Abstract
An attenuated derivative of simian immunodeficiency virus strain 239 deleted of V1-V2 sequences in the envelope gene (SIV239ΔV1-V2) was used for vaccine/challenge experiments in rhesus monkeys. Peak levels of viral RNA in plasma of 104 to 106.5 copies/ml in the weeks immediately following inoculation of SIV239ΔV1-V2 were 10- to 1,000-fold lower than those observed with parental SIV239 (∼107.3 copies/ml). Viral loads consistently remained below 200 copies/ml after 8 weeks of infection by the attenuated SIV239ΔV1-V2 strain. Viral localization experiments revealed large numbers of infected cells within organized lymphoid nodules of the colonic gut-associated lymphoid tissue at 14 days; double-labeling experiments indicated that 93.5% of the virally infected cells at this site were positive for the macrophage marker CD68. Cellular and humoral immune responses measured principally by gamma interferon enzyme-linked immunospot and neutralization assays were variable in the five vaccinated monkeys. One monkey had responses in these assays comparable to or only slightly less than those observed in monkeys infected with parental, wild-type SIV239. Four of the vaccinated monkeys, however, had low, marginal, or undetectable responses in these same assays. These five vaccinated monkeys and three naïve control monkeys were subsequently challenged intravenously with wild-type SIV239. Three of the five vaccinated monkeys, including the one with strong anti-SIV immune responses, were strongly protected against the challenge on the basis of viral load measurements. Surprisingly, two of the vaccinated monkeys were strongly protected against SIV239 challenge despite the presence of cellular anti-SIV responses of low-frequency and low-titer anti-SIV antibody responses. These results indicate that high-titer anti-SIV antibody responses and high-frequency anti-SIV cellular immune responses measurable by standard assays from the peripheral blood are not needed to achieve strong vaccine protection, even against a difficult, neutralization-resistant strain such as SIV239.
The characteristics of human immunodeficiency virus type 1 (HIV-1) infection suggest major difficulty for the development of a preventive vaccine (19, 23). Pessimism regarding the prospects for a vaccine is derived at least in part from the ability of HIV-1 to continually replicate in the face of apparently strong host immune responses, resistance to antibody-mediated neutralization, and the extensive sequence diversity in field strains of the virus. Lack of knowledge regarding the key components of a protective immune response also remains a major scientific obstacle. Vaccine/challenge experiments with macaque monkeys have been used to evaluate the properties and relative effectiveness of different vaccine approaches and to gauge the formidable nature of these difficulties.
One lesson that has been learned from vaccine/challenge experiments with macaque monkeys is the importance of challenge strain on outcome. Vaccinated monkeys that have been challenged with strains of simian immunodeficiency virus (SIV) with an HIV-1 envelope (SHIV) have almost invariably exhibited strong, long-term protection against disease, irrespective of the nature of the vaccine. Even peptide immunogens have protected against SHIV-induced disease (6, 12, 38). Vaccine approaches that have protected against SHIV challenge include DNA (5, 13), recombinant poxvirus (4), recombinant adenovirus (57), other viral recombinants (18, 55), prime and boost protocols (3, 53, 65), and purified protein (10, 64). Vaccine protection against pathogenic SIV strains such as SIV239, SIV251, and SIV-E660 has been much more difficult to achieve (2, 11, 27, 63). The identical replication-defective gag-recombinant adenovirus that provided strong protection against SHIV challenge (57) provided little or no protection against SIV239 challenge (11). Disappointing levels of protection against SIV have often been observed in the face of apparently robust vaccine-induced immune responses (see, for example, Vogel et al. [63] and Casimiro et al. [11]). Some partial vaccine protections against these SIV strains have been achieved by recombinant poxvirus (7, 50), replication-competent recombinant adenovirus (51), replication-defective adenovirus (66), recombinant poliovirus (15), recombinant Venezuelan equine encephalitis virus (18), and recombinant Sendai virus (44).
Differences between the biological properties of the SIV strains and those of the SHIV strains used for the above-mentioned studies provide clues as to what may be responsible for the differences in outcome. These SIV strains are difficult to neutralize (26, 34), use CCR5 as a coreceptor for entry into cells (21, 52), and induce a chronic, progressive disease course (17), and this course is independent of the infectious dose (17). The SHIV strains used for the above-mentioned studies are easier to neutralize, use CXCR4 for entry, and induce an acute decline in CD4 counts, and the disease course is dose dependent (29, 30, 48, 54). These SIV strains, like HIV-1 in humans, exhibit a marked preference for CD4+ CCR5+ memory cells, in contrast to the acutely pathogenic SHIV strains which principally target naïve cells (48).
Live, attenuated strains of SIV have provided the strongest vaccine protection by far against SIV challenge. Although clinical use of a live, attenuated HIV vaccine is not being considered, understanding the basis of the strong protection afforded by live, attenuated SIV strains remains an important research objective for the insights that can be provided. Most of the attenuated SIV strains that have been used lack a functional nef gene (16, 31, 58, 67). Shacklett et al. (56) used an attenuated SIV strain with modifications in the gp41 transmembrane protein for protection. Here, we describe strong vaccine protection by a replication-competent SIV strain lacking 100 amino acids from the essential gp120 envelope protein in the absence of overtly robust immune responses.
MATERIALS AND METHODS
Monkey infections.
Stocks of SIV were prepared by transfection of cultured cells with cloned DNA and harvest of the cell-free supernatant at or near the peak of virus production. The rhesus monkey T-cell line 221 has been described previously (1). Monkeys were infected by intravenous inoculation. When virus stock was diluted for inoculation, RPMI medium without serum was used. The concentrations of p27 were determined by antigen capture with a Coulter kit according to the manufacturer's recommendations.
Rhesus macaques (Macaca mulatta) were housed at the New England Primate Research Center in an animal biosafety level 3 containment facility in accordance with standards of the Association for Assessment and Accreditation of Laboratory Animal Care and the Harvard Medical School Animal Care and Use Committee. Research was conducted according to the principles described in the Guide for the Care and Use of Laboratory Animals and was approved by the Harvard Medical School Animal Care and Use Committee (47). Monkeys were typed for 10 Mamu class I alleles by sequence-specific PCR (35, 37). All animals were also tested and found to be free of simian retrovirus type D, SIV, simian T-lymphotrophic virus type 1, and herpes B virus prior to assignment.
Preparation of rhesus macaque PBMC.
Rhesus macaque peripheral blood mononuclear cells (PBMC) were isolated from fresh citrate blood by density gradient centrifugation (Ficoll 1077; Sigma). For analysis of viral replication in culture, lymphocytes in PBMC samples were activated for 72 h with 1 μg of phytohemagglutinin (Sigma, St. Louis, MO) per ml R10, washed in RPMI 1640, and incubated in R10 supplemented with 10% interleukin-2 overnight before infection.
Major histocompatibility complex (MHC) class I tetramer staining.
Peripheral blood was analyzed by four-color flow cytometry for SIV-specific CD8+ T cells. Whole blood (100 μl) was stained for 25 min at 37°C with CD3-fluorescein isothiocyanate (Becton Dickinson, San Jose, CA) and CD8-PerCP (Becton Dickinson) monoclonal antibodies and allophycocyanin-conjugated Mamu-A*01 Gag181-189 tetramers (generously provided by John Altman, Emory University). Samples were then treated for 10 min with fluorescence-activated cell sorter lysing solution (Becton Dickinson) to remove erythrocytes. Cells were washed and fixed in 2% paraformaldehyde-phosphate-buffered saline. Data were collected on a FACSCalibur flow cytometer (Becton Dickinson) and analyzed using the FlowJo software package (Tree Star, San Carlos, CA).
IFN-γ ELISPOT assays.
Gamma interferon (IFN-γ)-producing T-cell responses were enumerated using an enzyme-linked immunospot (ELISPOT) assay for detection of macaque IFN-γ (Mabtech, Mariemont, OH). PBMC were stimulated in duplicate wells at 3 × 105, 1 × 105, and 3 × 104 cells per well with peptide pools (15-mer peptides overlapping by 11 amino acids at 2.5 μg/ml each) representing the complete amino acid sequences of SIVmac239 proteins. Peptides were either synthesized by the Massachusetts General Hospital core peptide facility or obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. PBMC were incubated overnight in multiscreen plates (Millipore) coated with an IFN-γ capture antibody, and spots representing IFN-γ-producing T cells were detected in an enzyme-linked, colorimetric assay for bound IFN-γ. For analyses of purified CD8+ lymphocytes, PBMC were depleted by StemSep negative cell separation (Stem Cell Technologies, Vancouver, British Columbia), resulting in cell populations with less than 8% residual CD4+ T cells. Spots were counted using an automated ELISPOT plate reader (Zellnet Consulting, New York, NY). To determine the frequency of virus-specific IFN-γ-producing T cells per million PBMC, the number of background spots in medium control wells was subtracted from the number of spots in peptide-stimulated wells. Based on analysis of ELISPOT responses to Gag and Env in unimmunized control monkeys, >40 spot-forming cells (SFC) per 106 PBMC was considered significant.
