Abstract
A CHO-K1 cell mutant with a specific decrease in cellular phosphatidylethanolamine (PE) level was isolated as a variant resistant to Ro09–0198, a PE-directed antibiotic peptide. The mutant was defective in the phosphatidylserine (PS) decarboxylation pathway for PE formation, in which PS produced in the endoplasmic reticulum is transported to mitochondria and then decarboxylated by an inner mitochondrial membrane enzyme, PS decarboxylase. Neither PS formation nor PS decarboxylase activity was reduced in the mutant, implying that the mutant is defective in some step of PS transport. The transport processes of phospholipids between the outer and inner mitochondrial membrane were analyzed by use of isolated mitochondria and two fluorescence-labeled phospholipid analogs, 1-palmitoyl-2-{N-[6(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]caproyl}-PS (C6-NBD-PS) and C6-NBD-phosphatidylcholine (C6-NBD-PC). On incubation with the CHO-K1 mitochondria, C6-NBD-PS was readily decarboxylated to C6-NBD-PE, suggesting that the PS analog was partitioned into the outer leaflet of mitochondria and then translocated to the inner mitochondrial membrane. The rate of decarboxylation of C6-NBD-PS in the mutant mitochondria was reduced to ≈40% of that in the CHO-K1 mitochondria. The quantity of phospholipid analogs translocated from the outer leaflet of mitochondria into inner mitochondrial membranes was further examined by selective extraction of the analogs from the outer leaflet of mitochondria. In the mutant mitochondria, the translocation of C6-NBD-PS was significantly reduced, whereas the translocation of C6-NBD-PC was not affected. These results indicate that the mutant is defective in PS transport between the outer and inner mitochondrial membrane and provide genetic evidence for the existence of a specific mechanism for intramitochondrial transport of PS.
Keywords: antibiotic peptide, phospholipid transport, mitochondria
During mitochondrial biogenesis, massive imports of lipids and proteins into mitochondria occur, because mitochondria cannot produce most of these components. Although our understanding of the mechanisms of protein transport into mitochondria has progressed substantially (1, 2), limited information is available about the mechanism by which lipids are translocated into mitochondria. Phospholipid synthesis in mitochondria is restricted to the formation of phosphatidylglycerol, cardiolipin, and phosphatidylethanolamine (PE), and other lipids such as phosphatidylcholine (PC) and phosphatidylserine (PS) must be imported from sites of cellular lipid synthesis, the endoplasmic reticulum (ER) or closely related organelles such as mitochondria-associated membrane (MAM) (3, 4). PS imported to the outer mitochondrial membrane is then translocated to the inner mitochondrial membrane, where it is converted to PE by PS decarboxylase (PSD) (5, 6). It has been shown that the translocation of PS to mitochondria followed by its decarboxylation is a major pathway for the synthesis of PE in some cultured mammalian cells (7–10), suggesting that significant amounts of PE found in cell membranes are derived from mitochondria.
Because the enzymes involved in PS synthesis are located in ER or MAM, and PSD is exclusively located at the inner mitochondrial membrane, the conversion of PS to PE by PSD has been used as an indicator of PS translocation into the inner mitochondrial membrane (5, 6). Recent studies have shown that the transport of newly synthesized PS to the outer mitochondrial membrane requires no cytosolic proteins and is probably mediated by direct contact region between MAM and mitochondria (11–13). It is also suggested that the translocation of PS from the outer to inner mitochondrial membrane occurs through the contact sites where the two mitochondrial membranes are closely apposed and linked in a stable manner, because agents that disrupt the contact sites such as 1,4-dinitrophenol and doxorubicin inhibit the PS transport (14, 15). Louisot and co-workers (16) reported that when radiolabeled PS was introduced into isolated mitochondria in the presence of hydroxylamine, an inhibitor of PSD, the imported PS accumulated in the contact site. In addition, PE newly synthesized through the decarboxylation of PS in the inner mitochondrial membrane is exported very rapidly to the outer membrane, without mixing with inner membrane PE (16, 17). Although these observations imply the presence of a specific machinery for the intramitochondrial transport of PS, which is tightly coupled to their biosynthesis, little experimental evidence to support this assumption has been provided.
