Abstract
Dengue viruses (DV), composed of four distinct serotypes (DV1 to DV4), cause 50 to 100 million infections annually. Durable homotypic immunity follows infection but may predispose to severe subsequent heterotypic infections, a risk conferred in part by the immune response itself. Antibody-dependent enhancement (ADE), a process best described in vitro, is epidemiologically linked to complicated DV infections, especially in Southeast Asia. Here we report for the first time the ADE phenomenon in primary human dendritic cells (DC), early targets of DV infection, and human cell lines bearing Fc receptors. We show that ADE is inversely correlated with surface expression of DC-SIGN (DC-specific intercellular adhesion molecule-3-grabbing nonintegrin) and requires Fc gamma receptor IIa (FcγRIIa). Mature DC exhibited ADE, whereas immature DC, expressing higher levels of DC-SIGN and similar FcγRIIa levels, did not undergo ADE. ADE results in increased intracellular de novo DV protein synthesis, increased viral RNA production and release, and increased infectivity of the supernatants in mature DC. Interestingly, tumor necrosis factor alpha and interleukin-6 (IL-6), but not IL-10 and gamma interferon, were released in the presence of dengue patient sera but generally only at enhancement titers, suggesting a signaling component of ADE. FcγRIIa inhibition with monoclonal antibodies abrogated ADE and associated downstream consequences. DV versatility in entry routes (FcγRIIa or DC-SIGN) in mature DC broadens target options and suggests additional ways for DC to contribute to the pathogenesis of severe DV infection. Studying the cellular targets of DV infection and their susceptibility to ADE will aid our understanding of complex disease and contribute to the field of vaccine development.
Dengue virus (serotypes 1 to 4 [DV1 to DV4]) is a single-stranded positive-polarity RNA virus. Symptomatic infection ranges from a self-limited febrile illness (dengue fever [DF]) to a life-threatening syndrome (dengue hemorrhagic fever/dengue shock syndrome [DHF/DSS]). The pathogenesis of complicated DV infection is not clearly understood, but viral, host, and immune factors likely influence disease severity (12, 18, 48). Antibody-dependent enhancement (ADE) of DV infection is often implicated in the pathogenesis of DHF (24, 36). Presumably, subneutralizing concentrations of a heterologous antibody leads to ADE, which increases the intensity of infection and/or the number and/or types of cells infected, thereby increasing viremia and consequent disease severity (17, 49). DHF is recognized as the best example of in vivo ADE, largely based on key epidemiological studies showing increased risk of DHF in individuals with prior DV infections (6, 46, 49). The hypothesis described above was strengthened by the increased incidence of DHF associated with the recent introduction of DV2 into Cuba (19) after prior remote infection with DV1. DHF/DHS did not occur in individuals with primary infections, e.g., with DV1 or DV2 only. Passive transfer experiments conducted with nonhuman primates showed increased viremia in subsequent DV infections (14, 20); however, DHF has not been reproduced in the nonhuman-primate model (14). Further support for the role of ADE in complex disease is provided by the increased incidence of DHF during primary DV infection in the first year of life in infants, born to DV-immune mothers, who acquire DV antibody across the placenta (23).
One of the main challenges for DV vaccine development is the requirement for concurrent protective immunity to all four serotypes; otherwise, vaccination itself could pose additional risks. There are gaps in our understanding of antibody-mediated entry in susceptible cells and types of sera, antibodies, or other molecules promoting enhancement and downstream functional effects. While new animal models are being developed, we build upon the foundation of epidemiologic data and use in vitro cell-based studies to move forward. Many different primary cells and cell lines are reportedly infected by DV, including monocyte/macrophages, B cells, T cells, endothelial cells, hepatocytes, and neuronal cells (1). A new murine model confirms that both macrophages and dendritic cells (DC) are cellular targets (25). Human DC are the primary cells most susceptible to direct DV infection (unaided by antibody) and are considered early cellular targets (15, 33, 39, 51). The recently identified role of the DC-SIGN (DC-specific intercellular adhesion molecule-3-grabbing nonintegrin) molecule in facilitating viral entry further supports the concept of the involvement of DC in DV infection (37, 45).
Given the likelihood that DC are DV targets, and since DC-SIGN facilitates viral entry, we studied the relationship between DC-SIGN and ADE. We previously reported that immature DC (immDC) did not undergo ADE despite expressing levels of Fc gamma receptors (FcγRs) similar to those expressed by positive control K562 cells (33). We next studied the role of the C-type lectins in entry (45) and questioned whether the abundance of DC-SIGN on immDC overrides the effect of enhancing immune sera, either by preventing ADE altogether or by obscuring its effects. Since DC-SIGN levels are lower on mature DC (matDC), we evaluated their susceptibility to ADE. Fc receptors are identified as key molecules mediating ADE in DV infections (5, 22, 27, 43). We and others have previously shown that human DC mainly express FcγRII (3, 4, 28, 33). In this study, we tested DV-immune sera for a variety of ADE effects in a high-throughput and reproducible assay using relevant primary cell targets for DV infection, including primary DC and other Fc receptor- and non-Fc receptor-bearing cells.
Here we show that matDC display an enhanced infection pattern in the presence of DV-immune serum. We report for the first time that viral output on a per-cell basis is increased dramatically under conditions of ADE. This ADE pattern was detected after down-regulation of DC-SIGN upon DC maturation and requires cell surface expression of FcγRIIa. The data suggest that DV uses at least two routes of entry into the same cell type, depending on the milieu, with different outcomes. We show that the route of viral entry into matDC influences the intensity of cellular infection, viral output, transmissibility, and downstream cytokine secretion.
MATERIALS AND METHODS
Viral stocks and cell lines.
The Burma DV2 isolate S16803 (S. Halstead, personal communication) was used for all experiments. Cell lines bearing FcR included the human erythroleukemic cell line K562, the human monocytic cell line U937, and the human Raji B-cell line (ATCC, Manassas, VA). The K562, U937, and NIH 3T3 transfectants were graciously provided by Vineet Kewalramani (NCI, Ft. Detrick, MD). These were maintained in RPMI supplemented with 10% heat-inactivated fetal calf serum (Gemini Bio-Products, Sacramento, CA) with supplements of 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Quality Biological, Gaithersburg, MD). The non-FcR-bearing murine fibroblast cell line NIH 3T3 (ATCC) was maintained in Dulbecco's modified Eagle's medium (Quality Biological) with 10% heat-inactivated fetal calf serum and the supplements described above.
MAb and DV-immune serum.
Intracellular DV infection was measured by using the 2H2 monoclonal antibody (MAb) (kindly provided by Robert Putnak, WRAIR, Silver Spring, MD), a mouse-specific anti-prM immunoglobulin G2a (IgG2a) that is conserved for serotypes 1 to 4, indicating de novo protein production as described previously (45). We accessed small aliquots of a well-characterized DV1-immune serum collection from an institutional review board-approved Indonesian cohort obtained to study ADE. The DV1-immune sera were tested in a plaque reduction neutralization-70 assay and found to neutralize only DV1 (S.-J. Wu, personal communication). Purified 4G2 (IgG2a) MAb and 3H5 (IgG1) ascites MAb were obtained through the Naval Medical Research Center (Silver Spring, MD). Healthy human AB serum was purchased from Gemini Bio-Products, West Sacramento, CA.
