Abstract
It is unclear when the initial colonization by periodontal pathogens occurs in the oral cavity. Therefore, we report here the association between specific age groups and the time when the initial colonization by periodontal pathogens occurs in the oral cavity in such groups. Findings are based on an epidemiological analysis of the prevalence of five periodontal pathogens in the oral cavities of a wide range of age populations, from newborn to elderly, who were randomly selected in a geographic region of Brazil. These periodontal pathogens include Campylobacter rectus, Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Prevotella intermedia, and Tannerella forsythia and were analyzed in the bacterial samples isolated from gingival sulcus, the dorsum of the tongue, and cheek mucosa of diverse age groups, using a bacterial DNA-specific PCR method. Results indicated that there are distinct age-related groups where initial colonization by the five periodontal pathogens examined in this study can be detected and that the presence of teeth is a permissive factor for colonization by P. gingivalis, P. intermedia, and T. forsythia. Although it remains unclear exactly how or when target pathogens colonize healthy subjects, an understanding of age-related groups does provide a potentially useful tool in the early detection and prevention of periodontitis in healthy individuals.
Recent studies demonstrated that not only are periodontal pathogens, such as Porphyromonas gingivalis, Prevotella intermedia, and Tannerella forsythia, present in the subgingival pockets of periodontally diseased patients but they also colonize other oral sites, including, for example, the supragingival plaques of the same patients. This indicates that these gram-negative anaerobic bacteria are also present in an aerobic atmosphere (23). In addition, these pathogens were found in both sub- and supragingival plaques of periodontally healthy subjects (25). These findings imply that colonization by these pathogens may occur (i) in the aerobic environment of periodontally healthy subjects and (ii) in the oral cavity of healthy subjects as commensal bacteria. However, when commensal bacteria convert to a pathogenic state, a further significant implication is that such conversion may be causal for the onset of periodontal disease (10).
Based on the findings noted above, a novel, although still hypothetical, paradigm is emerging to explain the properties of bacteria associated with periodontal disease. These bacterial pathogens, including Porphyromonas gingivalis, Prevotella intermedia, and Tannerella forsythia, are opportunistic pathogens and, therefore, do not follow Koch's postulates (29). Opportunistic pathogens are infectious agents that usually do not induce disease in a healthy host, but they can affect people with a poorly functioning or suppressed immune defense system. Under these conditions, it would be useful to gain (i) an understanding of when initial colonization by these periodontal pathogens occurs in the oral cavity of healthy subjects and (ii) an ability to establish such colonization in site-specific tissue. This would lead to the development of relevant information for the development of diagnostic and prevention strategies for periodontal disease, even though the mechanisms underlying the onset and progression of periodontal disease remain unclear. Streptococcus mutans is a causal microorganism for dental caries, and it occurs in the oral cavity of infants aged between 3 and 24 months (21). As such, S. mutans provides a classic example illustrating a temporal gate for colonization by a bacterium, since it has been reported that early colonization by S. mutans occurs in approximately 80% of infants. To date, however, it has been unclear whether such age-related groups for the initial colonization by periodontal pathogens exist. Furthermore, it has not yet been reported what, if any, site-specific tissues may be associated with such initial colonization.
To address these questions, the present study conducted an epidemiological analysis of the prevalence of periodontal pathogens in the oral cavities of a wide range of age populations from newborn to elderly who were randomly selected in a geographic region of Brazil. In particular, study parameters dictated that the randomly selected subject population should have an incidence of periodontal diseases proportional to the whole population in the region. Since some anaerobic pathogens may require a deep (pathogenic) periodontal sulcus (>3 mm) for their initial colonization, both healthy and periodontally diseased subjects were recruited in this study. More specifically, the five species of periodontal pathogens, Campylobacter rectus, P. gingivalis, Aggregatibacter actinomycetemcomitans, P. intermedia, and T. forsythia, were screened from the bacteria sampled from subgingival sulcus, cheek mucosa, and the dorsum of the tongue by a bacterium-specific DNA sequence method using PCR. The overall aim of this study was to elucidate the age ranges when initial colonization by periodontal pathogens occurs in the oral cavity.
MATERIALS AND METHODS
Human subjects.
Participants included in the present epidemiological study were recruited from the region of Vale do Paraíba, São Paulo, Brazil. The eligible sample population was recruited from a total of 1,839 subjects who visited the Department of Dentistry, University of Taubaté, from August 2004 to July 2006. After screening for the exclusion criteria, which are described below, 330 Brazilian subjects representing multiple ethnic groups were finally recruited for the study. These subjects were allocated among seven different generational groups, as follows: (A) newborns with no teeth, (B) children aged 2.5 to 5 years, (C) children aged 6 to 12 years, (D) adolescents aged 13 to 18 years, (E) adults aged 19 to 44 years, (F) elderly over 55 years of age with teeth, and (G) elderly over 55 years with no teeth. A summary of the human subject data, including the number in each age-generational group, age range, and number of males and females, as well as smoking habits, is shown in Table 1.
TABLE 1.
