Abstract
The aim of this study was to investigate the prevalences of plasmid-mediated AmpC β-lactamases (PABLs) in isolates of Escherichia coli and Klebsiella spp. from a university hospital in China. A total of 1,935 consecutive nonrepeat clinical isolates of Escherichia coli, Klebsiella pneumoniae, and Klebsiella oxytoca were collected between January 2003 and July 2005. The isolates with cefoxitin zone diameters less than 18 mm (screen positive) were selected for PCR of the blaAmpC genes and sequencing. Fifty-four (2.79%) isolates harbored PABLs, as demonstrated by PCR and isoelectric focusing. Sequence analysis revealed the presence of blaDHA-1 and blaCMY-2 genes. The Southern blot hybridization studies confirmed that blaCMY-2 and blaDHA-1 were located on plasmids. Based on species, PABLs were detected in 4.29% (29 isolates of DHA-1 and 1 isolate of CMY-2) of K. pneumoniae, 1.91% (11 isolates of DHA-1 and 12 isolates of CMY-2) of E. coli, and 3.03% (1 isolate of DHA-1) of K. oxytoca isolates. In contrast to our anticipation, the occurrence rate of DHA-1-producing K. pneumonia significantly decreased (P < 0.01), from 7.54% in 2003 to 2.72% in 2004. The results of random amplified polymorphic DNA analysis indicate that the prevalences of DHA-1-producing K. pneumoniae and CMY-2-producing E. coli strains were not due to epidemic strains. In conclusion, DHA-1 was the most prevalent acquired AmpC beta-lactamase in this collection of isolates from a medical center in China, and DHA-1-producing K. pneumoniae was the most prevalent bacterium harboring a PABL. To the best of our knowledge, this is the first report of CMY-2-type AmpC β-lactamases in the Chinese mainland.
Plasmid-mediated AmpC β-lactamases (PABLs) are derived from chromosomal ampC genes of the family Enterobacteriaceae, such as those of Citrobacter freundii, Enterobacter cloacae, and Aeromonas species.
PABLs have been reported to occur in the United States, Korea, Japan, etc. (4, 14, 24), but there are seldom data indicating PABLs in the Chinese mainland (28, 29). The aims of this study were to establish the prevalence rate of this resistance mechanism in isolates of Klebsiella pneumoniae, Klebsiella oxytoca, and Escherichia coli from a university hospital in Shanghai, China. The first identification of the CMY-2 AmpC β-lactamase in the Chinese mainland is also described.
MATERIALS AND METHODS
Bacterial isolates.
A total of 1,935 nonduplicate clinical isolates of Escherichia coli (n = 1,203), Klebsiella pneumoniae (n = 699), and Klebsiella oxytoca (n = 33) were consecutively selected from the Sixth Affiliate Hospital of Shanghai Jiao Tong University between January 2003 and July 2005. Positive controls were used, including Klebsiella pneumoniae (strain MISC 304, containing MIR-1) and clinical isolates of C. freundii, E. cloacae, Hafinia alvei, and Morganella morganii. Identification was performed by the GNI card of the Vitek system (BioMèrieux, MO) or Walk Away 40 (Dade-Bering, Sacramento, CA).
Antimicrobial susceptibility testing and screening of β-lactamases.
Isolates were tested for susceptibility by the standard disk diffusion method, and the results were interpreted according to the guidelines of the CLSI (formerly NCCLS). Isolates with cefoxitin zone diameters less than 18 mm (4) were considered positive for the AmpC β-lactamase screening test and were selected for MIC, isoelectric focusing (IEF), PCR, and sequencing analyses. The MICs and the presence of extended-spectrum β-lactamases (ESBLs) were determined by using the ESBL confirmation panel (Dade-Behring, Sacramento, CA). The presence of ESBLs was also investigated by the CLSI-recommended disk screening and confirmatory tests.
PCR and sequencing.
Multiplex PCR was performed to amplify six PABL group genes (16, 17). A series of specific primers were used for the detection of DHA-1, CMY-2, MOX, ACC, EBC, FOX, TEM, SHV, and CTX-M (7, 16, 23, 27) (Table 1). The PCR products were purified with a QIAquick PCR purification kit (Qiagen, Hilden, Germany) and cloned into DH5α. Plasmid DNA was prepared by using Qiagen columns and was sequenced on an ABI PRISM 377 automated sequencer (Applied Biosystems, Foster City, CA).
