Abstract
High rates of Campylobacter fluoroquinolone resistance highlight the need to evaluate diagnostic strategies that can be used to assist with clinical management. Diagnostic tests were evaluated with U.S. soldiers presenting with acute diarrhea during deployment in Thailand. The results of bedside and field laboratory diagnostic tests were compared to stool microbiology findings for 182 enrolled patients. Campylobacter jejuni was isolated from 62% of the cases. Clinical and laboratory findings at the time of presentation were evaluated to determine their impact on the posttest probability, defined as the likelihood of a diagnosis of Campylobacter infection. Clinical findings, the results of tests for inflammation (stool occult blood testing [Hemoccult], fecal leukocytes, fecal lactoferrin, plasma C-reactive protein), and the numbers of Campylobacter-specific antibody-secreting cells in peripheral blood failed to increase the posttest probability above 90% in this setting of Campylobacter hyperendemicity when these findings were present. Positive results by a Campylobacter-specific commercial enzyme immunoassay (EIA) and, less so, a research PCR were strong positive predictors. The negative predictive value for ruling out Campylobacter infection, defined as a posttest probability of less than 10%, was similarly observed with these Campylobacter-specific stool-based tests as well the fecal leukocyte test. Compared to the other tests evaluated, the Campylobacter EIA is a sensitive and specific rapid diagnostic test that may assist with diagnostic evaluation, with consideration of the epidemiological setting, logistics, and cost.
Military personnel are frequently affected by short-term morbidity related to diarrheal diseases, with a potential adverse impact on the operational mission (24, 37). The use of empirical therapy without supplemental laboratory data is a feasible option; however, refinement of the management strategy by using laboratory testing may increase the cost-effectiveness and allow specific adjustments in antibiotic selection on the basis of regional susceptibility patterns. During military operations, the availability of a field laboratory with a microbiological testing capability is variable. Rapid, technically simple diagnostic tests need to be evaluated to determine their accuracy and acceptability in field settings. In Thailand, numerous surveys among deployed U.S. military personnel have shown that enteropathogenic Campylobacter species, Campylobacter jejuni and C. coli, account for as many as 60% of diarrheal cases (2, 4, 8, 27, 30, 34, 42), a very high prevalence compared to the prevalence detected in similar studies conducted in other regions (35). On the basis of this observation, the pathogen-specific diagnostic tests used in this study focused on Campylobacter. This study of military personnel presenting with acute diarrhea during deployment in Thailand evaluated the clinical findings in concert with bedside stool characterization and the results of field laboratory rapid diagnostic tests as components of an overall diagnostic approach.
(This work was conducted in partial support of a doctoral thesis in public health at the Uniformed Services University, Bethesda, MD [D.R.T.].)
MATERIALS AND METHODS
Study population and enrollment criteria.
Annual U.S. military training exercises were conducted in the Kingdom of Thailand in May 2000 and May 2001. Temporary medical units for the evaluation and management of personnel were in operation during the period of the exercise. Personnel presenting with acute diarrhea were requested to volunteer for participation. Enrollment criteria included the following: acute diarrhea duration of ≤96 h, onset of illness ≥24 h after arrival in Thailand, illness conforming to the definition of diarrhea, no antibiotic treatment (with the exception of doxycycline, used for malaria prophylaxis) in the previous 7 days, and the availability of a pretreatment stool culture. Diarrhea was defined as three or more loose stools in a 24-h period or two or more loose stools in a 24-h period with one or more associated complaints, including abdominal cramps, nausea, vomiting, or fever (oral temperature, ≥38°C).
Clinical evaluation and specimen collection.
A standardized questionnaire was used, and a medical examination was performed. The patients were asked to provide a stool specimen prior to administration of the first antibiotic dose. Stool characterization and bedside occult blood testing (Hemoccult; Beckman Coulter, Inc., Fullerton, CA) was completed by the study physician prior to transport of the specimen to the field laboratory. Stool specimens were graded on a scale from 1 to 5 (1, hard [normal]; 2, soft [normal]; 3, thick liquid; 4, opaque watery liquid; and 5, clear watery). Peripheral blood was collected directly into a Vacutainer tube containing EDTA (Beckton Dickinson Vacutainer Systems, Rutherford, NJ). Stool and blood specimens were transported to the field laboratory for immediate processing.
Stool microbiology (reference standard).
Primary plating of the stool specimens was undertaken at an on-site field laboratory, as described previously (38). Campylobacter species were isolated by a membrane filter method on nonselective blood agar before and after enrichment (39). Throughout this report, Campylobacter refers to both C. jejuni and C. coli. The isolates were transported to the Armed Forces Research Institute of Medical Sciences in Bangkok, Thailand, for species identification and susceptibility testing, as described previously (7, 9, 11). Five lactose-fermenting and five non-lactose-fermenting Escherichia coli colonies per specimen were tested by using DNA probes for the detection of enterotoxigenic E. coli (ETEC) toxins (heat-labile and heat-stable toxins), enteroinvasive E. coli(pMR17), enterohemorrhagic E. coli (Shiga-like toxin I and II producing), and enteropathogenic E. coli (eae and EAF plasmid positive) (10, 40). Microscopy evaluation of fresh stool specimens was used to evaluate the specimens for parasites. Aliquots of stool samples were frozen at −70°C on the day of receipt for viral antigen detection. The stool samples were evaluated for the presence of rotavirus and calicivirus antigens by a commercially available enzyme-linked immunosorbent assay (Rotazyme; Abbott Laboratories, North Chicago, IL) and a noncommercial antigen-capture calicivirus-specific enzyme-linked immunosorbent assay, respectively (26).
Diagnostic tests under evaluation (index tests).
Individuals performing the index tests were trained laboratory personnel masked to the results for the reference standard and clinical findings, with the exception of the results of the stool PCR assay, which was under development.
(i) Stool-based index tests.