Subject classification by age-related groups and clinical periodontal profiles in each groupa
| Group | Age (yr [mo for group A])
|
No. of subjects
|
Plaque index [mean ± SD (range)] | Gingival index [mean ± SD (range)] | PPD (mm) [mean ± SD (range)] | CAL (mm) [mean ± SD (range)] | ||
|---|---|---|---|---|---|---|---|---|
| Range | Mean ± SD | Per group | Male/female | |||||
| A | 0-4 | 2.84 ± 1.60 | 43 | 25/18 | NDb | ND | ND | ND |
| B | 2.5-5 | 3.88 ± 1.02 | 36 | 15/21 | ND | ND | ND | ND |
| C | 6-12 | 9.33 ± 1.99 | 33 | 18/15 | 0.40 ± 0.16 (0.04-0.76) | 0.02 ± 0.03 (0.0-0.1) | ND | ND |
| D | 13-18 | 14.73 ± 1.34 | 30 | 22/8 | 0.53 ± 0.31 (0.00-1.00) | 0.22 ± 0.27 (0.00-1.00) | 1.91 ± 0.85 (0.95-4.63) | 0.93 ± 0.76 (0.04-3.11) |
| E | 19-44 | 34.60 ± 7.49 | 63 | 12/51 | 0.65 ± 0.29 (0.00-1.05) | 0.51 ± 0.30 (0.00-1.05) | 2.90 ± 0.79 (1.53-4.69) | 2.94 ± 1.29 (0.03-5.95) |
| F | >55 (with teeth) | 59.75 ± 6.28 | 60 | 33/27 | 0.49 ± 0.36 (0.00-1.00) | 0.40 ± 0.29 (0.00-1.00) | 2.99 ± 0.83 (1.26-4.85) | 3.36 ± 1.55 (0.30-6.86) |
| G | >55 (without teeth) | 64.75 ± 8.49 | 65 | 33/32 | ND | ND | ND | ND |
Plaque and gingival index data represent mean values per age-related group determined on the basis of all clinical scores. A plaque index score of 0 represents the absence of supragingival plaque, and a score of 3 represents a large amount of plaque. A gingival index score of 0 represents the absence of gingival inflammation, and a score of 3 indicates severe gingival inflammation.
ND, not determined.
Data and personal information related to medical and dental histories of the subjects were obtained from responses by individuals or their parents to our questionnaire. Either subjects themselves, or their legal guardians, signed an informed consent form, which was previously approved by the Institutional Committee on Research Involving Human Subjects of the University of Taubaté (protocol 362/03).
Exclusion criteria for subject recruitment.
Subjects presenting any of the following conditions were excluded from the study: (i) antibiotic prophylaxis for dental treatment, (ii) uncontrolled systemic diseases, (iii) immunological compromise, and (iv) pregnancy or women currently breast-feeding. Also excluded were subjects (i) who were wearing orthodontic devices, (ii) who had been undergoing periodontal treatment 12 months before the beginning of the study or had been taking antibiotics within 6 months prior to the clinical and microbial examination, or (iii) whose first and second molars or mesial and lateral incisors were missing or who had fewer than three molars or only one incisor remaining. As discussed in “Human subjects,” the age limit for the older subjects was not specifically set in this study; rather, both elderly subjects over 55 years with teeth (F) and those without teeth (G) were included.
Clinical measurements and diagnosis.
One trained and certified examiner conducted all clinical measurements and collected the microbial samples. The calibration protocol for the optimization of intraexaminer difference followed methods similar to those published by Araujo et al. (2). Baseline data analysis was performed to determine if the intraexaminer reliability was calibrated. Using kappa statistics (K) for the categorical clinical measurement variables, such as periodontal probing depth (PPD) and clinical attachment level (CAL), the standard error of these measurements was monitored. The examiner's clinical measurement technique was considered calibrated if the standard error for the measurements was ≤0.8 and a K value ranged between 0.8 and 0.95. The reproducibility of the intraexaminer measurements was recalculated every 6 months.
The periodontal examinations were performed to determine periodontal status of all subjects with teeth. Subject groups representing individuals older than 2.5 years (i.e., those subjects in groups B, C, D, E, and F, as noted above) received radiographic examination to evaluate the presence of periodontal bone resorption. More specifically, a bite-wing X-ray examination was conducted for groups B and C, whereas a periapical radiograph was taken from groups D, E, and F. However, for subjects in group A, newborns (0 to 4 months old), and group G, elderly over 55 years of age with no teeth, only visual clinical examination was conducted without X-ray examination. For children from 6 to 12 years of age (group C), the clinical examination included plaque index (28) and gingival index (22). For adolescents from 13 to 18 years of age (group D) and adults and elderly with teeth (groups E and F), we performed plaque and gingival index measurements, radiographic examinations, and complete periodontal screening, including PPD and CAL measured in six sites per tooth, using a manual periodontal probe (PCPUNC15BR; Hu-Friedy).
A diagnosis of periodontal condition followed the criteria defined by Tanner et al. (31).
Participants in group B were all diagnosed as periodontally healthy subjects because they showed no evidence of periodontal bone resorption (as evaluated by bite-wing X ray) and showed no clinical signs of periodontal inflammation upon visual examination. All participants in group C were also considered periodontally healthy based on the lack of periodontal bone resorption and a gingival index of less than 1. For groups D, E, and F, healthy participants had a mean periodontal attachment level of <1.5 mm and no sites with >2-mm attachment loss. For early periodontitis 1 (EP1), patients in these groups also had a mean periodontal attachment level of <1.5 mm but at least one site with 2-mm attachment loss. For EP2, patients had >1.5-mm to <2.0-mm mean periodontal attachment loss. In addition, the subjects either who were healthy or who did not fit Tanner's criteria were classified according to the mean of attachment loss: chronic periodontitis 1 (CP1; CAL of >2 mm and <3 mm), CP2 (CAL of ≥3 mm and <5 mm), and CP3 (CAL of ≥5 mm).
Sampling of microorganisms.
A pooled subgingival sample was collected from each subject with teeth (153 subjects) from the mesiobuccal aspect of all first molars (n = 4 molars/subject) and mesial incisors (n = 2 incisors/subject, right maxillary or left mandibular) using sterile paper points inserted to the depth of the gingival sulcus after removal of supragingival plaque using sterile curettes. For subjects missing those teeth (first molars and mesial incisors), microbial samples were obtained from second molars and/or lateral incisors (see also “Exclusion criteria for subject recruitment”). After being placed in the sulcus for 60 seconds, paper points were removed and immediately transferred into a microtube containing reduced Ringer's solution (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom). In addition, microbial samples taken from the left side of the cheek and the dorsum of the tongue were obtained from all subjects included in the present study. These samples were taken from areas of approximately 1 cm2, using a swab with reduced Ringer's solution, rotated six times. Each swab was transferred into a microtube also containing reduced Ringer's solution (1 ml).
