Abstract
An immunochromatographic assay (Campy-ICA) using a newly generated single monoclonal antibody against a 15-kDa cell surface protein of Campylobacter jejuni was developed. When cell suspensions of 86 C. jejuni strains and 27 Campylobacter coli strains were treated with a commercially available bacterial protein extraction reagent and the resulting extracts were tested with the Campy-ICA, they all yielded positive results. The minimum detectable limits for the C. jejuni strains ranged from 1.8 × 104 to 8.2 × 105 CFU/ml of cell suspension, and those for the C. coli strains ranged from 1.4 × 105 to 4.6 × 106 CFU/ml of cell suspension. All 26 non-Campylobacter species tested yielded negative results with the Campy-ICA. To evaluate the ability of the Campy-ICA to detect C. jejuni and C. coli in human stool specimens, suspensions of 222 stool specimens from patients with acute gastroenteritis were treated with the bacterial protein extraction reagent, and the resulting extracts were tested with the Campy-ICA. The Campy-ICA results showed a sensitivity of 84.8% (28 of 33 specimens) and a specificity of 100% (189 of 189 specimens) compared to the results of isolation of C. jejuni and C. coli from the stool specimens by a bacterial culture test. The Campy-ICA was simple to perform and was able to detect Campylobacter antigen in a fecal extract within 15 min. These results suggest that Campy-ICA testing of fecal extracts may be useful as a simple and rapid adjunct to stool culture for detecting C. jejuni and C. coli in human stool specimens.
Campylobacter species are a major cause of human bacterial gastroenteritis in many industrialized countries (9, 10, 32). The majority (approximately 90%) of cases of Campylobacter gastroenteritis in humans are caused by Campylobacter jejuni, and most of the remainder are caused by Campylobacter coli (3, 12, 15). Among other Campylobacter species, although Campylobacter lari and Campylobacter upsaliensis have been reported to be associated with human gastroenteritis (4, 13), cases of human gastroenteritis caused by these species are very rare. C. jejuni and C. coli, therefore, are the most common causes of Campylobacter gastroenteritis in humans. Campylobacter gastroenteritis is a self-limited disease, and antimicrobial therapy is not generally required; however, treatment can decrease the duration and severity of illness if it is initiated early in the course of infection (16). Infants, elderly people, and immunocompromised individuals are at higher risk of developing more severe campylobacteriosis. In these cases, early antibiotic therapy is especially effective (33, 35). Rapid and appropriate identification of the etiologic agent of infectious gastroenteritis is important, since there are major differences in the treatments required for the different agents.
C. jejuni and C. coli in human stool specimens are commonly detected by a bacterial culture test. The test procedure includes culturing of the stool on selective agar medium and the subsequent identification of Campylobacter-suspected colonies. Enrichment medium is not required for recovery, since infected humans usually excrete 106 to 109 CFU of C. jejuni per g of stool (2). Stool culture on selective agar medium commonly requires at least 48 h due to the slow growth of Campylobacter organisms, and the identification of suspected colonies is a laborious and time-consuming procedure. A simple and rapid nonculture assay for the detection of C. jejuni and C. coli in human stool specimens, therefore, has been desired. Several methods, including a commercially available enzyme immunoassay (7, 8, 16, 36, 37), PCR-based assays (6, 22, 25, 26), and a DNA hybridization-based assay (28), have already been reported. Although these assays have excellent sensitivity, they require a comparatively complicated and time-consuming procedure. Their simplicity and rapidity appear to be insufficient for routine or emergency use in the microbiology laboratory.
The purpose of the present study was to develop a simple and rapid nonculture assay for detection of C. jejuni and C. coli in human stool specimens. For this purpose, a monoclonal antibody (MAb) against a 15-kDa cell surface protein of C. jejuni was newly generated. Using the combination of an immunochromatographic technique with the single MAb, a method that can detect Campylobacter antigen in a fecal extract within 15 min was successfully developed. Here we report in detail the development and evaluation of a new method using an immunochromatographic assay to detect Campylobacter antigen (Campy-ICA).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Eighty-six C. jejuni strains, derived from 56 outbreaks of food-borne disease, and 27 C. coli strains, derived from 24 different sporadic cases and 2 outbreaks of food-borne disease, were used in this study. These clinical strains were confirmed as Campylobacter species based on their Gram stain appearance (Campylobacter organisms are gram-negative, spiral rods), oxidase reactions, and latex agglutination in a Campylobacter confirmation test (Microscreen Campylobacter; Microgen Bioproducts, Ltd., Camberley, United Kingdom). Hippurate hydrolysis was used as a confirmatory test to identify C. jejuni (14), and hippurate-negative strains were identified to the species level by a species-specific PCR assay (22, 38, 40). Of the 86 C. jejuni strains, 55 were typeable by the Lior serotyping scheme (23). The typeable strains of C. jejuni belonged to 21 different Lior serotypes. All 27 C. coli strains were nontypeable by the serotyping scheme. The C. jejuni strains, the C. coli strains, and C. lari ATCC 43675 (American Type Culture Collection) were cultured on blood agar (blood agar base no. 2 [Oxoid Ltd., Basingstoke, United Kingdom] supplemented with 7% sterile defibrinated horse blood) at 42°C under a microaerobic atmosphere (Anaero Pack Campylo System; Mitubishi Gas Chemical Co., Inc., Tokyo, Japan). Campylobacter fetus subsp. fetus JCM2527T (Japan Collection of Microorganisms, Saitama, Japan), Campylobacter fetus subsp. venerealis JCM2528T, and C. upsaliensis ATCC 43954T were cultured on blood agar at 37°C under a microaerobic atmosphere.
