Abstract
Shiga toxin (Stx)-producing Escherichia coli (STEC) strains secrete toxins that are major virulence factors and diagnostic targets, but some STEC strains secrete Stx in amounts that cannot be detected using conventional cell cytotoxicity or immunological assays. Therefore, there is an urgent need for more-sensitive Stx detection methods. We describe the development of an assay that can detect low concentrations of Stx2 and its variants. An immuno-PCR Stx2 assay was developed based on an enzyme immunoassay (EIA) combining antibody capture and DNA amplification to increase the signal. The immuno-PCR assay detected 10 pg/ml of purified Stx2, compared to 1 ng/ml Stx2 detected by commercial EIA. Consequently, immuno-PCR detected Stx2 and its variants in STEC strains that produce the toxins at levels that are nondetectable by using the EIA, as well as the Stx2 in EIA-negative enriched stool cultures from patients. Our data demonstrate that the immuno-PCR developed here is a highly sensitive and specific method for the detection of trace amounts of Stx2 and Stx2 variants. It is therefore suitable for use by clinical microbiological laboratories to improve the toxin detection in clinical samples.
The Shiga toxin (Stx)-producing Escherichia coli (STEC) serotype O157:H7 and several non-O157:H7 serotypes have emerged worldwide as the causes of diarrhea, hemorrhagic colitis, and a life-threatening hemolytic-uremic syndrome (HUS) (3, 8, 9, 10, 13, 15, 17, 18, 22, 26, 28, 31, 43, 48, 52). The pathogenicity of STEC is based on the production and release of one or more Stx types (38), which include two major toxin types (Stx1 and Stx2) and a number of variants, of which Stx1c, Stx1d, Stx2c, Stx2c2, Stx2d, Stx2dactivatable, Stx2e, and Stx2f have been identified in STEC strains isolated from humans (7, 15, 17, 27, 32, 34, 39, 40, 41, 42, 50). Stx2 is clinically the most important Stx type, which is significantly associated with severe outcomes of human infections including HUS (9, 15, 43, 52).
Not only are Stx types the major virulence factors of STEC strains (47), but they and their encoding genes are also exploitable targets for laboratory diagnosis of these pathogens. Conventional PCR (20), real-time PCR (5, 27, 35, 36), and colony blot hybridization (19) have been used to detect the stx genes. Stx secreted by STEC strains is detected by using the Vero cell cytotoxicity assay (24), the enzyme immunoassay (EIA) (12), latex agglutination (23), and colony immunoblotting (21). Several assays are commercially available. A prerequisite for the successful detection of Stx is the production and secretion of the protein in amounts sufficient for detection.
We have recently shown that STEC strains that possess the stx2 gene variants secrete Stx in amounts that are below the level of detection by conventional techniques (51). Because such strains are not detected in clinical microbiological laboratories that rely solely on Stx detection, there is a need to improve the sensitivity of Stx detection procedures. The sensitivity of Stx detection can be enhanced by using the immuno-PCR technique, in which antibody capture is combined with DNA amplification. This method has been used to detect trace amounts of antigens such as cytokines, hormones, and viral antigens (1, 2, 4, 11, 14, 16, 29, 37). Here, we developed a highly sensitive immuno-PCR based on a direct EIA system and suitable for the detection of Stx2 and its variants.
MATERIALS AND METHODS
Bacterial strains.
STEC strains were isolated at the Institute of Hygiene and Microbiology, University of Würzburg, Würzburg, Germany, and at the Institute of Hygiene, University of Münster, Münster, Germany, using a protocol described previously (15, 31). The isolates were serotyped and characterized for their stx genotypes (51).
EIA for the detection of Stx.
Stx in stool cultures was detected using a commercial EIA (Ridascreen Verotoxin enzyme-linked immunoassay; R-Biopharm, Darmstadt, Germany) (35). The assay was performed with supernatants of stool cultures enriched overnight in tryptic soy broth (TSB) containing mitomycin C (50 ng/ml) (Sifin, Berlin, Germany), according to the manufacturer's instructions.
Purification of Stx.
Stx2 was purified as described previously (30) from E. coli C600 lysogenized with stx2-harboring bacteriophage 933W, originating from E. coli O157:H7 strain EDL933 (45). Briefly, the toxin was eluted from agar plates showing confluent lysis after inoculation with strain C600(Φ933W). After bacterial debris was removed by low-speed centrifugation, the supernatant was precipitated with 50% ammonium sulfate and applied to Sepharyl S200 columns. Fractions with cytotoxic activity in the molecular range between 30 and 80 kDa were concentrated by vacuum extraction. Toxin was further purified by using an Affi-Gel Blue column and further purged by chromatofocusing and high-performance liquid chromatography.
