Abstract
Herpesviruses are important pathogens of humans and other animals. Herpesvirus infectious clones that can reconstitute phenotypically wild-type (wt) virus are extremely valuable tools for elucidating the roles of specific genes in virus pathophysiology as well as for making vaccines. Ictalurid herpesvirus 1 (channel catfish herpesvirus [CCV]) is economically very important and is the best characterized of the herpesviruses that occur primarily in bony fish and amphibians. Here, we describe the cloning of the hitherto recalcitrant CCV genome as three overlapping subgenomic bacterial artificial chromosomes (BACs). These clones allowed us to regenerate vectorless wt CCVs with a phenotype that is indistinguishable from that of the wt CCV from which the BACs were derived. To test the recombinogenic systems, we next used the overlapping BACs to construct a full-length CCV BAC by replacing the CCV ORF5 with the BAC cassette and cotransfecting CCO cells. The viral progeny that we used to transform Escherichia coli and the resulting BAC had only one of the 18-kb terminal repeated regions. Both systems suggest that one of the terminal repeat regions is lost during the replicative stage of the CCV life cycle. We also demonstrated the feasibility of introducing a targeted mutation into the CCV BAC infectious clone by constructing a CCV ORF12 deletion mutant and showed that ORF12 encodes a nonessential protein for virus replication. This is the first report of the generation of an infectious BAC clone of a member of the fish and amphibian herpesviruses and its use to generate recombinants.
Channel catfish herpesvirus (CCV) can cause catastrophic losses in catfish farming, the largest aquaculture industry in the United States ($2 billion annually). CCV was the first fish herpesvirus isolated (13) and sequenced (9), and it is the best-characterized herpesvirus associated with bony fish and amphibians (10, 12). Still, little is known about how CCV interacts with its host, and there are no practical treatments for CCV disease. CCV, like other herpesviruses (32), can also be modified into an efficient live-vaccine vehicle (33, 39). Recombinant attenuated CCVs not only establish protective immunity against wild-type (wt) CCV challenge but also induce a strong immune response to foreign antigens (33, 39).
An infectious clone is a virus that is manipulated and propagated in a system other than its natural one and that is still capable of producing viable, replicating progeny in its natural system. Infectious clones are constructed because they are more amenable to molecular manipulation and may be modified to study molecular pathogenesis of the wt virus and/or to develop vaccines. The cloning of complex viral genomes as infectious clones in Escherichia coli has revolutionized the functional dissection of these genomes by harnessing powerful tools of bacterial genetics, which allow the efficient generation of desired mutants (6). The first herpesvirus infectious clone was constructed by cloning the Suid herpesvirus 1 genome as a set of overlapping cosmid clones in E. coli (34). The main advantage of overlapping cosmids is that recombination between overlapping fragments regenerates wt progeny in permissive cells or, after the successful introduction of a defined mutation(s), only mutant viruses. However, concerns about insert instability (18), the requirement for precise recombination between fragments, and the unavailability of simple E. coli recombination methods have resulted in the low adoption of the cosmid system.
Bacterial artificial chromosomes (BACs) were the next evolutionary step in herpesvirus infectious clone technology. BACs can maintain over 300 kb of foreign DNA (28) and are extremely stable (23, 28) and easily manipulated (5, 18, 23), and even the largest herpesvirus genomes can be cloned as single-unit BACs (23). BACs were initially developed as general cloning vectors with the intention of stably maintaining large DNA fragments in E. coli (28). BACs control their own replication and ensure low copy numbers and faithful segregation during cell division (28). To generate a full-length herpesvirus BAC, typically one (27, 29) or more (23) nonessential genes are replaced with a BAC vector. The disadvantages are that the inserted BAC can disrupt the expression of neighboring genes (30), viral progeny might be attenuated in vivo (23), and, most importantly, full-length herpesvirus BACs usually do not produce wt progeny because of the insertion of the BAC in their genome. This limits most of the single-unit herpesvirus BACs to applications not requiring wt viruses.
Here, we combine the advantages of overlapping clones and BACs to construct a set of three overlapping BAC clones that together regenerate wt CCV with no residual nucleotides and that allow us to rapidly and simply manipulate the CCV genome using standard molecular genetics. This is the first herpesvirus from a poikilotherm to be cloned as a BAC. To further simplify and accelerate the construction of CCV mutants for applications where the production of wt CCV is not required, such as vaccines, we also report the generation a single attenuated CCV BAC clone (the BAC is inserted into CCV ORF5) from our overlapping clones.
The functions of most genes from most herpesviruses have yet to be defined. In addition, it is axiomatic that comparative biology can be used to help infer conserved critical genetic functions, especially in herpesviruses that coevolve with their hosts. We chose to test our BAC system first on CCV ORF12. ORF12 encodes a predicted tegument protein (11) and contains a C3HC4 RING finger motif (9). Several other herpesviruses encode immediate-early (2, 3, 26) or early (21, 35, 36) RING finger proteins that have intrinsic substrate-specific ubiquitin protein ligase (E3) activities required to degrade host proteins during productive infections (3, 17, 21, 36). Herpesvirus RING finger proteins are important for reactivating quiescent genomes, stimulating lytic infection (3), and evading the immune system (17, 21, 36). We used our overlapping BAC system to demonstrate that ORF12 is not essential in vitro.
MATERIALS AND METHODS
Virus and cells.
CCV (Auburn 1 clone A; ATCC VR-665) was propagated at 2 50% tissue culture infective doses (TCID50)/cell in confluent monolayers of channel catfish ovary (CCO) (ATCC CRL-2772) cells. CCO cells were grown at 30°C in Dulbecco's modified Eagle's minimum essential medium supplemented with penicillin (5,000 U/ml), streptomycin (5,000 U/ml), and fetal calf serum (10%). To generate CCV stocks, infected CCO cells were harvested when cells were completely detached. Cells were lysed in media by three freeze-thaw cycles. Cell debris was pelleted (800 × g at 4°C for 5 min), and supernatants were stored at −80°C. Virus titers were determined in triplicate on CCO cells by end-point dilution and expressed as TCID50/ml (24).
Isolation of CCV DNA from eukaryotic cells.
Genomic CCV DNA was isolated from infected CCO cells grown in 25-cm2 culture flasks. Detached CCO monolayers were pelleted (800 × g at 4°C for 5 min); washed with ice-cold phosphate-buffered saline (pH 7.2); resuspended in 500 μl of a solution containing 100 mM NaCl, 10 mM Tris, and 1 mM EDTA at pH 8.0; and then lysed by adding 250 μl of sarcosine lysis buffer (75 mM Tris-HCl, 25 mM EDTA, and 3% [wt/vol] N-lauryl sarcosine [pH 8.0] for 15 min at 65°C). RNA was removed by using RNase A (5 μl of 10 mg/ml for 30 min at 37°C; Promega Corp., Madison, WI); protein was digested using proteinase K (2.5 μl of 10 mg/ml for 16 h at 55°C; Promega Corp., Madison, WI). Genomic CCV DNA was phenol-chloroform extracted three times, precipitated using standard ethanol precipitation, and redissolved in 10 mM Tris-HCl (pH 8.0).
Construction of overlapping subgenomic CCV BACs.