All stool-based index tests were performed with fresh specimens on the day of receipt. Fecal leukocytes were semiquantitatively determined by examining methylene blue-stained fecal smears under a microscope. The numbers of fecal leukocytes present per high-power field were categorized as follows: none, rare, 1 to 5, 6 to 10, and >10. Fecal lactoferrin was detected with the commercial Leuko-Test kit (TechLab, Blacksburg, VA), according to the manufacturer's instructions. The presence of lactoferrin in a 1:50-diluted stool specimen was detected by a visually read positive agglutination of ≥1+, as defined by the manufacturer. The presence of C. jejuni or C. coli was detected by using the commercial ProSpecT Campylobacter microplate assay (Alexon-Trend, Inc., Ramsey, MN), according to the manufacturer's instructions. Campylobacter-specific antigens were detected by a visually read color development of ≥1+, as defined by the manufacturer. A multiplex PCR for the detection of C. jejuni and C. coli in the stool specimens was included during the first year of the exercise, as described previously (21). The PCR assay detects the ceuE genes in C. jejuni and C. coli and is useful for primary detection and species differentiation. DNA templates from 10% stool suspensions in TE (10 mM Tris-HCl, 1 mM disodium EDTA, pH 8.0) were prepared by silicon dioxide extraction (20, 21). Oligonucleotide primer sequences derived from the ceuE genes and the PCR amplification conditions have been reported previously (21). All PCR tests were conducted in the presence of appropriate controls, and the results were considered valid only if the results for the controls were proven to be accurate; i.e., positive controls were PCR positive and negative controls were PCR negative.
(ii) Blood-based index tests.
Blood samples were evaluated for plasma C-reactive protein (CRP), an acute-phase protein whose level is elevated during inflammatory disease, at the time of initial presentation to the clinic (32). Fresh plasma was evaluated semiquantitatively by using the commercial RapiTex CRP test (Dade Behring, Marburg, Germany), according to the manufacturer's instructions. Mononuclear cells (MNCs) were isolated by Ficoll-Hypaque density gradient analysis (Organon Teknika Corp., Durham, NC) and were cryopreserved in the field laboratory (3). Assays for antibody-secreting cells (ASCs) were performed at the Naval Medical Research Center, Silver Spring, MD, as described previously (3). Campylobacter-specific immunoglobulin A ASC responses were evaluated at the initial presentation and at the 72-h clinical follow-up by using the enzyme-linked immunospot assay methodology (3). The specific antigens used included a C. jejuni strain 81-176 glycine extract, C. jejuni strain 81-176 whole cells, and a common Thai C. jejuni strain (Lior 36) whole-cell lysate preparation (28). The numbers of spots found in comparable wells were summed and adjusted to the number per 106 MNCs. A positive ASC response was defined as five or more Campylobacter antigen-specific spots per 106 MNCs.
Statistical analysis.
The physician-performed bedside diagnostic assays (stool characterization and occult blood testing) and laboratory technician-performed rapid diagnostic assays (fecal leukocyte smear, lactoferrin latex agglutination assay, Campylobacter-specific enzyme immunoassay [EIA], and CRP test) were compared with the “gold standard” stool microbiology assay results. The performance characteristics of each assay were assessed. Test performance characteristics (sensitivity, specificity, predictive values, and likelihood ratios) were evaluated for each clinical finding (such as fever, abdominal cramps, and severe diarrhea) and diagnostic assay with 95% confidence intervals. The probability that a test result would be accurately rated as positive or negative was quantitated by using the area under the receiver operating characteristic curve (ROC) (16). In order to compare the areas under the ROC curves obtained from various diagnostic tests derived from the same cases, an adjustment was made to account for correlations between areas (17). The likelihood ratio was evaluated in context with pre- and posttest probabilities for different scenarios (low- versus high-prevalence region). In addition, clinical findings and diagnostic assays were evaluated singly and in series by using likelihood ratios in order to determine the most accurate and efficient diagnostic algorithm. The results of the patient surveys, symptom diaries, physician findings, and microbiological testing results were entered into EpiInfo software (version 6.04) databases. Statistical analysis was performed with SPSS for Windows (version 10.1). All tests were two tailed, and P values of <0.05 were considered statistically significant.
RESULTS
A total of 182 U.S. military personnel presenting with acute diarrhea were enrolled in the study, and all individuals underwent reference standard testing. All index tests were performed prior to the administration of treatment and concurrent with the reference standard testing, with the exception of testing for ASCs (as described in Materials and Methods). The characteristics of the study population are provided in Table 1. The cases enrolled during the two exercise years had similar age and gender distributions. A higher proportion of individuals received malaria prophylaxis during the first exercise year; however, no difference in the time to presentation with illness, the characteristics of the illness, or the pathogen distribution was observed. Campylobacter was identified in initial cultures of stools from 62% of all cases, with 96% of the organisms found to be C. jejuni and C. coli accounting for the remainder. Salmonella and Plesiomonas were isolated from an additional 10 to 20% of the cases. The high rates of isolation of invasive bacterial pathogens were supported by the frequent clinical features of inflammatory enteritis in >50% of the enrolled cases. A notable exception to this pattern was the relatively low rate of positivity for fecal leukocytes observed in the first exercise year. This observation was not consistent with the results of concurrent fecal lactoferrin testing, which was consistent between exercise years, and likely represents variability in technician interpretation of fecal leukocyte stains.
TABLE 1.