TABLE 1.
Primers used for PCR of bla genes
| Target | Primer | Sequencea (5′ to 3′ as synthesized) | Product size (bp) | Annealing temp (°C) | Reference |
|---|---|---|---|---|---|
| CIT | CIT-MF | TGG CCA GAA CTG ACA GGC AAA | 462 | 64 | 17 |
| CIT-MR | TTT CTC CTG AAC GTG GCT GGC | ||||
| CMY25F1 | CAA TGT GTG AGA AGC AGT C | 1,432 | 50 | 7 | |
| CMY2DR1 | CGC ATG GGA TTT TCC TTG CTG | ||||
| DHA | DHA-MF | AAC TTT CAC AGG TGT GCT GGG T | 405 | 64 | 17 |
| DHA-MR | CCG TAC GCA TAC TGG CTT TGC | ||||
| DHA-1A | TGA TGG CAC AGC AGG ATA | 998 | 52 | 27 | |
| DHA-1B | GGC TTT GAC TCT TTC GGT A | ||||
| MOX | MOXMF | GCT GCT CAA GGA GCA CAG GAT | 520 | 64 | 17 |
| MOXMR | CAC ATT GAC ATA GGT GTG GTG C | ||||
| ACC | ACCMF | AAC AGC CTC AGC AGC CGG TTA | 346 | 64 | 17 |
| ACCMR | TTC GCC GCA ATC ATC CCT AGC | ||||
| EBC | EBCMF | TCG GTA AAG CCG ATG TTG CGG | 302 | 64 | 17 |
| EBCMR | CTT CCA CTG CGG CTG CCA GTT | ||||
| FOX | FOXMF | AAC ATG GGG TAT CAG GGA GAT G | 190 | 64 | 17 |
| FOXMR | CAA AGC GCG TAA CCG GAT TGG | ||||
| TEM | TEM-F | TCA ACA TTT CCG TGT CG | 860 | 42 | 13 |
| TEM-R | CTG ACA GTT ACC AAT GCT TA | ||||
| SHV | SHV-F | ATG CGT TAT ATT CGC CTG TG | 780 | 47 | 13 |
| SHV-R | AGA TAA ATC ACC ACA ATG CGC | ||||
| CTX | CTXMU1 | ATG TGC AGY ACC AGT AAR GT | 593 | 50 | 16 |
| CTXMU2 | TGG GTR AAR TAR GTS ACC AGA |
R, A or G; M, A or C; Y, C or T; K, G or T; S, G or C; I, inosine.
IEF.
IEF was performed as described previously (9). Crude beta-lactamase extracts were subjected to analytical IEF on an ampholine polyacrylamide gel (pH 3.5 to 9.5; Pharmacia, Uppsala, Sweden). Preparations from standard strains known to harbor TEM-12 (pI 5.25), TEM-10 (pI 5.6), TEM-29 (pI 6.1), SHV-1 (pI 7.6), and ACT-1 (pI 9) plasmid-mediated β-lactamases were used as controls. Beta-lactamases were visualized with a 0.2-mg/ml nitrocefin solution (Oxoid Ltd., Basingstoke, England). A 1 mM solution of potassium clavulanate and a 0.3 mM solution of cloxacilin were used to visualize general inhibitor characteristics.
DNA fingerprinting.
Random amplified polymorphic DNA (RAPD) analysis was applied to type the PABL-producing isolates with the primer ERIC2. Amplified PCR products were separated using 1.5% agarose gels and visualized by UV transillumination. DNA fingerprints were compared by visual inspection. RAPD patterns were regarded as different if there were different bands on visual inspection (10, 12, 21).
Conjugation.
E. coli C600, with rifamycin resistance (Rifr), was used as the recipient strain. Cultures of donor and recipient cells were grown to saturation, and 0.1 ml of each was added to the same medium (5 ml) and allowed to stand at 37°C for 2 h. The mixture was then incubated with shaking for 3 h, and 0.1-ml samples were streaked onto MacConkey agar containing 100 mg/liter rifampin and 10 mg/liter cefotaxime.
Southern hybridization.