Monocyte isolation.
Primary human monocytes were prepared by using the Dynal monocyte negative isolation kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Briefly, 107 peripheral blood mononuclear cells (PBMC) were incubated with blocking reagent and antibody mix for 10 min at 2°C to 8°C. Depletion Dynabeads (100 μl) were added and incubated for 15 min at 2°C to 8°C. The labeled cells were removed with a magnet (Dynal MPC), leaving untouched, highly purified monocytes (90 to 95% CD14+ as determined by flow cytometry).
Monocyte-derived DC.
PBMC were cultured as described previously (34, 47) with some modifications. PBMC isolated from leukapheresis products from healthy donors (BRT Laboratories, Baltimore, MD) were cryopreserved, allowing repeat experiments. PBMC were adhered to tissue culture dishes for 60 min, and after several RPMI washes, adherent cells were cultured in 10 ml of complete medium (CM) with 2 × 104 U/ml recombinant human granulocyte-macrophage colony-stimulating factor (Fisher Clinical Services, Allentown, PA) and 2 × 104 U/ml interleukin-4 (IL-4) (R&D Systems, Minneapolis, MN) for 7 days at 37°C with 5% CO2. On day 6, 50 μl of MCM mimic (15 μg/ml IL-6 [Peprotech, Rocky Hill, NJ], 500 ng/ml IL-1β, 500 ng/ml tumor necrosis factor alpha [TNF-α] [Sigma, St. Louis, MO], 100 μg/ml prostaglandin E2 [CaymanChemical, Ann Arbor, MI]) was added to mature the cells. The phenotypes of all DC were confirmed by flow cytometry before use. Specifically, DC lack CD3, CD19 or CD20, and CD14 but express high levels of HLA-DR and DC-SIGN. Mature cells additionally express CD25, CD83, and CD86 but much lower levels of DC-SIGN (34, 45).
ADE assay.
DV-immune serum, 4G2 MAb, or 3H5 MAb was serially diluted from 1/10 to 1/163,840 in a volume of 50 μl. Virus, at a multiplicity of infection (MOI) of 1, unless otherwise noted, was placed into the antibody dilution tubes and incubated for 60 min at 37°C with 5% CO2 to allow immune complex formation. The content of each tube was then added to 0.5 × 106 cells and incubated for 2 h. The exposed cells were washed with CM to remove excess DV-immune complexes. The cells were resuspended in CM and incubated for an additional 48 h. Cell viability was checked using trypan blue exclusion at 24 h and by flow cytometry using propidium iodide at 48 h. Tissue culture grade IgG1 (DAKO, Glostrup, Denmark), IgG2a, IgG2b (R&D Systems, Minneapolis, MN), and healthy human sera (Gemini Bio-Products, West Sacramento, CA) were included as additional negative controls for DV-immune sera.
Flow cytometry.
A FACSCalibur instrument (BD Biosciences, San Jose, CA) was used to monitor cell surface staining with a panel of phycoerythrin-conjugated MAb to HLA-DR, CD80, CD86, CD3, CD14, CD20, CD25, CD1a (BD Biosciences, San Jose, CA), and CD83 (Beckman Coulter, Fullerton, CA) and matched isotype controls. For detection of intracellular de novo DV protein production, cells were permeabilized with Cytofix/Cytoperm (BD Biosciences, San Jose, CA) and stained with 2H2 (anti-DV prM MAb) conjugated to AlexaFluor-488 (Invitrogen, Carlsbad, CA) 48 h after viral exposure.
Viral RNA quantification.
A viral RNA standard was prepared by amplifying a 170-bp fragment of the DV2 strain S16803 with primers 5′-AATATGCTGAAACGCGAGAGAAACCGCG-3′ (corresponding to genome position 136 to 163) and 5′-CACCAACAGCAGGGATATTG-3′ (corresponding to genome position 278 to 305). The resulting PCR product was ligated into a TA cloning vector using the pGEM-T Easy system (Promega, Madison, WI) and the sequence was confirmed using the BigDye Teminator cycle sequencing kit (Applied Biosystems, Foster City, CA). Plasmid DNA was linearized with EcoRI and RNA transcripts were generated using Megascript kit (Ambion, Austin, TX) according to the manufacturer's specifications. The concentration of transcribed RNA was estimated by UV spectrophotometry. Primers (Den-IF [5′-GCTGAAACGCGAGAGAAACC-3′] and Den_IR [5′-CAGTTTTAATGGTCCTCGTCCCT-3′]) and probe (Den_PR [5′FAM-CATTCCAAGTGAGAATCTCTTTGTCAACTGTTGT-BHQ1-3′ {where FAM refers to 6-carboxyfluorescein and BHQ1 refers to black hole quencher 1}]) were designed to target the region of the DV2 capsid gene which is highly conserved among the four DV but not in other flaviviruses (50). Amplification was performed using an ABI Prism 7700 or 7500 detection instrument (Applied Biosystems, Foster City, CA). The reverse transcription-PCR thermal cycles were performed as follows: 50°C for 30 min, 95°C for 15 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min. RNA copy numbers were calculated from a standard curve generated by an in vitro-transcribed RNA standard.
Vero cell plaque assay.
The Vero cell plaque assay was performed as described previously (11). Six 10-fold serial dilutions (10−1 to 10−6) were made from each supernatant sample and inoculated into quadruplicate wells of six-well tissue culture plates containing confluent Vero cell monolayers. After virus adsorption for 1 h, the Vero monolayer was overlaid with complete minimal essential medium (Cellgro, Manassas, VA) containing low-melting-point agarose (Invitrogen, Carlsbad, CA) to restrict dissemination of progeny virions. The cells were incubated for 5 days at 37°C for 5 days and overlaid with the vital stain neutral red (Sigma, St. Louis, MO). Plaques were counted by visual inspection at 24 h after neutral red overlay to determine the number of PFU of DV per milliliter of supernatant.
Measurement of cytokine levels.
Cytokines were measured in cell-free supernatants by using the cytometric bead array Flex set (BD Biosciences, San Jose, CA) per the manufacturer's instructions. Briefly, Multiscreen 1.2-μm hydrophilic filter plates (Millipore, Bedford, MA) were prewet with wash buffer and aspirated. Capture beads for each of the four examined cytokines (IL-6, IL-10, gamma interferon [IFN-γ], and TNF-α) were combined with 50 μl of supernatant obtained from the ADE experiments. The plate was incubated for 1 h at room temperature. Phycoerythrin detection reagent for each cytokine was pooled and added to the wells. The plate was incubated for 2 h at room temperature. Following incubation, the plate was washed and the beads were resuspended in wash buffer. Samples were read on an LSRII flow cytometer and analyzed using FCAP array software (BD Biosciences, San Jose, CA).
Blocking of FcγRs on matDC.
matDC were pretreated with 10 μg/ml anti-human FcγIIa blocking antibody (clone IV.3; ATCC, Manassas, VA), anti-human FcγIIb blocking antibody (clone 2B6; kindly provided by Macrogenics, Rockville, MD) or IgG1, IgG2a, or IgG2b controls for 1 h at 37°C. Treated cells were washed twice with CM before use in ADE assays.
