Abstract
Citrobacter rodentium, a murine model pathogen for enteropathogenic Escherichia coli, colonizes the colon utilizing attaching and effacing lesions to adhere specifically to the surfaces of intestinal epithelial cells and cause mucosal inflammation. CD4+ T cells, B cells, and immunoglobulin G (IgG), but not secretory IgA or IgM, play a critical role in eradicating this pathogen. Consistent with the importance of IgG in C. rodentium eradication, IgG transport by the neonatal Fc receptor for IgG within the intestinal epithelium also has a critical role in the regulation of C. rodentium infection. It remains to be determined, however, whether Fcγ receptors (FcγRs), the receptors for the Fc portion of IgG, regulate this bacterial infection within mucosal tissues. Therefore, we investigated the roles of FcγRs during C. rodentium infection. Fc receptor common gamma chain (FcRγ)-deficient mice were more susceptible to C. rodentium-induced colitis. This occurred through decreased efficiency of FcR-mediated endocytosis and maturation of dendritic cells and consequently T-cell activation of antigen-specific T cells. Moreover, in the absence of FcγRs, phagocytosis by macrophages was significantly diminished. Therefore, activating FcγRs play an important role in defending against C. rodentium infection, indicating that the critical role played by IgG in this infection is not mediated by IgG alone but is dependent upon this class of receptors.
Fcγ receptors (FcγRs), the receptors for the Fc portion of immunoglobulin G (IgG), are essential for antibody-dependent immune responses (14). FcγRs are detected on many hematopoietic cells, including macrophages, neutrophils, dendritic cells (DCs), eosinophils, basophils, mast cells, and NK cells (13). Functionally, there are two types of Fc receptors: activating receptors and inhibitory receptors, which transmit their signals via immunoreceptor tyrosine-based activation motifs or immunoreceptor tyrosine-based inhibitory motifs, respectively (15). In the mouse, the FcγRs that associate with a Fc receptor common gamma chain (FcRγ) homodimer that has immunoreceptor tyrosine-based activation motifs are Fcγ receptor I (FcγRI), FcγRIII, and FcγRIV, which lead to activation of downstream-signaling pathways. On the other hand, FcγRIIB is a unique FcγR with an immunoreceptor tyrosine-based inhibitory motif domain that directs an inhibitory program. The coexpression of activating and inhibitory FcγRs regulate the immune response by establishing a threshold for immune cell activation. In many murine model systems, the expression of aberrant FcγRs can result in uncontrolled immune responses and the initiation of autoimmune disease (2, 8, 19). Mice deficient in FcRγ (FcRγ−/−), a subunit common to FcγRI, FcγRIII, FcγRIV, FcɛRI, FcαRI, exhibit genetic inactivation of all activating FcγRs. This results in abrogated or heavily impaired immune complex-mediated immune responses, such as antibody-dependent cell-mediated cytotoxicity, release of inflammatory mediators, cytokine release, and phagocytosis of immune complexes (12, 20).
Enteric bacteria, such as enteropathogenic Escherichia coli, evade many mechanisms of systemic host defense by restricting their colonization to the luminal surface of the gut epithelium. Citrobacter rodentium, a murine model pathogen for enteropathogenic E. coli, colonize the epithelium of the colon utilizing attaching and effacing lesions to adhere to the surfaces of intestinal epithelial cells and cause mucosal inflammation. A few hours after oral challenge with 108 to 109 CFU of C. rodentium, initial colonization is observed at the cecum with colonization of the distal colon detectable at 2 or 3 days after infection. C. rodentium is usually spontaneously eradicated by day 28 after oral administration in wild-type mice (10). From the infection experiments using immune cell-deficient mice, CD4+ T cells, B cells, and IgG, but not secretory IgA or IgM, have been shown to play a critical role in eradicating this pathogen (3, 9, 17). It is speculated that C. rodentium-specific IgGs produced by B cells after stimulation of CD4+ T cells are necessary to eradicate this pathogen.
The neonatal Fc receptor for IgG, FcRn, is a critical molecule that is involved in the transport of IgG and its protection from catabolism. FcRn is structurally related to major histocompatibility complex (MHC) class I molecules and consists of a heterodimer composed of a glycosylated heavy chain in noncovalent association with β2-microglobulin (16). FcRn is known to have two cellular functions. One is the bidirectional transport of IgG across epithelial cells, and the other is the protection of IgG from catabolism by escape from lysosome degradation. It has been shown that human FcRn is the vehicle by which IgG is transported across the intestinal epithelium and can in turn also recycle the IgG together with its cognate antigen as an immune complex back across the intestinal epithelial barrier into the lamina propria for processing by DCs and presentation to CD4+ T cells (23).
IgG transport by FcRn may regulate immune responses to luminal pathogens. Specifically, it has been shown that the transport of IgG and antigen-IgG complexes by FcRn plays a role in the immune defense against C. rodentium infection (24). It has been speculated that the effects of the anti-bacterial IgG antibodies that are transported by FcRn are derived from the direct protection against bacterial invasion into lamina propria from the epithelium and indirectly by affecting antigen presentation to antigen-specific T cells, which leads to the activation and proliferation of antigen-specific CD4+ T cells that assist in the killing of invading bacteria or to the differentiation of immature B cells into plasma cells for the production of bacterial antigen-specific IgGs. Beyond FcRn, it is not known whether the protective effects of IgG in C. rodentium infection are also dependent upon FcγRs. It remains to be determined whether and how FcγRs regulate this bacterial infection of mucosal tissues. Therefore, in this report, we have investigated the roles of classical FcγRs during C. rodentium infection. Our results indicate that the elimination of activating FcRs for IgG, through deletion of FcRγ, and inhibitory FcRs for IgG, through deletion of FcRIIB, affect the susceptibility to C. rodentium infection.