ICS analysis.
Detection of antigen-specific T lymphocytes by use of intracellular cytokine staining (ICS) was performed as previously described (24) with minor modifications. Briefly, thawed cryopreserved PBMC were incubated with an SIV Gag peptide pool or R10 medium alone for 6 h at 37°C in the presence of cross-linked costimulatory anti-CD28 and anti-CD49d and brefeldin A (Sigma, 10 μg/ml), which was added to the culture for the last 4.5 h of stimulation. Stimulated cells were surface stained with PerCP-conjugated anti-CD4 antibody and subsequently fixed and permeabilized using FACS lysing solution (BD Biosciences) and FACS permeabilizing solution (BD Biosciences). Permeabilized cells were incubated with PE-conjugated anti-CD69 and an anti-tumor necrosis factor alpha (anti-TNF-α) antibody conjugated to allophycocyanin. Samples were analyzed on a FACSCalibur (BD Biosciences), lymphocytes were gated on forward and side scatter, and the proportions of CD3+ CD4+ and CD3+ CD4− T lymphocytes coexpressing CD69 and TNF-α were determined using FloJo (Tree Star, Ashland, OR). All values are reported with the frequency of TNF+ CD69+ events in unstimulated cells (which was <0.05%) subtracted.
Analysis of SIV-specific antibody responses.
Binding of antibodies to SIV proteins was analyzed by Western blot analysis. Commercially available SIV Western blot strips (ZeptoMetrix, Buffalo, NY) were probed with plasma samples at a dilution of 1:100 and developed according to the manufacturer's instructions. Titers of neutralizing antibodies to SIV239, to the laboratory-adapted strain SIV251, and to SIV239ΔV1-V2 were determined by the ability to block infection of CEMx174SIV-SEAP cells harboring a Tat-inducible SEAP reporter construct as described previously (45). Serial twofold dilutions of plasma were incubated with virus at 100 μl per well in 96-well plates for 1 h before the addition of 4 × 104 CEMx174SIVSEAP cells (100 μl) for a final volume of 200 μl. After 48 to 72 h for SIV239 and laboratory-adapted strain SIV251 and longer periods for SIV239ΔV1-V2, 18 μl of supernatant was removed and SEAP activity was determined using a Phospha-Light SEAP detection kit (Applied Biosystems, Foster City, CA) and a LumaPlate reader. Control wells for mock-infected cells and virus treated with pooled SIV-negative plasma were used to determine background and maximal SEAP production, respectively. The percent neutralization was calculated from the mean SEAP counts at each plasma dilution divided by the maximal SEAP counts after subtracting background activity. Fifty percent neutralization titers were calculated as the reciprocal of the plasma dilution at which infectivity was reduced by 50%.
Viral loads and CD4+ T-cell counts.
Viral loads in plasma were determined using a quantitative real-time reverse transcriptase PCR assay (41). CD4+ T-lymphocyte counts were calculated from the total lymphocyte population as determined by complete blood count analysis and the percentage of CD4 T cells as determined by flow cytometry (68).
Viral localization.
To examine distribution during acute viremia, immunohistochemistry and in situ hybridization for SIVmac were performed on formalin-fixed paraffin-embedded tissues as previously described (43). Immunofluorescence and confocal microscopy were performed using a previously described protocol (25). Macrophages were labeled using the anti-CD68 monoclonal antibody (KP1, 5 μg/ml; DAKO, Carpenteria, CA) followed by a fluorochrome-conjugated donkey anti-mouse immunoglobulin G secondary antibody (donkey anti-mouse 488; Molecular Probes, Eugene, OR). Sections were then stained for SIV by using an anti-SIVnef monoclonal antibody (KK75, 5 μg/ml; kindly provided by K. Kent and C. Arnold through the NIBSC Centre for AIDS Reagents) followed by a fluorochrome-conjugated donkey anti-goat immunoglobulin G secondary antibody (donkey anti-goat 568; Molecular Probes, Eugene, OR). Confocal microscopy was performed using a Leica TCS SP laser scanning microscope (Leica Microsystems, Exton, PA) fitted with a Leica objective (1.4 numerical aperture; PL APO) and using Leica image software. Colocalization for SIV and dendritic cell markers was examined by first performing in situ hybridization for SIV nucleic acid, using a 5-bromo,4-chloro,3-indolylphosphate (BCIP)-nitroblue tetrazolium chromogen, followed by immunohistochemistry for DC-SIGN (clone 120612; R & D Systems), using a gold label technique.
RESULTS
Vaccine phase.
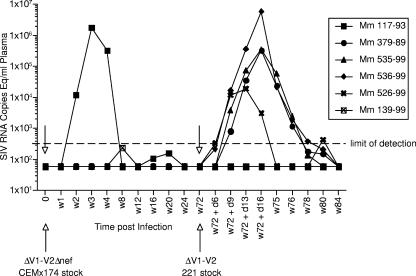
A derivative of SIV239 lacking 100 amino acids that encompass all of the V1-V2 region of gp120 Env is still replication competent but requires point mutations in Env, particularly in gp41, for optimal replication (32). We prepared a stock of SIV239ΔV1-V2Δnef by transfection of cloned DNA into CEMX174 cells and harvest of the supernatant 28 days following transfection. The deletion in nef has also been previously described (20, 36). This stock virus contained 72 ng/ml p27 as determined by antigen capture measurements. Each of six rhesus monkeys was inoculated intravenously with this SIV239ΔV1-V2Δnef stock, containing 50 ng p27. On the basis of viral loads in plasma, SIV recovery, and anti-SIV antibody responses as determined by an enzyme-linked immunosorbent assay, only one of the six inoculated monkeys showed clear evidence of a take of the infection (Fig. 1 and Table 1). This infected monkey, 117-93, exhibited peak viral loads and a strength of anti-SIV antibody response that were similar to the loads and responses in an initial two monkeys that were infected with SIV239ΔV1-V2 described previously (33).
FIG. 1.
Viral RNA loads in plasma during the vaccine phase. A stock of SIV239ΔV1-V2Δnef produced in CEMX174 cells was used for inoculation of all six monkeys on day (d) 0, and a stock of SIV239ΔV1-V2 produced in the rhesus monkey 221 cell line was used for inoculation of all six animals again on week 72 (w72). Virus containing 50 ng p27 was used for the inoculation of each animal on day 0, and virus containing 52 ng p27 was used for the inoculation of each animal at week 72. Plasma samples were obtained at the indicated times and used for quantitation of levels of viral RNA as described in Materials and Methods. The dashed line indicates the copy limit of detection for these analyses.
TABLE 1.
Summary of “takes” of SIV239ΔV1-V2 inoculations
| Animal no. | Virus loads after first inoculationa | Antibodies after first inoculation | Virus loads after second inoculationb | Antibodies after second inoculation |
|---|---|---|---|---|
| 117-93 | Yes | Yes (persisting) | No | Persisting |
| 379-89 | No | No | Yes | Yes |
| 535-99 | No | No | Yes | Yes |
| 536-99 | No | No | Yes | Yes |
| 526-99 | No | No | Yes | Yes |
| 139-99 | No | No | No | No |
SIV239ΔV1-V2Δnef (>400 viral RNA copy equivalents per ml of plasma).
SIV239ΔV1-V2 (>200 viral RNA copy equivalents per ml of plasma).
Because of the apparent lack of take in five of the six inoculated rhesus monkeys, we prepared a new stock by different means for reinoculation of these same monkeys. A stock of SIV239ΔV1-V2 with an intact nef gene was produced by transfection of cloned DNA into the rhesus monkey 221 T-cell line and harvest of the supernatant 27 days after transfection. This SIV239ΔV1-V2 stock contained 52 ng/ml p27. Each of the six rhesus monkeys was inoculated with this SIV239ΔV1-V2 stock, containing 52 ng p27, 72 weeks after the initial inoculation. On the basis of viral loads in plasma, SIV recovery, and anti-SIV antibody responses, four of the five monkeys that were refractory to the initial inoculation became infected following the second inoculation at week 72 (Fig. 1 and Table 1). One of the six monkeys, 139-99, showed no clear evidence of a take of infection from either of the two inoculations (Fig. 1 and Table 1). Monkey 117-93, which was clearly infected from the first inoculation, was apparently “protected” against the SIV239ΔV1-V2 challenge at week 72 in that viral RNA loads were not detected in the plasma of this animal after the second inoculation.
Challenge phase.