UV-irradiated DV preparation.
The DV viral stock was placed in a petri dish and exposed to short-wavelength UV light (540 nm) for 20 min. The lack of infectivity of UV-exposed DV was confirmed in highly susceptible DC-SIGN-tranfected Raji cells (Raji-DS cells) before use in the ADE assay.
Statistics.
The Student paired t test, Spearman's rank test, Mann-Whitney U test, and use of nonlinear-fit one-phase exponential decay for curve fitting were applied to the data with Prism software (GraphPad Software Inc., San Diego, CA).
RESULTS
A flow cytometry assay shows anti-DV1 immune serum enhancement of DV2 infection.
We refined a flow cytometry assay that permits high-throughput, quantitative, and reproducible assessment of the in vitro ADE phenomenon (33). As in earlier studies (16, 24, 36), we focused on two assay parameters: peak enhancement titer (PENT) and power of enhancement (Fig. 1A). We tested ADE in K562 cells by using the following antibodies: 4G2, a broadly reactive flavivirus MAb; 3H5, a DV2 envelope-specific MAb; and a well-characterized DV1-immune serum collection. We used a 2H2-AlexaFluor-488 conjugate to detect de novo intracellular DV prM antigen production as described previously (45). The DV-immune serum generally mediated an infection rate at the PENT at least twofold higher than that mediated by 4G2, while ADE was not observed with 3H5 (Fig. 1B). The concentration of 4G2 at the PENT ranged from 6.25 to 0.4 μg/ml. Similar results were observed using U937 cells (16; data not shown). Thus, we routinely used 4G2 to screen for ADE, conserving precious immune sera and accruing experience with a prediction model. Next, we looked for ADE in a variety of cell lines (U937, 3T3, and K562) with or without DC-SIGN transfection to test the role of DC-SIGN. The U937 and K562 wild-type (WT) cell lines were previously shown to undergo ADE (16, 24, 30, 33). All cells were phenotypically characterized by use of flow cytometry prior to virus exposure (Fig. 1C, insets). In the absence of enhancing antibodies, the flow cytometric assay showed very low baseline levels of infection in U937 WT cells (1%) (Fig. 1C, upper left panel). With subneutralizing concentrations of immune serum, U937 WT cells did undergo ADE (peak infection rate of 8.6%, as shown in Fig. 1C, upper left panel). Interestingly, we did not detect DV infection in NIH 3T3 WT cells under any experimental conditions, a cell line without Fc receptors (Fig. 1C, upper right panel). Under conditions of high DC-SIGN expression, high baseline infection rates were observed for both DC-SIGN-transfected U937 (U937-DS) and NIH 3T3 (3T3-DS) cell lines, i.e., 38% and 47% 2H2 expression, respectively (Fig. 1C, lower panels). However, ADE was not observed for cells with high levels of DC-SIGN at any immune serum dilution, suggesting that high levels of this molecule obscured the ADE phenomenon.
FIG. 1.
ADE of DV infection. (A) Schematic diagram of flow cytometry-based ADE assay results. The control value is the percentage of DV-infected cells in the absence of DV-immune serum (baseline infection). The PENT is the dilution at which the maximum infection rate occurs for the tested serum. The power is the ratio of the percent infection rate at the PENT divided by the percent infection rate at the control titer. (B) Comparison of ADE effects of DV2 in K562 cells by using anti-DV1 DV-immune serum, two commercially available anti-DV antibodies, 4G2 and 3H5, and healthy human IgG. Three independent experiments were performed in triplicate, and data shown are the means ± standard deviations for all three experiments. (C) Infection of U937 WT and 3T3 WT cells (upper panels) and U937-DS and 3T3-DS cells (lower panels) by DV2 S16803 (MOI = 1). The solid line represents the infectivity with DV-immune serum, and the dashed line represents infectivity without DV-immune serum in one experiment representative of three independent experiments. Surface expression levels of DC-SIGN for each cell line as measured by flow cytometry are shown as insets in the upper panels.
DC-SIGN levels influence ADE.
We next asked whether ADE occurred in the presence of lower levels of DC-SIGN in the Fc-bearing K562 cells. We compared the infection rates for K562 cells expressing high (Hi) and low (Lo) levels of DC-SIGN or not expressing DC-SIGN (WT). K562-Hi cells were 98% positive and showed a mean fluorescence intensity (MFI) of 331, and K562-Lo cells were 36% positive with an MFI of 88 for surface DC-SIGN expression (Fig. 2A). As expected, the K562 WT cells undergo ADE with a peak infection rate of 17.2% (power = 30) at a PENT of 1:640 (Fig. 2B, left column). Interestingly, K562-Lo cells showed enhancement, albeit at a lower power, with a peak infection rate of 11.6% (power = 8), and a PENT shift from 1:640 to 1:160 (Fig. 2B, middle column). Although K562-Hi cells showed the highest baseline infection (24.9%) without immune serum, we did not observe ADE at any tested serum dilution (Fig. 2B, right column). There is a strong negative correlation between surface levels of DC-SIGN and enhancement power (r = −0.98; P < 0.0001) as determined by Spearman's rank correlation (Fig. 2C, see the inset for the nonlinear-fit curve).
FIG. 2.
ADE in cell lines as a function of DC-SIGN expression. (A) DC-SIGN surface expression on K562 WT (shaded histogram), K562-Lo (dashed line), and K562-Hi (solid line) cells measured by using flow cytometry. (B) ADE patterns obtained from infection of K562 WT (left column), K562-Lo (middle column), and K562-Hi (right column) cells with or without serial dilutions of DV-immune sera. SSC, side scatter. (C) Percent surface expression of DC-SIGN (bars) versus power of enhancement (solid line) for K562 WT, K562-Lo, and K562-Hi cells. The inset graphs the power and the DC-SIGN MFI for each of the three cell types in three independent experiments, and the correlation (r2 = 0.96) was determined using a nonlinear curve fitting algorithm. Data shown are the means ± standard deviations from three independent experiments for each cell line.
ADE was observed in monocytes and matDC but not in immDC.
We extended the study of ADE to more physiologically relevant Fc receptor-bearing primary cells. Previous work showed that monocytes, but not autologous immDC, demonstrated ADE (33). Here we concurrently assessed ADE in immDC, matDC, and monocytes from multiple autologous donors. Figure 3A shows the relative levels of DC-SIGN expression on the three cell types from a representative donor. matDC express approximately 50% less DC-SIGN than immDC do, and as expected, monocytes do not express this molecule (Fig. 3A and B). We detected ADE in both monocytes (power, ∼10) and matDC (power, ∼2.5) (Fig. 3C). Despite the apparent lower power of enhancement in matDC, the overall enhancement effect was dramatic because the baseline infection rate for matDC is at least one-log-fold higher than that for K562 cells or monocytes (Fig. 3C, left and middle panels). No disease enhancement in immDC was observed under any conditions tested (Fig. 3C, right panel). Next, we extended the study of ADE by using additional samples of DV1-immune sera (n = 5) complexed with DV2 in immDC and matDC prepared from five different blood donors. A 5-by-5-by-2 matrix (sera by donors by cell types) was planned, although only 46/50 data points (92%) were collected, due to limited cell availability from one donor. matDC from all donors demonstrated ADE, to different degrees (power range, 2 to 10; mean, 4.4) (Table 1). Peak enhancement occurred at serum dilutions ranging from 1:640 to 1:2,560 (Table 1). Representative data from a single donor are shown in Fig. 3C. Although neutralization was seen at a dilution of 1:10 and a higher infection rate was shown for immDC in the absence of immune sera, ADE was not observed at any titer of immune sera (Fig. 3C, right panel).