Biotinylation of antibodies and conjugation of streptavidin to reporter DNA.
The Stx2 antigen was detected by using a monoclonal anti-Stx2 antibody (Sifin, Berlin, Germany); the antibody (clone VT136/8-H4) belongs to the immunoglobulin G1 class and reacts with the B subunit of Stx2. The antibody was conjugated to biotin [sulfosuccin-imidyl-6(biotinamido)hexanoate] according to the manufacturer's instructions (Molecular Biosciences, CO). Briefly, a solution of 50 μg of freshly prepared biotin was mixed with 500 μg of the anti-Stx2 antibody in 100 mM NaHCO3 (pH 8.5). After a 1-h incubation at room temperature, the antibody was stored at 4°C until use.
A biotinylated 296-bp DNA fragment originating from the plasmid pUC19 (Fermentas, St. Leon-Rot, Germany) was amplified by using PCR with the primers pUC-bio (5′-biotin-CCC GGA TCC CAG CAA TAA ACC AGC CAG CC-3′) and pUC-2 (5′-GCC AAC TTA CTT CTG ACA AC-3′) (synthesized by Sigma Genosys, Taufkirchen, Germany). A BamHI-restriction site was included in the first primer to enable a specific enzymatic restriction. The product was purified by using a QIAquick PCR purification kit (Qiagen, Hilden, Germany). The biotinylated DNA (1 μM) was mixed with 2 μM recombinant streptavidin (Roche, Mannheim, Germany) in Tris-buffered saline (TBS; 10 mM Tris [pH 7.3], containing 150 mM NaCl), incubated for 30 min at room temperature, and frozen at −20°C until used.
Immuno-PCR assay.
TopYield strip wells (Nunc, Roskilde, Denmark) were coated with the purified Stx2 preparation or bacterial supernatants (30 μl/well) and incubated for 16 h at 4°C. Ten-fold dilutions of Stx2 were prepared in sodium carbonate buffer (pH 9.5), starting from 1 μg/ml. Plates were washed five times with TBS, and nonadsorbed sites were blocked with 200 μl/well of blocking buffer (TBS containing 4.5% skim milk powder, 5 mM EDTA, and 1 mg/ml herring sperm DNA) for 1 h at room temperature. After wells were washed five times with TBS-TE (TBS containing 0.05% Tween 20 and 5 mM EDTA), 30 μl of biotinylated anti-Stx2 antibody (final concentration of 34 ng/ml) was added to each well. Incubation followed, for 30 min at 37°C, and wells were finally washed five times with TBS-TE. The biotinylated DNA conjugated with streptavidin was diluted 1:20 in TBS, and 30 μl was added to each well and incubated for 30 min at room temperature. After wells were washed eight times with TBS-TE, followed by five washes with TBS, the wells were incubated for 1 h with TBS to remove unbound DNA. Bound DNA was detached from the complex by incubating the wells with 30 μl of restriction buffer containing 1 U of BamHI (2 h, 37°C). The released DNA was collected into new tubes, and aliquots were used as templates in PCR.
Signal amplification by real-time PCR and by conventional PCR.
To amplify the signal of the immune complex, real-time PCR was performed with a LightCycler system (Roche, Mannheim, Germany) using a QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany). PCRs were prepared in microcapillary tubes in 10-μl volumes containing 1 μl of template DNA, 5 μl of 2× SYBR Green Master mixture, and 0.5 μM of the primers pUC-1 (5′-CAG CAA TAA ACC AGC CAG CC-3′) and pUC-2. Positive and negative controls (the PCR product created with the primers pUC-1/pUC-2 and sterile distilled water, respectively) were included in each run. Cycle parameters included a preliminary denaturation (95°C, 15 min), followed by 30 cycles of denaturation (95°C, 15 s), annealing (55°C, 30 s), and primer extension (72°C, 30 s).
Conventional PCR was accomplished in a volume of 30 μl, using a thermocycler (Biometra, Göttingen, Germany) and reagents from Peqlab Biotechnologie (Erlangen, Germany). The PCR mixture contained 3 μl of template DNA, 200 μM of each deoxynucleoside triphosphate, 15 pmol of the primers pUC-1 and pUC-2, 3 μl of 10-fold-concentrated polymerase synthesis buffer Y, 1.5 mM MgCl2, and 0.65 U of AmpliTaq DNA polymerase. DNA was amplified by 30 cycles of denaturation (95°C, 30 s), annealing (55°C, 1 min), and extension (72°C, 1 min), preceded by an initial denaturation (95°C, 5 min), and followed by a final extension (72°C, 5 min). Aliquots of 15 μl of the PCR products were analyzed by electrophoresis on a 1.5% agarose gel and visualized using ethidium bromide staining.
