Abstract
A large body of evidence has convincingly shown that Toll-like receptors are necessary sensors for infections with pathogens, but their activation was also suggested to generate autoimmunity. During experimental infections, the lack of these sensors or of their signaling molecules should lead to a deficient immune response. We found out that MyD88, the major adaptor of the Toll/interleukin-1 (Toll/IL-1) receptor signaling pathway, can actually act as a negative regulator of B-cell function in some settings. MyD88-deficient mice infected by Borrelia burgdorferi developed extreme hypergammaglobulinemia compared to wild-type animals, with high levels of immunoglobulin M (IgM) autoantibodies. In vivo, cell transfer experiments and cell blocking assays showed that this phenotype was not linked to the absence of MyD88 in B cells but rather to CD4 T-cell and likely dendritic cell dysfunctions leading to a Th1-to-Th2 cytokine switch. In addition, our results suggest a relative defect in the Ig class switch recombination process, since MyD88 knockout mice developed mostly IgM antibodies. Collectively, these data emphasize the complex role of the Toll/IL-1 receptor pathway in tuning the immune response against infection and avoiding autoimmunity.
Polyclonal lymphocyte activation is a general consequence of bacterial, viral, and parasitic infections. Generally speaking, three main consequences associated with polyclonal lymphocyte activation, i.e., protection from infection, immunosuppression, and autoimmunity, have been described, depending on the nature of the infectious agent. On the one hand, this nonspecific lymphocyte activation, largely exceeding the level of the specific antipathogen response, could control pathogen dissemination (33). On the other hand, it is able to inhibit the host-specific response to lymphocytic choriomeningitis virus, impairing virus neutralization (35), or to negatively influence the course of murine Trypanosoma cruzi infection (28). Finally, in humans, hypergammaglobulinemia and significant levels of autoantibodies, including rheumatoid factors (RFs) and antinuclear antibodies, are frequently described during the active phases of infectious states, sometimes leading to tissue damage. Experimentally, the mechanisms which govern pathogen-induced polyclonal B-cell activation seem diverse and include direct mitogenic properties of the experimental pathogens (possibly via membrane-bound Toll-like receptors [TLRs], but also via nonvariable regions of the membrane-bound immunoglobulin [Ig]) (2, 10, 36) as well as cognate CD4 T-cell help induced by B-cell-presented pathogen-derived peptides (14). Where analyzed, autoantibodies were frequently produced during the course of these experimental infections.
These general considerations could have important implications regarding the occurrence of autoimmune diseases. Indeed, in a scenario of a multistep process leading to overt autoimmunity, infection-induced polyclonal lymphocyte activation is regularly considered an early candidate event that can drive autoreactive B lymphocytes into an affinity maturation pathogenic process (41). If this scenario is correct, uncontrolled nonspecific B-cell activation during infection could be harmful. However, to date, very little is known about the mechanisms which control nonspecific B-cell activation during infection.
In order to understand the mechanisms of the autoreactive B-cell tolerance breakdown during experimental bacterial infection with Borrelia burgdorferi, we previously designed experimental infections of self-reactive B-cell transgenic mice (42). We showed in vitro that TLRs expressed on B cells were implicated not only in autoantibody production but also in nonspecific B-cell activation (42). We expected that, in vivo, TLR-deficient mice would have been devoid of such a nonspecific B-cell activation. However, to our surprise, mice deficient in the major adaptor protein of the Toll/interleukin-1 (Toll/IL-1) receptor signaling pathway, MyD88, were found to exhibit dramatic nonspecific B-cell activation, hypergammaglobulinemia, and high levels of autoantibodies during the course of B. burgdorferi infection. Thus, MyD88 appears to control potentially harmful nonspecific B-cell activation.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from Harlan (Gannat, France). Four-week-old MyD88 and TLR2 knockout (KO) and heterozygous (+/−) mice and IL-1 converting enzyme (ICE) KO mice (22) on a C57BL/6 background were originally supplied from the CDTA Institute (Orleans, France). Some MyD88 KO, MyD88+/−, TLR2 KO, and TLR2+/− mice were used directly for experiments. MyD88 KO mice were bred and maintained on a C57BL/6 background. All mice were housed in isolator cages in our institute's animal facility. MyD88 KO and MyD88+/− mice were selected by PCR genotyping as previously described (23). B. burgdorferi-inoculated mice and noninfected controls were housed in the Bacteriology Institute's animal facility. All animal experiments were performed with the approval of the Direction Départementale des Services Vétérinaires (Strasbourg, France).
B. burgdorferi infection.
The B. burgdorferi sensu stricto cN40 isolate was cultivated at low passage in Barbour-Stoenner-Kelly (BSK-H) medium (Sigma) supplemented with 6% normal rabbit serum (Sigma) at 33°C. Four- to 5-week-old mice were infected with 106 spirochetes by needle injection of 0.1 ml in the shaved back skin. Control mice were injected with an equal volume of sterile BSK-H medium and housed under the same conditions as infected animals. Mice were sacrificed 3 to 4 weeks after inoculation. In one experiment, mice of 8 to 10 weeks of age were used, with results similar to those for mice of 4 to 5 weeks of age (B- and T-cell statuses of MyD88 KO versus MyD88+/− mice, both infected and noninfected animals, with three mice in each group). The infectious status of the animals was evaluated by culture of different specimens (bladder, ear, heart, and spleen) in 7-ml tubes of BSK-H medium for up to 4 weeks at 33°C.
Quantitative PCR.
DNAs were extracted from the joints and lymph nodes (LN) of individual mice on a MagNA Pure system (Roche Diagnostics, France), using a MagNA Pure LC large-volume DNA isolation kit after external lysis by collagenase A and then proteinase K.
Quantification of the B. burgdorferi-specific fla gene was done on a LightCycler system (Roche Diagnostics, France). The primers used to amplify the fla gene were those previously described (16). Quantification of the mouse-specific gapdh gene was done on an ABI Prism 7000 instrument (Applera, Courtaboeuf, France), using a commercial kit (TaqMan rodent GADPH control reagent; Applera). External standards for fla and gapdh genes were developed as previously described (49). The number of B. burgdorferi cells in tissue specimens was calculated by comparing the crossing points of the samples with those of the standards and was normalized to 104 gapdh DNA copies.
Flow cytometry.
Preparation of LN and spleen cell suspensions, their staining for flow cytometry, and analysis of the B-cell phenotype have been described already (20, 42). T cells were monitored using double staining with anti-CD3-fluorescein isothiocyanate (anti-CD3-FITC), anti-CD4-phycoerythrin (anti-CD4-PE), or anti-CD8-FITC (Pharmingen) or triple staining with anti-CD4-PE, anti-CD25-biotin, and anti-CD45RB-FITC antibodies (Pharmingen) for regulatory T cells. Dendritic cells (DCs) were identified by anti-CD11c-biotin (Pharmingen) staining. Expression analysis of the activation marker CD86 was performed using anti-CD86-PE antibody (Pharmingen). Nonviable cells were excluded and identified by incorporation of propidium iodide (PI; 5 mg/ml). The analysis was performed with a FACSCalibur flow cytometer, using the CellQuest software package (BD Biosciences).
ELISA.
Mice were bled by retro-orbital puncture under anesthesia. Sera were centrifuged (12 min, 10,000 rpm) and stored at −20°C until enzyme-linked immunosorbent assay (ELISA) analysis.
ELISAs for serum IgM were done as described previously (10, 46).
