Abstract
Enteropathogenic Escherichia coli (EPEC) and the murine pathogen Citrobacter rodentium belong to the attaching and effacing (A/E) family of bacterial pathogens. These noninvasive bacteria infect intestinal enterocytes using a type 3 secretion system (T3SS), leading to diarrheal disease and intestinal inflammation. While flagellin, the secreted product of the EPEC fliC gene, causes the release of interleukin 8 (IL-8) from epithelial cells, it is unclear whether A/E bacteria also trigger epithelial inflammatory responses that are FliC independent. The aims of this study were to characterize the FliC dependence or independence of epithelial inflammatory responses to direct infection by EPEC or C. rodentium. Following infection of Caco-2 intestinal epithelial cells by wild-type and ΔfliC EPEC, a rapid activation of several proinflammatory genes, including those encoding IL-8, monocyte chemoattractant protein 1, macrophage inflammatory protein 3α (MIP3α), and β-defensin 2, occurred in a FliC-dependent manner. These responses were accompanied by mitogen-activated protein kinase activation, as well as the Toll-like receptor 5 (TLR5)-dependent activation of NF-κB. At later infection time points, a subset of these proinflammatory genes (IL-8 and MIP3α) was also induced in cells infected with ΔfliC EPEC. The nonmotile A/E pathogen C. rodentium also triggered similar innate responses through a TLR5-independent but partially NF-κB-dependent mechanism. Moreover, the EPEC FliC-independent responses were increased in the absence of the locus of enterocyte effacement-encoded T3SS, suggesting that translocated bacterial effectors suppress rather than cause the FliC-independent inflammatory response. Thus, we demonstrate that infection of intestinal epithelial cells by A/E pathogens can trigger an array of proinflammatory responses from epithelial cells through both FliC-dependent and -independent pathways, expanding our understanding of the innate epithelial response to infection by these pathogens.
Enteropathogenic Escherichia coli (EPEC) is a prominent cause of diarrheal disease, causing the deaths of hundreds of thousands of children each year in developing countries (8, 9). Belonging to a family of related gram-negative pathogenic bacteria that includes enterohemorrhagic E. coli (EHEC) and the mouse pathogen Citrobacter rodentium (28), EPEC strains are noninvasive, infecting their hosts by attaching to intestinal epithelial cells (IEC), effacing the epithelial microvilli, and producing pedestal-like structures (2, 29). The formation of attaching and effacing (A/E) lesions is required for these microbes to cause diarrheal disease (8, 34, 42); thus, many studies have characterized the virulence factors used by A/E bacteria to infect their hosts. The formation of A/E lesions depends on a type III secretion system (T3SS) encoded within the locus of enterocyte effacement (LEE), a pathogenicity island that contains all the genes required for A/E lesion formation (9). These pathogens use the LEE-encoded T3SS to inject an array of effector proteins, including the translocated intimin receptor (Tir), into host cells, where they interfere with normal cellular function (9).
Although the mechanisms by which A/E bacteria cause disease remain elusive, part of the symptomatology suffered by infected hosts reflects the subsequent host inflammatory and immune responses to infection. EPEC infection in vivo leads to intestinal-tissue damage, including neutrophil infiltration into the infected mucosa and damage to the gut epithelium (6, 26, 36). Much of this pathology has been linked to inflammatory responses by the infected epithelium, including the production of the chemokine interleukin 8 (IL-8). Although studies have implicated the EPEC T3SS in triggering IL-8 release by epithelial cells (13, 35), no effector protein has yet been identified to play this role. Instead, any possible proinflammatory role for the T3SS has been overshadowed by the discovery of bacterial flagellins, monomeric structural repeating proteins that comprise flagella. Like lipopolysaccharide (LPS), flagellins contain pathogen-associated molecular patterns that are recognized by the innate immune system, triggering an inflammatory response (17). A study by Zhou et al. demonstrated that flagellin is the major bacterial component responsible for the ability of EPEC supernatants to trigger IL-8 release from epithelial cells (47). Moreover, this inflammatory response required the activation of mitogen-activated protein kinases (MAPK), a group of serine-threonine kinases central to many host responses, including cytokine responses. Phosphorylation of MAPK and activation of NF-κB are required for induction of IL-8 production in epithelial cells (20).
While the discovery that EPEC flagellin causes IL-8 release has advanced our understanding of EPEC-induced gastroenteritis, other EPEC factors may also contribute to the inflammation, since bacterial pathogens can express more than one pathogen-associated molecular pattern. To date, the role of the innate immune response to EPEC flagellin, as well as the impact of this system on host defense or other inflammatory genes, is not well defined. Furthermore, studies based on bacterial supernatants alone do not account for any direct proinflammatory actions resulting from the intimate attachment of EPEC to host cells, including possible T3SS-dependent effects. This is particularly relevant, given that intimate attachment to epithelial cells is a hallmark of EPEC infection.
Understanding the scope of the inflammatory response elicited in epithelial cells by EPEC infection has also been limited by the human specificity of EPEC, with most researchers finding that it is unable to infect laboratory animals. The need for a relevant animal model to explore in vivo pathogenesis and disease mechanisms has been addressed through the use of related veterinary pathogens, including rabbit-specific strains of EPEC (27), as well as C. rodentium, a natural murine A/E pathogen (28). This bacterium colonizes the intestinal epithelium of mice using a set of T3SS effectors similar to those used by EPEC and EHEC. Interestingly, these infections are accompanied by an influx of many types of inflammatory cells, as well as the induction of antimicrobial peptides and enzymes, some of which we and others have localized to colonic epithelial cells (21, 28, 43). These findings suggest that infection by A/E pathogens may induce a more complex host response than has so far been attributed to EPEC flagellin.
Our goal in this study was to assess the impact of EPEC FliC, as well as its LEE-encoded T3SS, on the inflammatory response generated by IEC directly infected by EPEC, and further, to characterize the scope of the resulting inflammatory response generated by these virulence factors. We demonstrate that EPEC infection strongly activates Toll-like receptor 5 (TLR5), triggering a spectrum of innate chemokine and antimicrobial responses within epithelial cells in a predominantly FliC-dependent and MAPK-dependent manner. However, we also found that direct infection of epithelial cells induces a proinflammatory response, even in the absence of FliC, and while this response is more marked at later time points, it is not dependent on the LEE-encoded effectors. Interestingly, a similar inflammatory response is triggered in epithelial cells following infection by C. rodentium, as well as in the colons of C. rodentium-infected mice. These findings thus expand our current knowledge of the responses produced by epithelial cells following EPEC infection and indicate that while FliC may be the first and predominant proinflammatory stimulus, direct infection by these pathogens can also induce an innate inflammatory response in epithelial cells through other, non-FliC-dependent mechanisms.
MATERIALS AND METHODS
Cell culture.
Caco-2 IEC were obtained from the American Type Culture Collection (ATCC) and grown in Dulbecco's modified Eagle's minimal essential medium (DMEM) with 4.5 g/liter d-glucose, 1× nonessential amino acids, 2 mM glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal bovine serum (Sigma). Human embryonic kidney (HEK) 293 cells were obtained from the ATCC and maintained in MEM with 1× nonessential amino acids, 1 nM sodium pyruvate, penicillin/streptomycin, and 10% fetal bovine serum. The cells were seeded at high density in polystyrene T75cm2 culture flasks, 6-well plates, or 12-well plates and used for experiments 3 to 5 days after becoming confluent.
Bacterial strains and growth conditions.
The wild-type (WT) bacterial strains used in this study were EPEC strain 2348/69 and C. rodentium (formerly known as Citrobacter freundii biotype 4280) strain DBS100. ΔescN EPEC was generated by Gauthier et al. as described previously (15). ΔfliC EPEC was generated in our laboratory by using the sacB-based positive-selection suicide vector pCVD442. Three sets of primers were used for the generation of ΔfliC EPEC (Table 1). Two 1.5-kb fragments flanking the fliC gene in WT EPEC 2348/69 were amplified by primer sets F1 and R1, and F2 and R2. The third set of primers, F3 and R3, was used to amplify the fliC gene, including the two flanking fragments, to confirm deletion of the gene. The double mutant ΔfliC/ΔescN EPEC was generated using the same strategy as for ΔfliC in the ΔescN EPEC background strain (15). A similar approach was used to generate mutant strains of C. rodentium lacking the flagellin genes fliC (primers 1 to 3) and lafA (primers 4 to 6) in C. rodentium.
TABLE 1.
Primers used for generating EPEC and C. rodentium bacterial mutants used in this studya
| Primer no. | Sequence
|
|
|---|---|---|
| Forward | Reverse | |
| EPEC | ||
| 1 | 5′-GTCAAGCTTCAGGGTCTTACTAACGCCATCGG-3′ | 5′-CTGCTAGCCGACAGCGCAGACTGGTTCTTG-3′ |
| 2 | 5′-GCGCTAGCCAGCAGGCCGGTAACTCCGTAC-3′ | 5′-CCGAGCTCCGGCACAATGCCGCCCATGATTG-3′ |
| 3 | 5′-CAGGGTTGACGGCGATTGAG-3′ | 5′-CCTGATAAGCGCAGCGCATC-3′ |
| C. rodentium | ||
| 1 | 5′-GTGGTACCCAGCTGCGTAAACTGGGCGGTG-3′ | 5′-CCGCTAGCAGAGCCCAGTGCGGACTGAG-3′ |
| 2 | 5′-CGGCTAGCTCTGTTCTGGCGCAGGCTAACC-3′ | 5′-CCGAGCTCCGCATGATTAGAGATGCTGAAGG-3′ |
| 3 | 5′-CCTGAGCCTACGCCCAGCGAAG-3′ | 5′-CGCCAGTTGGTTCATGATGAACG-3′ |
| 4 | 5′-GTGGTACCACTTCCTGATACATAACAG-3′ | 5′-CCGCTAGCGTTGATGGCATTAACCGCTGC-3′ |
| 5 | 5′-CGGCTAGCCAGTCCAACAGCATGTCCAGC-3′ | 5′-CCGAGCTCGCCGTTGCTGGAGTCGAG-3′ |
| 6 | 5′-GCTATTAGAGCGGTGGCCAACG-3′ | 5′-GCTGGCGGTGTTGTGTAGCG-3′ |
Details in Materials and Methods.
