Abstract
Human (n = 33) and canine (n = 53) Campylobacter upsaliensis isolates from seven countries were genotyped by a new amplified fragment length polymorphism method. We observed 100% typeability and high overall diversity. The majority of human strains (23/33) clustered separately from canine strains, indicating that dogs may not be the main source of human infection.
Campylobacter upsaliensis is a cause of sporadic gastrointestinal disease in humans (13). The sources of human C. upsaliensis infections are not well described, but dogs and cats appear to be the only significant animal reservoirs (1). These pet animals are often healthy carriers, and more than 50% of individual dogs and cats can be colonized (7, 9). Dog ownership has been described as a risk factor for human campylobacteriosis (2, 10), and case reports have provided evidence of pet-borne zoonotic transmission of C. upsaliensis (5, 6). Studying the genetic similarity between human and animal isolates is useful for determining whether pathogens like C. upsaliensis can be transmitted between different host species. Amplified fragment length polymorphism (AFLP) is one of the tools available for this purpose, but so far, the usage of AFLP for C. upsaliensis has been limited by factors such as low reproducibility (3), low typeability (16), and lack of discriminatory power (11).
In this study, we developed a new AFLP method to study the genetic diversity of human and canine C. upsaliensis isolates. A total of 86 C. upsaliensis strains obtained between 1983 and 2006 were analyzed, including 33 human clinical strains from seven countries (Belgium, Denmark, Senegal, South Africa, Sweden, the United Kingdom, and the United States) and 53 canine strains from two countries (Denmark and Sweden). Strains were grown on 5% blood agar plates (48 to 72 h, 37°C) under microaerophilic conditions, followed by DNA extraction using a High Pure PCR template preparation kit (Roche Diagnostics). Three microliters of genomic DNA was digested at 37°C for 2 hours with 10 units of BspDI and 10 units of NheI (New England Biolabs) in 14 μl of restriction buffer (14). For ligation, 5 μl of restriction product was transferred to a new tube containing 1 unit T4 DNA ligase (GE Healthcare), 2 μl 10× T4 ligase buffer (supplied with the enzyme), 8 μl restriction buffer, 2 μl 20 μM double-stranded NheI adaptor, and 2 μl 20 μM double-stranded BspDI adaptor (Table 1). Double-stranded adaptors were assembled immediately prior to the ligation step by mixing equimolar amounts of corresponding adaptor oligonucleotides. The mixtures were heated at 65°C for 10 min and cooled for 10 min at room temperature. Ligation was carried out overnight at room temperature. PCR amplification was performed in a 50-μl reaction volume containing 2 μl ligation product diluted 1:10 in Milli-Q water, 200 μM of each of the four deoxynucleoside triphosphates, 1.5 U Taq DNA polymerase (GE Healthcare), 2.75 μM MgCl2, 10 μM Tris-HCl (pH 9.0), 50 mM KCl, 0.2 μM NheI primer, and 0.2 μM BspDI primer (Table 1). PCR was carried out on a GeneAmp 9700 system (Perkin-Elmer). The thermal program included initial denaturation at 94°C for 4 min succeeded by 30 cycles of 94°C for 60 s, 56°C for 60 s, and 72°C for 90 s and a 10-min final extension step at 72°C. For detection of AFLP fragments, 2 μl of PCR product was mixed with 2 μl formamide, 0.5 μl GeneScan 500-ROX size standard (Applied Biosystems), and 1.5 μl loading buffer (supplied with the size standard). The mixture was heated at 90°C for 2 min, cooled on ice, and loaded in aliquots of 1 μl on a 5% denaturing polyacrylamide gel. AFLP fragments were separated and detected on an ABI 377 automated sequencer (Perkin-Elmer) according to the manufacturer's instructions. AFLP profiles were analyzed with BioNumerics v. 4.5 (Applied Maths), using the Pearson product-moment correlation coefficient and clustering by the unweighted-pair group method with arithmetic averages. Band position tolerance and the optimization coefficient were set to 1.0% and 0.25%, respectively.
TABLE 1.
Adaptor and primer oligonucleotides used for AFLP reaction
| Oligonucleotide | Sequencea |
|---|---|
| BspDI adaptor | 5′-CAG GTA TCG CCG AGT TTA GAC A-3′ |
| 5′-CGT GTC TAA ACT CGG CGA TAC-3′ | |
| NheI adaptor | 5′-AGG TGG ATT GGC CGT ATT ATT C-3′ |
| 5′-CTA GGA ATA ATA CGG CCA ATC CA-3′ | |
| BspDI primer | 5′-GCC GAG TTT AGA CAC GAT-3′ |
| NheI primer | FAM-5′-GGC CGT ATT ATT CCT AGC-3′ |
FAM, 6-carboxyfluorescein.