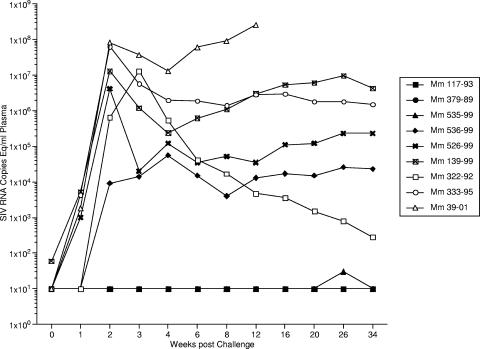
All six of these rhesus monkeys and three unvaccinated controls were challenged intravenously with 20 intravenous infectious doses of SIV239 110 weeks after the initial inoculation of SIV239ΔV1-V2Δnef, 38 weeks after the inoculation of SIV239ΔV1-V2. Blood samples were taken at regular intervals following the wild-type (WT) SIV239 challenge to monitor a number of parameters, including viral RNA loads in plasma. The three control monkeys showed a typical spike in viral RNA loads in plasma 14 to 20 days following the SIV239 inoculation (Fig. 2). The average value of 5 × 107 RNA copies per ml of plasma in these three control monkeys compares favorably with results for historical controls that have received this same challenge stock (3 × 107 RNA copies per ml) (33). One of three control monkeys (39-01) developed soaring viral loads, and this monkey was a rapid progressor in that it had to be sacrificed because of ill health 14 weeks after the WT SIV239 challenge. One of the other control monkeys (322-92) was unusual in that its viral loads were progressively brought under control, to fewer than 10,000 RNA copies per ml by 3 months after infection and fewer than 1,000 RNA copies per ml by 6 months after infection. Monkey 322-92 was Mamu-A*04 positive (Mamu-A*04+) but negative for the B*08 and B*17 alleles which have been associated with improved control of SIV replication (42, 69) (Table 2). The other control animal was more typical of Indian origin rhesus monkeys infected with SIV239 that are not rapid progressors (33) in that its viral loads 8 to 30 weeks after infection were in the 1 × 106 to 3 × 106 range (Fig. 2).
FIG. 2.
Viral RNA loads in plasma during the challenge phase. A stock of SIV239 was used for challenge of all six vaccinated monkeys and three naïve controls on day 0 of the challenge phase, 110 weeks after the initial inoculation of SIV239ΔV1-V2nef. Monkeys were challenged intravenously with 20 intravenous infectious doses of SIV239 (0.0031 ng p27). This titers of this stock were previously determined by the intravenous route in rhesus monkeys (39), and this stock was successfully used by our laboratories and the laboratories of others on numerous occasions (2, 22, 27, 63). Plasma samples were obtained at the indicated times relative to SIV239 challenge and used for quantitation of levels of viral RNA as described in Materials and Methods. Open symbols illustrate the data of the three naïve controls. The copy limit of detection for most of these assays was 30 or lower. Samples with RNA levels below the limit of reliable detection were assigned a value of 10 for the purpose of graphing.
TABLE 2.
MHC typinga
| Monkey no. | Vaccine | Result for indicated allele
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A*01 | A*02 | A*03 | A*04 | A*08 | A*11 | B*01 | B*03 | B*04 | B*08 | B*17 | ||
| 117-93 | ΔV1ΔV2 | + | − | − | − | + | − | + | − | − | − | − |
| 379-89 | ΔV1ΔV2 | − | + | − | + | − | − | − | − | − | − | − |
| 526-99 | ΔV1ΔV2 | + | − | − | − | + | − | − | − | − | − | − |
| 535-99 | ΔV1ΔV2 | − | − | − | + | − | − | + | − | − | + | − |
| 536-99 | ΔV1ΔV2 | − | − | − | − | + | − | − | − | − | − | − |
| 139-99 | ΔV1-V2 | − | − | − | + | − | − | − | − | − | − | − |
| 322-92 | None | − | − | − | + | − | − | − | − | − | − | − |
| 333-95 | None | + | − | − | − | − | + | − | − | − | − | − |
ND, not done.
Three of the five rhesus monkeys that had a “take” of V1-V2-deleted vaccine were strongly protected against the WT challenge. Monkeys 117-93, 379-89, and 535-99 had viral loads of fewer than 100 RNA copy equivalents per ml of plasma at all 11 samplings over an 8-month period, including weeks 1, 2, 3, and 4 postchallenge (Fig. 2). Vaccinated monkeys 536-99 and 526-99 showed partial and no protection, respectively. The postchallenge viral loads at peak height in 536-99 were 3 logs lower than those seen in unvaccinated control monkeys (Fig. 2). Vaccinated monkey 526-99 exhibited no discernible protective effects against subsequent challenge. Monkey 139-99, which exhibited no “take” of either of the vaccine strain inoculations, also exhibited postchallenge viral loads that were indistinguishable from those in unvaccinated control monkeys (Fig. 2).
Immune response measurements.
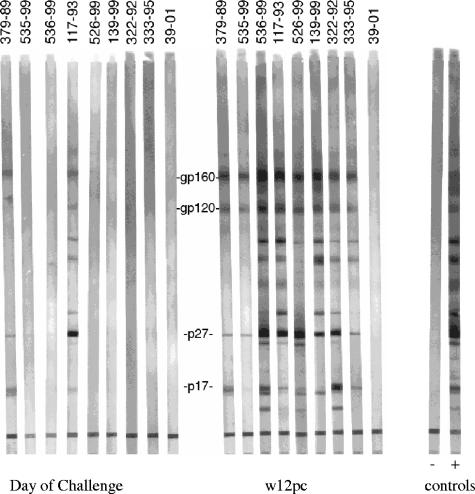
Plasma samples taken on the day of challenge with WT SIV239 were reacted with SIV proteins on commercial Western blot strips (Fig. 3). Only plasma samples from 117-93 and 379-99 scored positive for the presence of anti-SIV antibodies as determined by Western blot analysis at this time point. 117-93 showed the strongest reactivity. Monkeys that were not strongly protected against challenge (monkeys 536-99, 526-99, and 139-99) exhibited strong inductions of anti-SIV antibodies as determined by Western blot analysis by 12 weeks postchallenge (Fig. 3). Naïve control monkeys that were not rapid progressors (322-92 and 333-95) also showed strong inductions of anti-SIV antibodies as determined by Western blot analysis. Strongly protected monkeys 535-99 and 117-93 showed increases in anti-SIV antibodies as determined by Western blot analysis from the day of challenge to 12 weeks postchallenge (Fig. 3). Strongly protected monkey 379-99 showed little or no increase in anti-SIV antibodies as determined by Western blot analysis.
FIG. 3.
Western blot reactivity of plasma. Plasma samples taken on the day of challenge with WT SIV239 and 12 weeks postchallenge (w12pc) were reacted with SIV proteins on commercial Western blot strips.
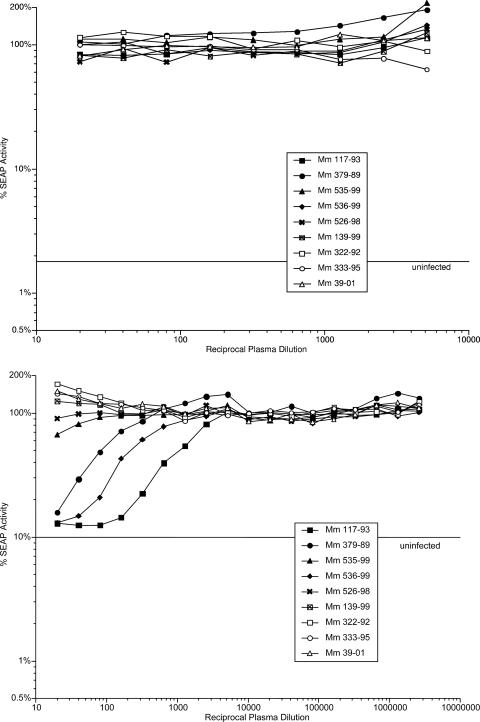
Plasma samples taken at various times during vaccine and challenge phases were used to assess the ability to neutralize the infectivity of SIV239, laboratory-adapted strain SIV251, and SIV239ΔV1-V2. None of the plasma samples tested had measurable neutralizing activity against the difficult-to-neutralize strain SIV239. The easier-to-neutralize, laboratory-adapted strain SIV251 was neutralized by many of the plasma samples (Fig. 4 and Table 3). Plasma from 117-93, but not that from the other monkeys, showed neutralizing activity against laboratory-adapted strain SIV251 after the SIV239ΔV1-V2Δnef inoculation, prior to the SIV239ΔV1-V2 inoculation. Low but consistent neutralizing activity was detected beginning 8 weeks after SIV239ΔV1-V2 inoculation of 379-89, and this activity persisted through the challenge phase at similar levels. Strongly increased levels of neutralizing activity were observed after WT SIV239 challenge in the vaccinated monkeys that were not strongly protected (536-99, 526-99, and the “no-vaccine-take” monkey 139-99) (Table 3). Protected monkey 535-99 exhibited a low titer of 1:25 at 8 weeks after SIV239ΔV1-V2 inoculation, but neutralizing activity greater than 50% at a 1:20 dilution was not observed again, even after WT SIV239 challenge. It should be noted that, except for 117-93, the vaccine phase neutralizing titers in the vaccinated monkeys were extremely low. Pools of SIV-positive (SIV+) plasma from infected monkeys yielded titers of 1:1,920 to 1:5,260 against laboratory-adapted strain SIV251 in the same assays run in parallel.
