Abstract
Naturally acquired antibody responses provide partial protection from clinical malaria, and blood-stage parasite vaccines under development aim to prime such responses. To investigate the determinants of antibody response longevity, immunoglobulin G (IgG) antibodies to several blood-stage vaccine candidate antigens in the sera of two cohorts of children of up to 6 years of age during the dry seasons of 2003 and 2004 in The Gambia were examined. The first cohort showed that most antibodies were lost within less than 4 months of the first sampling if a persistent infection was not present, so the study of the second-year cohort involved collecting samples from individuals every 2 weeks over a 3-month period. Antibody responses in the second cohort were also influenced by persistent malaria infection, so analysis focused particularly on children in whom parasites were not detected after the first time point. Antibodies to most antigens declined more slowly in children in the oldest age group (>5 years old) and more rapidly in children in the youngest group (<3 years old). However, antibodies to merozoite surface protein 2 were shorter lived than antibodies to other antigens and were not more persistent in older children. The age-specific and antigen-specific differences were not explained by different IgG subclass response profiles, indicating the probable importance of differential longevities of plasma cell populations rather than antibody molecules. It is likely that young children mostly have short-lived plasma cells and thus experience rapid declines in antibody levels but that older children have longer-lasting antibody responses that depend on long-lived plasma cells.
Immunity to mild Plasmodium falciparum malaria is acquired after repeated infections, although the longevity of the relevant components of the immune response that mediate this protection needs to be better determined (31). Passive transfer experiments have shown the importance of antibodies against blood-stage parasites in serum, and there are epidemiological associations between antibodies to particular P. falciparum antigens and protection from malaria, with some discrimination between those antibodies that are likely to be protective and those that are simply covariate (15, 27).
Levels of naturally acquired antibodies to P. falciparum antigens in sera have been shown previously to peak and decline rapidly after clinical malaria infections in young children (1, 7, 18, 19, 23). It is possible that the clearance of antibodies is particularly rapid during the resolution of a clinical malaria episode, and it is important that studies of antibody decline be conducted also with asymptomatic individuals who have previously resolved their infections. A model to explain cross-sectional age-specific serological profiles indicates that low levels of antibodies may be maintained for many years after infection (11), and early studies using crude malaria antigen preparations also indicated that antibodies can be detected for some years after infection (4, 9).
Antibody-secreting plasma cells can be short or long lived. Both types can be generated in the germinal center, and short-lived plasma cells can also be generated in the T-cell-rich extrafollicular regions. Short-lived plasma cells need to be replenished from a memory B-cell population, but long-lived plasma cells survive and secrete antibody for extended periods independently (20, 30). The longevity of antibody responses in the absence of continued antigenic presentation may provide an indication of the plasma cell populations responsible for antibody secretion.
To study the longevity of naturally acquired antibody responses to malaria antigens, children of up to 6 years of age in The Gambia were recruited into two longitudinal study cohorts and monitored during annual dry seasons when there was no detectable malaria transmission. We examined the duration of naturally acquired antibody responses to merozoite antigens apical merozoite antigen 1 (AMA1), erythrocyte binding antigen 175 (EBA175), merozoite surface protein 1 (MSP1), and MSP2, for which vaccine constructs have been developed and are under preclinical development or clinical testing (29, 34), as well as crude P. falciparum schizont extract. Associations among the longevity of antibody responses and the persistence of parasites, the ages of children, residential locations, and ethnicities were examined, as well as differences among the antigens.
MATERIALS AND METHODS
Study area.
Samples were collected during the dry seasons of 2003 and 2004 from children under 74 months of age living in The Gambia in the town of Farafenni and surrounding villages, an area situated approximately 130 km from the coast. Rainfall and the transmission of malaria are very rare during the dry season between November and June, so the study was conducted during this season in two different years when the risk of incident infections was minimal. Each year, consultations and open meetings with community leaders and traditional rulers were held to obtain community-wide consent prior to inviting individual participation and informed consent. The studies were reviewed and approved by the Medical Research Council Scientific Coordinating Committee and the Medical Research Council and Gambian Government Joint Ethics Committee.
Dry season cohort 1 (December 2002 to June 2003).
At the beginning of the dry season in December 2002, 129 children under 6 years of age whose parents provided written informed consent after the study and procedure were explained were enrolled for a prospective study of antibody levels. A finger prick blood sample (minimum of 200 μl) was collected from each child for the preparation of serum samples and thick-blood-smear slides. Later, during April, May, and June in the 2003 dry season, the children were visited every 2 weeks and a finger prick blood sample was requested upon every alternate visit (once per month) for serum sample and thick-blood-smear preparation. Each child, therefore, had a maximum of four blood samples collected, on day 0 in December 2002 and between days 129 and 141 (mean, day 133) in April, days 162 and 174 (mean, day 166) in May, and days 191 and 205 (mean, day 197) in June 2003. After the first time point, there were 17 refusals and 14 children were not located for follow-up, yielding 98 children with at least one follow-up sample. The analysis of antibody response longevity during the dry season was conducted with samples from 50 children that had enzyme-linked immunosorbent assay (ELISA) measurements of antibodies to at least one of the merozoite antigens of above 0.5 optical density (OD) units on day 0.