One powerful approach to understanding the molecular machinery involved in phospholipids transport is the isolation of mutants defective in a specific process in the transport. Trotter et al. (18) have recently isolated yeast mutants defective in the PS transport from ER to Golgi/vacuole and have shown that phosphatidylinositol 4-kinase, the STT4 gene product, is involved in the regulation of PS transport.
In this study, we isolated a CHO-K1 cell mutant with a decreased cellular PE level by screening for variants resistant to the cytotoxicity of a tetracyclic peptide, Ro09–0198 (Ro), which forms a tight equimolar complex with PE on biological membranes and subsequently induces cytolysis (19–23). The results presented herein indicate that the mutant is defective in intramitochondrial transport of PS and provide genetic evidence that a specific machinery is involved in intramitochondrial transport of PS, which is distinct from that used for PC transport.
Materials and Methods
Materials.
Egg PC was prepared by chromatography on aluminum oxide neutral and Iatrobeads (Iatron, Tokyo). PE, PS, C6-NBD-PS—where C6-NBD is 1-palmitoyl-2-{N-[6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]caproyl}, and C6-NBD-PC were purchased from Avanti Polar Lipids. l-[U-14C]serine and phosphatidyl-[3-14C]serine were from Amersham International (Buckinghamshire, England). [1,2-14C]Ethanolamine hydrochloride was from ICN. Ro was provided by H. Ishitsuka (Nippon Roch Research Center, Japan). Other chemicals were purchased from Sigma.
Cell Culture.
CHO-K1 fibroblasts was obtained from the American Type Culture Collection and cultured in Ham’s F12 medium containing 10% newborn calf serum (NCS) in a 5% CO2/95% air incubator. For mutagenesis, exponentially growing cells were treated with ethyl methanesulfonate (400 μg/ml) in growth medium at 37°C for 16 h, followed by incubation at 33°C for 3 days.
Isolation of CHO Mutants Resistant to Ro Peptide-Induced Cytolysis.
Mutagenized CHO-K1 cells were seeded into 100-mm diameter dishes at 5 × 103 cells per dish and cultivated at 33°C for 20 days. The mutagenized cell colonies were replicated onto polyester disks as described (8) for screening of mutant cells exhibiting a low binding activity to Ro peptide. The polyester discs were incubated for 24 h in growth medium at 39.5°C, washed twice with F-12 medium, and then incubated with 125I-labeled streptavidin–Ro peptide complex (125I-SA-Ro; 50,000 cpm/ml) (21) for 1h at 39.5°C. The radioactivities of 125I-SA-Ro bound to the colonies was analyzed by Fuji bioimage analyzer, and a mutant (designated as R-4) exhibiting a low binding activity (≈75% of that of the parental cell) to Ro peptide was isolated.
R-4 cells were mutagenized again, seeded in 100-mm diameter dishes at 2 × 105 cells per dish, and cultured at 33°C in the growth medium. After 2 days, the cells were further incubated at 39.5°C for 24 h. Cell monolayers were washed twice with 5 ml of F-12 medium and then incubated in F-12 medium containing 5 μM Ro peptide at 39.5°C for 1 h. The peptide-treated cells were washed three times with 5 ml of F-12 medium and cultured in the growth medium at 33°C for 20 days. Colonies of surviving cells were cloned and further purified by limiting dilution. Dose–response curves of mutants against the peptide-induced cytotoxicity were determined by measuring the activity of lactate dehydrogenase released from dead cells.
Enzyme Assay for PSD.
