Abstract
A retrospective survey of 93,775 samples testing positive in Candida species-specific PCR tests performed on cervicovaginal swabs over a 4-year period demonstrated consistent yearly distributions of Candida albicans (89%), C. glabrata (7.9%), C. parapsilosis (1.7%), and C. tropicalis (1.4%). However, the species distributions among different age groups revealed increases in the percentages of non-albicans species with increases in age.
Vulvovaginal candidiasis (VVC) is a common fungal infection that affects healthy women of all ages. At least 75% of women will develop one or more infections once during their lifetime, with 5 to 8% of those individuals developing recurrent infections (5, 7). Current literature examining the species distribution of Candida isolates involved in VVC is limited; however, several important observations have been made. For example, one study shows that Candida albicans accounts for 70 to 90% of all VVC cases, with a recent emergence of non-albicans species (10). The rise in VVC infections, more specifically in those caused by non-albicans species, could be due to several factors, ranging from an increase in over-the-counter antifungal use to an increase in high-risk patient populations (i.e., diabetics and menopausal women). Candida glabrata is the primary non-albicans species emerging in VVC, accounting for up to 14% of infections in immune-competent women (9, 10).
In addition to an increase in non-albicans species overall, it is becoming clear that certain patient populations may experience higher risks of infection from these non-albicans species, often leading to limited treatment options. Interestingly, in a few small studies, C. glabrata was found to be the primary species isolated from diabetic (61.3%) and elderly (51.2%) patients with VVC (2, 4, 6, 11). Often, these non-albicans species are associated with elevated MIC levels for the azoles, the most commonly prescribed class of antifungal drugs. It has been well documented that C. glabrata demonstrates both intrinsically low susceptibility to the azoles and the ability to develop frank resistance (8, 12, 13, 14, 15, 16). Moreover, a recent increase in the trailing phenotype, with low-level resistance to the azoles, has been observed for the Candida tropicalis isolates (1, 3). This highlights the importance of identifying Candida species within clinical samples in order to provide physicians with information concerning the proper treatment for their patients.
The purpose of this study was to examine the species distribution from a large collection of Candida isolates (n = 93,775) from cervicovaginal swabs of women suspected of having VVC in the United States. The species distributions among different age groups were also examined. The retrospective survey was performed on clinical cervicovaginal swab samples submitted to our clinical laboratory for PCR-based Candida identification between March 2003 and March 2007. All four Candida species-specific tests were performed on each sample, with informed consent as ordered by the evaluating physician, following the guidelines of the laboratory's federal, state, and Clinical Laboratory Improvement Amendments (CLIA) certifications. Internal validation procedures for the sensitivity, specificity, concordance, interference, accuracy, and precision of the individual and multiplexed PCR tests were performed. These validation procedures included comparison to culturing and determination of concordance with other published Candida species-specific and pan-Candida PCR tests. Patient anonymity was strictly protected in accordance with the federal Health Insurance Portability and Accountability Act (HIPPA) of 1996. Subjects were informed of the results of the testing procedures by their physicians. None of the samples were accompanied by information regarding the medical history or clinical presentation of the participant at the time of specimen collection. Only the patient's state of residence and age were obtained for the purposes of this retrospective study. The specimens were collected from a cervicovaginal sampling performed by the evaluating physician with a Cellmatics swab (BD, Sparks, MD) or OneSwab (Medical Diagnostic Laboratories L.L.C., Hamilton, NJ). Upon receipt, swabs were immediately processed for PCR analysis. The reference strains were obtained from the American Type Culture Collection (Manassas, VA) and include C. glabrata 66032, C. tropicalis 750, C. albicans 90028, and Candida parapsilosis 22019. Statistical analyses of the annual percent distribution of species-specific-PCR-positive patient samples were performed using Student's t test.