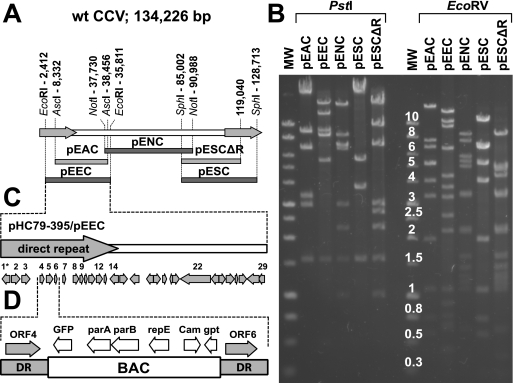
pEEC (Fig. 1A) was constructed by recloning the CCV DNA fragment of cosmid pHC79-395 into the HindIII-linearized plasmid pECBAC1 (14) (kindly provided by Richard Michelmore, University of California, Davis, CA). pHC79-395 was previously constructed by cloning a partially digested EcoRI fragment of CCV DNA (wt CCV DNA nucleotides [nt] 2412 to 38511) into the EcoRI site of cosmid pHC79 (16). To construct pENC and pESC (Fig. 1A), we digested wt CCV DNA with NotI or SphI and cloned the selected DNA fragments (a 53.3-kb NotI fragment at nt 37730 to 90988 and a 43.7-kb SphI fragment at nt 85002 to 128713) into NotI- and SphI-linearized pECBAC1, respectively. To construct pEAC (Fig. 1A), a synthetic dimer containing the AscI site (generated from two oligonucleotides, 5′-GATCggcgcgcc-3′ and 5′-AGCTggcgcgcc-3′ [lowercase type indicates the AscI site]) was first inserted into the unique BamHI and HindIII sites of vector pECBAC1, producing pECBAC1-AscI. The dimer was designed to preserve the reading frame of the beta-galactosidase gene of pECBAC1. pECBAC1-AscI was then linearized with AscI and ligated together with the 30.1-kb AscI fragment of CCV (nt 8331 to 38455) to produce pEAC.
FIG. 1.
Overlapping CCV BACs. (A) Positions of the CCV fragments that were used to generate overlapping CCV BAC clones. The CCV genome (134.2 kb) contains two identical terminal direct repeats (DR) (18.6 kb each) (arrows) that flank the unique region (97.1 kb). The CCV restriction fragments that were used to generate overlapping CCV BACs are shown below the CCV genome and are aligned with the regions of the CCV genome from which they were derived. pEEC contains the EcoRI fragment (positions 2412 to 35811), pEAC contains the AscI fragment (positions 8332 to 38456), pENC contains the NotI fragment (positions 7730 to 90988), and pESC contains the SphI fragment (positions 85002 to 128713). Nucleotide positions correspond to the wt CCV genome. pESCΔR is a derivative of pESC from which the majority of direct terminal repeat was removed. pESCΔR contains only the first 3,403 bp of the right repeat region. (B) Restriction enzyme analysis of CCV BACs. pEEC, pENC, pESC, pEAC, and pESCΔR were digested with PstI or EcoRV, and restriction DNA fragments were separated on a 0.7% agarose gel. Restriction patterns of CCV BACs correspond to the predicted ones. MW, molecular weight marker with band size in kb (1-kb DNA ladder; Promega Corp.). (C) CCV sequences carried by pEEC and pHC79-395. These clones contain a part of the direct terminal repeat and a portion of the unique region of the CCV genome. Arrows indicate positions and orientations of the predicted CCV ORFs. 1* is a 5′-terminal part of CCV ORF1. CCV ORF12 carried by pEEC was deleted to produce pΔ12. (D) CCV ORF5 carried by pHC79-395 was replaced with the BAC cassette of plasmid pHA2 (1). parA, parB, and repE are regulatory genes derived from the F factor of E. coli. GFP, green fluorescent protein; Cam, chloramphenicol resistance; gpt, guanosine phosphoribosyl transferase.
pESCΔR (Fig. 1A) is a pESC derivative that was truncated after the first 3,403 bp of the right repeat (after the ORF1R gene). A linear replacement fragment was designed to replace the majority of the direct repeat (wt CCV nt 119019 to 128713, including ORF2R to ORF8R) carried by pESC, with the kanamycin resistance gene flanked by directly repeated FRT sites. The replacement fragment was amplified from HindIII-linearized plasmid pKD13 (8) (provided by Barry L. Wanner, Purdue University, West Lafayette, IN) by using Taq polymerase (Boca Scientific Inc., FL) and primers delR-F and delR-R (Table 1). The 3′ ends of both primers (19 and 20 nt, respectively) are homologous to the targeting cassette used for PCR amplification. The 32-nt 5′ part of primer delR-F is homologous to the targeting region (71 bp downstream from the ORF1R stop codon), and the 35-nt 5′ part of primer delR-R is homologous to the pECBAC1 cloning vector. Primer delR-F was designed to carry an additional SphI site that would enable the straightforward excision of the CCV sequence from pESCΔR (Fig. 1A).
TABLE 1.
Oligonucleotidesa
| Primer | Sequence (5′-3′) | Template |
|---|---|---|
| delR-F | AAATGGCTACTGAGACGTTCTCTGTACAGCgcatgcGTGTAGGCTGGAGCTGCTT | pESC |
| delR-R | CCCGGGGATCCTCTAGAGTCGACCTGCAGgcatgcCTGTCAAACATGAGAATTAA | pESC |
| k12F | ACCGATCCACCAACAGGCCACTGAGTCGAGatgGTGTAGGCTGGAGCTGCTTC | pKD13 |
| k12R | GTATTTACATTAGTACAACAATCCGCTTATtcaCTGTCAAACATGAGAATTAA | pKD13 |
| d12F | GTCTCCAGTCTTTCCGTG | CCV ORF11 |
| d12R | ATGTTCCTCCCTGTGTGC | CCV ORF13 |
| kan1 | GGATTCATCGACTGTGGC | Knm |
| kan2 | GACAGGTCGGTCTTGACA | Knm |
| kan3 | AGTCATAGCCGAATAGCCT | Knm |
Italic type indicates homologous recombination sequences; lowercase type indicates restriction enzyme sites or ATG start or stop codons; boldface type indicates sequences annealing to a template. k12F and k12R have 33-nt 5′ extensions homologous up- and downstream of CCV ORF12, respectively (ATG and TCA are the CCV ORF12 start and stop codons, respectively). d12F and d12R are specific to CCV ORF12. kan1, kan2, and kan3 are specific to the kanamycin resistance gene of plasmid pKD13.
We used the λ recombination system to introduce a targeted mutation into the subgenomic CCV DNA fragment cloned into pESC essentially as described previously (20, 37). The 1,394-bp gel-purified replacement fragment was electroporated into electrocompetent and recombinogenic E. coli EL250 cells (20) containing pESC. Following electroporation, bacteria were spread onto chloramphenicol- and kanamycin-selective LB agar plates. BAC DNA (pESCΔR-Knm) was recovered from double-resistant colonies and transferred into E. coli DH10B cells. The restriction enzyme profiles of recombinant clones were examined following BglII, EcoRI, and SphI digestion and agarose electrophoresis (data not shown). Next, the kanamycin resistance gene was removed by Flpe recombination as described previously (20), producing pESCΔR. The proper structures of all CCV BAC clones were verified by digestion using several different restriction enzymes (Fig. 1B), PCR, and sequencing across the cloning regions.
Transfections.