For murine RF, plates were coated with total murine IgG (50 mg/ml; Jackson ImmunoResearch), and serum RF concentrations were revealed by adding a peroxidase-coupled anti-mouse IgM. For anti-double-stranded-DNA (anti-dsDNA) antibodies, calf thymus DNA was absorbed at 10 μg/liter (Sigma); single-stranded DNA was removed by digestion with S1 nuclease (100 IU/ml; Amersham), and bound antibodies were revealed with anti-mouse IgM-peroxidase (80 ng/ml; Jackson ImmunoResearch). For anti-thyroglobulin antibodies, plates were coated with thyroglobulin (50 μg/ml; Sigma), and binding of antibodies was revealed by adding anti-mouse IgM-peroxidase (80 ng/ml; Jackson ImmunoResearch). In each test (RF, anti-dsDNA, and anti-tyrosine), sera from MRL/lpr mice were used as positive controls; sera of MRL/lpr, MyD88+/−, and MyD88 KO mice were tested using 1/100, 1/250, 1/500, and 1/1,000 dilutions.
Mouse sera were tested for murine IgG antibodies to B. burgdorferi as previously described (42.) Anti-mouse IgG1, IgG2b, and IgG3 (Jackson ImmunoResearch) were used to reveal the different Ig isotypes (1/2,000 dilution).
For murine IgE, plates were coated with anti-murine IgE antibody (1 μg/ml; Pharmingen), and then a biotin-coupled anti-mouse IgE (0.5 μg/ml; Pharmingen) was added and serum concentrations of IgE were revealed using peroxidase-coupled streptavidin (1/20,000; Jackson ImmunoResearch).
ELISAs for IL-12, gamma interferon (IFN-γ), IL-4, and IL-10 were done using commercial kits (Bender Medsystems) as described by the manufacturer, using diluted sera (1/5 and 1/10 dilutions) and culture supernatants.
Cell purification.
Spleen or draining LN were harvested from 8- to 12-week-old mice.
Cells were isolated with a magnetic cell separation (MACS) depletion protocol (Miltenyi Biotec). Single-cell suspensions were depleted of non-B cells with anti-CD43 magnetic beads (Miltenyi Biotec). The purity of B cells was confirmed by staining with anti-CD19 antibody (>90% purity) and with anti-B220 antibody (>98% purity). T cells were purified using a pan-T-cell isolation kit (anti-CD11b, anti-CD45R, anti-DX5, and anti-Ter119 negative sorting; Miltenyi Biotec). In all experiments, >95% of recovered cells were CD3 positive.
B-cell proliferation assays.
Spleens or LN were removed from MyD88 KO mice, MyD88+/− mice, or control C57BL/6 mice and teased apart. The proliferation assays were done as previously described (42). Cells (106) were incubated in 24-well BD Falcon plates (final volume of 1 ml) with one of the following reagents: 10 μg/ml of lipopolysaccharide (LPS) from Salmonella enterica serovar Typhosa (Sigma), 10 μg/ml of an F(ab′)2 goat anti-mouse IgM (Jackson ImmunoResearch), or 10 μg/ml of sonicated B. burgdorferi. After 60 h of culture at 37°C, the phenotype of the cells was determined by flow cytometry analysis.
Preparation of sonicated B. burgdorferi.
Sonicated B. burgdorferi organisms were prepared as previously described (42). The protein content of the preparation was determined by Lowry assay.
B-cell-depleted LN cell culture.
LN cells from infected MyD88 KO or MyD88+/− mice were depleted of B cells (20 days after infection) by use of anti-CD19 magnetic beads (Miltenyi Biotec). In each well, 7 × 105 MyD88 KO or MyD88+/− B-cell-depleted LN cells were cultured in the presence of 10 μg/ml sonicated B. burgdorferi cells. Unstimulated cultures were used as controls.
B- and T-cell transfer.
μMT mice were given 5 × 107 MACS-purified B cells by intravenous transfer 3 days before B. burgdorferi infection. CD3ɛ KO mice received the transfer in the same way, but with 2 × 107 MACS-purified T cells.
Anti-CD4 treatment (8, 21, 42, 46).
Nondepleting mouse anti-CD4 antibody (YTS177.9.6.1), in ascites form and diluted in phosphate-buffered saline (PBS) (50 μl ascites plus 50 μl PBS per mouse per injection), was administered intraperitoneally 2 days before B. burgdorferi infection and then twice a week until sacrifice. Control animals were injected with PBS. CD4-T-cell blockade was visualized with an LN CD4-CD8 stain as previously described (46).
DC differentiation from bone marrow cells.
To obtain DCs, we used a modified version of an already described method (26). Bone marrow cells were cultured in bacterial petri dishes (100-mm diameter; Falcon) in RPMI 1640 supplemented with gentamicin (40 μg/ml; Gibco BRL), 10% fetal calf serum (Gibco BRL), β-mercaptoethanol (50 μM; Sigma), and granulocyte-macrophage colony-stimulating factor (20 ng/ml; Peprotech) for 9 days. The medium was changed on days 3 and 8. On day 6, half of the supernatant containing nonadherent cells was removed and centrifuged. The pellet was then resuspended in 10 ml culture medium and put back in a new petri dish. After 9 days, 70% of bone marrow-cultured cells expressed CD11c, as DCs do.
DC stimulation.
DCs (0.5 × 106 cells per culture well) were cultured for 24 h with anti-CD40 antibody (10 μg/ml; Pharmingen), LPS (1 μg/ml; Sigma), or B. burgdorferi (2.5 and 1 μg/ml). Cells were then removed, centrifuged, and stained for flow cytometry analysis.
B-cell stimulation with IL-4.
Purified B cells (anti-CD43 negative sorting; 0.5 × 106 B cells per well) were cultured for 60 h at 37°C in the presence of murine IL-4 (0.1 ng/ml; Roche Diagnostics), with or without sonicated B. burgdorferi (10 μg/ml). Unstimulated cells were used as controls.
RESULTS
B. burgdorferi infection in MyD88 KO mice.
B. burgdorferi induces a chronic infection in many inbred mouse strains, including C57BL/6. This infection is characterized by chronic bacteremia invading many tissues. The highest concentrations of spirochetes are found in the ankle joints, heart, and skin. Inflammation peaks about 2 to 4 weeks after infection and then regresses in the presence of B. burgdorferi-specific adaptive immune responses (6). Since B. burgdorferi lipoproteins activate innate immune cells via TLRs, two recent studies have checked the importance of the adaptor molecule MyD88 in B. burgdorferi-induced inflammation and infection resolution. MyD88-deficient mice developed carditis and arthritis like wild-type (WT) mice did, but with higher pathogen burdens (7, 24).
In the following experiments, 4- to 5-week-old MyD88 KO mice on the C57BL/6 background and MyD88+/− littermates were injected with B. burgdorferi or culture medium (BSK-H) and analyzed mostly 3 to 4 weeks after infection. Two weeks after B. burgdorferi infection, MyD88 KO mice developed severe arthritis as frequently as MyD88+/− mice did, indicating that MyD88-dependent signaling is not required for the onset of B. burgdorferi-induced disease. All infected mice had positive cultures from at least one tissue sample (heart, ear, or bladder) at 4 weeks. All KO mice mounted an anti-B. burgdorferi IgG response as well as MyD88+/− animals did (Table 1), as already described (7, 24).
TABLE 1.
B. burgdorferi infection in MyD88 KO mice and controlsa
| Mouse strain | No. of mice with result/total no. of mice
|
||
|---|---|---|---|
| Arthritis | Positive culture for B. burgdorferi | Anti-B. burgdorferi IgG | |
| MyD88 KO | 4/8 | 8/8 | 8/8 |
| MyD88+/− | 2/5 | 5/5 | 7/7 |
Mice were infected with 106 B. burgdorferi cells and necropsied at 3 to 4 weeks postinfection. Arthritis was considered an early marker of infection. The anti-B. burgdorferi IgG response was considered positive when optical density values reached the background level of +0.2.