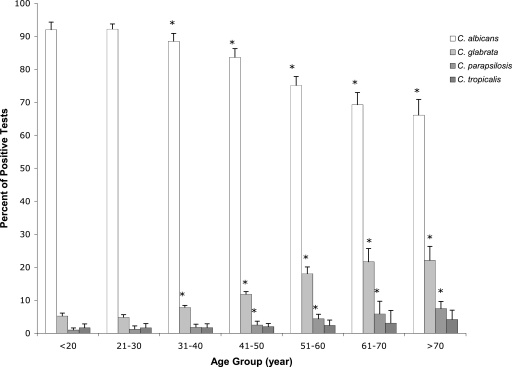
The evaluation of the prevalence of the four most common candidiasis-causing Candida species, C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis, within the cervicovaginal environments of patients suspected of VVC was performed. In addition, the survey evaluated potential changes of Candida species prevalence with age. The 93,775 samples testing positive in C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis species-specific PCR tests demonstrated a consistent yearly distribution of these Candida species over 4 years (Table 1). This distribution of Candida species is consistent with previously published surveys (8). Additionally, the distribution of Candida species encompassing the 48 continental states was consistent among the Northeast, South, Midwest, and West regions as defined by the U.S. Census Bureau (Table 1). Moreover, the distributions of Candida species-positive samples were consistent from year to year within the different age groups (Fig. 1). However, one interesting trend was noticed upon comparing the distributions of Candida species for the different age groups. The data showed significant (P ≤ 0.05) increases in the prevalences of non-albicans species, along with steady decreases in C. albicans, with increases in age. C. glabrata accounted for >18% of isolates in women of menopausal age and older (≥51 years), compared to <4.8% for young adults (<30 years) and <11.8% for middle-aged women (31 to 50 years). Significant increases in the prevalences of C. parapsilosis-positive samples were also observed with increases in age (Fig. 1). This increase in non-albicans species, specifically C. glabrata, is an important observation that was reported in smaller studies of the elderly (4). Similar increases in non-albicans species have been reported for diabetic patients, in which C. glabrata was the primary species isolated (2, 6, 11). Understanding the changes that occur in patient populations which exhibit higher prevalences of non-albicans species is warranted (i.e., menopausal and postmenopausal women and diabetic patients). Possible explanations include changes in patient physiology, hormone balance, and decrease in immune function.
TABLE 1.
Survey of samples testing positive in Candida species-specific PCR tests by species, annual distribution, and geographic location
| Species | No. of positive samples | Annual distribution (%) | Distribution (%) by region (United States)
|
|||
|---|---|---|---|---|---|---|
| Northeast | South | Midwest | West | |||
| C. albicans | 83,407 | 89.0 ± 2.0 | 17,065 (90.5) | 52,932 (88.1) | 8,626 (90.0) | 4,784 (91.1) |
| C. glabrata | 7,401 | 7.9 ± 0.26 | 1,224 (6.5) | 5,128 (8.5) | 671 (7.0) | 378 (7.2) |
| C. parapsilosis | 1,604 | 1.7 ± 1.0 | 362 (1.9) | 1,041 (1.8) | 155 (1.6) | 46 (0.9) |
| C. tropicalis | 1,363 | 1.4 ± 1.3 | 217 (1.1) | 971 (1.6) | 132 (1.4) | 43 (0.8) |
| Total isolates | 93,775 | 18,868 | 60,072 | 9,584 | 5,251 | |
FIG. 1.
Distribution of 93,775 Candida isolates by prevalence of species versus age. *, P was <0.05 for comparison to the <20 and 21-to-30 age groups. Error bars represent the standard deviations of the percentages of Candida species detected per year within each age group.
This large set of Candida species-specific PCR tests performed on cervicovaginal swabs over a 4-year period allowed us to investigate the species distribution of Candida isolates throughout the United States from women suspected of having VVC. Although C. albicans still remains the primary causative agent of VVC, this study shows a rise in the non-albicans species, which correlates with an increase in patient age. Given that non-albicans species, such as C. glabrata, have reduced susceptibilities to the azoles, a common VVC treatment, the importance of early species identification is necessary for proper treatment. The use of nonazole antifungals, such as boric acid and flucytosine, has been shown to be effective in treating non-albicans species, especially C. glabrata, which can often resist azole antifungal therapy.
Footnotes
Published ahead of print on 27 February 2008.