To reconstitute any CCV progeny from overlapping clones, cloned CCV sequences were first digested with appropriate restriction enzymes and then agarose gel purified. Mixtures of appropriate viral fragments (500 ng each) were transfected into CCO cells grown in six-well cell culture plates at 80% confluence (3.5 × 105 cells/well). Transfections were done using Lipofectin (Invitrogen Inc., Carlsbad, CA) according to the manufacturer's instructions.
Southern blotting.
wt and reconstituted CCV (rCCV-1) DNAs were digested with AscI, BamHI, BglII, EcoRI, EcoRV, KpnI, NotI, PstI, ScaI, SphI, or XbaI. pECBAC1 was digested with PstI. Restriction fragments were separated on a 0.7% agarose gel and transferred onto a positively charged nylon membrane (Hybond N+; GE Healthcare, Piscataway, NJ) by capillary blotting. The membrane was hybridized with horseradish peroxidase-labeled PstI-digested pECBAC1 as a probe (ECL direct nucleic acid labeling and detection system; GE Healthcare, Piscataway, NJ). CCV-ΔTK BAC DNA was digested with BamHI, BglII, or KpnI and separated by 0.7% agarose gel electrophoresis. DNA fragments were blotted onto a nylon membrane and hybridized with the labeled PstI-digested BAC vector of plasmid pHA2 (1) (provided by Martin Messerle, Ludwig Maximilians Universität, Munich, Germany).
Replication kinetics.
The abilities of wt CCV, rCCV-1, CCV-ΔTK, and CCV-ΔORF12 viruses to replicate in CCO cell culture were compared using both single- and multiple-step growth experiments. For single-step growth experiments, virus was inoculated at 2 TCID50/cell into six-well plates containing CCO monolayers. Virus was allowed to adsorb for 1 h at 30°C. The cells were then treated with a solution containing 40 mM sodium citrate, 10 mM KCl, and 135 mM NaCl (pH 3.0) for 1 min to inactivate any unadsorbed extracellular virus (22, 25). The cells were then washed three times with phosphate-buffered saline (pH 7.2) and overlaid with culture medium. At 6, 9, 12, 16, 20, and 24 h postinfection (hpi), the cells were harvested in the medium, lysed by three freeze-thaw cycles, and pelleted by centrifugation (800 × g at 4°C for 5 min), and supernatants (containing virions) were stored at −80°C. The TCID50 of each supernatant from each time point was then determined in triplicate by end-point dilution and titration on CCO cells. For multiple-step growth experiments, CCO cells grown in 24-well plates were infected at 0.01 TCID50/cell and harvested at 4, 8, 12, 16, 24, 32, and 40 hpi. Samples were processed, and infectious virus titers were determined as described above.
Channel catfish challenge.
Channel catfish fingerlings (5.25 ± 0.15 g [mean ± standard error of the mean]) were obtained from a specific-pathogen-free fish hatchery operated by the College of Veterinary Medicine, Mississippi State University. Fish were randomly stocked in 45 tanks supplied with flowing, nonchlorinated, aerated water (30°C ± 1°C) at a density of 22 to 23 fish per tank. Before the start of the experiment, catfish were acclimated to the basal diet for 7 days. Fish were fed three times a day ad libitum with commercial aquarium flake food (TetraMin Tropical Flakes; Spectrum Brands Inc.) and subjected to 12-h light-dark cycles. Fish in five tanks were challenged with each of the following doses: 1 × 104, 1 × 105, or 1 × 106 TCID50/liter of either wt CCV or rCCV-1 in 15 liters of water for 30 min. As a noninfected control, two tanks were mock challenged using the cell culture medium. Fish were observed five times per day, mortalities were recorded, and dead fish were removed. To confirm that fish died as a result of CCV disease, posterior kidneys were collected from the dead fish and homogenized in 300 μl of Dulbecco's modified Eagle's minimum essential medium supplemented with penicillin (20,000 U/ml), streptomycin (20,000 U/ml), and fetal calf serum (10%). Tissue remains were pelleted by centrifugation (15,000 × g at 4°C for 5 min), and 50 μl supernatant was transferred onto CCO monolayers plated in 96-well plates. Two days after the fish stopped dying (12 days postinfection [dpi]), surviving fish were killed by submersion in water containing 500 mg/liter 3-aminobenzoic acid ethyl ester methanesulfonate (Sigma-Aldrich Inc., St. Louis, MO).
Construction of CCV-ΔTK (BAC).
Plasmid pBSCV446 (38) is a pBluescript SK(−) (Stratagene, La Jolla, CA) construct containing the SacI-XbaI fragment of the wt CCV genome, nt 7269 to 10124. pBSCV457 (38) is a pBSCV446 derivative from which the 663 bp immediately following the thymidine kinase (TK) gene ATG start codon (wt CCV nt 8788 to 9453) was replaced by a linker containing BglII, PstI, SphI, and SpeI restriction enzyme cloning sites (38). We digested pBSCV457 with BglII and SpeI restriction enzymes and ligated the 5.1-kb BglII-SpeI fragment with a synthetic linker generated from the two oligonucleotides, 5′-GATCttaattaa-3′ and 5′-CTAGttaattaa-3′, containing the PacI cloning site (lowercase type), to generate pΔTK. pΔTK was then linearized with the PacI restriction enzyme and ligated with the BAC cassette released as a PacI fragment from plasmid pHA2 (1), resulting in vector pΔTK-BAC.
The TK replacement fragment containing a BAC cassette flanked by CCV sequences homologous to CCV ORF5 upstream (1,519 bp) and downstream (327 bp) was then released as a SacI restriction fragment of pΔTK-BAC. The gel-purified CCV ORF5 replacement fragment was electroporated into electrocompetent E. coli EL250 cells (20) containing cosmid pHC79-395, which had been induced recombinogenically (20). Following electroporation, the cells were plated onto chloramphenicol (17 μg/ml) and ampicillin (50 μg/ml) LB agar plates. pHC79-395-BAC DNA was recovered from 12 double-resistant colonies and transferred into E. coli DH10B cells. The proper structure of pHC79-395-BAC clones was verified by restriction enzyme digestion, PCR, and sequencing across the recombined regions.
To reconstitute the infectious CCV-ΔTK virus, pHC79-395-BAC, pENC, and pESC were digested with AscI, NotI, and SphI, respectively, and the overlapping CCV fragments were purified from 0.7% agarose gels and cotransfected into CCO cells. Because the SphI fragment of the pESC contains the CCV ORF5 derived from the right terminal repeat, we selected CCV-ΔTK virus (TK-negative progeny) with medium containing 45 mg of acyclovir per ml. Isolated viral genomic CCV-ΔTK DNA was electroporated into E. coli DH10B cells. Transformed bacteria were plated onto chloramphenicol (17 μg/ml)-selective LB agar plates. Colonies were allowed to develop for 24 to 48 h at 37°C. BAC DNA was prepared from cultures of the 24 colonies grown overnight and analyzed by restriction enzyme digestion. The correct structures of the two E. coli CCV-ΔTK BAC clones were confirmed by Southern blot hybridization (see Fig. 4A). The BAC cassette carries a gene for green fluorescent protein, which is under the control of the human cytomegalovirus immediate-early promoter (PCMV). This phenotypic marker can be used to monitor reconstitution and the level of infection in slowly growing mutant viruses or to screen for the presence of the BAC cassette in the CCV genome.