For routine cloning, transformation, and infections, bacteria were grown in Luria-Bertani (LB) agar or LB broth supplemented with appropriate antibiotics at 37°C. The antibiotics were used at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; and chloramphenicol, 30 μg/ml. When assaying for bacterial colonization in mice infected by C. rodentium, MacConkey lactose agar (Difco Laboratories), which is selective for gram-negative bacteria, was used to plate serial dilutions of mouse colonic contents for quantifying bacterial burdens. Complementation of flagellin-deficient EPEC was performed using the full-length FliC gene cloned into the pQE-30 UA vector (Qiagen). Briefly, flagellin-deficient competent EPEC was transformed by the heat shock method at 42°C with the pQE-30 UA vector carrying the full-length FliC gene, sequenced, and used in assessing proinflammatory responses.
Oligonucleotide sequences, molecular techniques, DNA cloning, and sequence analysis.
For PCR and cloning experiments, the proofreading Elongase amplification system (Gibco BRL/Life Technologies) was utilized to minimize the PCR error rate. The PCR products were cloned using the TOPO TA cloning kit (Invitrogen) with either pCR2.1-TOPO or pCRII-TOPO. The DNA sequence was determined at the Nucleotide and Protein Sequencing Unit of the University of British Columbia using the Taq dye terminator method and an automated 373A DNA sequencer (Applied Biosystems). M13 reverse and −20 (20 bp upstream of the target gene) primers complementary to the PCR cloning vectors and the subcloning vector pBluescript II SK(+) (Stratagene), as well as primers designed from available DNA sequences, were used for sequencing. Analysis of sequences was performed using software or web-based programs, such as DNA Strider, Gene Jockey, and CLUSTALW, as well as tools of the NCBI. NCBI's BLAST search server was used for nucleotide and protein sequence homology searches with the filter checked off. The oligonucleotide primer sequences referred to in this study are listed in Table 2.
TABLE 2.
Oligonucleotide primers used for chemokine gene expression in this study
| Target gene product | Sequence
|
Reference | |
|---|---|---|---|
| Forward | Reverse | ||
| GAPDH | 5′-ATGACCTTGCCCACAGCC-3′ | 5′-CCCATCACCATCTTCCAG-3′ | 5 |
| MIP-3α | 5′-GCAAGCAACTTTGACTGCTG-3′ | 5′-TGGGCTATGTCCAATTCCAT-3′ | |
| IL-8 | 5′-TCTGCAGCTCTGTGTGAAGGT-3′ | 5′-GCTTGAAGTTTCACTGGCATC-3′ | |
| MCP-1 | 5′-TCTGTGCCTGCTGCTCATAGC-3′ | 5′-GGGTAGAACTGTGGTTCAAGAGG-3′ | 31 |
| Beta Defensin-2 | 5′-CCAGCCATCAGCCATGAGGGT-3′ | 5′-GGAGCCCTTTCTGAATCCGCA-3′ | 30 |
| Murine MIP-2 | 5′-TCCTCGGGCACTCCAGAC-3′ | 5′-GCCTTGCCTTTGTTCAGTAT-3′ | 22 |
| Murine MIP-3α | 5′-TGCTCTTCCTTGCTTTGGCA-3′ | 5′-TCTGTGCAGTGATGTGCAGG-3′ | 22 |
Infection of Caco-2 IEC by A/E bacteria.
Caco-2 cells cultured in 6- and 12-well plates were infected by A/E pathogens in DMEM nutrient mixture-Ham F-12 (DMEM-F-12) for 3 to 4 h. At the end of 3 to 4 h of A/E infection, the multiplicity of infection ranged between 30 and 40. After infection, the cells were washed twice with DMEM-F-12, and gentamicin (1 μg/ml) was added to prevent host cell death and overgrowth of extracellular bacteria. At different time points after infection, the supernatant was collected for sandwich enzyme-linked immunosorbent assay (ELISA) (24 h) or cell lysates (2 to 8 h) were prepared for Western immunoblots.
Pharmacological inhibition of IL-8 and MIP-3α secretion.
SB-203580 (11) and Bay 11-7085 were purchased from Calbiochem and dissolved in dimethyl sulfoxide. Caco-2 cells cultured in a 6-well plate or a 12-well plate were fed with 2 ml of fresh warm DMEM-F-12 medium and treated with inhibitors for 1 h at 37°C prior to infection by A/E bacteria. Subsequently, 10 μl of A/E bacteria (grown overnight in LB broth) was then added to the medium and incubated for various times. IL-8 and macrophage inflammatory protein 3α (MIP-3α) were measured in the supernatant by sandwich ELISA using a kit, according to the manufacturer's instructions (BD OptEIA; BD Biosciences). SB-203580 is a cell-permeable specific inhibitor of p38 MAPK with a 50% inhibitory concentration of 34 nM, whereas Bay-11 inhibits NF-κB nuclear translocation by retaining it in the cytoplasm (31).
Assessment of bacterial adherence to Caco-2 cells.
To assess bacterial adhesion, Caco-2 cells were infected with bacteria for 3 to 4 h. The cells were then washed with warm DMEM three times and finally scraped into warm phosphate-buffered saline (PBS) and mixed by pipetting. Serial dilutions were performed, and aliquots of the scraped cells were streaked on agar plates and incubated at 37°C overnight. Bacterial colonies (CFU) were counted the next day. Similarly, Caco-2 cells pretreated with the above-described pharmacological inhibitors were infected with bacteria and scraped into PBS before being plated on agar.
Western blotting and immunoprecipitation.
Following infection with A/E bacteria, cells were washed twice with 2 ml of ice-cold Hank's balanced salt solution (Sigma). The cells were then lysed in 350 to 500 μl of lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride) on ice for 5 to 10 min and then scraped into microcentrifuge tubes. The tubes were centrifuged at 13,000 × g for 5 min to pellet debris, and the supernatant was transferred to another tube for Western blots and immunoprecipitation. Caco-2 proteins (50 μg in cleared cell lysate) were resolved by 9 to 11% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to 0.2-μm polyvinylidene difluoride membranes. The blots were then blocked for 1 h with 5% nonfat milk in Tris-buffered saline (20 mM Tris and 0.3 M NaCl, pH 7.4) with 0.05% Tween 20. The membranes were then incubated with primary antibody in Tris-buffered saline with 0.05% Tween 20 overnight at 4°C and probed with the respective secondary antibody the next day for 1 h at room temperature. Rabbit polyclonal phospho-p38 MAPK and I-κBα antibodies (Cell Signal Technology) were used in the study. Mouse monoclonal antibodies against phospho-ERK and phospho-JNK (Santa Cruz Biotechnology) were used in Western blots to examine the roles of these MAPK in Caco-2 cells.
Conventional semiquantitative RT-PCR.
Mouse colonic tissue (distal colon) was isolated and stored in RNAlater (Qiagen) according to the manufacturer's instructions. Sections of distal colon weighing 2 to 5 mg were homogenized in 2 ml of RLT lysis buffer (Qiagen) for 30 seconds in a 15-ml tube and centrifuged for 5 min at 13,000 × g at room temperature. One to 2 ml of supernatant was used for extraction of RNA using the RNeasy Protect Minikits (Qiagen) spin columns, according to the specifications of the manufacturer. Total RNA from Caco-2 cells grown in 24-well plates was extracted by RNeasy Protect Minikits according to the manufacturer's recommendations. One to 2 μg of RNA was reverse transcribed into cDNA for 60 min at 37°C in 20 μl reaction buffer containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 5.0 mM MgCl2, 100 μM pooled deoxynucleotide triphosphates U of Moloney murine leukemia virus reverse transcriptase (RT), 4-μg random-hexamer primers, and 20 U of RNase inhibitor. The random hexamers were used to provide a “one-step” RT reaction that yielded cDNA for amplification by PCR. A semiquantitative conventional PCR method of amplification was used because the target mRNA was expressed at low copy numbers. Aliquots of reverse-transcribed cDNA were added to PCR buffer to obtain a reaction mixture containing 20 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 100 μM pooled deoxynucleotide triphosphates, 1 to 2 U of Taq DNA polymerase, and 0.25 μM each of glyceraldehyde-3-phosphate dehydrogenase and gene-specific oligonucleotide primers (Nucleotide and Protein Sequencing Unit, University of British Columbia). The PCR conditions were optimized for different genes of interest.
TLR5 activation.