Bacterium-specific PCR.
The bacterial cells in the microtube were dispersed using a vortex mixer at maximal setting for 1 min and then maintained at −80°C until laboratory processing. The presence of C. rectus, P. gingivalis, A. actinomycetemcomitans, P. intermedia, and T. forsythia was determined by PCR, as described below.
In the Laboratory of Molecular Biology at the University of Taubaté (Sao Paulo State, Brazil), the bacterial suspensions were thawed and centrifuged at 12,000 × g for 3 min, and the DNA was extracted from the bacterial pellet using a DNA isolation kit, following the manufacturer's instructions (InstaGene purification matrix; Bio-Rad Laboratories, Hercules, CA). Briefly, 5 μl of the sample DNA was added to 45 μl of reaction mixture containing 5 μl of 10× PCR buffer (Promega, Madison, WI), 1.25 units of Taq DNA polymerase (Promega), and 0.2 mM of deoxyribonucleotide mixture (Pharmacia LKB, Piscataway, NJ). PCR amplification was performed using a thermal cycler (Perkin-Elmer, Wellesley, MA). The bacterium-specific primer (5′-3′) sequences used in this study are as follows: C. rectus, sense, 5′-TTTCGGAGCGTAAACTCCTTTTC-3′, and antisense, 5′-TTTCTGCAAGCAGACACTCTT-3′ (PCR product size 598 bp); A. actinomycetemcomitans, sense, 5′-AAACCCATCTCTGAGTTCTTCTTC-3′, and antisense, 5′-ATGCCAACTTGACGTTAAAT-3′ (550 bp); P. intermedia, sense, 5′-TTTGTTGGGGAGTAAAGCGGG-3′, and antisense, 5′-TCAACATCTCTGTATCCTGCGT-3′ (575 bp); P. gingivalis, sense, 5′-AGGCAGCTTGCCATACTGCGG-3′, and antisense, 5′-ACTGTTAGCAACTACCGATGT-3′ (404 bp); T. forsythia, sense, 5′-GCGTATGTAACCTGCCCGCA-3′, and antisense, 5′-TGCTTCAGTGTCAGTTATACCT-3′ (641 bp).
The detailed protocol for PCR-mediated DNA amplification was previously published by this group (Cortelli et al. [8]). PCR products were separated in a 1.5% agarose gel (Sigma, Dorset, United Kingdom) by an electrophoresis performed at 4 V/cm in Tris-acetate-EDTA buffer (Promega). The DNA bands present in the gel were stained with 0.5 μg/ml ethidium bromide (Amersham, Arlington Heights, IL) and photographed under 300-nm UV light illumination.
Statistical analysis.
The association between bacterial occurrence and genders and smoking habits was analyzed within each age range using the chi-squared test. Results were determined to be statistically significant at P < 0.05. All tests were performed using statistical software (SPSS for Windows Release 12.0; SPSS Inc., Chicago, IL).
RESULTS
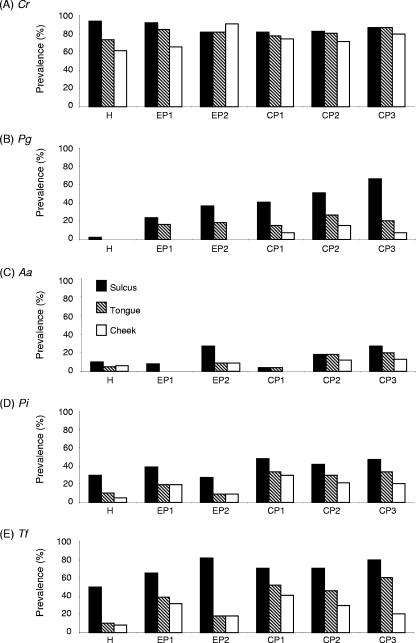
The prevalence of bacteria detected in the gingival sulcus, tongue, and cheek mucosa was analyzed for the subjects with teeth who were classified on the basis of their periodontal condition (Fig. 1). More specifically, subjects older than 2.5 years of age were grouped as follows: (i) healthy, (ii) EP1, (iii) EP2, (iv) CP1, (v) CP2, and (vi) CP3 (see the criteria for diagnosis described under “Clinical measurements and diagnosis” in Materials and Methods). Among the bacteria tested, C. rectus showed the highest prevalence (more than 80% in sulcus) throughout all subject groups. While the prevalence of C. rectus in the cheek mucosa was always slightly lower than that found in sulcus or tongue, at least 60% of subjects (healthy group) possessed C. rectus in the cheek mucosal surface. Therefore, C. rectus appeared to colonize ubiquitously in the oral cavity without preference to environmental oxygen represented by anaerobic (sulcus) or aerobic (tongue and cheek mucosa) atmosphere. P. gingivalis was prominently found in the subjects with periodontitis, while incidence of this bacterium in the healthy subjects was extremely low (2% in sulcus, 0% in tongue and cheek mucosa). Very interestingly, the incidence of P. gingivalis in the sulcus demonstrated an increasing trend, along with the severity of periodontitis in the subject groups (from EP1 to CP3). The prevalence of P. gingivalis in the tongue or cheek mucosa was always lower than that in the sulcus. Especially, P. gingivalis was not found in the cheek mucosa of EP1 and EP2 groups, whereas P. gingivalis was found at low prevalence (less than 16%) in the cheek mucosa of the CP1, CP2, and CP3 groups.
FIG. 1.