Dry season cohort 2 (February to May 2004).
Following the analysis of the results for cohort 1, a second cohort study with more frequent sampling during the dry season was designed to more precisely estimate antibody decline rates. Sampling every 2 weeks over a 3-month period was considered important for the capture of antibody decline, as was the presence of high antibody levels at the start. As cohort 1 showed that asymptomatic parasitemia substantially extended antibody response longevity, it was considered a priority to study the determinants of antibody longevity in children in cohort 2 who did not have parasitemia. Therefore, to provide an adequately large subcohort that would be parasite negative but have high antibody levels, children in cohort 2 were randomized so that half would receive antimalarial treatment to clear any asymptomatic parasitemia.
In early February 2004, 626 children under 7 years of age were enrolled and finger prick blood samples were collected for serum sample and thick-blood-smear preparation. Sera were screened by ELISA for levels of immunoglobulin G (IgG) antibodies to one or more of the five blood-stage antigens tested of greater than 1.0 OD units to identify 264 children with particularly high antibody levels who were potentially eligible for the longitudinal study. Approximately 21 days after this initial screening, 152 eligible children were relocated and consent for follow-up over a 12-week period was obtained. Venous blood samples of 5 ml were collected (the day 0 samples were taken in late February), and children were randomized with 50% probability for antimalarial treatment (regardless of slide positivity, as infections can be transiently undetectable) with a combination of sulfadoxine and pyrimethamine (Fansidar) and artesunate to clear parasitemia. Children were visited every week (between days 7 and 85), and finger prick blood samples were collected every 2 weeks, between days 11 and 19 (mean, day 16), days 24 and 35 (mean, day 30), days 39 and 50 (mean, day 45), days 52 and 62 (mean, day 57), days 66 and 76 (mean, day 71), and days 80 and 91 (mean, day 85), for serum sample and thick-blood-smear preparation. There were 124 children who had ELISA-determined levels of antibodies to one or more antigens of above 0.5 OD units on day 0 (late February) and who gave five or more samples at different time points from day 0 until the end of the study that were analyzed for antibody longevity. Of these children in the final analysis, 57 (46%) were female, 61 (49%) received antimalarial treatment, 97 (78%) lived in the town of Farafenni while 27 (22%) lived in surrounding villages, 81 (65%) were Mandinka, 18 (15%) were Fula, 15 (12%) were Wolof, and 10 (8%) were of other or mixed ethnicities. For the analysis of antibody longevity, ELISA OD values were transformed into standardized units by using a standard curve of IgG concentrations as described below.
Malaria parasite detection.
Giemsa-stained thick blood smears prepared from blood taken at each of the sampling time points were assessed by two experienced microscopists examining 100 high-powered fields (magnification, ×1,000) to determine the presence, the density, and the species of malaria parasites. Slides for which results differed by ≥log10 parasites/high-powered field were examined by a third microscopist to derive a final estimation. The analysis in this case considered only the presence or absence of P. falciparum on the slides (this was the only parasite species seen in this study).
Parasite antigens.
Antibodies were studied with five different recombinant proteins representing different blood-stage vaccine candidate antigens, AMA1, EBA175, MSP119, and MSP2 (two allelic types). The AMA1 antigen was an Escherichia coli-expressed full-length ectodomain comprising amino acids 83 to 531 of the P. falciparum strain 3D7 AMA1 (described previously as r-AMA1/E) (12). The EBA175 protein was a baculovirus-expressed His8-tagged antigen comprising amino acids 144 to 753 of the EBA175 sequence of the 3D7 strain, corresponding to the cysteine-rich region II (10). Three E. coli-expressed glutathione S-transferase fusion proteins were used, one based on the MSP119 sequence of the Wellcome P. falciparum strain (5) and two based on MSP2 amino acids 1 to 184 and 22 to 247 encoded by the CH150/9 (type A) and Dd2 (type B) alleles, respectively (27). The glutathione S-transferase fusion tag on its own was also expressed from the pGEX-2T vector as a negative control antigen. P. falciparum parasites from the 3D7 parasite clone were cultured to prepare schizont extract for a crude-antigen ELISA.
Antibody assays.