Southern blot hybridizations were performed by standard methods (22) with a blaCMY-2-specific and blaDHA-1-specific digoxigenin (DIG)-labeled probe. Briefly, purified plasmid DNA was transferred onto a positively charged nylon membrane (Millipore) by capillary action. DIG-labeled blaCMY-2-specific and blaDHA-1-specific detection probes were generated according to the directions of the manufacturer (Roche Diagnostics). After prehybridization, hybridization of the membrane with the denatured, DIG-labeled probe (25 ng/ml of hybridization solution) was done overnight at 65°C. The hybridized probes were immunodetected with anti-DIG-alkaline phosphatase.
Statistical analysis.
All statistical tests were two-tailed χ2 tests, and the results were considered statistically significant at a P value of <0.05. The data were stored and analyzed by using SPSS (version 12.0).
RESULTS
Three hundred twenty-seven (16.9%) of the 1,935 clinical isolates yielded cefoxitin zone diameters less than 18 mm (screen positive), 54 of which were demonstrated to harbor PABLs by multiplex PCR; 41 isolates carried bla genes of the DHA group, and 13 isolates carried genes belonging to the CIT group.
Two types of PABLs were detected by PCR with specific primers and confirmed as DHA-1 (41 isolates) and CMY-2 (13 isolates) by sequencing analysis. Each isolate produced one to four β-lactamases, with pI values of 5.4, 7.6, 8.2, 7.8, and 9.0. The pI 5.4 β-lactamase corresponds to the TEM-like enzyme (inhibited by clavulanate), and the pI 7.6 and 8.2 β-lactamases correspond to SHV-like and CTX-like enzymes (inhibited by clavulanate), respectively. The β-lactamases with pI values of 7.8 and 9.0 were inhibited by cloxacilin but not by clavulanate and were consistent with DHA-1 and CMY-2 enzymes, respectively.
Based on species, PABLs were detected in 30 (4.29%) of 699 K. pneumoniae, 23 (1.91%) of 1,203 E. coli, and 1 (3.03%) of 33 K. oxytoca isolates. The genotypes of PCR amplification showed that DHA-1 and CMY-2 were harbored by 29 and 1 of 30 PABL-producing K. pneumoniae isolates and 11 and 12 of the 23 E. coli isolates, respectively. The prevalence rate of DHA-1-producing K. pneumoniae was 7.54% in 2003, and this proportion significantly decreased, to 2.72%, in 2004 (P < 0.01) but was not significant between 2003 and 2005 (2.96%) (Table 2). ERIC2 PCR was performed to investigate whether the prevalence of DHA-1-producing K. pneumoniae was due to nosocomial outbreaks of infections caused by epidemic strains. The 15 isolates of DHA-1-producing K. pneumoniae from 2003 had seven different patterns (Fig. 1). Two of the DHA-1-producing K. pneumoniae isolates from 2004 displayed the same RAPD pattern. Only 5 of 13 DHA-1-producing E. coli strains showed one pattern (data not shown). Two CMY-2-producing E. coli isolates from 2003 and six from 2005 indicated very similar RAPD patterns, while four isolates from 2004 presented different types.
TABLE 2.
Prevalence of plasmid-mediated AmpC-producing clinical isolates in a Chinese university hospital from 2003 to 2005
| Organism and yr | No. of isolates
|
||||
|---|---|---|---|---|---|
| Total | Nonsusceptible to cefoxitin | Multiplex PCR positive | Producing indicated AmpC β-lactamase
|
||
| DHA-1 | CMY-2 | ||||
| E. coli | |||||
| 2003 | 400 | 75 | 7 | 5 | 2 |
| 2004 | 513 | 88 | 7 | 3 | 4 |
| 2005 | 290 | 79 | 9 | 3 | 6 |
| Total | 1,203 | 242 | 23 | 11 | 12 |
| K. pneumoniae | |||||
| 2003 | 199 | 30 | 15 | 15 | 0 |
| 2004 | 331 | 31 | 10 | 9 | 1 |
| 2005 | 169 | 23 | 5 | 5 | 0 |
| Total | 699 | 84 | 30 | 29 | 1 |
| K. oxytoca | |||||
| 2003 | 16 | 1 | 1 | 1 | 0 |
| 2004 | 11 | 0 | 0 | 0 | 0 |
| 2005 | 6 | 0 | 0 | 0 | 0 |
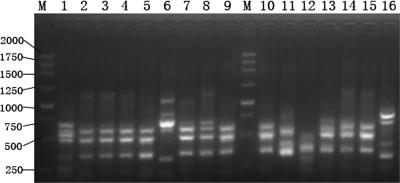
FIG. 1.