FIG. 4.
Neutralizing activity in plasma. Serial dilutions of plasma were used to measure the ability to neutralize laboratory-adapted strain SIV251 in the CEMX174 SIV-LTR-SEAP reporter cell line (45). Data from only selected time points are shown. Top, day of initial inoculation with SIV239ΔV1-V2Δnef; bottom, day of WT SIV239 challenge. Fifty percent neutralization titers are presented in Table 3.
TABLE 3.
Neutralization of laboratory-adapted strain SIV251a
| Monkey no. | Titer at indicated time pointb
|
||||||
|---|---|---|---|---|---|---|---|
| Day of ΔV1ΔV2 inoculationb | Wk 8 p.i. | Wk 28 p.i. | Day of SIV239 challengec | Wk 2 p.c. | Wk 12 p.c. | Wk 26 p.c. | |
| Mm 379-89 | Negative | 1:160 | 1:80 | 1:80 | 1:120 | 1:60 | 1:80 |
| Mm 535-99 | Negative | 1:25 | Negative | Negative | Negative | Negative | Negative |
| Mm 536-99 | Negative | 1:640 | 1:140 | 1:240 | 1:240 | 1:2,240 | 1:4,480 |
| Mm 117-93 | 1:320 | 1:560 | 1:480 | 1:1,120 | 1:1,120 | 1:640 | 1:1,920 |
| Mm 526-99 | Negative | 1:25 | Negative | Negative | Negative | 1:800 | 1:2,560 |
| Mm 139-99 | Negative | Negative | Negative | Negative | Negative | 1:2,560 | >1:5,120 |
| Mm 322-92 | ND | ND | ND | Negative | Negative | 1:1,280 | 1:2,560 |
| Mm 333-95 | ND | ND | ND | Negative | Negative | 1:1,280 | 1:4,480 |
| Mm 39-01 | ND | ND | ND | Negative | Negative | Negative | ND |
Dilutions that yielded 50% neutralization are shown. Different pools of SIV+ rhesus monkey plasma gave titers of 1:1,920 to 1:5,120 when tested in parallel with these assays. p.i., postinoculation; p.c., after WT SIV239 challenge; ND, not done.
16 July 2002.
9 April 2003.
Day-of-challenge plasma samples from all monkeys were also analyzed for neutralizing activity against the extremely sensitive strain SIV239ΔV1-V2. In this case, neutralizing activity was detected in all five vaccinated monkeys with a vaccine “take” (Table 4). The rank order of titers was the same as that observed with laboratory-adapted strain SIV251 (Tables 3 and 4). Again, except for 117-93, the titers were in general extremely low compared to the titers of 1:20,480 and 1:17,920 in two different pools of SIV+ rhesus monkey plasma in the same assay run in parallel. Monkey 536-99 had the second highest titer of neutralizing antibodies after 117-93 (Table 3 and 4), and 536-99 was not one of the three strongly protected monkeys.
TABLE 4.
Neutralization of SIV239ΔV1-V2a
| Monkey no. | Titer on day of SIV239 challenge |
|---|---|
| Mm 379-89 | 1:1,280 |
| Mm 535-99 | 1:160 |
| Mm 536-99 | 1:4,480 |
| Mm 117-93 | 1:17,920 |
| Mm 526-99 | 1:40 |
| Mm 139-99 | Negative |
| Mm 322-92 | Negative |
| Mm 333-95 | Negative |
| Mm 39-01 | Negative |
Dilutions that yielded 50% neutralization with plasma taken on the day of WT SIV239 challenge are shown. Different pools of SIV+ rhesus monkey plasma gave titers of 1:17,920 and 1:20,480 when tested in parallel with these assays.
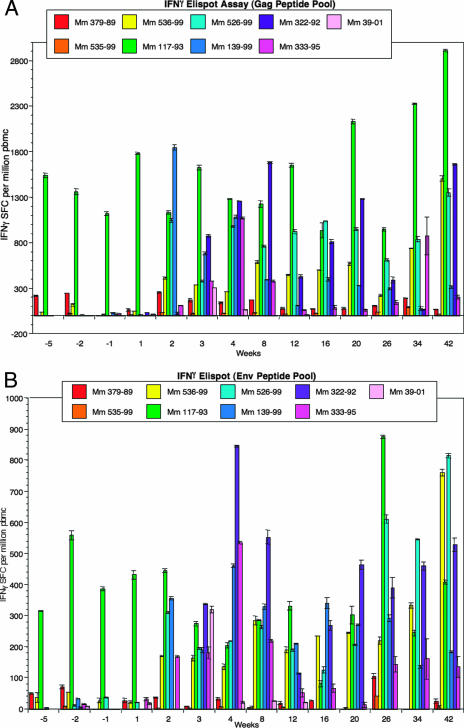
Anti-SIV IFN-γ ELISPOT responses were measured at 10 different time points, including time points prior to and after challenge (weeks −5 to +12 relative to the day of challenge), by using fresh PBMC samples. Separate assays were run with Gag peptide pools and with Env peptide pools. Strongly protected monkey 117-93 showed 1,100 to 1,500 SFC per million responsive to Gag prior to challenge and 1,200 to 1,700 SFC per million responsive to Gag after challenge (Fig. 5A). Strongly protected monkey 379-89 showed fewer than 50 to 200 SFC per million responsive to Gag prior to challenge and very similar levels after challenge (Fig. 5A). Strongly protected monkey 535-99 showed fewer than 50 SFC per million responsive to Gag both prior to and after challenge (Fig. 5A). Monkey 536-99, which exhibited little or no protection, showed 100 SFC per million responsive to Gag at only one of three time points prior to challenge but consistently exhibited 300 to 520 SFC per million responsive to Gag after challenge. Neither 526-99 nor 139-99 (the unprotected monkeys) had detectable ELISPOT reactivity prior to challenge, but both had relatively strong responses to Gag (1,000 to 1,800 SFC/106 PBMC) detectable 2 weeks after infection. The unvaccinated control monkeys 322-92, 333-95, and 39-01 developed lower levels of anti-IFN-γ ELISPOT responses (300 to 800 SFC/106 cells) in the weeks following challenge (Fig. 5A).
FIG. 5.
Cellular responses measured by IFN-γ ELISPOT. The abilities of SIV peptide pools to elicit IFN-γ secretion in cultured PBMC by ELISPOT assay were quantitated as described in Materials and Methods. The indicated weeks are relative to SIV239 challenge. (A) Gag peptide pool. (B) Env peptide pool.
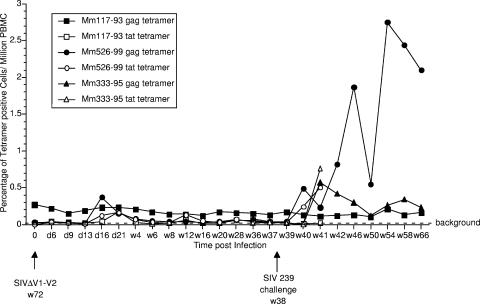
Vaccinated monkeys 117-93 and 526-99 and control monkey 333-95 were Mamu-A*01+, which allowed analysis of CD8 responses by staining with MHC class I tetramers. Monkey 117-93 showed consistent, persisting levels of Mamu-A*01 Gag-CM9 tetramer staining in the range of 0.15 to 0.3% of CD8+ peripheral blood T lymphocytes prior to and after challenge (Fig. 6). Monkey 117-93 showed a spike in Tat tetramer staining 21 days after the SIV239ΔV1-V2 inoculation (Fig. 6), suggesting that, despite the absence of measurable viral loads following this inoculation of this monkey, there was sufficient viral replication to stimulate a recall response to this epitope. Monkey 526-99 showed clear Gag-CM9 and Tat-SL8 responses, as measured by tetramer staining at multiple time points following the SIV239ΔV1-V2 inoculation, which subsequently waned to background levels. The persistence of the Gag tetramer response in 117-93 contrasts with its lack of persistence in 526-99. Both Gag and Tat tetramer responses increased in the unprotected monkey 526-99 by 2 weeks after the WT SIV239 challenge (Fig. 6).