Characteristics of U.S. military personnel presenting with acute diarrhea on deployment in Thailand
| Characteristic | Result in Cobra Gold exercise yr:
|
|
|---|---|---|
| 2000 | 2001 | |
| Thai city (base for training exercise) | Nakhon Sri Thammarat | Phitsanulok |
| Demographics | ||
| No. of enrolled cases | 109 | 73 |
| Median age (yr [IQRa]) | 26 (22-33) | 26 (23-32) |
| Gender (% male) | 90 | 90 |
| Malaria prophylaxis (% of cases)b | 91 | 75 |
| Median no. of days in country preillness (IQR) | 11 (7-16) | 12 (8-15) |
| Clinical presentation | ||
| Median no. of days of illness duration (IQR) | 1 (1-3) | 1 (1-2) |
| Median no. of diarrheal stools before 24 h (IQR) | 5 (4-10) | 5 (3-8) |
| Fever, by report (% of cases) | 51 | 49 |
| Vomiting (% of cases)b | 27 | 11 |
| Abdominal cramps (% of cases) | 89 | 85 |
| Bedside evaluation (% of cases) | ||
| Oral temp ≥100°F | 20 | 28 |
| Stool character, watery liquid | 37 | 41 |
| Visible gross blood | 13 | 14 |
| Stool occult blood testing positive | 35 | 36 |
| Field laboratory evaluation (% of cases) | ||
| Fecal leukocytes positiveb | 28 | 58 |
| Fecal lactoferrin positive | 79 | 80 |
| Serum CRP positive | 70 | NDc |
| Campylobacter EIA positive | 67 | 54 |
| Campylobacter PCR positive | 51 | ND |
| Campylobacter ASC positive | 44 | ND |
| Pathogen isolation (% of cases) | ||
| Campylobacter | 67 | 55 |
| Nontyphoidal Salmonella | 15 | 25 |
| Plesiomonas shigelloides | 9 | 6 |
| Noninvasive bacteria (ETEC, eae-positive E. coli) | 11 | 14 |
| Viral (rotavirus, calicivirus) | 6 | 7 |
| None identified | 17 | 26 |
IQR, interquartile range.
Differences in a given characteristic between exercise years (P < 0.05).
ND, the assay was not done during the exercise year.
Clinical and laboratory findings were evaluated to assess their potential as modalities for the detection of invasive enteropathogens, as well as the diagnosis of Campylobacter infection. The performance characteristics of the tests for the prediction of invasive enteropathogens are detailed in Table 2. In general, clinical findings were not sensitive, with the exception of abdominal cramping; however, this symptom had a specificity of less than 15%. Less frequent clinical findings, such as high-volume diarrhea, gross blood in stools, documented fever at presentation, and occult blood testing-positive stools, did yield greater specificities. However, the overall accuracy of these findings, as represented by the area under the ROC curve, was low (<0.65). The discordant findings in the fecal leukocyte test results between exercise years led to significant differences in test performance determination (Table 2). Despite these differences, the fecal leukocyte test sensitivity was less than 50% in both years. The lactoferrin latex agglutination and plasma CRP tests provided reasonable sensitivities but lacked specificity. Both tests yielded negative likelihood ratios, amenable to ruling out the presence of an invasive enteropathogen.
TABLE 2.
Clinical and laboratory findings as diagnostic modalities for invasive enteropathogensa
| Finding | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) | LR+ | LR− | AUC |
|---|---|---|---|---|---|---|---|
| Diarrhea frequency (past 24 h) | |||||||
| 6-9 loose stools | 52 (43, 60) | 70 (55, 82) | 36 (27, 46) | 82 (71, 89) | 1.7 (1.1, 2.7) | 0.7 (0.5, 0.9) | 0.61 (0.52, 0.70) |
| ≥10 loose stools | 28 (21, 37) | 86 (73, 94) | 32 (24, 40) | 84 (69, 93) | 2.0 (1.0, 4.3) | 0.8 (0.7, 1.0) | 0.57 (0.48, 0.66) |
| Gross blood in stools | 17 (11, 25) | 96 (84, 99) | 29 (23, 37) | 92 (72, 99) | 4.0 (1.0, 16) | 0.9 (0.8, 1.0) | 0.56 (0.47, 0.65) |
| Abdominal cramps | 88 (81, 93) | 14 (6, 28) | 30 (14, 53) | 73 (66, 80) | 1.0 (0.9, 1.2) | 0.9 (0.4, 2.0) | 0.51 (0.42, 0.61) |
| Vomiting (absence of finding) | 24 (17, 33) | 90 (77, 96) | 30 (23, 38) | 87 (70, 95) | 2.3 (1.0, 5.6) | 0.9 (0.7, 1.0) | 0.57 (0.48, 0.66) |
| Fever (by report) | 58 (49, 66) | 69 (54, 81) | 38 (28, 49) | 84 (74, 90) | 1.9 (1.2, 2.9) | 0.6 (0.5, 0.8) | 0.64 (0.55, 0.73) |
| Oral temp ≥100°F | 29 (21, 37) | 92 (80, 97) | 33 (25, 41) | 91 (77, 97) | 3.6 (1.4, 10) | 0.8 (0.7, 0.9) | 0.60 (0.52, 0.69) |
| Occult blood testing positive | 42 (33, 51) | 84 (70, 93) | 34 (25, 43) | 89 (77, 95) | 2.7 (1.3, 5.5) | 0.7 (0.6, 0.8) | 0.63 (0.54, 0.72) |
| Opaque/watery liquid stool | 42 (33, 51) | 69 (54, 81) | 31 (23, 41) | 78 (66, 87) | 1.4 (0.9, 2.2) | 0.8 (0.7, 1.1) | 0.56 (0.46, 0.65) |
| Dysentery/documented fever | 42 (33, 51) | 87 (74, 95) | 35 (27, 45) | 90 (79, 96) | 3.3 (1.5, 7.1) | 0.7 (0.6, 0.8) | 0.64 (0.56, 0.73) |
| Dysentery/documented fever or occult blood testing positive | 57 (48, 65) | 78 (63, 88) | 39 (29, 49) | 88 (79, 94) | 2.6 (1.4, 4.5) | 0.6 (0.5, 0.7) | 0.67 (0.58, 0.76) |
| Fecal leukocytesb | |||||||
| Any positive | 44 (35, 54) | 67 (52, 80) | 32 (23, 43) | 78 (66, 87) | 1.4 (0.9, 2.2) | 0.8 (0.6, 0.2) | 0.58 (0.49, 0.68) |
| ≥1-5/HPF | 33 (24, 42) | 85 (71, 93) | 33 (25, 42) | 84 (70, 93) | 2.1 (1.0, 4.4) | 0.8 (07, 1.0) | |
| Lactoferrin latex agglutination | |||||||
| Any positive | 93 (87, 97) | 56 (41, 70) | 75 (58, 87) | 85 (78, 90) | 2.1 (1.5, 2.9) | 0.1 (0.1, 0.2) | 0.77 (0.68, 0.86) |
| ≥2+ | 86 (79, 91) | 65 (49, 78) | 63 (48, 76) | 87 (80, 92) | 2.4 (1.7, 3.6) | 0.2 (0.1, 0.4) | |
| Serum CRP | 83 (73, 90) | 75 (53, 89) | 56 (38, 73) | 92 (83, 97) | 3.3 (1.7, 6.7) | 0.2 (0.1, 0.4) | 0.81 (0.71, 0.91) |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value; LR+, positive likelihood ratio; LR−, negative likelihood ratio; AUC, area under the ROC curve (AUC values represent true positive rate/true negative rate ratios); HPF, high-power field. The data in parentheses are 95% confidence intervals.