Blood samples for antibody analysis were collected in serum separator tubes (BD Vacutainer Systems, United Kingdom) and stored on ice during transfer from the field to the laboratory. Upon arrival at the laboratory, tubes were centrifuged at 1,100 × g for 5 min and sera were removed for storage at −20°C for cohort 1 and at −80°C for cohort 2. ELISAs were performed as described previously (6, 7, 27, 28). Briefly, each well of 96-well plates (Immulon 4 HBX; Dynatech) was coated with 50 ng of recombinant antigen or 10 μg (for cohort 1) and 5 μg (for cohort 2) of crude schizont extract antigen in 100 μl of coating buffer. Plates were incubated overnight at 4°C for antigen binding, after which the wells were washed four times in phosphate-buffered saline with 0.005% Tween 20 (PBS/T) and blocked for 4 to 5 h at room temperature with 200 μl of PBS/T per well and 1% milk powder. Wells were then washed three times in PBS/T, 100-μl aliquots of test sera at a 1/500 dilution in PBS/T with 1% milk powder were added in duplicate, and the plates were incubated overnight at 4°C. Wells were washed four times in PBS/T and then incubated for 3 h at room temperature with 100 μl of horseradish peroxidase-conjugated goat anti-human IgG (Dako UK, Ltd.) at 1:5,000 in PBS/T. Wells were washed four times in PBS/T, and then 100 μl of ortho-phenylene diamine solution (0.4 mg of ortho-phenylene diamine/ml, 0.1 M citric acid, 0.2 M Na2HPO4) was added, the plates were incubated at room temperature for up to 15 min before the reaction was stopped with 50 μl of 2 M sulfuric acid, and ODs at 490 nm for the wells were determined. To generate standardized units of specific IgG reactivity for cohort 2 data, additional wells were coated with purified myeloma-derived human IgG, IgG1, or IgG3 (The Binding Site, United Kingdom) in 12 doubling (1,000 to 0.5 μg ml−1) and 12 tripling (1,000 to 5 × 10−5 μg ml−1) dilutions, which were assayed alongside the test sera, generating a standard curve (a sigmoidal logistic four-parameter curve generated using SigmaPlot software) to allow OD units to be interpolated to linearized units of antibody concentrations (6). Whenever the OD for a test serum sample was above the level that could be clearly interpolated from the standard curve, the serum was retested at additional doubling dilutions (1/1,000, 1/2,000, and 1/4,000) alongside the range of standard IgG concentrations as necessary.
Statistical analysis.
The nonparametric Wilcoxon rank sum (Mann-Whitney) test was used to test for differences in continuous variables between two groups. To examine associations between continuous variables (antibodies in particular) and ordered categorical variables (age categories in particular), the nonparametric Kruskal-Wallis test was used. Pearson's chi-squared test for contingency tables, or Fisher's exact test when the expected value of any cell of a table was less than 5, was used to test for significant differences in proportions of categorical variables between groups.
Estimates of the longevity of antibody to each antigen in each individual were made with two different parameters, as follows: (i) determination of the lowest antibody level estimated at any time point as a proportion of the antibody level on day 0, termed the lowest ratio (high values represent little reduction in antibody during the follow-up period, while lower values represent more substantial antibody decline), and (ii) survival analysis of antibody levels above 50% of day 0 values, which can be considered to approximately represent serum antibody level half-life from the beginning of the follow-up (Kaplan-Meier plots and log rank tests were used to test the distributions over time and the significance of associated variables).
RESULTS
Dry season cohort 1.
For the first cohort studied, at the beginning of the dry season in December 2002, ELISA-determined OD levels of IgG antibodies against all P. falciparum antigens (five recombinant merozoite proteins, AMA1, EBA175, MSP119, MSP2 types A and B, and schizont extract) in sera from children with slides positive for P. falciparum were significantly higher than those in sera from children with negative slides (P < 0.05 for each antigen) (Fig. 1A). Antibody levels were also positively associated with age, as expected (P < 0.001 for all antigens, except MSP119, for which the association was not significant). Fifty children with ELISA-determined levels of antibody for at least one of the recombinant proteins of above 0.5 OD units and with at least one additional sample collected later in the dry season were included in an initial analysis of the longevity of antibodies. These children had a mean age of 3.4 years, and 16 (34%) of them had detectable parasites in December 2002.
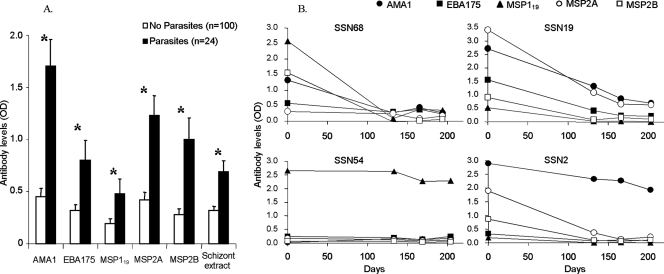
FIG. 1.
(A) ELISA-determined serum antibody levels (ODs) in cohort 1 children at the beginning of the dry season (December 2002), showing means and standard errors for those children with and without detected malaria parasites among the 124 children for whom a blood slide was examined. Antibody levels were significantly higher in those with parasites (*, P < 0.05 for all antigens). (B) Illustration of dry season changes in antibody levels in four representative 4-year-old children of cohort 1. Among these, only subject SSN2 had parasites on day 0 (December 2002) and none had parasites detected at the later time points.
Antibody ELISA OD levels in the later dry season samples (those from April to June 2003) were significantly lower than the starting levels (December 2002) for all individual antigens (P < 0.05 for each). As expected, there were generally larger reductions in ELISA OD levels for children with no parasites detected after December 2002 than for children with parasites detected at at least one of the later time points (P < 0.05 for AMA1, MSP2A, and schizont extract; similar trends for other antigens were not significant). However, there was also substantial variation in the persistence of antibodies among individuals that had no parasites detected, as illustrated by data for four of the children shown in Fig. 1B. The full data set for this cohort is available in the supplemental material (Table S1). The results for the first cohort thus indicated that a more intensive sampling schedule during the early and middle part of the dry season would be needed to track antibody declines more precisely over a period of less than 4 months and that the study should ideally include a larger sample group of children with high initial antibody levels that were parasite negative during follow-up.