CHO cells were seeded at 5 × 105 cells per 100-mm dish in growth medium at 39.5°C. After 3 days, cell extracts were prepared as described (8) and PSD activity was assayed, as described (11) with some modifications. The reaction mixture contained [14C]PS (1,750 cpm/nmol), 0.05% Triton X-100, 100 mM KH2PO4 (pH 6.8), and 10 mM EDTA in 0.2 ml. The reaction was initiated by adding of 300 μg of cell protein, allowed to proceed for 30 min at 39.5°C, and terminated by adding 3 ml of chloroform/methanol, 1:2 (vol/vol). Lipids were then extracted, separated, and analyzed as described (24).
Transient Expression of PSD.
PSD gene cDNA (25) inserted into the mammalian expression vector pSV-Sport1 (GIBCO/BRL) was introduced into cells with LipofectAMINE reagent (GIBCO/BRL).
Metabolic Labeling and Immunoprecipitation of PSD.
After transfection with the PSD gene, cells were incubated at 37°C for 1 h in methionine-deficient DMEM supplemented with 5% (vol/vol) dialyzed NCS before the addition of pulse medium containing [35S]methionine (0.5 mCi/ml; 1 Ci = 37 GBq) and 5% dialyzed NCS in methionine-deficient DMEM. Cells were incubated in pulse medium for 5 min and, subsequently for the chase, incubated in Ham’s F-12 medium supplemented with 10% NCS and 0.3 mM methionine. Immediately after the chase period, cells were lysed with 50 mM Tris⋅HCl (pH 8.0) containing 1% SDS. The lysate was boiled for 5 min, diluted 1:10 with Triton buffer (1% Triton X-100/150 mM NaCl/50 mM Tris⋅HCl, pH 8.0), and incubated with anti-PSD polyclonal antibody (25) for 1 h at 4°C. Protein A-Sepharose was added to the lysate. After incubation for 1 h, the Sepharose beads were collected by centrifugation and washed five times with Triton buffer. Proteins bound to the beads were fractionated by SDS/PAGE in 12% gels and examined by use of a bioimage analyzer (Fuji BAS2000).
Purification of Mitochondria.
Mitochondria were freshly prepared from both CHO-K1 and mutant cells as described (13) with some modifications. Briefly, cells were scraped from four 150-mm diameter dishes into 10 ml of PBS. Cellular materials were suspended in 5 ml of buffer A (250 mM mannitol/5 mM Hepes, pH 7.4/0.5 mM EGTA) and gently disrupted using 10 strokes of a motor-driven Potter–Elvejhem homogenizer. The homogenate was centrifuged twice at 600 × g for 10 min to remove nuclei and cell debris. The supernatant was subsequently centrifuged at 10,000 × g for 10 min to sediment the crude mitochondrial fraction. The resulting pellet was resuspended in 0.5 ml of buffer A, layered on top of 8 ml of Percoll buffer [225 mM mannitol/25 mM Hepes, pH 7.4/1 mM EGTA/30% (vol/vol) Percoll], and centrifuged for 30 min at 95,000 × g. The band containing the mitochondrial fraction was collected, and then the membranes were pelleted and washed twice in buffer A. The mitochondrial fraction was further purified by the same Percoll centrifugation procedure. The purity of mitochondrial fractions was assessed by measuring the activities of marker enzymes for mitochondria and other fractions. The specific activities of PS synthase in the mitochondrial fraction, which is enriched in MAM and microsome fractions (13, 25), were 8.1% and 9.6% of those in the respective MAM and microsome fractions in CHO-K1 cells, and 6.0% and 7.9% of those in the respective MAM and microsome fractions in R-41 mutant cells.
Determination of the Amount of C6-NBD-Lipids Associated with Mitochondria.