RAPD fingerprinting of isolates investigated in this work, carried out with primer ERIC2. Lanes 1 to 15, the 15 isolates of DHA-1-producing K. pneumoniae from 2003; lane 16, ATCC 25922; M, 250-bp ladder.
Twenty-seven DHA-1-producing isolates and 10 CMY-2-producing isolates were positive for ESBL production according to phenotypic ESBL confirmatory tests (Table 3). Based on species, 78.3% (18/23) of E. coli strains and 60% (18/30) of K. pneumoniae strains produced PABL and ESBL simultaneously. In order to determine the ESBL gene relatively exactly, we analyzed the beta-lactamases of these isolates by PCR experiments with a series of primers specific for blaTEM, blaSHV, and blaCTX-M. All 54 PABL-producing isolates harbored blaTEM-like genes. blaSHV-like and blaCTX-M-like genes were found in 37 and 29 of the 54 isolates, respectively. Thirty-seven of 41 DHA-1-producing isolates harbored blaSHV-like genes, and 20 of these 37 isolates harbored blaCTX-M-like genes simultaneously. Nine of 13 CMY-2-producing isolates harbored blaCTX-M-like genes. Four of 13 CMY-2-producing isolates transferred blaCMY-2 to transconjugants, 3 had blaCMY-2 alone, and 1 had blaCMY-2 and blaCTX-M. The Southern blot analysis further revealed that blaDHA-1 (data not shown) and blaCMY-2 were located on plasmids (Fig. 2).
TABLE 3.
Prevalence of AmpC and ESBL genes and correlation with phenotypes from ESBL tests
| Species and β-lactamase | No. of isolatesa
|
|||||
|---|---|---|---|---|---|---|
| Harboring genes in indicated group
|
Positive for ESBL production | Cefr | ||||
| AmpC | TEM | CTX-M | SHV | |||
| E. coli | ||||||
| DHA-1 | 11 | 11 | 5 | 8 | 8 | 8 |
| CMY-2 | 12 | 12 | 9 | 0 | 10 | 6 |
| K. pneumoniae | ||||||
| DHA-1 | 29 | 29 | 15 | 28 | 18 | 11 |
| CMY-2 | 1 | 1 | 0 | 0 | 0 | 0 |
| K. oxytoca | ||||||
| DHA-1 | 1 | 1 | 0 | 1 | 1 | 0 |
| Total | 54 | 54 | 29 | 37 | 37 | 25 |
Cefr represents resistance to cefepime.
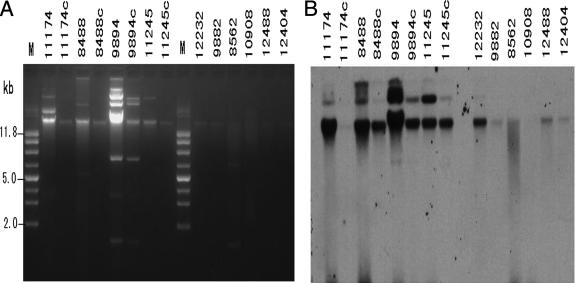
FIG. 2.
Plasmid profiles and Southern blotting. (A) plasmid DNA prepared from CMY-2-producing isolates and their transconjugants. Lane M, molecular size marker. (B) Southern blotting of the plasmid DNAs in panel A with the blaCMY-2 probe. The two photographs were aligned so that the size marker can apply to both.
The susceptibilities of the 54 isolates were as follows: piperacillin, 0%; cefazolin, 0%; cefotaxime, 13%; ceftazidime, 25.9%; cefepime, 53.7%; imipenem, 98%; and meropenem, 98%. The MICs and pI values of the isolates harboring CMY-2-type PABLs are given in Table 4.
TABLE 4.