Dry season cohort 2.
To maximize the amount of information on antibody longevity, a cohort of 152 children that had high levels of antibodies to one or more of the blood-stage antigens was recruited after the screening of 626 children in early February 2004. The selected cohort was then monitored prospectively from late February (day 0) for 12 weeks, with finger prick blood sampling every 2 weeks, yielding a final cohort for analysis comprising 124 children that had at least five samples taken over these 12 weeks. For each individual, relative levels of IgG antibody to each of the test antigens in serum at each time point were determined, with interpolation from a standard curve of IgG concentrations (antibody profiles of four individuals are shown in Fig. 2A). The full data set for this cohort is available in the supplemental material (Table S2). For each individual, the first time point at which the measured level of antibody to each antigen was less than 50% of the initial (day 0) level was determined, as well as the lowest antibody level measured at any time point as a proportion of the day 0 level (Fig. 2B).
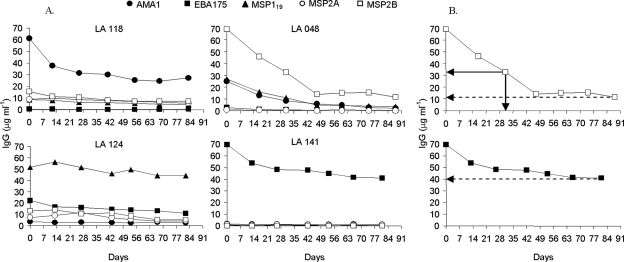
FIG. 2.
(A) Individual profiles of four study subjects in cohort 2 showing levels of IgG antibodies to merozoite antigens in the absence of parasites at different time points after day 0. Individual LA118 had antibodies to AMA1 that declined in the first few weeks and then remained at a stable level, while antibodies to other antigens remained at low levels throughout; individual LA048 had antibodies to MSP2B, AMA1, and MSP119 that declined over the first 6 weeks, with some residual antibodies to MSP2B remaining afterwards; individual LA124 had antibodies to MSP119 and lower levels of antibodies to other antigens that remained stable throughout; individual LA141 had antibodies only to EBA175 that declined slightly in the first few weeks and then remained at a stable level. (B) Illustration of antibody response longevity measurements, applied to two of the profiles (antibodies to MSP2B in individual LA148 and antibodies to EBA175 in individual LA141). The measurement of the lowest antibody concentration is indicated by dashed arrows to the y axes within the plots, and the decline to a level below 50% of the day 0 concentration and the estimated initial half-life are indicated by solid arrows to the x and y axes. (Top panel) MSP2B profile. Lowest ratio of antibody concentration to initial concentration, 12/69 (17%); estimated antibody half-life, 31 days. (Bottom panel) EBA175 profile. Lowest ratio of antibody concentration to initial concentration, 40/70 (57%); estimated initial half-life, undefined (beyond the period of the follow-up).
As expected, children with parasites detected at any time point after day 0 had longer-lasting antibody responses than children without parasites (Fig. 3). Parasite infection after day 0 was seen only in those children that were parasite slide positive on day 0, except for six children that were parasite negative on day 0 and were not randomized for antimalarial treatment (these children were presumed to have subpatent infections with subsequent recrudescence of parasitemia). A Kaplan-Meier survival analysis of the distribution of times for antibody levels in each individual to reach <50% of initial levels showed this difference between the parasite-positive and -negative groups to be significant for both allelic MSP2 antigens and the schizont extract (Fig. 3A). The difference was also significant for both MSP2 antigens in the analysis of the lowest measured antibody levels as proportions of initial levels (Fig. 3B). Neither analysis showed significant differences for other merozoite antigens (Fig. 3). Among children in whom parasite infection persisted, the antibodies to MSP2 and those to other antigens were similarly long lived (Fig. 3), but in the absence of infection the antibodies to MSP2 declined more rapidly than those to the other antigens (P < 0.05 by both methods of analysis for MSP2B compared with each of the other antigens except MSP2A; antibodies to MSP2A also declined more rapidly than those to the other antigens but only significantly in comparison to antibodies to EBA175 [P < 0.05]).
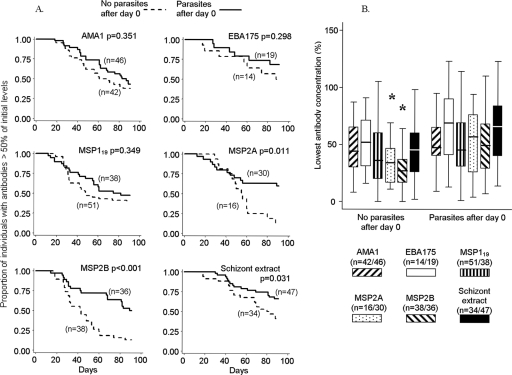
FIG. 3.