Surprisingly, B. burgdorferi infection induces considerable B-cell activation.
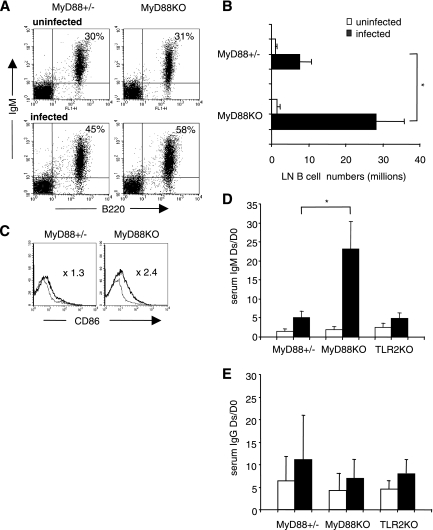
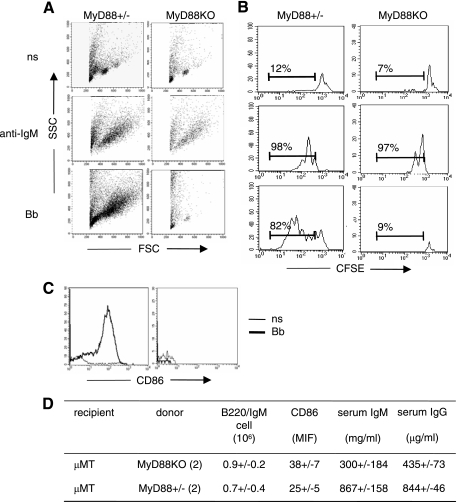
B. burgdorferi spirochetes and their lipoproteins have been shown in vitro to possess mitogenic activity on WT mouse B lymphocytes (27, 48). In vivo, B. burgdorferi induces polyclonal hypergammaglobulinemia in which B. burgdorferi-specific Ig represents only a minor fraction (7, 42). Previous work suggests that TLRs present on the B-cell surface, particularly TLR2 and TLR1, could be involved (3, 13, 47). Since most known mammalian TLRs, including TLR1/2 complexes, signal through the adaptor protein MyD88, one could expect that nonspecific B-cell activation would be abolished or seriously impaired in B. burgdorferi-infected MyD88 KO mice. Spleen and LN B cells from infected and noninfected MyD88 KO and MyD88+/− mice were analyzed by flow cytometry. Infection resulted in at least a threefold increase in LN B-cell numbers in MyD88+/− mice compared to those in control mice injected with culture medium alone. However, LN B-cell numbers of infected MyD88 KO mice reached at least 10 times the number of B cells in the noninfected animals (Fig. 1A and B and Table 2). This increase concerned mostly IgM+ IgD+ mature B cells (Table 2), as confirmed by the IgM/IgD/CD21/CD23 staining profile (not shown), with an abnormal activation state (CD86 staining intensity) (Fig. 1C). LN follicular organization (follicles, mantle zones, and germinal centers) appeared normal (not shown). In contrast, we found no significant modification in the splenic B-cell populations in both MyD88 KO and MyD88+/− mice, possibly reflecting the fact that the spleen only sporadically retains spirochetes (our culture results [not shown] and the PCR results of Wooten et al. [47]).
FIG. 1.
LN B cells in infected MyD88 KO mice compared with those in infected MyD88+/− mice. B. burgdorferi infection is associated with drastic B-cell activation and IgM production in MyD88 KO animals. (A) Flow cytometry analysis of infected and uninfected mouse LN cells. Viable lymphocytes were gated on forward scatter and side scatter parameters. B220+ IgM+ staining reflects most B cells. Numbers indicate mean percentages for five (MyD88+/−) to eight (MyD88 KO) mice. (B) Absolute numbers (106) of LN B220+ IgM+ cells in infected (black bars) and uninfected (open bars) MyD88+/− and MyD88 KO mice. Each value represents the mean ratio ± standard deviation (SD) for five to eight mice. Statistical difference is designated by an asterisk (P = 0.0015; Wilcoxon test). (C) Surface expression of CD86 on gated B220+ IgM+ cells in MyD88+/− and MyD88 KO (thick line) mice compared to that in uninfected mice (thin line). Mean values for CD86 mean fluorescence intensity ratios were as follows: for MyD88+/− mice (n = 5), 1.3 ± 0.29; and for MyD88 KO mice (n = 8), 2.4 ± 0.45 (P < 0.01; Wilcoxon test). (D) Levels of serum IgM in infected MyD88+/−, MyD88 KO, and TLR2 KO mice compared to those in noninfected controls. IgM levels were measured by ELISA. The values represent the mean ratios ± SD of the IgM concentrations between the day of sacrifice (Ds; 3 to 4 weeks after infection) and day 0 (D0) in infected mice (black bars) and uninfected mice (white bars). Numbers of MyD88+/− and MyD88 KO tested animals are those represented in panel A. Three infected TLR2 KO mice were compared with two noninfected controls (P = 0.01; Wilcoxon test). (E) Levels of total serum IgG in infected MyD88+/−, MyD88 KO, and TLR2 KO mice compared to those in noninfected controls. IgG levels were measured by ELISA. The values represent the mean ratios ± SD of the IgG concentrations between the day of sacrifice (Ds; 3 to 4 weeks after infection) and day 0 in infected mice (black bars) and uninfected mice (white bars).
TABLE 2.
LN lymphocytes in uninfected and infected MyD88 KO and MyD88+/− mice
| Mouse group | No. of cells (106)a
|
||||
|---|---|---|---|---|---|
| LN | B220+ IgM+ | IgM+ IgD+ | CD4+ | CD8+ | |
| MyD88+/−, uninfected | 4 ± 1.3 | 1.1 ± 0.43 | 0.9 ± 0.60 | 1.5 ± 0.50 | 1.0 ± 0.43 |
| MyD88+/−, infected | 13 ± 8.2 | 7.5 ± 3.27 | 6.6 ± 3.14 | 4.9 ± 1.75 | 3.0 ± 1.07 |
| MyD88 KO, uninfected | 5 ± 2.6 | 1.5 ± 0.80 | 0.9 ± 0.58 | 1.7 ± 0.92 | 1.2 ± 0.73 |
| MyD88 KO, infected | 46 ± 14.6 | 28.3 ± 7.55 | 23.1 ± 3.82 | 11.4 ± 4.35 | 7.7 ± 1.22 |
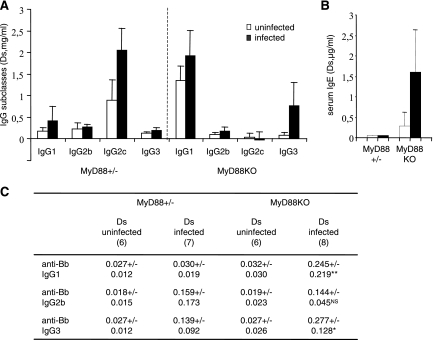
We detected a strong IgM hypergammaglobulinemia in MyD88 KO animals. Consistent with previous work, B. burgdorferi infection resulted in a two- to fourfold increase in the serum concentration of total IgM in infected MyD88+/− animals compared with uninfected MyD88+/− controls. In MyD88 KO mice, this increase was dramatic, reaching 20 times the IgM levels of uninfected MyD88 KO mice (Fig. 1D) (for noninfected MyD88+/− or MyD88 KO mice [n = 13], the mean IgM level was 0.200 ± 0.084 mg/liter; for infected MyD88+/− mice [n = 5], the mean IgM level was 0.536 ± 0.276 mg/liter; and for infected MyD88 KO mice [n = 8], the mean IgM level was 3.946 ± 2.302 mg/liter). Contrasting with IgM production, the increases in IgG levels were comparable in infected MyD88 KO and MyD88+/− animals (Fig. 1E), even if the relative contributions of the IgG subclasses were modified, suggesting that both T-cell-dependent (IgG1 and IgE) and, to a higher degree, T-cell-independent (IgG3) responses were activated in the absence of MyD88 during B. burgdorferi infection (Fig. 2A and B). Accordingly, both IgG1 and IgG3 anti-B. burgdorferi responses were enhanced in infected MyD88 KO mice (Fig. 2C).