HEK 293 cells were obtained from the ATCC and cultured at 37°C in a 5% CO2 incubator in DMEM containing 10% cosmic calf serum (HyClone, Logan, UT). The day prior to transfection, the HEK 293 cells were plated in a 96-well plate at a density of 5 × 104 per well. Transient transfection was performed using a Polyfect reagent (Qiagen). The cells were transfected with 150 ng NF-κB reporter (an endothelial leukocyte molecule 1 [ELAM-1]-firefly luciferase construct), 15 ng of thymidine kinase Renilla luciferase vector (Promega) to control for transfection efficiency, and 20 ng EF6 human TLR5. The empty vector pEF6 was used as a control and to normalize the DNA concentrations for all transfections to 250 ng per well. Twenty hours after transfection, the cells were stimulated with 10% supernatant from an overnight culture of EPEC or C. rodentium in LB broth, 10 ng/ml of Ultrapure Salmonella FliC (Calbiochem), or LB broth alone. The cells were lysed after 4 h using passive lysis buffer, and the luciferase activity was quantified using the dual-luciferase reporter assay system (Promega). Firefly luciferase units were divided by Renilla units to normalize for transfection efficiency between wells.
Immunofluorescence staining of colon tissues.
Immunofluorescence staining of infected tissues was performed in a fashion similar to that described previously (43). In brief, tissues were rinsed in ice-cold PBS, embedded in optimal cutting template compound (Sakura, Finetech), frozen with isopentane (Sigma) and liquid N2, and stored at −70°C. Serial sections were cut at a thickness of 6 μm and fixed in ice-cold acetone for 10 min. Tissue sections were directly blocked with 1% bovine serum albumin, followed by the addition of antibodies against the dendritic cell marker CD11c (Biolegend; dilution, 1:100) and Tir (dilution, 1:1,000) (43). Following extensive washing with Tris-buffered saline, Alexa 488-conjugated goat anti-mouse and Alexa 568-conjugated goat anti-rat immunoglobulin G antibodies (all at a dilution of 1:300) were added. Images were taken using a Zeiss Axioimager microscope.
Data presentation and statistical analysis.
All of the results are expressed as the mean value ± standard error of the mean. The results presented are from one representative infection out of at least three. Statistical analysis was performed using the one-way analysis of variance test and the Student t test, with a P value of <0.05 considered significant.
RESULTS
EPEC supernatants induce IL-8 secretion from Caco-2 cells.
Previous studies suggested that flagellin is the major factor within the EPEC supernatant that triggers IL-8 secretion from T84 human IEC (47). To determine if WT EPEC culture supernatant produces a similar response in LPS-hyporesponsive Caco-2 IEC, filter-sterilized bacterial culture supernatants were prepared from bacteria grown overnight in LB broth. Exposure to 200 to 400 μl of WT EPEC supernatant for 3 h produced a significant amount of IL-8 (1,007.5 ± 62 pg/ml) from Caco-2 cells (Fig. 1A) 24 h after initial infection. These levels were 40- to 50-fold higher than those of unstimulated controls and comparable to IL-1β-induced IL-8 secretion, included as a positive control. In contrast, ΔfliC EPEC supernatant caused only a modest IL-8 release (147.9 ± 27.3 pg/ml), approximately 10-fold lower than that seen with WT EPEC supernatant. EscN is an ATPase required for the functioning of the EPEC T3SS (13), which is a molecular needle-like structure used by EPEC to insert bacterial effector proteins into host cells and to subvert host cellular functions. We tested the ΔescN EPEC mutant, which is impaired in the generation of the T3SS and is thus unable to translocate bacterial effectors into host cells but is fully capable of producing flagellin. Exposure of Caco-2 cells to supernatant from ΔescN EPEC produced an induction of IL-8 secretion similar to that of WT EPEC (1,080 ± 79 pg/ml). These data confirm the prominent proinflammatory role played by EPEC flagellin and demonstrate that the T3SS does not affect the ability of EPEC supernatant to induce IL-8 in Caco-2 cells.
FIG. 1.
(A) EPEC supernatants induce IL-8 secretion from Caco-2 cells. Bacterial culture supernatant from overnight cultures of WT and mutant EPEC grown in LB broth were filter sterilized; 200 to 400 μl/well of supernatant was added to the growth medium of Caco-2 cell monolayers in 6- or 12-well plates for 3 h, cells were washed, and fresh medium was added. The cell culture supernatant was collected after 24 h, and the IL-8 concentration was quantified by ELISA. The bacteria were grown overnight in LB broth. IL-1β (10 ng/ml) was included as a positive control. The error bars indicate standard errors of the mean. *, P < 0.05 versus unstimulated. (B) Direct infection by EPEC bacteria triggers IL-8 secretion in a partially flagellin-dependent manner. WT EPEC, FliC-deficient EPEC (ΔfliC), complemented ΔfliC EPEC (Comp ΔfliC), T3SS-deficient EPEC (ΔescN), and a double mutant lacking FliC and T3SS (ΔfliC/ΔescN) were grown overnight in LB broth as described in Materials and Methods, and 10 μl of bacterial culture was used to infect Caco-2 cells in 2 ml of DMEM-F-12 in the absence of antibiotics and serum. After 3 h, the cells were washed twice with DMEM, and the medium was replaced with medium containing serum and antibiotics with gentamicin added to prevent the growth of extracellular bacteria. The cell culture supernatant was collected for IL-8 ELISA. The error bars indicate standard errors of the mean. *, P < 0.05 versus uninfected; **, P < 0.05 versus ΔfliC EPEC; ***, P < 0.05 versus WT EPEC; †, P < 0.05 versus ΔfliC EPEC.
Direct infection by EPEC can trigger IL-8 secretion in a FliC-independent manner.
While flagellin is the major factor in the EPEC supernatant that stimulates IL-8 secretion from epithelial cells, it is unknown if the same is true when IEC undergo direct infection by EPEC. To address this question, Caco-2 cells were infected by different EPEC strains for 3 h, followed by gentamicin treatment. As shown in Fig. 1B, WT EPEC infection induced 836.6 ± 97.4 pg/ml of IL-8 (24 h after initial infection), significantly less than the response to its supernatant in Fig. 1A (P < 0.05).
The flagellin-deficient ΔfliC EPEC infection produced a 20-fold-higher response than the uninfected control, and while this was twofold lower than WT, it was significantly higher than the IL-8 response to its supernatant in Fig. 1A (408.9 ± 42.6 versus 147.9 ± 27.3 pg/ml; P < 0.05). To confirm that the impaired inflammatory response elicited by ΔfliC EPEC was due to the loss of flagellin expression, we complemented the ΔfliC EPEC strain with a plasmid carrying the full-length fliC gene (46) and measured the IL-8 response from Caco-2 cells. As shown in Fig. 1B, levels of IL-8 were found to be significantly increased compared to the ΔfliC EPEC (671 ± 85.5 pg/ml versus 409 ± 42.6 pg/ml; P < 0.05). The amount of IL-8 released by Caco-2 cells due to complemented ΔfliC EPEC was approximately 80% of the amount induced by WT EPEC infection. This finding is consistent with that of Zhou et al. (47), who earlier reported partial restoration of IL-8 secretion from T84 IEC following complementation of flagellin-deficient EPEC. Our data indicate that flagellin is required for full induction of chemokine release from IEC.
The EPEC T3SS suppresses the FliC-independent IL-8 response.
Previous studies had implicated the LEE-encoded T3SS (and the bacterial effector proteins that are dependent on its function to translocate into host cells) in causing (13, 35), as well as suppressing (37), the inflammatory response by EPEC-infected epithelial cells. Interestingly, the loss of the LEE-encoded T3SS (ΔescN) did not attenuate IL-8 levels (Fig. 1B) but instead led to significantly greater IL-8 release than with WT EPEC (1,162.2 ± 71 versus 836.6 ± 97.4 pg/ml; P < 0.05). These data indicate that the mechanisms underlying the inflammatory response to direct EPEC infection are far more complex than that seen with EPEC supernatant.
To assess if the IL-8 response to direct infection by ΔfliC EPEC was linked either directly or indirectly to the LEE-encoded T3SS, a double ΔfliC/ΔescN mutant was generated. As shown in Fig. 1B, infection with the ΔfliC/ΔescN mutant bacteria yielded approximately twofold-higher levels of IL-8 than ΔfliC bacteria (783 ± 67 versus 408.9 ± 42.6 pg/ml; P < 0.05), indicating that the IL-8 response was augmented by loss of the T3SS. Considering that EPEC lacking escN does not translocate effector proteins, it is plausible that suppression of fliC-independent response is caused by a T3SS-dependent translocated effector. Moreover, these results demonstrate that even in the absence of flagellin, EPEC is capable of triggering a strong IL-8 response from infected Caco-2 cells that does not require the translocation of bacterial effectors into host cells.
p38 MAPK and NF-κB differentially regulate WT and ΔfliC EPEC-induced IL-8 secretion from Caco-2 cells.