MATERIALS AND METHODS
Animals.
FcRγ−/− mice (20), and FcRIIB−/− mice (21) were used. All mice were backcrossed more than six generations onto C57BL/6 mice. For ovalbumin (OVA) studies, OT-II mice were used (Jackson Laboratory, Bar Harbor, ME) (1). All mice were housed and bred in the Animal Unit of the Kobe University School of Medicine in a specific-pathogen-free facility under an approved experimental protocol.
Antibodies.
CD11c-biotin (clone N418), MHC class II-PE (MHC class II conjugated to phycoerythrin) (clone M5/114.15.2), CD86 conjugated to PE (CD86-PE) (clone GL-1), streptavidin-PerCP, T-cell receptor (TCR) Vβ5.1 or Vβ5.2-PE (clone MR9-4), CD69-PE (clone H1.2F3), Ly6G (unconjugated) (clone RB6-8C5), CD11b-PE (clone M1/70) were purchased from BD Bioscience (San Jose, CA). Alexa Fluor 488-conjugated goat anti-rat IgG (heavy and light chains) was purchased from Molecular Probes (Eugene, OR). Rabbit anti-OVA polyclonal antibody was purchased from Rockland (Gilbertsville, PA). Polyclonal anti-C. rodentium sera was obtained from wild-type mice 6 weeks after C. rodentium infection as described previously (3).
Protocol for induction of colitis by C. rodentium infection.
C. rodentium strain DB100 (catalog no. 51459; ATCC) was kindly provided by Gad Frankel (Division of Cell and Molecular Biology, Imperial College London, London, United Kingdom) and prepared by incubation with shaking at 37°C for 6 h in LB broth. After 6 h, the bacterial density was assessed by absorbance at an optical density of 600 nm and confirmed by plating of serial dilutions. Since the age and gut flora of mice at the time of oral administration are important in the susceptibility to C. rodentium infection, we used FcRγ+/− mice and FcRγ−/− mice born from the same parents. There were no observed differences between the susceptibility of FcRγ+/− mice and FcRγ+/+ mice (data not shown). Inoculation of 3-week-old mice was performed by oral administration of 5 × 108 CFU. Body weight, bacterial concentration in the feces, and histological findings of the colon were measured for 3 weeks after inoculation. Anti-C. rodentium IgG in the sera and feces was examined by enzyme-linked immunosorbent assays (ELISAs). Colonic tissues were evaluated by hematoxylin and eosin staining as described previously (11). Frozen sections were stained by anti-Ly-6G antibody and Alexa Fluor 488-conjugated goat anti-rat IgG and examined by confocal microscopy (LSM5Pascal; Carl Zeiss, Germany).
Preparation of bone marrow-derived DCs.
Bone marrow-derived DCs were prepared as described previously (7). Cells were placed at a concentration of 1 × 106 cells/well in each well (24 wells). The cells were plated in 1 ml of RPMI 1640 supplemented with 10% fetal calf serum, 0.1 U/ml penicillin, 0.1 μg/ml streptomycin, and GM-CSF (20 ng/ml) in each well. On day 6, aggregates were gently collected and used in some experiments.
Preparation of FITC-conjugated C. rodentium and evaluation of early endocytosis in FcR-deficient DCs.
C. rodentium was labeled with 1 mg/ml of fluorescein isothiocyanate (FITC) solution in phosphate-buffered saline at pH 7.4 for 15 min. Bone marrow-derived DCs from wild-type, FcRγ−/−, and FcRIIB−/− mice were cocultured with FITC-conjugated C. rodentium or C. rodentium-IgG complexes for 1 h, respectively. Cells were stained with anti-CD11c and anti-MHC class II and then analyzed by using a FACSCalibur flow cytometer (Becton-Dickinson, Franklin Lakes, NJ).
Evaluation of maturation in FcγR-deficient DCs.
Bone marrow-derived DCs from wild-type, FcRγ−/−, and FcRIIB−/− mice were incubated for 24 h in the absence or presence of C. rodentium or C. rodentium-IgG complexes. Cells were collected and stained with anti-CD86 or anti-MHC class II after gating anti-CD11c-positive cells and analyzed by flow cytometry. The supernatants of the culture medium were collected, and the amount of tumor necrosis factor alpha (TNF-α) measured by a standard ELISA kit (TNF-α Ready-Set-Go; e-Bioscience, San Diego, CA).
Establishment of C. rodentium producing OVA and GFP.
Constitutively OVA-expressing C. rodentium (OVA-C. rodentium) was created by electroporating the plasmid pUT-mini-Tn5 Km vector, including the chicken OVA construct (25, 26) under the control of the two gal operon promoters into C. rodentium strain DBS100. C. rodentium producing green fluorescent protein (GFP) (GFP-C. rodentium) was kindly provided by C. Sasakawa, Tokyo University, Japan.
Adoptive transfer with CFSE-labeled OVA-specific OT-II CD4+ T cells and antigenic challenge with OVA-C. rodentium.
Three-week-old FcRγ−/− mice and wild-type mice were orally inoculated with OVA-C. rodentium. The mice received 1 mg of anti-C. rodentium IgG or control IgG intravenously on day 2 after bacterial inoculation and received carboxyfluoroscein succinimidyl ester (CFSE)-labeled CD4+ OT-II cells on day 3. On day 5, mesenteric lymph node (MLN) cells were subjected to flow cytometric analysis for the evaluation of CFSE intensity of the CD4+ T cells gated on TCR Vβ5.1-positive cells. MLN cells were also stained on day 5 for CD69 expression using an anti-CD69 antibody (clone H1.2F3; BD Bioscience, San Jose, CA) to evaluate T-cell activation.
