Abstract
The fatal outcome of levofloxacin treatment in a patient with bacteremic pneumonia caused by Streptococcus pneumoniae with a preexisting parC mutation is reported. Failure was due to the emergence of a gyrA mutation after 4 days of therapy. Problems encountered in detecting first-step mutation isolates are discussed.
CASE REPORTS
A 79-year-old man with a history of chronic obstructive lung disease, hypertension, and transient ischemic attacks with residual dysarthria and right hemiparesis, was admitted to the emergency room with abrupt-onset fever, increased coughing, and dyspnea. On admission, the patient's temperature was 38.6°C and his white blood cell count was 18,000/μl (88.2% neutrophils). Arterial gasometry showed pH = 7.48, pO2 = 59 mm Hg, and pCO2 = 41 mm Hg, and chest radiography revealed condensation in the lower right lobe. The patient was hospitalized with a diagnosis of community-acquired pneumonia (CAP).
Blood cultures were taken before commencing empirical treatment with levofloxacin (500 mg [given intravenously]/24 h). At 24 h, growth of gram-positive cocci arranged in pairs was observed in the blood culture bottles. After subculturing, the microorganism was identified as Streptococcus pneumoniae susceptible to penicillin (Etest MIC, 0.06 μg/ml) and levofloxacin (Etest MIC, 1 μg/ml).
In view of the susceptibility results and because the patient's condition showed some clinical improvement (decrease in fever and total leukocyte number), treatment continued with levofloxacin at the same dosage. However, the fever reappeared on day 4 of hospitalization with the patient showing hypotension and tachycardia. A further blood culture was obtained, and it was decided to change the treatment to intravenous amoxicillin (1 g/8 h). The patient's clinical condition failed to improve, and he died in the hospital on day 5.
The S. pneumoniae isolate grown in the second blood culture was susceptible to penicillin (E-test MIC, 0.06 μg/ml) and resistant to levofloxacin (E-test MIC, >32 μg/ml). Both isolates were susceptible to trimethoprim-sulfamethoxazole, tetracycline, vancomycin, erythromycin, and clindamycin. No changes in the MICs were observed.
S. pneumoniae is the most common bacterial pathogen in CAP. The emergent resistance of these isolates to beta-lactams and macrolides has caused some concern about their use in the empirical treatment of CAP (11). Fluoroquinolones such as levofloxacin, with increased activity against S. pneumoniae, are now recommended for the empirical treatment of this infection (11, 13). The rate of fluoroquinolone-resistant S. pneumoniae infection remains low in most parts of the world, although an increase in fluoroquinolone use also implies a greater risk that resistant isolates will appear (10). Resistance to newer fluoroquinolones in S. pneumoniae may be emerging as a result of their increased use. In Spain, the reported resistance rate rose from 0.9% in the 1990s to 7.1% after the year 2000 (6, 14, 15), and significant increases have been noted in other countries such as the United States (7) and Canada (1). Higher rates of quinolone resistance have been found in isolates from the respiratory tracts of adult patients (14, 15).
Fluoroquinolone resistance in pneumococci has been reported previously as a cause of treatment failure, although in only a few cases has an in vivo selection for resistance been demonstrated (3, 8, 15). We describe here the emergence of a gyrA mutation and failure of levofloxacin treatment leading to death in a patient with bacteremic CAP caused by S. pneumoniae with a preexisting parC mutation.
