Abstract
Pneumolysin is a pore-forming cytolysin known as a major virulence determinant of Streptococcus pneumoniae. This protein toxin has also been shown to activate the Toll-like receptor 4 (TLR4) signaling pathway. In this study, a mutant S. pneumoniae strain deficient in pneumolysin (Δply) and a recombinant pneumolysin protein (rPLY) were constructed. Upon infection of macrophages in vitro, the ability to induce the production of interleukin-1α (IL-1α), IL-1β, and IL-18 was severely impaired in the Δply mutant, whereas there was no marked difference in the induction of tumor necrosis factor alpha (TNF-α) and IL-12p40 between the wild type and the Δply mutant of S. pneumoniae. When macrophages were stimulated with rPLY, the production of IL-1α, IL-1β, and IL-18 was strongly induced in a TLR4-dependent manner, whereas lipopolysaccharide, a canonical TLR4 agonist, hardly induced these cytokines. In contrast, lipopolysaccharide was more potent than rPLY in inducing the production of TNF-α, IL-6, and IL-12p40, the cytokines requiring no caspase activation. Activation of caspase-1 was observed in macrophages stimulated with rPLY but not in those stimulated with lipopolysaccharide, and the level of activation was higher in macrophages infected with wild-type S. pneumoniae than in those infected with the Δply mutant. These results clearly indicate that pneumolysin plays a key role in the host response to S. pneumoniae, particularly in the induction of caspase-1-dependent cytokines.
Streptococcus pneumoniae is a gram-positive bacterium that causes bacterial pneumonia, otitis media, bacterial meningitis, and septicemia (32). Due to the severe disease burden and mortality, the emergence of drug-resistant clinical isolates (14, 36), the lack of a universally effective vaccine (17, 39), and an increase in the number of immunocompromised patients, it is increasingly important to understand the pathogenic processes of pneumococcal disease in order to develop novel therapeutic modalities and an effective vaccine.
Pneumolysin (PLY), a 53-kDa protein toxin produced by virtually all clinical isolates of S. pneumoniae, has been regarded as a key virulence factor of this bacterium (7) and as one of the candidates for vaccine development against pneumococcal infection (21). PLY is one of the cholesterol-dependent cytolysins (24) that are known to form ring- or arc-shaped pores on cholesterol-containing membranes and whose activity is blocked by free cholesterol (40, 50). PLY is a multifunctional protein toxin that causes cytolysis and induces complement activation and the production of cytokines and nitric oxide (5, 9, 18, 33, 43). However, the mechanisms by which PLY affects the host defense are only partly understood.
In mammals, the sensing of microbial components by innate immune cells, such as macrophages and dendritic cells, is initiated by the recognition of conserved and unique pathogen-derived structures via pattern recognition molecules, such as Toll-like receptors (TLRs) (31). TLRs, transmembrane proteins belonging to the Toll/interleukin-1 (Toll/IL-1) receptor family (16), mediate host immune responses by inducing proinflammatory cytokines and costimulatory molecules through the activation of nuclear factor kappa B (NF-κB) and mitogen-activated protein kinases (MAPKs) after recognition of specific agonists (19). Among several TLRs, TLR4 plays a critical role in the recognition of lipopolysaccharide (LPS), a major component of the outer membranes of gram-negative bacteria, in cooperation with LPS-binding protein, CD14, and MD2 (1, 25, 51, 53), and plays a critical role in the host defense against gram-negative pathogens (8, 37). Several recent studies have shown that the association of TLR4 with PLY (and other cholesterol-dependent cytolysins) initiates an intracellular signaling cascade resulting in the activation of NF-κB (20, 27, 41, 49, 51). For pneumococcal infection, Malley et al. (27) reported an important role of TLR4 in the host defense with the finding that C3H/HeJ mice, which display mutant nonfunctional TLR4, were more susceptible than wild-type (wt) mice to invasive disease after pneumococcal colonization in the nasopharynx. Moreover, a protective role of TLR4 in the pneumococcal pneumonia model was also reported (8). Thus, it appears that PLY, a TLR4 agonist in this gram-positive pathogen (27), is involved in the induction of a host protective response against S. pneumoniae.
Several reports have shown that proinflammatory cytokines, such as IL-1, tumor necrosis factor alpha (TNF-α), IL-6, and IL-18, play protective roles against pneumococcal infection (22, 26, 48, 54, 55). Moreover, gamma interferon has been demonstrated to protect mice against infection with S. pneumoniae by promoting the accumulation of neutrophils in the infected lung (34). In addition, exogenous administration of IL-12 improved the innate defense against S. pneumoniae in the lung by inducing gamma interferon production (23). However, the involvement of PLY in the production of various cytokines induced in pneumococcal infections has yet to be clarified.
In the present study, we constructed an in-frame deletion mutant with a mutation in the S. pneumoniae ply gene and a recombinant protein of PLY to analyze the precise role for PLY in the host cytokine response to S. pneumoniae. Using an in vitro model of infection, we compared the levels of various proinflammatory cytokines secreted from macrophages, the cells comprising the front line of host defense and the innate immune response, after infection with the wt or ply-deficient mutant strain of S. pneumoniae. This study revealed a unique function of PLY in the induction of caspase-1-dependent cytokine production that could not be observed with LPS, a canonical TLR4 ligand.
MATERIALS AND METHODS
Experimental animals.
wt female C57BL/6 mice were purchased from Japan SLC (Shizuoka, Japan). C57BL/6 background TLR4 gene knockout (TLR4 KO) mice were purchased from Oriental Bioservices (Kyoto, Japan) and maintained in a specific-pathogen-free environment for use at 7 to 8 weeks of age. All animal experiments were approved by the Animal Ethics and Research Committee of Kyoto University Graduate School of Medicine.
Bacterial strains and growth conditions.
A serotype 2 strain of Streptococcus pneumoniae D39 was purchased from the National Collection of Type Cultures (NCTC 7466; Central Public Health Laboratory, London, United Kingdom). S. pneumoniae was grown on tryptic soy agar (Difco Laboratories, Detroit, MI) with 5% (vol/vol) defibrinated sheep blood (Nacalai Tesque, Kyoto, Japan) and in Todd-Hewitt broth (Difco) supplemented with 0.5% yeast extract (THY) at 37°C and 5% CO2 and subsequently stored at −80°C in THY plus 10% glycerol. For the preparation of bacterial stocks for macrophage stimulation, pneumococci were grown overnight on blood agar plates at 37°C and 5% CO2. Colonies were inoculated into the THY medium, grown until mid-logarithmic phase (optical density at 600 nm [OD600] = 0.5), and centrifuged at 6,000 × g for 15 min. The bacterial pellet was suspended in phosphate-buffered saline (PBS) and stocked at −80°C. The concentration was determined by viable cell counting on blood agar plates.
Construction of ply deletion mutant.
A deletion mutant of S. pneumoniae D39 for the PLY gene (ply) was constructed by using homologous recombination-based allelic exchange. To generate the ply deletion, the upstream (733 bp) and downstream (692 bp) flanking regions of ply were PCR amplified from D39 genomic DNA, using the primer sets P1/P2 and P3/P4, respectively (primer sequences are given in Table 1). Primers P1 and P4 carried one BamHI site, and P2 and P3 carried HindIII sites in their 5′ ends. Amplified fragments were digested with HindIII and ligated. The resulting fusion gene product was amplified by PCR using primers P1 and P4, digested with BamHI, and then ligated with BamHI-digested vector DNA (pTN-E18EM) (Ampr Emr). Plasmid pTN-E18EM is a pUC18-derived vector carrying ampicillin and erythromycin resistance genes and the multiple cloning site of pUC18. The erythromycin resistance gene (emrC) was amplified from plasmid pE194 by PCR and inserted into pUC18.