Preparation of peritoneal macrophages and evaluation of phagocytosis in FcγR-deficient macrophages.
Wild-type, FcγRγ−/−, and FcγRIIB−/− mice were injected with 1 ml of 3% thioglycolate into the peritoneal cavity. The peritoneal cells were harvested by peritoneal lavage with 10 ml cold sterile saline twice from each mouse. Macrophages from the peritoneal cavities of wild-type, FcRγ−/−, and FcRIIB−/− mice and GFP-C. rodentium were incubated for 30, 45, and 60 min with anti-C. rodentium IgG or control IgG and stained with TOPRO-3 and Alexa Fluor 546-conjugated phalloidin and then examined by confocal microscopy. Cells were also stained with a CD11b-specific antibody (clone M1/70; BD Bioscience, San Jose, CA), and phagocytic activity was measured as fluorescence intensity per CD11b-positive cells by flow cytometry.
Statistics.
Statistical significance was determined by a two-tailed Student's t test and analysis of variance (ANOVA). P values less than 0.05 were considered significant.
RESULTS
Susceptibility to C. rodentium in the absence of FcR gamma chain.
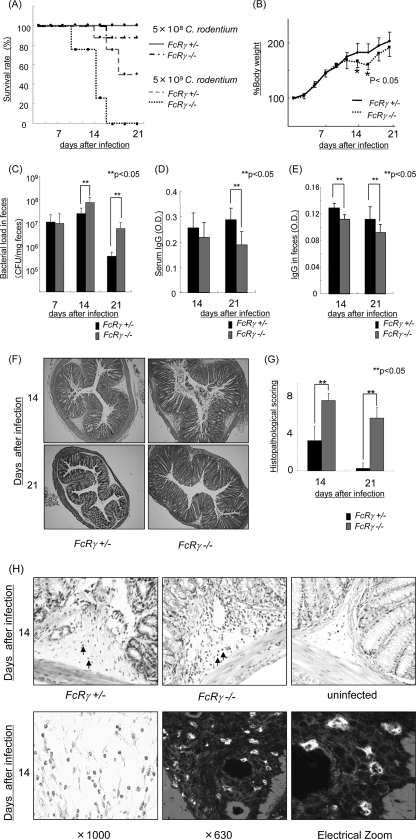
To evaluate the roles of IgG Fc receptors in mucosal infection, we used the Citrobacter rodentium model in a C57BL/6 mouse background (6). Previous studies have shown that C57BL/6 and BALB/c strains exhibit differing sensitivities to C. rodentium infection. C57BL/6 mice exhibit greater clinical evidence of disease and a fecal burden of bacteria compared with BALB/c mice. Three-week-old FcRγ−/− mice (C57BL/6) and control mice (FcRγ−/+ mice, C57BL/6) were orally inoculated with 5 × 109 or 5 × 108 CFU of C. rodentium. A dose of 5 × 109 CFU/mouse was lethal in 75% of FcRγ−/− mice on day 16 and 100% lethal on day 21. In contrast, the same dose was lethal in 12.5% of control mice on day 16 and 50% lethal on day 21. Even a dose of 5 × 108 CFU of C. rodentium was lethal in 12.5% of FcRγ−/− mice on day 21 after infection (Fig. 1A). These studies indicate that FcRγ−/− mice are more susceptible to C. rodentium infection.
FIG. 1.
Susceptibility to C. rodentium in the absence of FcR gamma chain. Susceptibility to infection with 5 × 108 CFU of C. rodentium in FcRγ−/− mice. (A) Survival rates in mice infected with 5 × 109 and 5 × 108 CFU of C. rodentium (eight mice per group). (B) Body weight changes in FcRγ−/− mice and control mice infected with 5 × 108 CFU of C. rodentium. The mean ± standard deviation (error bar) is shown. Values that are significantly different (P < 0.05) from the values for the control mice are indicated (*). (C) Bacterial loads (CFU/mg) of C. rodentium in feces 7, 14, and 21 days after infection. The mean ± standard deviation (error bar) is shown for each group (six mice per group). Values that are significantly different (P < 0.05) are indicated by brackets and two asterisks. (D and E) IgG on day 14 and day 21 after infection in the serum and feces. Values that are significantly different (P < 0.05) are indicated by brackets and two asterisks. O.D., optical density. (F and H) Histological findings of the colons of FcRγ−/− mice and control mice with C. rodentium infection 14 and 21 days after infection. Confocal microscopy analysis staining with an anti-Ly-6G antibody is shown in panel H. The arrows in panel H indicate neutrophil infiltration. Magnifications, ×10 (F), ×20 (H, top row), and ×1,000 (H, bottom row). (G) Histological score of colonic tissue in FcRγ−/−mice and control mice with C. rodentium infection 14 and 21 days after infection.