FIG. 6.
MHC class I tetramer staining of SIV-specific CD8+ cells. MCH class I tetramers for the Mamu A*01 gag CM9 epitope and the Mamu A*01 tat SL8 epitope were used to stain CD8+ cells from the Mamu-A*01+ monkeys in the study. Mm 333-95 is the naïve, unvaccinated control monkey that was Mamu-A*01+. d, day; w, week.
Lower numbers of Env-specific cells were observed with ELISPOT, but the results closely parallel those obtained with the Gag ELISPOT assays. Monkey 117-93 consistently had persistent anti-Env IFN-γ ELISPOT responses of 200 to 600 SFC/106 both before and after challenge (Fig. 5B). Before challenge, IFN-γ anti-Env ELISPOT responses in the other eight animals were around or below the limit of detection (Fig. 5B). Seven of the nine monkeys had readily detectable IFN-γ anti-Env ELISPOT responses by 3 weeks after challenge (Fig. 5B). However, strongly protected monkeys 379-89 and 535-99 did not have detectable anti-Env IFN-γ ELISPOT activity around the time of challenge (week −2) or at any time point immediately after challenge (Fig. 5B).
ELISPOT responses were also performed using CD8+ lymphocytes purified from PBMC that were cryopreserved on the day of challenge. Cells were stimulated with overlapping peptide pools corresponding to all SIV proteins. Significant responses from 117-93 were observed only for Gag and Env, and these responses were consistent with those obtained using fresh PBMC (Table 5). Macaque 379-89 had measurable responses to Gag and the Vif/Vpr/Vpx pool that were just above the limit of detection and a significant response to Nef. No significant responses to any protein were observed in 526-99, 535-99, or 536-99 (Table 5).
TABLE 5.
ELISPOT responses to SIV peptide pools by CD8+ T cells from vaccinated animalsa
| Monkey no. | No. of SPC for indicated peptide
|
||||||
|---|---|---|---|---|---|---|---|
| Gag | Env | Nef | Tat | Rev | V3b | Pol | |
| 535-99 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 117-93 | 264 | 53 | 0 | 0 | 0 | 0 | 0 |
| 379-89 | 0 | 0 | 172 | 0 | 0 | 40 | 0 |
| 526-99 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 536-99 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
ELISPOT assays were performed using thawed, cryopreserved PBMC from the day of challenge that were depleted of CD4+ T cells, resulting in >90% CD8+ T cells. Results are reported as numbers of SPC per 106 CD8+ T cells.
Pool of Vpr, Vpx, and Vif peptides.
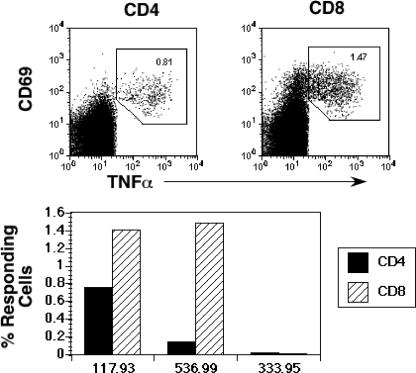
We also carried out ICS on cryopreserved samples of PBMC from selected vaccinated animals prior to challenge. As predicted from ELISPOT and tetramer assays, the strongly protected animal 117-93 had relatively strong CD4 (0.75%) and CD8 (1.7%) responses to Gag (Fig. 7). Significant CD8+ but not CD4+ T-cell responses were observed in the poorly protected macaque 536-99 (Fig. 7). No significant responses were observed prior to challenge in the unvaccinated animal 333-95. Lack of sufficient cryopreserved PBMC precluded analysis of prechallenge samples in the remaining animals.
FIG. 7.
ICS analysis of SIV Gag-specific responses in vaccinated and unvaccinated animals. Cryopreserved PBMC obtained 5 weeks before challenge were stimulated with an SIV Gag peptide pool and responding cells identified based on upregulation of CD69 and TNF-α. Background levels of CD69+ TNF-α+ cells in unstimulated samples ranged from 0.0% to 0.05% and were subtracted from the reported values of responding cells.
SIV239ΔV1-V2 tissue and cell distribution.
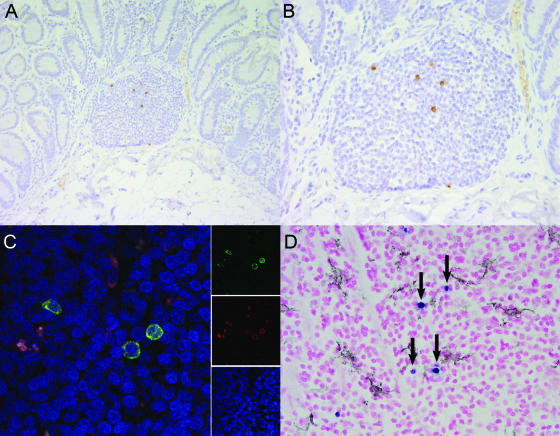
Distribution of SIV239ΔV1-V2 was independently investigated with a separate group of immunologically normal adult rhesus macaques following intravenous inoculation of SIV239ΔV1-V2 containing 100 ng of p27. At 2 weeks postinoculation, viral antigen was localized by immunohistochemistry and the presence of viral RNA was visualized by in situ hybridization using multiple organs, including thymus, peripheral and mesenteric lymph nodes, spleen, and colon. The greatest viral burden was found principally within the organized lymphoid follicles of colonic gut-associated lymphoid tissue (Fig. 8A and B). Virally infected cells were absent from the lamina propria of the small and large intestines. Within these organized lymphoid follicles, there were on average 4.5 viral positive cells per 40× field, compared to <0.1 positive cells for spleen or peripheral lymph node. With the SIVmac239 parental strain, large numbers of SIV-infected cells are typically found in the lamina propria of the large and small intestines by 14 days following inoculation (60, 62).
FIG. 8.
Tissue distribution of SIVmac239ΔV1V2 during acute viremia. (A and B) Localization by immunohistochemistry of SIVmac239ΔV1V2 in gut-associated lymphoid tissue revealed viral infection primarily of organized lymphoid nodules and absence from the lamina propria. (C) Colocalization of SIV and CD68 in the mesenteric lymph node of SIVmac239ΔV1V2 inoculated rhesus macaque revealed that 93.5% of SIV-infected cells are also CD68 positive. Images for individual panels (red, CD68 [KP-1]; green, SIV nef; and blue, ToPro-3 nuclear marker) are shown on the right and merged to form a composite image on the left. Yellow-to-orange areas indicate colocalization of SIV and CD68. (D) Colocalization failed to demonstrate that DC-SIGN-expressing dendritic cells were targeted. SIV-infected cells demonstrated by in situ hybridization are in blue (arrows); DC-SIGN-positive dendritic cells demonstrated by immunohistochemistry are in black.
The lamina propria contains predominantly activated and terminally differentiated lymphocytes and is considered an effector lymphoid tissue. Lymphocytes within the organized lymphoid follicles are mostly naïve and are considered to be within the inductive compartment of the lymphoid tissue. The initial targets of WT SIVmac239 infection in the gastrointestinal tract are believed to be terminally differentiated lymphocytes within the effector lymphoid compartment, the lamina propria (60, 62). In contrast, SIV239ΔV1-V2 was localized principally to the organized lymphoid follicles of gut-associated lymphoid tissue at 14 days in our current study. At this acute time point, virus could not be detected in brain, lung, liver, bone marrow, kidney, or male or female reproductive tract, suggesting that viral tropism had not been expanded by deletion of the V1-V2 loops. By 28 days following inoculation, SIV could not be localized by these means in any tissue of two additional animals examined; however, moderate-to-marked follicular hyperplasia was present in peripheral and mesenteric lymph nodes.
To further define infected cell types, confocal microscopy was performed to identify early targets of SIV239ΔV1-V2 infection. Double-label experiments for SIV and CD3 or CD68 were conducted on gastrointestinal and lymphoid tissues. These analyses revealed tissue macrophages to be the predominant cell type infected (Fig. 8C). A total of 93.5% of SIV-infected cells in gastrointestinal lymphoid tissue were also positive for the macrophage marker KP1. CD3+ T lymphocytes appeared to constitute the remainder of the SIV+ cells. This contrasts with the results for strains SIV239, SIVmac251, and SIVmac316, for which lymphocytes remain the principal target cell at early time points (8). Studies done to assess colocalization for a dendritic cell marker by immunohistochemistry and that for SIV nucleic acid by in situ hybridization failed to demonstrate productive infection of DC-SIGN-positive cells (Fig. 8D).