REFERENCES
- 1.Arthington-Skaggs, B. A., W. Lee-Wang, M. A. Ciblak, J. P. Frade, M. E. Brandt, R. A. Hajjeh, L. H. Harrison, A. N. Sofair, and D. W. Warnock. 2002. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantification method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob. Agents Chemother. 462477-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bader, M. S., S. M. Lai, V. Kumar, and D. Hinthorn. 2004. Candidemia in patients with diabetes mellitus: epidemiology and predictors of mortality. Scand. J. Infect. Dis. 36860-864. [DOI] [PubMed] [Google Scholar]
- 3.Chou, H.-H., H.-J. Lo, K.-W. Chen, M.-H. Liao, and S.-Y. Li. 2007. Multilocus sequence typing of Candida tropicalis shows clonal cluster enriched in isolates with resistance or trailing growth of fluconazole. Diagn. Microbiol. Infect. Dis. 58427-433. [DOI] [PubMed] [Google Scholar]
- 4.Dan, M., R. Segal, V. Marder, and A. Leibovitz. 2006. Candida colonization of the vagina in elderly residents of a long-term-care hospital. Eur. J. Clin. Microbiol. Infect. Dis. 25394-396. [DOI] [PubMed] [Google Scholar]
- 5.Fidel, P. L., and J. D. Sobel. 1996. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin. Microbiol. Rev. 9335-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goswami, D., R. Goswami, U. Banerjee, V. Dadhwal, S. Miglani, A. A. Lattif, and N. Kochupillai. 2006. Pattern of Candida species isolated from patients with diabetes mellitus and vulvovaginal candidiasis and their response to single dose oral fluconazole. J. Infect. 52111-117. [DOI] [PubMed] [Google Scholar]
- 7.Nyirjesy, P., S. M. Seney, M. H. T. Grody, C. A. Jordan, and H. R. Buckley. 1995. Chronic fungal vaginitis: the value of cultures. Am. J. Obstet. Gynecol. 173820-823. [DOI] [PubMed] [Google Scholar]
- 8.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Harold, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother 473149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozcan, S. K., F. Budak, G. Yucesoy, S. Susever, and A. Willke. 2006. Prevalence, susceptibility profile and proteinase production of yeasts causing vulvovaginitis in Turkish women. APMIS 114139-145. [DOI] [PubMed] [Google Scholar]
- 10.Paulitsch, A., W. Weger, G. Ginter-Hanselmayer, E. Marth, and W. Buzina. 2006. A 5-year (2000-2004) epidemiological survey of Candida and non-Candida yeast species causing vulvovaginal candidiasis in Graz, Austria. Mycoses 49471-475. [DOI] [PubMed] [Google Scholar]
- 11.Ray, D., R. Goswami, U. Banerjee, V. Dadhwal, D. Goswami, P. Mandal, V. Sreenivas, and N. Kochupillai. 2007. Prevalence of Candida glabrata and its response to boric acid vaginal suppositories in comparison with oral fluconazole in patients with diabetes and vulvovaginal candidiasis. Diabetes Care 30312-317. [DOI] [PubMed] [Google Scholar]
- 12.Redding, S. W., W. R. Kirkpatrick, S. Saville, B. J. Coco, W. White, A. Fothergill, M. Rinaldi, T. Eng, T. F. Patterson, and J. Lopez-Ribot. 2003. Multiple patterns of resistance to fluconazole in Candida glabrata isolates from a patient with oropharyngeal candidiasis receiving head and neck radiation. J. Clin. Microbiol. 41619-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richter, S. S., R. P. Galask, S. A. Messer, R. J. Hollis, D. J. Diekema, and M. A. Pfaller. 2005. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J. Clin. Microbiol. 432155-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safdar, A., D. Armstrong, E. W. Cross, and D. S. Perlin. 2002. Prospective epidemiologic analysis of triazole-resistant nosocomial Candida glabrata isolated from patients at a comprehensive cancer center. Int. J. Infect. Dis. 6198-201. [DOI] [PubMed] [Google Scholar]
- 15.Sanglard, D., F. Ischer, D. Calabrese, P. A. Majcherczyk, and J. Bille. 1999. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob. Agents Chemother. 432753-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vermitsky, J.-P., and T. D. Edlind. 2004. Azole resistance in Candida glabrata: coordinate upregulatioin of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrob. Agents Chemother. 483773-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]