To assess the role of the FcRγ chain in the adaptive immune response during C. rodentium infection in detail, we used a dose of 5 × 108 CFU/mouse in the following studies. FcRγ−/− mice exhibited more body weight loss (Fig. 1B) and higher bacterial concentrations in the feces at 14 and 21 days after infection (Fig. 1C) than littermate matched FcRγ−/+ mice. FcRγ−/+ mice exhibited a significantly greater increase of IgG levels on day 21 in sera and on days 14 and 21 in feces in comparison to FcRγ−/− mice (Fig. 1D and E). This increased production of IgG by FcRγ−/+ mice was associated with an approximately 50% survival on day 21 in response to a challenge with 5 × 109 CFU of C. rodentium in striking comparison to FcRγ−/− mice in which this dose was uniformly lethal (Fig. 1A). Consistent with this, macroscopic and microscopic injury was greater in FcRγ−/− mice than in FcRγ−/+ mice. The colons of FcRγ−/− mice were shorter and edematous compared with FcRγ−/+ mice (data not shown). FcRγ−/− mice exhibited greater histologic injury in comparison to FcRγ−/+ mice on days 14 and 21 after infection (Fig. 1F, G, and H). The submucosa and lamina propria of infected FcRγ−/− mice contained a greater number of polymorphonuclear leukocytes infiltrating the tissues as defined by Ly-6G expression (Fig. 1H), coincident with crypt hyperplasia 14 and 21 days after infection. In contrast, on day 21 after infection, there was no inflammation in FcRγ−/+ mice. These results indicate that FcRγ−/−mice are more susceptible to C. rodentium colitis than FcRγ−/+mice.
Decreased uptake of FITC-conjugated C. rodentium via FcγR in FcRγ−/− DCs.
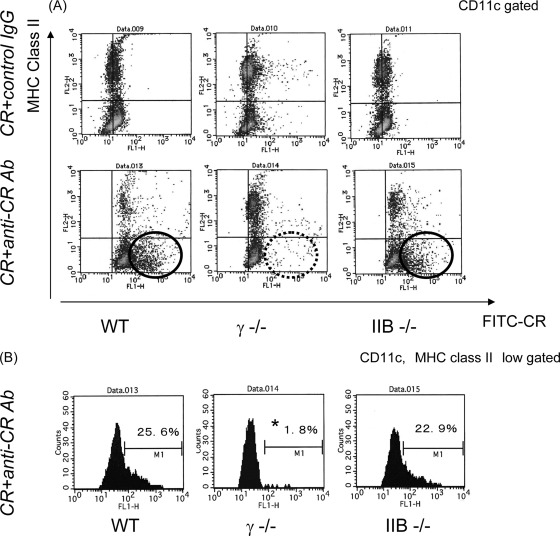
Since the above studies showed that activating FcγRs are necessary for protection against C. rodentium colitis, we then evaluated the function of FcγR-deficient DCs. At first, we evaluated FcR-mediated endocytosis by DCs. To do so, bone marrow-derived DCs from wild-type, FcRγ−/−, and FcRIIB−/− mice were incubated for 1 h with FITC-conjugated C. rodentium with anti-C. rodentium IgG or control IgG. The uptake of FITC-conjugated C. rodentium in the context of control IgG was comparable in each type of DC. In contrast, there was a remarkable decrease in FITC-conjugated C. rodentium uptake by FcRγ−/− DCs with anti-C. rodentium IgG compared with either wild-type or FcRIIB−/− DCs. This decrease in the FITC signal was especially evident in the MHC class II low fraction of DCs which are indicative of the immature subset of DCs (Fig. 2A and B). These observations are consistent with previous observations that unstimulated or immature DCs display a higher level of uptake in comparison to mature DCs (18). These studies indicate that uptake of immune complexes in relationship to C. rodentium infection is directly regulated by FcRγ.
FIG. 2.
Incorporation of C. rodentium or C. rodentium-IgG complexes into DCs from FcγR-deficient mice. (A) Bone marrow-derived DCs from wild-type, FcRγ−/− (γ −/−), or FcRIIB−/− (IIB −/−) mice were cocultured with FITC-conjugated C. rodentium with anti-C. rodentium IgG or control IgG for 1 h. Cells were labeled with anti-CD11c and anti-MHC class II antibodies and analyzed by flow cytometry gated on CD11c-positive cells. CR+anti-CR Ab, C. rodentium plus anti-C. rodentium antibody; CR+control IgG, C. rodentium plus control IgG; WT, wild type; FITC-CR, FITC-conjugated C. rodentium. (B) Analysis of incorporation of FITC-conjugated C. rodentium gated on CD11c-positive and MHC class II low-positive cells. Results are representative of three independent experiments.
Incorporation of C. rodentium-IgG complexes effectively induces DC maturation and production of proinflammatory cytokines.
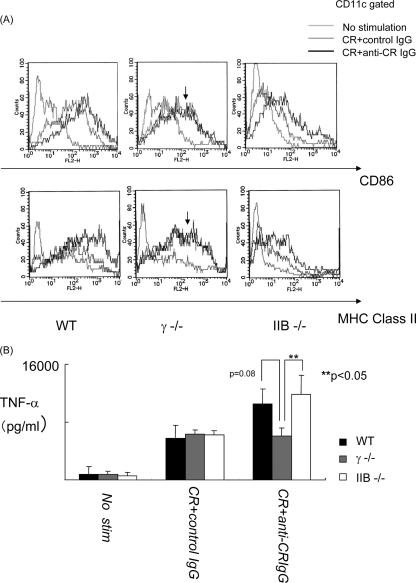
We next investigated the maturation of FcγR-deficient DCs after taking up bacterial antigens with IgG. Bone marrow-derived DCs from wild-type, FcRγ−/−, and FcRIIB−/− mice were incubated for 24 h with FITC-conjugated C. rodentium and anti-C. rodentium IgG or control IgG, and the expression levels of various surface markers (CD86 and MHC class II) indicative of activation on DCs were assessed by flow cytometry. Exposure of all types of DCs examined to C. rodentium with control IgG was observed to cause a slight up-regulation of CD86 and MHC class II expression presumed to result from direct stimulation caused by the bacterium alone. In contrast, exposure of the wild-type and FcRIIB−/− DCs to C. rodentium and anti-C. rodentium IgG, but not FcRγ−/− DCs to the same conditions, induced a further increase in CD86 and MHC class II expression beyond that caused by the bacterium with the control IgG.