Results for IEF and susceptibility testing of clinical isolates carrying the CMY-2-type blaAmpC gene
| Strain | pI values | Organismb | MIC (μg/ml)a for:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMP | CTN | MER | CPE | CAZ | CFT | CFX | PI | AZT | CPD | CAX | |||
| 8488 | 5.4, 8.2, 9.0 | ECO | ≤0.5 | 32 | ≤0.5 | >32 | 32 | >128 | >32 | >64 | 64 | >64 | >64 |
| 8562 | 5.4, 9.0 | ECO | ≤0.5 | 32 | ≤0.5 | 2 | 32 | 8 | >32 | >64 | 16 | >64 | 16 |
| 9882 | 5.4, 9.0 | KPN | ≤0.5 | 32 | ≤0.5 | <1 | 16 | 4 | >32 | >64 | 16 | >64 | 16 |
| 9894 | 5.4, 7.6, 9.0 | ECO | ≤0.5 | >32 | ≤0.5 | >32 | >128 | >128 | >32 | >64 | >64 | >64 | >64 |
| 10908 | 5.4, 9.0 | ECO | ≤0.5 | 4 | ≤0.5 | <1 | 8 | 4 | 32 | >64 | 4 | >64 | 4 |
| 11174 | 5.4, 8.2, 9.0 | ECO | ≤0.5 | >32 | ≤0.5 | >32 | 64 | >128 | >32 | >64 | 32 | >64 | >64 |
| 11245 | 5.4, 8.2, 9.0 | ECO | ≤0.5 | 32 | ≤0.5 | >32 | 128 | >128 | >32 | >64 | 64 | >64 | >64 |
| 12232 | 5.4, 8.2, 9.0 | ECO | ≤0.5 | 32 | ≤0.5 | >32 | 128 | >128 | >32 | >64 | >64 | >64 | >64 |
| 12404 | 5.4, 8.2, 9.0 | ECO | 16 | >32 | 16 | >32 | >128 | >128 | >32 | >64 | >64 | >64 | >64 |
| 12488 | 5.4, 8.2, 9.0 | ECO | ≤0.5 | 32 | ≤0.5 | >32 | 128 | >128 | >32 | >64 | 64 | >64 | >64 |
| 12535 | 5.4, 9.0 | ECO | ≤0.5 | 32 | ≤0.5 | >32 | 128 | >128 | >32 | >64 | 64 | >64 | >64 |
| 12575 | 5.4, 8.2, 9.0 | ECO | ≤0.5 | >32 | ≤0.5 | >32 | 64 | >128 | >32 | >64 | >64 | >64 | >64 |
| 13317 | 5.4, 8.2, 9.0 | ECO | ≤0.5 | 32 | ≤0.5 | <1 | 32 | 8 | >32 | >64 | 8 | >64 | 16 |
IMP, imipenem; CTN, cefotetan; MER, meropenem; CPE, cefepime; CAZ, ceftazidime; CFT, cefotaxime; CFX, cefoxitin; PI, piperacillin; AZT, aztreonam; CPD, cefpodoxime; CAX, ceftriaxone.
ECO, E. coli; KPN, K. pneumoniae.
DISCUSSION
Plasmid-mediated class C β-lactamases have been discovered most frequently in naturally AmpC-negative species, such as K. pneumoniae, K. oxytoca, Salmonella spp., and Proteus mirabilis, and also in E. coli, which normally weakly expresses the chromosomal AmpC enzyme by gene duplication or mutation in the ampC promoter or attenuator, with consequent enhanced gene expression. Since CMY-1 was first reported (isolated from South Korea in 1989), over 40 types of PABLs have been reported worldwide. The prevalence of PABLs among clinical isolates differs depending on the countries and institutions. In China, though PABL (ACT-1 type) was first reported in 2002 (3), the true rate of occurrence of PABLs remains unknown. In this study, the prevalence rate (2.79%) of PABLs in Escherichia coli and Klebsiella spp. is similar to that in Korea (3.1%) but higher than that in the United States (1.2%) (4, 25). The inducible PABL DHA-1 is the most prevalent PABL in China. DHA-1 is also the most prevalent PABL in other areas of Asia (25, 30).
Based on species, 1.9% of E. coli and 4.3% of K. pneumoniae isolates harbored PABLs in this study. In Korea, the occurrence rates for these species are 1.5% and 5.4% (or 2.9%), respectively (24, 25). But in the United States, they decreased to 1.6% (4) and 3.3% (13), respectively. The prevalence rate of DHA-1-producing K. pneumonia significantly decreased, from 7.54% to 2.72%, between 2003 and 2004 in this study. In contrast, it significantly increased in Korea, from 0.6% in 2002 to 2.8% in 2003 and to 4.3% in 2004 (24). The results of RAPD analysis indicate that the prevalences of DHA-1-producing K. pneumoniae (Fig. 1) and CMY-2-producing E. coli strains were due to a combination of clonal spread and horizontal transmission of plasmids (Fig. 3).