Of non-Campylobacter species, Arcobacter butzleri ATCC 49616T, Arcobacter skirrowii ATCC 51132T, and Helicobacter pylori JCM12093T were cultured on blood agar at 37°C under a microaerobic atmosphere. Arcobacter cryaerophilus ATCC 43158T was cultured on blood agar at 30°C under a microaerobic atmosphere. Strains of Citrobacter freundii, Escherichia coli, Plesiomonas shigelloides, Salmonella enterica serovar Enteritidis, Salmonella enterica serovar Typhimurium, Vibrio cholerae, and Vibrio parahaemolyticus were isolated from clinical samples. These bacterial strains and Acinetobacter calcoaceticus IAM12087T (Institute of Applied Microbiology culture collection, Tokyo, Japan), Aeromonas hydrophila IAM1646, Aeromonas sobria (IAM12333), Alcaligenes faecalis IAM12369T, Enterobacter aerogenes IAM12348T, Enterobacter cloacae IAM12349T, Enterobacter intermedius IAM14238T, Klebsiella oxytoca JCM1665, Klebsiella pneumoniae subsp. ozaenae (JCM1663), Morganella morganii JCM1672, Proteus vulgaris IAM12542T, Pseudomonas aeruginosa IAM1514T, Raoultella ornithinolytica ATCC 31898, Raoultella planticola ATCC 43176, and Raoultella terrigena ATCC 33628 were cultured on brain heart infusion agar plates (brain heart infusion [Becton Dickinson Microbiology Systems, Sparks, MD] supplemented with 1.5% Bacto agar [Becton Dickinson]) at 37°C.
Production of MAbs.
Cell extracts were prepared from bacterial strains according to the following procedure. Confluent bacterial growth on a single agar plate was harvested, washed twice with phosphate-buffered saline (PBS), and then suspended in 1 ml of B-PER II bacterial protein extraction reagent (Pierce, Rockford, IL). After stirring at room temperature for 15 min and centrifugation at 10,000 × g and 5°C for 5 min, the resulting supernatant (termed the cell extract) was filtered with a 33-mm-diameter Millex filter unit (pore size, 0.22 μm; Millipore, Bedford, MA).
The cell extract of the C. jejuni strain (Lior serotype 7) was used as an immunogen for the production of MAbs. Female BALB/c mice (8 weeks old) were immunized intraperitoneally with 60 μg of the immunogen emulsified in Freund's complete adjuvant (Wako Pure Chemical Industries, Osaka, Japan). After 3, 5, 7, and 11 weeks, the mice were boosted intraperitoneally with the immunogen emulsified in Freund's incomplete adjuvant (Wako). At 13 weeks, the mice were bled, and the antibody titer of each serum sample was determined by an enzyme-linked immunosorbent assay (ELISA) using the immunogen as the coating antigen. At 15 weeks, the mouse with the best antiserum was injected intraperitoneally with 600 μg of the immunogen alone. Three days after final immunization, spleen cells of the mouse and P3-X63-Ag8.U1 myeloma cells were mixed at a ratio of 5:1 and fused with 40% polyethylene glycol by a modification of the protocol of Galfre and Milstein (11). Cloning was performed using a hybridoma cloning kit (ClonaCell-HY; StemCell Technologies, Vancouver, British Columbia, Canada). Culture supernatants were screened for antibody production by an ELISA using the immunogen as the coating antigen. Subsequently, in order to select hybridomas secreting antibodies that react with various Campylobacter strains but not with non-Campylobacter strains, culture supernatants of antibody-secreting hybridomas were examined by ELISA using cell extracts from three C. jejuni strains (Lior serotypes 2, 7, and 9), one C. coli strain, and six non-Campylobacter strains (A. hydrophila, E. aerogenes, P. vulgaris, P. aeruginosa, Salmonella serovar Enteritidis, and V. cholerae) as coating antigens. The hybridomas selected by ELISA were amplified in the ascites of pristine (2,6,10,14-tetramethylpentadecane)-primed mice. MAbs were purified from the ascites supernatant by affinity chromatography on a protein A-agarose gel (Affi-Gel protein A MAPS II kit; Bio-Rad Laboratories, Hercules, CA) after being salted out with 50% saturated ammonium sulfate (pH 7.4). MAbs were isotyped with a mouse MAb isotyping kit (IsoStrip; Roche Diagnostics, Indianapolis, IN).
