Abstract
To test whether transgenic Epstein-Barr virus nuclear antigen 1 (EBNA1) expression in C57BL/6 mouse lymphocytes causes lymphoma, EBNA1 expressed in three FVB lineages at two or three times the level of latent infection was crossed up to six successive times into C57BL/6J mice. After five or six crosses, 14/36, (38%) EBNA1 transgenic mice, 11/31 (36%) littermate EBNA1-negative controls, and 9/25 (36%) inbred C57BL/6J mice housed in the same facility had lymphoma. These data indicate that EBNA1 does not significantly increase lymphoma prevalence in C57BL/6J mice.
Epstein-Barr Virus (EBV) nuclear protein 1 (EBNA1) is the only EBV protein expressed in all latent infections (27, 34). EBNA1 dimers bind to multiple cognate DNA repeats in the oriP segment of the EBV genome, thereby enhancing EBV episome replication, long-term persistence, and transcription (15, 16, 20, 32, 35, 49). In primary human infection, EBV replicates in oral epithelial cells and converts B lymphocytes to a latency III infection characterized by the expression of six EBNA proteins, two integral membrane proteins (LMPs), EBV-encoded small RNAs (EBER), microRNAs, rightward mRNAs of uncertain function, and cell proliferation (27). EBV infection of primary human B lymphocytes in vitro also results in latency III and cell proliferation; immortal lymphoblastoid cell lines (LCLs) result (27). While recombinant EBV lacking expression of EBNA2, EBNALP, EBNA3A, EBNA3C, LMP1, or EBNA1 are inefficient or null in LCL outgrowth (7, 21, 24, 31, 36), rare LCLs arise following infection with EBV lacking EBNA1, and these grow the same as wild-type-EBV-infected LCLs, indicating that EBNA1 may not be required for EBV effects on cell proliferation (21). Further, expression of a dominant-negative EBNA1 in an LCL with integrated EBV DNA, whose growth is dependent on EBNA2-induced Myc (8), inhibits EBNA1 enhancement of an oriP-dependent episome but does not alter cell growth or gene expression (22). Moreover, EBNA1 enhances oriP-dependent gene expression from episomes to levels similar to those of Gal4-VP16, but is much less active than Gal4-VP16 on gene expression from integrated oriP-dependent reporters (22). Although EBNA1 can increase expression from some integrated oriP-dependent promoter sites that have minimal expression (26), cognate sites have not been found in cell DNA (19). Thus, current evidence favors the hypothesis that EBNA1 does not interact with cognate cell DNA sequences to cause LCL growth or survival.
EBNA1 is expressed with LMP1 and LMP2 in latency II EBV infection in Hodgkin's disease Reed-Sternberg cells and in nasopharyngeal carcinoma and is the only protein expressed in latency I infection in most Burkitt's or other lymphoma cells (5, 10). The persistence of EBV genomes in all cells of these tumors is evidence that an EBV gene product supports tumor cell growth or survival (34). In support of this hypothesis, Akata latency I Burkitt's tumor cells induce tumors in nude mice, while Akata cells that have lost EBV episomes in cell culture do not induce tumors (42). However, EBER expression converts EBV-negative Akata cells to tumor induction in nude mice, whereas EBNA1 expression does not (28, 29, 38, 39). Nevertheless, EBNA1 associates with chromosomes, ubiquitin-specific protease 7, importin, EBP2, and p32TAP and could affect cell growth, survival, or gene expression through these associations (13, 17, 46). Indeed, EBNA1 expression in non-EBV-infected Burkitt tumor lymphoblasts has been associated with changes in cell growth or gene expression in some experiments (14, 25, 26, 48), but not in others (22, 23).