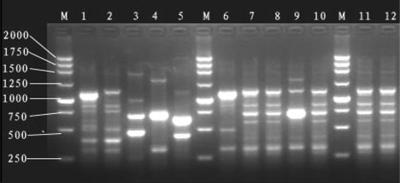
FIG. 3.
RAPD fingerprinting of isolates investigated in this work, carried out with primer ERIC2. Lanes 1 to 12, the 12 isolates of CMY-2-producing E. coli from 2003 to 2005. M, 250-bp ladder.
Because PABLs provided a broader spectrum of resistance than ESBLs and were not inhibited by commercially available inhibitors, the antimicrobial agents that could be used are limited. Previous studies suggested that cefepime might be effective for the treatment of infections caused by an AmpC-producing organism. However, in this study, only 53.7% (29 of 54) PABL producers were susceptible to cefepime. Song et al. reported that the association of PABLs with ESBLs may cause failure of treatment, and there is also a report indicating the high inoculum effect of cefepime in PABL-producing isolates (11, 20, 26). Thirty-seven (68.5%) of the 54 PABL-producing isolates also produced an ESBL, which was confirmed by CLSI-recommended confirmatory tests. By the PCR method, 47 (87.0%) of the 54 isolates were detected to harbor blaCTX-like or blaSHV-like genes. Based on species, 93.3% (28/30) of K. pneumoniae and 78.3% (18/23) of E. coli isolates harbored beta-lactamase genes of AmpC and blaCTX-like or blaSHV-like genes simultaneously. This rate of K. pneumoniae is far higher than that in Korea (8.7%) but is lower than that in Japan (100%) (14, 24). In this study, one isolate (no. 12404), which harbored blaCMY-2, blaTEM-like, and blaCTX-like genes, was simultaneously resistant to imipenem and meropenem. Poirel et al. reported that one E. coli strain with CMY-2 and without outer membrane proteins OmpF and OmpC was resistant to carbapenem and other broad-spectrum cephalosporins (19). The conjugation test of this isolate was not successful, and further research is in progress.
Southern blot hybridization revealed that the CMY-2 gene is located on plasmid. The different bands that hybridized to the CMY-2 probe, though the size of each band differed in each isolate, could presumably represent another gene that has homology to blaCMY-2 or partial or complete duplications of CMY-2.
The CMY-2 β-lactamase was first described in 1990 (2). It is the ancestor of the other C. freundii ampC alleles that have been found on plasmids since then (1). CMY-2 was detected not only in E. coli, K. pneumoniae, K. oxytoca, and P. mirabilis but also in Salmonella and Shigella (5, 8, 15), the last two having caused an outbreak. CMY-2 is the most prevalent and geographically the most widely distributed PABL (18). In China, the most prevalent type of PABL is DHA-1. The CMY-2 β-lactamase has not been previously reported to occur in China. In 2004, Guan et al. (6) reported a new CMY-type cephalosporinase which is 99% identical to the deduced amino acid sequences of CMY-2 and CMY-7. To the best of our knowledge, this is the first report of plasmid-mediated CMY-2-type AmpC β-lactamases in mainland China.
In conclusion, the prevalence rate of PABLs is 2.79% in Escherichia coli and Klebsiella spp. in this collection of isolates from a medical center in China. DHA-1 is the predominant PABL, and DHA-1-producing K. pneumoniae is the most prevalent bacterial species harboring PABLs. The results of this study reinforce the need for increasing concern for therapy for clinical infections caused by isolates that coproduce PABL and ESBL. This study also represents the first time that CMY-2 has been described to occur in China.
Footnotes
Published ahead of print on 27 February 2007.