Identification of antigens recognized by MAbs.
To determine whether the MAbs obtained react with Campylobacter cell surface antigens, MAbs were examined by ELISA using live whole cells of the C. jejuni strain (Lior serotype 7) as the coating antigen. Cellular components that react with MAbs were determined by immunoblot analysis. Briefly, components of the immunogen were separated on a slab gel by a modified Laemmli gel system (sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]) with a 4% stacking gel and a 15% separating gel. The separated components were transferred to a polyvinylidene difluoride membrane by a Trans-Blot SD cell (Bio-Rad) and proved with MAbs according to the protocol described previously (17).
Campy-ICA.
Test strips and MAb-colloidal-gold conjugates were prepared according to the protocol described previously (19). Briefly, to prepare the test strips, an absorbent pad (Millipore, Bedford, MA) was attached to a laminated membrane card (HiFlow Plus 135; Millipore) so that the pad slightly overlapped the membrane, and the assembly was then cut into strips consisting of the membrane (5 by 25 mm) with the absorbent pad (5 by 17 mm). A 1.0-μl aliquot of the MAb solution (1 mg/ml) was deposited on the membrane as a 1-mm-wide line at about the midpoint of the length of the membrane to serve as the detection zone of Campylobacter antigen. As the control zone, 1.0 μl of a goat anti-mouse immunoglobulin G solution (2 mg/ml) (Sigma Chemical Co., St. Louis, MO) was deposited on the membrane as a 1-mm-wide line 5 mm downstream from the detection zone. After drying, the test strips were stored at 4°C until use. To prepare the MAb-colloidal-gold conjugates, 1.0 ml of a MAb solution (0.2 mg/ml) was added to 10 ml of a colloidal-gold suspension (40 nm; BBI International, Cardiff, United Kingdom). After incubation and blocking, the MAb-gold conjugates were first suspended in 1.0 ml of 50 mM Tris-HCl-150 mM NaCl buffer, pH 8.2, containing 1% bovine serum albumin and 20% glycerol and were then stored at −25°C until use.
Samples were tested by the Campy-ICA according to the following procedure. To a well of a 96-well general assay plate (flat-bottom type) (Corning, Acton, MA), 30 μl of a sample and 30 μl of the MAb-gold conjugate (diluted 1:10 in 100 mM Tris-HCl buffer, pH 10.5, containing 2% bovine serum albumin and 2% Triton X-100) were added. After immediate mixing by repeated aspiration and ejection with a micropipette, the test strip was inserted into the mixture in the well. After migration of the mixture through the membrane for 15 min at room temperature, the appearance of red lines at both the detection zone and the control zone was interpreted as positive detection of the Campylobacter antigen.
Campy-ICA testing of cell extracts of Campylobacter and non-Campylobacter species.
Suspensions of test strains at various cell concentrations were prepared with PBS. To 50 μl of each of the cell suspensions, 50 μl of the B-PER II reagent (Pierce) was added. After mixing for 1 min, the mixtures were centrifuged at 10,000 × g and 5°C for 5 min. The resulting supernatants (cell extracts) were tested by the Campy-ICA. The cell concentrations of the suspensions were estimated by colony counting on agar plates.
Isolation and identification of Campylobacter species from stool specimens by a bacterial culture test.
A total of 222 stool specimens were obtained from different patients with acute gastroenteritis from 52 outbreaks of food-borne disease that occurred in Osaka prefecture, Japan. All stool specimens were obtained from patients within 5 days after the symptoms of gastroenteritis appeared. The stool specimens were transported to our laboratory from 13 local public health centers in Osaka prefecture and were processed within 4 h from the time they arrived in our laboratory. The stool specimens were streaked onto Skirrow (34) (blood agar base no. 2 supplemented with campylobacter selective supplement SR0069E [both from Oxoid Ltd.] and 7% sterile defibrinated horse blood), mCCDA (18) (Campylobacter blood-free selective agar base supplemented with CCDA selective supplement SR155 [both from Oxoid Ltd.]), and CAT (1) (Campylobacter blood-free selective agar base supplemented with CAT selective supplement SR174 [both from Oxoid Ltd.]) agar plates. CAT agar plates were included in order to isolate C. upsaliensis (1). Skirrow and mCCDA agar plates were incubated at 42°C for 48 h under a microaerobic atmosphere, and CAT agar plates were incubated at 37°C for 96 h under a microaerobic atmosphere. Morphologically suspected colonies were confirmed as Campylobacter species based on their Gram stain appearance (Campylobacter organisms are gram-negative spiral rods), oxidase reactions, and latex agglutination in a Campylobacter confirmation test (Microscreen Campylobacter; Microgen Bioproducts). Hippurate hydrolysis was used as a confirmatory test to identify C. jejuni (14), and hippurate-negative isolates were identified to the species level by a species-specific PCR assay (40). In parallel, bacteriological and virological examinations for other enteropathogens were also performed.