Isolated mitochondria were incubated with 10 μM of C6-NBD-lipids in 0.5 ml of buffer A at 30°C for the indicated periods. The mitochondria were then pelleted by centrifugation at 12,000 × g for 10 min at 4°C. The resulting pellet was washed with ice-cold buffer A twice. Then the fluorescent lipids associated with mitochondria were extracted as described by Bligh and Dyer (26) and separated by TLC using CHCl3/CH3OH/CH3COOH, 65:25:10 (vol/vol), as the developing solvent. The chromatograms were observed under UV illumination and the individual fluorescent lipids were quantified by fluorescence image analyzer (Fuji FLA-2000). As a parallel experiment, mitochondria incubated with C6-NBD-lipids were pelleted, then resuspended in 1 ml of ice-cold defatted 2% BSA-buffer A, and incubated for 30 min on ice to remove C6-NBD-lipids from the outer leaflet of the outer mitochondrial membrane (referred to as back exchange) (27, 28). Mitochondria were then pelleted by centrifugation at 12,000 × g for 10 min at 4°C. The resulting pellet was washed with 1 ml of ice-cold defatted 2% BSA in buffer A twice. Then the fluorescent lipids remaining in mitochondria were extracted and quantified as described above. Because C6-NBD-PS translocated into the inner mitochondrial membrane is decarboxylated to C6-NBD-PE by PSD, the amount of C6-NBD-PS associated with mitochondria was estimated as total amounts of C6-NBD-PS and C6-NBD-PE.
Other Procedures.
PS synthase was assayed as described (9). Turnover of newly synthesized PE was examined as described (29). Decarboxylation of exogenously added [14C]PS was determined as described (24). Proteins were measured according to Lowry et al. (30), using BSA as a standard.
Results
Isolation of a Mutant Defective in PE Biosynthesis.
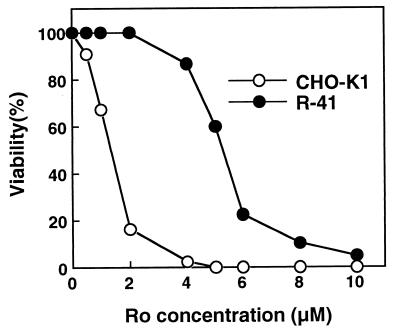
To isolate mutants with defects in PE biosynthesis, we selected CHO mutant cells resistant to an antibiotic peptide, Ro, that binds specifically to PE on biological membranes and subsequently induces cytolysis (19–23). One mutant, designated R-41, exhibited the most significant resistance to the peptide-induced cytolysis, and the LD50 of the peptide for R-41 mutant cells was about 4-fold higher than that required for CHO-K1 cells (Fig. 1). Cellular PE content of R-41 mutant cells was about half that of CHO-K1 cells, and the levels of other phospholipids including PS were not significantly changed, except for an elevation of the PC level (Table 1).
Figure 1.
Cytolytic sensitivity of CHO-K1 and R-41 mutant cells to Ro. Cells were seeded in a 96-well culture plate at 1 × 104 cells per well. After incubation for 2 days at 39.5°C, the cells were washed twice with F-12 medium and then incubated with various concentrations of Ro peptide for 1 h at 39.5°C. Cell viability was determined by measuring the activity of lactate dehydrogenase released from dead cells. Values are means from three experiments. ○, CHO-K1; ●, R-41.
Table 1.
Phospholipid compositions of CHO-K1 and R-41 mutant cells
| Strain | % total phospholipids
|
|||||
|---|---|---|---|---|---|---|
| PE | PS | PC | PI | SM | Others | |
| CHO-K1 | 16.1 | 5.6 | 59.1 | 5.8 | 10.3 | 3.0 |
| R-41 | 8.7 | 5.5 | 67.6 | 6.4 | 8.8 | 2.9 |
Cells were seeded at 5 × 105 cells per 100-diameter dish and then incubated for 3 days at 39.5°C. Phospholipids were extracted from cells by the procedure of Bligh and Dyer (26) and separated by two-dimensional thin-layer chromatography. Solvent systems used for chromatography were as follows: first dimension, chloroform/methanol/acetic acid, 65:25:10 (vol/vol); second dimension, chloroform/methanol/formic acid, 65:25:10 (vol/vol). Phospholipid phosphorus was chemically determined as described (7). Three experiments gave similar results, and one set of results is presented. PI, phosphatidylinositol; SM, sphingomyelin. Others include phosphatidylglycerol, phosphatidic acid, and cardiolipin.