RESULTS AND DISCUSSION
Development and optimization of immuno-PCR.
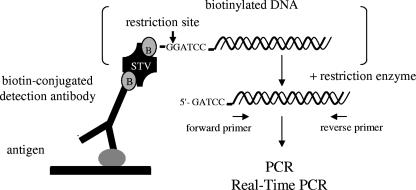
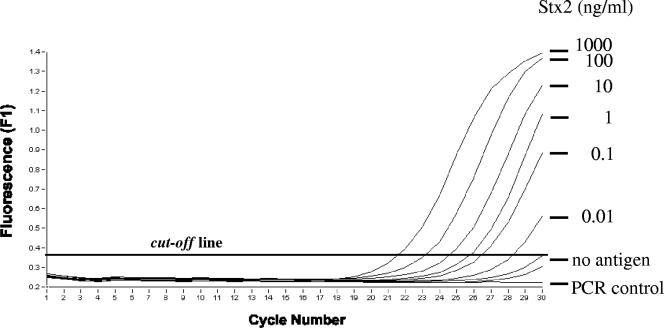
A highly sensitive immuno-PCR assay, which is based on a direct EIA system, was developed for the detection of Stx2 and its variants (Fig. 1). Because proteins and/or excess DNA can interfere with PCR, DNA bound to the antigen-antibody complex was detached by enzymatic restriction (Fig. 1), collected, and subsequently amplified with PCR. Under optimized conditions, the immuno-PCR allowed the detection of ≥10 pg/ml of purified Stx2 (Fig. 2), compared to 1 ng/ml of the toxin that was detectable with a commercial EIA. Because nonspecific signals would occur with an increase in the cycle numbers to more than 30 (Fig. 2), the number of cycles was limited to 30.
FIG. 1.
Schematic presentation of the immuno-PCR approach. B, biotin; STV, streptavidin.
FIG. 2.
Detection of purified Stx2 by using the immuno-PCR assay. Signals produced by decreasing concentrations in ng/ml of the purified Stx2 preparation with a real-time immuno-PCR assay are shown. Amplification results after 30 cycles are marked. Use of the coating buffer without antigen served as a negative control (no antigen). PCR was controlled using an assay without template DNA (PCR control).
Evaluation of the anti-Stx2 antibody used in the immuno-PCR to detect Stx2 variants.
To investigate the ability of the monoclonal anti-Stx2 antibody (clone VT136/8-H4) to recognize Stx2 and also Stx2 variants produced by STEC strains isolated from humans (15), reference STEC strains producing Stx2 or different Stx2 variants produced as a single Stx were tested by using a colony immunoblot assay (Shiga toxin [Verotoxin] colony immunoblot; Sifin, Berlin, Germany) which uses this antibody to detect Stx2. The anti-Stx2 antibody recognized Stx2c, Stx2d, Stx2dactivatable, and Stx2e but not Stx2f (Table 1), which displays only 57.4% nucleotide sequence homology of its B subunit to the B subunit of Stx2 (39). Moreover, the ability of the anti-Stx2 antibody was evaluated for the detection of the Stx2 variants in the immuno-PCR (Table 1). Again, all Stx2 variants except for Stx2f could be detected in culture supernatants of the reference STEC strains, up to the dilution of 1:100 (Table 1).
TABLE 1.
Detection of Stx2 variants in STEC reference strainsa
| Strain | Serotype | Stx types produced | Reference | Detection of the Stx2 variant using:
|
|
|---|---|---|---|---|---|
| Stx colony immunoblottingb | Immuno-PCRc | ||||
| E32511/HSC | O157:NM | Stx2c | 23 | + | 1:100 |
| EH250 | ONT:H12 | Stx2d | 34 | + | 1:100 |
| B2F1 | O91:H21 | Stx2dactivatable | 32 | + | 1:100 |
| 2771/97 | ONT:NM | Stx2e | 33 | + | 1:100 |
| T4/97 | O128:H2 | Stx2f | 39 | − | — |
Stx2 variants were detected in STEC reference strains by using an anti-Stx2 monoclonal antibody (clone VT136/8-H4) in a colony immunoblotting assay and immuno-PCR.
Shiga toxin (Verotoxin) colony immunoblotting (Sifin) was performed according to the manufacturers' instructions. +, positive signal; −, no signal.
The highest dilution of the supernatant which caused a positive signal is shown. —, no signal was observed with the undiluted supernatant.
Detection of Stx in low-Stx producers, using immuno-PCR.