Previous assays of transgene EBNA1 effects on lymphoma prevalence in inbred mice have been inconclusive. In C57BL/6 mice, EBNA1 expression in lymphocytes under an immunoglobulin (Ig) heavy-chain enhancer (Eμ) and a polyomavirus promoter resulted in one lineage with very low EBNA1 expression and uniform death from malignant lymphoma at 6 to 9 months of age and another lineage with higher EBNA1 expression, no premature death, and increased lymphoma histology in 19- to 24-month-old mice (44, 47). In another study, EBNA1 transgene expression in three FVB lineages under Eμ and promoter at levels higher than LCLs did not change survival, weight, serum IgG, IgM, total Ig, spleen size, lymphocyte numbers, lymphocyte types, lymphoid organ size, and spleen histology (23). A lymphoma and a histiocytic sarcoma were found in 111 autopsied EBNA1 transgenic mice versus none in 63 littermate control mice (23). However, FVB mice have a much lower propensity for lymphoma than C57BL/6J mice and may be less sensitive to an EBNA1 effect on lymphoma incidence (3, 4, 30).
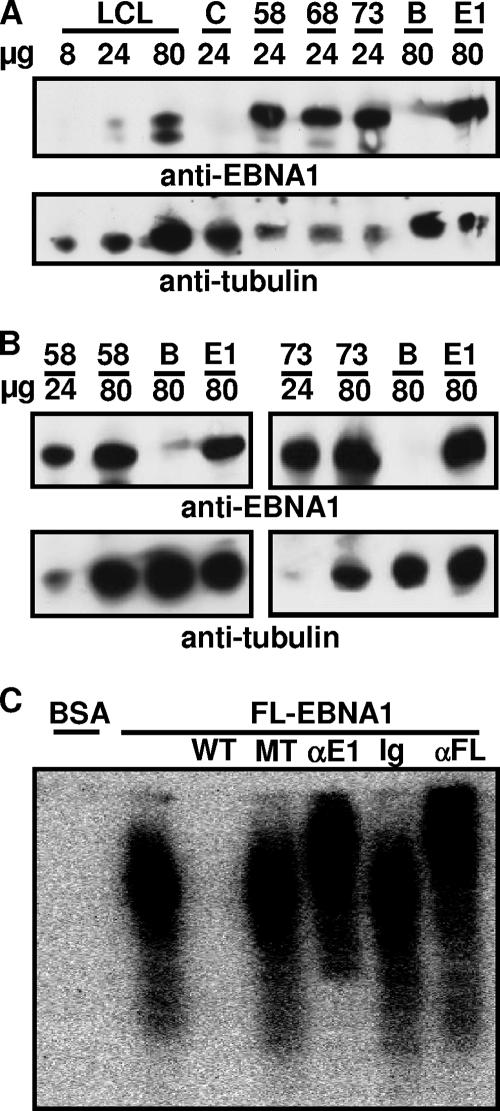
To further evaluate whether EBNA1 expression causes lymphoma in C57BL/6J mice, Flag-EBNA1 transgenic mice from the three FVB lineages were successively crossed up to six times to C57BL/6J inbred mice. EBNA1 transgene inheritance was identified by PCR, and EBNA1 effects on lymphoma incidence were assayed in the increasingly C57BL/6J genetic background (5, 10). At the level of the second cross into C57BL/6J, the lineage 58, 68, and 73 transgene EBNA1 DNA sequences, exclusive of the Gly-Ala-encoding repeat, were determined after PCR amplification from whole-genome DNA. The DNA sequences were identical to the initial transgene Flag-tagged EBNA1 DNA sequence (23). EBNA1 transgene expression in spleen extracts was two- to threefold higher than in LCLs relative to alpha tubulin and was comparable to Flag-EBNA1 expression in BJAB B lymphoma cells (Fig. 1A and B). EBNA1 in the three C57BL/6J lineages was also the same size as the starting EBNA1 (Fig. 1A and B) (23). Moreover, Flag-tagged EBNA1, from spleen extracts of lineage 68 and 73 C57BL/6J mice, specifically shifted cognate probe DNA and was supershifted by antibody to the Flag tag or to EBNA1 (Fig. 1C and data not shown).
FIG. 1.