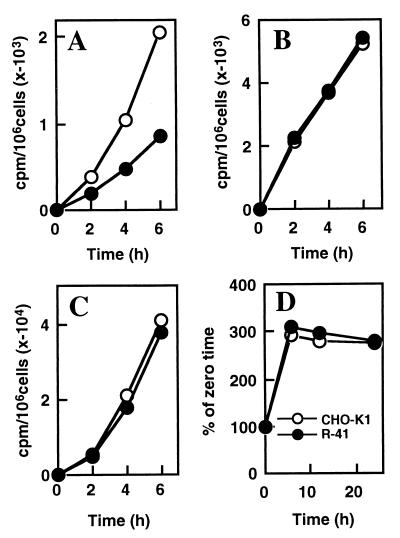
In mammalian cells, PE is synthesized via three distinct pathways: (i) the PS decarboxylation pathway, in which PS synthesized in the ER or the MAM, the closely related membranes, is imported into the inner mitochondrial membrane, where it is converted to PE by PSD (31); (ii) the CDP-ethanolamine pathway, in which ethanolamine is phosphorylated and converted to CDP-ethanolamine, followed by transfer of the phosphoethanolamine moiety to 1,2-sn-diacylglycerol (32); and (iii) the base exchange pathway, in which free ethanolamine is exchanged with the base moiety of preexisting phospholipids (33). To investigate PE biosynthesis in R-41 mutant cells, we metabolically labeled the mutant and CHO-K1 cells with either [14C]ethanolamine or [14C]serine. There was no appreciable difference in the rate of incorporation of [14C]ethanolamine into PE between the mutant and CHO-K1 cells, suggesting that PE biosynthesis through the CDP-ethanolamine and the base exchange pathways was not affected in the mutant (Fig. 2C). In cells labeled with [14C]serine, the rate of [14C]PE formation in the mutant cells was reduced to about 40% of that in CHO-K1 cells (Fig. 2A). The incorporation of [14C]serine into both PS and sphingomyelin (data not shown) in the mutant cells was similar to that in CHO-K1 cells (Fig. 2B). Furthermore, the rate of turnover of newly synthesized PE from the PS decarboxylation pathway in the mutant cells, determined by the loss of [14C]serine-labeled PE after removing the radioactive serine, was not affected (Fig. 2D). These results indicate that R-41 mutant was defective in the PS decarboxylation pathway for PE formation.
Figure 2.
R-41 mutant is defective in the PS decarboxylation pathway for PE formation. The time course of [14C]serine incorporation into PE (A) and PS (B) in CHO-K1 and R-41 mutant cells is shown. (C) The time course of [14C]ethanolamine incorporation into PE in CHO-K1 and R-41 mutant cells is shown. Cells were seeded in 60-mm diameter dishes and cultured to a density of ≈5 × 105 cells per dish in growth medium at 39.5°C. The cells were metabolically labeled at 39.5°C by replacing the medium with 2 ml of fresh growth medium containing l-[U-14C]serine (0.5 μCi/ml) or [1,2-14C]ethanolamine (0.5 μCi/ml). At the indicated times, cellular phospholipids were extracted from one dish of each strain and analyzed by TLC. The number of cells at zero time was determined and used to standardize the results. (D) Turnover of PE pulse-labeled with [14C]serine in CHO-K1 and R-41 mutant cells is shown. Cells were incubated with l-[U-14C]serine (2 μCi/ml) for 1 h at 39.5°C. After removing the radioactive medium (zero time), cells were washed and further incubated in chase medium containing 1 mM serine and 1 mM ethanolamine (29). At the indicated times, the cells were harvested, and phospholipids were extracted and analyzed by one-dimensional TLC. ○, CHO-K1; ●, R-41.
R-41 Mutant Cells Have Normal PS Synthase and PSD Activities.