FIG. 2.
IgG and IgE responses in B. burgdorferi-infected MyD88 KO and MyD88+/− mice compared to those in noninfected controls (A) Mean levels ± SD of total serum IgG1, IgG2b, IgG2c, and IgG3 in infected mice (black bars) and noninfected mice (white bars). Numbers of mice are indicated in panel C. Sera were collected from mice 4 weeks after infection. Ds, day of sacrifice. IgG isotypes were evaluated by ELISA. (B) Levels of IgE in infected and noninfected mice. Four mice were tested in each group. (C) Effect of MyD88 deficiency on B. burgdorferi-specific IgG isotype distribution (anti-B. burgdorferi Ig). Values represent the mean optical densities ± SD observed for a 1/200 serum dilution. Numbers of mice are indicated in each column. Statistical differences between the concentrations at the day of sacrifice (Ds) for infected MyD88 KO and MyD88+/− mice are designated by asterisks (**, P = 0.001; *, P = 0.03 [Wilcoxon test]). NS, no statistical difference.
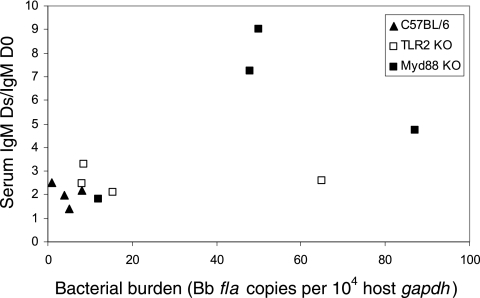
Recent evidence has demonstrated that MyD88 plays a significant role in the host defense against B. burgdorferi, with MyD88-deficient mice harboring larger numbers of spirochetes in the ankle joints, heart, and ears 2, 4, and 8 weeks after infection than do WT animals (7, 24). Thus, we wondered whether our results could be the consequence of a higher bacterial burden in infected MyD88 KO mice than in infected MyD88+/− mice. We first used real-time PCR to quantify the B. burgdorferi flagellin DNA (fla) in joints and LN of the infected mice. After 20 days of infection, the mean number of flagellin copies was indeed 100 times higher in MyD88 KO mouse joints than in MyD88+/− mouse joints, but the amounts of B. burgdorferi organisms in MyD88 KO and MyD88+/− infected LN were moderate and similar (not shown). One could argue that B cells circulate and could encounter antigens and/or be sensitive to growth factors that are due to the high level of bacteria. However, B. burgdorferi-infected TLR2-deficient mice have also been shown to harbor up to 20-fold more spirochetes in tissues than do TLR2+/− littermates (7, 47; our unpublished results), and IgM production in infected TLR2 KO animals was comparable to that seen in infected MyD88+/− (WT) mice (Fig. 1D). Finally, we injected increasing amounts of B. burgdorferi (103 to 107 spirochetes) into C57BL/6, TLR2 KO, and MyD88 KO mice in order to get various bacterial levels by the end of the 20-day experiment. There was no strict correlation between IgM production and the B. burgdorferi bacterial burden (Fig. 3), but we could not rule out that the high pathogen burden in infected MyD88 KO mice contributed to B-cell hyperactivation.
FIG. 3.
IgM levels are not directly correlated with B. burgdorferi burden. MyD88 KO, TLR2 KO, and C57BL/6 mice were inoculated with increased doses of B. burgdorferi (103 to 107) to get a panel of bacterial burdens. Ankle tissues were assessed by quantitative PCR for B. burgdorferi DNA levels in mice sacrificed at 20 days postinfection. Ds, day of sacrifice. Values reflect the numbers of B. burgdorferi fla gene copies normalized per 10,000 copies of a murine gene (gapdh).
B. burgdorferi-induced B-cell activation in MyD88 KO mice includes autoreactive B cells.
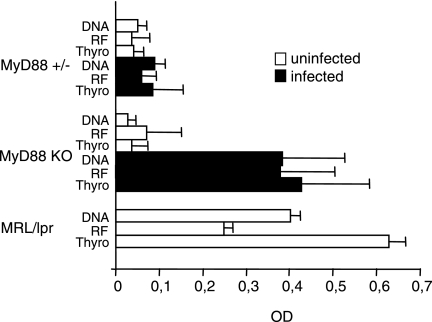
Clinical observations and different experimental models show that autoreactive B cells can be activated during microbial infections. B. burgdorferi-infected MyD88 KO mice produced high levels of several IgM autoantibodies, such as RF, anti-dsDNA, and anti-thyroglobulin antibodies, with levels similar to those found in the lupus-prone autoimmune strain MRL/lpr (Fig. 4). In contrast, no IgG autoantibodies could be detected in their sera. MyD88 KO mice appeared healthy 4 weeks after infection, except for the induced arthritis. Likewise, 3 months after B. burgdorferi infection, MyD88 KO mice still showed B-cell activation, IgM hypergammaglobulinemia, and autoantibody production, with no sign of autoimmune disease, in particular no proteinuria (not shown). Together, these data suggest that these autoantibodies are natural autoantibodies.
FIG. 4.
Induction of IgM autoantibody production after B. burgdorferi infection in Myd88 KO mice. RF, anti-dsDNA, and anti-thyroglobulin IgM antibody levels were measured by ELISA. Values represent the mean optical densities ± SD observed for the 1/100 (RF), 1/500 (anti-dsDNA), or 1/100 (anti-thyroglobulin) serum dilution, respectively. The same dilution for MRL/lpr pooled serum as that used for MyD88+/− and MyD88KO mice was used as a positive control. Five animals were tested in each group except for infected MyD88 KO mice (six animals).
In summary, although previous work strongly suggested the ability of B. burgdorferi to directly stimulate B cells via their TLRs, our results clearly indicate that the deficiency of the main adaptor of TLR signaling leads to B-cell expansion, IgM overproduction, and essentially no class switching after B. burgdorferi infection. In order to explain this phenotype, we explored different mechanistic hypotheses in vivo and in vitro, including an intrinsic B-cell defect and T-cell and DC dysfunctions.
B-cell hyperactivation in MyD88 KO mice during B. burgdorferi infection is not directly linked to an intrinsic B-cell defect.
Purified LN B cells were stimulated in vitro with sonicated B. burgdorferi. In keeping with previous results (42), B. burgdorferi directly stimulated MyD88+/− B cells, as assessed by cell size, CD86 expression, and carboxy-fluorescein diacetate succinimidyl ester staining intensity (Fig. 5A to C). However, MyD88 KO B cells did not present any sign of activation in the presence of B. burgdorferi, although they did respond to B-cell receptor stimulation by an anti-IgM antibody.
FIG. 5.