Importance of detected parasites as determinants of serum IgG antibody response longevity (cohort 2, days 0 to 91, February to May 2004). (A) Kaplan-Meier plots of survival of antibody levels above 50% of day 0 levels for each antigen among children with and without parasites detected after day 0. Antibodies declined to <50% of the initial levels more rapidly in children without parasites, significantly so for antibodies to MSP2A, MSP2B, and schizont extract. (B) Lowest ratios of antibody levels to starting values. Medians (horizontal lines) and interquartile ranges (boxes) are shown, and bars represent upper and lower adjacent values (*, P < 0.05 for comparison between groups). Antibodies to MSP2A and MSP2B declined to significantly lower levels (as proportions of initial levels) in children without parasites after day 0.
Further analysis of the determinants of antibody longevity focused on the children without parasites after day 0. In this parasite-negative subcohort, there were no differences in the longevity of antibodies to any antigen between males and females, between urban and rural children, between children receiving antimalarial treatment and those not receiving it, or among ethnic groups. To test for associations with age, comparisons among children below 3 years, 3 to 5 years, and older than 5 years of age were made. Antibodies declined more rapidly (Fig. 4A) and to lower levels (Fig. 4B) in younger children. For most of the antigens, the trends were similar and several individual tests were significant, but for MSP2, the antibody longevity showed no association with age (Fig. 4). These age-dependent differences in antibody decline were not dependent on differences in initial antibody levels, which were lower in younger children. Antibody decline parameters were similar in those with relatively low (OD < 1.0) and those with relatively high (OD > 1.0) reactivity at day 0.
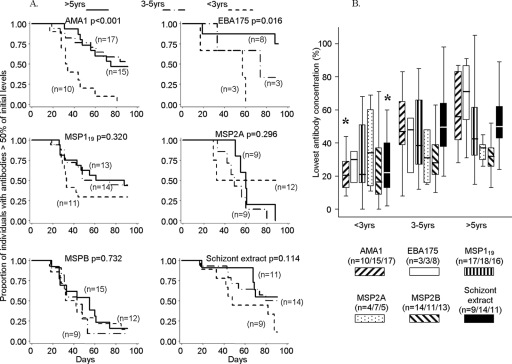
FIG. 4.
Importance of age as a determinant of antibody response longevity (cohort 2, days 0 to 91, February to May 2004) among children without parasites after day 0. (A) Kaplan-Meier plots of survival of antibody levels above 50% of day 0 levels for each antigen, with comparisons among three age categories: <3 years, 3 to 5 years, and >5 years. Antibodies declined to <50% of the initial levels more rapidly in younger children, significantly so for antibodies to AMA1 and EBA175. (B) Lowest ratios of antibody levels to starting values. Medians (horizontal lines) and interquartile ranges (boxes) are shown, and bars represent upper and lower adjacent values (*, P < 0.05 for comparison among age groups). Antibodies to AMA1 and schizont extract declined to significantly lower levels (as proportions of initial levels) in younger children.
Comparison between IgG subclasses.
Naturally acquired human IgG responses to these antigens have previously been shown to involve mostly IgG1 and IgG3, with the predominance of IgG1 against AMA1, MSP119, and EBA175 and that of IgG3 against MSP2 (6, 24, 27, 28, 32, 35), and it has been suggested previously that detectable levels of IgG3 may be particularly short lived due to more rapid clearance of this subclass from the circulation (6, 22). To investigate whether differences in IgG subclasses could explain the differences in antibody longevity seen here, sera from 35 of the children with high initial IgG levels that had been shown to decline were reassayed for levels of IgG1 and IgG3 antibodies to two of the antigens (AMA1 and MSP2). On day 0, there were detectable IgG1 or IgG3 antibodies to both antigens in 17 of the children, to MSP2 only in 10 children, and to AMA1 only in 8 children. The mean time to reach a 50% decrease of initial antibody levels was 39.4 days for IgG1 and 32.6 days for IgG3, while the mean lowest ratios were 27% for IgG1 and 30% for IgG3. There were no significant differences between the subclasses in an analysis of the 17 individuals that had both IgG1 and IgG3 reactivity (Wilcoxon signed-rank test: P = 0.84 by using data for both antigens).
In contrast to the lack of substantial differences in antibody longevity between the IgG1 and IgG3 subclasses, there were significant differences in antibody longevity with age for each of the subclasses considered separately (Table 1). Older children (4 to 6 years old) had mean times to 50% decline that were approximately three times longer (means of 52 days for IgG1 and 47 days for IgG3) than those for the younger group (means of 16 days for both subclasses), a difference that was significant for each of the subclasses separately (P = 0.002 for IgG1 and 0.03 for IgG3). The lowest ratio measurement also showed more antibody decline in the younger individuals, although this difference was not significant (Table 1).
TABLE 1.
Comparison of longevities of IgG1 and IgG3 antibodies in sera in selected children of different ages with high initial levels of IgG that declined during follow-upa
| Age group of children | Mean no. of days to decline to 50% of initial level of:
|
Mean lowest ratio (%) of antibody level to initial level of:
|
||
|---|---|---|---|---|
| IgG1 | IgG3 | IgG1 | IgG3 | |
| 1-3 yrs | 16 | 16 | 17 | 20 |
| 4-6 yrs | 52 | 47 | 31 | 37 |
| P value | 0.002 | 0.03 | 0.17 | 0.38 |
Antibodies were assayed with antigen AMA1 or MSP2. Values are not estimates for the population as a whole but for a subset selected for analysis (35 individuals with marked declines in levels of IgG antibodies to either or both of the two test antigens).