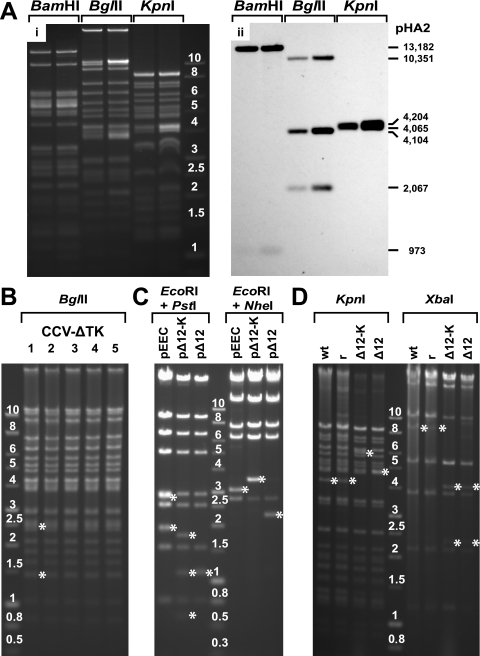
FIG. 4.
(A) BamHI, BglII, and KpnI digestion (i) and Southern blot analysis (hybridized with PstI fragments of the pHA2 vector) (ii) of the CCV-ΔTK genome as described in Materials and Methods. MW, molecular weight marker with band size in kb (1-kb DNA ladder; Promega Corp.). The reactive bands (973 and 13,182 bp for BamHI; 2,067, 4,104, and 10,351 bp for BglII; and 4,065 and 4,204 bp for KpnI) confirm the successful cloning of the entire CCV genome as a single-unit E. coli BAC containing one unique region and one repeat region. (B) BglII-digested DNA of five different CCV-ΔTK viruses (lanes 1 to 5). Asterisks identify fragments (1,436 and 2,446 bp) that were derived from the termini of the linear CCV-ΔTK DNA; these fragments demonstrate that both direct terminal repeats were restored in viral DNA isolated from nucleocapsids. (C) Structural analysis of pEEC and its derivatives, pΔ12-Knm and pΔ12. BACs were digested with EcoRI and PstI or with EcoRI and NheI. The fragments that are unique to different BACs are marked by asterisks. (D) Gel electrophoresis of KpnI- and XbaI-digested wt CCV (wt), rCCV-1 (r), CCV-Δ12-Knm (Δ12-K), and CCV-Δ12 (Δ12) virus DNA. Asterisks identify restriction DNA fragments that are unique to wt CCV (and rCCV-1) or to CCV ΔORF12 deletion mutants.
Construction of pΔ12.
We used a λ recombination system (20, 37) to delete CCV ORF12 from the pEEC essentially as described above. A 1,389-bp linear CCV ORF12 replacement fragment was amplified from HindIII-linearized plasmid pKD13 by PCR using primers k12F and k12R (Table 1). The replacement fragment was gel purified and electroporated into electrocompetent and recombinogenic E. coli EL250 cells (20) containing pEEC. Following electroporation, bacteria were allowed to recover and were spread onto chloramphenicol- and kanamycin-selective LB agar plates. BAC DNA (pΔ12-Knm) was recovered from 12 of the double-resistant colonies and transferred into E. coli DH10B cells. The restriction enzyme profiles of these clones were examined following BglII, EcoRI, or SphI restriction digestion and agarose gel electrophoresis (see Fig. 4C). Homologous recombination between the replacement fragment and pEEC resulted in the insertion of the kanamycin resistance gene and the deletion of ORF12 from nt 15394 to 16297. We further confirmed the correct insertion of the replacement fragment within the CCV genome by PCR using nearby locus-specific primers (d12F [ORF11 specific] and d12R [ORF13 specific]) (Table 1) and kanamycin-specific primers (kan1, kan2, and kan3) (Table 1) to verify newly formed junctions. A third PCR was conducted by nearby locus-specific primers alone to confirm the simultaneous loss of the parental fragment (2,326 bp) and gain of the new mutant-specific fragment (2,755 bp) (data not shown). Next, the kanamycin-selectable marker was removed from three different pΔ12-Knm clones by Flpe recombination as previously described (20), producing pΔ12. The correct removal of the kanamycin resistance gene was confirmed by restriction digestion (see Fig. 4C) and PCR (data not shown).
RESULTS
Construction of the infectious clone of CCV as a set of overlapping BAC clones.
Initially, we constructed three overlapping CCV BAC clones, pEEC, pENC, and pESC, which together contain all the coding sequences of the CCV genome (direct terminal repeat and the unique region) (Fig. 1A). These three overlapping CCV BACs can be used to regenerate wt CCV by recombination in cell culture. However, since pEEC and pESC contain identical DNA sequences (derived from the left and the right direct CCV repeats, respectively), construction of recombinant viruses with mutations in the region of overlap (10,630 bp) could be cumbersome. To simplify the process of generating mutants in repeat regions, we later constructed two additional CCV BACs, pEAC and pESCΔR (Fig. 1A). pEAC and pESCΔR contain shorter sequences for the repeat regions than pEEC and pESC, respectively. The correct structures of all CCV BAC clones were verified by restriction enzyme digestion analysis (Fig. 1B). The EcoRV BAC fragments (Fig. 1B) can be compared to the EcoRV fragments of CCV genomic DNA (Fig. 2A, ii, outside lanes).
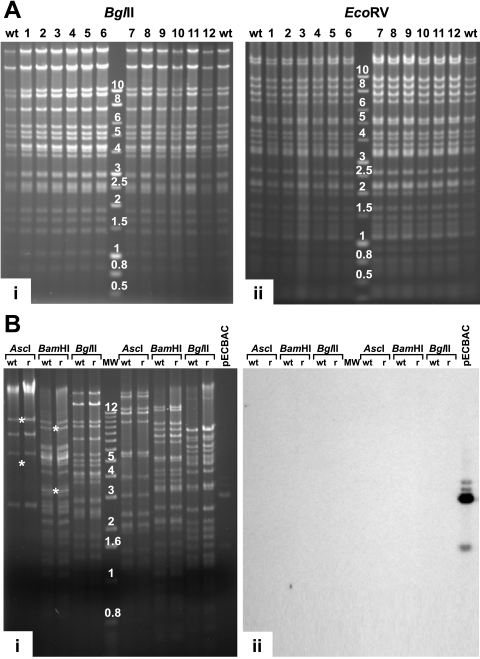
FIG. 2.
Restriction enzyme analysis of the rCCV genomes. (A) Parental wt CCV DNA (wt) and DNA of 12 independently regenerated and plaque-purified rCCV isolates (lanes 1 to 12) were digested with BglII (i) and EcoRV (ii) and separated by agarose gel electrophoresis. The molecular weight marker used was a 1-kb DNA ladder (Promega Corp.). (B) Restriction enzyme digestion and Southern blotting of rCCV-1 DNA. wt CCV (wt) and rCCV-1 DNA (r) were digested, separated by agarose gel electrophoresis (i), and hybridized with fragments of vector pECBAC1 (ii). *, AscI (5,597 and 10,224 bp) and BamHI (8,949 and 3,487 bp), DNA fragments which were derived from the termini of the linear virus demonstrating that rCCV-1 DNA isolated from virions contained both direct terminal repeats. pECBAC1, digested using PstI (1,541-, 2,949-, and 3,029-bp fragments), was used as a positive control. MW, molecular weight marker with band size in kb (1-kb DNA ladder; Invitrogen Inc.).