The absence of MyD88 in B cells is not sufficient to drive B-cell hyperactivation in infected MyD88 KO mice. One or more MyD88 KO cellular partners appear to be necessary. (A to C) Purified B cells (anti-CD43 negative sorting) from MyD88 KO or MyD88+/− pooled LN were cultured for 60 h in the presence of sonicated B. burgdorferi (10 μg/ml) or anti-IgM (10 μg/ml). Unstimulated cells were used as controls (ns). It should be noted that unstimulated purified B cells died under our culture conditions (60-h culture). (A) Side scatter versus forward scatter plots were used to monitor cell size. (B) Histograms show proliferation of carboxy-fluorescein diacetate succinimidyl ester-labeled B cells, with percentages indicating proliferating B cells. (C) Surface expression of CD86 activation on B220+ IgM+ cells. (D) Purified B cells (anti-CD43 negative sorting; 5 × 107 cells per mouse) from MyD88+/− and MyD88 KO mice were transferred to μMT mice (two per group). After 24 h, the recipient mice were infected with B. burgdorferi and analyzed 3 weeks after infection for LN B220+ IgM+ B-cell percentages, CD86 expression on LN B cells, and serum IgM and IgG levels.
In order to elucidate this apparent paradox, we tested B-cell activation in vivo by cellular transfer. Purified B cells from MyD88 KO or MyD88+/− animals were transferred into μMT mice, which lack mature B cells and are on a C57BL/6 background. Figure 5D summarizes the results of the transfer experiments. After B. burgdorferi infection, MyD88+/− B cells and MyD88 KO B cells were similarly represented in this C57BL/6 non-MyD88-deficient environment. More importantly, we did not observe any increase in IgM production in animals receiving MyD88 KO B cells compared to that in animals receiving MyD88+/− B cells. On the contrary, MyD88 KO B cells were even less able to produce IgM than were MyD88+/− B cells, suggesting that these cells are less responsive to B. burgdorferi when MyD88 is missing. Taken together, in vitro and in vivo results show that a MyD88-deficient non-B-cell partner is necessary to drive B-cell hyperactivation in response to B. burgdorferi infection.
MyD88 KO T cells.
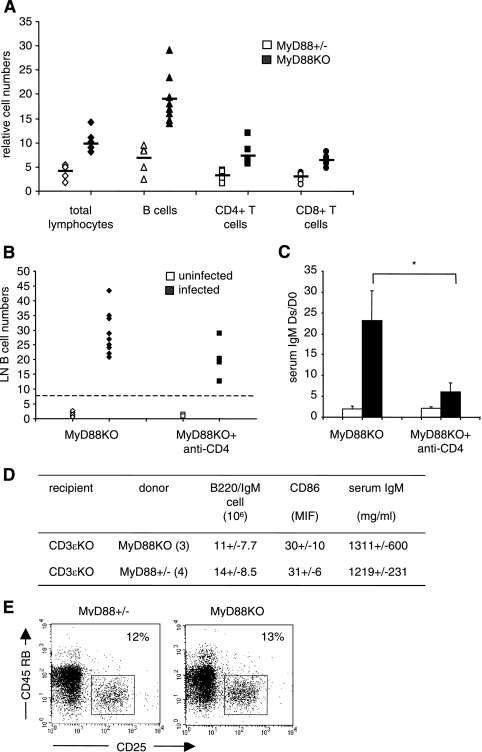
Do MyD88-deficient T cells drive B-cell hyperactivation? As shown in Table 2 and Fig. 6A, B. burgdorferi infection induces a significant increase of LN CD4 and CD8 T cells, although it is much less important than the B-cell increase, in MyD88 KO mice compared to MyD88+/− mice. To study the role of CD4 T lymphocytes, we treated MyD88 KO mice with a nondepleting anti-CD4 monoclonal antibody under conditions known to completely block CD4+ cells for 4 weeks (8, 21, 42, 46). As indicated in Fig. 6B, this treatment slightly reduced the number of LN B cells in infected MyD88 KO mice, although not significantly. However, the IgM levels were drastically reduced in anti-CD4-treated MyD88-deficient mice (Fig. 6C). Thus, CD4 T cells strongly influence the hyper-IgM production in MyD88 KO mice infected with B. burgdorferi.
FIG. 6.
Although it is dependent on CD4+ T-cell function, IgM hypergammaglobulinemia observed in infected MyD88 KO mice is not linked to the absence of MyD88 in T cells. (A) Numbers of CD4+ and CD8+ cells in LN of infected MyD88 KO (black symbols) and MyD88+/− (white symbols) animals, determined by flow cytometry, relative to uninfected controls. Each point represents one infected animal compared to the noninfected control mean value. (B) MyD88 KO mice were injected intraperitoneally with an anti-CD4 monoclonal antibody (YTS177) to block CD4+ cells for at least 4 weeks (see Materials and Methods). Each point represents a mouse. Infected and treated MyD88 KO mice were compared with infected and nontreated MyD88 KO mice. Uninfected mice were used as controls. (C) Serum IgM levels in anti-CD4-treated and untreated MyD88 KO mice 4 weeks after infection with B. burgdorferi. Bars represent the mean ratios ± SD of the IgM concentrations between the day of sacrifice (Ds; 3 to 4 weeks after infection) and day 0 (D0) in anti-CD4-treated or untreated, uninfected (white) or infected (black) MyD88 KO animals. Differences between anti-CD4-treated and untreated infected MyD88 KO mice are significant (*, P = 0.009; Wilcoxon test). (D) Purified T cells (anti-CD11b, anti-CD45R, anti-DX5, and anti-Ter119 negative sorting; 20 × 106 cells) from MyD88+/− and MyD88 KO mice were transferred to CD3ɛ ΚΟ mice (three and four per group). After 24 h, the recipient mice were infected with B. burgdorferi and analyzed 3 weeks after infection. All mice displayed significant numbers of transferred CD4 T cells (6%) in LN. The basal level of IgM in noninfected CD3 KO animals was 342 ± 10 μg/ml. (E) Regulatory T cells in uninfected MyD88 KO and MyD88+/− mice. LN cells were gated on forward scatter and side scatter parameters. Numbers indicate percentages of CD25high CD45RBlow cells among CD4+ cells.
We next wondered if the absence of MyD88 in T cells was responsible for the observed B-cell phenotype for infected MyD88 KO mice. Purified T cells from MyD88 KO or MyD88+/− mice were transferred into T-cell-deficient animals (CD3ɛ-deficient mice) on a C57BL/6 background. Figure 6D summarizes the results of these transfer experiments. The absence or presence of MyD88 in T cells did not influence the total number of B cells or the IgM level in infected recipients. We concluded from these experiments that the hyper-IgM observed in infected MyD88 KO mice is CD4 T cell dependent but independent of MyD88 signaling in T cells.
Recently, numbers of reports have suggested that CD4+ regulatory T cells could be involved in suppressing B-cell functions (17, 37). However, our data do not support a role for defective MyD88 KO regulatory T cells in the activated B-cell phenotype. Numbers of regulatory T cells, assessed by CD4+ CD25hi CD45RBlow staining, were similar in MyD88 KO and MyD88+/− LN and increased in similar proportions with B. burgdorferi infection (Fig. 6E and data not shown). In addition, transfer systems of MyD88 KO or MyD88+/− T cells included similar numbers of regulatory T cells, with no influence on B-cell activation.
MyD88 KO DCs.
TLR signaling activates DCs to secrete proinflammatory cytokines and upregulate costimulatory molecule expression, thereby linking innate and adaptive immunity (5, 9, 19). It has also been suggested that during infections, DCs could negatively regulate the Th2 response in a MyD88-dependent manner (44).