Consistent with these observations, the supernatants of the culture medium collected from these various types of incubations revealed that C. rodentium-specific IgG stimulated increased production of TNF-α by wild-type and FcRIIB−/− DCs, but not FcRγ−/− DCs, in comparison to that induced by control IgG complexes of C. rodentium (Fig. 3B). These results indicate that FcRγ expressed on DCs can induce maturation and activation of production of proinflammatory cytokines after taking up immune complexes of C. rodentium.
FIG. 3.
C. rodentium-IgG complexes incorporation effectively induced DC maturation via FcγR. (A) Bone marrow-derived DCs from FcγR-deficient mice were incubated for 24 h in the absence (white) or presence of C. rodentium and control IgG (CR+control IgG) (gray) or C. rodentium and anti-C. rodentium IgG complexes (CR+anti-CR IgG) (black). Cells from wild-type (WT), FcRγ−/− (γ −/−), and FcRIIB−/− (IIB −/−) mice are shown. DCs were stained with anti-CD86 or anti-MHC class II after gating on CD11c-positive cells and analyzed by flow cytometry. Results are from one representative experiment of three independent experiments. (B) Cytokine production of TNF-α was measured by an ELISA. The mean plus standard deviation (SD) are shown for each group (three mice per group). This experiment was evaluated by ANOVA. Values that are significantly different (P < 0.05) are indicated by brackets and two asterisks.
C. rodentium-IgG complexes effectively induce antigen-specific T-cell activation in MLNs.
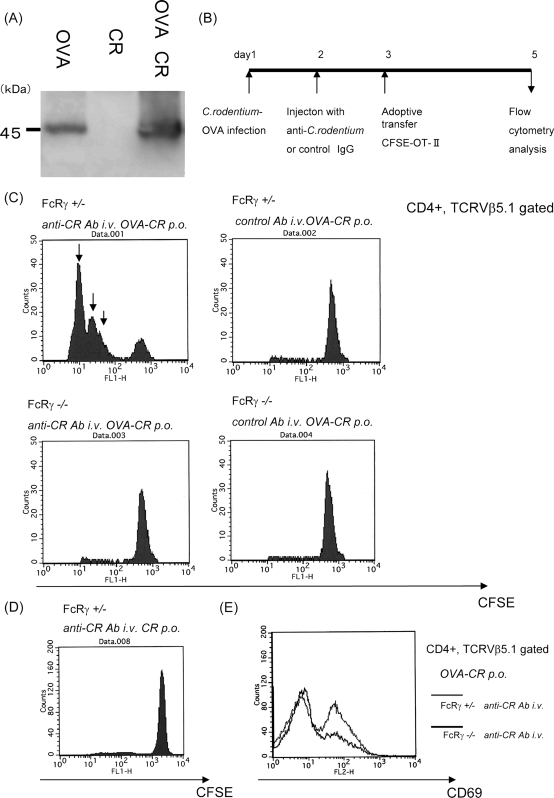
Next we evaluated antigen-specific T-cell activation in the mucosal lymphoid tissues and their regulation by Fcγ receptors. To examine whether FcRγ plays a role in infection-induced acquired immune responses, a genetically engineered C. rodentium strain was created that constitutively expressed OVA (OVA-C. rodentium). Immunoblot analysis confirmed the expression of OVA in the cell sonicates of OVA-C. rodentium but not control C. rodentium (Fig. 4A). To evaluate the antigen-specific T-cell responses, FcRγ−/− and FcRγ−/+ mice were infected with OVA-C. rodentium at 3 weeks of age. Anti-C. rodentium IgG or control IgG was injected on day 2 after infection. CFSE-labeled OT-II cells were transferred intravenously on day 3 after infection. Mononuclear cells were collected from the MLNs, and the cells derived from these nodes were examined by flow cytometry (Fig. 4B). Injection of C. rodentium-specific IgG dramatically increased the number of OVA-specific CD4+ T cells (actual total number of OVA-specific CD4+ T cells in MLN: FcRγ−/+ mice with anti-C. rodentium IgG: FcRγ−/− mice with anti-C. rodentium IgG = 9.6 × 104 ± 0.5 × 103: 2.8 × 104 ± 0.3 × 103), as demonstrated by an enhancement of cell divisions within the MLNs of FcRγ−/+ mice in comparison to FcRγ-deficient mice (Fig. 4C). In contrast, even in the presence of C. rodentium-specific IgG, no significant increase of OVA-specific CD4+ T cells was detected within the MLNs of FcRγ−/− mice. The same results were obtained by assessing the levels of CD69 expression on OT-II cells by flow cytometry wherein OT-II cells expressed higher levels of CD69 when adoptively transferred into FcRγ+/− mice in comparison to FcRγ−/− mice (Fig. 4E). As a control, C. rodentium without OVA expression did not enhance the proliferation of OT-II cells (Fig. 4D). These results reveal that FcRγ enhances the antigen-specific T-cell responses in the mucosa-associated lymphoid tissues in response to C. rodentium.
FIG. 4.