DISCUSSION
Immunization with live, attenuated strains of SIV has provided the most robust protection from challenge with pathogenic SIV demonstrated to date, but the basis of this protection and the immune correlates associated with it have remained obscure. In the current studies, we vaccinated animals with attenuated SIV strains in which 100 amino acids had been deleted from the V1-V2 loop coding sequences, with or without an intact nef coding sequence. Animals were initially inoculated with the ΔV1-V2Δnef construct but were subsequently immunized using a ΔV1-V2 stock after a failure to demonstrate a take of the ΔV1-V2Δnef virus in most of the animals. The basis for the variability of the take, particularly in the first set of SIV239ΔV1-V2Δnef inoculations, is not entirely clear at this time. There are several possible explanations or contributing factors. The absence of nef may have sufficiently debilitated this already attenuated strain such that its infectivity for rhesus monkeys is limited (59). Also, this stock was produced in the human TxB hybrid cell line CEMx174; it is possible that compensatory changes that adapted its replication to CEMx174 cells may not have served the virus well for replication in rhesus monkeys. However, the take of SIV239ΔV1-V2Δnef was exceptionally strong and durable in monkey 117-93. It is possible that a minor variant was responsible for the exceptionally good outgrowth in monkey 117-93. Further work will be needed to define the sequence changes that adapt V1-V2-deleted SIV to optimal replication in rhesus monkeys and the nature of the cells that support replication in vivo.
The vaccine protections in monkeys 379-89 and 535-99 are particularly remarkable considering how unimpressive the anti-SIV immune responses were in these two animals. The protection in these two cases cannot be explained by the lack of infectious WT SIV239 in the challenge inoculum. Over the several years previous to this challenge, we had inoculated nine naïve control rhesus monkeys with the same dose of the same stock by the same route as part of four separate studies and all became infected. The three naïve control monkeys challenged in parallel in the current experiments also became infected. Control monkeys receiving the same dose of the same challenge virus subsequent to these studies have also all become infected. In addition, monkey 535-99, together with 117-93, appeared to have increased levels of anti-SIV antibodies measurable by Western blot analysis following the challenge.
Monkey 535-99 had no detectable anti-SIV neutralizing activity even to the laboratory-adapted, neutralization-sensitive strain SIV251 on the day of challenge and a weak titer of 1:160 against the very neutralization-sensitive strain SIV239ΔV1-V2. This monkey also did not have anti-SIV antibodies detectable by Western blot analysis on the day of challenge. Monkey 379-89 had a paltry 50% neutralization titer of 1:80 for laboratory-adapted strain SIV251 on the day of challenge. This contrasts with the results for monkeys infected with WT SIV239 or nef-deleted SIV239, which typically exhibit titers of 1:3,000 to 1:6,000 against laboratory-adapted strain SIV251 in this same assay. Neither of these monkeys raised detectable neutralizing activity against SIV239. Monkeys 535-99 and 379-89 also had undetectable or low anti-SIV Gag and Env IFN-γ ELISPOT activity around the time of challenge and after challenge. This remarkable control in the absence of potent anti-SIV immune responses measurable by standard assays from the peripheral blood (Table 6) is reminiscent of the control exhibited in monkeys treated with the reverse transcriptase inhibitor tenofovir for 28 days starting 24 h after intravenous SIV inoculation (40, 41). However, this contrasts markedly with results from Horton et al. (27), Vogel et al. (63), and Casimiro et al. (11) in which anti-SIV immune responses of high magnitude were achieved by DNA prime and recombinant poxvirus or recombinant adenovirus boosting; these impressive anti-SIV immune responses measured with comparable assays provided little or no protection against the same challenge stock of homologous SIV239.
What could be responsible for the remarkable protections reported here and in the experiments of Lifson et al. (40, 41)? Quite clearly, there must be something special about the nature of the immune response in monkeys 535-99 and 379-89. Perhaps a relatively small number of viral-specific, reactive cells in the periphery have particularly good effector function, or perhaps the presence of appropriate viral-specific helper cell activity may be critical for containment of the challenge virus. Although we detected significant SIV-specific CD4+ T-cell responses to Gag in strongly protected monkey 117-93, these studies could not be performed with 535-99 and 379-89. The expression of all viral gene products by the vaccine could also be a critical factor; however, whole-proteome ELISPOT analysis with a subset of vaccinated animals revealed only limited responses to proteins other than Gag and Env. It is also possible that a localized immune response in gut-associated lymphoid tissue, not necessarily evident in the periphery, is critical for the protection. It is now generally believed that CCR5-using SIVs and HIVs replicate principally in the gut-associated lymphoid tissue in the early days and weeks following primary infection (9, 46, 60). This location is where activated, CCR5-positive memory cells that are the ideal targets for viral replication predominate. It is conceivable that early, effective containment at that site during the vaccine phase could provide effective immunity against a CCR5-using virus challenge even when the challenge virus is administered intravenously.
By 14 days after infection with SIV239ΔV1-V2, viral replication vastly predominated in the gut-associated lymphoid compartment on the basis of in situ hybridization and immunohistochemical staining. This is similar to what has been observed previously for CCR5-using SIV239 (60, 62) and more recently for early infection of humans with HIV-1 (9, 46). Although it was not specifically measured in this study, it is reasonable to think that significant immune responses were present in the gut where SIV239ΔV1-V2 was predominantly replicating in our studies described here. Recent analysis of a larger cohort of macaques infected with SIV239ΔV1-V2 has revealed detection of SIV-specific CD8+ T cells in rectal biopsies, in some cases at increased frequencies compared with those observed in peripheral blood (R. P. Johnson and R. C. Desrosiers, unpublished data). In any event, future comprehensive studies will be necessary in order to better define the range of adaptive immune responses induced by SIV239ΔV1-V2.
Lymphocytes within organized lymphoid follicles are mostly naïve and are considered to be within the inductive compartment of the gut lymphoid tissue. Our in situ measurements demonstrate a distribution of SIV239ΔV1-V2 principally within the inductive compartment of the gastrointestinal tract at 14 days. The lamina propria contains predominately activated and terminally differentiated lymphocytes and is considered effector lymphoid tissue. At 14 days of infection with WT SIV239, virus-positive cells have been found mostly in the lamina propria (60, 61). Only later do WT virus-positive cells predominate in the inductive sites. It thus appears that either SIV239ΔV1-V2 has an altered tropism for the preferential site of replication in the gut or this highly attenuated derivative is already largely cleared from the lamina propria by 14 days after infection.
The cell specificity of SIV239ΔV1-V2 in vivo also appeared to be highly unusual in that the vast majority of infected cells were macrophages at 14 days. Since SIV239ΔV1-V2 is able to infect cells to some extent in vitro in the absence of CD4 (32), the unusual cell specificity could reflect an altered cell tropism for SIV239ΔV1-V2. However, it is also possible that macrophages could represent long-lived reservoirs of SIV production in the face of effective immune responses to this highly attenuated derivative (28, 49).
Better understanding of the key factors responsible for the protection in our studies and those of Lifson et al. (40, 41) could potentially yield valuable insights for the design of successful vaccine approaches against HIV/AIDS. This is especially true in light of the recent failure of the Merck recombinant adenovirus product to demonstrate efficacy in field trials (14). Many of the vaccine design efforts to date have focused on increasing the magnitude of immune responses measured in the periphery by standard assays. Our results and those of Lifson et al. (40, 41) suggest that this may not be the appropriate tack. Perhaps all we need is to reproduce the nature of the immune responses, however low in magnitude, present in monkeys 379-89 and 535-99. Of course, even if we can figure out what those are and how best to induce them, we will still need to overcome the enormous problem of field strain diversity.
Acknowledgments
We thank Jennifer Bricker-Bolton, Amany Awad, Jackie Gillis, and the staff of the Division of Primate Resources at NEPRC for technical assistance. We also thank John Altman for providing peptide tetramers, David Watkins for advice on MHC typing, and Mike Piatak and his staff for viral load measurements.
This work was supported by PHS grants RR00168 (NEPRC), AI25328 (R.C.D.), AI35365 (R.C.D.), and AI62412 (R.P.J.) and by funding from the International AIDS Vaccine Initiative (IAVI). This work was also funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract NO1-CO-124000 (J.L.).
Footnotes
Published ahead of print on 13 February 2008.