Fecal leukocyte test performance differed significantly between exercise years, as follows, for the ≥1 to 5/high-power field classification (year 1 versus year 2): sensitivity, 28 versus 40%; specificity, 96 versus 75%; negative predictive value, 30 versus 38%; positive predictive value, 95 versus 76%; positive likelihood ratio, 6.1 versus 1.6; and negative likelihood ratio, both 0.8.
Clinical findings and bedside evaluation of the patient's stool specimen were assessed for their ability to support a diagnosis of Campylobacter enteritis (Table 3). A relatively poor test performance comparable to that seen for the other invasive enteropathogens was observed. Given the predominance of Campylobacter in this case series, it is not surprising that the findings were similar. Table 4 provides an assessment of the results of the field laboratory tests for systemic or intestinal inflammation, as well as those of the Campylobacter-specific rapid diagnostic tests. Fecal leukocyte determination and measurement of the circulating lymphocytes producing antibodies against Campylobacter-specific antigens in the enzyme-linked immunospot assay produced poor results in all measures of test performance. The lactoferrin latex agglutination test demonstrated a high sensitivity and a negative predictive value with a low specificity. The overall accuracy of this test was comparable to that of the plasma CRP test.
TABLE 3.
Clinical and bedside stool characteristics as Campylobacter diagnostic modalitiesa
| Finding | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) | LR+ | LR− | AUC |
|---|---|---|---|---|---|---|---|
| Diarrhea frequency (past 24 h) | |||||||
| 6-9 loose stools | 53 (44, 63) | 67 (54, 77) | 47 (37, 57) | 72 (61, 81) | 1.6 (1.1, 2.3) | 0.7 (0.5, 0.9) | 0.60 (0.51, 0.68) |
| ≥10 loose stools | 32 (23, 41) | 87 (76, 94) | 44 (36, 53) | 80 (64, 90) | 2.4 (1.2, 4.7) | 0.8 (0.7, 0.9) | 0.59 (0.51, 0.68) |
| Gross blood in stools | 20 (13, 29) | 97 (89, 100) | 42 (34, 50) | 92 (72, 99) | 6.5 (1.6, 27) | 0.8 (0.8, 0.9) | 0.58 (0.50, 0.67) |
| Abdominal cramps | 89 (82, 94) | 16 (9, 28) | 48 (27, 69) | 64 (56, 71) | 1.1 (0.9, 1.2) | 0.7 (0.3, 1.4) | 0.53 (0.44, 0.62) |
| Vomiting (absence of finding) | 27 (19, 36) | 90 (79, 95) | 42 (34, 51) | 81 (64, 91) | 2.5 (1.2, 5.5) | 0.8 (0.7, 0.9) | 0.58 (0.50, 0.67) |
| Fever (by report) | 64 (54, 72) | 72 (60, 82) | 54 (44, 65) | 79 (69, 87) | 2.3 (1.5, 3.4) | 0.5 (0.4, 0.7) | 0.68 (0.60, 0.76) |
| Oral temp ≥100°F | 33 (24, 42) | 93 (83, 97) | 46 (37, 54) | 88 (74, 96) | 4.5 (1.9, 11) | 0.7 (0.6, 0.8) | 0.63 (0.55, 0.71) |
| Occult blood testing positive | 47 (37, 57) | 86 (74, 93) | 48 (38, 57) | 85 (73, 93) | 3.3 (1.7, 6.2) | 0.6 (0.5, 0.8) | 0.66 (0.58, 0.74) |
| Opaque/watery liquid stool | 45 (36, 55) | 72 (60, 82) | 45 (35, 54) | 73 (60, 82) | 1.6 (1.1, 2.5) | 0.8 (0.6, 1.0) | 0.59 (0.50, 0.67) |
| Dysentery/documented fever | 48 (38, 57) | 89 (79, 95) | 50 (41, 60) | 88 (77, 95) | 4.5 (2.2, 9.3) | 0.6 (0.5, 0.7) | 0.69 (0.61, 0.76) |
| Dysentery/documented fever or occult blood testing positive | 63 (53, 72) | 79 (67, 88) | 55 (44, 65) | 84 (74, 91) | 3.1 (1.9, 5.1) | 0.5 (0.4, 0.6) | 0.71 (0.65, 0.79) |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value; LR+, positive likelihood ratio; LR−, negative likelihood ratio; AUC, area under the ROC curve (AUC values represent true positive rate/true negative rate ratios). The data in parentheses are 95% confidence intervals.
TABLE 4.