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Nucleotide sequence (5′-3′) |
|---|---|
| IL-1α-F | CTCTAGAGCACCATGCTACAGAC |
| IL-1α-R | TGGAATCCAG-GGGAAACACTG |
| IL-1β-F | AAGCTCTCCACCTCAATGGACAG |
| IL-1β-R | CTCAAACTCCAC-TTTGCTCTTGA |
| IL-18-F | ACTGTACAACCGCAGTAATACGG |
| IL-18-R | AGTGAACATTACAGATTTATCCC |
| TNF-α-F | GGCAGGTCTACTTTGGAGTCATTGC |
| TNF-α-R | ACATTCGAGGCTCCAGTGAATTCCA |
| IL-6-F | GAGGATACCACTCCCAACAGACC |
| IL-6-R | AAGTGCATCATCGTTGTTCATACA |
| IL-12p40-F | TCCGGAGTAATTTGGTGCTTCACA |
| IL-12p40-R | GCAAGAGACACAGTCCTGGG |
| GAPDH-F | TGCCCAGAACATCATCCCTG |
| GAPDH-R | AACACGGAAGGCCATGCCAG |
| PLY-F | CGATGGATCCTATGGCAAATAAAGCAGTAA |
| PLY-R | ACGCGGTACCCTAGTCATTTTTCTACCTTAT |
| P1 | ACACGGATCCTCAACAGGCACTCATCCACA |
| P2 | GCGCAAGCTTGGAGAATGCTTGCGACAAAA |
| P3 | GCGCAAGCTTAAATCAGCCGTGGTTGGACT |
| P4 | ACACGGATCCCGCAAAGCCCTTTTTCTAGC |
Transformation into S. pneumoniae and selection of ply deletion mutant.
To carry out the transformation of the recombinant plasmid, frozen stocks of S. pneumoniae were thawed and diluted 1:20 in competence medium (tryptic soy broth [Difco], pH 8.0, 10% glycerol, 0.16% bovine serum albumin, 0.01% CaCl2) containing competence-stimulating peptide 1 (100 ng/ml; Invitrogen, Carlsbad, CA). S. pneumoniae D39 was preincubated for 20 min at 37°C and 5% CO2 and then incubated for 1 h with approximately 1 μg of DNA. The cells were plated on blood agar containing erythromycin, and transformants were obtained. For the selection of the ply deletion mutant, transformants were grown in THY medium without antibiotics and plated on blood agar without antibiotics, and then colonies were plated on replica plates with or without erythromycin. Erythromycin-sensitive colonies were selected, and ply-negative mutants were confirmed by PCR to have an absence of ply, using primers PLY-F and PLY-R (Table 1), and the presence of the upstream and downstream sequences of the ply gene. The absence of PLY in the ply deletion mutant was verified by Western blotting using a monoclonal antibody against PLY (NovoCastra Laboratories Ltd., Newcastle upon Tyne, United Kingdom).
Production and purification of rPLY.
Full-length recombinant PLY (rPLY) was prepared as described previously (4). Briefly, the ply gene was cloned into the pQE-31 vector (Qiagen, Hilden, Germany), and the recombinant vector was transformed into Escherichia coli SG13009 (Qiagen) harboring a pREP4 plasmid, which contains lacI and kanamycin resistance genes. rPLY was produced in E. coli cells as a six-His-tagged protein by incubation of the transformants with 2 mM isopropyl-β-d-thiogalactopyranoside (Nacalai Tesque) at 25°C for 6 h. The E. coli cells were then harvested by centrifugation, incubated with lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, 1 mg/ml lysozyme, 200 U DNase I, pH 8.0), and disrupted by vortexing with 0.1-mm zirconia-silica beads (Bio-Spec Products, Inc., Bartlesville, OK). rPLY was then purified from the soluble fraction by use of a nickel-nitrilotriacetic acid column (Qiagen) under native conditions according to the manufacturer's instructions. Contaminating LPS was extensively removed using a Detoxi-Gel endotoxin-removing gel (Pierce Chemical Co., Rockford, IL). The level of LPS in the rPLY preparation was determined by the Limulus Color KY test (Wako Pure Chemical Industries, Osaka, Japan) and was found to be <0.4 pg/ml when the preparation was suspended in PBS at a protein concentration of 1 μg/ml. The purity was analyzed by Coomassie brilliant blue staining and immunoblotting using an anti-His-tag monoclonal antibody (penta-His antibody; Qiagen) after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
To inhibit its cytolytic activity, rPLY was treated with 20 μg/ml of cholesterol for 30 min on ice (35). Heat-treated PLY and LPS were prepared by heating the aqueous stock suspensions, in 50% glycerol-water (vol/vol) for PLY and in PBS for LPS, in a boiling water bath for 60 min (27).
Isolation and stimulation of peritoneal macrophages.
Peritoneal exudate cells were collected from C57BL/6 wt and TLR4 KO mice 3 days after an intraperitoneal injection of 4% thioglycolate medium (Eiken Chemical, Tokyo, Japan). Cells were cultured in 48-well plates (2 × 105 cells per well) in medium consisting of RPMI 1640 (Gibco-BRL Life Technologies, Rockville, MD) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Gibco) for 2 h at 37°C and 5% CO2. After removal of nonadherent cells, the adherent cells were used as peritoneal macrophages and infected with wt S. pneumoniae and its isogenic ply deletion mutant (Δply) at a multiplicity of infection (MOI) of 10 in gentamicin-free medium. To inhibit the growth of bacteria in the medium, 100 μg/ml of gentamicin (Gibco) was added to the cultures 8 h after infection, when the largest number of bacteria were associated with cells, and the cells were incubated for an additional 16 h. Supernatants were collected and stored at −80°C until they were assayed for cytokines. Similarly, peritoneal macrophages were stimulated with rPLY, LPS (from E. coli O55:B5; Sigma-Aldrich, St. Louis, MO), or Pam3CSK4 (Invivogen, San Diego, CA). In some experiments, polymyxin B (PMB; Nacalai Tesque), z-VAD-fmk (Peptide Institute, Osaka, Japan), and z-YVAD-cmk (R&D Systems, Minneapolis, MN) were added to the cultures 30 min before stimulation with rPLY or LPS.
Reverse transcription-PCR.
Total cellular RNA was extracted from peritoneal macrophages by using Nucleospin RNA II (Macherey-Nagel, Düren, Germany). Total RNA (0.2 μg) was treated with RNase-free DNase (Promega, Madison, WI) to eliminate contaminating DNA and then subjected to reverse transcription using random primers (Invitrogen) and ReverTra Ace (Toyobo, Osaka, Japan). PCR was performed using KOD-Plus DNA polymerase (Toyobo) under the following PCR conditions: 94°C for 15 s, 60°C for 30 s, and 68°C for 30 s. The reaction was extended by incubation at 68°C for 7 min. The samples were amplified for 28 to 30 cycles. The most appropriate number of amplification cycles for each cytokine was determined by preliminary experiments. PCR products were analyzed in 2% agarose gels. Primer sequences used for the amplification of specific genes by reverse transcription-PCR are shown in Table 1.
Cytokine measurement.
Levels of secreted cytokines in culture medium were determined by two-site sandwich enzyme-linked immunosorbent assay (ELISA). ELISA kits for IL-1α and IL-1β were purchased from BD Biosciences (San Diego, CA). IL-6, TNF-α, and IL-12p40 kits were obtained from eBioscience (San Diego, CA), and an IL-18 kit was obtained from MBL (Nagoya, Japan). All samples were assayed according to the respective manufacturer's instructions.
Western blot analysis.
For Western blotting, cells were lysed in 2× SDS-PAGE sample buffer (62.5 mM Tris-HCl, 2% SDS, 10% glycerol, 50 mM dithiothreitol, 0.1% bromophenol blue) and subjected to brief ultrasonication and boiling for 5 min. The lysates were resolved by SDS-PAGE and then transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). Antibodies specific for mouse IκBα, p38 MAPK, phospho-p38 MAPK (Thr180/Tyr182), and phospho-ERK1/ERK2 (Thr202/Tyr204) were purchased from Cell Signaling Technology (Danvers, MA) and were used as recommended by the manufacturer.
Caspase-1 activation assay.