The EBNA1 transgene in FVB cross C57BL/6J mouse spleen cells. (A and B) After the second cross of the EBNA1 transgene from FVB to C57BL/6J mice, spleen extracts from each of three Flag-tagged EBNA1 transgenic lineages (58, 68, and 73) or control C57BL/6J mice (C) were assayed for protein with Bradford reagent and dissolved in sodium dodecyl sulfate sample buffer at 95°C, and 8, 24, or 80 μg of protein was loaded onto a denaturing polyacrylamide gel, along with similar amounts of protein from IB4 LCLs (LCL), from Flag-tagged EBNA1-expressing BJAB cells (E1), or from BJAB cells (B), and was blotted with anti-EBNA1 or anti-alpha tubulin antibody (23). (C) Spleen extracts were incubated with labeled family of repeats probe in the absence or presence of 500-fold excess unlabeled wild-type (WT) or mutant (MT) competitor or anti-EBNA1 (αE1), anti-Flag (αFL), or Ig isotype (Ig) control antibody (23). BSA, bovine serum albumin.
Progeny mice from generations 3 to 6 of successive EBNA1 mouse matings with C57BL/6J mice (87% to 98.5% C57BL/6J) were maintained for 17 to 20 months under microisolator barrier conditions in a biosafety level 1 facility. Premature deaths were recorded, and postmortem gross and histological analyses were done, when tissues were adequately preserved (Table 1). The mice were sacrificed at 17 to 20 months to assess changes in spleen and lymph node anatomy and histology that would be indicative of lymphoma. Postmortem examinations included dissection and microscopic analysis of spleen, lung, and kidney and of liver, lymph node, or gastrointestinal tissues that appeared abnormal.
TABLE 1.
EBNA1 and lymphoma prevalences in C57BL6/J mice
| Parameter
|
Value
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| C57BL/6J | Littermate control | EBNA1 | |||||||
| No. of FVB × C57BL/6J crosses | 3 | 4 | 5 | 6 | 3 | 4 | 5 | 6 | |
| Total no. | 28 | 7 | 21 | 6 | 30 | 19 | 16 | 13 | 27 |
| No. that died | 9 | 5 | 3 | 3 | 12 | 6 | 3 | 0 | 12 |
| No. with histology | 25 | 4 | 18 | 6 | 25 | 14 | 13 | 13 | 23 |
| No. with lymphoma | 9 | 0 | 0 | 2 | 9 | 0 | 0 | 5 | 9 |
At the fifth and sixth C57BL/6J crosses, 17- to 20-month-old EBNA1 transgenic and littermate control mice were assessed for body weight and spleen size and weight. At the fifth C57BL/6J cross, EBNA1 transgenic mice (n = 9) did not differ from littermate control (n = 6) mice in body weight (47.19 ± 7.03 g versus 45.22 ± 10.31 g), spleen size (1.47 ± 0.15 cm versus 1.58 ± 0.14 cm), or spleen weight (0.12 ± 0.02 g versus 0.12 ± 0.02 g). Similarly, at the sixth C57BL/6J cross, EBNA1 (n = 16) and littermate control (n = 12) mice were similar in body weight (39.7 ± 7.5 g versus 41.8 ± 7.5 g), spleen size (1.76 ± 0.62 cm versus 1.91 ± 0.59 cm), and spleen weight (0.73 ± 0.87 g versus 0.84 ± 0.98 g). Thus, 17- to 20-month-old transgenic EBNA1 mice after five or six crosses with C57BL/6J mice did not differ from littermate control mice in body weight or spleen size or weight.
C57BL/6J mice purchased for mating and housed in the same room had spontaneous mortality of 9/28 (31%) before sacrifice at 17 to 20 months. Of the nine prematurely dead mice, six were adequate for postmortem histology. Histology of these 6 and the remaining 19, which were sacrificed at 17 to 20 months, revealed 9/25 (36%) with lymphoma and 1/25 (4%) with pulmonary adenoma (Table 1).