Fluoroquinolone resistance in S. pneumoniae is the result of target mutations in the quinolone resistance-determining regions (QRDRs) of parC and gyrA, which encode topoisomerase IV and DNA gyrase, respectively (1, 10, 18). Low-level resistance occurs after a first-step mutation in one of the target genes, and high-level resistance occurs after a subsequent mutation in the other one. Isolates with a single first-step parC mutation are frequently susceptible to fluoroquinolones because the MICs are at or below the Clinical and Laboratory Standards Institute (CLSI) breakpoints (4). However, a first-step parC mutation increases the likelihood of a subsequent gyrA mutation, high-level resistance, and therapeutic failure (1, 18). At the same time, it has also been observed that once a parC mutation has taken place in an S. pneumoniae strain, the mutation prevention concentration (MPC) increases dramatically for all fluoroquinolones (17). Reports by Croisier et al. and Allen et al. showed that after levofloxacin or moxifloxacin treatment, mutants occur in vivo if there is a preexisting parC mutation since the drug concentrations fall below the MPCs of the strains. Increasing both the drug concentration and exposure to exceed the MPC may prevent mutant strains from emerging, although this strategy did not prevent the selection of secondary mutants in strains with preexisting mutations (2, 5).
According to the recommendations of the CLSI, levofloxacin susceptibility in S. pneumoniae is defined as a MIC of ≤2 μg/ml (4). This breakpoint, however, only allows the detection of high-level resistant mutants affecting at least two targets; the prevalence of pneumococci which are not fluoroquinolone susceptible may therefore be underestimated when the present CLSI breakpoints are used. This case report provides disturbing evidence that fluoroquinolone-resistant pneumococci are able to emerge within only a few days of the start of treatment.
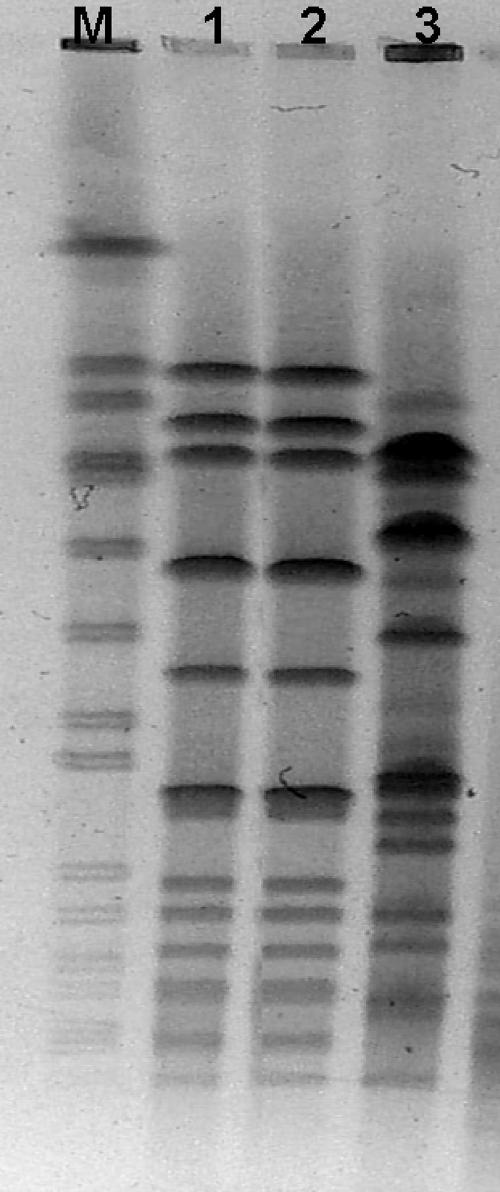
Both of the blood culture isolates belonged to serotype 8 (a non-seven-valent vaccine serotype), and their clonal relationship was demonstrated by pulsed-field gel electrophoresis (PFGE) (Fig. 1) with the SmaI endonuclease (6). By PCR and sequencing (12), the first isolate showed a mutation in the QRDR of parC (Ser79Phe), and the second one had an additional gyrA mutation (Ser81Phe). These mutations are most commonly found in fluoroquinolone-resistant S. pneumoniae strains (1, 18). In addition, genetic relatedness was supported by several polymorphisms identified in the QRDRs of the two isolates: Met1Leu in parC and Ile460Val in parE. Using PCR, we investigated the presence of the qnrA, qnrB, and qnrS genes that cause plasmid-mediated quinolone resistance in gram-negative rods (16). The result was negative for both isolates.