Reconstitution of the infectious CCV in CCO cells from three overlapping BAC clones.
To test whether the three overlapping BAC clones pEEC, pENC, and pESC can reconstitute infectious wt CCV, the cloned CCV sequences were first excised from the BAC clones: pEEC was digested with AscI (wt CCV nt 8832 and 38456), and pENC and pESC were digested with NotI and SphI, respectively. CCV DNA fragments were gel purified and then cotransfected into in vitro-cultured CCV-permissive CCO cells (4). Twelve independent transfections resulted in productive viral infections. At 24 h posttransfection, a typical CCV cytopathic effect (CPE) occurred in the cultured cells; 36 h posttransfection, we observed generalized CPE followed by the complete destruction and detachment of cell monolayers. The morphology of syncytial plaques formed by rCCV viruses was indistinguishable from those of plaques formed by wt CCV on CCO cells (Fig. 3B). At 100% CPE, we filtered (0.22 μm) culture medium and transferred this into new flasks of CCO cells, and CPE appeared 6 h later, demonstrating that rCCV formed infectious virions. rCCV isolates were then purified from each of the 12 populations by three rounds of plaque purification.
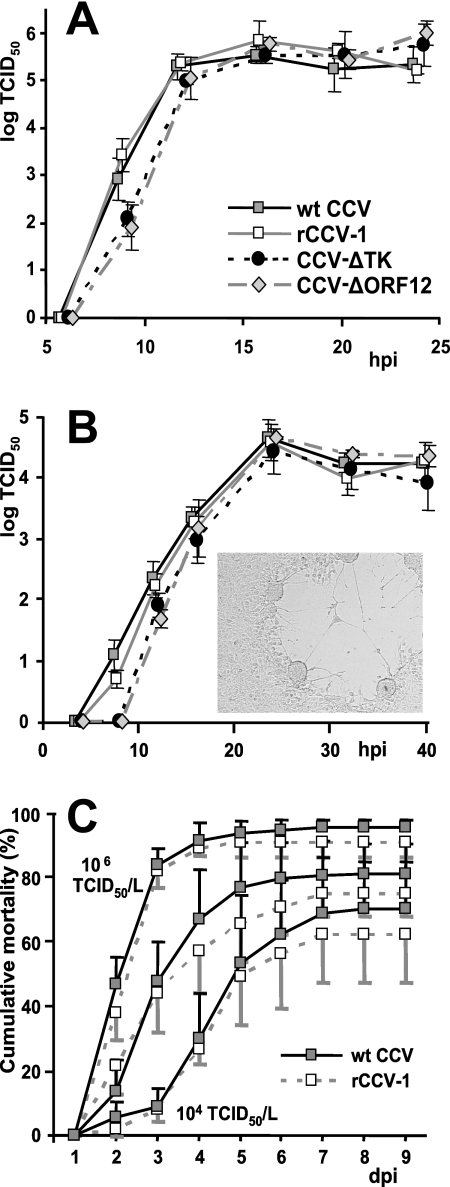
FIG. 3.
Single-step (A) and multiple-step (B) growth curves of wt CCV, rCCV-1, CCV-ΔTK, and CCV-ΔORF12. Virus was harvested from infected CCO cells at the indicated times postinfection (hpi), and titers were determined in triplicate by end-point dilution and titration on CCO cells. Averaged titers and 95% confidence intervals are illustrated. The morphology of syncytial plaques formed on CCO cells by rCCV-1, CCV-ΔTK, or CCV-ΔORF12 viruses was indistinguishable from that of wt CCV (inset). (C) Mean cumulative mortality of catfish fingerlings infected with wt CCV or rCCV-1 viruses at a dose of 1 × 104, 1 × 105, or 1 × 106 TCID50/liter. Although mortality significantly (P < 0.05) differed by CCV dose, mortality was not significantly different between the groups infected with the same dose of each different virus (P > 0.05).
Regenerated CCV contains the full-length CCV genome.
To characterize the genomic structure of rCCV viruses, DNAs from the 12 different rCCVs as well as wt CCV DNA were digested using BglII or EcoRV and separated on agarose gels (Fig. 2A). Restriction enzyme fragment patterns of all 12 rCCV viruses were indistinguishable from that of the wt CCV parent, indicating that rCCV genomes regenerated without genomic rearrangements (Fig. 2A). Although the three overlapping BAC subgenomic fragments that were combined to produce the rCCV do not contain the entire direct repeat regions, restriction digestion analysis of rCCV DNA demonstrates that unit-length molecules (with both direct terminal repeats restored) are present in infected CCO cells (Fig. 2A). This is consistent with the finding that shortly after infection, the CCV genome changes from a linear unit-length structure into a circular/concatameric structure by recombination through the repeated sequences (and one copy of the terminal repeats is lost) (7). Unit-length virion DNA reappears again in the infected cells during the late stages of CCV replication, when a genome with both direct repeats is generated by excision from the concatameric DNA.
One of the rCCVs (isolate 1, rCCV-1) was selected for further characterization by additional restriction digestion analysis using AscI, BamHI, BglII, EcoRI, EcoRV, or KpnI and Southern blotting (Fig. 2B). wt CCV and rCCV-1 DNAs exhibited identical restriction enzyme fragment patterns. To further confirm that CCV-1 did not contain any pECBAC1 sequences, wt CCV, rCCV-1, and PstI-digested pECBAC-1 DNA fragments were transferred from the gel (Fig. 2B, i) onto a nylon membrane and hybridized with pECBAC1 DNA labeled with horseradish peroxidase (Fig. 2B, ii). There were no hybridizing fragments with the rCCV-1 DNA fragments, indicating that rCCV-1 progeny did not contain any large pECBAC1 sequences (Fig. 2B). In addition, two other possible combinations of the three appropriate overlapping CCV clones (pEEC, pENC, and pESCΔR; pEAC, pENC, and pESC) produced infectious virus with wt characteristics (data not shown).
rCCV-1 and wt CCV are biologically indistinguishable.
Restriction digestion reveals larger selections or rearrangements, and Southern hybridization indicates if larger BAC vector sequences are incorporated into the genome of progeny virus, but only complete sequencing of rCCV genomes would detect more subtle mutations. Resequencing of the large herpesvirus genomes is impractical; however, because the herpesvirus genomes are densely packed with coding and regulatory sequences, analysis of the biological characteristics provides a reliable assessment of overall biological stability (6). To confirm that rCCV-1 maintains biological characteristics of wt CCV, we examined its growth properties in tissue culture (Fig. 3A and B) and measured rCCV-1 virulence by a channel catfish challenge trial (Fig. 3C) compared to parental wt CCV.
The ability of rCCV-1 to replicate in CCO cell culture was assessed using single-step (Fig. 3A) and multiple-step (Fig. 3B) growth experiments and was compared to that of wt CCV. rCCV-1 replicated in vitro indistinguishably from the wt CCV parent, as both viruses replicated to equivalent titers at each time point postinfection with equivalent kinetics (Fig. 3A and B).