Campy-ICA testing of fecal extracts.
Suspensions (10%, wt/wt) of stool specimens were prepared with PBS. To 50 μl of each of the fecal suspensions, 50 μl of the B-PER II reagent (Pierce) was added. After 1 min of mixing, the mixtures were centrifuged at 10,000 × g and 5°C for 5 min. The resulting supernatants (termed fecal extracts) were tested by the Campy-ICA.
RESULTS
Production and characterization of MAbs.
A total of 1,714 hybridoma colonies were obtained by the fusion of spleen cells from the immunized mouse and P3U1 myeloma cells. Of these colonies, culture supernatants of 55 colonies reacted with the immunogen in ELISAs. Of the antibody-secreting colonies, culture supernatants of 12 colonies reacted with all of the C. jejuni and C. coli strains tested but not with the non-Campylobacter strains tested when examined by ELISAs using cell extracts of the bacterial strains as coating antigens. Coating and labeling antibody combinations of MAbs from the 12 colonies were screened for reactivity to the immunogen by a sandwich-type immunochromatographic assay. Of 144 sandwich MAb combinations, only 1, which used MAb 4B4 as both the coating and the labeling antibody, yielded a positive result. MAb 4B4 was therefore used as both the coating and the labeling antibody of an immunochromatographic assay to detect Campylobacter antigen (Campy-ICA).
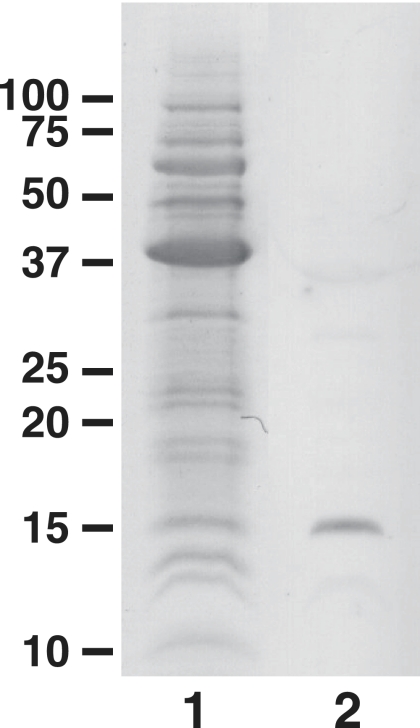
MAb 4B4 belongs to the immunoglobulin G1(κ) subclass. MAb 4B4 reacted with live whole cells of C. jejuni when examined by ELISA using live whole cells as the coating antigen. This ELISA result means that MAb 4B4 binds to a cell surface-exposed antigen of C. jejuni. As shown in Fig. 1, immunoblot analysis revealed that the cell surface antigen recognized by MAb 4B4 is a protein component with an approximate molecular mass of 15 kDa.
FIG. 1.
SDS-PAGE of the immunogen and immunoblotting with MAb 4B4. Lane 1, SDS-PAGE of the immunogen stained with Coomassie blue; lane 2, immunoblot with MAb 4B4. The sizes of molecular weight standards (prestained standards; Bio-Rad) (marker lane) are given on the left (in thousands).
Campy-ICA testing of cell extracts of Campylobacter and non-Campylobacter species.
It is necessary to examine the reactivities of the Campy-ICA with various C. jejuni and C. coli strains and other bacterial species before using the Campy-ICA as a method to detect C. jejuni and C. coli in human stool specimens. For this purpose, cell suspensions of the 86 C. jejuni strains, 27 C. coli strains, and strains of the 4 other Campylobacter species and 26 non-Campylobacter species were treated with B-PER II reagent, and the resulting cell extracts were tested by the Campy-ICA. The results are summarized in Table 1. Of the 86 C. jejuni strains and 27 C. coli strains tested, all yielded positive results with the Campy-ICA. The minimum detectable limits for C. jejuni strains ranged from 1.8 × 104 to 8.2 × 105 CFU/ml of cell suspension, and those for C. coli strains ranged from 1.4 × 105 to 4.6 × 106 CFU/ml of cell suspension. Among other Campylobacter species tested, C. lari and C. upsaliensis, which are also associated with human gastroenteritis, yielded positive results, with minimum detectable limits of 5.3 × 105 and 5.0 × 105 CFU/ml of cell suspension, respectively. All of the 26 non-Campylobacter species tested yielded negative results by the Campy-ICA.
TABLE 1.