Field laboratory tests as Campylobacter diagnostic modalitiesa
| Finding | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) | LR+ | LR− | AUC |
|---|---|---|---|---|---|---|---|
| Fecal leukocytes | |||||||
| Any positive | 45 (35, 55) | 65 (52, 76) | 44 (34, 54) | 66 (53, 77) | 1.3 (0.9, 1.9) | 0.9 (0.7, 1.1) | 0.57 (0.48, 0.66) |
| ≥1-5/HPF | 34 (25, 44) | 82 (70, 90) | 45 (36, 54) | 73 (58, 85) | 1.8 (1.0, 3.3) | 0.8 (0.7, 1.0) | |
| Lactoferrin latex agglutination | |||||||
| Any positive | 96 (91, 99) | 48 (36, 60) | 89 (73, 96) | 75 (67, 82) | 1.9 (1.5, 2.3) | 0.1 (0.1, 0.2) | 0.80 (0.73, 0.87) |
| ≥2+ | 91 (84, 95) | 58 (46, 70) | 80 (65, 89) | 78 (70, 85) | 2.2 (1.6, 2.9) | 0.2 (0.1, 0.3) | |
| Serum CRP | 89 (79, 95) | 69 (51, 83) | 75 (56, 88) | 85 (75, 92) | 2.8 (1.7, 4.6) | 0.1 (0.1, 0.2) | 0.83 (0.75, 0.91) |
| Campylobacter EIA | 95 (88, 98) | 94 (84, 98) | 91 (81, 96) | 96 (90, 99) | 16 (6.0, 40) | 0.1 (0.1, 0.2) | 0.94 (0.89, 0.98) |
| Campylobacter PCR | 82 (65, 92) | 90 (72, 97) | 79 (61, 90) | 91 (75, 98) | 7.9 (2.7, 23) | 0.2 (0.1, 0.4) | 0.86 (0.76, 0.95) |
| Campylobacter-specific ASCs | 46 (55, 74) | 60 (42, 76) | 38 (25, 52) | 68 (52, 81) | 1.2 (0.7, 1.9) | 0.9 (0.6, 1.3) | 0.53 (0.41, 0.65) |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value; LR+, positive likelihood ratio; LR−, negative likelihood ratio; AUC, area under the ROC curve (AUC values represent true positive rate/true negative rate ratios); HPF, high-power field. The data in parentheses are 95% confidence intervals.
The two stool-based Campylobacter-specific tests, PCR and EIA, yielded the highest specificities and positive likelihood ratios of all tests under evaluation. More cases were evaluated by EIA than by PCR, by which the stool specimens were evaluated only in the second exercise year. False-negative results by the PCR assay led to a lower sensitivity than that observed for the EIA. A total of seven culture-positive Campylobacter cases had negative results by the PCR test. The simultaneously tested positive controls included for the detection of DNA in the stool specimen were positive for all but one of the specimens, ruling out the presence of nonspecific inhibitors as the primary explanation for the false-negative results. Of these seven culture-positive, PCR-negative specimens, three were also negative by the Campylobacter-specific EIA. The remaining four specimens had 4+ reactions by the EIA. There were three culture-negative, PCR-positive specimens. Two of these specimens were 4+ positive by the EIA. A total of six culture-positive, EIA-negative specimens were observed. Follow-up EIAs were undertaken for all of these cases by using stool specimens collected either 3 or 7 days after the initial treatment. None of the follow-up EIAs were positive, nor were any of the follow-up stool cultures. Four culture-negative, EIA-positive specimens were observed. Two of these cases had follow-up stool specimens posttreatment in which one was culture negative and EIA negative and the other was culture positive and EIA positive. Stool cultures and EIAs performed 3 and 7 days after administration of the first antibiotic dose detected no new EIA-positive cases. Among the cases initially EIA positive, there continued to be positive responses for 31% at day 3 and 23% at day 7. The semiquantitative test results trended toward a high proportion of 3+ and 4+ results in 80% of pretreatment specimens compared to 57% of posttreatment specimens.
DISCUSSION
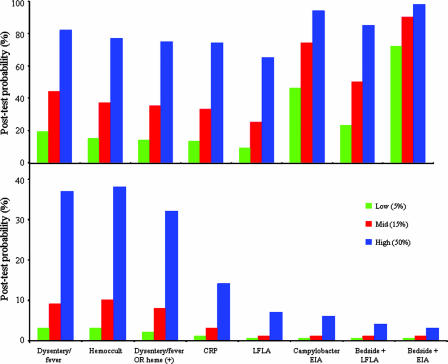
Rapid diagnostic tests range from the inexpensive Gram stain for the presumptive identification of Campylobacter spp. (sensitivity, 60 to 90%) to the more technically complex and more expensive PCR (1, 5, 19, 29, 31). The utility of a diagnostic test is dependent upon the prevalence of the disease in the population. In Fig. 1, the posttest probability of Campylobacter-associated illness is presented in various settings of endemicity and is separated by the effect that a positive or a negative result has on ruling in or ruling out a diagnosis of campylobacteriosis, respectively. The presence of certain clinical features, including gross blood in stools or documented fever at presentation, is very specific (≥93%) for Campylobacter infection; however, this diagnosis will be missed in 70 to 80% of cases. The inability of the clinical presentation to guide therapy for individuals with traveler's diarrhea was documented by Ericsson and coworkers (13). Reliance on specific yet insensitive clinical features would lead to the withholding of therapy in individuals who may benefit from early treatment. The ability of positive test findings to alter the probability of the presence of Campylobacter infection is most effective when a pathogen-specific stool-based test, such as the EIA, is used. In a setting of hyperendemicity, such as Thailand, with prevalence estimates of 50%, a positive EIA result yields a 94% posttest probability of disease. Concurrent findings of dysentery or fever further increase the posttest probability of a positive EIA result to 98%. The impact of a positive EIA result in a region with a lower prevalence of Campylobacter is less dramatic. An estimated prevalence of 5%, as may be observed in U.S. clinics, has a posttest probability of 46% in the event of a positive EIA result. Unlike the setting in Thailand, bedside clinical findings of dysentery or fever provide additive benefit in increasing the probability to 72% in this setting.
FIG. 1.
Posttest probability of Campylobacter-associated illness based on the diagnostic approach used in various settings of endemicity (% prevalence). (Top panel) Results based on a diagnostic approach yielding a positive test finding (rule in disease); (bottom panel) results based on a negative test finding (rule out disease).