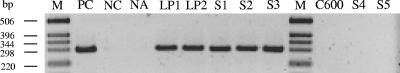
The optimized immuno-PCR was subsequently adapted for conventional PCR and used to reassay Stx in six STEC strains, which, despite the presence of intact stx genes, tested Stx negative by using conventional toxin detection assays including the Vero cell test, EIA, latex agglutination, and Stx colony immunoblotting (51). Bacterial supernatants from cultures in TSB containing mitomycin C were used to coat the plates, and Stx2 detection was performed as described above. This permitted the detection of Stx in supernatant dilutions of up to 1:10 (Table 2; Fig. 3, lanes LP1 and LP2). Supernatants of strains that produced Stx at levels that were detectable by the conventional assays were used for comparison, and they tested positive with the immuno-PCR at dilutions of up to 1:100 (Table 2). Thus, using the sensitive immuno-PCR technique, all six STEC strains that were not detected with conventional assays were identified as Stx producers, notwithstanding their low levels of Stx production. In contrast, culture supernatants of the 20 stx gene-negative E. coli strains, including the laboratory E. coli strain C600 (Fig. 3), which were tested as controls to determine the specificity of the observed immuno-PCR signals, were negative with the immuno-PCR (Table 2).
TABLE 2.
Detection of Stx in STEC strain supernatants and enriched stool cultures by conventional assays and by immuno-PCR
| Sample (no. of samples) | stx genotype | Conventional assaysa | Immuno-PCR detection of Stxb
|
||
|---|---|---|---|---|---|
| Undiluted | 1:10 | 1:100 | |||
| STEC low producers (6) | stx2dc | − | + | + | − |
| stx2ed | − | + | + | − | |
| STEC controls (4) | stx2de | + | + | + | + |
| stx2ef | + | + | + | + | |
| stx1, stx2g | + | + | + | + | |
| Nonproducers (20) | —h | − | − | − | − |
| Stool samples (3) | stx2 | − | + | ND | ND |
| Stool samples (10) | — | − | − | ND | ND |
Conventional assays include the Vero cell cytotoxicity assay, the Stx enzyme immunoassay (R-Biopharm), the latex agglutination assay (VTEC-RPLA; Denka Seiken, Coventry, United Kingdom), and Stx colony immunoblotting (Sifin). +, positive result; −, negative result. Identical results were obtained with all of the conventional assays for each strain.
Immuno-PCR detection response to various supernatant dilutions. +, PCR product present; −, PCR product absent; ND, not done.
Serotypes O40:H8 (three samples), O86:H−, and Orough:HNT.
Serotype ONT (not typeable):H−.
Serotypes O91:H− and ONT:H12.
Serotype ONT:H−.
Strain EDL933 serotype O157:H7.
—, no stx gene was detected.
FIG. 3.
Agarose gel electrophoresis of PCR amplicons pUC-1/pUC-2 produced with a conventional immuno-PCR assay with culture supernatants of the low Stx producers (LP1 and LP2), the stx-negative E. coli strain C600, the stx-positive enriched stool samples (S1 to S3), and the stx-negative enriched stool samples (S4 and S5). DNA ladder (lanes M, 1-kb ladder; Invitrogen) and PCR controls in the presence (PC, positive control) or absence of template DNA (NC, negative control) were included. The negative control of the immuno-PCR was coating buffer without antigen (NA, no antigen).
Detection of Stx in enriched stool cultures, using immuno-PCR.
Next, we investigated the ability of the immuno-PCR to detect Stx2 in enriched stool cultures that were stx2 positive by PCR screening but Stx negative by using a commercial EIA (Ridascreen Verotoxin enzyme-linked immunoassay; R-Biopharm), which is widely used to screen for STEC strains in clinical and environmental samples (6, 10, 35). The samples were enriched in TSB medium containing mitomycin C, and supernatants of the enriched cultures were tested using the Ridascreen EIA and the immuno-PCR. The immuno-PCR detected Stx2 in all three stool cultures (Fig. 3, lane S1 to S3; Table 2) that tested negative with the EIA. In contrast, none of 10 stool cultures that were stx negative with PCR screening produced any signal in the immuno-PCR (Table 2 and Fig. 3, lanes S4 and S5), demonstrating the specificity of Stx detection in stool cultures.
Our data demonstrate that the immuno-PCR method developed in this study is ∼100-fold more sensitive than the EIA for the detection of purified Stx2 and can detect the toxin and its variants Stx2d and Stx2e in STEC isolates, as well as in patients' enriched stool cultures, which tested negative for Stx with the EIA. Moreover, we show that the anti-Stx2 antibody used in the immuno-PCR is also able to recognize, in addition to the Stx2 types listed above, other presently known Stx2 variants associated with human disease, specifically Stx2c and Stx2dactivatable (Table 1). Therefore, the immuno-PCR is also potentially useful for the detection of, in addition to Stx2d and Stx2e, low amounts of Stx2c and Stx2dactivatable in STEC isolates, as well as in patients' enriched stool cultures. The failure of the antibody to recognize Stx2f (in both the immuno-PCR and the EIA), likely as a consequence of the low amino acid sequence homology between the B subunits of Stx2f and Stx2 (39), is not critical for the applicability of the immuno-PCR in clinical laboratories because STEC strains producing Stx2f are only extremely rarely associated with human diseases (15, 41).
