. Giansanti, A. Boffi, M. Ajello, P. Valenti, E. Chiancone, and G. Antonini. 2002. Ca2+-binding to bovine lactoferrin enhances protein stability and influences the release of bacterial lipopolysaccharide. Biochem. Cell Biol. 8041-48. [DOI] [PubMed] [Google Scholar]
- 54.Saito, H., H. Miyakawa, Y. Tamura, S. Shimamura, and M. Tomita. 1991. Potent bactericidal activity of bovine lactoferrin hydrolysate produced by heat treatment at acidic pH. J. Dairy Sci. 743724-3730. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez, L., M. Calvo, and J. H. Brock. 1992. Biological role of lactoferrin. Arch. Dis. Child. 67657-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaper, M., S. K. Hollingshead, W. H. Benjamin, and D. E. Briles. 2004. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin. Infect. Immun. 725031-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi, Y., W. Kong, and K. Nakayama. 2000. Human lactoferrin binds and removes the haemoglobin receptor protein of the periodontopathogen Porphyromonas gingivalis. J. Biol. Chem. 27530002-30008. [DOI] [PubMed] [Google Scholar]
- 58.Singh, P. K., M. R. Parsek, E. P. Greenberg, and M. J. Walsh. 2002. A component of innate immunity prevents bacterial biofilm development. Nature 417552-555. [DOI] [PubMed] [Google Scholar]
- 59.Sullivan, M. A., R. E. Yasbin, and F. E. Young. 1984. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene 2921-26. [DOI] [PubMed] [Google Scholar]
- 60.Taylor, J. M., and D. E. Heinrichs. 2002. Transferrin binding in Staphylococcus aureus: involvement of a cell wall-anchored protein. Mol. Microbiol. 431603-1614. [DOI] [PubMed] [Google Scholar]
- 61.van Renswoude, J., K. R. Bridges, J. B. Harford, and R. D. Klausner. 1982. Receptor-mediated endocytosis of transferrin and the uptake of Fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc. Natl. Acad. Sci. USA 796186-6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Veen, H. A., M. E. J. Geerts, P. H. C. van Berkel, and J. H. Nuijens. 2004. The role of N-linked glycosylation in the protection of human and bovine lactoferrin against tryptic proteolysis. Eur. J. Biochem. 271678-684. [DOI] [PubMed] [Google Scholar]
- 63.Vermeiren, C. L., M. Pluym, J. Mack, D. E. Heinrichs, and M. J. Stillman. 2006. Characterization of the heme binding properties of Staphylococcus aureus IsdA. Biochemistry 4512867-12875. [DOI] [PubMed] [Google Scholar]
- 64.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344505-509. [DOI] [PubMed] [Google Scholar]
- 65.Waldvogel, F. A. 1995. Staphylococcus aureus (including toxic shock syndrome), p. 1754-1777. In G. L. Mandell, J. E. Bennett, and R. Dolio (ed.), Principles and practice of infectious diseases. Churchill Livingstone, New York, NY.
- 66.Ward, P. P., S. Uribe-Luna, and O. M. Conneely. 2002. Lactoferrin and host defence. Biochem. Cell Biol. 8095-102. [DOI] [PubMed] [Google Scholar]
- 67.Ward, P. P., M. Mendoza-Meneses, G. A. Cunningham, and O. M. Conneely. 2003. Iron status in mice carrying a targeted disruption of lactoferrin. Mol. Cell. Biol. 23178-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ward, P. P., E. Paz, and O. M. Conneely. 2005. Multifunctional roles of lactoferrin: a critical overview. Cell. Mol. Life Sci. 622540-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weinberg, E. D. 2001. Human lactoferrin: a novel therapeutic with broad spectrum potential. J. Pharm. Pharmacol. 531303-1310. [DOI] [PubMed] [Google Scholar]
- 70.Weinstein, H. J. 1959. The relationship between nasal staphylococcal carriage state and the incidence of post-operative complications. N. Engl. J. Med. 2601303-1308. [DOI] [PubMed] [Google Scholar]
- 71.Wellnitz, O., and D. E. Kerr. 2004. Cryopreserved bovine mammary cells to model epithelial response to infection. Vet. Immunol. Immunopathol. 101191-202. [DOI] [PubMed] [Google Scholar]
- 72.Wiltshire, M. D., and S. J. Foster. 2001. Identification and analysis of Staphylococcus aureus components expressed by a model system of growth in serum. Infect. Immun. 695198-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu, V. L., A. Goetz, M. Wagener, P. B. Smith, J. D. Rihs, J. Hanchett, and J. J. Zuravleff. 1986. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N. Engl. J. Med. 31591-96. [DOI] [PubMed] [Google Scholar]
- 74.Zheng, J., J. L. Ather, T. S. Sonstegard, and D. E. Kerr. 2005. Characterization of the infection-responsive bovine lactoferrin promoter. Gene 353107-117. [DOI] [PubMed] [Google Scholar]