Prevalence of periodontal pathogens in gingival sulcus, tongue, and cheek mucosa of different subject groups classified on the basis of their periodontal disease status. The subject groups B to F shown in Table 1 (aged >2.5 years, a total of 222 subjects who have teeth) were employed in this analysis. The presence of DNA specific to C. rectus (A), P. gingivalis (B), A. actinomycetemcomitans (C), P. intermedia (D), and T. forsythia (E) in the samples isolated from subgingival sulcus, the dorsum of the tongue, and cheek mucosa of each subject was determined by PCR, following the method described in Materials and Methods.
The prevalence of P. intermedia and T. forsythia showed similar profiles of bacterial recovery from all groups examined. These two bacteria were detected from patient groups as well as healthy groups. On average, the incidence of T. forsythia in the sulcus was slightly higher than that of P. intermedia for all groups examined. The incidences of P. intermedia and T. forsythia in the tongue and cheek mucosa were always lower than those in sulcus, and the cheek mucosa demonstrated the lowest prevalence. A. actinomycetemcomitans, a putative pathogen for localized aggressive periodontitis, was found in sulcus, tongue, and cheek mucosa in all healthy and patient groups at a lower frequency than that of the other four species of bacteria examined (less than 28% in sulcus). The prevalence of A. actinomycetemcomitans found in tongue and cheek mucosa was equal to, or lower than, its prevalence in sulcus. These data indicated that colonization by P. gingivalis is tightly associated with periodontitis and that the colonization by the other four bacteria can also be found in the healthy oral cavity. However, while these data do conform to the conventional format for etiological studies involving periodontal bacterial infection, they do not offer any clues which would concretely establish the time of onset of initial bacterial colonization. Therefore, to achieve the primary goal of this study, i.e., to examine an age-related group which corresponds to the detection of initial bacterial colonization, additional epidemiological analysis was conducted using these patient data with the addition of periodontally healthy groups (A, B, and G in Table 1).
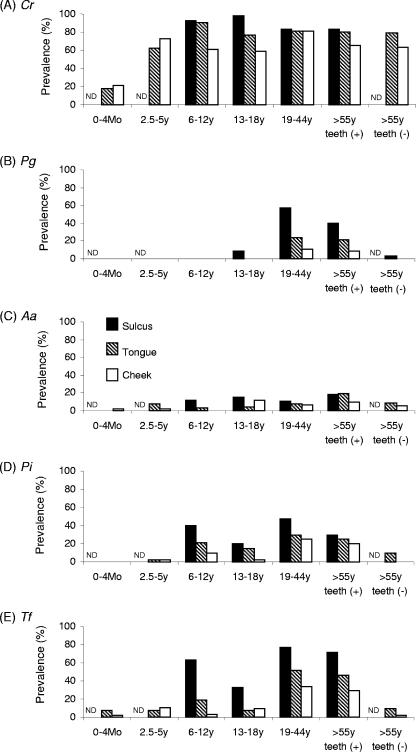
Subjects recruited for this study were grouped on the basis of their age (Fig. 2). In particular, two groups, 0 to 4 months old and elderly subjects (>55 years) without any teeth (tongue and cheek mucosa data only), were additionally included in the population shown in Fig. 1. According to these statistics, the prevalence of C. rectus was still the highest among the bacteria examined in the present study, irrespective of the age groups. However, the prevalence of C. rectus in tongue and cheek mucosa was lower in the 0- to 4-month newborn group than in the rest of the groups (tongue: 0 to 4 months, 18%; >2.5 years, intergroup average, 78.3% ± 9.4% [standard deviation]; cheek: 0 to 4 months, 21.5%; >2.5 years, intergroup average, 66.9% ± 8.3% [standard deviation]), indicating that colonization by C. rectus starts in the oral cavity as early as 0 to 4 months. Very interestingly, in the edentulous elderly subjects (group G), the prevalence of C. rectus remained high (79% in tongue, 63% in cheek mucosa) compared to the other four species of bacteria, which showed a prevalence of less than 10% in both tongue and cheek mucosa, indicating that the presence of teeth (or sulcus) is not permissive for colonization by C. rectus.
FIG. 2.
Prevalence of periodontal pathogens in gingival sulcus, tongue, and cheek mucosa of different subject groups classified on the basis of their age and presence of teeth. The subject groups A to G shown in Table 1 (aged >0 months, a total of 330 subjects with or without teeth) were employed in this analysis. The presence of DNA specific to C. rectus (A), P. gingivalis (B), A. actinomycetemcomitans (C), P. intermedia (D), and T. forsythia (E) in the samples isolated from subgingival sulcus, the dorsum of the tongue, and cheek mucosa of each subject was determined by PCR, following the method described in Materials and Methods. ND, not determined.
The incidence of P. gingivalis in group C (Fig. 2) was sporadic (13 to 18 years old: sulcus, 9%; tongue, 0%; and cheek mucosa, 0%), while a prominent emergence of P. gingivalis was found in subjects older than 19 years (groups E and F). The prevalence of P. gingivalis in the edentulous elderly subjects (group G) was remarkably low compared to that in the same age group with teeth (group F), suggesting that colonization by P. gingivalis requires the presence of teeth (or sulcus).
A. actinomycetemcomitans was found sporadically at an incidence of less than 20% through all age groups (Fig. 2). Although colonization by A. actinomycetemcomitans was found in younger age groups (groups A to D), it is unclear if such colonization at younger ages leads to localized aggressive periodontitis. Only a longitudinal cohort study will elucidate this question. It is noteworthy that none of the subjects in this study was diagnosed with localized aggressive periodontitis, in which A. actinomycetemcomitans is accepted as a major pathogen.
The initial colonization by P. intermedia appeared to occur from the ages of 6 to 12 years (Fig. 2). While only sporadic colonization by T. forsythia was found in groups A and B (ages 0 to 4 months and 2.5 to 5 years, respectively), a major increase in T. forsythia colonization was detected in group C (6 to 12 years). The prevalence of P. intermedia and T. forsythia in the older age groups with teeth (13 to 18 years, 19 to 44 years, and >55 years) remains at a level similar to that in group C (6 to 12 years). However, the prevalence of these two bacteria, P. intermedia and T. forsythia, in group G (>55 years old without teeth) remarkably decreased in the tongue and cheek mucosa, indicating that teeth (or sulcus) is a permissive factor for colonization by P. intermedia and T. forsythia.
