Abstract
Bloodstream infections are an important cause of morbidity and mortality. Physician orders for blood cultures often specify that blood specimens be collected at or around the time of a temperature elevation, presumably as a means of enhancing the likelihood of detecting significant bacteremia. In a multicenter study, which utilized retrospective patient chart reviews as a means of collecting data, we evaluated the timing of blood culture collection in relation to temperature elevations in 1,436 patients with bacteremia and fungemia. The likelihood of documenting bloodstream infections was not significantly enhanced by collecting blood specimens for culture at the time that patients experienced temperature spikes. A subset analysis based on patient age, gender, white blood cell count and specific cause of bacteremia generally also failed to reveal any associations.
Bloodstream infections (BSIs) occur more than 200,000 times annually in the United States, with associated mortality rates of 35 to 60% (20, 33, 34). Prompt administration of appropriate antimicrobial therapy plays an important role in reducing the mortality associated with this condition (9, 12, 14, 15, 16, 18, 22, 26, 31). In patients with bacteremia, the optimization of therapy is ultimately predicated on rapid documentation of positive blood cultures, expeditious performance of in vitro antimicrobial susceptibility tests, and timely reporting of results (2, 23, 33). Numerous factors influence the likelihood of detecting bacteremia. These factors include the volume of blood specimens cultured and the number of blood cultures performed (1, 8, 10, 11, 17, 21, 25, 27, 29). The conventional practice has been to obtain blood specimens at or around the time of a temperature elevation as a means of enhancing the likelihood of documenting bacteremia (5, 32). This practice is based on the principle that the presence of organisms in the intravascular space leads to the elaboration of cytokines, which in turn causes body temperatures to rise.
The value of attempting to time the collection of blood for culture around temperature elevations is complicated by the fact that many patients with bacteremia, especially those that are elderly, may be hypothermic at the time that they are bacteremic or may be unable to mount a febrile response to infection (7). Furthermore, there are numerous causes of fever other than bacteremia, e.g., ischemia, drug reactions, immunological conditions, and malignancy (4). This issue is further complicated by the observation made more than 50 years ago by Bennett and Beeson: bacteremia actually precedes temperature elevations by 1 or 2 h (3). These researchers noted that blood cultures were frequently negative at the time of the temperature spike and concluded that, ideally, blood cultures should be drawn some time prior to elevations in temperature. More recently, Jaimes et al. found that fever was not a useful independent predictor of bacteremia and needed to be considered in light of other factors, such as hypotension, white blood cell (WBC) counts, and the presence or absence of shaking chills (13).
We know of only one previous study that has attempted to objectively address the utility of collecting blood cultures around the time of temperature elevations. Thomson and colleagues found that rates of bacteremia detection were not enhanced by collecting blood cultures at the time that patients were noted to have temperature spikes. This investigation, however, was limited in size, was conducted in only one medical center, and has not been reported in the peer-reviewed literature (30).
In order to systematically determine whether the timing of specimen collection for blood cultures vis-à-vis the occurrence of temperature elevations optimizes the detection of bacteremia and fungemia, we performed a seven-center study, based on retrospective reviews of medical records, among a large number of patients (18 years of age and older) with BSIs.
MATERIALS AND METHODS
The medical records of 1,436 patients, 18 years of age or older, with significant bacteremia or fungemia in seven different U.S. medical centers were reviewed retrospectively during 2006. Four large, tertiary care, university-affiliated medical centers (the University of Iowa Hospital and Clinics, Iowa City, IA; Johns Hopkins University Medical Center, Baltimore, MD; Barnes-Jewish Hospital, Washington University School of Medicine, St. Louis, MO; and the University of Texas Health Science Center, San Antonio, TX), two Veterans Affairs medical centers (VA Boston Healthcare System, West Roxbury, MA, and the VA Medical Center, Portland, OR), and one non-university-affiliated tertiary care referral center (the Geisinger Medical Center, Danville, PA) participated in this study. At each participating institution, medical records from 200 to 250 consecutive unique patients with BSIs were reviewed. The clinical significance of blood culture isolates was determined in each participating center according to the criteria used in each center for assessing positive blood cultures.
