Abstract
The first human case of fulminant gas gangrene caused by Clostridium chauvoei, a pathogen causing ruminant blackleg, was confirmed for a 58-year-old man suffering from diabetes mellitus. The patient developed conspicuous emphysematous gangrene in the right chest wall as well as intravascular gas entrapments and died 2 h after hospital arrival.
CASE REPORT
A 58-year-old man sustained a minor trauma to his anterior to right lateral chest region from accidentally running into an iron pipe at a construction site in late February 2006. He also had a 2-day history of fever over 39°C, cough, and nasal discharge prior to the chest injury. On the morning of the day after his chest injury, the man sought medical attention at a local hospital for a persistent high fever and an increase in pain and swelling of the bruised regions. He was diagnosed with two cracked ribs. In the evening, he suddenly collapsed while going to the bathroom. Upon the arrival at his home of an ambulance with a doctor, he went into cardiopulmonary arrest. Spontaneous circulation was restored, and he was transported to the emergency department of our medical center. He had earlier been treated for mild diabetes mellitus and hypertension with oral medication.
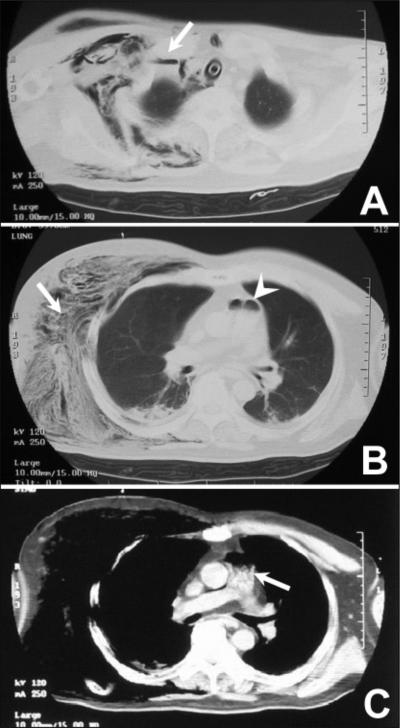
On arrival, he had a Glasgow Coma Scale score of 3, bilateral pupils dilated 6 mm in diameter with no light reflex. His heart rate was 108 beats/min, and blood pressure was undetectable except for palpable slight pulsation of the common carotid artery. Examination of the patient's chest revealed dark red cutaneous ecchymoses measuring approximately 5 by 5 cm in the right anterolateral chest with marked swelling. A computed tomographic (CT) scan was done immediately while the patient was supported with fluid resuscitation. The chest CT scan showed striking subcutaneous emphysema and destruction of muscle tissue centering around the anterior to lateral region of the right chest and rich gaseous contamination in the right subclavian vein and pulmonary artery (Fig. 1A to C).
FIG. 1.
Chest CT scan imaging 20 min after arrival. (A) Right subclavian vein filled with entrapped gas (arrow). (B) Marked subcutaneous emphysema with destruction of muscle tissue around right chest (arrow) and massive gas entrapped in the pulmonary artery (arrowhead). (C) CT scan revealing many bubbles (arrow) below the massive gas entrapment in the pulmonary artery.
Laboratory tests showed a mildly elevated leukocyte count and elevation of C-reactive protein (CRP), creatinine kinase, and blood glucose levels. Arterial blood gas analysis revealed severe mixed acidosis (Table 1). The patient died 2 h after arrival. No autopsy was performed. Culture of the antemortem specimen from the subcutaneous emphysematous lesions by needle aspiration later grew clostridia.
TABLE 1.
Patient clinical laboratory data
| Variable (unit) | Value |
|---|---|
| Hematology | |
| Leukocyte count (cells/μl) | 8,800 |
| Erythrocyte count (cells/μl) | 365 × 104 |
| Hemoglobin (g/dl) | 12.4 |
| Hematocrit (%) | 39.2 |
| Platelet count (cells/μl) | 89,000 |
| Blood chemistry | |
| Aspartate aminotransferase (U/liter) | 213 |
| Alanine aminotransferase (U/liter) | 72 |
| Lactate dehydrogenase (U/liter) | 870 |
| Creatine kinase (U/liter) | 9,985 |
| Amylase (U/liter) | 86 |
| Creatinine (mg/dl) | 1.90 |
| Urea nitrogen (mg/dl) | 20 |
| Albumin (g/dl) | 3.0 |
| Total bilirubin (mg/dl) | 1.1 |
| Sodium (mEq/liter) | 137 |
| Potassium (mEq/liter) | 6.0 |
| Chloride (mEq/liter) | 90 |
| Glucose (mg/dl) | 384 |
| CRP (mg/dl) | 35.80 |
| Arterial-blood gases | |
| pH | 6.471 |
| Partial pressure of carbon dioxide (mm Hg) | 96.1 |
| Partial pressure of oxygen (mm Hg) | 57.8 |
| Bicarbonate (mmol/liter) | 6.6 |
| Base excess (mmol/liter) | −28.1 |
| Detection of influenza virus antigens from nasal swab specimena | Negative |
Conducted at a local hospital.