Smoking is known as a risk factor promoting the incidence of periodontitis. Therefore, we have further classified the subjects into smokers and nonsmokers (Table 2). Especially, in the group of subjects 19 to 44 years old (E) and those subjects aged >55 years (F), nearly one-half of recruited subjects were identified as smokers. However, when the association between smoking and the presence of all bacteria was evaluated, there was no statistically significant difference observed in the sulcus, tongue, or cheek mucosa in either group E or group F (chi-squared test; data not given). Therefore, smoking did not appear to be a factor that influences the prevalence of bacteria tested in this study. The influence of gender difference on the prevalence of bacteria within each age-related group (all groups, A to G) also did not show any statistical difference as analyzed by chi-square test (data not shown).
TABLE 2.
Distribution of different statuses of periodontitis in the groups of subjects with teeth
| Periodontal status | No. of subjects by groupa and smoking status with periodontal status
|
||||||
|---|---|---|---|---|---|---|---|
| B (2.5-5 yr; never smoked) | C (6-12 yr; never smoked) | D (13-18 yr; never smoked) | E (19-44 yr)
|
F (>55 yr; with teeth)
|
|||
| Smokerb | Never smoked | Smokerb | Never smoked | ||||
| Healthy | 36c | 33c | 15 | 2 | 0 | 0 | 0 |
| EP1 | 0 | 0 | 9 | 3 | 4 | 4 | 6 |
| EP2 | 0 | 0 | 2 | 5 | 2 | 2 | 0 |
| CP1: CAL, >2 mm and <3 mm | 0 | 0 | 1 | 5 | 10 | 4 | 7 |
| CP2: CAL, >3 mm and <5 mm | 0 | 0 | 3 | 12 | 14 | 16 | 12 |
| CP3: CAL, ≥5 mm | 0 | 0 | 0 | 3 | 3 | 4 | 5 |
| Total | 36 | 33 | 30 | 30 | 33 | 30 | 30 |
Groups of subjects who have teeth are shown. Groups B to F correspond to the age-related groups shown in Table 1. Newborn and edentulous elderly groups are not shown because of the lack of periodontal tissues.
No significant difference in bacterial prevalence was detected between the smokers and nonsmokers in group E and group F.
Participants in group B were all diagnosed as periodontally healthy subjects because they showed no evidence of periodontal bone resorption (as evaluated by bite-wing X ray) and showed no clinical signs of periodontal inflammation upon visual examination. All participants in group C were also considered periodontally healthy based on the lack of periodontal bone resorption and a gingival index of less than 1.
DISCUSSION
Several studies have shown an association between the onset and progression of periodontal disease and the presence of periodontal pathogens in the periodontal sulcus of periodontally diseased patients in Brazil and many other countries, as well. Those pathogens found in Brazilian patients with periodontal diseases include A. actinomycetemcomitans, P. gingivalis, P. intermedia, T. forsythia, C. rectus, Eikenella corrodens, Treponema denticola, Streptococcus spp., and some others (3, 4, 6, 7, 8, 9, 24, 26, 32, 33, 34). In particular, these previous studies focused on the prevalence of periodontal pathogens in diseased subjects representing four different stages of clinical treatment, as follows: (i) before periodontal treatment, (ii) after nonsurgical mechanical therapy (scaling and root planing), (iii) after antibiotic therapy, and (iv) after surgical treatment. However, none of the studies using a Brazilian population has yet addressed the question of when initial colonization by periodontal pathogens occurs in the oral cavity. For this reason, the present study utilized an epidemiological approach to specifically examine the prevalence of five types of periodontal pathogens in the oral cavities of subjects in diverse age groups ranging from newborns to elderly, with or without teeth.
Data from this study were analyzed and used to achieve the major aim of this report: to establish a link between particular age-related groups and the time when the initial colonization by periodontal pathogens might occur in the oral cavities of subjects. These subjects were randomly chosen from a geographic region in Brazil in order to include both healthy and periodontally diseased individuals in the study. The data in the present study demonstrated that (i) initial colonization by T. forsythia was found in tongue and cheek mucosa of newborns (0 to 4 months), and more than 60% of healthy subjects possessed T. forsythia in gingival sulcus at ages 6 to 12 years; (ii) initial colonization by C. rectus was found as early as ages 0 to 4 months and remained high through all ages, irrespective of the presence of teeth; (iii) initial colonization by P. intermedia in the oral cavity appeared to occur in periodontal sulcus during ages 6 to 12 years; (iv) initial colonization by P. gingivalis seemed to start during the ages of 19 to 44 years; and (v) the presence of teeth in the oral cavity is a permissive factor for colonization by P. intermedia, T. forsythia, and P. gingivalis.
Previous studies showed that the incidence of T. forsythia in the periodontal sulcus positively correlates with the clinical signs of periodontal disease and can be regarded as a risk indicator for attachment and bone loss (15, 16, 17). According to the work of Tanner et al. (31), the incidence of P. gingivalis and T. forsythia in subgingival and tongue samples is associated with early periodontitis compared to the samples isolated from healthy subjects. For the latter, Tanner et al. found that T. forsythia is present in the subgingival samples isolated from healthy subjects, which agrees with the findings of the present study. However, they did not detect T. forsythia in the tongues of healthy adults (aged 20 to 40 years), which is contrary to our finding that T. forsythia can be found in the tongue of healthy subjects at low incidence, about 11% (Fig. 1). More importantly, the present study demonstrated that colonization by T. forsythia can be detected in the tongue as early as age 0 to 4 months, in individuals who obviously have no teeth (Fig. 2). Therefore, our data indicate that T. forsythia is an opportunistic pathogen whose colonization of the oral cavity does not necessarily result in the onset of periodontal disease.