REFERENCES
- 1.Barlow, M., and B. G. Hall. 2002. Origin and evolution of the AmpC beta-lactamases of Citrobacter freundii. Antimicrob. Agents Chemother. 461190-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauernfeind, A., I. Stemplinger, R. Jungwirth, and H. Giamarellou. 1996. Characterization of the plasmidic beta-lactamase CMY-2, which is responsible for cephamycin resistance. Antimicrob. Agents Chemother. 40221-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, Y. L., H. Wang, W. Y. Wu, and M. J. Chen. 2002. Identification of a plasmid-mediated AmpC beta-lactamase (ACT-1) from a clinical isolate of Escherichia coli. Chin. J. Infect. Chemother. 2158-161. [Google Scholar]
- 4.Coudron, P. E., E. S. Moland, and K. S. Thomson. 2000. Occurrence and detection of AmpC beta-lactamases among Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates at a veterans medical center. J. Clin. Microbiol. 381791-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doublet, B., A. Carattoli, J. M. Whichard, D. G. White, S. Baucheron, E. Chaslus-Dancla, and A. Cloeckaert. 2004. Plasmid-mediated florfenicol and ceftriaxone resistance encoded by the floR and bla(CMY-2) genes in Salmonella enterica serovars Typhimurium and Newport isolated in the United States. FEMS Microbiol. Lett. 233301-305. [DOI] [PubMed] [Google Scholar]
- 6.Guan, X. Z., Y. N. Liu, Y. P. Luo, D. Y. She, G. Zhou, L. A. Chen, and Y. P. Xu. 2004. A new member of CMY type cephalosporinase prevailing in Escherichia coli. Zhonghua Yi Xue Za Zhi 841872-1875. (In Chinese.) [PubMed] [Google Scholar]
- 7.Hanson, N. D., E. S. Moland, A. Hossain, S. A. Neville, I. B. Gosbell, and K. S. Thomson. 2002. Unusual Salmonella enterica serotype Typhimurium isolate producing CMY-7, SHV-9 and OXA-30 beta-lactamases. J. Antimicrob. Chemother. 491011-1014. [DOI] [PubMed] [Google Scholar]
- 8.Huang, I. F., C. H. Chiu, M. H. Wang, C. Y. Wu, K. S. Hsieh, and C. C. Chiou. 2005. Outbreak of dysentery associated with ceftriaxone-resistant Shigella sonnei: first report of plasmid-mediated CMY-2-type AmpC beta-lactamase resistance in S. sonnei. J. Clin. Microbiol. 432608-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang, X., Y. Ni, Y. Jiang, F. Yuan, L. Han, M. Li, H. Liu, L. Yang, and Y. Lu. 2005. Outbreak of infection caused by Enterobacter cloacae producing the novel VEB-3 beta-lactamase in China. J. Clin. Microbiol. 43826-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson, J. R., and T. T. O'Bryan. 2000. Improved repetitive-element PCR fingerprinting for resolving pathogenic and nonpathogenic phylogenetic groups within Escherichia coli. Clin. Diagn. Lab. Immunol. 7265-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang, C. I., H. Pai, S. H. Kim, H. B. Kim, E. C. Kim, M. D. Oh, and K. W. Choe. 2004. Cefepime and the inoculum effect in tests with Klebsiella pneumoniae producing plasmid-mediated AmpC-type beta-lactamase. J. Antimicrob. Chemother. 541130-1133. [DOI] [PubMed] [Google Scholar]
- 12.Manges, A. R., J. R. Johnson, B. Foxman, T. T. O'Bryan, K. E. Fullerton, and L. W. Riley. 2001. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N. Engl. J. Med. 3451007-1013. [DOI] [PubMed] [Google Scholar]
- 13.Moland, E. S., N. D. Hanson, J. A. Black, A. Hossain, W. Song, and K. S. Thomson. 2006. Prevalence of newer beta-lactamases in gram-negative clinical isolates collected in the United States from 2001 to 2002. J. Clin. Microbiol. 443318-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muratani, T., T. Kobayashi, and T. Matsumoto. 2006. Emergence and prevalence of beta-lactamase-producing Klebsiella pneumoniae resistant to cephems in Japan. Int. J. Antimicrob. Agents 27491-499. [DOI] [PubMed] [Google Scholar]
- 15.Navarro, F., E. Perez-Trallero, J. M. Marimon, R. Aliaga, M. Gomariz, and B. Mirelis. 2001. CMY-2-producing Salmonella enterica, Klebsiella pneumoniae, Klebsiella oxytoca, Proteus mirabilis and Escherichia coli strains isolated in Spain (October 1999-December 2000). J. Antimicrob. Chemother. 48383-389. [DOI] [PubMed] [Google Scholar]