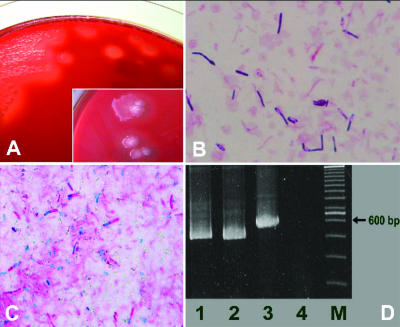
Small to medium rough, grayish white colonies with beta-hemolysis were grown anaerobically in pure culture (Fig. 2A) from a needle aspirate specimen on Brucella HK (hemin, vitamin K1) RS (rabbit, sheep) agar (Kyokuto Pharmaceutical, Tokyo, Japan) by 48-h incubation at 37°C. Abundant gas production and motility were noted with thioglycolate medium (Becton Dickinson Microbiology Systems, Cockeysville, MD). Staining with Gram and spore stains (Fig. 2B and C) demonstrated pleomorphic gram-positive rods with ovoid, subterminal endospores. The organism gave varied results with biochemical identification systems; some yielded Clostridium septicum and others Clostridium clostridioforme or Clostridium histolyticum. The 1,441-base nucleotide sequence of the 16S rRNA gene of the microbe tested was completely identical to that of the 16S rRNA gene of Clostridium chauvoei ATCC 10092 (GenBank accession no. U51843) by a BLAST homology search (www.ncbi.nlm.nih.gov/BLAST/). But 13 base mismatches (99% identity) were found between the 1,441-base sequence and that of C. septicum ATCC 12464 (GenBank accession no. U59278) (6). Then the 16S-23S rRNA gene intergenic spacer region was amplified by PCR as reported previously (9, 10), generating a 522-bp Clostridium chauvoei-specific product coinciding with that of C. chauvoei ATCC 10092, as shown in Fig. 2D. Bacterial swarming was indeed observed on soft agar, but no amplicon was observed by PCR using a set of primers for detecting the flagellin gene of C. chauvoei (4, 8). This observation may suggest the production of genetically different flagella by this strain.
FIG. 2.
Microbiological and PCR amplification findings for Clostridium chauvoei. (A) Grayish white, rough colonies with beta-hemolysis around bacterial colonies after 48 h of culture on Brucella HK RS agar under anaerobic conditions. (B and C) Gram stain morphology and Wirtz-Conklin spore stain, respectively, from growth on Brucella HK RS agar (magnification, ×1,000). Gram-positive rods with subterminal endospores were noted. (D) Five percent polyacrylamide gel electrophoresis of PCR products of the 16S-23S rRNA gene intergenic spacer region. Lane 1, patient's isolate; lane 2, Clostridium chauvoei ATCC 10092; lane 3, Clostridium septicum ATCC 12464; lane 4, negative control; lane M, 100-bp ladder as a molecular marker. A 522-bp amplicon is observed in both lanes 1 and 2.
Clostridial gas gangrene or myonecrosis, characterized by rapid progress of tissue necrosis with gas formation, is a life-threatening infection caused by toxin- and gas-producing clostridia. The most common histotoxic clostridia in humans include Clostridium perfringens, Clostridium novyi, and Clostridium septicum, followed by Clostridium histolyticum and Clostridium bifermentans (1). These clostridia mainly inhabit the soil and the intestinal tracts of humans and animals (1). Human clostridial gas gangrene often results from spore contamination in wound infections or in surgical operations under nonsterile conditions, while spontaneous, nontraumatic, or intrinsic infections from a bowel source have been increasingly reported recently (5, 11). Spontaneous infection is mostly associated with predisposing factors of hematologic or colorectal malignancies and with diabetes mellitus (1, 5).
C. chauvoei is of veterinary importance as a causative pathogen of blackleg, a highly fatal gas gangrenous infection of cattle and sheep (7). Other susceptible hosts reported so far are limited to animals, including goats and swine (2). No human gas gangrene case caused by this bacterial species has been recognized to date. So it is the rare clinician who notices the presence of C. chauvoei as a possible causative pathogen of human gas gangrene and invasive clostridial infections. C. septicum and C. chauvoei are genetically very similar, making them difficult to distinguish in biochemical testing. In the present study, however, the human fulminant gas gangrene case was confirmed to be caused by C. chauvoei after precise genetic characterizations including analyses of the 16S rRNA gene (6) and the 16S-23S rRNA gene intergenic spacer region (9, 10). Therefore, strains from human patients with devastating clostridial infections that have been identified as C. septicum are worth checking out by further genetic identification, as was done in the present study.
In the present case, we initially suspected that cardiac arrest might have been caused by cardiac tamponade, massive bleeding of the chest wall, and/or hemopneumothorax triggered by trauma, which was not evident by CT examination. Although subcutaneous emphysema was noted and the immediate cause of sudden cardiac arrest was thought to be pulmonary air embolism, it was still difficult to consider that the minor trauma observed in the contusion area led to cardiac arrest. Moreover, clinical findings of high fever before and after trauma accompanied by suspected respiratory infection, an abnormal CRP level, and severe mixed acidosis prompted us to perform a microbiological examination of the needle aspirate specimen from the subcutaneous emphysematous lesions. Finally, the diagnosis of gas gangrene was exactly confirmed by isolation and identification of C. chauvoei from the specimen.