Additional findings revealed in the present study demonstrated the significance of the association between age-related groups and the prevalence of periodontal bacteria colonizing particular affected tissues. To illustrate, the initial colonization by C. rectus, P. gingivalis, A. actinomycetemcomitans, P. intermedia, and T. forsythia was first noted in all ages of dentate subjects, but it was also observed that the prevalence of each bacterium remained at high levels in subgingival plaque, tongue, and cheek mucosa of elderly subjects (>55 years). However, a major difference between C. rectus and the other bacteria, including P. gingivalis, A. actinomycetemcomitans, P. intermedia, and T. forsythia, was found with respect to their level of incidence in the elderly group without teeth. Specifically, the prevalence of P. gingivalis, A. actinomycetemcomitans, P. intermedia, and T. forsythia in the oral cavity (tongue and cheek) remarkably diminished in the edentulous elderly, whereas the prevalence of C. rectus in these edentulous elderly remained high. Considering the scarcity of data regarding the prevalence of periodontal pathogens in the edentulous elderly (30), we believe that the present study is the first to demonstrate that the presence of teeth appeared to be a permissive factor for colonization by species, such as T. forsythia, A. actinomycetemcomitans, P. gingivalis, and P. intermedia (Fig. 2, group F versus group G).
On the other hand, while the presence of teeth appeared to be “permissive,” it is not “requisite” for colonization by T. forsythia, A. actinomycetemcomitans, P. gingivalis, and P. intermedia because all these microorganisms were still present at a low prevalence in the dorsum of the tongue and in the cheek of newborn and edentulous elderly subjects. Therefore, our findings contradict the report by Danser et al. (11), who demonstrated that A. actinomycetemcomitans and P. gingivalis disappear from the oral cavity after the extraction of all teeth. Additionally, our data also disagree with the work of Könönen et al. (19), who did not detect A. actinomycetemcomitans in 51 edentulous subjects (44 to 92 years; mean age, 74 years) who used complete dentures. However, in the present study, P. gingivalis was not detected in any subject under 12 years of age, and the first occurrence of this microorganism was observed at a very low incidence (6.67%) in subgingival plaque, but not in tongue or cheek samples, of adolescents (13 to 18 years). It is noteworthy that this finding contradicts the report by Lamell et al. (20), who detected P. gingivalis DNA in the samples of tongue, buccal mucosa, or mesial sulcus of all teeth at rates of 36% (first examination) to 43% (second examination at recall after 1 to 3 years) between the ages of 0 and 18 years. They therefore concluded that P. gingivalis inhabits the oral cavities of child subjects of any age (0 to 18 years) and usually colonizes only transiently. It is conceivable that the difference between the present study and the report by Lamell et al. (20) in the ability to detect periodontal pathogens on mucosal surfaces may depend on the sensitivity of the method used for bacterial DNA detection. Nonetheless, there should be factors, other than the presence of teeth, which affect colonization by P. gingivalis or transitory habitation by P. gingivalis. Such factors may change the properties of P. gingivalis through the activation of a two-component system (13), which would allow the bacterium to survive in the aerobic environment of tongue and cheek surfaces.
The diversity of bacterial composition in the plaque isolated from different sites of the oral cavity has been well documented. These sites include supra- and subgingival sulcus, tongue, cheek, and other areas of the oral cavity. For example, Aas et al. (1), utilizing culture-independent molecular techniques, defined the bacterial diversity of the healthy human oral cavity. This study examined the bacterial composition in nine sites, including supra- and subgingival plaque, the lip vestibule, soft palate, hard palate, lateral side of the tongue, tongue dorsum, buccal epithelium, and tonsils. The results showed that some species, such as those of Gemella, Granilicatella, Streptococcus, and Veillonella, were common to all nine sites, whereas Rothia dentocariosa, Actinomyces spp., Streptococcus sanguis, Streptococcus gordonii, and Abiotrophia defectiva were detected only in supragingival plaque on the teeth. The results also demonstrated that, in general, Streptococcus salivarius was present on the tongue dorsum, indicating that certain strains of microorganisms detected in subgingival plaque could also be detected in other areas of the oral cavity.
The present study further addressed the question of whether microorganisms present in subgingival plaque could also be present in the cheek and dorsum of the tongue (Fig. 1 and 2). The subject population randomly recruited in this study showed a high prevalence of C. rectus through all age groups, as well as at the three sampling sites of periodontal sulcus, tongue, and cheek mucosa. Moreover, since the presence of C. rectus in the subgingival pocket appeared to be associated with its detection in the tongue and cheek, tongue sampling may offer a convenient, as well as efficient, means of detecting C. rectus in the oral cavity. Aside from C. rectus, the probability of simultaneous occurrence of the other bacteria used in this study in the subgingival plaque, tongue, and cheek was low (data not shown). Taken together, these findings suggested that sampling of microorganisms from different sites of the oral cavity is required to identify a particular microorganism in the oral cavity of human subjects.
While several studies have implicated the effects of smoking in the composition of the subgingival microbial flora (14, 18, 35, 36), others failed to show any differences in the prevalences of periodontal pathogens for either smokers or nonsmokers (5, 12). The present study found no correlation between smoking and the prevalence of the five tested bacteria. It is true that Shiloah et al. (27) showed that the prevalence of pathogenic bacteria in the periodontal sulcus of periodontitis-free individuals is related to their daily consumption of cigarettes and duration of cigarette smoking. However, it is not realistic to monitor the daily consumption of cigarettes by younger smokers, even if this factor may affect the early colonization by periodontal pathogens in the periodontal sulcus of those in the younger populations. Since it is known that environmental factors, such as nutrition and lifestyle, affect the onset and progression of periodontal disease, epidemiological studies, which involve more study arms of environmental factors in addition to smoking, will provide insight into the effect of smoking on colonization by periodontal pathogens.