Because of its high sensitivity, the immuno-PCR is suitable for routine use in clinical microbiological laboratories, especially for patients with suspected STEC infections who test Stx negative with conventional toxin assays. This is particularly important also because E. coli O157:H7, the STEC strain with the strongest association with outbreaks and severe illness (15, 47), might be overlooked using EIAs (26, 28, 49), probably because the in vitro Stx production is not consistently above the level of detection by such methods (28, 49). Moreover, we believe that after optimization and standardization for a particular purpose, the immuno-PCR has additional potential applications such as for the detection of low amounts of Stx in body fluids such as urine. Specifically, using urine samples spiked with purified Stx2, we could show that the immuno-PCR was able to detect ≥10 pg of Stx2 per ml of urine, the level of sensitivity comparable to that for the detection of Stx2 in culture supernatants (authors' unpublished data). This suggests that the immuno-PCR might be useful for the detection of Stx2 (and its variants) in the urine in those rare cases of HUS which follow a urinary tract infection (25, 44, 46), enabling a more precise evaluation of the etiological association of the STEC isolated from the patient's urine with the underlying HUS. In contrast, our immuno-PCR protocol in its present form was not able to detect Stx2 in filtrates of stool samples spiked with various Stx2 concentrations ranging from 100 ng/ml to 10 pg/ml (unpublished data). The absence of a measurable signal even in the presence of 100 ng/ml of Stx2, which could be detected by a commercial EIA, probably results from the inability of the Stx2 present in the filtrate to bind the anti-Stx2 antibody, likely as a consequence of a blocking effect of other proteins and/or other substances present in the stool. Because the highest Stx2 concentration (100 ng/ml) could be detected by using a commercial EIA, in which an anti-Stx2 antibody rather than a sample is used to coat the microtiter plate, we believe that a modification of the immuno-PCR to a sandwich system similar to that used in the EIA will allow the detection of Stx2 in stool filtrates and will increase the sensitivity of the Stx2 detection compared to that of the EIA. This project is currently under way in our laboratory.
Because of its ability to detect, with a high sensitivity, the presence of Stx, which in turn, suggests the presence of living STEC organisms in the stool sample, the immuno-PCR represents a useful adjunct to DNA-based STEC screening methods, such as various PCR protocols, which also provide positive signals with nonliving bacteria. However, in every case, the positive result obtained with a screening method needs to be confirmed by the isolation of the infecting STEC strain from the stool sample. Only such an approach enables us to confirm the specificity of the nonculture test and to perform further, thorough characterizations of the isolate, which is necessary for microbiological, diagnostic, and epidemiological purposes.
Acknowledgments
This work was supported in part by research grants from the German Federal Ministry for Education and Science (project 0312733), from the Ministry for Education, Science and Research of North Rhine Westphalia (project ZM760374), and from the EU Network ERA-NET PathoGenoMics (project number 0313937C).
We thank Phillip I. Tarr (Washington University School of Medicine, St. Louis, MO) for critical reading of the manuscript and fruitful discussions.
Footnotes
Published ahead of print on 13 February 2008.