REFERENCES
- 1.Alexander, L., Z. Du, M. Rosenzweig, J. U. Jung, and R. C. Desrosiers. 1997. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 Nef alleles in lymphocyte activation. J. Virol. 716094-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., L. Mortara, B. R. Mothe, M. Liebl, P. Jing, B. Calore, M. Piekarczyk, R. Ruddersdorf, D. H. O'Connor, X. Wang, C. Wang, D. B. Allison, J. D. Altman, A. Sette, R. C. Desrosiers, G. Sutter, and D. I. Watkins. 2002. Tat-vaccinated macaques do not control simian immunodeficiency virus SIVmac239 replication. J. Virol. 764108-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 29269-74. [DOI] [PubMed] [Google Scholar]
- 4.Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 755151-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290486-492. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov, I. M., Z. Hel, B. Kelsall, V. A. Kuznetsov, J. D. Ahlers, J. Nacsa, D. I. Watkins, T. M. Allen, A. Sette, J. Altman, R. Woodward, P. D. Markham, J. D. Clements, G. Franchini, W. Strober, and J. A. Berzofsky. 2001. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 71320-1326. [DOI] [PubMed] [Google Scholar]
- 7.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 724170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borda, J. T., X. Alvarez, I. Kondova, P. Aye, M. A. Simon, R. C. Desrosiers, and A. A. Lackner. 2004. Cell tropism of simian immunodeficiency virus in culture is not predictive of in vivo tropism or pathogenesis. Am. J. Pathol. 1652111-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cafaro, A., A. Caputo, C. Fracasso, M. T. Maggiorella, D. Goletti, S. Baroncelli, M. Pace, L. Sernicola, M. L. Koanga-Mogtomo, M. Betti, A. Borsetti, R. Belli, L. Akerblom, F. Corrias, S. Butto, J. Heeney, P. Verani, F. Titti, and B. Ensoli. 1999. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 5643-650. [DOI] [PubMed] [Google Scholar]
- 11.Casimiro, D. R., F. Wang, W. A. Schleif, X. Liang, Z. Q. Zhang, T. W. Tobery, M. E. Davies, A. B. McDermott, D. H. O'Connor, A. Fridman, A. Bagchi, L. G. Tussey, A. J. Bett, A. C. Finnefrock, T. M. Fu, A. Tang, K. A. Wilson, M. Chen, H. C. Perry, G. J. Heidecker, D. C. Freed, A. Carella, K. S. Punt, K. J. Sykes, L. Huang, V. I. Ausensi, M. Bachinsky, U. Sadasivan-Nair, D. I. Watkins, E. A. Emini, and J. W. Shiver. 2005. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 7915547-15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, X., G. Scala, I. Quinto, W. Liu, T. W. Chun, J. S. Justement, O. J. Cohen, T. C. vanCott, M. Iwanicki, M. G. Lewis, J. Greenhouse, T. Barry, D. Venzon, and A. S. Fauci. 2001. Protection of rhesus macaques against disease progression from pathogenic SHIV-89.6PD by vaccination with phage-displayed HIV-1 epitopes. Nat. Med. 71225-1231. [DOI] [PubMed] [Google Scholar]
- 13.Cherpelis, S., I. Shrivastava, A. Gettie, X. Jin, D. D. Ho, S. W. Barnett, and L. Stamatatos. 2001. DNA vaccination with the human immunodeficiency virus type 1 SF162ΔV2 envelope elicits immune responses that offer partial protection from simian/human immunodeficiency virus infection to CD8+ T-cell-depleted rhesus macaques. J. Virol. 751547-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen, J. 2007. Did Merck's failed HIV vaccine cause harm? Science 3181048-1049. [DOI] [PubMed] [Google Scholar]
- 15.Crotty, S., C. J. Miller, B. L. Lohman, M. R. Neagu, L. Compton, D. Lu, F. X. Lu, L. Fritts, J. D. Lifson, and R. Andino. 2001. Protection against simian immunodeficiency virus vaginal challenge by using Sabin poliovirus vectors. J. Virol. 757435-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live-attenuated SIV vaccine with a deletion in the nef gene. Science 2581938-1941. [DOI] [PubMed] [Google Scholar]
- 17.Daniel, M. D., N. L. Letvin, P. K. Sehgal, G. Hunsmann, D. K. Schmidt, N. W. King, and R. C. Desrosiers. 1987. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J. Gen. Virol. 683183-3189. [DOI] [PubMed] [Google Scholar]
- 18.Davis, N. L., I. J. Caley, K. W. Brown, M. R. Betts, D. M. Irlbeck, K. M. McGrath, M. J. Connell, D. C. Montefiori, J. A. Frelinger, R. Swanstrom, P. R. Johnson, and R. E. Johnston. 2000. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J. Virol. 74371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desrosiers, R. C. 2004. Prospects for an AIDS vaccine. Nat. Med. 10221-223. [DOI] [PubMed] [Google Scholar]
- 20.Desrosiers, R. C., J. D. Lifson, J. S. Gibbs, S. C. Czajak, A. Y. M. Howe, L. O. Arthur, and R. P. Johnson. 1998. Identification of highly attenuated mutants of simian immunodeficiency virus. J. Virol. 721431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edinger, A. L., J. E. Clements, and R. W. Doms. 1999. Chemokine and orphan receptors in HIV-2 and SIV tropism and pathogenesis. Virology 260211-221. [DOI] [PubMed] [Google Scholar]
- 22.Evans, D. T., J. E. Bricker, H. B. Sanford, S. Lang, A. Carville, B. A. Richardson, M. Piatak, J. D. Lifson, K. G. Mansfield, and R. C. Desrosiers. 2005. Immunization of macaques with single-cycle simian immunodeficiency virus (SIV) stimulates diverse virus-specific immune responses and reduces viral loads after challenge with SIVmac239. J. Virol. 797707-7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garber, D. A., G. Silvestri, and M. B. Feinberg. 2004. Prospects for an AIDS vaccine: three big questions, no easy answers. Lancet Infect. Dis. 4397-413. [DOI] [PubMed] [Google Scholar]
- 24.Gauduin, M. C., A. Kaur, S. Ahmad, T. Yilma, J. D. Lifson, and R. P. Johnson. 2004. Optimization of intracellular cytokine staining for the quantitation of antigen-specific CD4+ T cell responses in rhesus macaques. J. Immunol. Methods 28861-79. [DOI] [PubMed] [Google Scholar]
- 25.Hendricks, E. E., K. C. Lin, K. Boisvert, D. Pauley, and K. G. Mansfield. 2004. Alterations in expression of monocyte chemotactic protein-1 in the simian immunodeficiency virus model of disseminated Mycobacterium avium complex. J. Infect. Dis. 1891714-1720. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch, V., D. Adger-Johnson, B. Campbell, S. Goldstein, C. Brown, W. R. Elkins, and D. C. Montefiori. 1997. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J. Virol. 711608-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 767187-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Igarashi, T., C. R. Brown, Y. Endo, A. Buckler-White, R. Plishka, N. Bischofberger, V. Hirsch, and M. A. Martin. 2001. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA 98658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Igarashi, T., Y. Endo, G. Englund, R. Sadjadpour, T. Matano, C. Buckler, A. Buckler-White, R. Plishka, T. Theodore, R. Shibata, and M. Martin. 1999. Emergence of a highly pathogenic simian/human immunodeficiency virus in a rhesus macaque treated with anti-CD8 mAb during a primary infection with a nonpathogenic virus. Proc. Natl. Acad. Sci. USA 9614049-14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joag, S. V., Z. Li, L. Foresman, E. B. Stephens, L.-J. Zhao, I. Adany, D. M. Pinson, H. M. McClure, and O. Narayan. 1996. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J. Virol. 703189-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson, R. P., J. D. Lifson, S. C. Czajak, K. S. Cole, K. H. Manson, R. Glickman, J. Yang, D. C. Montefiori, R. Montelaro, M. Wyand, and R. C. Desrosiers. 1999. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relation of degree of protection with level of attenuation. J. Virol. 734952-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson, W., J. Morgan, J. Reitter, B. Puffer, S. Czajak, R. Doms, and R. C. Desrosiers. 2002. A replication-competent, neutralization-sensitive variant of simian immunodeficiency virus lacking 100 amino acids of envelope. J. Virol. 762075-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson, W. E., J. D. Lifson, S. M. Lang, R. P. Johnson, and R. C. Desrosiers. 2003. Importance of B-cell responses for immunological control of variant strains of simian immunodeficiency virus. J. Virol. 77375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson, W. E., H. Sanford, L. Schwall, D. R. Burton, P. W. Parren, J. E. Robinson, and R. C. Desrosiers. 2003. Assorted mutations in the envelope gene of simian immunodeficiency virus lead to loss of neutralization resistance against antibodies representing a broad spectrum of specificities. J. Virol. 779993-10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaizu, M., G. J. Borchardt, C. E. Glidden, D. L. Fisk, J. T. Loffredo, D. I. Watkins, and W. M. Rehrauer. 2007. Molecular typing of major histocompatibility complex class I alleles in the Indian rhesus macaque which restrict SIV CD8+ T cell epitopes. Immunogenetics 59693-703. [DOI] [PubMed] [Google Scholar]