To summarize, the prevalence of C. rectus, P. gingivalis, A. actinomycetemcomitans, P. intermedia, and T. forsythia demonstrated differences in their initial colonizations in both the types of tissues and temporal ranges for the diverse age groups examined in this study. C. rectus was found in subjects aged 0 to 4 months and remained at high levels in the oral cavity (tongue and cheek) of elderly subjects, irrespective of their possession of teeth. Initial colonization by P. intermedia and T. forsythia in subgingival sulcus was detected in a very young group (6 to 12 years), whereas P. gingivalis was first detected in a much older group (19 to 44 years). However, the prevalence of all three bacteria declined in the elderly individuals without teeth but not in elderly individuals with teeth. The prevalence of P. gingivalis, P. intermedia, and T. forsythia found in tongue and cheek mucosa was always lower than their prevalence detected in the subgingival sulcus. In conclusion, the findings related to the association between age-related groups and the time of initial colonization by the five major periodontal pathogens may provide a potentially useful set of markers for the early detection of opportunistic pathogens during their benign state. This could then ultimately provide new insights into the mechanisms underlying pathogenic conversion of these opportunistic pathogens, resulting in the identification of targets for novel preventive and therapeutic approaches.
Acknowledgments
We are grateful to Ângela Maria Quintão Lana (UFMG) for her help with the statistical analysis.
This study was supported by a grant, 04/00256-6, from the FAPESP (São Paulo Foundation for Research) and by DE18310 from NIDCR.
Footnotes
Published ahead of print on 20 February 2008.
REFERENCES
- 1.Aas, J. A., B. J. Paster, L. N. Stokes, I. Olsen, and F. E. Dewhirst. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 435721-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo, M. W. B., K. M. Benedek, J. R. Benedek, S. G. Grossi, J. Dorn, J. Wactawski-Wende, R. J. Genco, and M. Trevisan. 2003. Reproducibility of probing depth measurements using a constant force electronic probe: analysis of inter- and intra-examiner variability. J. Periodontol. 741736-1740. [DOI] [PubMed] [Google Scholar]
- 3.Avila-Campos, M. J., I. N. Rivera, and V. Nakano. 2006. Genetic diversity of oral Fusobacterium nucleatum isolated from patients with different clinical conditions. Rev. Inst. Med. Trop. Sao Paulo 4859-63. [DOI] [PubMed] [Google Scholar]
- 4.Avila-Campos, M. J., and G. Velasquez-Melendez. 2002. Prevalence of putative periodontopathogens from periodontal patients and healthy subjects in Sao Paulo, SP, Brazil. Rev. Inst. Med. Trop. Sao Paulo 441-5. [DOI] [PubMed] [Google Scholar]
- 5.Boström, L., J. Bergström, G. Dahlén, and L. E. Linder. 2001. Smoking and subgingival microflora in periodontal disease. J. Clin. Periodontol. 28212-219. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho, L. H., G. B. D'Avila, A. Leao, C. Goncalves, A. D. Haffajee, S. S. Socransky, and M. Feres. 2005. Scaling and root planing, systemic metronidazole and professional plaque removal in the treatment of chronic periodontitis in a Brazilian population. II. Microbiological results. J. Clin. Periodontol. 32406-411. [DOI] [PubMed] [Google Scholar]
- 7.Colombo, A. P., R. P. Teles, M. C. Torres, W. Rosalem, M. C. Mendes, R. M. Souto, and M. Uzeda. 2005. Effects of non-surgical mechanical therapy on the subgingival microbiota of Brazilians with untreated chronic periodontitis: 9-month results. J. Periodontol. 76778-784. [DOI] [PubMed] [Google Scholar]
- 8.Cortelli, J. R., S. C. Cortelli, S. Jordan, V. I. Haraszthy, and J. J. Zambon. 2005. Prevalence of periodontal pathogens in Brazilians with aggressive or chronic periodontitis. J. Clin. Periodontol. 32860-866. [DOI] [PubMed] [Google Scholar]
- 9.Cortelli, S. C., M. Feres, A. A. Rodrigues, D. R. Aquino, J. A. Shibli, and J. R. Cortelli. 2005. Detection of Actinobacillus actinomycetemcomitans in unstimulated saliva of patients with chronic periodontitis. J. Periodontol. 76204-209. [DOI] [PubMed] [Google Scholar]
- 10.Dalwai, F., D. A. Spratt, and J. Pratten. 2006. Modeling shifts in microbial populations associated with health or disease. Appl. Environ Microbiol. 723678-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danser, M. M., A. J. van Winkelhoff, and U. van der Venden. 1997. Periodontal bacteria colonizing oral mucous membranes in edentulous patients wearing dental implants. J. Periodontol. 68209-216. [DOI] [PubMed] [Google Scholar]
- 12.Darby, I. B., P. J. Hodge, M. P. Riggio, and D. F. Kinane. 2000. Microbial comparison of smoker and non-smoker adult and early-onset periodontitis patients by polymerase chain reaction. J. Clin. Periodontol. 27417-424. [DOI] [PubMed] [Google Scholar]
- 13.Duran-Pinedo, A. E., K. Nishikawa, and M. J. Duncan. 2007. The Rpr 4 response regulator of Porphyromonas gingivalis. Mol. Microbiol. 641061-1074. [DOI] [PubMed] [Google Scholar]