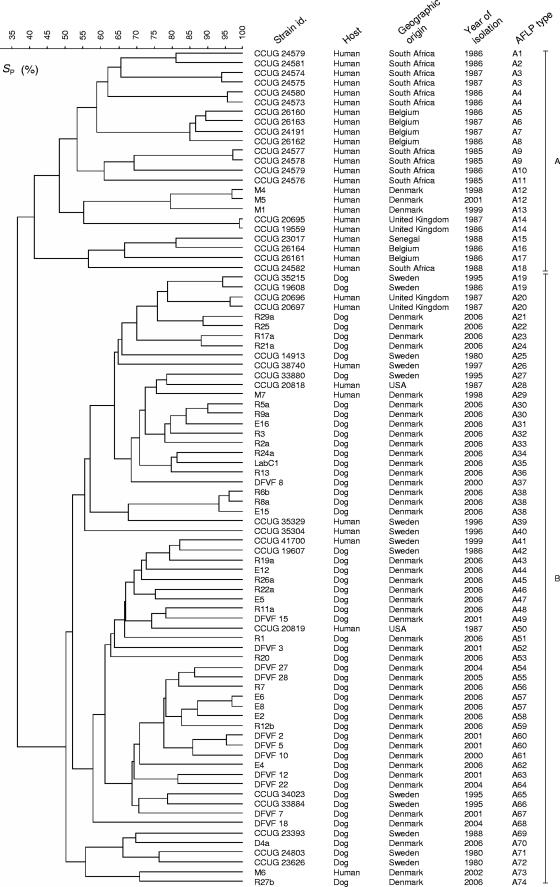
All strains were typeable by AFLP analysis, and duplicate runs revealed a reproducibility of at least 90%. At the cutoff level of 90%, 74 different profiles were detected (Fig. 1). The AFLP fingerprints clustered into two main groups (A and B), sharing only 37% similarity (Fig. 1), thus confirming the high genetic heterogeneity typical for Campylobacter spp. (15). Group A was exclusively composed of human strains, whereas group B encompassed strains of both animal and human origins. This finding may be interpreted in various ways. First, C. upsaliensis subpopulations may be host specific, as previously suggested by Stanley et al. (12). However, the rare examples of healthy human carriers and the sporadic nature of C. upsaliensis infections dispute the existence and maintenance of such strains. Secondly, dogs may not be the main source of human infection and human strains belonging to group A might originate from cats or other yet undiscovered sources of C. upsaliensis infection. Finally, a biased sample population may have influenced the results. In fact, only human strains from Belgium, Senegal, South Africa, and the United Kingdom were analyzed and it cannot be excluded that canine strains from these countries would belong to group A.
FIG. 1.
Dendrogram showing genotypic similarities of 86 C. upsaliensis strains based on AFLP fingerprints. SP, similarity calculated by the Pearson product-moment correlation coefficient.
The presence of certain indistinguishable profiles in group B gave insight into colonization and transmission dynamics among dogs. For example, the detection of indistinguishable AFLP patterns in strains isolated from dogs living within the same household (R17a and R21a) or rescue kennel (R25 and R29a) indicates that C. upsaliensis can be transmitted between dogs living in close contact (Fig. 1). Furthermore, it appears that dogs can be colonized with the same strain for relatively long periods, as indicated by the recovery of identical AFLP patterns in strains isolated from the same dog with a 1-month difference (DFVF 2 and DFVF 5) (Fig. 1). Some strains had indistinguishable AFLP profiles despite their being isolated several years apart. Among these were two Swedish dog strains (CCUG 19608 and CCUG 35215) from 1986 and 1995. These strains may represent stable clonal lineages, as previously suggested by other authors describing Campylobacter (8, 11).
In order to evaluate the typeability and discriminatory power of the AFLP protocol, a subset of 21 human and 21 canine strains were analyzed by a previously described pulsed-field gel electrophoresis (PFGE) method (7). Five strains showed no visible bands after two attempted runs, but results from the remaining 37 strains were in overall accordance with those obtained by AFLP. In some cases, AFLP was able to discriminate between strains sharing the same PFGE profile, and on one occasion, PFGE discriminated between two strains having the same AFLP profile (data not shown).