The first significant positive blood culture obtained from an individual patient was defined as the index positive blood culture (IPBC). The time that the specimen for this culture was obtained was defined as the time of the IPBC (T-IPBC). Three temperatures posted in the patient's medical record, together with the time that they were obtained, were noted: the highest temperature recorded during the 24-h period prior to the T-IPBC, the temperature recorded on the medical record closest to the T-IPBC, and the highest temperature recorded during the 24-h period after the T-IPBC. A time to maximum concentration of drug in serum (Tmax) was determined as having been recorded when one of these three temperatures was at least 0.5°C above the higher of the other two (19, 24, 28). The following additional information was recorded: the identity of the organism recovered from the IPBC, patient age and gender, and WBC count determined at the time closest to the T-IPBC. Data were collected, and this study was performed in accordance with the dictates of the institutional review board of each participating institution. The significance of differences between various comparison groups was assessed using the Chi-square goodness-of-fit test (6).
RESULTS AND DISCUSSION
Among the total of 1,436 patients assessed in this study, 67% were men and 33% were women. The average age for all patients was 58.9 years (range, 18 to 97). The organisms recovered from the IBPCs are listed in Table 1. Among all IPBCs in this study, 54.1% yielded gram-positive bacteria, 38.2% yielded gram-negative bacteria, 2.6% grew anaerobes, and 5.0% grew yeast. The most commonly recovered organisms were Staphylococcus aureus (n = 382), coagulase-negative staphylococci (n = 160), Enterococcus spp. (n = 139), Escherichia coli (n = 196), Pseudomonas aeruginosa (n = 62), and Klebsiella pneumoniae (n = 108).
TABLE 1.
Organisms recovered from blood cultures from patients with significant bacteremia or fungemiaa
| Organism | No. of isolates recovered in:
|
Totals | ||||||
|---|---|---|---|---|---|---|---|---|
| Ctr 1 | Ctr 2 | Ctr 3 | Ctr 4 | Ctr 5 | Ctr 6 | Ctr 7 | ||
| S. aureus | 67 | 45 | 61 | 38 | 51 | 57 | 63 | 382 |
| CONS | 15 | 13 | 9 | 43 | 65 | 9 | 6 | 160 |
| Enterococcus spp. | 18 | 24 | 15 | 25 | 13 | 23 | 21 | 139 |
| Other GPC | 10 | 13 | 10 | 6 | 9 | 20 | 28 | 96 |
| E. coli | 24 | 24 | 33 | 28 | 19 | 30 | 38 | 196 |
| Enterobacter cloacae | 8 | 5 | 5 | 4 | 3 | 4 | 4 | 33 |
| P. aeruginosa | 8 | 13 | 7 | 4 | 12 | 4 | 14 | 62 |
| K. pneumoniae | 18 | 20 | 12 | 20 | 8 | 20 | 10 | 108 |
| Other GNB | 27 | 31 | 15 | 7 | 19 | 23 | 28 | 150 |
| Anaerobic bacteria | 4 | 7 | 10 | 0 | 10 | 1 | 6 | 38 |
| Yeast | 0 | 16 | 22 | 4 | 13 | 15 | 2 | 72 |
| Totals | 199 | 211 | 199 | 179 | 222 | 206 | 220 | 1,436 |
CONS, coagulase-negative staphylococci; Ctr, center; GB, gram-negative bacilli; GPC, gram-positive cocci.
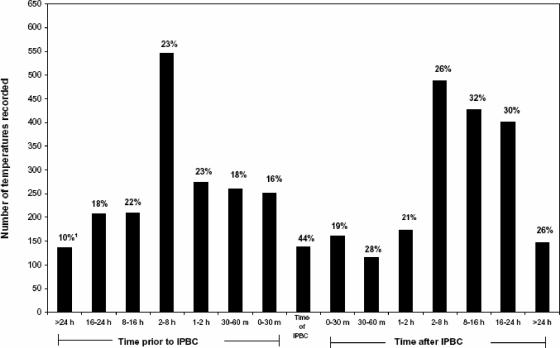
Among the 1,436 episodes of BSI examined in this study, a total of 3,937 temperatures recorded within ±24 h of the T-IPBC were excerpted for analysis. The times these temperatures were recorded relative to the T-IPBC are depicted in Fig. 1. In 933 patients (65%), one of the three temperatures recorded within the 48-h time frame was found to represent a Tmax. In the remaining 503 patients, none of the three temperatures recorded was judged to be a Tmax. The percentage of temperatures recorded during a specific interval that was found to be a Tmax is also presented in Fig. 1. In 138 patients (9.6%), a temperature was recorded at exactly the same time that the IPBC was obtained. This accounted for 3.5% of all temperatures recorded. Among these 138 temperatures, 44% were found to be Tmaxs. Larger numbers of temperatures were recorded at all of the other intervals defined in this study both before and after the T-IPBC; the percentages of these temperatures which were found to be Tmaxs varied between 10 and 31%. None of these percentages were found to be statistically significantly different from any other percentage, including the 45% of temperatures recorded at the T-IPBC that were found to be Tmaxs. Interestingly, in general, slightly higher percentages of Tmaxs were noted among temperatures obtained beginning 2 h after the T-IPBC. As stated above, however, these percentages were not found to be significantly different from those noted at any other time interval.