REFERENCES
- 1.Adler, M., R. Wacker, and C. M. Niemeyer. 2003. A real-time immuno-PCR assay for routine ultrasensitive quantification of proteins. Biochem. Biophys. Res. Commun. 308240-250. [DOI] [PubMed] [Google Scholar]
- 2.Adler, M., S. Schulz, R. Fischer, and C. M. Niemeyer. 2005. Detection of rotavirus from stool samples using a standardized immuno-PCR (“Imperacer”) method with end-point and real-time detection. Biochem. Biophys. Res. Commun. 3331289-1294. [DOI] [PubMed] [Google Scholar]
- 3.Banatvala, N., P. M. Griffin, K. D. Greene, T. J. Barrett, W. F. Bibb, J. H. Green, and J. G. Wells. 2001. The United States national prospective hemolytic uremic syndrome study: microbiologic, serologic, clinical, and epidemiologic findings. J. Infect. Dis. 1831063-1070. [DOI] [PubMed] [Google Scholar]
- 4.Barletta, J. M., D. C. Edelman, and N. T. Constantine. 2004. Lowering the detection limits of HIV-1 viral load using real-time immuno-PCR for HIV-1 p24 antigen. Am. J. Clin. Pathol. 12220-27. [DOI] [PubMed] [Google Scholar]
- 5.Bellin, T., M. Pulz, A. Matussek, H. G. Hempen, and F. Gunzer. 2001. Rapid detection of enterohemorrhagic Escherichia coli by real-time PCR with fluorescent hybridization probes. J. Clin. Microbiol. 39370-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutin, L., H. Steinruck, G. Krause, K. Steege, S. Haby, G. Hultsch, and B. Appel. 2007. Comparative evaluation of the Ridascreen verotoxin enzyme immunoassay for detection of Shiga-toxin producing strains of Escherichia coli (STEC) from food and other sources. J. Appl. Microbiol. 102630-639. [DOI] [PubMed] [Google Scholar]
- 7.Bielaszewska, M., A. W. Friedrich, T. Aldick, R. Schurk-Bulgrin, and H. Karch. 2006. Shiga toxin activatable by intestinal mucus in Escherichia coli isolated from humans: predictor for a severe clinical outcome. Clin. Infect. Dis. 431160-1167. [DOI] [PubMed] [Google Scholar]
- 8.Bielaszewska, M., R. Köck, A. W. Friedrich, C. von Eiff, L. B. Zimmerhackl, H. Karch, and A. Mellmann. 2007. Shiga toxin-mediated hemolytic uremic syndrome: time to change the diagnostic paradigm? PLoS ONE 2e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks, J. T., E. G. Sowers, J. G. Wells, K. D. Greene, P. M. Griffin, R. M. Hoekstra, and N. A. Strockbine. 2005. Non-O157 Shiga toxin-producing Escherichia coli infections in the United States, 1983-2002. J. Infect. Dis. 1921422-1429. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2007. Laboratory-confirmed non-O157 Shiga toxin-producing Escherichia coli - Connecticut, 2000-2005. MMWR Morb. Mortal. Wkly. Rep. 5629-31. [PubMed] [Google Scholar]
- 11.Chao, H. Y., Y. C. Wang, S. S. Tang, and H. W. Liu. 2004. A highly sensitive immuno-polymerase chain reaction assay for Clostridium botulinum neurotoxin type A. Toxicon 4327-34. [DOI] [PubMed] [Google Scholar]
- 12.Donohue-Rolfe, A., M. A. Kelley, M. Bennish, and G. T. Keusch. 1986. Enzyme-linked immunosorbent assay for shigella toxin. J. Clin. Microbiol. 2465-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott, E. J., R. M. Robins-Browne, E. V. O'Loughlin, V. Bennett-Wood, J. Bourke, P. Henning, G. G. Hogg, J. Knight, H. Powell, D. Redmond, and Contributors to the Australian Paediatric Surveillance Unit. 2001. Nationwide study of haemolytic uraemic syndrome: clinical, microbiological, and epidemiological features. Arch. Dis. Child. 85125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer, A., C. von Eiff, T. Kuczius, K. Omoe, G. Peters, and K. Becker. 2007. A quantitative real-time immuno-PCR approach for detection of staphylococcal enterotoxins. J. Mol. Med. 85461-469. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich, A. W., M. Bielaszewska, W. Zhang, M. Pulz, T. Kuczius, A. Ammon, and H. Karch. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J. Infect. Dis. 18574-84. [DOI] [PubMed] [Google Scholar]
- 16.Hendrickson, E. R., T. M. Truby, R. D. Joerger, W. R. Majarian, and R. C. Ebersole. 1995. High sensitivity multianalyte immunoassay using covalent DNA-labeled antibodies and polymerase chain reaction. Nucleic Acids Res. 23522-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jelacic, J. K., T. Damrow, G. S. Chen, S. Jelacic, M. Bielaszewska, M. Ciol, H. M. Carvalho, A. R. Melton-Celsa, A. D. O'Brien, and P. I. Tarr. 2003. Shiga toxin-producing Escherichia coli in Montana: bacterial genotypes and clinical profiles. J. Infect. Dis. 188719-729. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, K. E., C. M. Thorpe, and C. L. Sears. 2006. The emerging clinical importance of non-O157 Shiga toxin-producing Escherichia coli. Clin. Infect. Dis. 431587-1595. [DOI] [PubMed] [Google Scholar]