To compare the virulence of rCCV-1 to that of wt CCV, we used immersion challenge-lethal dose trials on fingerling channel catfish. Both wt CCV and rCCV-1 were capable of causing lethal disease, and no deaths occurred in the mock-infected control fish. Catfish infected with both wt CCV and rCCV-1 started dying 2 dpi, and the highest mortality occurred between 3 and 4 dpi. Catfish infected with the highest doses of either virus (1 × 106 TCID50/liter) suffered the highest overall mortality (91.3% for rCCV-1 and 96.4% for wt CCV). Catfish from subgroups infected with a lesser amount of CCV (1 × 105 TCID50/liter or 1 × 104 TCID50/liter) had lower rates of mortality (75.7% and 62.6%, respectively, for rCCV-1 and 81.8% and 70.9%, respectively, for wt CCV). All dead fish had signs of CCV disease including exophthalmia and hemorrhagic lesions on the ventral surface and at the base of fins. Infectious CCV was detected in kidneys of 566 of 573 dead fish (98.8%) in both groups. There were no significant differences in mean cumulative mortality or total mortality between the catfish groups infected with the same dose of each CCV (Fig. 3C). Overall, rCCV-1 derived from overlapping BACs was phenotypically identical to parental wt CCV.
Whole-genome CCV BAC (CCV-ΔTK).
To simplify and accelerate the construction of CCV mutants for applications where the production of wt CCV is not required, we constructed a single-unit CCV BAC. We utilized the overlapping CCV BAC system and constructed a full-length infectious clone of CCV (CCV-ΔTK) from three overlapping CCV fragments by homologous recombination in cell culture. The whole genome of CCV was cloned as a single BAC by replacing CCV ORF5, which encodes TK, with the BAC cassette of plasmid pHA2 (1). We chose the CCV ORF5 insertion site because it is nonessential for CCV replication and because ORF5 deletion attenuates CCV in vivo (38).
Infectious CCV-ΔTK (BAC) virus was reconstituted in CCO cells. Plaque-purified CCV-ΔTK DNA was then isolated and electroporated into E. coli DH10B cells. The BamHI, BglII, or KpnI digestion profiles of CCV-ΔTK BAC E. coli clones exhibited the predicted restriction fragment patterns (Fig. 4A). As expected, CCV-ΔTK BACs had a circular structure (crucial for replication in E. coli) composed of the entire unique region and only one of the terminal repeats of the CCV genome. The correct structures of two E. coli CCV-ΔTK BAC clones were further confirmed by Southern blot hybridizations using a labeled PstI-digested BAC vector of plasmid pHA2 (1) as a probe (Fig. 4A). We observed the expected reaction pattern in BamHI-, BglII-, or KpnI-digested DNA.
Reconstitution of infectious CCV-ΔTK virus from CCV-ΔTK BAC.
DNA isolated from five different E. coli CCV-ΔTK BAC clones was transfected into CCO cells. Each of the clones reconstituted infectious virus and produced CPE typical of CCV. We next examined the stability of the BAC-derived CCV-ΔTK viruses and identified whether or not both terminal repeats were restored in the linear CCV-ΔTK DNA. The progenies of five different reconstituted CCV-ΔTK viruses were serially passaged six times. Virion DNA was isolated and analyzed by restriction enzyme digestion (Fig. 4B). We observed the expected restriction patterns and confirmed that both terminal repeats were restored in the unit-length molecules (Fig. 4B). We concluded that the BAC cassette is stably inserted into the CCV-ΔTK viruses reconstituted from individual CCV-ΔTK BAC clones.
To determine if the deletion of CCV ORF5 and insertion of the BAC vector into the CCV genome had any effect on viral replication in vitro, we assessed the single- and multiple-step replication kinetics of the CCV-ΔTK virus reconstituted from the BAC clone and compared them to those of wt CCV (Fig. 3A and B). We did not detect statistically significant differences (P < 0.05) in virus titers between wt CCV and CCV-ΔTK by single-step growth experiments (Fig. 3A), as expected, because no significant difference in growth kinetics or progeny production was detected between a previous TK-negative CCV and parentally wt CCV by single-step growth experiments (38). However, multiple-step growth experiments did show that CCV-ΔTK had less virus than wt CCV during the first hours postinfection, when cells were infected with lower multiplicities of infection (0.01 TCID50/cell) (Fig. 3B). CCV-ΔTK virus titers were significantly lower than those of wt CCV at 8 and 12 hpi (P = 0.004 and P = 0.044, respectively). The impairment in replication efficiency of the CCV-ΔTK virus was most probably caused by the deletion of the ORF5 gene. No statistically significant differences (P < 0.05) in CCV titers were detected between two viruses at 16 hpi, when both viruses produced equivalent amounts of total progeny virions. Based on these results, we conclude that the insertion of the BAC vector into the CCV genome, along with the abolition of TK activity, did not significantly affect the in vitro growth kinetics of CCV-ΔTK or its ability to produce progeny.
CCV-ΔORF12.
We examined whether or not CCV ORF12 is essential for virus replication in vitro by constructing CCV ORF12 deletion mutants, CCV-ΔORF12-Knm and CCV-ΔORF12. To generate these mutants, we first deleted CCV ORF12 from pEEC by replacing it with the kanamycin resistance gene, producing pΔ12-Knm. We then removed the kanamycin gene from pΔ12-Knm by Flpe recombination in E. coli, resulting in pΔ12. The correct structures of pΔ12-Knm and pΔ12 were confirmed by restriction enzyme digestion analysis (Fig. 4C).
Unit-length virion CCV DNA contains two copies of the ORF12 gene (right and left terminal repeats). We showed previously that CCV progeny reconstituted from three overlapping CCV fragments restores both terminal repeats. Since pESC does not contain the second copy of the ORF12 gene, the CCV progeny which arises from recombination between pEEC-Δ12, pENC, and pESC will be ORF12 negative. To examine whether ORF12 is essential for CCV replication in cell culture, we released subgenomic CCV fragments from pENC, pESC, and pΔ12-Knm (or from pENC, pESC, and pΔ12) and transfected them in appropriate mixtures into CCO cells. In both cases, plaques typical of CCV developed within 2 days posttransfection. Plaque formation was followed by productive CCV infection and the complete destruction of cell monolayers within the next 2 days. Isolates of both CCV-ΔORF12-Knm and CCV-ΔORF12 were plaque purified, and DNA was isolated. Restriction enzyme digestion of CCV-ΔORF12-Knm and CCV-ΔORF12 DNA demonstrated that ORF12 was successfully deleted from the CCV genome (Fig. 4D), indicating that the ORF12 gene product was not essential for CCV replication in cell culture.
To determine if the deletion of the ORF12 gene from the CCV genome had any effect on replication in cell culture, we compared the replication of CCV-ΔORF12 to that of wt CCV by single- and multiple-step growth experiments (Fig. 3A and B). The ΔORF12-CCV titers were statistically lower (P < 0.05) than those of wt CCV at 9 hpi in the single-step growth experiments (Fig. 3A) and at 8 hpi and 12 hpi in the multiple-step growth experiments (Fig. 3B). Titers of both viruses were not statistically different at 12 hpi in the single-step growth experiments and at 16 hpi in the multiple-step growth experiments. There was no difference in infectious virus yield between the two viruses. Based on these results, we conclude that the CCV ORF12 gene product is beneficial but not essential for CCV replication in vitro.