The Campylobacter-specific EIA has previously been evaluated with frozen stool sample collections and in clinic-based series in the United States and Europe (6, 18, 41). Positive EIA results have been documented out to 5 years from Campylobacter-positive stool specimens stored at −20°C, with a detection threshold of 3 × 106 CFU/g of stool (12). Real-time assessment under routine conditions has documented a sensitivity of 89% and a specificity of 98 to 99% in settings with a prevalence of Campylobacter ranging from 3 to 8% (6, 18). The positive likelihood ratio exceeded 30 and the negative likelihood ratio was less than 0.15 in both previous evaluations. These findings are comparable to the likelihood ratios observed in this study, which is the first evaluation of this test under field conditions and outside of an established hospital. The ceuE-based multiplex PCR evaluated in this study had previously been evaluated by using frozen stool specimens, with comparisons of the culture and PCR results based on microbiological recovery from stored specimens (21). The previous study documented much higher positivity rates for PCR than for culture (77 and 56%, respectively). On the basis of the test performance observed in this study with fresh specimens, the earlier observation was likely due to the presence of nonviable organisms in frozen specimens rather than a significant difference in the sensitivities of the PCRs. The rates of PCR false-negative results for fresh specimens observed in this study were higher than those observed for the frozen specimens in the previous study (21) (18 and 8%, respectively). This finding has been reported previously and in certain cases has been postulated to be secondary to the presence in the specimen of inhibitors that affect the PCR test (25, 43). This may also be explained by a PCR detection limit in the range of 105 campylobacters per ml stool (33), which may be higher than the detection limits of culture methods. Further development of this test is needed before it may gain clinical utility. The Campylobacter-specific ASC assay lacked test performance parameters supportive of its clinical use. Potential improvements in this test may derive from the use of more purified antigens that are broadly cross-reactive with Campylobacter species yet not cross-reactive with other bacterial enteropathogens; however, the kinetics of the transient circulation of these lymphocytes following mucosal infection may limit the diagnostic potential of this test at the time of clinical presentation (14).
Rapid diagnostic tests based on the detection of an inflammatory state, blood-based CRP, and stool-based lactoferrin rather than on the detection of a specific pathogen were unable to increase the posttest probability beyond 75% in the event of a positive result (Fig. 1). However, the CRP and lactoferrin tests performed well at ruling out campylobacteriosis. A negative finding resulted in posttest probabilities of 14% for CRP and 7% for the lactoferrin assay (Fig. 1) in a setting of hyperendemicity, such as Thailand, which has a 50% prevalence (pretest probability). Given the improved ability to rule out Campylobacter infection and the infrequency with which blood specimens for the clinical management of diarrhea are obtained, the use of the fecal lactoferrin assay was preferable for screening for inflammatory enteritis. These findings are consistent with a systematic analysis of fecal screening tests (22). A meta-analysis stratified fecal screening test performance on the basis of the population studied, that is, populations from resource-poor regions and populations from developed countries, in order to account for differences in pathogen prevalence and disease spectrum (15). In developing countries, the rapid stool-based markers of inflammatory enteritis performed poorly in ruling in disease, possibly due to the high degree of endemicity of enteropathogens, asymptomatic carriage, frequent findings of inflammatory markers, and comorbid noninfectious conditions that may lead to positive findings, as postulated by the authors (15). The fecal lactoferrin assay has demonstrated value as a negative predictor of the presence of invasive enteropathogens in children in the developing world with acute diarrhea (23, 36), as was also shown in the deployed population in the present study. In this study, the fecal lactoferrin test would have failed to identify nine invasive pathogens (7%) or four Campylobacter cases (4%).
The regional predominance of Campylobacter previously documented in Thailand limits the broad application of these results across operational platforms in various regions. The results of the present study, coupled with analyses from an area where ETEC is predominant (with some contribution from Shigella species, which were not observed in this study), will better permit generalization. The Campylobacter-specific EIA provided desirable performance in ruling in or ruling out infection under field conditions, with results available within 2 h. Given the prevalence of Campylobacter in this setting and the high rates of fluoroquinolone resistance, this test most aids the clinician in determining the most appropriate type of patient management.
Acknowledgments
We thank the technicians and staff of the Naval Medical Research Center (Silver Spring, MD) and the Armed Forces Research Institute of Medical Sciences (Bangkok, Thailand) for their microbiology expertise and assistance with the conduct of the study. We also thank the medical staff during the Cobra Gold exercises and the voluntary participation of U.S. military personnel.
This work was supported by Work Unit number 643807A.849.D.A0002.
This study was approved by the ethical review committees of the Naval Medical Research Center (protocol 31528), the Walter Reed Army Institute of Research (WRAIR 792), and the Uniformed Services University of the Health Sciences (G187MT) and complies with all federal regulations governing the protection of human subjects.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the U.S. Department of Defense, or the U.S. government. D. Tribble is an employee of the U.S. government. This work was prepared as part of his official duties. Title 17 U.S.C. 101 defines U.S. government work as work prepared by a military service member or employee of the U.S. government as part of that person's official duties.
Footnotes
Published ahead of print on 30 January 2008.