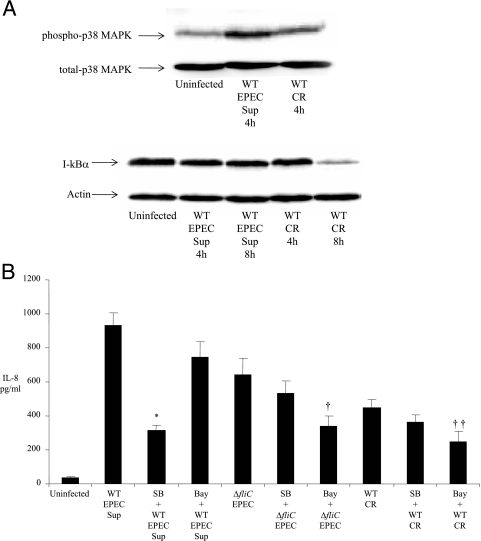
The next study examined which signaling pathways were involved in the IL-8 response to infection by WT and ΔfliC EPEC, focusing on p38 MAPK and NF-κB activation. Previous studies had shown that purified A/E flagellin and bacterial supernatants containing flagellin activate both pathways (4). Using a rabbit polyclonal antibody that detected the active phosphorylated tyrosine and threonine p38 MAPK, EPEC infection was observed to cause significant activation of p38 MAPK after 2 h of infection (Fig. 2A), as indicated by an intense 43-kDa band. Furthermore, the ΔfliC EPEC also activated p38 MAPK more than an uninfected control. IL-1β treatment for 2 h caused p38 activation and was included as a positive control. To assess activation of the NF-κB signaling pathway by EPEC, we examined the stability of I-κB protein following infection with WT and ΔfliC EPEC. A time course of I-κB stability was studied beginning at 2 h and followed until 8 h postinfection (p.i.). As shown in Fig. 2A, both strains caused degradation of I-κB, beginning at 4 h and reaching the maximal level at 8 h after infection, as indicated by loss of band intensity. Next, we investigated if ERK and JNK MAPK were activated at between 1 and 8 h during WT EPEC infection. Using specific antibodies against the phosphorylated species of these kinases, we found that ERK and JNK were not significantly activated in Caco-2 cells at the time points studied (data not shown).
FIG. 2.
(A) WT and flagellin-deficient EPEC strains activate p38 MAPK and induce I-κB degradation in Caco-2 cells. Caco-2 cells were infected with WT and flagellin-deficient (ΔfliC) EPEC for 2 to 8 h. Cell lysates were prepared as described in Materials and Methods. Fifty micrograms of cleared whole-cell lysate was subjected to SDS-PAGE in Western blots. The membranes were probed with rabbit polyclonal primary antibodies against human phospho-p38 MAPK, total p38 MAPK (top), and I-κB (bottom). Mouse monoclonal antibody was used for detection of actin (internal loading control). The blots were finally developed by enhanced chemiluminescence. (B) IL-8 secretion by flagellin-deficient EPEC is mediated preferentially by NF-κB in Caco-2 cells. Cells were pretreated with 10 μM SB-203580 (p38 MAPK inhibitor) and 20 μM Bay 11-7085 for 1 h and infected with WT and flagellin-deficient (ΔfliC) EPEC for 3 h. The cells were washed twice, and fresh warm medium was added. The supernatant was collected as described in the text for IL-8 ELISA. The error bars indicate standard errors of the mean. * and **, P < 0.05 versus WT; ***, P < 0.05 versus ΔfliC EPEC. SB-203580 and Bay-11 were prepared in dimethyl sulfoxide (vehicle), which did not induce IL-8 from Caco-2 cells (data not shown). (C) Outline of TLR5-mediated NF-κB and MAPK signaling pathway in a human IEC undergoing infection by WT EPEC.
We next examined the effect of pharmacological inhibition of these pathways on IL-8 secretion caused by EPEC infection. We pretreated Caco-2 cells with the p38 MAPK pharmacological inhibitor SB-203580 (10 μM) and the NF-κB inhibitor Bay 11-7082 (20 μM) for 1 h and then infected these cells with WT EPEC for 3 h. As shown in Fig. 2B, p38 MAPK inhibition decreased IL-8 secretion significantly (threefold) (882 ± 69 versus 290 ± 81 pg/ml; P < 0.05). Inhibition of NF-κB also caused a 30% decrease in IL-8 secretion compared to inhibition of p38 MAPK (882 ± 69 versus 290.4 ± 34 pg/ml; P < 0.05). These results indicate that p38 MAPK plays a critical role in EPEC-induced IL-8 secretion, with NF-κB activation contributing modestly to chemokine secretion. We next examined the roles of these two pathways in the FliC-independent response. As described above, Caco-2 cells were pretreated with pharmacological inhibitors, followed by infection with ΔfliC EPEC. As indicated in Fig. 2B, NF-κB inhibition produced a significant (50%) decrease in IL-8 levels following ΔfliC EPEC (432.4 ± 43 versus 219.3 ± 38; P < 0.05) infection, whereas p38 MAPK inhibition did not significantly affect IL-8 secretion (375.6 ± 87 versus 432.4 ± 43), clarifying that although the IL-8 response to WT EPEC infection is predominantly MAPK dependent, the IL-8 response to ΔfliC EPEC is predominantly NF-κB dependent. A summary of the NF-κB and p38 MAPK signaling pathways is depicted in Fig. 2C.
Flagellin is expressed by EPEC, but not C. rodentium, in LB broth culture.
C. rodentium is a related A/E pathogen that infects mice, and it has recently provided a valuable animal model of human EHEC and EPEC infection and disease. While C. rodentium is described as nonmotile, its genome was recently released, and genes for two flagellins (FliC and Flag-2) were found within the C. rodentium genome (www.sanger.ac.uk/Projects/C_rodentium/) (33). To clarify whether C. rodentium is truly flagellin deficient, we assessed flagellin expression through immunoblotting using a mouse monoclonal antibody raised against pathogenic-E. coli flagella (and broadly cross-reactive against most flagellin types). Blotting of culture supernatants of WT EPEC and ΔescN EPEC grown overnight in LB broth (Fig. 3A) detected a single intense 65-kDa band corresponding to FliC protein. In contrast, we saw no expression of flagellin in the culture supernatant of ΔfliC EPEC or in the culture supernatant from C. rodentium. The motilities of these microbes were then assessed using 0.3% motility agar plates. Both WT and ΔescN EPEC strains were motile (Fig. 3B), whereas the ΔfliC EPEC and C. rodentium were nonmotile, on agar plates. These data confirm that the ΔfliC EPEC generated in our laboratory was deficient in flagellin protein and indicate that C. rodentium does not express detectable flagellin or flagella when grown in LB broth.
FIG. 3.
(A) Flagellin expression in LB broth culture supernatant of WT and mutant EPEC and WT C. rodentium. Flagellin expression was assessed in WT EPEC, flagellin-deficient EPEC (ΔfliC EPEC), T3SS-deficient EPEC (ΔescN EPEC), and C. rodentium (CR) grown overnight in LB broth as described in the text. The bacterial culture supernatants were filter sterilized, and 100 μg of protein was subjected to SDS-PAGE in Western blots. The blots were probed with a mouse monoclonal antibody raised against E. coli flagellin and subsequently developed in enhanced chemiluminescence. (B) A/E bacterial motility assay in 0.3% agar. LB agar (0.3%) plates were inoculated with WT EPEC, FliC-deficient (ΔfliC) EPEC, T3SS-deficient (ΔescN) EPEC, and C. rodentium (CR) and incubated for 48 h at 37°C. (C) Bacterial adherence assay in Caco-2 cells. WT EPEC, ΔfliC EPEC, and C. rodentium (CR) were used to infect Caco-2 cells for 3 to 4 h. After infection, the cells were washed with DMEM three times and scraped into sterile warm PBS. Serial dilutions were prepared, plated on agar plates, and incubated overnight. y axis, CFU; x axis, bacterial strains plated at a dilution of 10−5. The error bars indicate standard errors of the mean. *, P < 0.05 versus ΔfliC EPEC.
To compare how closely bacterial strains adhered to IEC, we performed a bacterial-adherence assay using Caco-2 cells. We infected these cells with WT EPEC, ΔfliC EPEC, and C. rodentium for 3 to 4 h. The Caco-2 cells were then washed repeatedly and collected in PBS. An aliquot of the cells was streaked on agar plates, and bacterial colonies (CFU) were counted the following day. As shown (Fig. 3C), all strains were found to adhere to Caco-2 cells, with maximum adherence (105.3 × 105 ± 6 × 105 CFU) noted for WT EPEC. Interestingly, while both ΔfliC EPEC and C. rodentium also adhered to Caco-2 cells, their adherence was attenuated compared to WT EPEC. In particular, the adherence of C. rodentium was significantly delayed and was impaired even compared to ΔfliC EPEC. These findings suggest that adherence to IEC occurs by different mechanisms among A/E pathogens and may be attributed to structures such as flagella, pili, and fimbriae during the infectious cycles of these microbes in their hosts.
Infection by ΔfliC EPEC and C. rodentium, but not exposure to supernatants, induces IL-8 secretion.
It is not known if C. rodentium is able to trigger a proinflammatory response in IEC in vitro. To assess this, culture supernatants and whole ΔfliC EPEC and C. rodentium bacteria were added to Caco-2 cells for 3 h. As shown in Fig. 4, infection with ΔfliC EPEC bacteria caused significantly (10-fold) greater IL-8 secretion than the culture supernatant alone (420.5 ± 45 versus 42.3 ± 7 pg/ml; P < 0.05). Similarly, infection with C. rodentium also yielded IL-8 secretion threefold higher than the culture supernatant alone (246.3 ± 33.3 versus 79.4 ± 10 pg/ml; P < 0.05). The IL-8 response elicited by ΔfliC EPEC and C. rodentium bacteria was significantly greater than that caused by the laboratory E. coli strain K-12 (C. rodentium versus K-12, 242.7 ± 38 versus 165.7 ± 20 pg/ml; P < 0.05). Even so, exposure of Caco-2 cells to K-12 induced IL-8 release that was higher than that for the uninfected control (127 ± 18 versus 38 ± 12 pg/ml; P < 0.05). These data suggest that direct infection by ΔfliC-deficient EPEC and C. rodentium, but not their supernatants, induces a significant release of IL-8 from Caco-2 cells.
FIG. 4.