- 19.Karch, H., and T. Meyer. 1989. Evaluation of oligonucleotide probes for identification of Shiga-like-toxin-producing Escherichia coli. J. Clin. Microbiol. 271180-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karch, H., and T. Meyer. 1989. Single primer pair for amplifying segments of distinct Shiga-like-toxin genes by polymerase chain reaction. J. Clin. Microbiol. 272751-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karch, H., N. A. Strockbine, and A. D. O'Brien. 1986. Growth of Escherichia coli in the presence of trimethoprim-sulfamethoxazole facilitates detection of Shiga-like toxin-producing strains by colony blot assay. FEMS Microbiol. Lett. 35141-145. [Google Scholar]
- 22.Karch, H., P. I. Tarr, and M. Bielaszewska. 2005. Enterohaemorrhagic Escherichia coli in human medicine. Int. J. Med. Microbiol. 295405-418. [DOI] [PubMed] [Google Scholar]
- 23.Karmali, M. A., M. Petric, and M. Bielaszewska. 1999. Evaluation of a microplate latex agglutination method (Verotox-F assay) for detecting and characterizing verotoxins (Shiga toxins) in Escherichia coli. J. Clin. Microbiol. 37396-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karmali, M. A., M. Petric, C. Lim, P. C. Fleming, G. S. Arbus, and H. Lior. 1985. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J. Infect. Dis. 151775-782. [DOI] [PubMed] [Google Scholar]
- 25.Kater, A. P., A. M. Westermann, M. R. de Groot, A. E. von dem Borne, and E. J. Kuijper. 2000. Toxin-mediated haemolytic uraemic syndrome without diarrhoea. J. Intern. Med. 248263-265. [DOI] [PubMed] [Google Scholar]
- 26.Klein, E. J., J. R. Stapp, C. R. Clausen, D. R. Boster, J. G. Wells, X. Qin, D. L. Swerdlow, and P. I. Tarr. 2002. Shiga toxin-producing Escherichia coli in children with diarrhea: a prospective point-of-care study. J. Pediatr. 141172-177. [DOI] [PubMed] [Google Scholar]
- 27.Kuczius, T., M. Bielaszewska, A. W. Friedrich, and W. Zhang. 2004. A rapid method for the discrimination of genes encoding classical Shiga toxin (Stx) 1 and its variants, Stx1c and Stx1d, in Escherichia coli. Mol. Nutr. Food Res. 48515-521. [DOI] [PubMed] [Google Scholar]
- 28.Manning, S. D., R. T. Madera, W. Schneider, S. E. Dietrich, W. Khalife, W. Brown, T. S. Whittam, P. Somsel, and J. T. Rudrik. 2007. Surveillance for Shiga toxin-producing Escherichia coli, Michigan, 2001-2005. Emerg. Infect. Dis. 13318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKie, A., D. Samuel, B. Cohen, and N. A. Saunders. 2002. A quantitative immuno-PCR assay for the detection of mumps-specific IgG. J. Immunol. Methods 270135-141. [DOI] [PubMed] [Google Scholar]
- 30.Meisen, I., A. W. Friedrich, H. Karch, U. Witting, J. Peter-Katalinic, and J. Muthing. 2005. Application of combined high-performance thin-layer chromatography immunostaining and nanoelectrospray ionization quadrupole time-of-flight tandem mass spectrometry to the structural characterization of high- and low-affinity binding ligands of Shiga toxin 1. Rapid Commun. Mass Spectrom. 193659-3665. [DOI] [PubMed] [Google Scholar]
- 31.Mellmann, A., M. Bielaszewska, L. B. Zimmerhackl, R. Prager, D. Harmsen, H. Tschape, and H. Karch. 2005. Enterohemorrhagic Escherichia coli in human infection: in vivo evolution of a bacterial pathogen. Clin. Infect. Dis. 41785-792. [DOI] [PubMed] [Google Scholar]
- 32.Melton-Celsa, A. R., S. C. Darnell, and A. D. O'Brien. 1996. Activation of Shiga-like toxins by mouse and human intestinal mucus correlates with virulence of enterohemorrhagic Escherichia coli O91:H21 isolates in orally infected, streptomycin-treated mice. Infect. Immun. 641569-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muniesa, M., J. Recktenwald, M. Bielaszewska, H. Karch, and H. Schmidt. 2000. Characterization of a Shiga toxin 2e-converting bacteriophage from an Escherichia coli strain of human origin. Infect. Immun. 684850-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piérard, D., G. Muyldermans, L. Moriau, D. Stevens, and S. Lauwers. 1998. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J. Clin. Microbiol. 363317-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pulz, M., A. Matussek, M. Monazahian, A. Tittel, E. Nikolic, M. Hartmann, T. Bellin, J. Buer, and F. Gunzer. 2003. Comparison of a Shiga toxin enzyme-linked immunosorbent assay and two types of PCR for detection of Shiga toxin-producing Escherichia coli in human stool specimens. J. Clin. Microbiol. 414671-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reischl, U., M. T. Youssef, J. Kilwinski, N. Lehn, W. L. Zhang, H. Karch, and N. A. Strockbine. 2002. Real-time fluorescence PCR assays for detection and characterization of Shiga toxin, intimin, and enterohemolysin genes from Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 402555-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito, K., D. Kobayashi, M. Sasaki, H. Araake, T. Kida, A. Yagihashi, T. Yajima, H. Kameshima, and N. Watanabe. 1999. Detection of human serum tumor necrosis factor-alpha in healthy donors, using a highly sensitive immuno-PCR assay. Clin. Chem. 45665-669. [PubMed] [Google Scholar]