DISCUSSION
Virus infectious clones have revolutionized viral pathogenesis studies and recombinant vaccine production. Here, we describe an extension of the strategy of cloning of herpesvirus genomes as single infectious BACs in E. coli (23). We first attempted to clone CCV using such “traditional” approaches, but CCV proved to be recalcitrant. As an alternative, we combined the principle of homologous recombination used in the cloning of viruses using cosmids with BAC technology to develop the method of overlapping BAC clones. This hybrid method combines advantages of overlapping clones with the advantages of BACs. This simple strategy not only allowed us to regenerate wt virus progeny within 2 months of starting from CCV DNA but also, unlike other strategies, enabled us to regenerate the viral progeny that was genetically indistinguishable from its wt parent. Also, after the successful introduction of the defined mutation(s), recombination between overlapping fragments produces only mutant viral progeny, obviating lengthy counterselection against nonrecombinant wt viruses. Furthermore, BACs ensure extensive genetic stability and enable rapid and reliable manipulation of cloned sequences with the techniques established for E. coli.
The design of the CCV infectious clone presented here is based on the observations of “endless” CCV DNA, which is present in infected cells (7). Shortly after infection, the CCV genome changes from a linear unit-length structure (a unique region flanked by two direct repeats) into circles or concatemers (or a combination of both) by intragenomic or intergenomic recombination through the repeated sequences. During this fusion of the molecular ends of the linear genome, one copy of the terminal repeats is lost (7). Unit-length virion DNA reappears again in the infected cells during the late stages of viral replication, when the CCV genome with both direct repeats is generated by excision from concatameric DNA structures (7). Although no combination of any three subgenomic clones that we constructed contains the whole unit-length virion CCV DNA (e.g., pEEC, pENC, and pESC) (Fig. 1A), each progeny rCCV had both terminal repeats. This demonstrated that genetic recombination between any three appropriate overlapping subgenomic CCV fragments in transfected cells reconstituted infectious virus with terminally repetitive CCV DNA genomes. Furthermore, the reconstituted single BAC was generated by transforming E. coli with DNA from infected cells. This can produce viable BAC clones only when the genome is circular, as occurs during virus infection and DNA replication. The finding that the infectious single BAC lacks one of the repeats supports the suggestion that one of the repeats is lost during the replicative process.
In principle, the strategy of overlapping BACs is applicable, and particularly valuable, to other viruses intransigent to cloning as single-unit BACs. In addition, combinatorial architecture of overlapping fragments should allow the easy construction of viruses with multiple mutations. All herpesviruses contain repeat regions. Overlapping BACs may also facilitate the analysis of herpesvirus repeats, since repeats constructed in different clones can be manipulated independently. Reconstitution of the CCV genome from overlapping fragments leads directly to the generation of wt virus. However, prior to transfection, subgenomic fragments must be released from the BAC vector, and a mix of fragments must be used in transfections. A single-unit CCV BAC simplifies and accelerates the construction of CCV recombinants for applications for which the presence of the BAC vector in the CCV genome is of no significance. In addition, this approach should allow the construction of full-length herpesvirus BACs without the deletion of any viral sequences (a BAC cassette can be inserted into an intergenic region). We used our overlapping fragments to construct a full-length BAC virus.
The BAC cassette in the CCV-ΔTK virus is also flanked by loxP sites. Thus, if necessary, the whole BAC could be removed from CCV-ΔTK, or any of its future descendants, by targeted, site-specific recombination by using cre recombinase to produce vector-free progeny (only one 34-bp loxP site will be left in the CCV genome). A mutant similar to CCV-ΔTK, TK-negative CCV (CCVTK−), was constructed previously (38) by homologous recombination in CCO cells. CCVTK− is attenuated in vivo, and approximately 100-fold more CCVTK− is required to kill the same number of fish as the wt CCV (38). We expect that CCV-ΔTK would also be attenuated similar to the previously reported (and genetically equivalent) CCV TK− (38).
Relative to other economically important herpesviruses of animals, and certainly compared with herpesviruses of medical importance, little molecular virology has been done on CCV. This sparsity of information is due to the small size of the research community and the relatively small research investment. One of the advantages of our new CCV BAC infectious clone system, however, is that it democratizes CCV molecular virology by making CCV molecular virology much easier and less expensive. We wished to demonstrate this utility and chose to first target CCV ORF12 by constructing a deletion mutant. We chose ORF12 because although it is unclear whether CCV ORF12 is a kinetically immediate-early (19) or early (31) gene, CCV ORF12 was identified to have a putative RING finger motif (9). We speculated that the CCV ORF12 gene product, like other herpesvirus RING finger proteins (17, 21, 36), may be beneficial for virus replication in vivo. Other herpesvirus RING finger proteins are not “essential”; i.e., they can be deleted, and the virus will still replicate in vitro. We thus used our CCV infectious clone to rapidly test our hypothesis that CCV ORF12 is nonessential for viral replication in cell culture, and this proved to be the case. In the future, we can quickly produce desired CCV ORF12 gene mutants to test further hypotheses about ORF12 that are directly relevant to CCV pathogenesis and, because of the unique evolutional niche of CCV, of comparative interest. This could include whether ORF12, as other herpesvirus RING finger proteins (15, 17, 21, 36), is important for CCV immune escape.
Ours is the first report of the generation of an infectious BAC system for the group of herpesviruses that are phylogenetically distant from the better-characterized herpesviruses of birds and mammals (12). Furthermore, we demonstrated the ability to generate wt CCV from the BAC system and applied highly efficient recombinogenic methods to our BAC. These combined systems provide powerful tools to evaluate functions of the genes in replication and pathogenesis. Many CCV genes have homology to other genes in fish and amphibian herpesviruses. Therefore, the application of these tools will strengthen the use of CCV as a model virus for basic studies on this group of pathogens.
Acknowledgments
We thank Richard Michelmore (University of California, Davis, CA) for plasmid pECBAC1, Martin Messerle (Ludwig Maximilians Universität, Munich, Germany) for plasmid pHA2, Neal Copeland (Center for Cancer Research, National Cancer Institute, Frederick, MD) for the EL250 bacteria, and Barry Wanner (Purdue University, West Lafayette, IN) for the gene disruption kit. We thank Fiona McCarthy, Mark Lawrence, Lorelei Ford (all from the College of Veterinary Medicine, MSU), and Karen Coats (Department of Biology, MSU) for valuable discussions.
D.K. was supported by research assistantship from the CVM DBS.
S.C.B., D.K., and L.A.H. designed research; D.K., S.V.H., and I.F.N. performed research; S.C.B. and L.A.H. contributed biological material and reagents; and D.K., S.C.B., and L.A.H. wrote the paper.
This paper is Mississippi Agricultural and Forestry Experiment Station publication number J-11305.
Footnotes
Published ahead of print on 30 January 2008.