In EBNA1-negative littermate control mice (n = 36) from five and six crosses of mice from the three FVB lineages with C57BL/6J mice, the spontaneous mortality was 15/36 (42%). Among these 15, 10 were adequate for postmortem histology. Histology of these 10 and the remaining 21, which were sacrificed at 17 to 20 months, revealed 11/31 (35%) with lymphoma and 1/31 (3%) with pulmonary adenoma, very similar to the 36% lymphoma and 4% pulmonary adenoma prevalences in the inbred control C57BL/6J mouse group (Table 1). Thus, C57BL/6J and EBNA1-negative littermate control mice had 35% to 36% lymphoma prevalence, an appropriate prevalence to observe an EBNA1 effect.
In EBNA1 transgene-positive mice from five and six crosses with C57BL6/J mice (n = 40), the spontaneous mortality was 12/40 (30%), similar to the 9/28 (31%) in C57BL/6J mice and the 15/36 (42%) in littermate control mice. Of the 12 spontaneous deaths in EBNA1 transgenic mice, 8 allowed postmortem histology. Histology of these 8 and the remaining 28, which were sacrificed at 17 to 20 months, revealed 14/36 (39%) with lymphoma and 2/36 (5.6%) with pulmonary adenoma (Table 1). Thus, lymphoma developed in 14/36 (39%) EBNA1 transgene-positive mice at the fifth and sixth crosses to C57BL/6J mice, similar to the 11/31 (35%) with lymphoma in EBNA1-negative littermate mice, the 9/25 (36%) in C57BL/6J mice, and the 31 to 36% reported for C57BL/6 mice (3, 4, 12). Fisher's exact test comparison of EBNA1 transgenic mice with littermate control mice yielded values of 0.51 (one tailed) and 1 (two tailed), as expected for nearly identical rates. These data indicate that EBNA1 does not significantly increase lymphomas in C57BL/6J mice.
Lymphomas were not observed in EBNA1 transgenic or littermate control mice until the fifth and sixth crosses to C57BL/6J mice; 5/13 transgenic EBNA1 mice at the fifth cross had lymphoma, and at the sixth cross, 9/23 had lymphoma, while 2/6 littermate control mice at the fifth cross had lymphoma and 9/25 at the sixth cross had lymphoma. Also, although the numbers were smaller, the incidences of lymphoma at the sixth cross among mice from the original EBNA1 transgenic FVB lineages was similar, for example, 39% and 39% in lineage 58 and 73 EBNA1-positive mice, respectively, and 36% and 33% in lineage 58 and 73 littermate control mice. Thus, in at least two separate lineages, we have sufficient data to exclude the uniform mortality observed previously in one lineage (47) or the frequent lymphoma histology observed in aged mice in another lineage (47).
This study was undertaken to evaluate whether EBNA1 expression causes lymphoma in lymphoma-prone C57BL/6J inbred mice. Our data indicate that EBNA1 expression at a higher level than is ordinarily associated with latent EBV infection does not increase lymphoma prevalence in C57BL/6J mice. These data are in marked contrast to those obtained in a previous study in which EBNA1 RNA expression with very low protein expression was associated with uniform mortality in 6- to 9-month-old C57BL/6 mice and higher EBNA1 protein expression was associated with only increased prevalence of lymphoma histology in aged mice (47). The uniform mortality in one lineage in the previous study is similar to that with transgenic c-myc overexpression and was likely due to an insertion effect on a host proto-oncogene (1, 2, 9, 41, 45), whereas the increase in lymphoma histology in the second lineage was likely due to the increased variability in C57BL/6 mice in an open (47) versus a barrier (23) facility (37).