Infection by flagellin-deficient EPEC and C. rodentium (CR), but not exposure to supernatants, induces IL-8 secretion. Bacterial culture supernatant (Sup) and whole bacteria were prepared as described in the legend to Fig. 1. Ten microliters of bacterial culture and 200 μl of filter-sterilized bacterial culture supernatant were added to Caco-2 cells in growth medium for 3 h. Subsequently, the cells were washed, and the medium was replaced with fresh warm medium treated with gentamicin. The cell culture supernatant was subsequently collected for IL-8 ELISA. The error bars indicate standard errors of the mean. *, P < 0.05 versus uninfected cells and cells exposed to K-12; **, P < 0.05 versus WT C. rodentium supernatant and K-12.
TLR5 is activated by WT EPEC but not ΔfliC EPEC or C. rodentium supernatants.
We next assessed whether mammalian TLR5 senses and is activated by bacterial products from EPEC and C. rodentium. HEK 293 cells were transiently transfected with an ELAM-luciferase reporter to measure NF-κB activity and cotransfected with TLR5. We assessed NF-κB activity by measuring the luminescence of transfected cells following exposure to purified FliC or to the supernatant taken from the overnight cultures of WT EPEC, ΔfliC EPEC, and C. rodentium. Ultrapure FliC (10 ng/ml; Calbiochem) activated NF-κB in TLR5-transfected cells 50- to 60-fold compared to unstimulated cells or cells transfected with an empty vector (Fig. 5). Similarly, EPEC supernatants activated the TLR5 reporter by 20- to 25-fold relative to TLR5 transfectants treated with an equal volume of LB broth. In contrast, ΔfliC EPEC supernatant activated TLR5 by only threefold, whereas C. rodentium supernatant did not cause any significant increase in reporter activity, demonstrating that only WT EPEC supernatant activates cells through TLR5. This activation was specific, as WT EPEC supernatant did not activate NF-κB in HEK 293 cells transfected with TLR8, while stimulation with a TLR8 agonist, R484, led to a five- to eightfold increase in NF-κB-dependent luciferase activity (data not shown). No significant activity was measured in cells transfected with an empty vector and stimulated under the same conditions.
FIG. 5.
NF-κB is activated by the supernatant of WT EPEC but not that of ΔfliC EPEC or C. rodentium. HEK 293 cells transiently transfected with ELAM-luciferase NF-κB reporter human TLR5 were exposed to WT EPEC, FliC-deficient EPEC (ΔfliC EPEC), or WT C. rodentium (CR) or exposed to an ultrapure S. enterica serovar Typhimurium flagellin preparation. NF-κB activity is represented as induction.
To further clarify whether the inflammatory response generated against C. rodentium is flagellin independent, we generated a double mutant deficient in the two flagellin genes (for FliC and Flag-2) known to exist in C. rodentium. This mutant strain was then compared to WT C. rodentium for IL-8 response from Caco-2 cells. We observed that IL-8 secretion from the mutant strain was similar to that from WT C. rodentium (429 ± 54 versus 393.5 ± 69 pg/ml) (data not shown). This led us to conclude that flagellin is unlikely to be involved in IL-8 secretion from cultured IEC infected with C. rodentium.
EPEC infection increases MCP-1, MIP-3α, and β-defensin-2 gene expression.
To date, most studies examining epithelial inflammatory responses to EPEC infection have focused on secretion of the neutrophil chemoattractant IL-8, since EPEC infection is associated with neutrophil infiltration (36). Less is known about the recruitment of other inflammatory cells within the human intestine during EPEC infection. Based on the in vivo C. rodentium model, infection may also lead to the influx of macrophages (22) and the upregulated expression of antimicrobial peptides (21). While dendritic cells play an important role in innate responses at the mucosal barrier (10), their role and function in the C. rodentium model of infectious colitis (39) has not been assessed. To address whether epithelial cells could initiate a similar inflammatory response following EPEC infection, the expression levels of the monocyte chemoattractant protein MCP-1, the dendritic cell chemoattractant MIP-3α, and the antimicrobial peptide β-defensin-2 were assessed and compared to IL-1β treatment as our positive control. As shown in Fig. 6, infection led to the increased expression of both MCP-1 and β-defensin-2 in an almost completely FliC-dependent manner. In contrast, significant expression of MIP-3α expression was noted following both WT and ΔfliC EPEC infection. Interestingly, infection with ΔescN EPEC was accompanied by the exaggerated expression of all three genes to levels exceeding the expression due to WT EPEC infection, confirming the suppressive role of the LEE-encoded T3SS.
FIG. 6.
Infection with WT EPEC increases MCP-1 and β-defensin-2 gene expression in Caco-2 cells. Cells were grown to confluence in 12-well plates and subsequently infected with WT EPEC, FliC-deficient (ΔfliC) EPEC, or T3SS-deficient (ΔescN) EPEC for 3 h in 2 ml DMEM without antibiotics and serum. After infection, the cells were washed twice, and fresh medium was added with gentamicin treatment. Total RNA was extracted 6 h after initial infection and subjected to semiquantitative RT-PCR to measure gene expression as described in Materials and Methods. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was included as an internal control.
ΔfliC EPEC and C. rodentium induce IL-8 and MIP-3α secretion after prolonged infection.
Since we observed a delayed attachment of ΔfliC EPEC and C. rodentium to Caco-2 cells, we assayed the inflammatory response induced after a longer infection of 4 h, focusing on IL-8 and MIP-3α secretion from Caco-2 cells. The double mutant ΔfliC/ΔescN EPEC was included to verify our earlier data and to exclude possible effects of the T3SS. As predicted, we found increased IL-8 secretion following ΔfliC EPEC infection compared to uninfected controls (584.1 ± 41 versus 48 ± 23; P < 0.05) (Fig. 7A). Similarly, C. rodentium-induced IL-8 secretion was also significantly higher than in controls (409 ± 92 versus 48 ± 23 pg/ml; P < 0.05). Importantly, infection for 4 h with both ΔfliC and C. rodentium resulted in substantial increases in IL-8 secretion compared to 3 h of infection (Fig. 4) (ΔfliC, 584.1 ± 41 versus 420.5 ± 45 pg/ml, P < 0.05, and C. rodentium, 409 ± 92 versus 246.3 ± 33.3, P < 0.05). As expected, the ΔfliC/ΔescN EPEC infection yielded an even greater IL-8 response than both ΔfliC EPEC and C. rodentium. Interestingly, WT EPEC infection for 4 h (compared to a 3-h infection earlier [Fig. 1B and 2B]) did not significantly affect IL-8 release from Caco-2 cells, suggesting it had reached a plateau.
FIG. 7.
(A) IL-8 secretion by flagellin-deficient (ΔfliC) EPEC and C. rodentium after 4 h of infection in Caco-2 cells. Cells grown in six-well plates were infected with WT EPEC, FliC-deficient EPEC (ΔfliC EPEC), a double EPEC mutant lacking FliC and T3SS (ΔfliC/escN EPEC), and WT C. rodentium (CR) for 4 h. After infection, the cells were washed and fresh medium was added with gentamicin as described in the text. The cell culture supernatant was collected for IL-8 ELISA. The error bars indicate standard errors of the mean. *, P < 0.05 versus uninfected. (B) MIP-3α secretion by flagellin-deficient EPEC and C. rodentium after 4 h of infection in Caco-2 cells. MIP-3α protein levels in cell culture supernatants was quantified by ELISA using the same samples as for panel A. *, P < 0.05 versus uninfected.
The secretion of MIP-3α from Caco-2 cells after 4 h of infection followed a trend similar to that of the IL-8 response (Fig. 7B). Whereas WT EPEC caused a 10-fold increase in MIP-3α (1,048.3 ± 43 versus 125 ± 24 pg/ml; P < 0.05), ΔfliC infection produced a 7-fold increase over the control (717.5 ± 78 pg/ml; P < 0.05) and C. rodentium infection caused a 4-fold increase (479 ± 56 pg/ml; P < 0.05). As expected, the double mutant yielded a greater response, similar to that triggered by WT EPEC infection. We conclude that EPEC FliC triggers a rapid inflammatory response but that given sufficient time to attach to host cells, both ΔfliC EPEC and C. rodentium can cause significant IL-8 and MIP-3α release from epithelial cells.
C. rodentium activates both p38 MAPK and NF-κB in epithelial cells.
Based on the similarities in the inflammatory responses triggered by ΔfliC EPEC and C. rodentium, we tested the possibility that C. rodentium may also preferentially signal through NF-κB rather than p38 MAPK. As shown in Fig. 8A, while C. rodentium activated p38 MAPK with an increased 43-kDa band, the activation was modest, and as expected, it was much less than that induced by the supernatant from WT EPEC. In contrast, while both WT EPEC supernatant and C. rodentium decreased I-κB stability in Caco-2 cells after 8 h of infection, the band corresponding to C. rodentium infection was of lower intensity than that for the supernatant, suggesting that NF-κB is a major contributor to the inflammatory response to C. rodentium infection.
FIG. 8.