C. chauvoei is a common microbe both in soil and in the guts of ruminants. Blackleg usually appears to be a nontraumatic endogenous infection in cattle harboring the organism in their gastrointestinal tracts (2), probably due to ingestion of spores. The spores or trophozoites may translocate from the intestine into humoral circulation and lie dormant in muscle and other tissues until some ectogenic causes induce bacterial proliferation. In sheep, the disease is frequently associated with wounding caused by shearing, castrating, or tail docking (3). Since gas gangrene occurred at the site of blunt trauma in our case, it was likely the result of wound contamination. However, there was no apparent open wound at the contusion area, so the possibility of a nontraumatic or intrinsic infection could not be excluded. It is also not known whether C. chauvoei resides in the intestinal tracts of humans as transitory microbial flora. The patient had suffered from mild diabetes mellitus prior to the onset of gas gangrene, but no obvious colon cancer, a possible means of entrance of microbes, was found on the basis of the abdominal CT scan. It was also unclear whether the high fever and symptoms of upper respiratory tract infections preceding the blunt trauma were associated with the gas gangrene.
In conclusion, we encountered a case of human fulminant gas gangrene caused by C. chauvoei that was exactly identified by genetic characterization. Thus, the possibility of C. chauvoei should be considered hereafter in human gas gangrene cases diagnosed as caused by C. septicum, a species that is difficult to distinguish from C. chauvoei by the routine clinical microbiology laboratory tests. C. chauvoei may well be recognized as a new zoonotic pathogen.
Acknowledgments
This work was supported by research expenditures from the Ministry of Health, Labor and Welfare of Japan.
We are indebted to Shun Ozawa of the Funabashi Municipal Medical Center for incisive medical advice.
Footnotes
Published ahead of print on 6 February 2008.
REFERENCES
- 1.Allen, S. D., C. L. Emery, and D. M. Lyerly. 2003. Clostridium, p. 835-856. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 2.Cato, E. P., W. L. George, and S. M. Finegold. 1986. Genus Clostridium. Prazmowski, 1880, 23AL, p. 1141-1200. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. The Williams & Wilkins Co., Baltimore, MD. [Google Scholar]
- 3.Hatheway, C. L. 1990. Toxigenic clostridia. Clin. Microbiol. Rev. 366-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kojima, A., I. Uchida, T. Sekizaki, Y. Sasaki, Y. Ogikubo, and Y. Tamura. 2001. Rapid detection and identification of Clostridium chauvoei by PCR based on flagellin gene sequence. Vet. Microbiol. 78363-371. [DOI] [PubMed] [Google Scholar]
- 5.Kornbluth, A. A., J. B. Danzig, and L. H. Bernstein. 1989. Clostridium septicum infection and associated malignancy: report of two cases and review of the literature. Medicine 6830-37. [DOI] [PubMed] [Google Scholar]
- 6.Kuhnert, P., S. E. Capaul, J. Nicolet, and J. Frey. 1996. Phylogenetic positions of Clostridium chauvoei and Clostridium septicum based on 16S rRNA gene sequences. Int. J. Syst. Bacteriol. 461174-1176. [DOI] [PubMed] [Google Scholar]
- 7.Maclennan, J. D. 1962. The histotoxic clostridial infections of man. Bacteriol. Rev. 26177-276. [PMC free article] [PubMed] [Google Scholar]
- 8.Sasaki, Y., A. Kojima, H. Aoki, Y. Ogikubo, N. Takikawa, and Y. Tamura. 2002. Phylogenetic analysis and PCR detection of Clostridium chauvoei, Clostridium haemolyticum, Clostridium novyi types A and B, and Clostridium septicum based on the flagellin gene. Vet. Microbiol. 86257-267. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki, Y., K. Yamamoto, K. Amimoto, A. Kojima, Y. Ogikubo, M. Norimatsu, H. Ogata, and Y. Tamura. 2001. Amplification of the 16S-23S rDNA spacer region for rapid detection of Clostridium chauvoei and Clostridium septicum. Res. Vet. Sci. 71227-229. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki, Y., K. Yamamoto, A. Kojima, Y. Tetsuka, M. Norimatsu, and Y. Tamura. 2000. Rapid and direct detection of Clostridium chauvoei by PCR of the 16S-23S rDNA spacer region and partial 23S rDNA sequences. J. Vet. Med. Sci. 621275-1281. [DOI] [PubMed] [Google Scholar]
- 11.Stevens, D. L., D. M. Musher, D. A. Watson, H. Eddy, R. J. Hamil, F. Gyorkey, H. Rosen, and J. Mader. 1990. Spontaneous, nontraumatic gangrene due to Clostridium septicum. Rev. Infect. Dis. 12286-296. [DOI] [PubMed] [Google Scholar]