The absence of an EBNA1 protein effect on tumor incidence in C57BL/6J mice is important for an understanding of EBNA1 effects in EBV latency-associated cell growth. Together with the recent finding that EBV lacking EBNA1 is inefficient in initiation of LCL outgrowth but that integration of EBV DNA into cells results in otherwise-typical latency III LCL outgrowth (21), these data support the hypothesis that EBNA1 is not a substantial cause of increased lymphocyte growth or survival. Relative safety is important for the use of EBNA1 and an oriP episome vector system in human gene therapy. Retroviral vectors have been associated with rare leukemias due to integration near LMO2 (6, 33, 43), and adeno-associated virus has been associated with hepatocellular carcinomas in mice (11).
We cannot exclude the possibility that EBNA1 overexpression at these levels could have an effect on cell survival or growth. EBNA1 transgenic FVB mice had a 39% prevalence of pulmonary adenomas versus 7% in littermate control animals (23). At the third transgenic EBNA1 cross with C57BL/6J mice, 14 EBNA1 transgenic mice had three pulmonary adenomas (Table 1), whereas 4 littermate control animals had zero and 25 C57BL/6J mice had one. With crosses 4 to 6, only two pulmonary adenomas were found in 49 EBNA1 transgenic animals, and one was found in 49 control animals. These limited data are consistent with an EBNA1 increase in pulmonary adenoma prevalence in pulmonary adenoma-prone FVB mice and not in lymphoma-prone C57BL/6J mice. The three pulmonary adenomas in sections of 14 EBNA1 transgenic mice at cross 3 into the C57BL/6J line could be due to persistence of FVB susceptibility in these animals, which are still 12.5% FVB. EBNA1 may interact with the specific FVB predisposition to pulmonary adenoma to increase adenoma prevalence, since there was no effect in the higher-cross C57BL/6J background. Since the previous FVB data may have been biased by a more thorough search for tumors in the EBNA1 transgenic FVB mice, a blinded study of EBNA1 effects on FVB predisposition to pulmonary adenoma is needed to substantiate the putative pulmonary adenoma affect. EBNA1 overexpression can displace p53 from USP7, enhance p53 degradation, and protect cells from apoptosis (18, 40), consistent with the possibility that even threefold EBNA1 overexpression might increase pulmonary adenoma prevalence in FVB mice. Whether such effects are evident at EBNA1 levels in latent EBV infections can be determined by comparing p53 levels and cell survival in LCLs or other cells infected with EBV versus an EBV that has a null mutation in the EBNA1-USP7 interaction domain.
Acknowledgments
We gratefully acknowledge the support of grant CA47006 from the National Cancer Institute and National Institutes of Health and advice from Ellen Cahir McFarland and Arlene Sharpe.
Footnotes
Published ahead of print on 6 February 2008.
REFERENCES
- 1.Adams, J. M., A. W. Harris, W. Y. Langdon, C. A. Pinkert, R. L. Brinster, R. D. Palmiter, L. Corcoran, W. S. Alexander, M. W. Graham, and S. Cory. 1986. c-myc-induced lymphomagenesis in transgenic mice and the role of the Pvt-1 locus in lymphoid neoplasia. Curr. Top. Microbiol. Immunol. 1321-8. [DOI] [PubMed] [Google Scholar]
- 2.Adams, J. M., A. W. Harris, C. A. Pinkert, L. M. Corcoran, W. S. Alexander, S. Cory, R. D. Palmiter, and R. L. Brinster. 1985. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318533-538. [DOI] [PubMed] [Google Scholar]
- 3.Babbitt, J. T., A. I. Kharazi, J. M. Taylor, C. B. Bonds, S. G. Mirell, E. Frumkin, D. Zhuang, and T. J. Hahn. 2000. Hematopoietic neoplasia in C57BL/6 mice exposed to split-dose ionizing radiation and circularly polarized 60 Hz magnetic fields. Carcinogenesis 211379-1389. [PubMed] [Google Scholar]