To determine the activation of caspase-1, peritoneal exudate cells were seeded in 24-well tissue culture plates at 5 × 105 cells per well. After removal of nonadherent cells, the adherent cells were incubated with 30 μM biotinylated YVAD-cmk (Alexis Biochemicals, San Diego, CA) for 1 h. The cells were then either left unstimulated or stimulated with rPLY or LPS for 3 h at different concentrations. In the case of LPS plus ATP stimulation, cells were primed with LPS for 2.5 h and subsequently stimulated with 1 mM ATP (Amersham) for 30 min. Similarly, peritoneal macrophages were infected with wt S. pneumoniae and the Δply mutant at an MOI of 10 for 3 or 6 h. Cells were then washed with PBS three times and lysed with 1 ml lysis buffer consisting of 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 10% glycerol, 1% Triton X-100, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 5 μg/ml pepstatin, and 1 mM phenylmethylsulfonyl fluoride. Lysates were centrifuged at 12,000 ×g for 1 min to remove cell debris. To adjust the amount of lysate recovered from each well, 10 μl of cleared lysate was subjected to Western blotting using a specific antibody for β-actin (Sigma), and the rest of the lysate was used for pull-down assay for detecting the active form of caspase-1. Activated caspase-1 (bound to biotinylated YVAD-cmk) was concentrated with tetrameric avidin resin (Promega) and detected by Western blotting using anti-caspase-1 polyclonal antibody (MBL).
Statistical analysis.
For comparisons between two groups, the Mann-Whitney U test was used, and statistical significance was determined as a P value of <0.05. Multigroup comparisons of mean values were conducted by the Kruskal-Wallis test and the Games-Howell post hoc test (P < 0.05) after the confirmation of homogeneity of variances among the groups by using Bartlett's test.
RESULTS
Role of PLY in cytokine production by macrophages infected with S. pneumoniae in vitro.
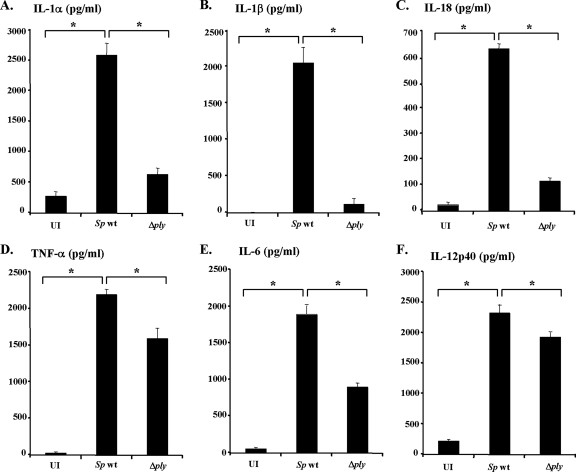
To evaluate the involvement of PLY in inducing proinflammatory cytokines in response to S. pneumoniae, peritoneal macrophages from C57BL/6 mice were infected with live wt S. pneumoniae or the Δply mutant, and the levels of cytokines in supernatants were assayed. wt S. pneumoniae potently induced IL-1α, IL-1β, and IL-18, whereas the Δply mutant was unable to induce these cytokines, indicating a critical role of PLY in inducing IL-1α, IL-1β, and IL-18. In contrast, no marked difference was observed between the two strains in the ability to induce TNF-α, IL-6, and IL-12p40, suggesting a minor role of PLY in induction of these cytokines (Fig. 1). The impaired ability of the Δply mutant to induce cytokine production was not due to any enhanced cytotoxicity, as the release of lactate dehydrogenase from macrophages infected with the mutant never exceeded the level induced by wt S. pneumoniae (data not shown).
FIG. 1.
Pneumolysin is essential for the secretion of IL-1α, IL-1β, and IL-18 in response to Streptococcus pneumoniae. Peritoneal macrophages were left uninfected (UI) or infected with wt S. pneumoniae (Sp wt) or the ply mutant (Δply) at a macrophage/bacterium ratio of 1:10 for 8 h. Cells were cultured for an additional 16 h in the presence of gentamicin (100 μg/ml), and culture supernatants were then collected. The amounts of IL-1α (A), IL-1β (B), IL-18 (C), TNF-α (D), IL-6 (E), and IL-12p40 (F) were determined using ELISAs specific for each cytokine. The results are representative of three similar experiments. The data are the means ± standard deviations for three determinations. *, P < 0.05 for uninfected cells compared to wt S. pneumoniae-infected cells and for wt S. pneumoniae-infected cells compared to Δply mutant-infected cells.
PLY strongly induces IL-1α, IL-1β, and IL-18.
To confirm whether the ply gene product was responsible for the difference in cytokine induction between the ply-positive wt and ply-deficient mutant strains, we next examined the profiles of various cytokines induced by rPLY stimulation. Although it was difficult to determine the exact amount of PLY released from wt S. pneumoniae in our in vitro infection system, we tried to estimate the amount based on a previous report on the relationship between bacterial number and the amount of PLY protein (18). The amount of PLY released from the infection dose of wt S. pneumoniae used in this study was calculated to be 0.04 μg/ml. In a study by Malley et al. (27), rPLY was used at concentrations of 0.1 μg/ml to 10 μg/ml to determine TLR4-dependent cytokine-inducing activity. Taking these findings into consideration, we used rPLY in the present study at cytolytic (1 μg/ml) and sublytic (0.1 μg/ml) concentrations, which caused 53.0% and 5.1% release of lactate dehydrogenase from macrophages, respectively. Both doses of rPLY exhibited a stronger activity to induce IL-1α, IL-1β, and IL-18 than did LPS (Fig. 2) and TLR2 ligands, such as Pam3CSK4 (data not shown). In contrast, the ability of rPLY to induce IL-6, IL-12p40, and TNF-α was significantly lower than that of LPS. A treatment of 1 μg/ml or 0.1 μg/ml of rPLY with cholesterol resulted in the reduction of the cytolytic activity determined by lactate dehydrogenase release assay, by 95.3% and 100%, respectively. However, the cytokine-inducing activity was not affected by cholesterol pretreatment, even after the abolishment of cytolytic activity. Both cholesterol-treated rPLY and untreated rPLY induced similar levels of all cytokines tested (Fig. 2), indicating that cytokine-inducing activity and cytolytic activity of PLY may be dissociated. The absence of any stimulating or toxic activity was confirmed in the medium containing cholesterol alone.
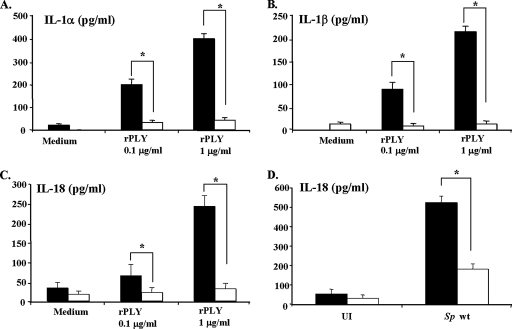
FIG. 2.
Pneumolysin is a strong inducer of IL-1α, IL-1β, and IL-18 production. Peritoneal macrophages were left unstimulated (M), treated with cholesterol only (C), or stimulated with rPLY, cholesterol-treated rPLY (CT-rPLY), or LPS at a concentration of 0.1 μg/ml (white bars) or 1 μg/ml (black bars) for 24 h, and culture supernatants were then collected. The levels of cytokines in supernatants were determined by ELISA. The results are representative of three similar experiments. The data are the means ± standard deviations for three determinations.
To eliminate the possibility that cytokine induction by rPLY was due to contamination of LPS from E. coli, we added PMB to the cell culture before stimulation with rPLY and LPS. PMB did not affect rPLY-induced production of IL-1β or IL-6 (Fig. 3) as well as IL-1α, IL-18, TNF-α, and IL-12p40 (data not shown), whereas the same amount of PMB completely abolished the production of cytokines induced by LPS. Furthermore, when rPLY was heated at 100°C for 60 min, the ability to induce these cytokines was completely abrogated. Based on these findings, the possibility of contaminating LPS-induced cytokine production was ruled out.