In conclusion, the AFLP method proved to be an easy, reproducible, and discriminatory tool for genotyping of C. upsaliensis. Spatial and temporal associations between epidemiologically related strains were detected, and the high genetic diversity of C. upsaliensis was confirmed. These results, combined with the perception that AFLP is less susceptible to genetic recombination than PFGE and other typing methods (4, 15), make this method suitable for both short- and long-term epidemiological studies of C. upsaliensis. The finding of a human cluster unrelated to canine strains emphasizes the need for more investigation to understand the epidemiology and population genetic structure of this bacterial species.
Acknowledgments
We thank Enevold Falsen for provision of strains from the CCUG collection in Gothenburg and Leif Percival Andersen for provision of strains from the University Hospital of Copenhagen.
Footnotes
Published ahead of print on 20 February 2008.
REFERENCES
- 1.Bourke, B., V. L. Chan, and P. Sherman. 1998. Campylobacter upsaliensis: waiting in the wings. Clin. Microbiol. Rev. 11440-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrique-Mas, J., Y. Andersson, M. Hjertqvist, A. Svensson, A. Torner, and J. Giesecke. 2005. Risk factors for domestic sporadic campylobacteriosis among young children in Sweden. Scand. J. Infect. Dis. 37101-110. [DOI] [PubMed] [Google Scholar]
- 3.Duim, B., P. A. Vandamme, A. Rigter, S. Laevens, J. R. Dijkstra, and J. A. Wagenaar. 2001. Differentiation of Campylobacter species by AFLP fingerprinting. Microbiology 1472729-2737. [DOI] [PubMed] [Google Scholar]
- 4.Duim, B., T. M. Wassenaar, A. Rigter, and J. Wagenaar. 1999. High-resolution genotyping of Campylobacter strains isolated from poultry and humans with amplified fragment length polymorphism fingerprinting. Appl. Environ. Microbiol. 652369-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figura, N. 1991. Campylobacter spp isolated from dog faeces. Lancet 3381403. [DOI] [PubMed] [Google Scholar]
- 6.Goossens, H., L. Vlaes, J. P. Butzler, A. Adnet, P. Hanicq, S. N′Jufom, D. Massart, G. de Schrijver, and W. Blomme. 1991. Campylobacter upsaliensis enteritis associated with canine infections. Lancet 3371486-1487. [DOI] [PubMed] [Google Scholar]
- 7.Hald, B., K. Pedersen, M. Waino, J. C. Jorgensen, and M. Madsen. 2004. Longitudinal study of the excretion patterns of thermophilic Campylobacter spp. in young pet dogs in Denmark. J. Clin. Microbiol. 422003-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manning, G., B. Duim, T. Wassenaar, J. A. Wagenaar, A. Ridley, and D. G. Newell. 2001. Evidence for a genetically stable strain of Campylobacter jejuni. Appl. Environ. Microbiol. 671185-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno, G. S., P. L. Griffiths, I. F. Connerton, and R. W. Park. 1993. Occurrence of campylobacters in small domestic and laboratory animals. J. Appl. Bacteriol. 7549-54. [DOI] [PubMed] [Google Scholar]
- 10.Neimann, J., J. Engberg, K. Molbak, and H. C. Wegener. 2003. A case-control study of risk factors for sporadic campylobacter infections in Denmark. Epidemiol. Infect. 130353-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.On, S. L., and C. S. Harrington. 2000. Identification of taxonomic and epidemiological relationships among Campylobacter species by numerical analysis of AFLP profiles. FEMS Microbiol. Lett. 193161-169. [DOI] [PubMed] [Google Scholar]
- 12.Stanley, J., C. Jones, A. Burnens, and R. J. Owen. 1994. Distinct genotypes of human and canine isolates of Campylobacter upsaliensis determined by 16S rRNA gene typing and plasmid profiling. J. Clin. Microbiol. 321788-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steele, T. W., N. Sangster, and J. A. Lanser. 1985. DNA relatedness and biochemical features of Campylobacter spp. isolated in Central and South Australia. J. Clin. Microbiol. 2271-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. van de Lee, M. Hornes, A. Frijters, J. Pot, J. Peleman, M. Kuiper, et al. 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 234407-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wassenaar, T. M., and D. G. Newell. 2000. Genotyping of Campylobacter spp. Appl. Environ. Microbiol. 661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieland, B., G. Regula, J. Danuser, M. Wittwer, A. P. Burnens, T. M. Wassenaar, and K. D. Stark. 2005. Campylobacter spp. in dogs and cats in Switzerland: risk factor analysis and molecular characterization with AFLP. J. Vet. Med. B 52183-189. [DOI] [PubMed] [Google Scholar]