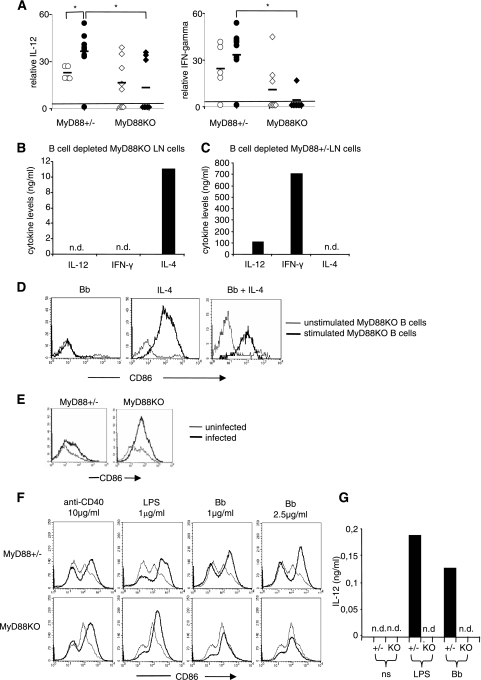
Sera from infected and noninfected mice were analyzed for IL-4 (Th2 response), IL-12, and IFN-γ levels (Th1 response). IL-4 was not detectable. The results for IL-12 and IFN-γ are shown in Fig. 7A. Noninfected MyD88 KO animals displayed significantly less IL-12 and IFN-γ, and in contrast to the case for MyD88+/− mice, B. burgdorferi infection did not increase the production of these cytokines. These results suggest that MyD88 KO mice are defective in Th1 cell differentiation and that this defect is amplified during B. burgdorferi infection.
FIG. 7.
Th1-Th2 cytokine imbalance and DCs could drive B-cell activation and IgM production in vivo in infected MyD88 KO mice. (A) Serum IL-12 and IFN-γ were measured by ELISA in infected (black symbols) and uninfected (white symbols) MyD88 KO and MyD88+/− mice and compared to the detectability threshold of the ELISA method used (black lines; 15.6 pg/ml and 16 pg/ml, respectively). Numbers of mice were as follows: for uninfected MyD88+/− mice, n = 5; for infected MyD88+/− mice, n = 10; for uninfected MyD88 KO mice, n = 8; and for infected MyD88 KO mice, n = 8. (B and C) B-cell-depleted MyD88 KO (B) or MyD88+/− (C) in vivo-activated LN cells (anti-CD19 negative sorting) were cultured in the presence of sonicated B. burgdorferi (10 μg/ml). Supernatants were tested for IL-12, IFN-γ, and IL-4 levels by ELISA. n.d., nondetectable. (D) Purified LN MyD88 KO B cells were cultured for 60 h with sonicated B. burgdorferi (10 μg/ml), IL-4 (0.1 ng/ml), or both. Diagrams show expression of CD86 in stimulated (thick lines) and control unstimulated (thin lines) cells. (E) Expression of CD86 on DCs in MyD88 KO and MyD88+/− infected mice. Histograms show CD86 staining on CD11c-positive cells from infected (thick lines) and uninfected (thin lines) mice. (F) Bone marrow-derived DCs from MyD88+/− and MyD88 KO mice were cultured for 60 h with anti-CD40 antibody (10 μg/ml), LPS (1 μg/ml), or B. burgdorferi (1 or 2.5 μg/ml). Diagrams show expression of CD86 on stimulated (thick lines) and unstimulated (thin lines) CD11c+ cells. (G) After stimulation as depicted in panel F, the IL-12 concentration in the supernatants was evaluated by ELISA. n.d., nondetectable.
The Th2 differentiation of T cells leads to IL-4 production. In our model, we tested the responses of MyD88+/− and MyD88 KO non-B cells to B. burgdorferi. LN from infected MyD88 KO animals were B cell depleted. The non-B cells, including T cells and antigen-presenting cells representing the B-cell environment, were secondarily stimulated in vitro with B. burgdorferi. As shown in Fig. 7B, and confirming previous data (24), supernatants contained IL-4 but no IFN-γ and no IL-12, which was not the case for supernatants originating from cultures of MyD88+/− non-B cells under the same conditions (Fig. 7C). Finally, we verified that IL-4 was indeed able to stimulate MyD88 KO B cells in vitro (Fig. 7D).
DC numbers in LN, based on CD11c expression, were similar in infected MyD88+/− and MyD88 KO mice (not shown). B. burgdorferi infection was associated with an increase in CD86 activation marker expression on DC surfaces in both MyD88+/− and MyD88 KO mice (Fig. 7E). To further characterize the MyD88 KO DC response to B. burgdorferi, we generated mature DCs from bone marrow cultures with granulocyte-macrophage colony-stimulating factor and analyzed the activation status of DCs in terms of the expression of the activation marker CD86 and IL-12 production with direct B. burgdorferi stimulation. As a positive control, compared to MyD88+/− DCs, MyD88 KO DCs upregulated CD86 in response to CD40 cross-linking but displayed an altered response to LPS, as already described. The direct stimulation of MyD88 KO DCs with B. burgdorferi did not upregulate CD86 (Fig. 7F) or induce IL-12 production (Fig. 7G), in contrast to the case for MyD88+/− DCs. These results show that although DCs from infected MyD88 KO mice upregulate CD86 in vivo, they are not directly activated by B. burgdorferi. Thus, we can speculate that a DC defect in MyD88 KO mice might contribute to the Th1-Th2 imbalance.
DISCUSSION
We have examined the polyclonal B-cell activation induced by B. burgdorferi infection in MyD88-deficient mice and have made the important observation that it is dramatically increased compared to what is observed in heterozygous control animals. We checked the level of the defect and found that it is not intrinsic to B cells, since (i) MyD88 deficiency rendered B cells unresponsive in vitro to B. burgdorferi and (ii) MyD88 KO and MyD88+/− B cells behaved similarly in a reconstituted WT environment. We also show that while CD4 cell function is required to drive polyclonal IgM overproduction, the MyD88 status of the T cells is not relevant. Finally, our experiments suggest that MyD88 KO DCs could be the source of the expansion of B cells in infected MyD88 KO mice.
It has already been suggested that Th1 effector responses require TLR-mediated recognition and signaling, whereas Th2 responses are independent of the TLR pathway (34, 40). Our results agree with this view and with data showing impaired IL-12 and IFN-γ production in MyD88 KO mice during various experimental infections (11, 31, 39). At least two factors could be implicated in the activation of CD4 Th2 cells in B. burgdorferi-infected MyD88 KO mice, including the lack of IL-12 secretion and the production of IL-4 (30, 32). Indeed, it was proposed that in the absence of IL-12, Th2 differentiation becomes a default pathway by derepression of GATA3, a key transcription factor for Th2 differentiation. In fact, DCs from MyD88 KO mice have lost the ability to produce proinflammatory cytokines such as IL-12 in response to a large number of pathogen-associated molecular patterns (39, 40), and in vitro studies have suggested that MyD88 in DCs could be critical for Th cell differentiation (18, 40). On the other hand, IL-4, which is produced in minute amounts by naïve T cells and activates T cells along an autocrine loop, also promotes GATA3 expression. Other possibilities must be considered, among them the direct B. burgdorferi-induced activation of the Notch pathway leading to Th2 differentiation (4), T-bet deregulation (25), and the direct activation by B. burgdorferi of other MyD88-deficient nonlymphoid cells able to produce cytokines.