FIG. 3.
Effects of PMB and heating on rPLY-induced IL-1β and IL-6 production. Peritoneal macrophages were stimulated with cholesterol-treated rPLY or LPS for 24 h, and the culture supernatant was then collected. The amounts of IL-1β (A) and IL-6 (B) in the culture supernatant were measured by ELISA. The effects of the addition of 0.5 μg of PMB/ml and heating at 100°C for 1 h were examined. The results are representative of three similar experiments. The data are the means ± standard deviations for three determinations.
Supplementation with rPLY enhanced IL-1α, IL-1β, and IL-18 production by macrophages infected with the Δply mutant.
Although rPLY demonstrated a significantly higher activity in inducing IL-1α, IL-1β, and IL-18 than did LPS (Fig. 2), the levels were significantly lower than those induced by infection of macrophages with wt S. pneumoniae (Fig. 1). To examine whether high levels of these cytokines induced upon infection with wt S. pneumoniae were due to simultaneous stimulation with PLY and other bacterial components, we infected macrophages with the Δply mutant in the presence of rPLY. Costimulation with rPLY and the Δply mutant induced a greatly enhanced production of IL-1α, IL-1β, and IL-18, which was comparable to that induced by wt S. pneumoniae (Fig. 4). In the absence of rPLY, an increase in the dose of the Δply mutant up to an MOI of 50 did not result in such an enhancement (data not shown). These findings clearly indicate that the PLY-dependent cytokine response is enhanced by other stimuli from bacterial cells but that PLY, not any other bacterial components, is solely responsible for the induction of IL-1α, IL-1β, and IL-18 production, which requires the activation of cleaving enzymes, including caspase-1. However, costimulation with rPLY and purified TLR agonists, such as LPS or Pam3CSK4, resulted in no marked enhancement of the production of IL-1α, IL-1β, and IL-18 (data not shown), suggesting that the mechanism of enhancement might be more complex than just a simultaneous stimulation with rPLY and TLR agonists from S. pneumoniae.
FIG. 4.
Costimulation of rPLY with the Δply mutant significantly increased IL-1α, IL-1β, and IL-18 production by the Δply mutant. Peritoneal macrophages were left uninfected (UI) or infected with wt S. pneumoniae (Sp wt), the Δply mutant, or rPLY (1 μg/ml) plus the Δply mutant at a macrophage/bacterium ratio of 1:10. The amounts of IL-1α (A), IL-1β (B), and IL-18 (C) in culture supernatants were determined by ELISAs specific for each cytokine. The results are representative of three similar experiments. The data are the means ± standard deviations for three determinations. *, P < 0.05 for the Δply mutant versus rPLY plus the Δply mutant.
Critical role of TLR4 in IL-1α, IL-1β, and IL-18 production induced by PLY.
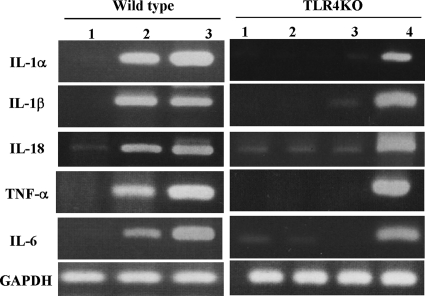
In a previous study, it was shown that PLY induces TNF-α and IL-6 production through TLR4 (27). To investigate whether TLR4 is also required for the production of IL-1α, IL-1β, and IL-18 induced by rPLY, we compared the levels of these cytokines produced by macrophages from C57BL/6 background TLR4-deficient mice and wt mice in response to rPLY. In addition to that of TNF-α and IL-6, the production of IL-1α, IL-1β, and IL-18 induced by rPLY was dependent on TLR4, as these cytokines were produced only by wt macrophages upon stimulation with rPLY (Fig. 5A to C). The production of IL-18 induced by infection with wt S. pneumoniae was also critically dependent on the presence of TLR4, suggesting that production of the cytokine in response to S. pneumoniae is due to the recognition of PLY via TLR4 (Fig. 5D). Because the production of IL-1α, IL-1β, and IL-18 is regulated both transcriptionally and posttranscriptionally, we next examined the involvement of TLR4 in the rPLY-induced gene expression of these cytokines. rPLY induced or up-regulated the expression of IL-1α, IL-1β, and IL-18 in macrophages from wt mice but not in those from TLR4 KO mice (Fig. 6). These results indicate that TLR4 plays a role in the production of IL-1α, IL-1β, and IL-18 induced by rPLY, at least at the gene expression level. In addition, the difference between rPLY and LPS in inducing the production of IL-1α, IL-1β, and IL-18 resulted from a posttranscriptional process, not the ability to induce gene expression, because LPS induced gene expression of these cytokines at levels comparable to those induced by rPLY.
FIG. 5.
TLR4-dependent induction of inflammatory cytokines by PLY. Peritoneal macrophages from C57BL/6 wt mice (black bars) and TLR4 KO mice (white bars) were stimulated with cholesterol-treated rPLY (A to C) or infected with wt S. pneumoniae at a macrophage/bacterium ratio of 1:10 (D) for 24 h, and the culture supernatants were then collected. The amounts of IL-1α, IL-1β, and IL-18 in culture supernatants were determined by ELISA. The results are representative of three similar experiments. The data are the means ± standard deviations for three determinations. Asterisks indicate that the value is significantly different from that of wt cells (P < 0.05).
FIG. 6.
Expression of mRNAs for various cytokines induced by rPLY was TLR4 dependent. Peritoneal macrophages of C57BL/6 wt and TLR4 KO mice were stimulated with rPLY, LPS, and Pam3CSK4 (1 μg/ml) for 6 h. Total RNA was extracted and subjected to reverse transcription-PCR for detection of cytokine mRNAs for IL-1α, IL-1β, IL-18, TNF-α, IL-6, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Lanes: 1, PBS; 2, rPLY; 3, LPS; 4, Pam3CSK4. Representative results are shown. Similar results were obtained in three separate experiments.
Activation of TLR4 downstream signals by rPLY.
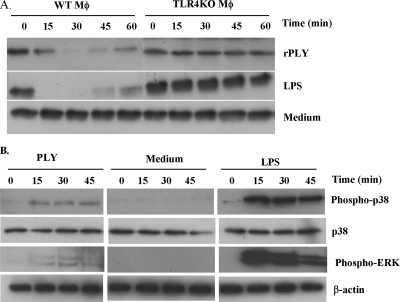
As shown in Fig. 2 and 6, there was dissociation between the gene expression and production of TNF-α after stimulation of macrophages with rPLY. Moreover, the levels of rPLY-induced gene expression and production of TNF-α and IL-6 were significantly lower than those induced by LPS (Fig. 2 and 6). These findings may be due to some difference in the events downstream of TLR4 ligation with rPLY or LPS. Therefore, we first examined the proteolysis of IκBα, a regulator of NF-κB, to estimate the activation of NF-κB. Both rPLY and LPS induced the degradation of IκBα, but some differences were observed in its strength and kinetics. The LPS-induced degradation of IκBα was observed as early as 5 min after stimulation (data not shown), whereas that induced by rPLY took place later (Fig. 7A). The degradation of IκBα was not observed in TLR4-deficient macrophages after stimulation with both rPLY and LPS. Next, the activation of MAPKs was compared. LPS strongly induced the phosphorylation of p38 MAPK and extracellular signal-regulated kinases (ERKs). In contrast, rPLY induced only weak phosphorylation of p38 MAPK and hardly induced ERK phosphorylation (Fig. 7B). These results indicate that the activation of the signaling process after sensing by TLR4 differs between stimulation with rPLY and LPS, which may account for the different profiles of the cytokine response to stimulation with these two TLR4 agonists.
FIG. 7.