- 14.Eggert, M., M. H. McLeod, and G. Flowerdew. 2001. Effects of smoking and treatment status on periodontal bacteria: evidence that smoking influences control of periodontal bacteria at the mucosal surface of the gingival crevice. J. Periodontol. 721210-1220. [DOI] [PubMed] [Google Scholar]
- 15.Grossi, S. G., J. J. Zambon, A. W. Ho, G. Koch, R. G. Dunford, E. E. Machtei, O. M. Norderyd, and R. J. Genco. 1994. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J. Periodontol. 65260-267. [DOI] [PubMed] [Google Scholar]
- 16.Grossi, S. G., R. J. Genco, E. E. Machtei, A. W. Ho, G. Koch, R. G. Dunford, J. J. Zambon, and E. Hausmann. 1995. Assessment of risk for periodontal disease. II. Risk indicators for alveolar bone loss. J. Periodontol. 6623-29. [DOI] [PubMed] [Google Scholar]
- 17.Hamlet, S., R. Ellwood, M. Cullinan, H. Worthington, J. Palmer, P. Bird, D. Narayanan, R. Davies, and G. Seymour. 2004. Persistent colonization with Tannerella forsythensis and loss of attachment in adolescents. J. Dent. Res. 83232-235. [DOI] [PubMed] [Google Scholar]
- 18.Kamma, J. J., M. Nakou, and P. C. Baehni. 1999. Clinical and microbiological characteristics of smokers with early onset periodontitis. J. Periodontal Res. 3425-33. [DOI] [PubMed] [Google Scholar]
- 19.Könönen, E., S. Asikainen, S. Alaluusua, M. Könönen, P. Summanen, A. Kanervo, and H. Jousimies-Somer. 1991. Are certain oral pathogens part of normal oral flora in denture-wearing edentulous subjects? Oral Microbiol. Immunol. 6119-122. [DOI] [PubMed] [Google Scholar]
- 20.Lamell, C. W., A. L. Griffen, D. L. McClellan, and E. J. Leys. 2000. Acquisition and colonization stability of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in children. J. Clin. Microbiol. 381196-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law, V., W. K. Seow, and G. Townsend. 2007. Factors influencing oral colonization of mutans streptococci in young children. Aust. Dent. J. 5293-100. [DOI] [PubMed] [Google Scholar]
- 22.Löe, H., and J. Silness. 1963. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol. Scand. 21533-551. [DOI] [PubMed] [Google Scholar]
- 23.Mager, D. L., L. A. Ximenez-Fyvie, A. D. Haffajee, and S. S. Socransky. 2003. Distribution of selected bacterial on intraoral surfaces. J. Clin. Periodontol. 30644-654. [DOI] [PubMed] [Google Scholar]
- 24.Malheiros, V. J., and M. J. Avila-Campos. 2004. Detection of pathogens from periodontal lesions. Rev. Saude Publica 38723-728. [DOI] [PubMed] [Google Scholar]
- 25.Mayanagi, G., T. Sato, H. Shimauchi, and N. Takahashi. 2004. Detection frequency of periodontitis-associated bacteria by polymerase chain reaction in subgingival and supragingival plaque of periodontitis and healthy subjects. Oral Microbiol. Immunol. 19379-385. [DOI] [PubMed] [Google Scholar]
- 26.Missailidis, C. G., J. E. Umeda, C. Ota-Tsuzuki, D. Anzai, and M. P. Mayer. 2004. Distribution of fimA genotypes of Porphyromonas gingivalis in subjects with various periodontal conditions. Oral Microbiol. Immunol. 19224-229. [DOI] [PubMed] [Google Scholar]
- 27.Shiloah, J., M. R. Patters, and M. B. Waring. 2000. The prevalence of pathogenic periodontal microflora in healthy young adult smokers. J. Periodontol. 71562-567. [DOI] [PubMed] [Google Scholar]
- 28.Silness, J., and H. Löe. 1964. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 22121-135. [DOI] [PubMed] [Google Scholar]
- 29.Socransky, S. S. 1979. Criteria for the infectious agents in dental caries and periodontal disease. J. Clin. Periodontol. 616-21. [DOI] [PubMed] [Google Scholar]
- 30.Socransky, S. S., and A. D. Haffajee. 2005. Periodontal microbial ecology. Periodontology 2000 38135-187. [DOI] [PubMed] [Google Scholar]
- 31.Tanner, A. C. R., B. J. Paster, S. C. Lu, E. Kanasi, J. R. R. Kent, T. Van Dyke, and S. T. Sonis. 2006. Subgingival and tongue microbiota during early periodontitis. J. Dent. Res. 85318-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teixeira, R. E., E. N. Mendes, M. A. Roque de Carvalho, J. R. Nicoli, L. M. Farias, and P. P. Magalhaes. 2006. Actinobacillus actinomycetemcomitans serotype-specific genotypes and periodontal status in Brazilian subjects. Can. J. Microbiol. 52182-188. [DOI] [PubMed] [Google Scholar]
- 33.Tinoco, E. M., M. I. Beldi, F. Campedelli, M. Lana, C. A. Loureiro, H. T. Bellini, T. E. Rams, N. M. Tinoco, P. Gjermo, and H. R. Preus. 1998. Clinical and microbiological effects of adjunctive antibiotics in treatment of localized juvenile periodontitis. A controlled clinical trial. J. Periodontol. 691355-1363. [DOI] [PubMed] [Google Scholar]
- 34.Tinoco, E. M., M. I. Beldi, C. A. Loureiro, M. Lana, F. Campedelli, N. M. Tinoco, P. Gjermo, and H. R. Preus. 1997. Localized juvenile periodontitis and Actinobacillus actinomycetemcomitans in a Brazilian population. Eur. J. Oral Sci. 1059-14. [DOI] [PubMed] [Google Scholar]
- 35.Umeda, M., C. Chen, I. Bakker, A. Contreras, J. L. Morrison, and J. Slots. 1998. Risk indicators for harboring periodontal pathogens. J. Periodontol. 691111-1118. [DOI] [PubMed] [Google Scholar]
- 36.van Winkelhoff, A. J., C. J. Bosch-Tijhof, E. G. Winkel, and W. A. van der Reijden. 2001. Smoking affects the subgingival microflora in periodontitis. J. Periodontol. 72666-671. [DOI] [PubMed] [Google Scholar]