REFERENCES
- 1.Amar, C. F., C. L. East, J. Gray, M. Iturriza-Gomara, E. A. Maclure, and J. McLauchlin. 2007. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: re-examination of the English case-control Infectious Intestinal Disease Study (1993-1996). Eur. J. Clin. Microbiol. Infect. Dis. 26311-323. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, J. D., P. Echeverria, G. D. Shanks, J. Karwacki, L. Bodhidatta, and J. E. Brown. 1990. A comparative study of gastrointestinal infections in United States soldiers receiving doxycycline or mefloquine for malaria prophylaxis. Am. J. Trop. Med. Hyg. 43608-613. [DOI] [PubMed] [Google Scholar]
- 3.Baqar, S., A. A. Nour El Din, D. A. Scott, A. L. Bourgeois, A. S. Mourad, M. T. Kleinosky, M. J. Oplinger, and J. R. Murphy. 1997. Standardization of measurement of immunoglobulin-secreting cells in human peripheral circulation. Clin. Diagn. Lab. Immunol. 4375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beecham, H. J., III, C. I. Lebron, and P. Echeverria. 1997. Short report: impact of traveler's diarrhea on United States troops deployed to Thailand. Am. J. Trop. Med. Hyg. 57699-701. [DOI] [PubMed] [Google Scholar]
- 5.Collins, E., M. Glennon, S. Hanley, A. M. Murray, M. Cormican, T. Smith, and M. Maher. 2001. Evaluation of a PCR/DNA probe colorimetric membrane assay for identification of Campylobacter spp. in human stool specimens. J. Clin. Microbiol. 394163-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dediste, A., O. Vandenberg, L. Vlaes, A. Ebraert, N. Douat, P. Bahwere, and J. P. Butzler. 2003. Evaluation of the ProSpecT microplate assay for detection of Campylobacter: a routine laboratory perspective. Clin. Microbiol. Infect. 91085-1090. [DOI] [PubMed] [Google Scholar]
- 7.Echeverria, P., C. W. Hoge, L. Bodhidatta, C. Tungtaem, J. Herrmann, S. Imlarp, and K. Tamura. 1994. Etiology of diarrhea in a rural community in western Thailand: importance of enteric viruses and enterovirulent Escherichia coli. J. Infect. Dis. 169916-919. [DOI] [PubMed] [Google Scholar]
- 8.Echeverria, P., L. R. Jackson, C. W. Hoge, M. K. Arness, G. R. Dunnavant, and R. R. Larsen. 1993. Diarrhea in U.S. troops deployed to Thailand. J. Clin. Microbiol. 313351-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Echeverria, P., O. Sethabutr, and C. Pitarangsi. 1991. Microbiology and diagnosis of infections with Shigella and enteroinvasive Escherichia coli. Rev. Infect. Dis. 13(Suppl. 4)S220-S225. [DOI] [PubMed] [Google Scholar]
- 10.Echeverria, P., O. Sethabutr, and O. Serichantalergs. 1993. Modern diagnosis (with molecular tests) of acute infectious diarrhea. Gastroenterol. Clin. N. Am. 22661-682. [PubMed] [Google Scholar]
- 11.Echeverria, P., D. N. Taylor, U. Lexsomboon, M. Bhaibulaya, N. R. Blacklow, K. Tamura, and R. Sakazaki. 1989. Case-control study of endemic diarrheal disease in Thai children. J. Infect. Dis. 159543-548. [DOI] [PubMed] [Google Scholar]
- 12.Endtz, H. P., C. W. Ang, N. van den Braak, A. Luijendijk, B. C. Jacobs, P. de Man, J. M. van Duin, A. van Belkum, and H. A. Verbrugh. 2000. Evaluation of a new commercial immunoassay for rapid detection of Campylobacter jejuni in stool samples. Eur. J. Clin. Microbiol. Infect. Dis. 19794-797. [DOI] [PubMed] [Google Scholar]
- 13.Ericsson, C. D., T. F. Patterson, and H. L. Dupont. 1987. Clinical presentation as a guide to therapy for travelers' diarrhea. Am. J. Med. Sci. 29491-96. [DOI] [PubMed] [Google Scholar]
- 14.Forrest, B. D. 1988. Identification of an intestinal immune response using peripheral blood lymphocytes. Lancet i81-83. [DOI] [PubMed] [Google Scholar]
- 15.Gill, C. J., J. Lau, S. L. Gorbach, and D. H. Hamer. 2003. Diagnostic accuracy of stool assays for inflammatory bacterial gastroenteritis in developed and resource-poor countries. Clin. Infect. Dis. 37365-375. [DOI] [PubMed] [Google Scholar]
- 16.Hanley, J. A., and B. J. McNeil. 1982. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 14329-36. [DOI] [PubMed] [Google Scholar]
- 17.Hanley, J. A., and B. J. McNeil. 1983. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148839-843. [DOI] [PubMed] [Google Scholar]
- 18.Hindiyeh, M., S. Jense, S. Hohmann, H. Benett, C. Edwards, W. Aldeen, A. Croft, J. Daly, S. Mottice, and K. C. Carroll. 2000. Rapid detection of Campylobacter jejuni in stool specimens by an enzyme immunoassay and surveillance for Campylobacter upsaliensis in the greater Salt Lake City area. J. Clin. Microbiol. 383076-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho, D. D., M. J. Ault, M. A. Ault, and G. H. Murata. 1982. Campylobacter enteritis: early diagnosis with Gram's stain. Arch. Intern. Med. 1421858-1860. [DOI] [PubMed] [Google Scholar]
- 20.Houng, H. S., O. Sethabutr, and P. Echeverria. 1997. A simple polymerase chain reaction technique to detect and differentiate Shigella and enteroinvasive Escherichia coli in human feces. Diagn. Microbiol. Infect. Dis. 2819-25. [DOI] [PubMed] [Google Scholar]
- 21.Houng, H. S., O. Sethabutr, W. Nirdnoy, D. E. Katz, and L. W. Pang. 2001. Development of a ceuE-based multiplex polymerase chain reaction (PCR) assay for direct detection and differentiation of Campylobacter jejuni and Campylobacter coli in Thailand. Diagn. Microbiol. Infect. Dis. 4011-19. [DOI] [PubMed] [Google Scholar]
- 22.Huicho, L., M. Campos, J. Rivera, and R. L. Guerrant. 1996. Fecal screening tests in the approach to acute infectious diarrhea: a scientific overview. Pediatr. Infect. Dis. J. 15486-494. [DOI] [PubMed] [Google Scholar]