FIG. 1.
Temperature records and Tmaxs from 1,436 patients with bacteremia or fungemia. 1, The percentage of temperatures at the indicated time interval that were Tmaxs.
The results obtained from the seven individual medical centers that participated in this study are presented in Table 2. As can be seen, although there were differences between centers with respect to the percentage of temperatures recorded at various intervals before and after the IPBCs that were found to be Tmaxs, substantial variability was noted. No one time interval consistently yielded the highest proportion of Tmaxs.
TABLE 2.
Distribution of Tmaxs at various intervals before and after an IPBC was obtained from 1,436 adult patients in seven different United States medical centers
| Time interval | No. of temps recorded at each time interval (% temps that were a Tmax) fora:
|
Totals | ||||||
|---|---|---|---|---|---|---|---|---|
| Ctr 1 | Ctr 2 | Ctr 3 | Ctr 4 | Ctr 5 | Ctr 6 | Ctr 7 | ||
| Amt of time before the T-IPBC | ||||||||
| More than 24 h | 27 (11) | 12 (17) | 1 (0) | 4 (25) | 7 (0) | 31 (16) | 55 (5) | 137 (10) |
| Sixteen to 24 h | 35 (23) | 23 (26) | 20 (5) | 39 (18) | 39 (18) | 27 (22) | 24 (8) | 207 (18) |
| Eight to 16 h | 38 (26) | 23 (13) | 26 (27) | 28 (14) | 37 (35) | 41 (15) | 17 (24) | 210 (22) |
| Two to 8 h | 78 (21) | 77 (26) | 60 (22) | 86 (15) | 72 (38) | 97 (22) | 76 (17) | 546 (23) |
| One or 2 h | 31 (3) | 38 (18) | 32 (22) | 46 (37) | 58 (29) | 41 (20) | 28 (21) | 274 (23) |
| Thirty to 60 min | 40 (0) | 38 (26) | 22 (18) | 48 (21) | 46 (17) | 32 (25) | 34 (24) | 260 (18) |
| Zero to 30 min | 41 (2) | 44 (25) | 43 (14) | 35 (14) | 33 (18) | 28 (16) | 28 (29) | 252 (16) |
| T-IPBC | 16 (38) | 28 (36) | 9 (0) | 38 (74) | 11 (27) | 2 (0) | 4 (0) | 138 (44) |
| Amt of time after the T-IPBC | ||||||||
| Zero to 30 min | 20 (0) | 33 (18) | 12 (42) | 38 (13) | 31 (19) | 17 (29) | 9 (44) | 160 (19) |
| Thirty to 60 min | 7 (14) | 22 (32) | 11 (18) | 24 (17) | 28 (7) | 15 (60) | 9 (78) | 116 (28) |
| One or 2 h | 15 (13) | 28 (21) | 7 (14) | 34 (12) | 29 (14) | 26 (31) | 34 (32) | 173 (21) |
| Two to 8 h | 62 (26) | 70 (31) | 54 (17) | 79 (24) | 76 (18) | 86 (31) | 61 (31) | 488 (26) |
| Eight to 16 h | 63 (41) | 52 (27) | 57 (12) | 58 (40) | 64 (44) | 75 (24) | 59 (37) | 428 (32) |
| Sixteen to 24 h | 38 (32) | 51 (43) | 45 (13) | 58 (43) | 79 (42) | 39 (28) | 91 (14) | 401 (30) |
| More than 24 h | 36 (28) | 30 (40) | 9 (22) | 2 (50) | 8 (25) | 15 (13) | 47 (19) | 147 (26) |
Ctr, center.
