Abstract
Given the low sensitivity of amoebal coculture, we developed a specific real-time PCR for the detection of Parachlamydia. The analytical sensitivity was high, and the inter- and intrarun variabilities were low. When the PCR was applied to nasopharyngeal aspirates, it was positive for six patients with bronchiolitis. Future studies should assess the role of Parachlamydia in bronchiolitis.
Parachlamydia acanthamoebae is an obligate intracellular bacterium that belongs to the order Chlamydiales (1). Epidemiological (2), serological (11, 17), and molecular (6, 7, 10) studies support a potential role of Parachlamydia acanthamoebae as an agent of pneumonia. P. acanthamoebae has been shown to enter and replicate within human macrophages (13, 14) and to enter and persist within pneumocytes and lung fibroblasts (4). We recently established an animal model of lung infection that confirmed the third and fourth Koch's postulates for the role of P. acanthamoebae in pneumonia (3). Taken together, these studies suggest that human exposure to P. acanthamoebae may lead to bronchitis, community-acquired pneumonia, and aspiration pneumonia.
Diagnostic methods for the detection of human Parachlamydia infection are limited by the inability of these agents to grow on axenic medium. In addition, amoebal coculture is time-consuming and is available in only a few specialized laboratories (12). Serologic diagnosis is also limited by possible cross-reactivity and by the time necessary to seroconvert against an invading pathogen. For these reasons, molecular diagnostic approaches are warranted. Broad-range PCR assays for the members of the Chlamydiales, which include P. acanthamoebae, have been described (8, 18), but their sensitivities are limited. An additional sequencing step is required, which is directly achievable (without cloning) only for samples containing a minimum of 1,000 DNA copies (G. Greub et al., unpublished data). We therefore developed a real-time PCR assay for the specific detection of Parachlamydia acanthamoebae from clinical samples and applied it to samples taken from pediatric patients with bronchiolitis.
Using the primer express software (Applied Biosystems, Darmstadt, Germany), we selected probe PacS (5′-tetrachloro-6-carboxyfluorescein-TTCCACATGTAGCGGTGAAATGCGTAGATATG-Black Hole Quencher 1-3′), as well as primers PacF (5′-CTCAACTCCAGAACAGCATTT-3′) and PacR (5′-CTCAGCGTCAGGAATAAGC-3′), which amplify a 103-bp part of the 16S rRNA-encoding gene. The reactions were performed with 0.2 μM of each primer, 0.1 μM of probe, iTaq Supermix (Bio-Rad, Rheinach, Switzerland), and 5 μl of DNA sample. The cycling conditions were 2 min at 50°C and 10 min at 95°C, followed by 45 cycles of 15 s at 95°C and 1 min at 60°C. The PCR products were detected with an ABI Prism 7000 instrument (Applied Biosystems). Each sample was amplified in duplicate. Inhibition control, negative PCR mixture control, and extraction controls were systematically tested. To allow quantification, a plasmid containing the target gene was constructed, as described previously (5).
The analytical sensitivity of the real-time PCR was 10 copies of plasmidic control DNA per reaction mixture. This sensitivity is similar to that of a quantitative TaqMan PCR targeting the ADP/ATP translocase encoding gene (tlc) of Parachlamydia (10) (data not shown) and is 100-fold more sensitive than the 16SigF-Rp2Chlam broad-range PCR (18). Use of this real-time PCR has an additional advantage, in that gel electrophoresis is not needed. The risk of amplicon contamination is highly limited since the PCR microplates are not opened after amplification.
The real-time PCR was highly specific, since no cross-amplification was observed when the genomic DNA of humans, fungi (Candida albicans ATCC 10231, Aspergillus fumigatus clinical isolate), Acanthamoeba castellanii (ATCC 30010), and the bacteria listed in Table 1 were tested.
TABLE 1.