- 4.Babbitt, J. T., A. I. Kharazi, J. M. Taylor, C. B. Bonds, D. Zhuang, S. G. Mirell, E. Frumkin, and T. J. Hahn. 2001. Increased body weight in C57BL/6 female mice after exposure to ionizing radiation or 60Hz magnetic fields. Int. J. Radiat. Biol. 77875-882. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, L., Q. Y. Yao, A. B. Rickinson, and L. S. Young. 1992. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J. Virol. 662689-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinen, J., and J. M. Puck. 2004. Successes and risks of gene therapy in primary immunodeficiencies. J. Allergy Clin. Immunol. 113595-603. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, J. I., F. Wang, J. Mannick, and E. Kieff. 1989. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc. Natl. Acad. Sci. USA 869558-9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, A., E. Johannsen, S. Maruo, E. Cahir-McFarland, D. Illanes, D. Davidson, and E. Kieff. 2003. EBNA3A association with RBP-Jκ down-regulates c-myc and Epstein-Barr virus-transformed lymphoblast growth. J. Virol. 77999-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cory, S., A. W. Harris, W. Y. Langdon, W. S. Alexander, L. M. Corcoran, R. D. Palmiter, C. A. Pinkert, R. L. Brinster, and J. M. Adams. 1987. The myc oncogene and lymphoid neoplasia: from translocations to transgenic mice. Haematol. Blood Transfus. 31248-251. [DOI] [PubMed] [Google Scholar]
- 10.Deacon, E. M., G. Pallesen, G. Niedobitek, J. Crocker, L. Brooks, A. B. Rickinson, and L. S. Young. 1993. Epstein-Barr virus and Hodgkin's disease: transcriptional analysis of virus latency in the malignant cells. J. Exp. Med. 177339-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donsante, A., D. G. Miller, Y. Li, C. Vogler, E. M. Brunt, D. W. Russell, and M. S. Sands. 2007. AAV vector integration sites in mouse hepatocellular carcinoma. Science 317477. [DOI] [PubMed] [Google Scholar]
- 12.Festing, M. F. 1996. What can gerontologists tell us about the choice of rat strain for toxicological research and screening? Hum. Exp. Toxicol. 15602-604. [PubMed] [Google Scholar]
- 13.Fischer, N., E. Kremmer, G. Lautscham, N. Mueller-Lantzsch, and F. A. Grasser. 1997. Epstein-Barr virus nuclear antigen 1 forms a complex with the nuclear transporter karyopherin α2. J. Biol. Chem. 2723999-4005. [DOI] [PubMed] [Google Scholar]
- 14.Flavell, J. R., K. R. Baumforth, V. H. Wood, G. L. Davies, W. Wei, G. M. Reynolds, S. Morgan, A. Boyce, G. L. Kelly, L. S. Young, and P. G. Murray. 2008. Down-regulation of the TGF-beta target gene, PTPRK, by the Epstein-Barr virus encoded EBNA1 contributes to the growth and survival of Hodgkin's lymphoma cells. Blood 111292-301. [DOI] [PubMed] [Google Scholar]
- 15.Frappier, L., and M. O'Donnell. 1992. EBNA1 distorts oriP, the Epstein-Barr virus latent replication origin. J. Virol. 661786-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frappier, L., and M. O'Donnell. 1991. Epstein-Barr nuclear antigen 1 mediates a DNA loop within the latent replication origin of Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 8810875-10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holowaty, M. N., Y. Sheng, T. Nguyen, C. Arrowsmith, and L. Frappier. 2003. Protein interaction domains of the ubiquitin-specific protease, USP7/HAUSP. J. Biol. Chem. 27847753-47761. [DOI] [PubMed] [Google Scholar]
- 18.Holowaty, M. N., M. Zeghouf, H. Wu, J. Tellam, V. Athanasopoulos, J. Greenblatt, and L. Frappier. 2003. Protein profiling with Epstein-Barr nuclear antigen-1 reveals an interaction with the herpesvirus-associated ubiquitin-specific protease HAUSP/USP7. J. Biol. Chem. 27829987-29994. [DOI] [PubMed] [Google Scholar]