FIG. 3.
Analysis of ADE pattern in monocytes, immDC, and matDC. (A) DC-SIGN surface expression on immDC, matDC, and monocytes from a single representative donor. (B) MFI of surface DC-SIGN expression from paired immDC and matDC (n = 5). (C) Infection and ADE patterns obtained for monocytes, immDC, and matDC prepared from a single representative donor in the absence or presence of DV-immune serum. All three cell types (monocyte, immDC, and matDC) were tested in three independent experiments with three different donors.
TABLE 1.
Enhancement of DV2 infection by anti-DV1 immune serum in multiple donorsd
| Serum no. | ADE observed (peak enhancement titer)a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BC266
|
BC284
|
BC287
|
BC291
|
BC295
|
||||||
| immDC (25 ± 1.5)b | matDC (10 ± 2.1)b | immDC (28 ± 2.1)b | matDC (12.7 ± 2.7)b | immDC (17 ± 2.0)b | matDC (8.2 ± 1.5)b | immDC (30 ± 1.8)b | matDC (7.7 ± 2.3)b | immDC (17, 22)c | matDC (10, 7.4)c | |
| 94501283 | − | ++ (640) | − | + (640) | − | + (640) | − | + (2,560) | − | + (640) |
| 94501286 | − | + | − | + (640) | − | ± (2,560) | − | ++ (640) | NT | NT |
| 94501310 | − | ++ | − | + (2,560) | − | ± (640) | − | + (2,560) | − | + (640) |
| 94501326 | − | ± | − | + (2,560) | − | + (640) | − | ± (2,560) | NT | NT |
| 94501333 | − | ++ | − | + (640) | − | + (640) | − | + (2,560) | NT | NT |
ADE responses are indicated as ++(power, >5), +(power, 2 to 5), ±(power, <2), or −(no ADE observed).
The numbers represent the mean percent baseline infection rate (no sera) ± standard deviation calculated from at least three independent infections from each donor.
The two numbers represent the percent baseline infection rates (no sera) from two independent infections.
We planned a 5-by-5-by-2 matrix to test five donors, with five sera across a dilution range (1:10 to 1:163,840), comparing two different cell types (immDC and matDC) for a total of 50 data points. Due to limited cell availability, only 46 of 50 data points are shown. NT, not tested. Experiments were repeated if cells and sera permitted (serum 94501283 was used in repeated experiments for all five donors, and serum 94501333 was used in repeated experiments for four donors).
ADE in matDC increases viral output and proinflammatory cytokines.
Results from our ADE assay demonstrated enhanced infection of DV2 under the influence of DV1 immune serum in matDC. We next looked for changes in viral production by using absolute quantitative real-time PCR to measure the viral RNA copy number in the culture supernatants from the ADE assays. We observed a 100-fold increase in viral RNA production in the comparison of baseline infection (no immune serum) to infection at the PENT (2.1 × 106 versus 5.6 × 108) (Fig. 4A). This result indicated that viral production was also enhanced by DV-immune serum. Parallel studies were performed to further investigate if the released virions in these supernatants retained infectivity. Raji-DS cells are highly susceptible to DV infection and were used previously in a standardized DV neutralization assay (35). To assess infectivity, Raji-DS cells were exposed to the supernatants collected from each condition in the ADE assays in matDC (Fig. 4B). The pattern of Raji-DS infection after exposure to the supernatants correlates closely with the enhancement effects observed in matDC. A similar infectivity pattern was observed with the standard Vero cell plaque assay (Fig. 4C). We next asked whether ADE in matDC was accompanied by cytokine release, as cytokine storms are implicated in the pathogenesis of DHF/DSS (40). We measured the cytokine levels in culture supernatants from matDC exposed to serum alone, DV alone, and different concentrations of DV-immune serum complexes. Increased levels of TNF-α and IL-6 coincided with the PENT (Fig. 4D). IL-10 and IFN-γ were undetectable under any conditions tested (data not shown).
FIG. 4.
Increased viral production and proinflammatory cytokines with ADE in matDC. (A) Detection of viral output in supernatant using quantitative real-time PCR compared with intracellular viral antigen detection using 2H2 MAb in matDC undergoing ADE. (B) Culture supernatants collected from matDC undergoing ADE (as described for panel A) were tested for productive infection by culturing with Raji-DS cells (filled circles). (C) Supernatants from matDC described for panel A were tested in parallel in Vero cell plaque assays to confirm Raji-DS infectivity data. (D) Enhanced proinflammatory cytokines (TNF-α and IL-6) were detected in culture supernatants from matDC undergoing ADE only at enhancement titers. The dashed line indicates the lower limit of detection for the assay (20 pg/ml). Results from an experiment representative of four independent experiments performed in triplicate are shown. All data points shown are means ± standard deviations. Similar cytokine production patterns were obtained from five donors tested across five different sera demonstrating DV ADE (Table 1).
FcγRIIa mediates ADE in matDC.
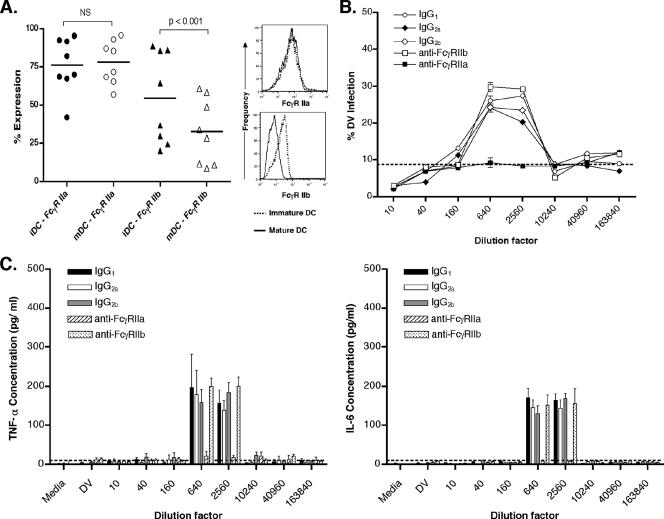
FcγRs reportedly mediate ADE in monocytes/macrophages and cell lines (43). FcγRs are expressed constitutively on monocyte-derived DC, and FcγRII expression predominates over FcγRI or FcγRIII in both immDC and matDC. (2, 4, 33). Interestingly, DC express both FcγRIIa, with an intracytoplasmic activation internalization motif, and FcγRIIb, with an inhibitory motif. Figure 5A shows the percent expression of FcγRIIa and FcγRIIb in paired DC donors. FcγRIIa levels were maintained throughout DC maturation, but FcγRIIb was significantly down-regulated (P < 0.001) (the histogram in Fig. 5A shows results for a representative donor), consistent with previous reports (4, 28). Because K562 cells express only FcγRIIa, we suspected, and confirmed with blocking studies, that it was the sole mediator of ADE in K562 cells (data not shown). We next studied the roles of FcγRIIa and FcγRIIb on ADE in matDC. We pretreated matDC with MAb against either FcγRIIa or FcγRIIb, before subjecting them to the ADE assay. matDC treated with the FcγRIIb-specific MAb or the IgG1, IgG2a, and IgG2b control antibody exhibited similar ADE patterns (Fig. 5B). However, the ADE effect was abrogated when matDC were pretreated with the FcγRIIa-specific MAb. Finally, we tested the supernatants collected from these blocking studies for cytokine production (Fig. 5C). As expected, TNF-α and IL-6 were detected in the control antibody and the FcγRIIb studies at the PENT, but no cytokines were detected if ADE was inhibited with FcγRIIa-blocking MAb.