Reactivity of the Campy-ICA for Campylobacter and non-Campylobacter species
| Test bacterium | No. of strains tested | No. (%) of strains positive by Campy-ICA | Minimum detectable limit (CFU/ml of cell suspension) |
|---|---|---|---|
| C. jejuni | 86 | 86 (100) | 1.8 × 104-8.2 × 105 |
| C. coli | 27 | 27 (100) | 1.4 × 105-4.6 × 106 |
| C. lari | 1 | 1 (100) | 5.3 × 105 |
| C. upsaliensis | 1 | 1 (100) | 5.0 × 105 |
| C. fetus subsp. fetus | 1 | 0 (0) | >1.5 × 109 |
| C. fetus subsp. venerealis | 1 | 0 (0) | >1.5 × 109 |
| Non-Campylobacter species | 26a | 0 (0) | >1.1 × 1011 |
Strains of 26 non-Campylobacter species were tested by the Campy-ICA.
Campy-ICA testing of fecal extracts.
To evaluate the ability of the Campy-ICA to detect C. jejuni and C. coli in human stool specimens, extracts of 222 stool specimens obtained from different patients with acute gastroenteritis as a result of outbreaks of food-borne disease were tested by the Campy-ICA. The Campy-ICA results were compared with the results of isolation of Campylobacter species from the stool specimens by a bacterial culture test. The results are summarized in Table 2. Of the 222 stool specimens cultured, 33 were positive for either C. jejuni or C. coli. Other human gastroenteritis-related Campylobacter species, C. lari and C. upsaliensis, were not isolated. Of the 33 Campylobacter culture-positive stool specimens, 28 were positive but 5 were negative by the Campy-ICA. All of the 189 Campylobacter culture-negative stool specimens were negative by the Campy-ICA. The Campy-ICA results therefore showed a sensitivity of 84.8% and a specificity of 100% relative to the bacterial culture test. Of the 189 Campylobacter culture-negative specimens, 29, 43, 6, 4, and 2 were positive for Salmonella species, norovirus, Staphylococcus aureus, Escherichia coli O157:H7, and Vibrio parahaemolyticus, respectively.
TABLE 2.
Comparison between Campy-ICA results and results of isolation of Campylobacter species for 222 stool specimens from patients with acute gastroenteritis
| No. of specimens | Results of isolation of Campylobacter speciesa
|
No. of specimens with the following result by Campy-ICA:
|
|||
|---|---|---|---|---|---|
| C. jejuni | C. coli | Others | Positive | Negative | |
| 30 | + | − | − | 26 | 4 |
| 3 | − | + | − | 2 | 1 |
| 189 | − | − | − | 0 | 189 |
+, culture positive; −, culture negative.
DISCUSSION
The purpose of the present study was to develop a simple and rapid method for direct detection of C. jejuni and C. coli in human stool specimens. We developed the Campy-ICA by combining an immunochromatographic technique with a single MAb, 4B4, against the 15-kDa cell surface protein of C. jejuni. C. jejuni and C. coli exhibit antigenic diversity in their cellular components, as demonstrated by the existence of numerous different serotypes (23, 31, 39). Therefore, antigenic diversity in the 15-kDa cell surface protein, which is a target antigen in the Campy-ICA, was also expected among clinical strains of C. jejuni and C. coli. The Campy-ICA will not react broadly with various clinical strains of C. jejuni and C. coli if the antigenic determinant, which is recognized by MAb 4B4, on the 15-kDa cell surface protein varies highly among the clinical strains. In the present study, we examined whether the Campy-ICA reacts broadly with various clinical strains of C. jejuni and C. coli. The Campy-ICA yielded positive results for all clinical strains of C. jejuni and C. coli tested, which include 21 different Lior serotypes. This result suggests that the antigenic determinant, which is recognized by MAb 4B4, on the 15-kDa cell surface protein is broadly conserved among various clinical strains of C. jejuni and C. coli. Several cell surface proteins of C. jejuni have been identified so far (5, 24, 30), some of which have been characterized genetically, immunologically, and functionally (5, 20, 21, 27, 29, 30); however, to our knowledge, there are no reports on the 15-kDa cell surface protein of C. jejuni. We therefore speculate that the 15-kDa protein is a newly identified cell surface component of C. jejuni. In the present study, the 15-kDa cell surface protein was characterized as a component containing the antigenic determinant that is broadly conserved among various clinical strains of C. jejuni and C. coli. Although it is desirable to characterize the 15-kDa cell surface protein further, it appears to be not strictly necessary for the purposes of the present study. We will therefore characterize the 15-kDa cell surface protein fully in future studies.
Before the Campy-ICA can be applied for detecting C. jejuni and C. coli in human stool specimens, it is very important to examine the reactivities of the Campy-ICA with other Campylobacter and non-Campylobacter species. In addition to C. jejuni and C. coli, the Campy-ICA cross-reacted with C. lari and C. upsaliensis. This result means that the Campylobacter-specific antigen detected by the Campy-ICA is also shared by C. lari and C. upsaliensis. C. lari and C. upsaliensis have been reported to be associated with human gastroenteritis (4, 13), although cases are very rare. The detection limits of the Campy-ICA for reference strains of C. lari and C. upsaliensis were comparable to those for clinical strains of C. jejuni and C. coli. This result suggests that the Campy-ICA has sufficient ability to detect C. lari and C. upsaliensis in human stool specimens. In the present study, although 222 stool specimens from patients with acute gastroenteritis were examined, no stool specimens containing C. lari or C. upsaliensis could be found. This result is consistent with that obtained for 631 patient stool specimens from the greater Salt Lake City area in a previous study (16). Cases of human gastroenteritis caused by C. lari or C. upsaliensis are very rare, and therefore we could not evaluate the reactivities of the Campy-ICA with various clinical strains of C. lari and C. upsaliensis or the ability of the Campy-ICA to detect these two species in human stool specimens.