Temperature associations were also analyzed according to specific organism recovered from IPBCs (Table 3) and according to patient age, gender, and WBC count (Table 4). For the purposes of these analyses, the percentages of temperatures recorded during three time periods that were determined to be Tmaxs were compared. The three time periods were >24 to 1 h prior to the IPBC, 1 h before to 1 h after the IPBC, and 1 to >24 h after the IPBC. No statistically significant associations were noted among the different organism groups which comprised the bloodstream infections characterized in this study (Table 3). It should be noted, however, that the number of patients with blood cultures yielding yeast was too small to permit meaningful analysis. Larger numbers of fungemias would be required before definitive conclusions could be drawn about the value of collecting blood specimens around the time of temperature spikes in patients with fungemia. Similarly, with one exception, an analysis of the data according to patient age, gender, and WBC count failed to reveal any statistically significant associations. (Table 4). The single exception concerned patients in the younger age group (i.e., 18 to 30 years old) where a Tmax was significantly more likely to have occurred in the period 1 to >24 h following the IPBC than in the other two defined time periods. Unfortunately, we were not able to assess the effect of clinical service, the presence or absence of underlying diseases, or patient medication histories on the relationship between timing of blood culture collection and the likelihood of documenting significant bacteremia, as this information was not available to us.
TABLE 3.
Distribution of Tmaxs at various intervals before and after the time of collection of an IPBC sorted according to the specific cause of the bacteremiaa
| Time interval | No. of temps recorded at each time interval (% temps that were Tmaxs) among isolates of:
|
||||||
|---|---|---|---|---|---|---|---|
| S. aureus | CONS | Enterococcus spp. | E. coli | Enterobacter cloacae | P. aeruginosa | K. pneumoniae | |
| More than 24 h to 1 h prior to the T-IPBC | 342 (23) | 169 (22) | 146 (20) | 173 (20) | 24 (25) | 61 (18) | 98 (23) |
| One hour prior to 1 h after the T-IPBC | 222 (23) | 88 (16) | 85 (22) | 121 (23) | 23 (22) | 41 (29) | 62 (21) |
| One hour to >24 h after the T-IPBC | 425 (29) | 183 (30) | 153 (29) | 232 (27) | 34 (32) | 72 (26) | 127 (25) |
CONS, coagulase-negative staphylococci. P values for the number of temperatures recorded at each time interval were 0.619 for S. aureus, 0.113 for CONS, 0.389 for Enterococcus spp., 0.589 for E. coli, 0.368 for Enterobacter cloacae, 0.265 for P. aeruginosa, and 0.840 for K. pneumoniae.
TABLE 4.
Distribution of Tmaxs at various intervals before or after an IPBC was drawn in patients with bloodstream infections analyzed with respect to patient age, gender, and WBC counta
| Time interval | No. of temps recorded at each time interval (% temps that were Tmaxs) analyzed according to:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Age (yr)
|
Gender
|
WBC count (per mm3)
|
||||||
| 18-30 | 30-65 | >65 | Male | Female | <3,700 | 3,700-10,500 | >10,500 | |
| More than 24 h to 1 h prior to the T-IPBC | 107 (17) | 777 (19) | 481 (22) | 934 (19) | 431 (20) | 176 (23) | 444 (19) | 761 (18) |
| One hour prior to 1 h after the T-IPBC | 86 (26) | 522 (22) | 270 (27) | 557 (26) | 317 (20) | 117 (27) | 295 (25) | 454 (22) |
| One hour to >24 h after the T-IPBC | 125 (38) | 930 (28) | 582 (26) | 1136 (27) | 499 (30) | 238 (20) | 533 (24) | 868 (29) |
P values for the number of temperatures recorded at each time interval were 0.016 for patients 18 to 30 years old, 0.401 for patients 30 to 65 years old, 0.756 for patients more than 65 years old, 0.240 for males, 0.453 for females, 0.589 for WBC counts of <3,700, 0.634 for WBC counts of 3,700 to 10,500, and 0.260 for WBC counts of >10,500.