FIG. 5.
Influence of FcγRIIa on ADE in matDC. (A) Scatter plots show the percentages of FcγRIIa-positive cells and FcγRIIb-positive cells in immDC (iDC) and matDC (mDC) from eight paired donors. The black horizontal lines represent means. FcγRIIb expression decreases significantly between immDC and matDC (paired t test; P < 0.001). The histogram shows a single representative donor's changes in FcγRIIa and FcγRIIb expression levels with maturation. (B) Infection and ADE pattern in matDC treated with control IgG1, control IgG2a, control IgG2b, specific anti-FcγRIIa MAb, and anti-FcγRIIb MAb. The dashed line represents the baseline infection rate without DV-immune serum. (C) TNF-α (left panel) and IL-6 (right panel) in matDC (white bar) undergoing ADE, under the influence of control IgG1, IgG2a, IgG2b, specific anti-FcγRIIa MAb, and specific anti-FcγRIIb MAb. The dashed line indicates the lower limit of detection for the assay (20 pg/ml). Results of one experiment representative of three independent experiments using three donors with two different DV-immune sera are shown. The experiments were performed in triplicate and the bars represent means ± standard deviations.
Cytokine production required active virus.
We questioned whether the cytokine production detected at the PENT required live, replicating virus or was simply a result of FcR cross-linking by DV-immune complexes. DV2 was inactivated with UV irradiation before use in the ADE assay. Figure 6A shows loss of infectivity (and no ADE) when UV-irradiated DV2 was used, while the active virus showed enhancement in matDC. The supernatants from matDC infected with active virus contained TNF-α and IL-6 but not IL-10 or IFN-γ at the enhancement titer, while UV-irradiated DV2 did not elicit production of these cytokines (Fig. 6B). These results suggest that viral replication after antibody-enhanced entry, and not solely FcR cross-linking, is required for enhanced cytokine production. In the absence of DV-immune sera, DC-SIGN facilitates viral entry into DC (Fig. 6C, left panel). TNF-α and IL-6 levels were detectable, but there was no consistent increase in cytokines with higher infection rate's under these conditions (Fig. 6C, right panel). Next, we conducted a head-to-head comparison of the infection rates, cytokine production levels, and infectivities of progeny virions from supernatants of matDC undergoing ADE or a matched direct infection (no sera) by using a higher MOI. Similar infectivity rates were observed when matDC were infected with high inocula (MOI = 5) or an MOI of 1 plus 1/640 immune serum at the PENT (Fig. 7A). The MFIs of intracellular 2H2 levels, the levels of de novo DV antigen production with a high MOI (5) and an MOI of 1 plus 1/640 immune sera (209.1 ± 21.7 and 196.3 ± 16.1, respectively), were comparable as well (data not shown). However, the same patterns of elevated IL-6 and TNF-α were observed under ADE conditions (Fig. 7B), indicating that the cytokine signaling component required both live DV and DV-immune sera at subneutralizing concentrations. Finally, in a Vero cell plaque assay comparing the supernatants, we found that virions released from matDC under ADE conditions (MOI of 1 plus 1/640 serum) and those inoculated at an MOI of 5 showed similar levels of infection (plaques) in Vero cells (5.9 × 107 versus 8.0 × 107 PFU/ml, respectively) (Fig. 7C). However, the infections with matched MOI (i.e., MOI of 1) with or without DV-immune sera showed a substantial 2.3-log increase in ADE viral output (2.6 × 105 versus 5.9 × 107 PFU/ml). These data indicate that while the percentage of DC infected increased threefold (from 9 to 25% 2H2 positive) (Fig. 7A) under ADE conditions, there was a much greater output of virus on a per-cell basis (1.5- to 2.0-log-fold increase in plaque formation and viral copy number).
FIG. 6.
ADE requires active virus. mDC, matDC. (A) Percent infection in ADE assay in matDC using active DV2 versus UV-irradiated DV2. (B) Cytokine production (TNF-α [left panel] and IL-6 [right panel]) in ADE assay supernatants with active DV2 versus UV-irradiated DV2. (C) DV infection without DV-immune serum (left panel) in matDC alone and matDC treated with 10 μg/ml anti-DC-SIGN MAb. TNF-α and IL-6 production from matDC and anti-DC-SIGN-treated matDC (right panel). Experiments were performed in triplicate, and results are expressed as means ± standard errors of the means for three different donors. The dashed line indicates the lower limit of detection for the assay (20 pg/ml).
FIG. 7.
Comparison of infection patterns in the presence and absence of DV-immune serum. mDC, matDC. (A) Percent DV infection under the influence of DV-immune serum at the PENT and without immune sera (MOI of 1 or 5). (B) TNF-α (white bar) and IL-6 (gray bar) production from matDC under ADE conditions and without DV-immune serum. (C) Vero cell plaque assay from supernatants of matDC undergoing ADE (MOI of 1 plus 1/640 sera) or inoculated with DV2 at an MOI of 1 or 5. Experiments were performed in quadruplicate, and results are expressed as means ± standard errors of the means for three different donors.
DISCUSSION
This is the first report of ADE in human primary DC. Prior studies emphasized the high susceptibility of DC to DV infection, largely owing to their very high levels of DC-SIGN expression (29, 37, 45). Here we show an inverse correlation between DC-SIGN expression levels and ADE effects when tested in an array of cell types, both cell lines and primary cells, including monocytes, immDC, and matDC. ADE was not observed in the presence of high DC-SIGN levels, as in K562-Hi and U937-DS cells (Fig. 1 and 2) or immDC (Fig. 3). The negative effect of DC-SIGN on ADE was best demonstrated using K562 cells expressing various levels of DC-SIGN, as shown in Fig. 2B. K562 WT cells naturally undergo ADE (33), with the maximal rate of ADE of infection observed here being about 17%. Low levels of DC-SIGN (K562-Lo) reduced the peak infection rate by about half. High levels of DC-SIGN (K562-Hi) effectively obscured ADE (maximal infection rate of 20%). These results suggest (i) that the virus preferentially uses DC-SIGN when sufficient levels are present and (ii) that Fc receptor-mediated entry is not operational under conditions of high levels of DC-SIGN. This effect of high DC-SIGN levels obscuring ADE was recently reported for another flavivirus infection model (14, 41).