Bacterial species used to determine specificity of the real-time PCR
| Bacterial species | Source or strain |
|---|---|
| Bordetella pertussis | Clinical specimen |
| Chlamydia trachomatis | Clinical specimen |
| Chlamydophila pneumoniae | ATCC VR-1310 |
| Criblamydia sequanensis | CRIB-18 |
| Enterococcus faecalis | ATCC 29212 |
| Escherichia coli | ATCC 35218 |
| Gardnerella vaginalis | Clinical specimen |
| Haemophilus influenzae | ATCC 49247 |
| Klebsiella pneumoniae | ATCC 27736 |
| Lactobacillus spp. | Clinical specimen |
| Legionella pneumophila | Clinical specimen |
| Listeria monocytogenes | Clinical specimen |
| Moraxella catarrhalis | Clinical specimen |
| Mycobacterium tuberculosis | Clinical specimen |
| Neisseria lactamica | Clinical specimen |
| Neisseria weaveri | Clinical specimen |
| Protochlamydia amoebophila | |
| strain UWE25 | ATCC PRA-7 |
| Pseudomonas aeruginosa | ATCC 27853 |
| Rhabdochlamydia crassificans | CRIB-01 |
| Simkania negevensis | ATCC VR-1471 |
| Staphylococcus epidermidis | Clinical specimen |
| Streptococcus agalactiae | ATCC 13813 |
| Streptococcus mutans | Clinical specimen |
| Streptococcus pneumoniae | Clinical specimen |
| Streptococcus pyogenes | ATCC 19615 |
| Waddlia chondrophila | ATCC VR-1470 |
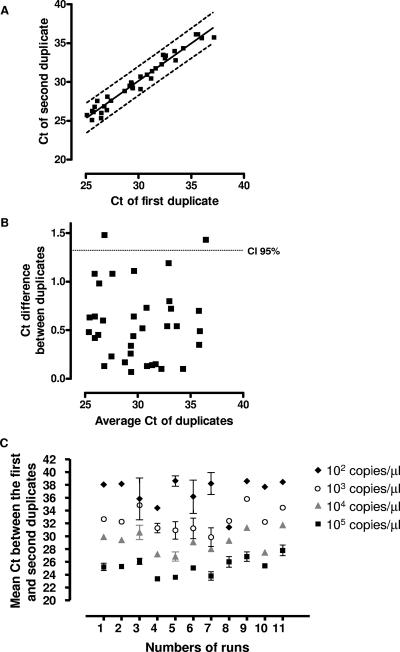
The reproducibility of the threshold cycle (CT) results was determined by testing duplicates of 10-fold serial dilutions of the plasmid in 11 independent experiments. The intrarun reproducibility was good, as shown in Fig. 1, with the CT results for both duplicates being relatively similar and with a correlation coefficient (r2) of 0.961 (Fig. 1A). By using the Bland-Altman test, the 95% confidence interval was 1.32 cycles (Fig. 1B). The interrun reproducibility is shown in Fig. 1C. The interrun variability was relatively low at high concentrations, being 1.43, 1.68, and 1.95 cycles for 105, 104, and 103 plasmidic copies μl−1, respectively. The interrun variability was, however, relatively high at a lower concentration (2.63 cycles for 102 plasmidic copies μl−1) (Fig. 1C).
FIG. 1.
(A) Plots of the CT values of the first and second duplicates, showing the intrarun and interrun variabilities of the real-time PCR between duplicates of the positive control. The dashed lines show the 95% confidence interval. (B) Bland-Altman graph showing the difference in the CT values of both duplicates according to the mean of the CT values of the duplicates. The dashed line shows the 95% confidence interval (i.e., the limit of agreement). (C) Intra- and interrun reproducibilities of the real-time PCR assessed with duplicates of plasmidic positive controls performed at 10-fold dilutions from 105 to 102 plasmid μl−1 in 11 successive runs. Standard deviations show the intrarun reproducibility of the real-time PCR.
Since several lines of evidence support the role of Parachlamydia acanthamoebae as a potential agent of lower respiratory tract infections (reviewed in references 6 and 15), the real-time PCR was applied to 39 nasopharyngeal aspirates obtained from children with respiratory syncytial virus-negative bronchiolitis. DNA was extracted from 200 μl of thawed samples by using the AquaPure genomic DNA extraction kit (Bio-Rad). DNA was eluted in a final volume of 100 μl of the elution buffer provided with the kit. A negative extraction control was tested for each extraction run. The results for positive samples were confirmed by the tlc real-time PCR (10).