- 23.Huicho, L., V. Garaycochea, N. Uchima, R. Zerpa, and R. L. Guerrant. 1997. Fecal lactoferrin, fecal leukocytes and occult blood in the diagnostic approach to childhood invasive diarrhea. Pediatr. Infect. Dis. J. 16644-647. [DOI] [PubMed] [Google Scholar]
- 24.Hyams, K. C., A. L. Bourgeois, B. R. Merrell, P. Rozmajzl, J. Escamilla, S. A. Thornton, G. M. Wasserman, A. Burke, P. Echeverria, K. Y. Green, et al. 1991. Diarrheal disease during Operation Desert Shield. N. Engl. J. Med. 3251423-1428. [DOI] [PubMed] [Google Scholar]
- 25.Iijima, Y., N. T. Asako, M. Aihara, and K. Hayashi. 2004. Improvement in the detection rate of diarrhoeagenic bacteria in human stool specimens by a rapid real-time PCR assay. J. Med. Microbiol. 53617-622. [DOI] [PubMed] [Google Scholar]
- 26.Jiang, X., N. Wilton, W. M. Zhong, T. Farkas, P. W. Huang, E. Barrett, M. Guerrero, G. Ruiz-Palacios, K. Y. Green, J. Green, A. D. Hale, M. K. Estes, L. K. Pickering, and D. O. Matson. 2000. Diagnosis of human caliciviruses by use of enzyme immunoassays. J. Infect. Dis. 181(Suppl. 2)S349-S359. [DOI] [PubMed] [Google Scholar]
- 27.Kuschner, R. A., A. F. Trofa, R. J. Thomas, C. W. Hoge, C. Pitarangsi, S. Amato, R. P. Olafson, P. Echeverria, J. C. Sadoff, and D. N. Taylor. 1995. Use of azithromycin for the treatment of Campylobacter enteritis in travelers to Thailand, an area where ciprofloxacin resistance is prevalent. Clin. Infect. Dis. 21536-541. [DOI] [PubMed] [Google Scholar]
- 28.Loeb, M. R., A. L. Zachary, and D. H. Smith. 1981. Isolation and partial characterization of outer and inner membranes from encapsulated Haemophilus influenzae type b. J. Bacteriol. 145596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahendru, M., K. N. Prasad, T. N. Dhole, and A. Ayyagari. 1997. Rapid identification of Campylobacter jejuni strains by polymerase chain reaction & their restriction fragment length polymorphism analysis. Indian J. Med. Res. 1059-14. [PubMed] [Google Scholar]
- 30.Murphy, G. S., Jr., P. Echeverria, L. R. Jackson, M. K. Arness, C. LeBron, and C. Pitarangsi. 1996. Ciprofloxacin- and azithromycin-resistant Campylobacter causing traveler's diarrhea in U.S. troops deployed to Thailand in 1994. Clin. Infect. Dis. 22868-869. [DOI] [PubMed] [Google Scholar]
- 31.Oyofo, B. A., S. A. Thornton, D. H. Burr, T. J. Trust, O. R. Pavlovskis, and P. Guerry. 1992. Specific detection of Campylobacter jejuni and Campylobacter coli by using polymerase chain reaction. J. Clin. Microbiol. 302613-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepys, M. B. 1981. C-reactive protein fifty years on. Lancet i653-657. [DOI] [PubMed] [Google Scholar]
- 33.Persson, S., and K. E. Olsen. 2005. Multiplex PCR for identification of Campylobacter coli and Campylobacter jejuni from pure cultures and directly on stool samples. J. Med. Microbiol. 541043-1047. [DOI] [PubMed] [Google Scholar]
- 34.Petruccelli, B. P., G. S. Murphy, J. L. Sanchez, S. Walz, R. DeFraites, J. Gelnett, R. L. Haberberger, P. Echeverria, and D. N. Taylor. 1992. Treatment of traveler's diarrhea with ciprofloxacin and loperamide. J. Infect. Dis. 165557-560. [DOI] [PubMed] [Google Scholar]
- 35.Riddle, M. S., J. W. Sanders, S. D. Putnam, and D. R. Tribble. 2006. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am. J. Trop. Med. Hyg. 74891-900. [PubMed] [Google Scholar]
- 36.Ruiz-Pelaez, J. G., and S. Mattar. 1999. Accuracy of fecal lactoferrin and other stool tests for diagnosis of invasive diarrhea at a Colombian pediatric hospital. Pediatr. Infect. Dis. J. 18342-346. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez, J. L., J. Gelnett, B. P. Petruccelli, R. F. Defraites, and D. N. Taylor. 1998. Diarrheal disease incidence and morbidity among United States military personnel during short-term missions overseas. Am. J. Trop. Med. Hyg. 58299-304. [DOI] [PubMed] [Google Scholar]
- 38.Sanders, J. W., D. W. Isenbarger, S. E. Walz, L. W. Pang, D. A. Scott, C. Tamminga, B. A. Oyofo, W. C. Hewitson, J. L. Sanchez, C. Pitarangsi, P. Echeverria, and D. R. Tribble. 2002. An observational clinic-based study of diarrheal illness in deployed United States military personnel in Thailand: presentation and outcome of Campylobacter infection. Am. J. Trop. Med. Hyg. 67533-538. [DOI] [PubMed] [Google Scholar]
- 39.Steele, T. W., and S. N. McDermott. 1984. The use of membrane filters applied directly to the surface of agar plates for the isolation of Campylobacter jejuni from feces. Pathology 16263-265. [DOI] [PubMed] [Google Scholar]
- 40.Taylor, D. N., P. Echeverria, O. Sethabutr, C. Pitarangsi, U. Leksomboon, N. R. Blacklow, B. Rowe, R. Gross, and J. Cross. 1988. Clinical and microbiologic features of Shigella and enteroinvasive Escherichia coli infections detected by DNA hybridization. J. Clin. Microbiol. 261362-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tolcin, R., M. M. LaSalvia, B. A. Kirkley, E. A. Vetter, F. R. Cockerill, III, and G. W. Procop. 2000. Evaluation of the Alexon-trend ProSpecT Campylobacter microplate assay. J. Clin. Microbiol. 383853-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tribble, D. R., J. W. Sanders, L. W. Pang, C. Mason, C. Pitarangsi, S. Baqar, A. Armstrong, P. Hshieh, A. Fox, E. A. Maley, C. Lebron, D. J. Faix, J. V. Lawler, G. Nayak, M. Lewis, L. Bodhidatta, and D. A. Scott. 2007. Traveler's diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin. Infect. Dis. 44338-346. [DOI] [PubMed] [Google Scholar]
- 43.Wilson, I. G. 1997. Inhibition and facilitation of nucleic acid amplification. Appl. Environ. Microbiol. 633741-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]