DISCUSSION
The longevity of IgG antibody responses to P. falciparum antigens in sera of young children was here shown to be age dependent, increasing in early childhood through the stage of development when immunity to clinical malaria is normally acquired in the population studied (16, 21). There were differences among antigens, as concentrations of antibodies to the MSP2 antigen declined more rapidly than those of antibodies to the other antigens tested and did not increase in duration with the age of the children. It is not known why antibodies to the MSP2 antigen were shorter lived, but it is possible that tandem repeated epitope sequences in this antigen influence the ability to stimulate effective memory B-cell responses (there are no such repeat sequences in AMA1, EBA175, or MSP119). A high level of within-population sequence polymorphism in the MSP2 antigen is also likely to slow down the acquisition of memory B- and T-cell responses and thus lead to more transient antibody production, although there is considerable cross-reactivity among antibody epitopes in the variants within each of the two major serological types (14).
Although antibody responses to P. falciparum antigens have previously been shown to decline within a few weeks after the clearance of parasites in many individuals (1, 7, 18, 19, 23), the magnitude and profile of the decline have not been well understood and have varied among studies. A detailed study of Kenyan children with clinical malaria showed that the estimated time for a 50% decline of antibodies to the same recombinant proteins studied here was less than 10 days for both IgG1 and IgG3 (19). This time was noted to be shorter than the normally reported catabolic half-lives of these antibodies (particularly the reported half-life of 21 days for IgG1) (22) and indicated that there may be particularly rapid clearance of antibodies during and immediately after the resolution of a clinical malaria episode. The present study enrolled individuals that had various and unrecorded times since their most recent clinical episode, and most individuals had antibodies that persisted at more than 50% of initial levels for much longer than the catabolic half-lives of antibodies would allow. During the whole follow-up period (a mean of 85 days) among those who did not have a persistent infection, the levels of antibodies to the different antigens in the youngest age group declined to a mean of approximately 20 to 30% of initial levels, but residual levels in the oldest group (>5 years old) were approximately twice as high, so antibody production is clearly sustained to various extents in different individuals in the absence of infection. Although declines in specific antibody levels occurred in most children, a substantial minority had levels that were very stable throughout, suggesting steady-state antibody production in these individuals.
Persistent antigen is needed to generate persistent antibody responses resulting from short-lived plasma cells, either by stimulating the proliferation of memory B cells to replenish short-lived plasma cell populations or by stimulating the production of non-germinal center short-lived plasma cells (20). Young children may have plasma cells that are mostly short lived and thus experience rapid decline in antibody levels after infections are cleared. In contrast, the longer-lasting antibody responses in older children may result from having some long-lived plasma cells derived from the germinal center. Other age-related differences have been noted previously, including the observation that antigen presentation and helper T-cell function among young children increase with age (8). During an acute episode of measles, children older than 24 months are more likely to make an effective measles-specific antibody response than younger children (13). The persistence of vaccination-induced antibodies to a capsular polysaccharide of group A Neisseria meningitidis among children also increases with age (17).
Differences between adults and children in antibody response longevity have been suggested from previous epidemiological analyses in Kenya (2, 3) and The Gambia (33). The differences among young children described herein substantially support and extend the evidence for significant age effects. Apart from cumulative exposure to malaria antigens, it is possible that age directly determines the ability to sustain high antibody levels. A lack of ability to generate long-lived plasma cells among infant mice and observed differences in vaccination-induced antibody responses between infant and adult mice indicate inherent age-related differences (25, 26). Following naturally acquired malaria infections, short-lived plasma cells may predominate among younger children and require continuous antigen stimulation for persistent antibody responses. The ability of the immune environment to support long-lived plasma cells may gradually increase with age, such that older children have higher proportions of long-lived plasma cells, enabling antibodies to be continually produced without restimulation by persistent antigen.
Further studies among older children and adults and populations associated with different endemicity patterns should investigate whether persistent antibody responses continue to develop with increasing age or whether differences are restricted to young children. If there is an inherent limitation in the ability of many infants and very young children to produce sustained antibody responses to malaria vaccine candidate antigens, this limitation would contribute to the difficulty of developing an effective blood-stage parasite vaccine for populations in which malaria is endemic.
Supplementary Material
Acknowledgments
We thank the children and their parents for involvement in this study and appreciate the support of many staff members at the Medical Research Council Laboratories in The Gambia, including fieldworkers (Demba Sambou and colleagues) and laboratory technicians (Kintinu Dampha and colleagues). We are grateful to Eleanor Riley, Patrick Corran, Sam Kinyanjui, Kevin Marsh, Lars Hviid, Tony Holder, Jana McBride, David Cavanagh, and Neal Alexander for helpful discussions on antibody response duration and measurement and in some cases also for comments on the manuscript.