C. rodentium-IgG complexes effectively induce T-cell activation. (A) Establishment of a genetically engineered C. rodentium strain that constitutively produces OVA. The immunoblot confirms the expression of OVA by C. rodentium. CR, C. rodentium; OVA CR, C. rodentium strain that constitutively produces OVA. (B) Summary of the experimental protocol with the inoculation of OVA-C. rodentium, injection of anti-C. rodentium IgG or control IgG, and the adoptive transfer of CD4+ OVA-specific T cells from OT-II mice. (C) The number of OVA-specific T cells in the MLNs in FcRγ−/− mice and control mice increased in the presence of anti-C. rodentium IgG or control IgG (three mice per group). Arrows indicate increasing rounds of cell division. anti-CR Ab i.v. OVA-CR p.o., anti-C. rodentium antibody given intravenously and OVA-CR given per os (orally). (D) Evaluation of CFSE-labeled OT-II cells in mice receiving C. rodentium without OVA expression. (E) Evaluation of CD69 expression on OT-II cells after in vivo stimulation with OVA-C. rodentium together with anti-C. rodentium IgG. Results are representative of two independent experiments.
Macrophage phagocytosis of opsonized bacteria is regulated by specific IgG via FcRγ chain in response to C. rodentium.
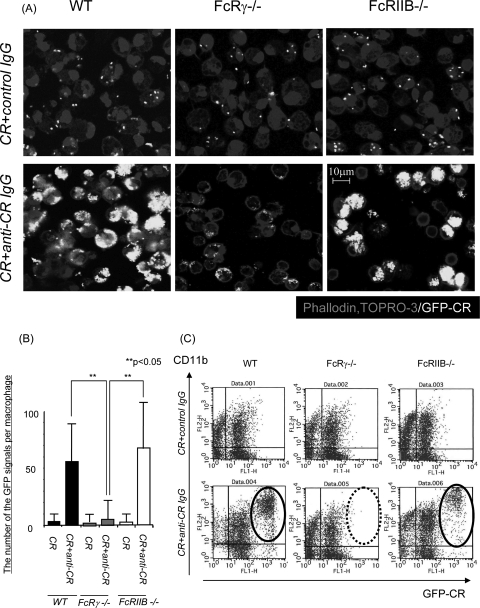
Antigen-specific T-cell activation via FcRγ induces differentiation of B cells to produce antigen-specific IgG. In turn, antigen-specific IgG can stimulate phagocytosis by macrophages via FcγRs. Therefore, peritoneal macrophages from wild-type, FcRγ−/−, and FcRIIB−/− mice were collected after peritoneal injection with thioglycolate and then cultured with GFP-C. rodentium for 45 min with anti-C. rodentium IgG or control IgG. Cells were stained by TOPRO-3 and phalloidin, and the number of GFP signals per macrophage was determined by confocal microscopy. The number of C. rodentium bacteria that were incorporated into macrophages was increased in wild-type and FcRIIB−/− mice treated with anti-C. rodentium IgG, but not in FcRγ−/− macrophages treated in the same manner (Fig. 5A and B). The same results were obtained by measurement of phagocytic activity as defined by flow cytometry (Fig. 5C). These results indicate that FcRγ stimulates phagocytosis by macrophages.
FIG. 5.
Macrophages phagocytose opsonized bacteria via the FcR gamma chain. (A) Macrophages from the peritoneal cavities of wild-type (WT), FcRγ−/−, or FcRIIB−/− mice and GFP-expressing C. rodentium (white) were incubated for 45 min with control IgG or anti-C. rodentium IgG and stained for nuclei and actin (phalloidin) (gray). Samples were examined by confocal microscopy. CR+anti-CR IgG, C. rodentium plus anti-C. rodentium IgG. Magnification, ×630. (B) The number of C. rodentium bacteria in each macrophage was counted by confocal microscopy. The mean plus standard deviation (error bar) is shown for each group (n = 100). This experiment was evaluated by ANOVA. Values that are significantly different (P < 0.05) are indicated by brackets and two asterisks. (C) Flow cytometry analysis of phagocytic activity in macrophages. Results are representative of three independent experiments. GFP-CR, GFP-expressing C. rodentium.
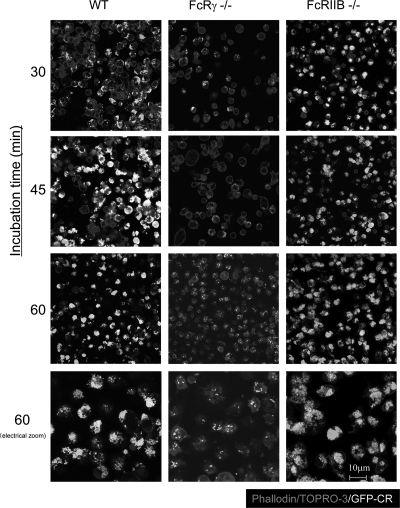
Therefore, to evaluate the phagocytic activity of each type of FcγR-deficient macrophage in detail, macrophages and GFP-C. rodentium were incubated for 30, 45, and 60 min with anti-C. rodentium IgG. Consistent with the previous results as described above, only a few bacteria that were opsonized with specific IgG could be detected in FcRγ−/− macrophages even at 60 min after incubation (Fig. 6). Interestingly, the GFP signals were higher in the FcRIIB−/− macrophages than in the wild-type macrophages 60 min after incubation, which is consistent with the loss of this inhibitory receptor (Fig. 6). These results indicate that phagocytosis of immune complexes by macrophages are regulated in the opposite manner by activating and inhibitory FcγRs in response to C. rodentium infection.
FIG. 6.
Phagocytosis of immune complexes by macrophages are regulated by activating and inhibitory FcγRs. Macrophages from the peritoneal cavities of wild-type (WT), FcRγ−/−, or FcRIIB−/− mice and GFP-expressing C. rodentium (GFP-CR) (white) were incubated for 30, 45, and 60 min with anti-C. rodentium IgG, stained for nuclei and actin (phalloidin) (gray), and then examined by confocal microscopy. Results are representative of two independent experiments. Magnification, ×630.