In a preliminary study, when fecal suspensions were tested by the Campy-ICA, the migration of the mixture of the fecal suspension and the MAb 4B4-colloidal-gold conjugate through the test strip was very slow and often stopped. We speculated that this interference with migration was caused by large fecal particles, and we therefore attempted to remove these from the fecal suspensions prior to the assay. However, when centrifugation was used, most of the Campylobacter cells were also removed, because the weights of the Campylobacter cells were similar to those of the large fecal particles. In this case, most fecal extracts of Campylobacter culture-positive stool specimens yielded false-negative results by the Campy-ICA (data not shown). To resolve this difficulty, we developed a new fecal sample preparation method for the Campy-ICA. The aim of this sample preparation method was to separate the 15-kDa cell surface protein, which is a target antigen in the Campy-ICA, from Campylobacter cells by treatment with a commercially available bacterial protein extraction reagent prior to the assay. Since the 15-kDa protein components separated from Campylobacter cells are very small molecules compared to the large fecal particles, they are expected to remain in the resulting supernatant (fecal extract), even after centrifugation. The 15-kDa protein can therefore be detected by the Campy-ICA. When suspensions of 33 Campylobacter culture-positive stool specimens were treated according to the protocol of the sample preparation method and the resulting extracts were tested by the Campy-ICA, most (84.8%) yielded positive results. This result demonstrates that the sample preparation method is appropriate for the Campy-ICA.
The detection limits of the Campy-ICA for the C. jejuni strains tested ranged from 1.8 × 104 to 8.2 × 105 CFU/ml. On the other hand, the sensitivity of culture for the detection of C. jejuni has been reported to be 3 × 102 CFU/ml (16). This difference between the detection sensitivities of the Campy-ICA and culture for C. jejuni might explain why 4 of 30 C. jejuni culture-positive stool specimens yielded false-negative results by the Campy-ICA. Although a large number of organisms is required for the Campy-ICA to give a positive result, patients infected with C. jejuni usually excrete 106 to 109 CFU of C. jejuni per g of stool (2). The Campy-ICA is therefore sensitive enough to detect C. jejuni in human stool specimens. Moreover, the Campy-ICA also appears to be suitable for detecting C. coli in human stool specimens, since the detection limits of the Campy-ICA for C. coli strains were comparable to those for C. jejuni strains. In fact, two of three C. coli culture-positive stool specimens yielded positive results by the Campy-ICA in the present study.
In terms of assay performance, Campy-ICA testing of fecal extracts is less sensitive (sensitivity, 84.8%) than the “gold standard,” stool culture; however, since the Campy-ICA is able to detect Campylobacter antigen in a fecal extract within 15 min, all examination processes can be completed rapidly and simply within 30 min, including the time for preparation of a fecal extract. The total time for the assay is considerably shorter than that (at least 2 days) required for stool culture. In addition, since Campy-ICA testing of fecal extracts shows high specificity (100%), the test result is accurate when it is positive.
Currently, a commercial enzyme immunoassay (ProSpecT Campylobacter microplate assay; Alexon-Trend, Ramsey, MN) is available for the detection of Campylobacter species in human stool specimens. In previous studies, although one study group reported that the commercially available enzyme immunoassay showed a sensitivity of 96% relative to stool culture (37), other groups reported that the assay showed sensitivities of 80% to 89% (7, 8, 16). Since the sensitivity of the Campy-ICA was 84.8%, we consider that the Campy-ICA showed a sensitivity comparable to that of the commercial enzyme immunoassay. The sensitivity of the Campy-ICA therefore appears to be sufficient for use as an adjunct to stool culture. The commercial enzyme immunoassay offers an opportunity to diagnose Campylobacter gastroenteritis in approximately 2 h (7). Campy-ICA testing of a fecal extract is more rapid than the commercial enzyme immunoassay, since it can be completed within 30 min, including the time for preparation of the fecal extract. In addition, Campy-ICA testing of a fecal extract can be completed with one-step incubation after the test strip is inserted into the mixture of the fecal extract and the MAb 4B4-colloidal-gold conjugate, whereas the commercial enzyme immunoassay requires multiple incubations and washing steps after a fecal sample is added to the microplate well (7). The Campy-ICA appears to be easier to perform than the commercial immunoassay. We consider that the rapidity and simplicity of the Campy-ICA make it more suitable for use in routine screening, and especially for emergency use, in the clinical microbiology laboratory. The results of the present study suggest that Campy-ICA testing of fecal extracts may be useful as a simple and rapid adjunct to stool culture for detection of C. jejuni and C. coli in human stool specimens.