MyD88, originally described as a myeloid differentiation primary response protein, is an adaptor molecule in IL-1 receptor (IL-1R)/TLR signaling by interacting with the Toll/IL-1R domain (15). Thereafter, IL-1R-associated kinases (IRAK-1, IRAK-2, IRAK-M, and IRAK-4) are recruited and, in turn, recruit TNFR-associated factor 6 (TRAF-6), leading to the activation of NF-κB and AP-mediated genes. In addition to its role in the TLR activation pathway, MyD88 is an adaptor molecule for the IL-1 and IL-18 receptor signaling pathways (1). Responses of IL-1R-deficient mice to several antigenic stimuli have already been described: IL-1R KO mice develop a Th2-like response following infection with the Th1-inducing pathogen Leishmania major as well as after immunization with keyhole limpet hemocyanin, although the mechanisms of this Th2 shift are not clearly understood (38). IL-18 also stimulates Th1 differentiation, promoting IFN-γ and IL-12 production and NK cell activation, and this Th1 cell development is altered in IL-18R-deficient mice (29). However, the lack of B. burgdorferi-induced hyper-IgM in ICE-deficient mice (A. Woods, unpublished results) suggests that this phenomenon hinges on a TLR pathway deficiency. Several MyD88-dependent TLRs are involved in the B-cell response to B. burgdorferi (2, 3, 13, 47), including TLR2 via the recognition of lipoproteins and TLR5 and TLR9 via the bacterial flagellin and DNA, explaining how MyD88 deficiency abolishes direct activation of B cells and DCs in response to B. burgdorferi. Indeed, the lack of hyper-IgM in B. burgdorferi-infected TLR2 KO mice could be the consequence of the binding of B. burgdorferi antigens to multiple TLRs. Thus, our results could reflect either (i) the blockade of the MyD88-dependent TLR cascade or (ii) the alteration of another unknown MyD88-dependent pathway (43). This issue will require further work.
Regarding the consequences of such high levels of polyclonal hypergammaglobulinemia in infected MyD88 KO mice, one could be worried about the possible occurrence of autoimmunity. These mice have increased levels of different autoantibodies, including anti-DNA and RFs of the IgM class, compared to control WT mice. The lack of autoantibody class switching, combined with a possible lack of affinity maturation, could explain the absence of overt autoimmunity in these animals, at least within the time frame of our observation. In fact, compared to the IgM levels, the slightly increased level of total IgG in MyD88 KO B. burgdorferi-infected mice, as previously described (7), is similar to the IgG levels of infected MyD88+/− mice. What could be the interpretation of extremely high levels of IgM with only slightly elevated IgG levels? IgM overproduction in infected MyD88 KO mice is dependent on CD4 T cells and likely on IL-4 production. Thus, the partial class switch defect is probably linked to CD4 T-cell IL-4 production without cognate T-cell-B-cell cooperation (which should have induced a more pronounced Ig switch), circumventing the CD40 ligand role. Also, these results may reflect a defect in the Ig class switch recombination (CSR) process in MyD88 KO mice. Recently, in a mouse model of vaccination against human papillomavirus type 16, MyD88 KO mice developed only IgM. In this model, human papillomavirus type 16 caused naïve purified B cells to undergo CSR and to express γ1, γ2, and γ3 H chain transcripts in WT animals, but only the μ transcript was detected in MyD88 KO mice (49). However, MyD88 KO B cells still produced IgG in response to CD40 ligation and IL-4, demonstrating that they are able to undergo CSR. Thus, in B. burgdorferi-infected mice, CSR is partially allowed and, owing to the Th2 profile, accounts for the slightly increased IgG1 production in MyD88 KO mice.
Our data contrast with those published recently by Pasare and Medzhitov (34) and with their conclusion that in addition to CD4+ T-cell help, the generation of T-cell-dependent antigen-specific antibody responses requires activation of TLRs in B cells. Neither T-cell-dependent polyclonal nor specific antibody production is prevented by MyD88 deficiency in B. burgdorferi infection. However, Pasare and Medzhitov mostly considered the anti-human serum albumin response in MyD88-deficient B cells. Some differences in the results could be explained by the antigen and the condition of antigenic stimulation used (in our model, in vivo chronic bacterial infection).
In the end, we are left with a contrasting picture of MyD88's roles in this infectious model: on the one hand, it controls the potentially harmful hypergammaglobulinemia and autoantibody production, and on the other hand, it authorizes the production of hazardous class-switched autoantibodies. These results also alert us to the relevance of therapeutic selective inhibition targeting TLR signaling. Although TLRs have clearly been implicated in the genesis of autoimmune processes, the effect of the disruption of MyD88 interactions should be considered with caution (12).
Acknowledgments
We thank La Fondation pour la Recherche Médicale for giving a grant to P. Soulas-Sprauel and A. Woods.
We thank B. Ryffel and S. Akira for giving us access to MyD88 and TLR2 KO mice, B. Ryffel for giving us access to ICE KO mice for our latest experiments, E. Collin for assistance with B. burgdorferi quantitative PCR and anti-B. burgdorferi antibody dosage, A. Soley for technical support, and C. Benoist and J. L. Imler for discussions and critical readings of the manuscript.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 28 January 2008.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9143-150. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-receptor signalling. Nat. Rev. Immunol. 4499-511. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulou, L., T. Venetta, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8878-884. [DOI] [PubMed] [Google Scholar]
- 4.Amsen, D., J. M. Blander, G. R. Lee, K. Tanigaki, T. Honjo, and R. A. Flavell. 2004. Instruction of distinct CD4 T helper cell fates by different Notch ligands on antigen presenting cells. Cell 117515-526. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. [DOI] [PubMed] [Google Scholar]
- 6.Barthold, S. W., D. S. Beck, G. H. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162133-138. [DOI] [PubMed] [Google Scholar]
- 7.Bolz, D. D., S. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirshning, J. F. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 1732003-2010. [DOI] [PubMed] [Google Scholar]
- 8.Cobbold, S. P., G. Martin, and H. Waldmann. 1990. The induction of skin graft tolerance in major histocompatibility complex-mismatched or primed recipients: primed T cells can be tolerized in the periphery with anti-CD4 and anti-CD8 antibodies. Eur. J. Immunol. 202747-2755. [DOI] [PubMed] [Google Scholar]
- 9.Degli-Esposti, M. Z. A., and M. J. Smyth. 2005. Close encounters of different kinds: dendritic cells and NK cells take center stage. Nature 5112-124. [DOI] [PubMed] [Google Scholar]
- 10.Donati, D., L. P. Zhang, Q. Chen, A. Chêne, K. Flick, M. Nyström, M. Wahlgren, and M. T. Bejarano. 2004. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect. Immun. 725412-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelson, B. T., and E. R. Unanue. 2002. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 1693869-3875. [DOI] [PubMed] [Google Scholar]
- 12.Emery, P., and F. Ponchel. 2006. Inhibiting Toll-like receptors in inflammatory disease. Lancet 368821-822. [DOI] [PubMed] [Google Scholar]