Activation of TLR4 downstream signals by rPLY. Peritoneal macrophages from C57BL/6 (WT) and TLR4 KO mice were stimulated with medium alone, rPLY, or LPS (1 μg/ml). (A) At the indicated times, cell lysates were collected and IκBα degradation was analyzed by Western blotting. (B) The cell lysates described in panel A were then subjected to Western blotting using antibodies specific to p38, phospho-p38, phospho-ERK, and β-actin. The results are representative of at least three independent experiments.
Caspase-1 dependency of PLY-induced IL-1β and IL-18 production.
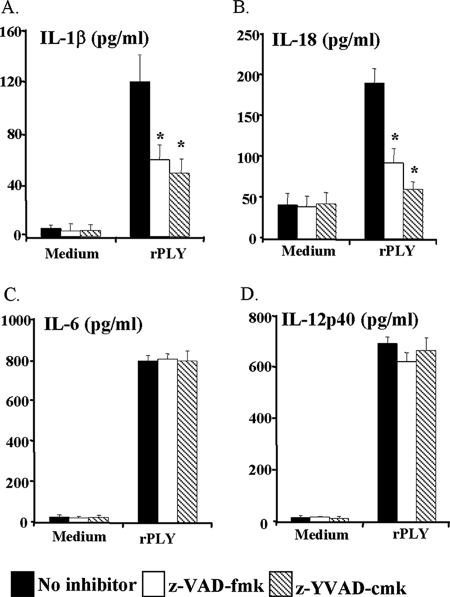
Intracellular IL-1β and IL-18 are not secreted from cells as active forms until they are cleaved by caspase-1. To understand whether PLY-induced macrophage production of IL-1β and IL-18 is due to the activation of caspase-1, the effects of caspase inhibitors were examined. Peritoneal macrophages were pretreated with the caspase-1-specific inhibitor z-YVAD-cmk or the broad-spectrum caspase inhibitor z-VAD-fmk. Both inhibitors effectively reduced the secretion of IL-1β and IL-18 in the culture supernatant, suggesting that caspase-1 activation was induced by rPLY stimulation (Fig. 8). Nonspecific effects of the two inhibitors could be ruled out because the production of IL-6 and IL-12p40 was unaffected by either inhibitor.
FIG. 8.
Pneumolysin-induced IL-1β and IL-18 production is caspase-1 dependent. Peritoneal macrophages were stimulated with cholesterol-treated rPLY (1 μg/ml) in the presence or absence of a broad-spectrum caspase inhibitor (z-VAD-fmk [30 μM]) and a caspase-1-specific inhibitor (z-YVAD-cmk [30 μM]) for 24 h, and the culture supernatant was collected. The amounts of IL-1β (A), IL-18 (B), IL-6 (C), and IL-12p40 (D) were determined by ELISA. The results are representative of three separate experiments. The data are the means ± standard deviations for three or four determinations. *, P < 0.05 compared to no-inhibitor treatment.
Activation of caspase-1 by PLY.
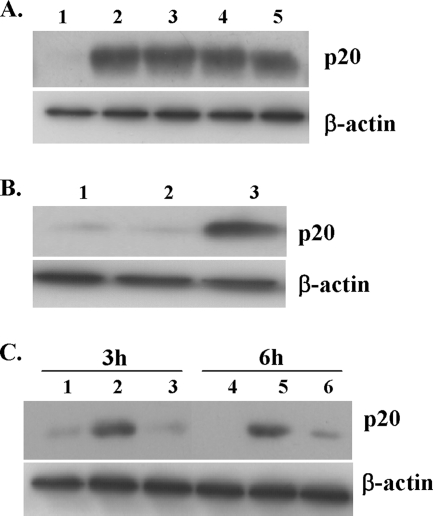
Since the production of IL-1β and IL-18 induced by rPLY was dependent on caspase-1, to confirm the PLY-induced caspase activation, we first tried to detect the active form of caspase-1 in macrophages stimulated with rPLY by means of Western blotting using a polyclonal antibody against the cleaved p20 fragment of caspase-1. This approach was not successful, however, because the amount of p20 fragment inside cells was too small, probably due to a rapid secretion of the active form soon after the cleavage (28). For this reason, we concentrated intracellular activated caspase-1 by a pull-down method using biotinylated YVAD-cmk, which binds to the active form of caspase-1, as previously described, with slight modification (29), and then applied the samples to Western blotting. The activated form of caspase-1 could be detected in macrophages stimulated by both cholesterol-treated rPLY and untreated rPLY (Fig. 9A). Caspase-1 activation was induced sufficiently with even a low dose (0.1 μg/ml) of rPLY, whereas 1 μg/ml LPS was not capable of inducing caspase-1 activation. As previously reported (47), an additional stimulation with ATP was required for the activation of caspase-1 in macrophages stimulated with LPS (Fig. 9B). To rule out the contamination of our rPLY preparations with ATP, the level of ATP was determined using the Enliten ATP assay system (Promega). The ATP level in 1 μg/ml of rPLY was <17.9 pM, and this concentration of ATP never enhanced caspase-1 activation in LPS-stimulated macrophages (data not shown). These results suggest that rPLY induces the activation of caspase-1 without any other additional stimuli and that this ability is independent of its cytolytic activity (Fig. 9A). When macrophages were infected with wt S. pneumoniae, caspase-1 activation was strongly induced, whereas the Δply mutant was unable to induce it effectively (Fig. 9C), suggesting that PLY is also required for the activation of caspase-1 in macrophages infected with S. pneumoniae.
FIG. 9.
Activation of caspase-1 by rPLY and Streptococcus pneumoniae infection. Peritoneal macrophages were incubated with biotinylated YVAD-cmk (30 μM) for 1 h and subsequently stimulated with rPLY, cholesterol-treated rPLY, or LPS for 3 h, using different concentrations of rPLY or LPS. Activated caspase-1 was precipitated using streptavidin beads. Precipitates were subsequently analyzed for the presence of active caspase-1 (p20 subunit) by Western blotting using a caspase-1 antibody (A and B). (A) Lanes: 1, unstimulated; 2, 1 μg/ml rPLY; 3, 0.1 μg/ml rPLY; 4, 1 μg/ml cholesterol-treated rPLY; 5, 0.1 μg/ml cholesterol-treated rPLY. (B) Lanes: 1, unstimulated; 2, 1 μg/ml LPS; 3, LPS plus ATP (1 mM). (C) Similarly, peritoneal macrophages were incubated with biotinylated YVAD-cmk (30 μM) for 1 h and infected with wt S. pneumoniae and the Δply mutant at an MOI of 10, and cell lysates were collected at the indicated time points. Activated caspase-1 was detected as mentioned above. Lanes 1 and 4, uninfected cells; lanes 2 and 5, wt S. pneumoniae-infected cells; lanes 3 and 6, Δply mutant-infected cells. For all panels, results are representative of at least three separate experiments.
DISCUSSION
In the present study, we examined the role of PLY in the induction of various proinflammatory cytokines in response to S. pneumoniae infection. Among a number of different cytokines induced in macrophages infected with S. pneumoniae, the production of IL-1α, IL-1β, and IL-18 was exclusively dependent on the presence of the ply gene, as clearly shown using an in-frame deletion mutant of ply. In contrast, deletion of ply did not result in any severe change in the ability of S. pneumoniae to induce the production of TNF-α, IL-6, and IL-12. This finding strongly implied that the PLY protein itself is an effective ligand that activates caspase-1, which is required for the maturation of IL-1β and IL-18. This possibility was confirmed by the fact that the rPLY protein alone did induce the production of these cytokines. The reduced production of IL-1α, IL-1β, and IL-18 observed in macrophages infected with the Δply mutant recovered when it was supplemented with rPLY, to a level comparable with that in macrophages infected with wt S. pneumoniae. Taken together, these findings clearly show that the profile of the S. pneumoniae-induced cytokine response strongly depends on PLY and that PLY is unique in terms of activity for inducing caspase-1 activation, which is not observed with LPS.