One limitation of this study was the fact that we examined only the distribution of temperature elevations during time intervals before and after an IPBC was obtained. It would have been informative to have compared this cohort of patients to a matched cohort of patients whose blood cultures remained negative. The omission of patients with negative blood cultures does not, however, change our overall findings that irrespective of subset analysis, the percentages of temperatures obtained that were found to be Tmaxs were essentially comparable over the entire time period examined, i.e., 24 h before through 24 h after the time an IPBC was obtained.
We conclude from the results of this investigation that is not necessary in routine practice to collect blood for culture at the time that adult patients are experiencing a temperature elevation as a means for optimizing the detection of bacteremia. In individuals 18 years of age and older, the timing of collection of blood specimens for culture can be predicated on convenience. The emphasis should be on obtaining specimens of adequate volume, the performance of suitable numbers of blood cultures, and the use of strict aseptic technique.
Footnotes
Published ahead of print on 27 February 2008.
REFERENCES
- 1.Arpi, M., M. W. Bentzon, J. Jensen, and W. Frederiksen. 1989. Importance of blood volume cultured in the detection of bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 8838-842. [DOI] [PubMed] [Google Scholar]
- 2.Beekmann, S. E., D. J. Diekema, K. C. Chapin, and G. V. Doern. 2003. Effects of rapid detection of bloodstream infections on length of hospitalization and hospital charges. J. Clin. Microbiol. 413119-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett, I. L., and R. B. Beeson. 1954. Bacteremia: a consideration of some experimental and clinical aspects. Yale J. Biol. Med. 262241-262. [PMC free article] [PubMed] [Google Scholar]
- 4.Bossink, A. W., A. B. Groeneveld, C. E. Hack, and L. G. Thijs. 1999. The clinical host response to microbial infection in medical patients with fever. Chest 116380-390. [DOI] [PubMed] [Google Scholar]
- 5.Bryan, C. S. 1989. Clinical implications of positive blood cultures. Clin. Microbiol. Rev. 2329-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chernoff, H., and E. L. Lehmann. 1954. The use of maximum likelihood estimates in χ2 tests for goodness-of-fit. Ann. Math. Stat. 25579-586. [Google Scholar]
- 7.Clemmer, T. P., C. J. Fisher, R. C. Bone, G. J. Slotman, C. A. Metz, and F. O. Thomas for the Methylprednisolone Severe Sepsis Study Group. 1992. Hypothermia in the sepsis syndrome and clinical outcome. Crit. Care Med. 201395-1401. [DOI] [PubMed] [Google Scholar]
- 8.Cockerill, F. R., G. S. Reed, J. G. Hughes, C. A. Torgerson, E. A. Vetter, W. S. Harmsen, J. C. Dale, G. D. Roberts, D. M. Ilstrup, and N. K. Henry. 1997. Clinical comparison of BACTEC 9240 Plus Aerobic/F resin bottles and the Isolator aerobic culture system for detection of bloodstream infections. J. Clin. Microbiol. 351469-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross, P. A., T. L. Barrett, E. P. Dellinger, P. J. Krause, W. J. Martone, J. E. McGowan, R. L. Sweet, and R. P. Wenzel. 1994. Quality standard for the treatment of bacteremia. Clin. Infect. Dis. 18428-430. [DOI] [PubMed] [Google Scholar]
- 10.Hall, M. M., D. M. Ilstrup, and J. A. Washington. 1976. Effect of volume of blood cultured on detection of bacteremia. J. Clin. Microbiol. 3643-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilstrup, D. M., and J. A. Washington. 1983. The importance of volume of blood cultured in the detection of bacteremia and fungemia. Diagn. Microbiol. Infect. Dis. 1107-110. [DOI] [PubMed] [Google Scholar]
- 12.Ispahani, P., N. J. Pearson, and D. Greenwood. 1987. An analysis of community and hospital acquired bacteraemia in a large teaching hospital in the United Kingdom. Q. J. Med. 63427-440. [PubMed] [Google Scholar]
- 13.Jaimes, F., C. Arango, G. Ruiz, J. Cuervo, J. Botero, G. Velez, N. Upegui, and F. Machado. 2004. Predicting bacteremia at the bedside. Clin. Infect. Dis. 38357-362. [DOI] [PubMed] [Google Scholar]