FIG. 1.
PFGE of S. pneumoniae isolates. Lane M, molecular weight marker; lane 1, levofloxacin-susceptible S. pneumoniae (MIC = 1 μg/ml); lane 2, levofloxacin-resistant S. pneumoniae (MIC, >32 μg/ml); lane 3, unrelated clone.
There is no currently available test for accurately identifying isolates with low-level fluoroquinolone resistance. In common with other authors, we consider it an urgent task to develop a phenotypic test which will permit the accurate detection of first-step mutants associated with treatment failure in some pneumonia cases.
The Comité de l'Antibiogramme of the Societé Française de Microbiologie (CA-SFM) recommends the use of norfloxacin, similar to the use of the oxacillin disk test, as a marker for detecting low-level fluoroquinolone-resistant pneumococci. According to the CA-SFM, when the inhibition zone around a disk containing 5 μg of norfloxacin is <10 mm or the MIC is >16 μg/ml, the risk of “in vivo” selection of fluoroquinolone-resistant mutants and of therapeutic failure is high (CA-SFM [http://www.sfm.asso.fr/publi/general.php?pa=1]).
Another proposed screening test is the MIC for ciprofloxacin, where an MIC of ≥4 μg/ml defines nonsusceptible isolates. However, about 30% of the isolates in the susceptible category harbor first-step mutations (10).
A number of risk factors have been identified as identifying patients who are likely to be colonized or infected with fluoroquinolone-resistant pneumococci, i.e., patients who are >64 years of age and have a history of chronic obstructive lung disease and/or prior fluoroquinolone exposure (9). In our case, we do no know whether the patient had previously received fluoroquinolone treatment. However, the risk factors associated with infections caused by first-step quinolone-resistant S. pneumoniae mutants remain to be studied so that future research into the effects of these factors may well determine the full clinical implications of this trend.
Because currently available phenotypic diagnostic tests are unable to identify pneumococci with a first-step mutation, empirical monotherapy using fluoroquinolones may be inappropriate for patients with CAP whose risk factors include infection with fluoroquinolone-resistant pneumococci.
Acknowledgments
This study was supported by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III—FEDER, Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008).
We thank Carmen Ardanuy for collaboration in the PFGE analysis.
Footnotes
Published ahead of print on 20 February 2008.
REFERENCES
- 1.Adam, H. J., K. N. Schurek, K. A. Nichol, C. J. Hoban, T. J. Baudry, N. M. Laing, D. J. Hoban, and G. G. Zhanel. 2007. Molecular characterization of increasing fluoroquinolone resistance in Streptococcus pneumoniae isolates in Canada, 1997 to 2005. Antimicrob. Agents Chemother. 51198-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, G. P., G. W. Kaatz, and M. J. Rybak. 2003. Activities of mutant prevention concentration-targeted moxifloxacin and levofloxacin against Streptococcus pneumoniae in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 472606-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlavilla, A. B., F. López-Medrano, F. Chaves, V. Villena, J. Echave-Sustaeta, and J. M. Aguado. 2005. Failure of levofloxacin therapy in two cases of community-acquired pneumonia caused by fluoroquinolone-resistant Streptococcus pneumoniae and complicated with emphysema. Enferm. Infecc. Microbiol. Clin. 23270-273. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing. Fifteenth informational supplement. M-100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Croisier, D., M. Etienne, E. Bergoin, P. E. Charles, C. Lequeu, L. Piroth, H. Portier, and P. Chavanet. 2004. Mutant selection window in levofloxacin and moxifloxacin treatments of experimental pneumococcal pneumonia in a rabbit model of human therapy. Antimicrob. Agents Chemother. 