(A) Infection by C. rodentium activates an NF-κB-dependent pathway in Caco-2 cells. Caco-2 cells grown to confluence were exposed to filter-sterilized WT EPEC bacterial culture supernatant (Sup WT EPEC) grown overnight in LB broth. Similarly, an inoculum of 10 μl from WT C. rodentium (WT CR) culture grown overnight in LB broth was used to infect Caco-2 cells for 4 h. Subsequently, cell lysates were prepared after 4 h and 8 h of infection as described in the text. Fifty micrograms of cleared cell lysates was subjected to SDS-PAGE and probed with rabbit polyclonal antibodies to phospho-p38 MAPK, total MAPK (top), and I-κBα (bottom). Mouse monoclonal antibody was used for detection of actin (internal loading control). The blots were developed by enhanced chemiluminescence. (B) IL-8 secretion by flagellin-deficient EPEC requires NF-κB in Caco-2 cells. Caco-2 cells grown to confluence were pretreated with p38 MAPK inhibitor (SB-203580 [SB];10 μM) and 20 μM NF-κB inhibitor (Bay 11-7085) for 1 h. The cells were subsequently exposed to filter-sterilized supernatant from WT EPEC (Sup WT) or infected with FliC-deficient EPEC (ΔfliC) or C. rodentium (CR) for 4 h. The cells were washed as described in the text and treated with gentamicin. IL-8 was quantified in the cell culture supernatant 24 h after infection. Uninf., uninfected. *, P < 0.05 versus Sup WT; †, P < 0.05 versus ΔfliC; ‡, P < 0.05 versus CR.
To test this hypothesis, we assessed the impact of p38 MAPK and NF-κB inhibition on the IL-8 response in Caco-2 cells infected with C. rodentium for 4 h, comparing the results to those obtained from cells stimulated for the same duration with ΔfliC EPEC, as well as the supernatant from WT EPEC cultures. Exposure to WT EPEC supernatant induced significant IL-8 release that declined threefold due to MAPK inhibition (931.8 ± 73 versus 315.4 ± 28; P < 0.05) (Fig. 8B), whereas NF-κB inhibition caused only a modest decrease in IL-8 (931.8 ± 73 versus 747 ± 92), similar to the results obtained with 3-h stimulation (Fig. 2B). NF-κB inhibition yielded a more dramatic decline in the IL-8 release due to 4 h of ΔfliC EPEC and C. rodentium infection; the ΔfliC EPEC IL-8 response to infection decreased to approximately 50% in the presence of NF-κB inhibitor (644 ± 97 versus 339 ± 58; P < 0.05) compared to ΔfliC alone. Inhibition of NF-κB also abrogated the C. rodentium IL-8 response approximately 45% (450 ± 48 versus 247.7 ± 60; P < 0.05). In contrast, MAPK inhibition of cells exposed to ΔfliC EPEC or C. rodentium caused only a 20% decrease in IL-8 levels. The above data support our hypothesis that, like ΔfliC EPEC, NF-κB contributes to the C. rodentium-induced proinflammatory response in Caco-2 cells.
In vivo C. rodentium infection is associated with rapid MIP3α induction and dendritic cell recruitment.
As outlined above, EPEC and C. rodentium infection of Caco-2 cells led to increased expression and release of the dendritic cell chemokine MIP-3α; however, it is unclear if a similar response occurs during in vivo A/E bacterial infection, particularly since reports have yet to characterize dendritic cell recruitment into the intestinal mucosa during these infections. To determine the potential in vivo relevance of these findings, C3H/HeJ mice were infected with C. rodentium, and their tissues were assessed for chemokine responses at day 2 p.i. Compared to uninfected tissues, MIP3α expression showed a fivefold increase in the distal colon by day 2 p.i. (Fig. 9A). Since mice do not express IL-8, we also assessed MIP2, another neutrophil chemokine that is expressed in mice, and observed a similar fivefold increase in expression (Fig. 9A). We previously demonstrated that neutrophil recruitment into the colons of C. rodentium-infected mice coincides with an upregulation in MIP2α expression (22). To assess whether the increased MIP3α expression had a functional impact, colonic tissues were immunostained for the dendritic cell marker CD11c and the bacterial Tir protein, which is required for the attachment of A/E pathogens to epithelial cells. As shown in Fig. 9B, few CD11c-positive (CD11c+) cells were identified within uninfected colonic tissues, and no immunoreactivity to Tir was seen, but by day 2 p.i., numerous CD11c+ cells were observed within the mucosa, often just beneath apical epithelial cells, as well as at the bases of colonic crypts and in the submucosa (Fig. 9C). As expected, we detected bacterial Tir in the infected colon, mostly localized at the apical surfaces of epithelial cells and often in proximity to the CD11c+ cells. These results clarify that in vivo C. rodentium infection is associated with the recruitment of dendritic cells, putatively in response to increased expression of MIP3α during infection.
FIG. 9.
(A) C. rodentium infection is associated with increased chemokine gene expression in the infected colon. MIP3α (open bars) and MIP2α (filled bars) expression in control and day 2 p.i. mice was assessed by quantitative RT-PCR. As shown, MIP3α and MIP2α expression at day 2 p.i. was significantly increased (*, P < 0.05) over control levels. (B and C) C. rodentium infection leads to dendritic cell recruitment to the infected colon in contrast to control colon tissues (B), where few if any CD11c+ cells were detected (green) and no bacterial Tir (a T3SS protein) was seen (red). However, by day 2 p.i. (C), numerous CD11c+ cells were detected in the infected colon (arrows) and immunoreactive bacterial Tir protein was seen on the apical surface. These putative dendritic cells were predominantly localized near the colonic lumen, as well as at the bases of crypts.
DISCUSSION
Our data confirm that EPEC flagellin is the major proinflammatory molecule found within the EPEC culture supernatant; however, aside from inducing IL-8 release from epithelial cells, EPEC infection also activates a spectrum of proinflammatory genes, including the dendritic cell chemokine MIP-3α, MCP-1, and the antimicrobial peptide β-defensin-2. Moreover, our studies demonstrate that direct infection by WT EPEC leads to a complex host response involving both the epithelial response to flagellin and a delayed inflammatory response that is FliC independent. This FliC-independent response includes a subset of the FliC-induced inflammatory genes, including IL-8 and MIP3α. Like many other gram-negative bacterial pathogens, both EPEC and C. rodentium possess a T3SS that acts like a molecular syringe to deliver bacterial effector proteins into host cells (13). While earlier studies raised the possibility that the EPEC LEE-encoded effectors contribute to proinflammatory-chemokine secretion (12, 13), our results instead demonstrate that the LEE-encoded T3SS does not cause the FliC-independent response, but rather, it inhibits both the FliC-dependent and -independent epithelial inflammatory responses. Interestingly, infection with the related mouse-specific A/E pathogen C. rodentium also generated an inflammatory response in epithelial cells similar to that raised against ΔfliC EPEC. Based on these findings, we were able to identify an early MIP-3 response in vivo that correlated with a rapid influx of dendritic cells into the colonic mucosa of C. rodentium-infected mice.
Since the discovery that flagellin from enteroaggregative E. coli induces IL-8 release from IEC (40), flagellins from many bacterial pathogens, including EPEC flagellin, have been assessed for their proinflammatory roles. These earlier studies found that purified EPEC flagellin induces IL-8 expression in IEC (47), while similar findings were made with EHEC flagellin (4). While these studies correctly highlighted the importance of EPEC flagellin as the major proinflammatory bacterial product within the supernatant, it has remained unclear whether A/E pathogens can cause significant inflammatory responses from epithelial cells in the absence of flagellin expression. We propose that simple exposure of epithelial cells to bacterial supernatants is not representative of an actual A/E bacterial infection. While epithelial cells undergo direct infection by A/E bacteria, the cells are also exposed to numerous bacterial products, as well as the LEE-encoded T3SS-dependent injection of translocated bacterial effector proteins into host cells. Addressing the potential for non-FliC-dependent inflammation is important, since several studies have suggested that EPEC deregulates FliC expression when exposed to culture media and/or epithelial cells. For example, integration host factor was found to repress the expression of flagellin in EPEC and EHEC when grown in DMEM (45). Similarly, studies of other bacterial pathogens suggest that while flagellin expression may occur at early stages of infection, or at specific tissue sites, flagellin expression may be reduced or even absent at later stages of infection, as was recently described for Salmonella enterica serovar Typhimurium (11).
While our demonstration of an inflammatory response occurring in the absence of FliC was not surprising, the fact that over time it reached an intensity similar to that seen with FliC expressing WT EPEC was unexpected, suggesting that Caco-2 cells may rapidly reach a plateau in their inflammatory responses. At present, it is unclear what bacterial products are responsible for the IL-8 response elicited by ΔfliC EPEC. Our studies demonstrate that T3SS-translocated effector proteins are not responsible, since the ΔescN strain induces a strong IL-8 response yet is unable to translocate effector proteins into host cells. EPEC does elaborate other virulence factors, including bundle-forming pilin and fimbriae (25, 41); thus, it is tempting to speculate that these bacterial factors, as well as others, may be involved.
It is also unclear what host factors are involved in triggering the FliC-independent IL-8 response. Our assessment of TLR5 activation suggests that neither ΔfliC EPEC nor C. rodentium significantly induces TLR5 activation. Moreover, we ruled out the possibility that the host response against C. rodentium was directed against flagella by generating C. rodentium mutants deficient in both FliC and Flag-2 flagellin genes and testing them for the ability to induce an IL-8 response that was similar to that of WT C. rodentium infection. Other TLRs could be involved, and in a previous study, we showed that both C. rodentium and EPEC activate the LPS receptor TLR4 (20). While we reported that TLR4 activation did contribute to the inflammatory response to C. rodentium, the role of TLR4 in the inflammatory response by IEC during enteric infections remains controversial. Moreover, TLR4 is unlikely to be involved in the IL-8 response we detected in Caco-2 cells, since this cell line is known to be hyporesponsive to LPS (1). Other studies have also found that TLR4 expression is very low in Caco-2 cells, and these cells lack MD-2, which is required for activation of TLR4 (14). In addition, we found that Caco-2 cells secrete only insignificant levels of IL-8 when exposed to LPS (data not shown). Therefore the IL-8 response against ΔfliC EPEC and C. rodentium infection is unlikely to involve TLR4.