The present data reveal in vitro ADE susceptibility in an important target cell of DV infection, primary matDC. The significance of this observation lies in the fact that DC are increasingly recognized as early targets in infection (25, 37, 51). DC show uniquely high susceptibility to DV infection in the absence of serum (51), generally 20 to 50% baseline infection rates (Fig. 3C). The demonstration of enhancement in matDC suggests an even greater role for viral infection within this cell compartment. While we did not observe ADE in immDC here, consistent with prior work (33), we know these cells express high levels of DC-SIGN. Thus, the effect of DC-SIGN observed in cell lines was confirmed in relevant primary human cells.
Increased infections under the influence of immune sera were consistently observed for matDC, with some donor-to-donor variability in power (2- to 10-fold increases in infection; Fig. 3C and Table 1). These variations could be influenced by genetic polymorphisms between donors. For example, polymorphisms of CD209 (DC-SIGN) were shown to be associated with severity of dengue disease (44). While the 2- to 10-fold increases in usual ADE effects may seem small, the baseline infection rates of DC must be taken into consideration. It is helpful to consider the area under the curve and the large increase in cellular infection in the presence of a subneutralizing concentration of antibody. The disease status of serum donors also may have contributed to the different levels of enhancement. Further work using different sources of immune sera and different virus serotypes will directly address this question.
We showed associated ADE effects, including a more productive infection of matDC, as judged by the accumulation of disproportionately higher copy numbers of viral RNA (2-log increase, for threefold power) and the release of infectious virus into cell supernatants (Fig. 4). The ability of DV-immune sera to modulate infection rates for matDC expands the potential role of DC in dengue pathogenesis. immDC, bearing high levels of DC-SIGN, can act as early direct targets for DV independent of antibody effects. Under the influence of DV-immune serum, modeling secondary infection, matDC could become a viral factory, especially in the presence of waning or fluctuating concentrations of heterologous antibody.
In addition to high levels of viremia, cytokine cascades are thought to contribute to severe dengue disease (8, 13, 42). Induction of cytokine signaling was recently reported in postentry events of DV infection under ADE conditions in monocytic cell lines (7, 33). Interestingly, in our study, TNF-α and IL-6 were detected only at PENT in matDC (Fig. 4D, 5C, 6B, and 7B). Despite similar levels of infection in the absence of immune sera (baseline infection) when a higher MOI was used, minimal levels of cytokines were detected, suggesting additional signaling in postentry ADE in primary DC. This trend of cytokine production correlates closely with other ADE effects measured by viral antigen production, accumulation of viral RNA in supernatants, and the transmissibility of infection to Raji-DS cells via supernatants and in classic Vero cell-based plaque assays. Of note, matDC typically produce no cytokines or much lower levels of cytokines than immDC infected with DV (21). The ability of DV-immune sera to increase infection rates and viral and proinflammatory cytokine production levels in matDC suggests additional viral entry mechanisms (e.g., mediated by FcR) with different signaling components and downstream functional effects.
Fc receptors are often implicated in ADE infection (5, 15, 30, 31). DC constitutively express these receptors, predominantly FcγRII (4, 27, 28, 33). Our study examined the role of two different forms of FcγRII expressed on DC in ADE: the activating (FcγRIIa) and inhibitory (FcγRIIb) isoforms (Fig. 5A and B). We and others (4, 28, 41) reported that the ratio of FcγRIIa to FcγRIIb is increased in matDC and propose that these changes regulate DC function and control cellular responses (9, 10, 38). Blocking experiments (Fig. 5B) using specific anti-FcγRIIa or -FcγRIIb MAb illustrate the critical function of FcγRIIa on ADE in matDC. We observed a similar role for FcγRIIa in ADE of K562 cells, as these cells express only FcγRIIa (data not shown). This raises the possibility that, in addition to DC-SIGN down-regulation, increasing the FcγRIIa/FcγRIIb ratio facilitates ADE in DV infection in matDC. DC maturation may have independent effects not studied here that play a role in facilitating ADE. Additionally, FcγRIIa blocking also clearly inhibited TNF-α and IL-6 production at enhancement titers (Fig. 5C), suggesting that FcγRIIa mediated entry of DV-immune complexes leads to signaling for cytokine production. Cytokine storms are linked to disease severity in severe DV infections, e.g., high serum levels of TNF-α in patients suffering from DHF/DSS (52). Identifying a new cellular compartment susceptible to ADE with a capacity for increased viral replication and proinflammatory cytokine release at PENT links key pathogenesis concepts.
The cytokine signaling cascade could be triggered simply as a result of cross-linking of FcγRs. However, we identified a requirement for active virus to elicit cytokine production that is biologically plausible and supported in the literature (26, 31, 32). UV-irradiated DV did not cause ADE nor induce cytokine production (Fig. 6). Furthermore, comparable levels of infection of matDC unaided by antibody with higher-input virus, a DC-SIGN-mediated process, did not elicit such cytokine responses (Fig. 6C). Therefore, we propose that the ADE phenomenon described here as measured by increased de novo DV antigen production and cytokine release requires viral replication after antibody-facilitated entry, not solely FcγR cross-linking. Importantly, these results show the versatility of DV to exploit multiple routes to gain access to the same cellular target.
Limiting access to cellular targets during DV infection is a reasonable goal for a DV vaccine. Therefore, identifying conditions favoring ADE would contribute to the field of vaccine development. Data presented here substantiate a major role for FcRγIIa in ADE in matDC. Though not formally studied here, our results raise the possibility that different isotypes and relative affinities of these isotypes for FcγRII could be further investigated for differential enhancement potential (e.g., 4G2-positive control is an IgG2a isotype, and both negative controls 3H5 and control sera are IgG1 isotypes). Our results with the DV2-specific 3H5 ascites (no ADE) differ from those from an early report (27) showing 3H5 enhancement of DV2 infection in K562 cells (Fig. 1B). There may be differences in the actual cell line used in this early study given the very high baseline infection rate in their K562 cells (10 to 20%) compared to the typical baseline infection rate reported for the K562 cells, <0.5% (16, 33). In addition, we used a different strain of DV2 in our study, and the type of virus may play an important role. Well-controlled blocking studies whose results are shown in Fig. 5B indicate that DV specificity is required for antibody-mediated enhancement.
We propose that the K562 DC-SIGN model can be readily used to study serum, antibody, or other immunologic effects (e.g., complement) for DV vaccine advancement. We now routinely monitor ADE by using an automated plate-based assay run in replicate with excellent reproducibility. We are currently evaluating ADE in primary DC by using all four DV serotypes with additional sources of immune sera, as this pilot study focused only on the effects of anti-DV1 immune sera against DV2 infection. While this infection sequence (DV1 followed by DV2) is a recognized risk for predisposition to DHF (18, 20, 48), much more information will be gained by studying different virus sequences, serotypes, and serum sources to understand and mitigate risks for immune enhancement of disease.