Parachlamydia DNA was detected in 13 of the 39 samples, 6 of which were confirmed to be positive by the tlc quantitative PCR. The clinical and microbiological characteristics of these six patients are summarized in Table 2. We successfully sequenced the product of the 16SigF-Rp2Chlam PCR (18) only once, consistent with a bacterial burden of <1,000 copies in the five other samples. The sequence shared 99.6% (577/579) similarity with P. acanthamoebae strain Hall's coccus and 100% (577/577) similarity with P. acanthamoebae strain BN9.
TABLE 2.
Characteristics of six patients with bronchiolitis whose nasopharyngeal samples had positive Parachlamydia PCR resultsa
| Patient no. | Age | Sex | Signs and symptoms | X-ray findings | Paraclinical findings | Underlying condition(s) | Other etiology | New qPCR result (mean CT value [no. of copies/μl]) | tlc qPCR result (mean CT value) | 16SigF- Rp2Chlam PCR result |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 mo | M | Increased work of breathing, desaturation, hypercapnia | Lung infiltrate, emphysema | Normal leukocyte count and C-reactive protein level | Surgically corrected congenital heart defect, tracheomalacia | Respiratory syncytial virus detected 1 wk later | 33.48 (1,049) | 32.49 | Positiveb |
| 2 | 14 yr | M | Increased work of breathing, desaturation | Diffuse infiltrates | 34.79 (641) | 38.79 | Negative | |||
| 3 | 26 mo | F | Increased work of breathing, cough | Peribronchial infiltrates | Asthma | 36.28 (220) | 36.96 | Negative | ||
| 4 | 9 mo | M | Increased work of breathing, low-grade fever | Right upper lobe atelectasis | Normal leukocyte count and C-reactive protein level | 35.33 (289) | 36.10 | Negative | ||
| 5 | 2.5 yr | M | Increased work of breathing, cough, rhinorrhea | X ray not done | 36.75 (157) | 38.09 | Negative | |||
| 6 | 11 mo | F | Increased work of breathing, fever, cough, rhinorrhea | Right lower lobe pneumonia and infiltrate | Increased C-reactive protein level, leucocytosis with left shift | Acute otitis media | 37.86 (81) | 40.17 | Negative |
qPCR, quantitative TaqMan real-time PCR; M, male; F, female.
The sequence shared 99.6% (577/579) similarity with P. acanthamoebae strain Hall's coccus.
The seven patients with a positive result by the PacF-PacR PCR (the new real-time PCR) but a negative result by the real-time PCR targeting the tlc gene were also negative by the 16SigF-Rp2Chlam PCR. Thus, the positive PacF-PacR PCR results may represent either false-positive results due to PCR contamination or false-negative results by both of the other PCRs.
The fact that another agent of bronchiolitis was identified in only one patient positive for Parachlamydia (Table 2) supports a possible role of Parachlamydia in the pathogenesis of bron-chiolitis. However, we cannot exclude the possibility that Parachlamydia is only a colonizer of the lower respiratory tract. Since Simkania negevensis, another member of the order Chlamydiales related to Parachlamydia, has been associated with bronchiolitis in infants (9, 16), further studies should investigate a possible pathogenic role of P. acanthamoebae in this setting. This new quantitative PCR may be useful for the better definition of the pathogenicity of Parachlamydia in both animals and humans.
Acknowledgments
This work was supported by Swiss National Science Foundation grant FN3200BO-116445. Gilbert Greub is supported by the Leenards Foundation through a career award (Bourse Leenards pour la Relève Académique en Médecine Clinique à Lausanne).
We thank P. Tarr (Medizinische Universitätsklinik, Kantonsspital Bruderholz, Bruderholz, Switzerland) for reviewing the manuscript, as well as S. Aeby (Institute of Microbiology, Lausanne, Switzerland) for technical help.
Footnotes
Published ahead of print on 30 January 2008.