Another explanation for the flagellin-independent responses seen in this study may be that they occur through stimulation of innate nucleotide oligomerization domain 2 (NOD2) (22) receptors that detect bacterial muramyl dipeptide, a component of bacterial pepditoglycan (3), in Caco-2 cells. Since both EPEC and Citrobacter species have muramyl dipeptide in their cell walls (7, 16), these components may be shed by bacteria and sensed by NOD2 in Caco-2 cells. Outer membrane proteins of EPEC have also been implicated in inducible nitric oxide synthase (iNOS) induction in Caco-2 cells (23); however, the receptor for recognition of these proteins has yet to be identified. One may surmise that the inflammatory response we detect is multifactorial, and it will require significant additional studies to tease out the specific roles of bacterial, as well as host, factors.
Previous studies and our results suggest that the release of chemokines following EPEC infection is dependent on activation of p38 MAPK and NF-κB in Caco-2 cells (Fig. 2C). Other groups have also shown activation of ERK and JNK, in addition to p38 MAPK, due to EPEC infection in T84 cells (12). In contrast, we found that p38 MAPK is the major MAPK activated by direct EPEC infection in Caco-2 cells, while only modest activation of ERK and JNK was detected. These results may be explained by the specific characteristics of Caco-2 IEC. While ERK and JNK were not significantly activated by EPEC in Caco-2 cells, these MAPK could be important in the innate responses elicited in other types of IEC, such as T84 or HT-29 cells, which are LPS responsive. It is known that p38 MAPK activates AP-1 and stabilizes IL-8 mRNA transcripts during cytokine-induced IL-8 secretion from epithelial cells (20). Interestingly, ΔfliC EPEC and C. rodentium both induced IL-8 and MIP-3α secretion in epithelial cells that involved the NF-κB pathway. Activation of the NF-κB pathway during C. rodentium infection was also demonstrated in a recent study investigating hyperplasia of colonic epithelial cells (44). In our experiments, the adherence of bacterial strains to Caco-2 cells was unaffected by the pharmacological inhibitors we used to assess the roles of MAPK and NF-κB.
Based on our results, we propose a paradigm in which flagellin monomers released by EPEC during infection activate p38 MAPK and NF-κB pathways, whereas later, non-FliC-dependent responses largely involve NF-κB signaling. We also show that EPEC is able to suppress both FliC-dependent and -independent epithelial responses in a LEE-encoded T3SS-dependent manner. Our results further suggest that the LEE-encoded T3SS-mediated suppression of both types of inflammatory responses seen in our study could be mediated by suppression of NF-κB signaling, since this transcription factor is involved in both flagellin-dependent and -independent responses. Moreover, Maresca et al. (24) showed that EPEC suppression of antimicrobial iNOS in Caco-2 cells is mediated by alteration of NF-κB activity. These authors also found that suppression of iNOS is dependent on EPEC adherence, as nonadherent bacteria were unable to modulate NF-κB.
The aim of our study was to determine the scope and nature of epithelial proinflammatory responses to EPEC and C. rodentium. We also asked whether these responses are triggered solely by bacterial flagellin or if other virulence factors, including the LEE-encoded T3SS, were involved. For our studies, we used the Caco-2 human intestinal epithelial cell line, in which the innate mechanisms and TLR signaling are well characterized. Moreover, these cells are hyporesponsive to LPS, which is thought to be reflective of enterocytes in vivo. While C. rodentium would normally infect murine epithelial cells, not much is known about the expression of TLR signaling pathways in murine IEC. Furthermore, results obtained from murine IEC are difficult to interpret due to the lack of a specific IL-8 homolog that is characteristic of the innate response in human IEC. Therefore, we reasoned that since C. rodentium can infect human IEC in vitro, infection of Caco-2 cells and direct comparison with the proinflammatory response against EPEC was warranted. It will be interesting, however, to determine in future studies if C. rodentium induces any differential responses in murine IEC.
We should note that previous studies have also examined the contributions of virulence factors in EPEC that induce inflammatory responses from human IEC. For example, Czerucka et al. (12) tested the roles of EPEC LEE-encoded T3SS and intimin in IEC responses, focusing on phosphoactivation of MAPK p38 and ERK. However, their studies assessed IL-8 release from T84 cells, which are LPS responsive. Further, these studies were limited, as other important chemokines, such as MIP-3α and antimicrobial peptides, were not tested.
Several groups have found that C. rodentium colitis is characterized by significant intestinal inflammation in murine models of colitis (18, 19, 39), including inflammatory responses by epithelial cells (22, 38). Considering the important role attributed to flagellin in intestinal inflammation, and since C. rodentium has been described as a nonmotile pathogen, it has remained uncertain how this pathogen causes intestinal inflammation. We confirmed that C. rodentium is not motile in vitro, nor does it activate TLR5 under in vitro conditions. Despite this, our results indicate that C. rodentium infection of IEC leads to significant chemokine secretion, comparable to that observed in response to flagellin-deficient EPEC. Since C. rodentium does not activate TLR5 in vitro, another TLR or innate receptor may well be involved in this response. As FliC plays an important role in the recruitment of neutrophils in mucosal inflammation and bacterial colitis (46), the absence of FliC protein expression in C. rodentium may explain why infection by this murine pathogen causes a modest granulocyte infiltration of the infected colon (19).
Our findings support the putative role of the intestinal epithelium as the cell population that initiates and directs the inflammatory response against A/E pathogens. While the host response to these pathogens is not well understood, the increased expression of both chemokines, as well as antimicrobial peptides, has been detected in infected tissues (32, 47). Our data suggest that the expression of these mediators during A/E pathogen infections may be initially due to epithelial cells. Moreover, although neutrophils have been associated with A/E bacterial infections, our data show that EPEC- and C. rodentium-infected epithelial cells are capable of releasing additional chemokines, which signal to and attract dendritic cells to the site of colonic infection and inflammation. At present, the role of dendritic cells in C. rodentium infection is unclear, but these host cells are known to often play a key role in the development of adaptive immunity and have previously been associated with the phagocytosis of other intestinal bacterial pathogens, including Salmonella enterica serovar Typhimurium.
In summary, our data advance the current knowledge regarding epithelial cell inflammatory responses to A/E pathogens, clarifying that EPEC FliC can activate TLR5 and induce chemokine and antimicrobial gene expression, as well as demonstrating that these pathogens can trigger a robust epithelial inflammatory response even in the absence of FliC expression. Dissecting what will undoubtedly be a complex mixture of bacterial and host factors involved in this FliC-independent response will be the focus of future studies. Our data also support findings by Sharma et al. (37) that the presence of the T3SS inhibits rather than induces epithelial inflammatory responses. Thus, A/E pathogens not only trigger epithelial inflammatory responses, they may also be able to locally limit these responses, creating protected niches that permit the growth and survival of these bacteria in the face of the host response. This protection and the development of prolonged infections may lead to greater intestinal inflammation and tissue damage in infected hosts. Addressing both actions and clarifying their in vivo relevance will provide us with a better understanding of the pathogenesis of these infections and perhaps provide novel approaches for therapies or vaccination against A/E pathogens.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 28 January 2008.