- 19.Horner, D., M. Lewis, and P. J. Farrell. 1995. Novel hypotheses for the roles of EBNA-1 and BHRF1 in EBV-related cancers. Intervirology 38195-205. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh, D. J., S. M. Camiolo, and J. L. Yates. 1993. Constitutive binding of EBNA1 protein to the Epstein-Barr virus replication origin, oriP, with distortion of DNA structure during latent infection. EMBO J. 124933-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humme, S., G. Reisbach, R. Feederle, H. J. Delecluse, K. Bousset, W. Hammerschmidt, and A. Schepers. 2003. The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proc. Natl. Acad. Sci. USA 10010989-10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang, M. S., S. C. Hung, and E. Kieff. 2001. Epstein-Barr virus nuclear antigen 1 activates transcription from episomal but not integrated DNA and does not alter lymphocyte growth. Proc. Natl. Acad. Sci. USA 9815233-15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang, M. S., H. Lu, T. Yasui, A. Sharpe, H. Warren, E. Cahir-McFarland, R. Bronson, S. C. Hung, and E. Kieff. 2005. Epstein-Barr virus nuclear antigen 1 does not induce lymphoma in transgenic FVB mice. Proc. Natl. Acad. Sci. USA 102820-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaye, K. M., K. M. Izumi, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 909150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy, G., J. Komano, and B. Sugden. 2003. Epstein-Barr virus provides a survival factor to Burkitt's lymphomas. Proc. Natl. Acad. Sci. USA 10014269-14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy, G., and B. Sugden. 2003. EBNA-1, a bifunctional transcriptional activator. Mol. Cell. Biol. 236901-6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2574. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 28.Komano, J., S. Maruo, K. Kurozumi, T. Oda, and K. Takada. 1999. Oncogenic role of Epstein-Barr virus-encoded RNAs in Burkitt's lymphoma cell line Akata. J. Virol. 739827-9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komano, J., M. Sugiura, and K. Takada. 1998. Epstein-Barr virus contributes to the malignant phenotype and to apoptosis resistance in Burkitt's lymphoma cell line Akata. J. Virol. 729150-9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahler, J. F., W. Stokes, P. C. Mann, M. Takaoka, and R. R. Maronpot. 1996. Spontaneous lesions in aging FVB/N mice. Toxicol. Pathol. 24710-716. [DOI] [PubMed] [Google Scholar]
- 31.Maruo, S., E. Johannsen, D. Illanes, A. Cooper, and E. Kieff. 2003. Epstein-Barr virus nuclear protein EBNA3A is critical for maintaining lymphoblastoid cell line growth. J. Virol. 7710437-10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Middleton, T., and B. Sugden. 1992. EBNA1 can link the enhancer element to the initiator element of the Epstein-Barr virus plasmid origin of DNA replication. J. Virol. 66489-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nienhuis, A. W., C. E. Dunbar, and B. P. Sorrentino. 2006. Genotoxicity of retroviral integration in hematopoietic cells. Mol. Ther. 131031-1049. [DOI] [PubMed] [Google Scholar]
- 34.Oudejans, J. J., D. F. Dukers, N. M. Jiwa, A. J. van den Brule, F. A. Grasser, P. C. de Bruin, A. Horstman, W. Vos, J. van Gorp, J. M. Middeldorp, and C. J. Meijer. 1996. Expression of Epstein-Barr virus encoded nuclear antigen 1 in benign and malignant tissues harbouring EBV. J. Clin. Pathol. 49897-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rawlins, D. R., G. Milman, S. D. Hayward, and G. S. Hayward. 1985. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell 42859-868. [DOI] [PubMed] [Google Scholar]