The research was funded by the Medical Research Council of the United Kingdom.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 22 January 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Boutlis, C. S., P. K. Fagan, D. C. Gowda, M. Lagog, C. S. Mgone, M. J. Bockarie, and N. M. Anstey. 2003. Immunoglobulin G (IgG) responses to Plasmodium falciparum glycosylphosphatidylinositols are short lived and predominantly of the IgG3 subclass. J. Infect. Dis. 187862-865. [DOI] [PubMed] [Google Scholar]
- 2.Branch, O. H., A. J. Oloo, B. L. Nahlen, D. Kaslow, and A. A. Lal. 2000. Anti-merozoite surface protein-1 19-kDa IgG in mother-infant pairs naturally exposed to Plasmodium falciparum: subclass analysis with age, exposure to asexual parasitaemia, and protection against malaria. V. The Asembo Bay Cohort Project. J. Infect. Dis. 1811746-1752. [DOI] [PubMed] [Google Scholar]
- 3.Branch, O. H., V. Udhayakumar, A. W. Hightower, A. J. Oloo, W. A. Hawley, B. L. Nahlen, P. B. Bloland, D. C. Kaslow, and A. A. Lal. 1998. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kilodalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitaemia, and anaemia. Am. J. Trop. Med. Hyg. 58211-219. [DOI] [PubMed] [Google Scholar]
- 4.Bruce-Chwatt, L. J., J. S. Dodge, C. C. Draper, E. Topley, and A. Voller. 1972. Sero-epidemiological studies on population groups previously exposed to malaria. Lancet i512-515. [DOI] [PubMed] [Google Scholar]
- 5.Burghaus, P. A., and A. A. Holder. 1994. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein. Mol. Biochem. Parasitol. 64165-169. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh, D. R., C. Dobano, I. M. Elhassan, K. Marsh, A. Elhassan, L. Hviid, E. A. T. G. Khalil, T. G. Theander, D. E. Arnot, and J. S. McBride. 2001. Differential patterns of human immunoglobulin G subclass responses to distinct regions of a single protein, the merozoite surface protein 1 of Plasmodium falciparum. Infect. Immun. 691207-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanagh, D. R., I. M. Elhassan, C. Roper, V. J. Robinson, H. Giha, A. A. Holder, L. Hviid, T. G. Theander, D. E. Arnot, and J. S. McBride. 1998. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J. Immunol. 161347-359. [PubMed] [Google Scholar]
- 8.Clerici, M., L. DePalma, E. Roilides, R. Baker, and G. M. Shearer. 1993. Analysis of T-helper and antigen presenting cell functions in cord blood and peripheral blood leukocytes from healthy children of different ages. J. Clin. Investig. 912829-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, W. E., J. C. Skinner, and G. M. Jeffery. 1968. Studies on the persistence of malarial antibody response. Am. J. Epidemiol. 87592-598. [DOI] [PubMed] [Google Scholar]
- 10.Daugherty, J. R., C. I. Murphy, L. A. Doros-Richert, A. Barbosa, L. O. Kashala, W. R. Ballou, N. J. Snellings, C. F. Ockenhouse, and D. E. Lanar. 1997. Baculovirus-mediated expression of Plasmodium falciparum erythrocyte binding antigen 175 polypeptides and their recognition by human antibodies. Infect. Immun. 653631-3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drakeley, C. J., P. H. Corran, P. G. Coleman, J. E. Tongren, S. L. McDonald, I. Carneiro, R. Malima, J. Lusingu, A. Manjurano, W. M. Nkya, M. M. Lemnge, J. Cox, H. Reyburn, and E. M. Riley. 2005. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc. Natl. Acad. Sci. USA 1025108-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutta, S., P. V. Lalitha, L. A. Ware, A. Barbosa, J. K. Moch, M. A. Vassell, B. B. Fileta, S. Kitov, N. Kolodny, D. G. Heppner, J. D. Haynes, and D. E. Lanar. 2002. Purification, characterization, and immunogenicity of the refolded ectodomain of the Plasmodium falciparum apical membrane antigen 1 expressed in Escherichia coli. Infect. Immun. 703101-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forthal, D. N., G. Landucci, A. Habis, M. Laxer, M. Javato-Laxer, J. G. Tilles, and E. J. Janoff. 1995. Age, sex, and household exposure are associated with the acute measles-specific antibody-dependent cellular cytotoxicity antibody response. J. Infect. Dis. 1721587-1591. [DOI] [PubMed] [Google Scholar]
- 14.Franks, S., L. Baton, K. Tetteh, E. Tongren, D. Dewin, B. D. Akanmori, K. A. Koram, L. Ranford-Cartwright, and E. M. Riley. 2003. Genetic diversity and antigenic polymorphism in Plasmodium falciparum: extensive serological cross-reactivity between allelic variants of merozoite surface protein 2. Infect. Immun. 713485-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray, J. C., P. H. Corran, E. Mangia, M. W. Gaunt, Q. Li, K. K. A. Tetteh, S. D. Polley, D. J. Conway, A. A. Holder, T. Bacarese-Hamilton, E. M. Riley, and A. Crisanti. 2007. Profiling the antibody immune response against blood stage malaria vaccine candidates. Clin. Chem. 531244-1253. [DOI] [PubMed] [Google Scholar]