REFERENCES
- 1.Abreu, M. T., M. Fukata, and M. Arditi. 2005. TLR signaling in the gut in health and disease. J. Immunol. 1744453-4460. [DOI] [PubMed] [Google Scholar]
- 2.Allen-Vercoe, E., M. C. Toh, B. Waddell, H. Ho, and R. DeVinney. 2005. A carboxy-terminal domain of Tir from enterohemorrhagic Escherichia coli O157:H7 (EHEC O157:H7) required for efficient type III secretion. FEMS Microbiol. Lett. 243355-364. [DOI] [PubMed] [Google Scholar]
- 3.Barnich, N., J. E. Aguirre, H. C. Reinecker, R. Xavier, and D. K. Podolsky. 2005. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor-κB activation in muramyl dipeptide recognition. J. Cell Biol. 17021-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berin, M. C., A. Darfeuille-Michaud, L. J. Egan, Y. Miyamoto, and M. F. Kagnoff. 2002. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-κB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell Microbiol. 4635-648. [DOI] [PubMed] [Google Scholar]
- 5.Bramley, A. M., M. A. Khan, H. E. Manson, and R. G. Hegele. 2003. Development of respiratory syncytial virus “bronchiolitis” in guinea pigs does not reflect an allergic predisposition in the host. Chest 124671-681. [DOI] [PubMed] [Google Scholar]
- 6.Chakravortty, D., and K. S. Kumar. 1999. Interaction of lipopolysaccharide with human small intestinal lamina propria fibroblasts favors neutrophil migration and peripheral blood mononuclear cell adhesion by the production of proinflammatory mediators and adhesion molecules. Biochim. Biophys. Acta 1453261-272. [DOI] [PubMed] [Google Scholar]
- 7.Chart, H., and B. Rowe. 1989. The outer membrane protein of enteropathogenic Escherichia coli, described as the ‘localised adherence factor’, is OmpF and probably not involved in adhesion to HEp-2 cells. FEMS Microbiol. Lett. 52291-295. [DOI] [PubMed] [Google Scholar]
- 8.Chen, H. D., and G. Frankel. 2005. Enteropathogenic Escherichia coli: unravelling pathogenesis. FEMS Microbiol. Rev. 2983-98. [DOI] [PubMed] [Google Scholar]
- 9.Clarke, S. C., R. D. Haigh, P. P. Freestone, and P. H. Williams. 2003. Virulence of enteropathogenic Escherichia coli, a global pathogen. Clin. Microbiol. Rev. 16365-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colonna, M., B. Pulendran, and A. Iwasaki. 2006. Dendritic cells at the host-pathogen interface. Nat. Immunol. 7117-120. [DOI] [PubMed] [Google Scholar]
- 11.Cummings, L. A., W. D. Wilkerson, T. Bergsbaken, and B. T. Cookson. 2006. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol. Microbiol. 61795-809. [DOI] [PubMed] [Google Scholar]
- 12.Czerucka, D., S. Dahan, B. Mograbi, B. Rossi, and P. Rampal. 2001. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect. Immun. 691298-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Grado, M., C. M. Rosenberger, A. Gauthier, B. A. Vallance, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli infection induces expression of the early growth response factor by activating mitogen-activated protein kinase cascades in epithelial cells. Infect. Immun. 696217-6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckmann, L. 2004. Innate immunity and mucosal bacterial interactions in the intestine. Curr. Opin. Gastroenterol. 2082-88. [DOI] [PubMed] [Google Scholar]
- 15.Gauthier, A., J. L. Puente, and B. B. Finlay. 2003. Secretin of the enteropathogenic Escherichia coli type III secretion system requires components of the type III apparatus for assembly and localization. Infect. Immun. 713310-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genereux, C., D. Dehareng, B. Devreese, J. Van Beeumen, J. M. Frere, and B. Joris. 2004. Mutational analysis of the catalytic centre of the Citrobacter freundii AmpD N-acetylmuramyl-l-alanine amidase. Biochem. J. 377111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gewirtz, A. T. 2006. Flag in the crossroads: flagellin modulates innate and adaptive immunity. Curr. Opin. Gastroenterol. 228-12. [DOI] [PubMed] [Google Scholar]
- 18.Hansen, K. K., P. M. Sherman, L. Cellars, P. Andrade-Gordon, Z. Pan, A. Baruch, J. L. Wallace, M. D. Hollenberg, and N. Vergnolle. 2005. A major role for proteolytic activity and proteinase-activated receptor-2 in the pathogenesis of infectious colitis. Proc. Natl. Acad. Sci. USA 1028363-8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins, L. M., G. Frankel, I. Connerton, N. S. Goncalves, G. Dougan, and T. T. MacDonald. 1999. Role of bacterial intimin in colonic hyperplasia and inflammation. Science 285588-591. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann, E., O. Dittrich-Breiholz, H. Holtmann, and M. Kracht. 2002. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 72847-855. [PubMed] [Google Scholar]
- 21.Iimura, M., R. L. Gallo, K. Hase, Y. Miyamoto, L. Eckmann, and M. F. Kagnoff. 2005. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J. Immunol. 1744901-4907. [DOI] [PubMed] [Google Scholar]
- 22.Khan, M. A., C. Ma, L. A. Knodler, Y. Valdez, C. M. Rosenberger, W. Deng, B. B. Finlay, and B. A. Vallance. 2006. Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect. Immun. 742522-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malladi, V., M. Puthenedam, P. H. Williams, and A. Balakrishnan. 2004. Enteropathogenic Escherichia coli outer membrane proteins induce iNOS by activation of NF-κB and MAP kinases. Inflammation 28345-353. [DOI] [PubMed] [Google Scholar]
- 24.Maresca, M., D. Miller, S. Quitard, P. Dean, and B. Kenny. 2005. Enteropathogenic Escherichia coli (EPEC) effector-mediated suppression of antimicrobial nitric oxide production in a small intestinal epithelial model system. Cell Microbiol. 71749-1762. [DOI] [PubMed] [Google Scholar]
- 25.Melo, A. R., E. B. Lasunskaia, C. M. de Almeida, A. Schriefer, T. L. Kipnis, and W. Dias da Silva. 2005. Expression of the virulence factor, BfpA, by enteropathogenic Escherichia coli is essential for apoptosis signalling but not for NF-κB activation in host cells. Scand. J. Immunol. 61511-519. [DOI] [PubMed] [Google Scholar]
- 26.Michail, S. K., D. R. Halm, and F. Abernathy. 2003. Enteropathogenic Escherichia coli: stimulating neutrophil migration across a cultured intestinal epithelium without altering transepithelial conductance. J. Pediatr. Gastroenterol. Nutr. 36253-260. [DOI] [PubMed] [Google Scholar]
- 27.Milon, A., E. Oswald, and J. De Rycke. 1999. Rabbit EPEC: a model for the study of enteropathogenic Escherichia coli. Vet. Res. 30203-219. [PubMed] [Google Scholar]
- 28.Mundy, R., T. T. MacDonald, G. Dougan, G. Frankel, and S. Wiles. 2005. Citrobacter rodentium of mice and man. Cell Microbiol. 71697-1706. [DOI] [PubMed] [Google Scholar]
- 29.Nougayrede, J. P., P. J. Fernandes, and M. S. Donnenberg. 2003. Adhesion of enteropathogenic Escherichia coli to host cells. Cell Microbiol. 5359-372. [DOI] [PubMed] [Google Scholar]
- 30.O'Neil, D. A., E. M. Porter, D. Elewaut, G. M. Anderson, L. Eckmann, T. Ganz, and M. F. Kagnoff. 1999. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 1636718-6724. [PubMed] [Google Scholar]
- 31.Parhar, K., A. Ray, U. Steinbrecher, C. Nelson, and B. Salh. 2003. The p38 mitogen-activated protein kinase regulates interleukin-1β-induced IL-8 expression via an effect on the IL-8 promoter in intestinal epithelial cells. Immunology 108502-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramirez, K., R. Huerta, E. Oswald, C. Garcia-Tovar, J. M. Hernandez, and F. Navarro-Garcia. 2005. Role of EspA and intimin in expression of proinflammatory cytokines from enterocytes and lymphocytes by rabbit enteropathogenic Escherichia coli-infected rabbits. Infect. Immun. 73103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren, C. P., S. A. Beatson, J. Parkhill, and M. J. Pallen. 2005. The Flag-2 locus, an ancestral gene cluster, is potentially associated with a novel flagellar system from Escherichia coli. J. Bacteriol. 1871430-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothbaum, R. J., R. A. Giannella, and J. C. Partin. 1982. Diarrhea caused by adherent enteropathogenic E. coli. J. Pediatr. 101486. [DOI] [PubMed] [Google Scholar]
- 35.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 36.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1996. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect. Immun. 644480-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma, R., S. Tesfay, F. L. Tomson, R. P. Kanteti, V. K. Viswanathan, and G. Hecht. 2006. Balance of bacterial pro- and anti-inflammatory mediators dictates net effect of enteropathogenic Escherichia coli on intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 290G685-694. [DOI] [PubMed] [Google Scholar]
- 38.Sherman, M. A., and D. Kalman. 2004. Initiation and resolution of mucosal inflammation. Immunol. Res. 29241-252. [DOI] [PubMed] [Google Scholar]
- 39.Spahn, T. W., C. Maaser, L. Eckmann, J. Heidemann, A. Lugering, R. Newberry, W. Domschke, H. Herbst, and T. Kucharzik. 2004. The lymphotoxin-beta receptor is critical for control of murine Citrobacter rodentium-induced colitis. Gastroenterology 1271463-1473. [DOI] [PubMed] [Google Scholar]
- 40.Steiner, T. S., J. P. Nataro, C. E. Poteet-Smith, J. A. Smith, and R. L. Guerrant. 2000. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J. Clin. Investig. 1051769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tatsuno, I., R. Mundy, G. Frankel, Y. Chong, A. D. Phillips, A. G. Torres, and J. B. Kaper. 2006. The lpf gene cluster for long polar fimbriae is not involved in adherence of enteropathogenic Escherichia coli or virulence of Citrobacter rodentium. Infect. Immun. 74265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulshen, M. H., and J. L. Rollo. 1980. Pathogenesis of Escherichia coli gastroenteritis in man—another mechanism. N. Engl. J. Med. 30299-101. [DOI] [PubMed] [Google Scholar]
- 43.Vallance, B. A., W. Deng, M. De Grado, C. Chan, K. Jacobson, and B. B. Finlay. 2002. Modulation of inducible nitric oxide synthase expression by the attaching and effacing bacterial pathogen Citrobacter rodentium in infected mice. Infect. Immun. 706424-6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, Y., G. S. Xiang, F. Kourouma, and S. Umar. 2006. Citrobacter rodentium-induced NF-κB activation in hyperproliferating colonic epithelia: role of p65 (Ser536) phosphorylation. Br. J. Pharmacol. 148814-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yona-Nadler, C., T. Umanski, S. Aizawa, D. Friedberg, and I. Rosenshine. 2003. Integration host factor (IHF) mediates repression of flagella in enteropathogenic and enterohaemorrhagic Escherichia coli. Microbiology 149877-884. [DOI] [PubMed] [Google Scholar]
- 46.Yu, Y., S. Sitaraman, and A. T. Gewirtz. 2004. Intestinal epithelial cell regulation of mucosal inflammation. Immunol. Res. 2955-68. [DOI] [PubMed] [Google Scholar]
- 47.Zhou, X., J. A. Giron, A. G. Torres, J. A. Crawford, E. Negrete, S. N. Vogel, and J. B. Kaper. 2003. Flagellin of enteropathogenic Escherichia coli stimulates interleukin-8 production in T84 cells. Infect. Immun. 712120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]