DISCUSSION
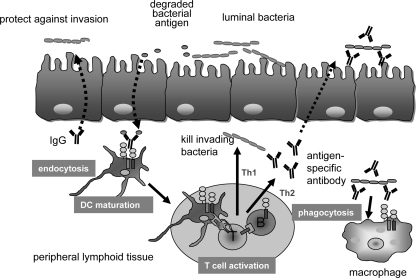
In this work, we examined the roles of FcγRs in mucosal colonic inflammation induced by C. rodentium infection. IgG, but not secretory IgA or IgM, has been shown to play a critical role in prevention against infection by the attaching and effacing pathogen C. rodentium. This model is therefore appropriate to evaluate the function of IgG and its relationship to FcRγ in defending against bacterial infection within mucosal tissues. In these studies, we show that deletion of the FcRγ chain causes decreased efficiency of various aspects of DC and macrophage function relevant to antimicrobial immunity, including endocytosis, phagocytosis, and antigen-presenting cell activation, leading to a less mature phenotype of the latter types of cells. Consistent with this, in the absence of FcRγ expression, there is less stimulation of antigen-specific T cells and thus adaptive immunity. This stimulation of T cells is associated with increased B-cell production of bacterium-specific IgGs. The results of these studies thus support an important role of FcRγ in coordinating innate and adaptive immune responses to invasive bacteria (Fig. 7). The susceptibility to C. rodentium infection in FcRγ−/− mice is thus explained by a broad decrease in the efficacy of the immune response associated with defense against this mucosal pathogen. Moreover, the effects of FcRγ shown here are likely related to its effects on Fcγ receptors rather than Fcα receptors, since neither IgA or IgM has any role to play in protection against C. rodentium infection (3, 9, 17).
FIG. 7.
Summary of FcγR-mediated protection against C. rodentium infection. FcRγ−/− mice are susceptible to C. rodentium infection by the broad decrease in the efficacy of antigen-presenting cell function, including early endocytosis and maturation of dendritic cells, antigenic activation of T cells, and phagocytosis of C. rodentium by macrophages when bacterium-specific IgG exists.
We examined the uptake of bacterium-IgG complexes in various FcγR-deficient DCs. Many studies have shown that the FcRγ chain has a critical role in the early endocytosis of antigen-IgG complexes (5). As shown with other bacterial infections and model antigens, such as OVA, we now show that the FcRγ chain also plays an important role in endocytosis of C. rodentium. We also examined the effect of FcRγ on the maturation of DCs in the context of C. rodentium infection. Yada et al. have previously shown that the maturation of FcRγ−/− DCs is delayed compared with wild-type or FcRIIB−/− DCs using OVA as an immune complex (22). We expected that bacterium-IgG complexes would cause a different effect because bacterial lipopolysaccharide, CpG, and other Toll receptor ligands would cause significant maturation of DCs. However, our results indicate that IgG Fc receptors, such as FcRγ, elicit unique and significant signals to DCs beyond that which is delivered by Toll-like receptor-derived signals and are important to mediating protection against C. rodentium infection.
The role of FcRIIB in bacterial infection is not well-known. Some studies have shown that FcRIIB−/− mice exhibit less inflammation than wild-type mice do during bacterial infection (4, 5). In preliminary studies, FcRIIB−/− mice exhibited less inflammation of the distal colon during C. rodentium infection (data not shown). Consistent with this, we show here that macrophages from FcRIIB−/− mice displayed increased phagocytic function in comparison to wild-type macrophages which we speculate is one mechanism by which FcRIIB−/− mice exhibit less inflammation during C. rodentium infection compared to that observed with wild mice. This point certainly deserves examination in future studies but suggests that FcRIIB−/− mice clear C. rodentium infection more effectively.
In summary, we show that the activating FcγR, FcRγ, plays an important role in defending against a mucosal pathogen, C. rodentium. FcRγ regulates a variety of important cellular processes within DCs and macrophages that enhance their microbiocidal activity and augmentation of both innate and adaptive immune pathways in response to this mucosal pathogen. As such, it can be predicted that the known protective effect of IgG in C. rodentium infection is mediated in large part through the properties of this activating FcγR.
Acknowledgments
M.Y. was supported by grants from the Nagao Memorial Fund, a Grant-in-Aid for Scientific Research for Scientific Research in Priority Areas “Membrane Traffic” from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Foundation of Advancement of International Science. A.M. was supported by grants from the Research Leader Program of the Kobe University School of Medicine and Sinryokukai Fund. R.S.B. was supported by grants from the National Institutes of Health (RO1 DK53056, DK51362, and DK44319).
Statistics were kindly performed by Daisuke Sugiyama, Kobe University Graduate School of Medicine, Department of Evidence-Based Laboratory Medicine (Sysmex).
Editor: S. R. Blanke
Footnotes
Published ahead of print on 28 January 2008.