- 36.Robertson, E. S., S. Grossman, E. Johannsen, C. Miller, J. Lin, B. Tomkinson, and E. Kieff. 1995. Epstein-Barr virus nuclear protein 3C modulates transcription through interaction with the sequence-specific DNA-binding protein J. kappa. J. Virol. 693108-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Cuesta, J., F. Vidal-Vanaclocha, L. Mendoza, M. Valcarcel, N. Gallot, and G. Martinez de Tejada. 2005. Effect of asymptomatic natural infections due to common mouse pathogens on the metastatic progression of B16 murine melanoma in C57BL/6 mice. Clin. Exp. Metastasis 22549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruf, I. K., P. W. Rhyne, C. Yang, J. L. Cleveland, and J. T. Sample. 2000. Epstein-Barr virus small RNAs potentiate tumorigenicity of Burkitt lymphoma cells independently of an effect on apoptosis. J. Virol. 7410223-10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruf, I. K., P. W. Rhyne, H. Yang, C. M. Borza, L. M. Hutt-Fletcher, J. L. Cleveland, and J. T. Sample. 1999. Epstein-Barr virus regulates c-Myc, apoptosis, and tumorigenicity in Burkitt lymphoma. Mol. Cell. Biol. 191651-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saridakis, V., Y. Sheng, F. Sarkari, M. N. Holowaty, K. Shire, T. Nguyen, R. G. Zhang, J. Liao, W. Lee, A. M. Edwards, C. H. Arrowsmith, and L. Frappier. 2005. Structure of the p53 binding domain of HAUSP/USP7 bound to Epstein-Barr nuclear antigen 1: implications for EBV-mediated immortalization. Mol. Cell 1825-36. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt, E. V., P. K. Pattengale, L. Weir, and P. Leder. 1988. Transgenic mice bearing the human c-myc gene activated by an immunoglobulin enhancer: a pre-B-cell lymphoma model. Proc. Natl. Acad. Sci. USA 856047-6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimizu, N., A. Tanabe-Tochikura, Y. Kuroiwa, and K. Takada. 1994. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt's lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J. Virol. 686069-6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Themis, M., S. N. Waddington, M. Schmidt, C. von Kalle, Y. Wang, F. Al-Allaf, L. G. Gregory, M. Nivsarkar, M. V. Holder, S. M. Buckley, N. Dighe, A. T. Ruthe, A. Mistry, B. Bigger, A. Rahim, T. H. Nguyen, D. Trono, A. J. Thrasher, and C. Coutelle. 2005. Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice. Mol. Ther. 12763-771. [DOI] [PubMed] [Google Scholar]
- 44.Tsimbouri, P., M. E. Drotar, J. L. Coy, and J. B. Wilson. 2002. bcl-xL and RAG genes are induced and the response to IL-2 enhanced in EmuEBNA-1 transgenic mouse lymphocytes. Oncogene 215182-5187. [DOI] [PubMed] [Google Scholar]
- 45.Vaux, D. L., S. Cory, and J. M. Adams. 1988. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335440-442. [DOI] [PubMed] [Google Scholar]
- 46.Wang, Y., J. E. Finan, J. M. Middeldorp, and S. D. Hayward. 1997. P32/TAP, a cellular protein that interacts with EBNA-1 of Epstein-Barr virus. Virology 23618-29. [DOI] [PubMed] [Google Scholar]
- 47.Wilson, J. B., J. L. Bell, and A. J. Levine. 1996. Expression of Epstein-Barr virus nuclear antigen-1 induces B cell neoplasia in transgenic mice. EMBO J. 153117-3126. [PMC free article] [PubMed] [Google Scholar]
- 48.Wood, V. H., J. D. O'Neil, W. Wei, S. E. Stewart, C. W. Dawson, and L. S. Young. 2007. Epstein-Barr virus-encoded EBNA1 regulates cellular gene transcription and modulates the STAT1 and TGFβ signaling pathways. Oncogene 264135-4147. [DOI] [PubMed] [Google Scholar]
- 49.Wysokenski, D. A., and J. L. Yates. 1989. Multiple EBNA1-binding sites are required to form an EBNA1-dependent enhancer and to activate a minimal replicative origin within oriP of Epstein-Barr virus. J. Virol. 632657-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]