REFERENCES
- 1.Adler, H., M. Messerle, M. Wagner, and U. H. Koszinowski. 2000. Cloning and mutagenesis of the murine gammaherpesvirus 68 genome as an infectious bacterial artificial chromosome. J. Virol. 746964-6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutell, C., M. Canning, A. Orr, and R. D. Everett. 2005. Reciprocal activities between herpes simplex virus type 1 regulatory protein ICP0, a ubiquitin E3 ligase, and ubiquitin-specific protease USP7. J. Virol. 7912342-12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowser, P. R., and J. A. Plumb. 1980. Fish cell lines: establishment of a line from ovaries of channel catfish. In Vitro 16365-368. [DOI] [PubMed] [Google Scholar]
- 5.Brune, W., C. Menard, U. Hobom, S. Odenbreit, M. Messerle, and U. H. Koszinowski. 1999. Rapid identification of essential and nonessential herpesvirus genes by direct transposon mutagenesis. Nat. Biotechnol. 17360-364. [DOI] [PubMed] [Google Scholar]
- 6.Brune, W., M. Messerle, and U. H. Koszinowski. 2000. Forward with BACs: new tools for herpesvirus genomics. Trends Genet. 16254-259. [DOI] [PubMed] [Google Scholar]
- 7.Cebrian, J., D. Bucchini, and P. Sheldrick. 1983. “Endless” viral DNA in cells infected with channel catfish virus. J. Virol. 46405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davison, A. J. 1992. Channel catfish virus: a new type of herpesvirus. Virology 1869-14. [DOI] [PubMed] [Google Scholar]
- 10.Davison, A. J. 1998. The genome of salmonid herpesvirus 1. J. Virol. 721974-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davison, A. J., and M. D. Davison. 1995. Identification of structural proteins of channel catfish virus by mass spectrometry. Virology 2061035-1043. [DOI] [PubMed] [Google Scholar]
- 12.Davison, A. J., W. Sauerbier, A. Dolan, C. Addison, and R. G. McKinnell. 1999. Genomic studies of the Lucke tumor herpesvirus (RaHV-1). J. Cancer Res. Clin. Oncol. 125232-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fijan, N. 1968. Progress report on acute mortality of channel catfish fingerlings caused by a virus. Bull. Off. Int. Epizoot. 691167-1168. [PubMed] [Google Scholar]
- 14.Frijters, A. C. J., Z. Zhang, M. van Damme, G.-L. Wang, P. C. Ronald, and R. W. Michelmore. 1997. Construction of a bacterial artificial chromosome library containing large EcoRI and HindIII genomic fragments of lettuce. Theor. Appl. Genet. 94390-399. [Google Scholar]
- 15.Hagglund, R., C. Van Sant, P. Lopez, and B. Roizman. 2002. Herpes simplex virus 1-infected cell protein 0 contains two E3 ubiquitin ligase sites specific for different E2 ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 99631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson, L. A., K. G. Kousoulas, and R. L. Thune. 1994. Channel catfish herpesvirus (CCV) encodes a functional thymidine kinase gene: elucidation of a point mutation that confers resistance to Ara-T. Virology 202659-664. [DOI] [PubMed] [Google Scholar]
- 17.Hewitt, E. W., L. Duncan, D. Mufti, J. Baker, P. G. Stevenson, and P. J. Lehner. 2002. Ubiquitylation of MHC class I by the K3 viral protein signals internalization and TSG101-dependent degradation. EMBO J. 212418-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horsburgh, B. C., M. M. Hubinette, and F. Tufaro. 1999. Genetic manipulation of herpes simplex virus using bacterial artificial chromosomes. Methods Enzymol. 306337-352. [DOI] [PubMed] [Google Scholar]
- 19.Huang, S., and L. A. Hanson. 1998. Temporal gene regulation of the channel catfish virus (ictalurid herpesvirus 1). J. Virol. 721910-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, E. C., D. Yu, J. Martinez de Velasco, L. Tessarollo, D. A. Swing, D. L. Court, N. A. Jenkins, and N. G. Copeland. 2001. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 7356-65. [DOI] [PubMed] [Google Scholar]
- 21.Lorenzo, M. E., J. U. Jung, and H. L. Ploegh. 2002. Kaposi's sarcoma-associated herpesvirus K3 utilizes the ubiquitin-proteasome system in routing class I major histocompatibility complexes to late endocytic compartments. J. Virol. 765522-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahony, T. J., F. M. McCarthy, J. L. Gravel, L. West, and P. L. Young. 2002. Construction and manipulation of an infectious clone of the bovine herpesvirus 1 genome maintained as a bacterial artificial chromosome. J. Virol. 766660-6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messerle, M., I. Crnkovic, W. Hammerschmidt, H. Ziegler, and U. H. Koszinowski. 1997. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc. Natl. Acad. Sci. USA 9414759-14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 25.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 768939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saira, K., Y. Zhou, and C. Jones. 2007. The infected cell protein 0 encoded by bovine herpesvirus 1 (bICP0) induces degradation of interferon response factor 3 and, consequently, inhibits beta interferon promoter activity. J. Virol. 813077-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schumacher, D., B. K. Tischer, W. Fuchs, and N. Osterrieder. 2000. Reconstitution of Marek's disease virus serotype 1 (MDV-1) from DNA cloned as a bacterial artificial chromosome and characterization of a glycoprotein B-negative MDV-1 mutant. J. Virol. 7411088-11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shizuya, H., B. Birren, U. J. Kim, V. Mancino, T. Slepak, Y. Tachiiri, and M. Simon. 1992. Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc. Natl. Acad. Sci. USA 898794-8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith, G. A., and L. W. Enquist. 1999. Construction and transposon mutagenesis in Escherichia coli of a full-length infectious clone of pseudorabies virus, an alphaherpesvirus. J. Virol. 736405-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, G. A., and L. W. Enquist. 2000. A self-recombining bacterial artificial chromosome and its application for analysis of herpesvirus pathogenesis. Proc. Natl. Acad. Sci. USA 974873-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stingley, R. L., and W. L. Gray. 2000. Transcriptional regulation of the channel catfish virus genome direct repeat region. J. Gen. Virol. 812005-2010. [DOI] [PubMed] [Google Scholar]
- 32.Trapp, S., J. von Einem, H. Hofmann, J. Kostler, J. Wild, R. Wagner, M. Beer, and N. Osterrieder. 2005. Potential of equine herpesvirus 1 as a vector for immunization. J. Virol. 795445-5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanderheijden, N., L. A. Hanson, E. Thiry, and J. A. Martial. 1999. Channel catfish virus gene 50 encodes a secreted, mucin-like glycoprotein. Virology 257220-227. [DOI] [PubMed] [Google Scholar]
- 34.van Zijl, M., W. Quint, J. Briaire, T. de Rover, A. Gielkens, and A. Berns. 1988. Regeneration of herpesviruses from molecularly cloned subgenomic fragments. J. Virol. 622191-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, X., L. Lybarger, R. Connors, M. R. Harris, and T. H. Hansen. 2004. Model for the interaction of gammaherpesvirus 68 RING-CH finger protein mK3 with major histocompatibility complex class I and the peptide-loading complex. J. Virol. 788673-8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, X., Y. Ye, W. Lencer, and T. H. Hansen. 2006. The viral E3 ubiquitin ligase mK3 uses the Derlin/p97 endoplasmic reticulum-associated degradation pathway to mediate down-regulation of major histocompatibility complex class I proteins. J. Biol. Chem. 2818636-8644. [DOI] [PubMed] [Google Scholar]
- 37.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 975978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, H. G., and L. A. Hanson. 1995. Deletion of thymidine kinase gene attenuates channel catfish herpesvirus while maintaining infectivity. Virology 209658-663. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, H. G., and L. A. Hanson. 1996. Recombinant channel catfish virus (Ictalurid herpesvirus 1) can express foreign genes and induce antibody production against the gene product. J. Fish Dis. 19121-128. [Google Scholar]