- 38.Sandvig, K. 2001. Shiga toxins. Toxicon 391629-1635. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt, H., J. Scheef, S. Morabito, A. Caprioli, L. H. Wieler, and H. Karch. 2000. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl. Environ. Microbiol. 661205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt, C. K., M. L. McKee, and A. D. O'Brien. 1991. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H− strain E32511. Infect. Immun. 591065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonntag, A. K., E. Zenner, H. Karch, and M. Bielaszewska. 2005. Pigeons as a possible reservoir of Shiga toxin 2f-producing Escherichia coli pathogenic to humans. Berl. Munch. Tierarztl. Wochenschr. 118464-470. [PubMed] [Google Scholar]
- 42.Sonntag, A. K., M. Bielaszewska, A. Mellmann, N. Dierksen, P. Schierack, L. H. Wieler, M. A. Schmidt, and H. Karch. 2005. Shiga toxin 2e-producing Escherichia coli isolates from humans and pigs differ in their virulence profiles and interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 718855-8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonntag, A. K., R. Prager, M. Bielaszewska, W. Zhang, A. Fruth, H. Tschape, and H. Karch. 2004. Phenotypic and genotypic analyses of enterohemorrhagic Escherichia coli O145 strains from patients in Germany. J. Clin. Microbiol. 42954-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starr, M., V. Bennett-Wood, A. K. Bigham, T. F. de Koning-Ward, A. M. Bordun, D. Lightfoot, K. A. Bettelheim, C. L. Jones, and R. M. Robins-Browne. 1998. Hemolytic-uremic syndrome following urinary tract infection with enterohemorrhagic Escherichia coli: case report and review. Clin. Infect. Dis. 27310-315. [DOI] [PubMed] [Google Scholar]
- 45.Strockbine, N. A., L. R. Marques, J. W. Newland, H. W. Smith, R. K. Holmes, and A. D. O'Brien. 1986. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encodes antigenically distinct toxins with similar biologic activities. Infect. Immun. 53135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarr, P. I., L. S. Fouser, A. E. Stapleton, R. A. Wilson, H. H. Kim, J. C. Vary, Jr., and C. R. Clausen. 1996. Hemolytic-uremic syndrome in a six-year-old girl after a urinary tract infection with Shiga-toxin-producing Escherichia coli O103:H2. N. Engl. J. Med. 335635-638. [DOI] [PubMed] [Google Scholar]
- 47.Tarr, P. I., C. A. Gordon, and W. L. Chandler. 2005. Shiga toxin-producing Escherichia coli and the haemolytic uraemic syndrome. Lancet 3651073-1086. [DOI] [PubMed] [Google Scholar]
- 48.Tozzi, A. E., A. Caprioli, F. Minelli, A. Gianviti, L. De Petris, A. Edefonti, G. Montini, A. Ferretti, Z. De Palo, M. Gaido, and G. Tizzoni; Hemolytic Uremic Syndrome Study Group. 2003. Shiga toxin-producing Escherichia coli infections associated with hemolytic uremic syndrome, Italy, 1988-2000. Emerg. Infect. Dis. 9106-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yam, W. C., D. N. Tsang, T. L. Que, M. Peiris, W. H. Seto, and K. Y. Yuen. 1998. A unique strain of Escherichia coli O157:H7 that produces low verocytotoxin levels not detected by use of a commercial enzyme immunoassay kit. Clin. Infect. Dis. 27905-906. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, W., M. Bielaszewska, T. Kuczius, and H. Karch. 2002. Identification, characterization, and distribution of a Shiga toxin 1 gene variant (stx1c) in Escherichia coli strains isolated from humans. J. Clin. Microbiol. 401441-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, W., M. Bielaszewska, A. W. Friedrich, T. Kuczius, and H. Karch. 2005. Transcriptional analysis of genes encoding Shiga toxin 2 and its variants in Escherichia coli. Appl. Environ. Microbiol. 71558-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang, W., A. Mellmann, A. K. Sonntag, L. Wieler, M. Bielaszewska, H. Tschape, H. Karch, and A. W. Friedrich. 2007. Structural and functional differences between disease-associated genes of enterohaemorrhagic Escherichia coli O111. Int. J. Med. Microbiol. 29717-26. [DOI] [PubMed] [Google Scholar]