Various bacterial ligands are recognized by several TLRs, with a strict specificity of each TLR and the pattern of each ligand. TLR4, which plays an essential role in LPS recognition, is also reported to be involved in the recognition of PLY (27, 51) and other related bacterial cytolysins, including anthrolysin (41) and Listeria-derived cytolysins (20). In the present study, using rPLY, we demonstrated that TLR4 was essential for the PLY-induced production of IL-1α, IL-1β, and IL-18. Interestingly, in spite of their similar and exclusive dependence on TLR4, rPLY and LPS induced quite different profiles of cytokine production. This suggested that PLY and LPS differentially activated the downstream signaling cascades. Indeed, there was some difference in the timing and duration of NF-κB activation between macrophages stimulated with rPLY and LPS. Furthermore, PLY only weakly activated ERK and p38 MAPK, whereas LPS strongly activated these downstream molecules. Because both NF-κB and MAPKs contribute to the induction of various cytokines, the different modes of activation of these signaling molecules might account for the difference observed in cytokine-inducing activity between PLY and LPS. For example, the production of TNF-α is regulated by NF-κB and MAPKs during and after transcription. ERK is involved particularly in the posttranscriptional transport of TNF-α mRNA from the nucleus to the cytoplasm in response to LPS (15). Although the exact reason that rPLY induced just a small amount of TNF-α is not yet clear, it is possible that TNF-α production was down-regulated posttranscriptionally, because rPLY clearly induced the gene expression of TNF-α but hardly activated ERK.
Although both LPS and PLY are TLR4 agonists, the precise interaction of each ligand with TLR4 seems to be different. Malley et al. (27) showed that MD2, which associates with TLR4 and plays a critical role in the cytokine response to LPS, was not required for PLY-dependent activation of NF-κB. Furthermore, Srivastava et al. (51) demonstrated that there is a specific and strong physical interaction between TLR4 and PLY by using a solid-phase binding assay. It is likely that cofactors required for LPS sensing via TLR4, such as MD2, LPS-binding protein, and CD14, are not so important for the recognition of PLY by TLR4. Sa15-21, which is an anti-TLR4 monoclonal antibody, was reported to bind to the N-terminal leucine-rich repeat of TLR4 and to act as an agonistic antibody. This antibody strongly induced NF-κB activation but modestly induced TNF-α production in vivo and in vitro (2). Therefore, it is conceivable that the observed difference in the activation of the downstream signaling cascade and the overall difference in the profile of cytokine production may result from the different interaction between TLR4 and LPS or PLY.
LPS directly binds to MD2 associated with the extracellular domain of TLR4 and then induces clustering of TLR4/MD2, which leads to the activation of two main signaling pathways downstream of TLR4, namely, the MyD88/Toll-IL-1 receptor (TIR) domain-containing adaptor protein (TIRAP)-dependent pathway and the TIR domain-containing adaptor inducing beta interferon (IFN-β) (TRIF)/TRIF-related adaptor molecule (TRAM)-dependent pathway (46). The MyD88/TIRAP-dependent pathway is involved preferentially in the induction of proinflammatory cytokines, whereas the TRIF/TRAM-dependent pathway is involved in the induction of IFN-β rather than proinflammatory cytokines (3). Although it remains unclear whether TLR4/MD2 clustering occurs in response to PLY, our data suggested that MyD88 was required for the rPLY-induced expression of proinflammatory cytokines. A finding that rPLY did not induce the expression of IFN-β (data not shown) implied that PLY may activate the MyD88/TIRAP-dependent but not the TRIF/TRAM-dependent pathway via its binding to TLR4. It has been reported that the integrity of lipid rafts is essential for the cellular response to LPS and that TLR4 is recruited to lipid rafts after stimulation with LPS (52). Do TLR4/MD2 clustering and/or recruitment of TLR4 to lipid rafts occur upon stimulation with PLY? Are lipid rafts required for the TLR4-dependent cytokine response to PLY? Which part of TLR4 interacts with PLY, and does binding result in some conformational change of TLR4 which leads to activation of the downstream signals? Although there are a number of questions to be addressed, a PLY-induced, noncanonical pathway may provide a new insight into the study of TLR signaling, especially that via TLR4.
An interesting finding of this study is that the caspase-1-dependent cytokines, IL-1β and IL-18 were strongly induced by stimulation with rPLY but hardly induced by LPS alone. Our study confirmed that PLY-induced production of IL-1β and IL-18 was caspase-1 dependent, and the active form of caspase-1 was detected in macrophages stimulated with rPLY. The question of whether TLR4 is involved in the activation of caspase-1 arose. To clarify this point, we tested the involvement of TLR4 in PLY-induced caspase-1 activation. When macrophages were stimulated with rPLY at a sublytic concentration (0.1 μg/ml), our result suggested a requirement for TLR4 in caspase-1 activation, but TLR4 dependency was not observed when cells were stimulated with rPLY at a lytic concentration (1 μg/ml) (data not shown). Our assumption is that both TLR4-dependent and -independent pathways might be involved in PLY-induced activation of caspase-1 and that TLR4 might potentiate the activation of caspase-1. The decrease in intracellular K+ level induced by danger signals and toxins, such as ATP and nigericin, results in the enhancement of caspase-1 activation (44). Furthermore, a cytolytic concentration of PLY caused a K+ efflux without inhibiting the activity of Na+,K+-ATPase (11). It is therefore possible that a PLY-formed pore may mediate K+ efflux and consequently modulate the caspase-1 processing pathway. However, the ability of rPLY to induce the production of IL-1β and IL-18 was not affected, even when its pore-forming activity was blocked by cholesterol pretreatment. Moreover, rPLY-induced activation of caspase-1 was not abolished by cholesterol pretreatment. Because cholesterol pretreatment completely blocked the cytolytic activity of 0.1 μg/ml rPLY and this concentration of cholesterol-treated rPLY clearly induced caspase-1 activation and the production of caspase-1-dependent cytokines, pore formation does not appear to be essential for the induction of caspase-1 activation by PLY. Nonetheless, we cannot completely rule out the possibilities that pore-forming activity of rPLY might remain at a level under the detection limit of the lactate dehydrogenase release assay, even after cholesterol treatment, and that the level of pore formation might be sufficient to mediate K+ efflux that can induce caspase-1 activation. In a future study, we plan to clarify whether the PLY-formed pore is essentially involved in the activation of caspase-1 by using a truncated rPLY that completely lacks cytolytic activity. Indeed, a noncytolytic mutant of PLY has been reported to retain its ability to activate TLR4 signaling and to induce IL-6, TNF-α, and IFN-γ (5, 27), and therefore such a mutant protein would be useful for this line of investigation. PLY is a multifunctional protein and its mechanisms for cytolysis have been investigated, but how this cytolysin induces caspase-1 activation remains to be clarified.
Both IL-1 and IL-18 have been reported to play a key role in infections caused by S. pneumoniae. Zwijnenburg et al. (55) demonstrated that endogenous IL-1 is essential for an adequate host defense in pneumococcal meningitis, as reflected by impaired bacterial clearance and reduced survival of IL-1 receptor-deficient (IL-1R−/−) mice. Similarly, in a murine pneumococcal pneumonia model, IL-1R−/− mice showed an impaired early host defense (45). Studies have also shown that IL-18 has a protective role in the early immune response in a murine pneumococcal pneumonia model by promoting bacterial clearance from the lung and delaying the progression to a systemic infection (26), although the contribution of an inflammatory response to the detrimental effect was also reported for a meningitis model (56). In the present study, PLY was identified as an essential factor that contributes to IL-1α, IL-1β, and IL-18 production in response to S. pneumoniae.
It is therefore possible that PLY-dependent caspase-1 activation and subsequent production of IL-1β and IL-18 protect the host against pneumococcal infection. The present finding that IL-18 production induced by S. pneumoniae was dependent on TLR4 may account for the increased susceptibility to S. pneumoniae of mice that lack functional TLR4 (27). A previous report demonstrated that the interaction between PLY and TLR4 resulted in the induction of caspase-dependent host cell apoptosis in mice infected with S. pneumoniae (51), although several reports demonstrated that apoptosis-like cell death induced by S. pneumoniae or PLY in various types of cells is independent of caspases (6, 10, 12). In that report (51), it appeared that S. pneumoniae-induced apoptosis contributed to the host defense, because administration of the broad-spectrum caspase inhibitor z-VAD-fmk to mice increased the mortality rate after pneumococcal colonization in the nasopharynx. Because PLY-induced IL-18 production was also inhibited by z-VAD-fmk in our study, IL-18 production in addition to apoptosis may participate in the host defense mechanism induced by the TLR4-PLY interaction.