- 36.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for the development of AIDS. Cell 65651-662. [DOI] [PubMed] [Google Scholar]
- 37.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50657-661. [DOI] [PubMed] [Google Scholar]
- 38.Letvin, N. L., S. Robinson, D. Rohne, M. K. Axthelm, J. W. Fanton, M. Bilska, T. J. Palker, H. X. Liao, B. F. Haynes, and D. C. Montefiori. 2001. Vaccine-elicited V3 loop-specific antibodies in rhesus monkeys and control of a simian-human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate envelope. J. Virol. 754165-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis, M. G., S. Bellah, K. McKinnon, J. Yalley-Ogunro, P. M. Zack, W. R. Elkins, R. C. Desrosiers, and G. E. Eddy. 1994. Titration and characterization of two rhesus derived SIVmac challenge stocks. AIDS Res. Hum. Retrovir. 10213-220. [DOI] [PubMed] [Google Scholar]
- 40.Lifson, J. D., J. L. Rossio, R. Arnaout, L. Li, T. L. Parks, D. K. Schneider, R. F. Kiser, V. J. Coalter, G. Walsh, R. J. Imming, B. Fisher, B. M. Flynn, N. Bischofberger, M. Piatak, Jr., V. M. Hirsch, M. A. Nowak, and D. Wodarz. 2000. Containment of simian immunodeficiency virus infection: cellular immune responses and protection from rechallenge following transient postinoculation antiretroviral treatment. J. Virol. 742584-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 7510187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loffredo, J. T., J. Maxwell, Y. Qi, C. E. Glidden, G. J. Borchardt, T. Soma, A. T. Bean, D. R. Beal, N. A. Wilson, W. M. Rehrauer, J. D. Lifson, M. Carrington, and D. I. Watkins. 2007. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J. Virol. 818827-8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mansfield, K. G., R. S. Veazey, A. Hancock, A. Carville, M. Elliott, K. C. Lin, and A. A. Lackner. 2001. Induction of disseminated Mycobacterium avium in simian AIDS is dependent upon simian immunodeficiency virus strain and defective granuloma formation. Am. J. Pathol. 159693-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matano, T., M. Kobayashi, H. Igarashi, A. Takeda, H. Nakamura, M. Kano, C. Sugimoto, K. Mori, A. Iida, T. Hirata, M. Hasegawa, T. Yuasa, M. Miyazawa, Y. Takahashi, M. Yasunami, A. Kimura, D. H. O'Connor, D. I. Watkins, and Y. Nagai. 2004. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J. Exp. Med. 1991709-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Means, R. E., T. Greenough, and R. C. Desrosiers. 1997. Neutralization sensitivity of cell culture passaged simian immunodeficiency virus. J. Virol. 717895-7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Research Council. 1996. Guide for the care and use of laboratory animals, p. 86-123. National Academy Press, Washington, DC.
- 48.Nishimura, Y., T. Igarashi, O. K. Donau, A. Buckler-White, C. Buckler, B. A. Lafont, R. M. Goeken, S. Goldstein, V. M. Hirsch, and M. A. Martin. 2004. Highly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc. Natl. Acad. Sci. USA 10112324-12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orenstein, J. M., C. Fox, and S. M. Wahl. 1997. Macrophages as a source of HIV during opportunistic infections. Science 2761857-1861. [DOI] [PubMed] [Google Scholar]
- 50.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. VanCott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nacsa, M. Klein, J. Tartaglia, and G. Franchini. 2002. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patterson, L. J., N. Malkevitch, D. Venzon, J. Pinczewski, V. R. Gomez-Roman, L. Wang, V. S. Kalyanaraman, P. D. Markham, F. A. Robey, and M. Robert-Guroff. 2004. Protection against mucosal simian immunodeficiency virus SIVmac251 challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J. Virol. 782212-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puffer, B. A., S. Pohlmann, A. L. Edinger, D. Carlin, M. D. Sanchez, J. Reitter, D. D. Watry, H. S. Fox, R. C. Desrosiers, and R. W. Doms. 2002. CD4 independence of simian immunodeficiency virus Envs is associated with macrophage tropism, neutralization sensitivity, and attenuated pathogenicity. J. Virol. 762595-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramsburg, E., N. F. Rose, P. A. Marx, M. Mefford, D. F. Nixon, W. J. Moretto, D. Montefiori, P. Earl, B. Moss, and J. K. Rose. 2004. Highly effective control of an AIDS virus challenge in macaques by using vesicular stomatitis virus and modified vaccinia virus Ankara vaccine vectors in a single-boost protocol. J. Virol. 783930-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I.-W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 706922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106539-549. [DOI] [PubMed] [Google Scholar]
- 56.Shacklett, B. L., K. E. Shaw, L. A. Adamson, D. T. Wilkens, C. A. Cox, D. C. Montefiori, M. B. Gardner, P. Sonigo, and P. A. Luciw. 2002. Live, attenuated simian immunodeficiency virus SIVmac-M4, with point mutations in the Env transmembrane protein intracytoplasmic domain, provides partial protection from mucosal challenge with pathogenic SIVmac251. J. Virol. 7611365-11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415331-335. [DOI] [PubMed] [Google Scholar]
- 58.Stott, J., S. L. Hu, and N. Almond. 1998. Candidate vaccines protect macaques against primate immunodeficiency viruses. AIDS Res. Hum. Retrovir. 14(Suppl. 3)S265-S270. [PubMed] [Google Scholar]
- 59.Sugimoto, C., K. Tadakuma, I. Otani, T. Moritoyo, H. Akari, F. Ono, Y. Yoshikawa, T. Sata, S. Izumo, and K. Mori. 2003. nef gene is required for robust productive infection by simian immunodeficiency virus of T-cell-rich paracortex in lymph nodes. J. Virol. 774169-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280427-431. [DOI] [PubMed] [Google Scholar]
- 61.Veazey, R. S., and A. A. Lackner. 2004. Getting to the guts of HIV pathogenesis. J. Exp. Med. 200697-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veazey, R. S., J. D. Lifson, I. Pandrea, J. Purcell, M. Piatak, Jr., and A. A. Lackner. 2003. Simian immunodeficiency virus infection in neonatal macaques. J. Virol. 778783-8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vogel, T. U., M. R. Reynolds, D. H. Fuller, K. Vielhuber, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, M. L. Marthas, V. Erfle, S. M. Wolinsky, C. Wang, D. B. Allison, E. W. Rud, N. Wilson, D. Montefiori, J. D. Altman, and D. I. Watkins. 2003. Multispecific vaccine-induced mucosal cytotoxic T lymphocytes reduce acute-phase viral replication but fail in long-term control of simian immunodeficiency virus SIVmac239. J. Virol. 7713348-13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Voss, G., K. Manson, D. Montefiori, D. I. Watkins, J. Heeney, M. Wyand, J. Cohen, and C. Bruck. 2003. Prevention of disease induced by a partially heterologous AIDS virus in rhesus monkeys by using an adjuvanted multicomponent protein vaccine. J. Virol. 771049-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willey, R. L., R. Byrum, M. Piatak, Y. B. Kim, M. W. Cho, J. L. Rossio Jr., J. Bess Jr., T. Igarashi, Y. Endo, L. O. Arthur, J. D. Lifson, and M. A. Martin. 2003. Control of viremia and prevention of simian-human immunodeficiency virus-induced disease in rhesus macaques immunized with recombinant vaccinia viruses plus inactivated simian immunodeficiency virus and human immunodeficiency virus type 1 particles. J. Virol. 771163-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson, N. A., J. Reed, G. S. Napoe, S. Piaskowski, A. Szymanski, J. Furlott, E. J. Gonzalez, L. J. Yant, N. J. Maness, G. E. May, T. Soma, M. R. Reynolds, E. Rakasz, R. Rudersdorf, A. B. McDermott, D. H. O'Connor, T. C. Friedrich, D. B. Allison, A. Patki, L. J. Picker, D. R. Burton, J. Lin, L. Huang, D. Patel, G. Heindecker, J. Fan, M. Citron, M. Horton, F. Wang, X. Liang, J. W. Shiver, D. R. Casimiro, and D. I. Watkins. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 805875-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wyand, M. S., K. Manson, D. C. Montefiori, J. D. Lifson, R. P. Johnson, and R. C. Desrosiers. 1999. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J. Virol. 738356-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wyand, M. S., K. H. Manson, M. Garcia-Moll, D. Montefiori, and R. C. Desrosiers. 1996. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J. Virol. 703724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yant, L. J., T. C. Friedrich, R. C. Johnson, G. E. May, N. J. Maness, A. M. Enz, J. D. Lifson, D. H. O'Connor, M. Carrington, and D. I. Watkins. 2006. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 805074-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]