- 14.Kang, C.-I., S.-H. Kim, H.-B. Kim, S.-W. Park, Y.-J. Choe, M.-D. Oh, E.-C. Kim, and K.-W. Choe. 2003. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin. Infect. Dis. 37745-751. [DOI] [PubMed] [Google Scholar]
- 15.Kang, C.-I., S.-H. Kim, W. B. Park, K.-D. Lee, H.-B. Kim, E.-C. Kim, M.-D. Oh, and K.-W. Choe. 2005. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob. Agents Chemother. 49760-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreger, B. E., D. E. Craven, and W. R. McCabe. 1980. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am. J. Med. 68344-355. [DOI] [PubMed] [Google Scholar]
- 17.Lee, A., S. Mirrett, L. B. Reller, and M. P. Weinstein. 2007. Detection of bloodstream infections in adults: how many blood cultures are needed? J. Clin. Microbiol. 453546-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leibovici, L., H. Konisberger, and S. D. Pitlik. 1992. Bacteremia and fungemia of unknown origin in adults. Clin. Infect. Dis. 14436-439. [DOI] [PubMed] [Google Scholar]
- 19.Mackowiak, P. A. 1998. Concepts of fever. Arch. Intern. Med. 1581870-1881. [DOI] [PubMed] [Google Scholar]
- 20.Martin, G. S., D. M. Mannino, S. Eaton, and M. Moss. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 3481546-1554. [DOI] [PubMed] [Google Scholar]
- 21.Mermel, L. A., and D. G. Maki. 1993. Detection of bacteremia in adults: consequences of culturing an inadequate volume of blood. Ann. Intern. Med. 119270-272. [DOI] [PubMed] [Google Scholar]
- 22.Micek, S. T., A. E. Lloyd, D. J. Ritchie, R. M. Reichley, V. J. Fraser, and M. H. Kollef. 2005. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob. Agents Chemother. 491306-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munson, E. L., D. J. Diekema, S. E. Beekmann, K. C. Chapin, and G. V. Doern. 2003. Detection and treatment of bloodstream infection: laboratory reporting and antimicrobial management. J. Clin. Microbiol. 41495-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Grady, N. P., P. S. Barie, and J. Bartlett. 1998. Practice parameters for evaluating new fever in critically adult patients. Crit. Care Med. 26392-408. [DOI] [PubMed] [Google Scholar]
- 25.Plorde, J. J., F. C. Tenover, and L. G. Carlson. 1985. Specimen volume versus yield in the BACTEC blood culture system. J. Clin. Microbiol. 22292-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rayner, B. L., and P. A. Willcox. 1988. Community-acquired bacteremia: a prospective survey of 239 cases. Q. J. Med. 69907-919. [PubMed] [Google Scholar]
- 27.Reimer, L. G., M. L. Wilson, and M. P. Weinstein. 1997. Update on detection of bacteremia and fungemia. Clin. Microbiol. Rev. 10444-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saper, C. B., and C. D. Breder. 1994. The neurologic basis of fever. N. Engl. J. Med. 3301880-1886. [DOI] [PubMed] [Google Scholar]
- 29.Tenney, J. H., L. B. Reller, S. Mirrett, W.-L. Wang, and M. P. Weinstein. 1982. Controlled evaluation of the volume of blood cultured in detection of bacteremia and fungemia. J. Clin. Microbiol. 15558-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson, R. B., C. Corbin, and J. S. Tan. 1989. Timing of blood culture collection from febrile patients, abstr. C-227, p. 431. Abstr. 89th Annu. Meet. Am. Soc. Microbiol. 1989. American Society for Microbiology, Washington, DC.
- 31.Vallés, J., J. Rello, A. Ochagavia, J. Garnacho, and M. A. Alcala. 2003. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest 1231615-1624. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein, M. P., J. R. Murphy, L. B. Reller, and K. A. Lichtenstein. 1983. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. II. Clinical observations, with special reference to factors influencing prognosis. Rev. Infect. Dis. 554-70. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein, M. P., M. L. Towns, S. M. Quartey, S. Mirrett, L. G. Reimer, G. Parmigiani, and L. B. Reller. 1997. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin. Infect. Dis. 24584-602. [DOI] [PubMed] [Google Scholar]
- 34.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39309-317. [DOI] [PubMed] [Google Scholar]