PE synthesis via the PS decarboxylation pathway requires the combined actions of PS synthase and PSD (7–10, 31). PS synthase activity in a homogenate of R-41 mutant cells was similar to that of CHO-K1 cells (CHO-K1, 38.2 pmol per min per mg of protein; R-41, 42.1 pmol per min per mg of protein). This result and the finding above that PS content and biosynthetic rate in R-41 mutant cells were similar to those in CHO-K1 cells clearly showed that the mutant has a normal activity to synthesize PS. PSD activity in the mutant homogenate was also similar to that in CHO-K1 homogenate at various concentrations of the substrate PS (Fig. 3A), suggesting that the defect of R-41 mutant in the PS decarboxylation pathway was not due to a mutation of PSD. For further confirmation, we examined the effect of expression of the PSD cDNA from CHO-K1 cells on PE formation via the PS decarboxylation pathway. Transient transfection with the PSD cDNA caused striking increases in the PSD activities in the homogenates of CHO-K1 and R-41 mutant cells: from 0.52 to 11.9 nmol per min per mg and 0.48 to 9.81 nmol per min per mg, respectively. In cells labeled with [14C]serine, the rate of [14C]PE formation in the PSD cDNA-transfected CHO-K1 cells was elevated to about 2-fold that of the mock-transfected cells, whereas the rate in the PSD cDNA-transfected R-41 cells remained at low level, similar to that in the mock-transfected R-41 cells (Fig. 3B). These results indicate that the primary lesion in R-41 mutant is not at the level of PS biosynthesis or enzymatic activity of PSD.
Figure 3.
PSD activity in CHO-K1 and R-41 mutant cells. (A) Cells seeded at 5 × 105 cells per 100-mm dish were grown at 39.5°C for 3 days. The harvested cells were sonically disrupted in 0.25 M sucrose, 10 mM Hepes (pH 7.5), and 1 mM EDTA, and the cell lysate were assayed for PSD activity as described (25). The error for duplicate determinations was less than 5%. ○, CHO-K1; ●, R-41. (B) Effect of expression of a CHO PSD cDNA on PE formation via the PS decarboxylation pathway. CHO-K1 (○, ▵) and R-41 mutant (●, ▴) cells transfected either with control vector (mock; ○, ●) or with vector containing cDNA of PSD gene (PSD; ▵, ▴) were cultured for 2 days at 39.5°C. The cells were metabolically labeled with l-[U-14C]serine (0.5 μCi/ml). At the indicated times, cellular phospholipids were extracted from one dish of each strain and analyzed by TLC.
Localization of PSD in R-41 Mutant Cells.
One possible explanation for the defect in PS decarboxylation of R-41 mutant cells is the mislocalization of PSD in the mutant because of mutation(s) affecting the protein sorting system: PSD of mammalian cells is exclusively located in the inner mitochondrial membrane (34). To examine whether PSD in R-41 mutant is imported into mitochondria, we purified mitochondria from R-41 mutant and CHO-K1 cells. The specific activities of PSD in the mitochondria from the mutant (2.22 nmol per min per mg of protein) and CHO-K1 cells (2.43 nmol per min per mg of protein) were similar to each other, suggesting that PSD is imported into mitochondria in the mutant cells. Next, we examined the posttranslational processing of PSD gene product in R-41 mutant cells. The 46-kDa primary translation product of the PSD gene has the general motif of the mitochondrial targeting signal and the inner membrane sorting signal, which are sequentially cleaved by mitochondrial processing enzymes, in addition to an autocatalytic cleavage site for production of α and β subunits of PSD (25, 31). The pulse–chase experiment revealed no significant difference between R-41 mutant and CHO-K1 cells in the processing rate of the PSD gene product (Fig. 4), implying that the translocation of PSD into mitochondria is normal in the mutant. These results suggest that PSD in R-41 mutant cells is correctly located in mitochondria.
Figure 4.
Posttranslational processing of the PSD gene product. CHO-K1 and R-41 mutant cells transfected with the PSD gene were pulse-labeled with [35S]methionine for 5 min and then chased in the presence of excess unlabeled methionine for the periods indicated. Radioactive proteins were precipitated with anti-PSD antibody, separated by SDS/PAGE on 12% gels, and examined by use of a bioimage analyzer.