IL-1 and IL-18 are proinflammatory cytokines that can induce inflammatory reactions and are involved in the development of systemic and local inflammatory illnesses, including arthritis, asthma, sepsis, pneumonia, and meningitis caused by various agents (13, 30, 38, 42, 56). From our finding that PLY, which is a toxin highly associated with the pathogenicity of S. pneumoniae, plays an essential role in the activation of caspase-1 and subsequent induction of IL-1 and IL-18 production upon S. pneumoniae infection, it is thus suggested that the PLY-dependent cytokine response comprises part of the pathophysiological mechanism, in addition to cytolytic activity and other functions of the cytolysin as a virulence factor. Indeed, the absence of IL-1R or IL-18 affected the histopathology of the lungs and brains in murine models of S. pneumoniae infection (26, 55). Analysis of infection models using mice deficient for caspase-1 or both IL-1R and IL-18 would elucidate the significance of PLY-dependent cytokines in the aspects of host defense and disease progression. For better control of the diseases caused by S. pneumoniae infection, more detailed investigations into the virulence of this pathogen, host defense mechanisms, and their influences on pathophysiology are required. A full understanding of the functions and physiological roles of PLY may pave the way for the control of harmful cytokines during pneumococcal infection.
Acknowledgments
This study was supported by a grant-in-aid for scientific research on priority areas from The Ministry of Education, Science, Culture and Sports of Japan and by a grant-in-aid for scientific research (B and C) and a grant-in-aid for young scientists (B) from The Japan Society for the Promotion of Science.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 14 January 2008.
REFERENCES
- 1.Akashi, S., S. Saitoh, Y. Wakabayashi, T. Kikuchi, N. Takamura, Y. Nagai, Y. Kusumoto, K. Fukase, S. Kusumoto, Y. Adachi, A. Kosugi, and K. Miyake. 2003. Lipopolysaccharide interaction with cell surface Toll-like receptor4-MD-2: higher affinity than that with MD-2 or CD14. J. Exp. Med. 1981035-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akashi-Takamura, S., T. Furuta, K. Takahashi, N. Tanimura, Y. Kusumoto, T. Kokayashi, S. I. Saitoh, Y. Adachi, T. Doi, and K. Miyake. 2006. Agonistic antibody to TLR4/MD2 protects mice from acute lethal hepatitis induced by TNF-alpha. J. Immunol. 1764244-4251. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., and K. Takeda. 2004. Toll-like receptor signaling. Nat. Rev. Immunol. 4499-511. [DOI] [PubMed] [Google Scholar]
- 4.Baba, H., I. Kawamura, C. Kohda, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, S. Ichiyama, and M. Mitsuyama. 2001. Essential role of domain 4 of pneumolysin from S. pneumoniae in cytolytic activity as determined by truncated proteins. Biochem. Biophys. Res. Commun. 28137-44. [DOI] [PubMed] [Google Scholar]
- 5.Baba, H., I. Kawamura, C. Kohda, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, S. Ichiyama, and M. Mitsuyama. 2002. Induction of gamma interferon and nitric oxide by truncated pneumolysin that lacks pore-forming activity. Infect. Immun. 70107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermpohl, D., A. Halle, D. Freyer, E. Dagand, J. S. Braun, I. Bechmann, N. W. Schroder, and J. R. Weber. 2005. Bacterial programmed cell death of cerebral endothelial cells involves dual death pathways. J. Clin. Investig. 1151607-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry, A. M., A. D. Ogunniyi, D. C. Miller, and J. C. Paton. 1999. Comparative virulence of Streptococcus pneumoniae strains with insertion-duplication, point, and deletion mutations in the pneumolysin gene. Infect. Immun. 67981-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branger, J., S. Knapp, S. Weijer, J. C. Leemans, J. M. Pater, P. Spleelman, S. Florquin, and T. van der Poll. 2004. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect. Immun. 72788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun, J. S., R. Novak, G. Gao, P. J. Murray, and J. L. Shenep. 1999. Pneumolysin, a protein toxin of Streptococcus pneumoniae, induces nitric oxide production from macrophages. Infect. Immun. 673750-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun, J. S., R. Novak, P. J. Murray, C. M. Eischen, S. A. Sustin, G. Kroemer, A. Halle, J. R. Weber, E. I. Tuomanen, and J. L. Cleveland. 2001. Apoptosis-inducing factor mediates microglial and neuronal apoptosis caused by pneumococcus. J. Infect. Dis. 1841300-1309. [DOI] [PubMed] [Google Scholar]
- 11.Cockeran, R., A. J. Theron, H. C. Steel, N. M. Matlola, T. J. Mitchell, C. Feldman, and R. Anderson. 2001. Proinflammatory interactions of pneumolysin with human neutrophils. J. Infect. Dis. 183604-611. [DOI] [PubMed] [Google Scholar]
- 12.Colino, J., and C. M. Snapper. 2003. Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. J. Immunol. 1712354-2365. [DOI] [PubMed] [Google Scholar]
- 13.Dinarello, C. A. 1996. Biologic basis for interleukin-1 in disease. Blood 872095-2147. [PubMed] [Google Scholar]
- 14.Doern, G. V., A. B. Brueggemann, H. Huynh, E. Wingert, and P. Rhomberg. 1999. Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997-98. Emerg. Infect. Dis. 5757-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumitru, C. D., J. D. Ceci, C. Tsatsanis, D. Kontoyiannis, K. Stamatakis, J. H. Lin, C. Patriotis, N. A. Jenkins, N. G. Copeland, G. Kollias, and P. N. Tsichlis. 2000. TNF-α induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 1031071-1083. [DOI] [PubMed] [Google Scholar]
- 16.Dunne, A. O., and A. J. Luke. 2003. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Science STKE 1701-17. [DOI] [PubMed] [Google Scholar]
- 17.French, N., J. Nakiyingi, L. M. Carpenter, E. Lugada, C. Watera, K. Moi, M. Moore, D. Antvelink, D. Mulder, E. N. Janoff, J. Whitworth, and C. F. Gilks. 2000. 23-Valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 3552106-2111. [DOI] [PubMed] [Google Scholar]
- 18.Houldworth, S., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infect. Immun. 621501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inohara, N., and G. Nunez. 2003. NOD-LRRs: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3371-382. [DOI] [PubMed] [Google Scholar]
- 20.Ito, Y., I. Kawamura, C. Kodha, K. Tsuchiya, T. Nomura, and M. Mitsuyama. 2005. Seeligeriolysin O, a protein toxin of Listeria seeligeri stimulates macrophage cytokine production via Toll-like receptors in a profile different from that induced by other bacterial ligands. Int. Immunol. 171597-1606. [DOI] [PubMed] [Google Scholar]
- 21.Jedrzejas, M. J. 2001. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, M. R., B. T. Simms, M. M. Lupa, M. S. Kogan, and J. P. Mizgend. 2005. Lung NF-kappa B activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J. Immunol. 175530-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keer, S., S. L. Salmon, S. A. Lotz, and D. W. Metzger. 2007. Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect. Immun. 751196-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly, S. J., and M. J. Jedrzejas. 2000. Structure and molecular mechanism of a functional form of pneumolysin: a cholesterol-dependent cytolysin from Streptococcus pneumoniae. J. Struct. Biol. 13272-81. [DOI] [PubMed] [Google Scholar]