REFERENCES
- 1.Barnden, M. J., J. Allison, W. R. Heath, and F. R. Carbone. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 7634-40. [DOI] [PubMed] [Google Scholar]
- 2.Bolland, S., and J. V. Ravetch. 2000. Spontaneous autoimmune disease in FcγRIIB-deficient mice results from strain-specific epistasis. Immunity 13277-285. [DOI] [PubMed] [Google Scholar]
- 3.Bry, L., and M. B. Brenner. 2004. Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J. Immunol. 172433-441. [DOI] [PubMed] [Google Scholar]
- 4.Clatworthy, M. R., and K. G. Smith. 2004. FcgammaRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J. Exp. Med. 199717-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gjertsson, I., S. Kleinau, and A. Tarkowski. 2002. The impact of Fcgamma receptors on Staphylococcus aureus infection. Microb. Pathog. 33145-152. [PubMed] [Google Scholar]
- 6.Higgins, L. M., G. Frankel, G. Douce, G. Dougan, and T. T. MacDonald. 1999. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect. Immun. 673031-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1761693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioan-Facsinay, A., S. J. de Kimpe, S. M. Hellwig, P. L. van Lent, F. M. Hofhuis, H. H. van Ojik, C. Sedlik, S. A. da Silveira, J. Gerber, Y. F. de Jong, R. Roozendaal, L. A. Aarden, W. B. van den Berg, T. Saito, D. Mosser, S. Amigorena, S. Izui, G. J. van Ommen, M. van Vugt, J. G. van de Winkel, and J. S. Verbeek. 2002. FcgammaRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity 16391-402. [DOI] [PubMed] [Google Scholar]
- 9.Maaser, C., M. P. Housley, M. Iimura, J. R. Smith, B. A. Vallance, B. B. Finlay, J. R. Schreiber, N. M. Varki, M. F. Kagnoff, and L. Eckmann. 2004. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect. Immun. 723315-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mundy, R., T. T. MacDonald, G. Dougan, G. Frankel, and S. Wiles. 2005. Citrobacter rodentium of mice and man. Cell. Microbiol. 71697-1706. [DOI] [PubMed] [Google Scholar]
- 11.Neurath, M. F., B. Weigmann, S. Finotto, J. Glickman, E. Nieuwenhuis, H. Iijima, A. Mizoguchi, E. Mizoguchi, J. Mudter, P. R. Galle, A. Bhan, F. Autschbach, B. M. Sullivan, S. J. Szabo, L. H. Glimcher, and R. S. Blumberg. 2002. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J. Exp. Med. 1951129-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nimmerjahn, F., and J. V. Ravetch. 2006. Fcgamma receptors: old friends and new family members. Immunity 2419-28. [DOI] [PubMed] [Google Scholar]
- 13.Qiu, W. Q., D. de Bruin, B. H. Brownstein, R. Pearse, and J. V. Ravetch. 1990. Organization of the human and mouse low-affinity Fc gamma R genes: duplication and recombination. Science 248732-735. [DOI] [PubMed] [Google Scholar]
- 14.Ravetch, J. V., and J. P. Kinet. 1991. Fc receptors. Annu. Rev. Immunol. 9457-492. [DOI] [PubMed] [Google Scholar]
- 15.Ravetch, J. V., and L. L. Lanier. 2000. Immune inhibitory receptors. Science 29084-89. [DOI] [PubMed] [Google Scholar]
- 16.Simister, N. E., and K. E. Mostov. 1989. An Fc receptor structurally related to MHC class I antigens. Nature 337184-187. [DOI] [PubMed] [Google Scholar]
- 17.Simmons, C. P., S. Clare, M. Ghaem-Maghami, T. K. Uren, J. Rankin, A. Huett, R. Goldin, D. J. Lewis, T. T. MacDonald, R. A. Strugnell, G. Frankel, and G. Dougan. 2003. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 715077-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman, R. M., D. Hawiger, K. Liu, L. Bonifaz, D. Bonnyay, K. Mahnke, T. Iyoda, J. Ravetch, M. Dhodapkar, K. Inaba, and M. Nussenzweig. 2003. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann. N. Y. Acad. Sci. 98715-25. [DOI] [PubMed] [Google Scholar]
- 19.Sylvestre, D. L., and J. V. Ravetch. 1994. Fc receptors initiate the Arthus reaction: redefining the inflammatory cascade. Science 2651095-1098. [DOI] [PubMed] [Google Scholar]
- 20.Takai, T., M. Li, D. Sylvestre, R. Clynes, and J. V. Ravetch. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell 76519-529. [DOI] [PubMed] [Google Scholar]
- 21.Takai, T., M. Ono, M. Hikida, H. Ohmori, and J. V. Ravetch. 1996. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature 379346-349. [DOI] [PubMed] [Google Scholar]
- 22.Yada, A., S. Ebihara, K. Matsumura, S. Endo, T. Maeda, A. Nakamura, K. Akiyama, S. Aiba, and T. Takai. 2003. Accelerated antigen presentation and elicitation of humoral response in vivo by FcgammaRIIB- and FcgammaRI/III-mediated immune complex uptake. Cell. Immunol. 22521-32. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida, M., S. M. Claypool, J. S. Wagner, E. Mizoguchi, A. Mizoguchi, D. C. Roopenian, W. I. Lencer, and R. S. Blumberg. 2004. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 20769-783. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida, M., K. Kobayashi, T. T. Kuo, L. Bry, J. N. Glickman, S. M. Claypool, A. Kaser, T. Nagaishi, D. E. Higgins, E. Mizoguchi, Y. Wakatsuki, D. C. Roopenian, A. Mizoguchi, W. I. Lencer, and R. S. Blumberg. 2006. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J. Clin. Investig. 1162142-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida, M., Y. Shirai, T. Watanabe, M. Yamori, Y. Iwakura, T. Chiba, T. Kita, and Y. Wakatsuki. 2002. Differential localization of colitogenic Th1 and Th2 cells monospecific to a microflora-associated antigen in mice. Gastroenterology 1231949-1961. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida, M., Y. Wakatsuki, Y. Kobayashi, T. Itoh, K. Murakami, A. Mizoguchi, T. Usui, T. Chiba, and T. Kita. 1999. Cloning and characterization of a novel membrane-associated antigenic protein of Helicobacter pylori. Infect. Immun. 67286-293. [DOI] [PMC free article] [PubMed] [Google Scholar]