Acknowledgments
This work was supported by the Pediatric Dengue Vaccine Initiative and in part by the cooperative agreement DAMD17-98-2-8007 between the U.S. Army Medical Research and Materiel Command, the Henry M. Jackson Foundation for the Advancement of Military Medicine, and the Military Infectious Disease Research Program. K.B. and P.P. were financially supported by the Thailand Research Fund through Thai Royal Golden Jubilee Ph.D. Program.
The views and opinions expressed herein are those of the authors and do not purport to reflect the official policy or position of the Department of Defense.
We appreciate the critical review of the manuscript and provision of helpful comments by Mark DeSouza, AFRIMS, Bangkok, Thailand.
Footnotes
Published ahead of print on 13 February 2008.
REFERENCES
- 1.Anderson, R. 2003. Manipulation of cell surface macromolecules by flaviviruses. Adv. Virus Res. 59229-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajtay, Z., E. Csomor, N. Sandor, and A. Erdei. 2006. Expression and role of Fc- and complement-receptors on human dendritic cells. Immunol. Lett. 10446-52. [DOI] [PubMed] [Google Scholar]
- 3.Bergtold, A., D. D. Desai, A. Gavhane, and R. Clynes. 2005. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity 23503-514. [DOI] [PubMed] [Google Scholar]
- 4.Boruchov, A. M., G. Heller, M. C. Veri, E. Bonvini, J. V. Ravetch, and J. W. Young. 2005. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J. Clin. Investig. 1152914-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, M. G., C. A. King, C. Sherren, J. S. Marshall, and R. Anderson. 2006. A dominant role for FcgammaRII in antibody-enhanced dengue virus infection of human mast cells and associated CCL5 release. J. Leukoc. Biol. 801242-1250. [DOI] [PubMed] [Google Scholar]
- 6.Burke, D. S., A. Nisalak, D. E. Johnson, and R. M. Scott. 1988. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 38172-180. [DOI] [PubMed] [Google Scholar]
- 7.Chareonsirisuthigul, T., S. Kalayanarooj, and S. Ubol. 2007. Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and pro-inflammatory cytokine production, in THP-1 cells. J. Gen. Virol. 88365-375. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi, U. C., R. Agarwal, E. A. Elbishbishi, and A. S. Mustafa. 2000. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol. Med. Microbiol. 28183-188. [DOI] [PubMed] [Google Scholar]
- 9.Desai, D. D., S. O. Harbers, M. Flores, L. Colonna, M. P. Downie, A. Bergtold, S. Jung, and R. Clynes. 2007. Fc gamma receptor IIB on dendritic cells enforces peripheral tolerance by inhibiting effector T cell responses. J. Immunol. 1786217-6226. [DOI] [PubMed] [Google Scholar]
- 10.Dhodapkar, K. M., and M. V. Dhodapkar. 2005. Recruiting dendritic cells to improve antibody therapy of cancer. Proc. Natl. Acad. Sci. USA 1026243-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckels, K. H., W. E. Brandt, V. R. Harrison, J. M. McCown, and P. K. Russell. 1976. Isolation of a temperature-sensitive dengue-2 virus under conditions suitable for vaccine development. Infect. Immun. 141221-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endy, T. P., A. Nisalak, S. Chunsuttitwat, D. W. Vaughn, S. Green, F. A. Ennis, A. L. Rothman, and D. H. Libraty. 2004. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J. Infect. Dis. 189990-1000. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Mestre, M. T., K. Gendzekhadze, P. Rivas-Vetencourt, and Z. Layrisse. 2004. TNF-alpha-308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens 64469-472. [DOI] [PubMed] [Google Scholar]
- 14.Goncalvez, A. P., R. E. Engle, M. St. Claire, R. H. Purcell, and C. J. Lai. 2007. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. USA 1049422-9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green, S., and A. Rothman. 2006. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr. Opin. Infect. Dis. 19429-436. [DOI] [PubMed] [Google Scholar]
- 16.Guy, B., P. Chanthavanich, S. Gimenez, C. Sirivichayakul, A. Sabchareon, S. Begue, S. Yoksan, C. Luxemburger, and J. Lang. 2004. Evaluation by flow cytometry of antibody-dependent enhancement (ADE) of dengue infection by sera from Thai children immunized with a live-attenuated tetravalent dengue vaccine. Vaccine 223563-3574. [DOI] [PubMed] [Google Scholar]
- 17.Guzman, M. G., M. Alvarez, R. Rodriguez-Roche, L. Bernardo, T. Montes, S. Vazquez, L. Morier, A. Alvarez, E. A. Gould, G. Kouri, and S. B. Halstead. 2007. Neutralizing antibodies after infection with dengue 1 virus. Emerg. Infect. Dis. 13282-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzman, M. G., G. Kouri, J. Bravo, M. Soler, L. Morier, S. Vazquez, A. Diaz, R. Fernandez, A. Ruiz, A. Ramos, et al. 1988. Dengue in Cuba: history of an epidemic. Rev. Cubana Med. Trop. 4029-49. (In Spanish.) [PubMed] [Google Scholar]
- 19.Guzman, M. G., G. P. Kouri, J. Bravo, M. Soler, S. Vazquez, and L. Morier. 1990. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am. J. Trop. Med. Hyg. 42179-184. [DOI] [PubMed] [Google Scholar]
- 20.Halstead, S. B. 1979. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J. Infect. Dis. 140527-533. [DOI] [PubMed] [Google Scholar]
- 21.Ho, L. J., J. J. Wang, M. F. Shaio, C. L. Kao, D. M. Chang, S. W. Han, and J. H. Lai. 2001. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 1661499-1506. [DOI] [PubMed] [Google Scholar]
- 22.Huang, K. J., Y. C. Yang, Y. S. Lin, J. H. Huang, H. S. Liu, T. M. Yeh, S. H. Chen, C. C. Liu, and H. Y. Lei. 2006. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J. Immunol. 1762825-2832. [DOI] [PubMed] [Google Scholar]
- 23.Kliks, S. C., S. Nimmanitya, A. Nisalak, and D. S. Burke. 1988. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg. 38411-419. [DOI] [PubMed] [Google Scholar]
- 24.Kliks, S. C., A. Nisalak, W. E. Brandt, L. Wahl, and D. S. Burke. 1989. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 40444-451. [DOI] [PubMed] [Google Scholar]
- 25.Kyle, J. L., P. R. Beatty, and E. Harris. 2007. Dengue virus infects macrophages and dendritic cells in a mouse model of infection. J. Infect. Dis. 1951808-1817. [DOI] [PubMed] [Google Scholar]
- 26.Lidbury, B. A., and S. Mahalingam. 2000. Specific ablation of antiviral gene expression in macrophages by antibody-dependent enhancement of Ross River virus infection. J. Virol. 748376-8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Littaua, R., I. Kurane, and F. A. Ennis. 1990. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J. Immunol. 1443183-3186. [PubMed] [Google Scholar]