Footnotes
Published ahead of print on 6 February 2008.
REFERENCES
- 1.Aspinall, S. T., D. R. Wareing, P. G. Hayward, and D. N. Hutchinson. 1993. Selective medium for thermophilic campylobacters including Campylobacter upsaliensis. J. Clin. Pathol. 46829-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser, M. 1999. Campylobacter jejuni and related species, p. 2276-2285. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practices of infectious diseases, vol. 2. Churchill Livingstone, Philadelphia, PA. [Google Scholar]
- 3.Bolton, F. J., D. N. Hutchinson, and G. Parker. 1987. Isolation of Campylobacter: what are we missing? J. Clin. Pathol. 40702-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broczyk, A., S. Thompson, D. Smith, and H. Lior. 1987. Water-borne outbreak of Campylobacter laridis-associated gastroenteritis. Lancet i164-165. [DOI] [PubMed] [Google Scholar]
- 5.Burnens, A., U. Stucki, J. Nicolet, and J. Frey. 1995. Identification and characterization of an immunogenic outer membrane protein of Campylobacter jejuni. J. Clin. Microbiol. 332826-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, E., M. Glennon, S. Hanley, A. M. Murray, M. Cormican, T. Smith, and M. Maher. 2001. Evaluation of a PCR/DNA probe colorimetric membrane assay for identification of Campylobacter spp. in human stool specimens. J. Clin. Microbiol. 394163-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dediste, A., O. Vandenberg, L. Vlaes, A. Ebraert, N. Douat, P. Bahwere, and J. P. Butzler. 2003. Evaluation of the ProSpecT Microplate Assay for detection of Campylobacter: a routine laboratory perspective. Clin. Microbiol. Infect. 91085-1090. [DOI] [PubMed] [Google Scholar]
- 8.Endtz, H. P., C. W. Ang, N. van den Braak, A. Luijendijk, B. C. Jacobs, P. de Man, J. M. van Duin, A. van Belkum, and H. A. Verbrugh. 2000. Evaluation of a new commercial immunoassay for rapid detection of Campylobacter jejuni in stool samples. Eur. J. Clin. Microbiol. Infect. Dis. 19794-797. [DOI] [PubMed] [Google Scholar]
- 9.Friedman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter. ASM Press, Washington, DC.
- 10.Frost, J. A., I. A. Gillespie, and S. J. O'Brien. 2002. Public health implications of campylobacter outbreaks in England and Wales, 1995-9: epidemiological and microbiological investigations. Epidemiol. Infect. 128111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galfre, G., and C. Milstein. 1981. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 733-46. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie, I. A., S. J. O'Brien, J. A. Frost, G. K. Adak, P. Horby, A. V. Swan, M. J. Painter, and K. R. Neal. 2002. A case-case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses. Emerg. Infect. Dis. 8937-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goossens, H., L. Vlaes, M. De Boeck, B. Pot, K. Kersters, J. Levy, P. De Mol, J. P. Butzler, and P. Vandamme. 1990. Is “Campylobacter upsaliensis” an unrecognised cause of human diarrhoea? Lancet. 335584-586. [DOI] [PubMed] [Google Scholar]
- 14.Harvey, S. M. 1980. Hippurate hydrolysis by Campylobacter fetus. J. Clin. Microbiol. 11435-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healing, T. D., M. H. Greenwood, and A. D. Pearson. 1992. Campylobacters and enteritis. Rev. Med. Microbiol. 3159-167. [Google Scholar]
- 16.Hindiyeh, M., S. Jense, S. Hohmann, H. Benett, C. Edwards, W. Aldeen, A. Croft, J. Daly, S. Mottice, and K. C. Carroll. 2000. Rapid detection of Campylobacter jejuni in stool specimens by an enzyme immunoassay and surveillance for Campylobacter upsaliensis in the greater Salt Lake City area. J. Clin. Microbiol. 383076-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husson, M. O., and H. Leclerc. 1991. Detection of Helicobacter pylori in stomach tissue by use of a monoclonal antibody. J. Clin. Microbiol. 292831-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchinson, D. N., and F. J. Bolton. 1984. Improved blood free selective medium for the isolation of Campylobacter jejuni from faecal specimens. J. Clin. Pathol. 37956-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawatsu, K., M. Ishibashi, and T. Tsukamoto. 2006. Development and evaluation of a rapid, simple, and sensitive immunochromatographic assay to detect thermostable direct hemolysin produced by Vibrio parahaemolyticus in enrichment cultures of stool specimens. J. Clin. Microbiol. 441821-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kervella, M., J. M. Pages, Z. Pei, G. Grollier, M. J. Blaser, and J. L. Fauchere. 1993. Isolation and characterization of two Campylobacter glycine-extracted proteins that bind to HeLa cell membranes. Infect. Immun. 