REFERENCES
- 1.Amann, R., N. Springer, W. Schonhuber, W. Ludwig, E. N. Schmid, K. D. Muller, and R. Michel. 1997. Obligate intracellular bacterial parasites of acanthamoebae related to Chlamydia spp. Appl. Environ. Microbiol. 63115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birtles, R. J., T. J. Rowbotham, C. Storey, T. J. Marrie, and D. Raoult. 1997. Chlamydia-like obligate parasite of free-living amoebae. Lancet 349925-926. [DOI] [PubMed] [Google Scholar]
- 3.Casson, N., J. M. Entenza, N. Borel, A. Pospischil, and G. Greub. 2007. Murine model of pneumonia caused by Parachlamydia acanthamoebae. Proc. 5th Annu. Workshop COST Action 855 Animal Chlamydioses Zoonotic Implications, Pulawy, Poland.
- 4.Casson, N., N. Medico, J. Bille, and G. Greub. 2006. Parachlamydia acanthamoebae enters and multiplies within pneumocytes and lung fibroblasts. Microbes Infect. 81294-1300. [DOI] [PubMed] [Google Scholar]
- 5.Casson, N., R. Michel, K. D. Muller, J. D. Aubert, and G. Greub. 2008. Protochlamydia naegleriophila as etiologic agent of pneumonia. Emerg. Infect. Dis. 14168-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corsaro, D., and G. Greub. 2006. Pathogenic potential of novel Chlamydiae and diagnostic approaches to infections due to these obligate intracellular bacteria. Clin. Microbiol. Rev. 19283-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corsaro, D., D. Venditti, A. Le Faou, P. Guglielmetti, and M. Valassina. 2001. A new Chlamydia-like 16S rDNA sequence from a clinical sample. Microbiology 147515-516. [DOI] [PubMed] [Google Scholar]
- 8.Everett, K. D., R. M. Bush, and A. A. Andersen. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49415-440. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg, D., A. Banerji, M. G. Friedman, C. H. Chiu, and S. Kahane. 2003. High rate of Simkania negevensis among Canadian Inuit infants hospitalized with lower respiratory tract infections. Scand. J. Infect. Dis. 35506-508. [DOI] [PubMed] [Google Scholar]
- 10.Greub, G., P. Berger, L. Papazian, and D. Raoult. 2003. Parachlamydiaceae as rare agents of pneumonia. Emerg. Infect. Dis. 9755-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greub, G., I. Boyadjiev, B. La Scola, D. Raoult, and C. Martin. 2003. Serological hint suggesting that Parachlamydiaceae are agents of pneumonia in polytraumatized intensive care patients. Ann. N. Y. Acad. Sci. 990311-319. [DOI] [PubMed] [Google Scholar]
- 12.Greub, G., B. La Scola, and D. Raoult. 2004. Amoebae-resisting bacteria isolated from human nasal swabs by amoebal coculture. Emerg. Infect. Dis. 10470-477. [DOI] [PubMed] [Google Scholar]
- 13.Greub, G., J. L. Mege, J. P. Gorvel, D. Raoult, and S. Meresse. 2005. Intracellular trafficking of Parachlamydia acanthamoebae. Cell. Microbiol. 7581-589. [DOI] [PubMed] [Google Scholar]
- 14.Greub, G., J. L. Mege, and D. Raoult. 2003. Parachlamydia acanthamoebae enters and multiplies within human macrophages and induces their apoptosis. Infect. Immun. 715979-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greub, G., and D. Raoult. 2002. Parachlamydiaceae: potential emerging pathogens. Emerg. Infect. Dis. 8625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahane, S., D. Greenberg, M. G. Friedman, H. Haikin, and R. Dagan. 1998. High prevalence of “Simkania Z,” a novel Chlamydia-like bacterium, in infants with acute bronchiolitis. J. Infect. Dis. 1771425-1429. [DOI] [PubMed] [Google Scholar]
- 17.Marrie, T. J., D. Raoult, B. La Scola, R. J. Birtles, and E. de Carolis. 2001. Legionella-like and other amoebal pathogens as agents of community-acquired pneumonia. Emerg. Infect. Dis. 71026-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas, V., N. Casson, and G. Greub. 2006. Criblamydia sequanensis, a new intracellular Chlamydiales isolated from Seine river water using amoebal co-culture. Environ. Microbiol. 82125-2135. [DOI] [PubMed] [Google Scholar]