- 16.Greenwood, B. M., A. K. Bradley, A. M. Greenwood, P. Byass, K. Jammeh, K. Marsh, S. Tuloch, F. S. J. Oldfield, and R. Hayes. 1987. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans. R. Soc. Trop. Med. Hyg. 81478-485. [DOI] [PubMed] [Google Scholar]
- 17.Kayhty, H., V. Karanko, H. Peltola, S. Sarna, and P. H. Makela. 1980. Serum antibodies to capsular polysaccharide vaccine of group A Neisseria meningitidis followed for three years in infants and children. J. Infect. Dis. 142861-868. [DOI] [PubMed] [Google Scholar]
- 18.Kinyanjui, S. M., P. Bull., C. I. Newbold, and K. Marsh. 2003. Kinetics of antibody responses to Plasmodium falciparum-infected erythrocyte variant surface antigens. J. Infect. Dis. 187667-674. [DOI] [PubMed] [Google Scholar]
- 19.Kinyanjui, S. M., D. J. Conway, D. E. Lanar, and K. Marsh. 2007. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar. J. 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manz, R. A., A. E. Hauser, F. Hiepe, and A. Radbruch. 2005. Maintenance of serum antibody levels. Annu. Rev. Immunol. 23367-386. [DOI] [PubMed] [Google Scholar]
- 21.Marsh, K., L. Otoo, R. J. Hayes, D. C. Carson, and B. M. Greenwood. 1989. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 83293-303. [DOI] [PubMed] [Google Scholar]
- 22.Morell, A., W. D. Terry, and T. A. Waldmann. 1970. Metabolic properties of IgG subclasses in man. J. Clin. Investig. 49673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ofori, M. F., D. Dodoo, T. Staalso, J. A. L. Kurtzhals, K. Koram, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens. Infect. Immun. 702982-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okenu, D. M. N., E. M. Riley, Q. D. Bickle, P. U. Agomo, A. Barbosa, J. R. Daugherty, D. E. Lanar, and D. J. Conway. 2000. Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infect. Immun. 685559-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pihlgren, M., M. Friedli, C. Tounge, A. F. Rochat, P. H. Lambert, and C. A. Siegrist. 2006. Reduced ability of neonatal and early-life bone marrow stromal cells to support plasmablast survival. J. Immunol. 176165-172. [DOI] [PubMed] [Google Scholar]
- 26.Pihlgren, M., N. Schallert, C. Tougne, P. Bozzotti, J. Kovarik, A. Fulurija, M. Kosco-Vilbois, P. H. Lambert, and C. A. Siegrist. 2001. Delayed and deficient establishment of the long-term bone marrow plasma cell pool during early life. Eur. J. Immuol. 31939-946. [DOI] [PubMed] [Google Scholar]
- 27.Polley, S. D., D. J. Conway, D. R. Cavanagh, J. S. McBride, B. Lowe, T. N. Williams, T. Mwangi, and K. Marsh. 2006. High levels of serum antibodies to merozoite surface protein 2 of Plasmodium falciparum are associated with reduced risk of clinical malaria in coastal Kenya. Vaccine 244233-4246. [DOI] [PubMed] [Google Scholar]
- 28.Polley, S. D., T. Mwangi, C. H. Kocken, A. W. Thomas, S. Dutta, D. E. Lanar, E. Remarque, A. Ross, T. N. Williams, G. Mwambingu, B. Lowe, D. J. Conway, and K. Marsh. 2004. Human antibodies to recombinant protein constructs of Plasmodium falciparum apical membrane antigen 1 (AMA1) and their associations with protection from malaria. Vaccine 23718-728. [DOI] [PubMed] [Google Scholar]
- 29.Richie, T. 2007. High road, low road? Choices and challenges on the pathway to a malaria vaccine. Parasitology 133S113-S144. [DOI] [PubMed] [Google Scholar]
- 30.Slifka, M. K., R. Antia, J. K. Whitmire, and R. Ahmed. 1998. Humoral immunity due to long-lived plasma cells. Immunity 8363-372. [DOI] [PubMed] [Google Scholar]
- 31.Struik, S. S., and E. M. Riley. 2004. Does malaria suffer from a lack of memory? Immunol. Rev. 201268-290. [DOI] [PubMed] [Google Scholar]
- 32.Taylor, R. R., S. J. Allen, B. M. Greenwood, and E. M. Riley. 1998. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am. J. Trop. Med. Hyg. 58406-413. [DOI] [PubMed] [Google Scholar]
- 33.Taylor, R. R., A. Egan, D. McGuinness, A. Jepson, R. Adair, C. Drakeley, and E. Riley. 1996. Selective recognition of malaria antigens by human serum antibodies is not genetically determined but demonstrated some features of clonal imprinting. Int. Immunol. 8905-915. [DOI] [PubMed] [Google Scholar]
- 34.Todryk, S. M., and A. V. S. Hill. 2007. Malaria vaccines: the stage we are at. Nat. Rev. Microbiol. 5487-489. [DOI] [PubMed] [Google Scholar]
- 35.Tongren, J. E., C. J. Drakeley, S. L. McDonald, H. G. Reyburn, A. Manjurano, W. M. Nkya, M. M. Lemnge, C. D. Gowda, J. E. Todd, P. H. Corran, and E. M. Riley. 2006. Target antigen, age and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect. Immun. 74257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.