481699-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Campa, A. G., L. Balsalobre, C. Ardanuy, A. Fenol, E. Pérez-Trallero, J. Liñares, and the Spanish Pneumococcal Infection Study Network G03/103. 2004. Fluoroquinolone resistance in penicillin-resistant Streptococcus pneumoniae clones, Spain. Emerg. Infect. Dis. 101751-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doern, G. V., S. S. Richter, A. Miller, N. Miller, C. Rice, K. Heilmann, and S. Bekmann. 2005. Antimicrobial resistance among Streptococcus pneumoniae in the United States: have we begun to turn the corner on resistance to certain antimicrobial classes? Clin. Infect. Dis. 41139-148. [DOI] [PubMed] [Google Scholar]
- 8.Endimiani, A., G. Brigante, A. A. Bettaccini, F. Luzzaro, P. Grossi, and A. Q. Toniolo. 2005. Failure of levofloxacin treatment in community-acquired pneumococcal pneumonia. BMC Infect. Dis. 5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho, P. L., W. S. Tse, K. W. Tsang, T. K. Kwok, T. K. Ng, V. C. Cheng, and R. M. Chan. 2001. Risk factors for acquisition of levofloxacin resistant Streptococcus pneumoniae: a case-control study. Clin. Infect. Dis. 32701-707. [DOI] [PubMed] [Google Scholar]
- 10.Low, D. E. 2004. Quinolone resistance among pneumococci: therapeutic and diagnostic implications. Clin. Infect. Dis. 38S357-S362. [DOI] [PubMed] [Google Scholar]
- 11.Mandell, L. A., R. G. Wunderink, A. Anzueto, J. G. Bartlett, G. D. Campbell, N. C. Dean, S. F. Dowell, T. M. File, D. M. Musher, M. S. Niederman, A. Torres, and C. G. Whitney. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44S27-S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrissey, I., and J. George. 1999. Activities of fluoroquinolone against Streptococcus pneumoniae type II topoisomerases purified as recombinant proteins. Antimicrob. Agents Chemother. 432579-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niederman, M. S., L. A. Mandell, A. Anzueto, J. B. Bass, W. A. Broughton, G. D. Campbell, N. Dean, T. File, M. J. Fine, P. A. Gross, F. Martinez, T. J. Marrie, J. F. Plouffe, J. Ramirez, G. A. Sarosi, A. Torres, R. Wilson, and V. L. Yu. 2001. American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am. J. Respir. Crit. Care Med. 1631730-1754. [DOI] [PubMed] [Google Scholar]
- 14.Pérez-Trallero, E., C. Fernández-Mazarrasa, C. García-Rey, E. Bouza, L. Aguilar, J. García-de-Lomas, F. Baquero, and the Spanish Surveillance Group for Respiratory Pathogens. 2001. Antimicrobial susceptibilities of 1,684 Streptococcus pneumoniae and 2,039 Streptococcus pyogenes isolates and their ecological relationships: results of a 1-year (1998-1999) multicenter surveillance study in Spain. Antimicrob. Agents Chemother. 453334-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez-Trallero, E., J. A. Marimón, L. Iglesias, and J. Larruskain. 2003. Fluoroquinolone and macrolide treatment failure in pneumococcal pneumonia and selection of multidrug-resistant isolates. Emerg. Infect. Dis. 91159-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robicsek, A., G. A. Jacoby, and D. C. Hooper. 2006. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect. Dis. 6629-640. [DOI] [PubMed] [Google Scholar]
- 17.Smith, H. J., M. Walters, T. Hisanaga, G. G. Zhanel, and D. J. Hoban. 2004. Mutant prevention concentrations for single-step fluoroquinolone-resistant mutants of wild-type, efflux-positive, or parC or gyrA mutation-containing Streptococcus pneumoniae isolates. Antimicrob. Agents Chemother. 483954-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varon, E., S. Houssage, S. Grondin, L. Gutmann, and the Groupe des Observatoires de la Resistance du Pneumocoque. 2006. Nonmolecular test for detection of low-level resistance to fluoroquinolones in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 50572-579. [DOI] [PMC free article] [PubMed] [Google Scholar]