- 13.Guerau-de-Arellano, M., and B. T. Huber. 2005. Chemokines and Toll-like receptors in Lyme disease pathogenesis. Trends Mol. Med. 11114-120. [DOI] [PubMed] [Google Scholar]
- 14.Hunziker, L., M. Recher, A. J. Macpherson, A. Ciurea, S. Freigang, H. Hengartner, and R. M. Zinkernagel. 2003. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat. Rev. Immunol. 4343-349. [DOI] [PubMed] [Google Scholar]
- 15.Imler, J.-L., and J. A. Hoffmann. 2001. Toll receptors in innate immunity. Trends Cell Biol. 11304-311. [DOI] [PubMed] [Google Scholar]
- 16.Jaulhac, B., R. Heller, F. X. Limbach, Y. Hansmann, D. Lipsker, H. Monteil, J. Sibilia and Y. Piemont. 2000. Direct molecular typing of Borrelia burgdorferi sensu lato species in synovial samples from patients with Lyme arthritis. J. Clin. Microbiol. 381895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang, H., and L. Chess. 2006. Regulation of immune responses by T cells. N. Engl. J. Med. 3541166-1176. [DOI] [PubMed] [Google Scholar]
- 18.Kaisho, T., K. Hoshino, T. Iwabe, O. Takeuchi, T. Yasui, and S. Akira. 2002. Endotoxin can induce MyD88-deficient dendritic cells to support Th2 cell differentiation. Int. Immunol. 14695-700. [DOI] [PubMed] [Google Scholar]
- 19.Kapsenberg, M. L. 2003. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3984-993. [DOI] [PubMed] [Google Scholar]
- 20.Koenig-Marrony, S., P. Soulas, S. Julien, A.-M. Knapp, J.-C. Garaud, T. Martin, and J.-L. Pasquali. 2001. Natural autoreactive B cells in transgenic mice reproduce an apparent paradox to the clonal tolerance theory. J. Immunol. 1661463-1470. [DOI] [PubMed] [Google Scholar]
- 21.Kouskoff, V., A. S. Korganow, V. Duchatelle, C. Degott, C. Benoist, and D. Mathis. 1996. Organ-specific disease provoked by systemic autoimmunity. Cell 87811-822. [DOI] [PubMed] [Google Scholar]
- 22.Kuida, K., A. Lippke, G. Ku, M. W. Harding, D. J. Livingston, M. S. Su, and R. A. Flavell. 1995. Altered cytokine export and apoptosis in mice deficient in interleukine-β converting enzyme. Science 2672000-2003. [DOI] [PubMed] [Google Scholar]
- 23.Leadbetter, E. A., I. R. Rifkin, A. M. Hohlbaum, B. C. Beaudette, M. J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416603-607. [DOI] [PubMed] [Google Scholar]
- 24.Liu, N., R. R. Montgomery, S. W. Barthold, and L. K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 723195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, N., N. Ohnishi, L. Ni, S. Akira, and K. B. Bacon. 2003. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat. Immunol. 4687-693. [DOI] [PubMed] [Google Scholar]
- 26.Lutz, M. B., N. Kulutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romai, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 22377-92. [DOI] [PubMed] [Google Scholar]
- 27.Ma, Y., and J. J. Weis. 1993. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect. Immun. 613843-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minoprio, P., S. Itohara, C. Heusser, S. Tonegawa, and A. Coutinho. 1989. Immunobiology of murine T. cruzi infection: the predominance of parasite-non-specific responses and the activation of TcRI T cells. Immunol. Rev. 112183-207. [DOI] [PubMed] [Google Scholar]
- 29.Monteforte, G. M., K. Takeda, M. Rodriguez-Sosa, S. Akira, J. R. David, and A. R. Satoskar. 2000. Genetically resistant mice lacking IL-18 gene develop Th1 response and control cutaneous Leishmania major infection. J. Immunol. 1645890-5893. [DOI] [PubMed] [Google Scholar]
- 30.Moser, M., and K. M. Murphy. 2000. Dendritic cell regulation of Th1-Th2 development. Nat. Immunol. 1199-205. [DOI] [PubMed] [Google Scholar]
- 31.Muraille, E., C. De Trez, M. Brait, P. De Baetselier, L. Oberdan, and Y. Carlier. 2002. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J. Immunol. 1704237-4241. [DOI] [PubMed] [Google Scholar]
- 32.Murphy, K. M., and S. L. Reiner. 2000. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2933-944. [DOI] [PubMed] [Google Scholar]
- 33.Ochsenbein, A. F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R. M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 2862156-2159. [DOI] [PubMed] [Google Scholar]
- 34.Pasare, C., and R. Medzhitov. 2005. Control of B-cell responses by Toll-like receptors. Nature 438364-368. [DOI] [PubMed] [Google Scholar]
- 35.Recher, M., K. S. Lang, L. Hunziker, S. Freigang, B. Eschli, N. L. Harris, A. Navarini, B. M. Senn, K. Fink, M. Löstcher, L. Hangartner, R. Zellweger, M. Hersberger, A. Theocharides, H. Hengartner, and R. M. Zinkernagel. 2004. Deliberate removal of T cell help improves virus-neutralizing antibody production. Nat. Immunol. 5934-942. [DOI] [PubMed] [Google Scholar]
- 36.Reina-San-Martin, B., W. Degrave, C. Rougeot, A. Cosson, N. Chamond, A. Cordeiro-Da-Silva, M. Arala-Chaves, A. Coutinho, and P. Minoprio. 2000. A B-cell mitogen from a pathogenic trypanosome is a eukaryotic proline racemase. Nat. Med. 6890-897. [DOI] [PubMed] [Google Scholar]
- 37.Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101455-458. [DOI] [PubMed] [Google Scholar]
- 38.Satoskar, A. R., M. Okano, S. Connaughton, A. Raisanen-Sokolwski, J. R. David, and M. Labow. 1998. Enhanced Th2-like responses in IL-1 type 1 receptor-deficient mice. Eur. J. Immunol. 282066-2074. [DOI] [PubMed] [Google Scholar]
- 39.Scanga, C. A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E. Y. Denkers, R. Medzhitov, and A. Sher. 2002. MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite induced IL-12 production by dendritic cells. J. Immunol. 1685997-6001. [DOI] [PubMed] [Google Scholar]
- 40.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptative immune responses. Nat. Immunol. 10947-950. [DOI] [PubMed] [Google Scholar]
- 41.Silverstein, A. M., and N. R. Rose. 2003. On the implications of polyclonal B cell activation. Nat. Immunol. 4931-932. [DOI] [PubMed] [Google Scholar]
- 42.Soulas, P., A. Woods, B. Jaulhac, A. M. Knapp, J.-L. Pasquali, T. Martin, and A.-S. Korganow. 2005. Autoantigen, innate immunity, and T cells cooperate to break B cell tolerance during bacterial infection. J. Clin. Investig. 82257-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun, D., and A. Ding. 2006. MyD88-mediated stabilization of interferon-γ-induced cytokine and chemokine mRNA. Nat. Immunol. 7375-381. [DOI] [PubMed] [Google Scholar]
- 44.Sun, J., M. Walsch, A. V. Villarino, L. Cervi, C. A. Hunter, Y. Choi, and E. J. Pearce. 2005. TLR ligands can activate dendritic cells to provide a MyD88-dependent negative signal for Th2 cell development. J. Immunol. 174742-751. [DOI] [PubMed] [Google Scholar]
- 45.Reference deleted.
- 46.Woods, A., F. Monneaux, P. Soulas-Sprauel, S. Muller, T. Martin, A. S. Korganow, and J. L. Pasquali. 2007. Influenza virus-induced type I interferon leads to polyclonal B-cell activation but does not break down B-cell tolerance. J. Virol. 8112525-12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired host defense to Borrelia burgdorferi. J. Immunol. 168348-355. [DOI] [PubMed] [Google Scholar]
- 48.Yang, L., Y. Ma, R. Schoenfeld, M. Griffiths, E. Eichwald, B. Araneo, and J. J. Weiss. 1992. Evidence for B-lymphocyte mitogenic activity in Borrelia burgdorferi-infected mice. Infect. Immun. 603033-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang, R., F. M. Murillo, M. J. Delannoy, R. L. Blosser, W. H. Yutzy, S. Uematsu, K. Takeda, S. Akira, R. P. Viscidi, and R. B. Roden. 2005. B lymphocyte activation by human papilloma virus-like particles directly induces Ig class switch recombination via TLR4-MyD88. J. Immunol. 1747912-7919. [DOI] [PubMed] [Google Scholar]