- 16.Pagani, L., E. Dell'Amico, R. Migliavacca, M. M. D'Andrea, E. Giacobone, G. Amicosante, E. Romero, and G. M. Rossolini. 2003. Multiple CTX-M-type extended-spectrum beta-lactamases in nosocomial isolates of Enterobacteriaceae from a hospital in northern Italy. J. Clin. Microbiol. 414264-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-Pérez, F. J., and N. D. Hanson. 2002. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 402153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philippon, A., G. Arlet, and G. A. Jacoby. 2002. Plasmid-determined AmpC-type beta-lactamases. Antimicrob. Agents Chemother. 461-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poirel, L., C. Heritier, C. Spicq, and P. Nordmann. 2004. In vivo acquisition of high-level resistance to imipenem in Escherichia coli. J. Clin. Microbiol. 423831-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Queenan, A. M., B. Foleno, C. Gownley, E. Wira, and K. Bush. 2004. Effects of inoculum and beta-lactamase activity in AmpC- and extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae clinical isolates tested by using NCCLS ESBL methodology. J. Clin. Microbiol. 42269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasschaert, G., K. Houf, H. Imberechts, K. Grijspeerdt, L. De Zutter, and M. Heyndrickx. 2005. Comparison of five repetitive-sequence-based PCR typing methods for molecular discrimination of Salmonella enterica isolates. J. Clin. Microbiol. 433615-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 23.Schlesinger, J., S. Navon-Venezia, I. Chmelnitsky, O. Hammer-Munz, A. Leavitt, H. S. Gold, M. J. Schwaber, and Y. Carmeli. 2005. Extended-spectrum beta-lactamases among Enterobacter isolates obtained in Tel Aviv, Israel. Antimicrob. Agents Chemother. 491150-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song, W., J. S. Kim, H. S. Kim, D. Yong, S. H. Jeong, M. J. Park, and K. M. Lee. 2006. Increasing trend in the prevalence of plasmid-mediated AmpC beta-lactamases in Enterobacteriaceae lacking chromosomal ampC gene at a Korean university hospital from 2002 to 2004. Diagn. Microbiol. Infect. Dis. 55219-224. [DOI] [PubMed] [Google Scholar]
- 25.Song, W., J. S. Kim, M. N. Kim, E. C. Kim, Y. J. Park, D. Yong, K. Lee, W. G. Lee, S. H. Jeong, and K. M. Lee. 2002. Occurrence and genotypic distributions of plasmid-mediated AmpC β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Korea. Korean J. Lab. Med. 22410-416. [Google Scholar]
- 26.Song, W., E. S. Moland, N. D. Hanson, J. S. Lewis, J. H. Jorgensen, and K. S. Thomson. 2005. Failure of cefepime therapy in treatment of Klebsiella pneumoniae bacteremia. J. Clin. Microbiol. 434891-4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, Y., X. F. Jiang, J. Y. Sun, L. Z. Han, and Y. X. Ni. 2003. Detection of plasmid-mediated ampC gene, blaDHA-1 from clinical isolates of Klebsiella pneumoniae. Shanghai J. Med. Lab. Sci. 18331-335. [Google Scholar]
- 28.Wang, Y., and Z. H. Li. 2005. Identification of a plasmid-mediated AmpC beta-lactamase from clinical isolates of Escherichia coli and Klebsiella pneumoniae. Zhonghua Jie He He Hu Xi Za Zhi 28685-688. (In Chinese.) [PubMed] [Google Scholar]
- 29.Wei, Z. Q., Y. G. Chen, Y. S. Yu, W. X. Lu, and L. J. Li. 2005. Nosocomial spread of multi-resistant Klebsiella pneumoniae containing a plasmid encoding multiple beta-lactamases. J. Med. Microbiol. 54885-888. [DOI] [PubMed] [Google Scholar]
- 30.Yan, J. J., W. C. Ko, H. M. Wu, S. H. Tsai, C. L. Chuang, and J. J. Wu. 2004. Complexity of Klebsiella pneumoniae isolates resistant to both cephamycins and extended-spectrum cephalosporins at a teaching hospital in Taiwan. J. Clin. Microbiol. 425337-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]