- 28.Liu, Y., X. Gao, E. Masuda, P. B. Redecha, M. C. Blank, and L. Pricop. 2006. Regulated expression of FcgammaR in human dendritic cells controls cross-presentation of antigen-antibody complexes. J. Immunol. 1778440-8447. [DOI] [PubMed] [Google Scholar]
- 29.Lozach, P. Y., L. Burleigh, I. Staropoli, E. Navarro-Sanchez, J. Harriague, J. L. Virelizier, F. A. Rey, P. Despres, F. Arenzana-Seisdedos, and A. Amara. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J. Biol. Chem. 28023698-23708. [DOI] [PubMed] [Google Scholar]
- 30.Mady, B. J., I. Kurane, D. V. Erbe, M. W. Fanger, and F. A. Ennis. 1993. Neuraminidase augments Fc gamma receptor II-mediated antibody-dependent enhancement of dengue virus infection. J. Gen. Virol. 74839-844. [DOI] [PubMed] [Google Scholar]
- 31.Mahalingam, S., and B. A. Lidbury. 2003. Antibody-dependent enhancement of infection: bacteria do it too. Trends Immunol. 24465-467. [DOI] [PubMed] [Google Scholar]
- 32.Mahalingam, S., and B. A. Lidbury. 2002. Suppression of lipopolysaccharide-induced antiviral transcription factor (STAT-1 and NF-kappa B) complexes by antibody-dependent enhancement of macrophage infection by Ross River virus. Proc. Natl. Acad. Sci. USA 9913819-13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marovich, M., G. Grouard-Vogel, M. Louder, M. Eller, W. Sun, S. J. Wu, R. Putvatana, G. Murphy, B. Tassaneetrithep, T. Burgess, D. Birx, C. Hayes, S. Schlesinger-Frankel, and J. Mascola. 2001. Human dendritic cells as targets of dengue virus infection. J. Investig. Dermatol. Symp. Proc. 6219-224. [DOI] [PubMed] [Google Scholar]
- 34.Marovich, M. A., J. R. Mascola, M. A. Eller, M. K. Louder, P. A. Caudrelier, R. El-Habib, S. Ratto-Kim, J. H. Cox, J. R. Currier, B. L. Levine, C. H. June, W. B. Bernstein, M. L. Robb, B. Schuler-Thurner, R. M. Steinman, D. L. Birx, and S. Schlesinger-Frankel. 2002. Preparation of clinical-grade recombinant canarypox-human immunodeficiency virus vaccine-loaded human dendritic cells. J. Infect. Dis. 1861242-1252. [DOI] [PubMed] [Google Scholar]
- 35.Martin, N. C., J. Pardo, M. Simmons, J. A. Tjaden, S. Widjaja, M. A. Marovich, W. Sun, K. R. Porter, and T. H. Burgess. 2006. An immunocytometric assay based on dengue infection via DC-SIGN permits rapid measurement of anti-dengue neutralizing antibodies. J. Virol. Methods 13474-85. [DOI] [PubMed] [Google Scholar]
- 36.Morens, D. M., and S. B. Halstead. 1990. Measurement of antibody-dependent infection enhancement of four dengue virus serotypes by monoclonal and polyclonal antibodies. J. Gen. Virol. 712909-2914. [DOI] [PubMed] [Google Scholar]
- 37.Navarro-Sanchez, E., R. Altmeyer, A. Amara, O. Schwartz, F. Fieschi, J. L. Virelizier, F. Arenzana-Seisdedos, and P. Despres. 2003. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 4723-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nimmerjahn, F., and J. V. Ravetch. 2008. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 834-47. [DOI] [PubMed] [Google Scholar]
- 39.Palucka, A. K. 2000. Dengue virus and dendritic cells. Nat. Med. 6748-749. [DOI] [PubMed] [Google Scholar]
- 40.Pang, T., M. J. Cardosa, and M. G. Guzman. 2007. Of cascades and perfect storms: the immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS). Immunol. Cell Biol. 8543-45. [DOI] [PubMed] [Google Scholar]
- 41.Pierson, T. C., Q. Xu, S. Nelson, T. Oliphant, G. E. Nybakken, D. H. Fremont, and M. S. Diamond. 2007. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe 1135-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinto, L. M., S. A. Oliveira, E. L. Braga, R. M. Nogueira, and C. F. Kubelka. 1999. Increased pro-inflammatory cytokines (TNF-alpha and IL-6) and anti-inflammatory compounds (sTNFRp55 and sTNFRp75) in Brazilian patients during exanthematic dengue fever. Mem. Inst. Oswaldo Cruz 94387-394. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigo, W. W., X. Jin, S. D. Blackley, R. C. Rose, and J. J. Schlesinger. 2006. Differential enhancement of dengue virus immune complex infectivity mediated by signaling-competent and signaling-incompetent human FcγRIA (CD64) or FcγRIIA (CD32). J. Virol. 8010128-10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakuntabhai, A., C. Turbpaiboon, I. Casademont, A. Chuansumrit, T. Lowhnoo, A. Kajaste-Rudnitski, S. M. Kalayanarooj, K. Tangnararatchakit, N. Tangthawornchaikul, S. Vasanawathana, W. Chaiyaratana, P. T. Yenchitsomanus, P. Suriyaphol, P. Avirutnan, K. Chokephaibulkit, F. Matsuda, S. Yoksan, Y. Jacob, G. M. Lathrop, P. Malasit, P. Despres, and C. Julier. 2005. A variant in the CD209 promoter is associated with severity of dengue disease. Nat. Genet. 37507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tassaneetrithep, B., T. H. Burgess, A. Granelli-Piperno, C. Trumpfheller, J. Finke, W. Sun, M. A. Eller, K. Pattanapanyasat, S. Sarasombath, D. L. Birx, R. M. Steinman, S. Schlesinger, and M. A. Marovich. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thein, S., M. M. Aung, T. N. Shwe, M. Aye, A. Zaw, K. Aye, K. M. Aye, and J. Aaskov. 1997. Risk factors in dengue shock syndrome. Am. J. Trop. Med. Hyg. 56566-572. [DOI] [PubMed] [Google Scholar]
- 47.Thurner, B., C. Roder, D. Dieckmann, M. Heuer, M. Kruse, A. Glaser, P. Keikavoussi, E. Kampgen, A. Bender, and G. Schuler. 1999. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods 2231-15. [DOI] [PubMed] [Google Scholar]
- 48.Vaughn, D. W. 2000. Dengue lessons from Cuba. Am. J. Epidemiol. 152800-803. [DOI] [PubMed] [Google Scholar]
- 49.Vaughn, D. W., S. Green, S. Kalayanarooj, B. L. Innis, S. Nimmannitya, S. Suntayakorn, T. P. Endy, B. Raengsakulrach, A. L. Rothman, F. A. Ennis, and A. Nisalak. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 1812-9. [DOI] [PubMed] [Google Scholar]
- 50.Wang, W. K., C. N. Lee, C. L. Kao, Y. L. Lin, and C. C. King. 2000. Quantitative competitive reverse transcription-PCR for quantification of dengue virus RNA. J. Clin. Microbiol. 383306-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu, S. J., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6816-820. [DOI] [PubMed] [Google Scholar]
- 52.Yadav, M., K. R. Kamath, N. Iyngkaran, and M. Sinniah. 1991. Dengue haemorrhagic fever and dengue shock syndrome: are they tumour necrosis factor-mediated disorders? FEMS Microbiol. Immunol. 445-49. [DOI] [PubMed] [Google Scholar]