- 25.Latz, E., A. Visintin, E. Lien, K. A. Fitzgerald, B. G. Monks, E. A. Kurt-Jones, D. T. Golenbock, and T. Espevik. 2002. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the Toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J. Biol. Chem. 27747834-47843. [DOI] [PubMed] [Google Scholar]
- 26.Lauw, F. N., J. Branger, S. Florquin, P. Speelman, S. J. van Deventer, S. Akira, and T. van der Poll. 2002. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J. Immunol. 168372-378. [DOI] [PubMed] [Google Scholar]
- 27.Malley, R., P. Henneke, S. C. Morse, M. J. Cieslewicz, M. Lipsitch, C. M. Thompson, E. Kurt-Jones, J. C. Paton, M. R. Wessels, and D. T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 1001966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinon, F., K. Burns, and J. Tschopp. 2002. The inflamasome: a molecular platform triggering the activation of inflammatory caspases and processing of proIL-1 beta. Mol. Cell 10417-426. [DOI] [PubMed] [Google Scholar]
- 29.Martinon, F., L. Agostini, E. Meylan, and J. Tshopp. 2004. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflamasome. Curr. Biol. 141929-1934. [DOI] [PubMed] [Google Scholar]
- 30.Matsui, K., H. Tsutsui, and K. Nakanishi. 2003. Pathological role for IL-18 in inflammatory arthritis. Expert Opin. Ther. Targets 7701-724. [DOI] [PubMed] [Google Scholar]
- 31.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1135-145. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell, T. J., J. E. Alexander, P. J. Morgan, and P. W. Andrew. 1997. Molecular analysis of virulence factors of Streptococcus pneumoniae. Soc. Appl. Bacteriol. Symp. Ser. 2662S-71S. [PubMed] [Google Scholar]
- 33.Mitchell, T. J., P. W. Andrew, F. K. Saunders, A. N. Smith, and G. J. Boulnois. 1991. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol. Microbiol. 51883-1888. [DOI] [PubMed] [Google Scholar]
- 34.Nakamatsu, M., N. Yamamoto, M. Hotta, C. Nakasone, T. Kinjo, K. Miyagi, K. Uezu, K. Nakamura, M. Taniguchi, Y. Iwakura, M. Kaku, J. Fujita, and K. Kawakami. 2007. Role of interferon-gamma in Valpha 14+ natural killer T cell-mediated host defense against Streptococcus pneumoniae infection in murine lungs. Microbes Infect. 9364-374. [DOI] [PubMed] [Google Scholar]
- 35.Nomura, T., I. Kawamura, K. Tsuchiya, C. Kohda, H. Baba, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2002. Essential role of interleukin-12 (IL-12) and IL-18 for gamma interferon production induced by listeriolysin O in mouse spleen cells. Infect. Immun. 701049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novak, R., B. Henriques, E. Charpentier, S. Normark, and E. Tuomanen. 1999. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature 399590-593. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien, A. D., D. L. Rosenstreich, I. Scher, G. H. Campbell, R. P. MacDermott, and S. B. Formal. 1980. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J. Immunol. 12420-24. [PubMed] [Google Scholar]
- 38.Okamura, H., K. Nagata, T. Komatsu, T. Tanimoto, Y. Nukata, F. Tanabe, K. Akita, K. Torigoe, T. Okura, S. Fukuda, and M. Kurimoto. 1995. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect. Immun. 633966-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortqvist, A., J. Hedlund, L. A. Burman, E. Elbel, M. Hofer, M. Leinonen, I. Lindblad, B. Sundelof, and M. Kalin. 1998. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet 351399-403. [DOI] [PubMed] [Google Scholar]
- 40.Palmer, M. 2001. The family of thiol activated, cholesterol-binding cytolysins. Toxicon 391681-1689. [DOI] [PubMed] [Google Scholar]
- 41.Park, J. M., V. H. Ng, S. Maeda, R. F. Rest, and M. Karin. 2004. Anthrolysin O and other gram-positive cytolysins are Toll-like receptor 4 agonists. J. Exp. Med. 2001647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paterson, G. K., C. E. Blue, and T. J. Mitchell. 2005. Role of IL-18 in experimental infections with Streptococcus pneumoniae. J. Med. Microbiol. 54323-326. [DOI] [PubMed] [Google Scholar]
- 43.Paton, J. C. 1996. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 4103-106. [DOI] [PubMed] [Google Scholar]
- 44.Perregaux, D., and C. A. Gabel. 1994. Interleukin-1 beta maturation and release in response to ATP and nigricin: evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 26915195-15203. [PubMed] [Google Scholar]
- 45.Rijneveld, A. W., S. Florquin, J. Branger, P. Speelman, S. J. Van Deventer, and T. van der Poll. 2001. TNF-α compensates for the impaired host defense of IL-1 type I receptor-deficient mice during pneumococcal pneumonia. J. Immunol. 1675240-5246. [DOI] [PubMed] [Google Scholar]
- 46.Saitoh, S., and K. Miyake. 2006. Mechanism of regulating cell surface expression and activation of Toll-like receptor 4. Chem. Rec. 6311-319. [DOI] [PubMed] [Google Scholar]
- 47.Sanz, J. M., and F. Di Virgilio. 2000. Kinetics and mechanism of ATP-dependent IL-1β release from microglial cells. J. Immunol. 644893-4898. [DOI] [PubMed] [Google Scholar]
- 48.Schaaf, B., J. Rupp, M. Muller-Steinhardt, J. Kruse, F. Boehmke, M. Maass, P. Zabel, and K. Dalhoff. 2005. The interleukin-6-174 promoter polymorphism is associated with extrapulmonary bacterial dissemination in Streptococcus pneumoniae infection. Cytokine 31324-328. [DOI] [PubMed] [Google Scholar]
- 49.Schmeck, B., S. Huber, K. Moog, J. Zahlten, A. C. Hocke, B. Opitz, S. Hammerschmidt, T. J. Mitchell, M. Kracht, S. Rosseau, N. Suttorp, and S. Hippenstiel. 2006. Pneumococci induced TLR- and Rac1-dependent NF-κB-recruitment to the IL-8 promoter in lung epithelial cells. Am. J. Physiol. Lung Cell Physiol. 290L730-L737. [DOI] [PubMed] [Google Scholar]
- 50.Shumway, C. N., and S. J. Klebanoff. 1971. Purification of pneumolysin. Infect. Immun. 4388-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srivastava, A., P. Henneke, A. Visintin, S. C. Morse, V. Martin, C. Watkins, J. C. Paton, M. R. Wessels, D. T. Golenbock, and R. Malley. 2005. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect. Immun. 736479-6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Triantafilou, M., K. Miyake, D. T. Golenbock, and K. Triantafilou. 2002. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 1152603-2611. [DOI] [PubMed] [Google Scholar]
- 53.Visintin, A., E. Latz, B. G. Monks, T. Espevik, and D. T. Golenbock. 2003. Lysines 128 and 132 enable lipopolysaccharide binding to MD-2, leading to Toll like receptor-4 aggregation and signal transduction. J. Biol. Chem. 27848313-48320. [DOI] [PubMed] [Google Scholar]
- 54.Wellmer, A., J. Gerber, J. Ragheb, G. Zysk, T. Kunst, A. Smirnov, W. Bruck, and R. Nau. 2001. Effect of deficiency of tumor necrosis factor or both of its receptors on Streptococcus pneumoniae central nervous system infection and peritonitis. Infect. Immun. 696881-6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zwijnenburg, P. J., T. van der Poll, S. Florquin, K. Takeda, J. J. Roord, and A. M. van Furth. 2003. IL-1 receptor type-1 gene-deficient mice demonstrate an impaired host defense against pneumococcal meningitis. J. Immunol. 1704724-4730. [DOI] [PubMed] [Google Scholar]
- 56.Zwijnenburg, P. J., T. van der Poll, S. Florquin, S. Akira, K. Takeda, J. J. Roord, and A. M. van Furth. 2003. Interleukin-18 gene-deficient mice show enhanced defense and reduced inflammation during pneumococcal meningitis. J. Neuroimmunol. 13831-37. [DOI] [PubMed] [Google Scholar]