613440-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konkel, M. E., D. J. Mead, and W. Cieplak, Jr. 1996. Cloning, sequencing, and expression of a gene from Campylobacter jejuni encoding a protein (Omp18) with similarity to peptidoglycan-associated lipoproteins. Infect. Immun. 641850-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linton, D., A. J. Lawson, R. J. Owen, and J. Stanley. 1997. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. J. Clin. Microbiol. 352568-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lior, H., D. L. Woodward, J. A. Edgar, L. J. Laroche, and P. Gill. 1982. Serotyping of Campylobacter jejuni by slide agglutination based on heat-labile antigenic factors. J. Clin. Microbiol. 15761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Logan, S. M., and T. J. Trust. 1983. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect. Immun. 42675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maher, M., C. Finnegan, E. Collins, B. Ward, C. Carroll, and M. Cormican. 2003. Evaluation of culture methods and a DNA probe-based PCR assay for detection of Campylobacter species in clinical specimens of feces. J. Clin. Microbiol. 412980-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metherell, L. A., J. M. Logan, and J. Stanley. 1999. PCR-enzyme-linked immunosorbent assay for detection and identification of Campylobacter species: application to isolates and stool samples. J. Clin. Microbiol. 37433-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nachamkin, I., and A. M. Hart. 1986. Common and specific epitopes of Campylobacter flagellin recognized by monoclonal antibodies. Infect. Immun. 53438-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olive, D. M., M. Johny, and S. K. Sethi. 1990. Use of an alkaline phosphatase-labeled synthetic oligonucleotide probe for detection of Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 281565-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pei, Z., and M. J. Blaser. 1993. PEB1, the major cell-binding factor of Campylobacter jejuni, is a homolog of the binding component in gram-negative nutrient transport systems. J. Biol. Chem. 26818717-18725. [PubMed] [Google Scholar]
- 30.Pei, Z. H., R. T. Ellison III, and M. J. Blaser. 1991. Identification, purification, and characterization of major antigenic proteins of Campylobacter jejuni. J. Biol. Chem. 26616363-16369. [PubMed] [Google Scholar]
- 31.Penner, J. L., and J. N. Hennessy. 1980. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J. Clin. Microbiol. 12732-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rautelin, H., and M. L. Hanninen. 2000. Campylobacters: the most common bacterial enteropathogens in the Nordic countries. Ann. Med. 32440-445. [DOI] [PubMed] [Google Scholar]
- 33.Salazar-Lindo, E., R. B. Sack, E. Chea-Woo, B. A. Kay, Z. A. Piscoya, R. Leon-Barua, and A. Yi. 1986. Early treatment with erythromycin of Campylobacter jejuni-associated dysentery in children. J. Pediatr. 109355-360. [DOI] [PubMed] [Google Scholar]
- 34.Skirrow, M. B. 1977. Campylobacter enteritis: a “new” disease. Br. Med. J. 29-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snijders, F., E. J. Kuijper, B. de Wever, L. van der Hoek, S. A. Danner, and J. Dankert. 1997. Prevalence of Campylobacter-associated diarrhea among patients infected with human immunodeficiency virus. Clin. Infect. Dis. 241107-1113. [DOI] [PubMed] [Google Scholar]
- 36.Tissari, P., and H. Rautelin. 2007. Evaluation of an enzyme immunoassay-based stool antigen test to detect Campylobacter jejuni and Campylobacter coli. Diagn. Microbiol. Infect. Dis. 58171-175. [DOI] [PubMed] [Google Scholar]
- 37.Tolcin, R., M. M. LaSalvia, B. A. Kirkley, E. A. Vetter, F. R. Cockerill III, and G. W. Procop. 2000. Evaluation of the Alexon-trend ProSpecT Campylobacter microplate assay. J. Clin. Microbiol. 383853-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winters, D. K., and M. F. Slavik. 1995. Evaluation of a PCR based assay for specific detection of Campylobacter jejuni in chicken washes. Mol. Cell. Probes 9307-310. [DOI] [PubMed] [Google Scholar]
- 39.Woodward, D. L., and F. G. Rodgers. 2002. Identification of Campylobacter heat-stable and heat-labile antigens by combining the Penner and Lior serotyping schemes. J. Clin. Microbiol. 40741-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamazaki-Matsune, W., M. Taguchi, K. Seto, R. Kawahara, K. Kawatsu, Y. Kumeda, M. Kitazato, M. Nukina, N. Misawa, and T. Tsukamoto. 2007. Development of a multiplex PCR assay for identification of Campylobacter coli, Campylobacter fetus, Campylobacter hyointestinalis subsp. hyointestinalis, Campylobacter jejuni, Campylobacter lari and Campylobacter upsaliensis. J. Med. Microbiol. 561467-1473. [DOI] [PubMed] [Google Scholar]