R-41 Mutant Cells Are Defective in Intramitochondrial Transport of PS.
Another explanation for the defect in PS decarboxylation of R-41 mutant cells is that PS synthesized in ER or MAM in the mutant cells is not effectively transported to the inner mitochondrial membrane, where PSD is located. When cells were incubated with [14C]serine-labeled PS for 8 h at 39.5°C, the formation of [14C]serine-labeled PE in the mutant was significantly reduced ([14C]PE converted from [14C]PS was 25.6% and 15.3% of total incorporated [14C]PS in CHO-K1 and R-41 mutant cells, respectively), although there was no significant difference in the uptake of the radioactive PS between CHO-K1 and R-41 mutant cells. This result was consistent with the above-mentioned explanation. Next, to examine whether or not R-41 mutant is defective in intramitochondrial transport of PS, mitochondria purified from CHO-K1 and mutant cells were incubated with a PS analog, C6-NBD-PS, which is readily inserted into accessible membranes and does not undergo spontaneous transmembrane movement (11), and its translocation-dependent decarboxylation was determined. When mitochondria from CHO-K1 cells were incubated with C6-NBD-PS, the PS analog was efficiently converted to C6-NBD-PE in a time-dependent manner (Fig. 5A), suggesting that C6-NBD-PS was transported to the inner mitochondrial membrane and then decarboxylated by the inner membrane enzyme PSD. In R-41 mutant mitochondria, the rate of C6-NBD-PE formation was ≈40% of that in CHO-K1 mitochondria. Furthermore, when mitochondrial membrane structure was disrupted by sonication, the disrupted mitochondria of CHO-K1 and R-41 mutant cells exhibited similar activities of the conversion of C6-NBD-PS to C6-NBD-PE (Fig. 5B). These results suggest that R-41 mutant is defective in PS transport between the outer and inner mitochondrial membrane.
Figure 5.
Translocation-dependent decarboxylation of C6-NBD-PS in isolated mitochondria from CHO-K1 and R-41 mutant cells. Intact (A) or disrupted (B) mitochondria from either CHO-K1 or R-41 mutant cells were incubated with 10 μM C6-NBD-PS in 1 ml of buffer A (250 mM mannitol/5 mM Hepes, pH 7.4/0.5 mM EGTA) at 39.5°C for various periods. The reaction was terminated by adding 3.75 ml of chloroform/methanol, 1:2 (vol/vol), and then the lipids were extracted and separated by TLC. C6-NBD-PE spots were scraped from TLC plates, and then the lipids were extracted as described by Bligh and Dyer (26). The relative fluorescence of the lipid extracts was measured with a fluorescence spectrophotometer F-2000 (Hitachi, Tokyo). Mitochondria were disrupted by exposure five times to ultrasound on ice for 30 sec at setting 1 in a Branson ultrasonic disrupter. ○, CHO-K1; ●, R-41. R.F.U., relative fluorescent unit(s).
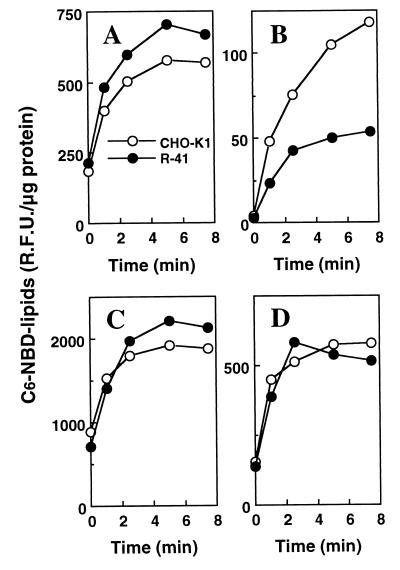
C6-NBD-lipids, such as C6-NBD-PS and C6-NBD-PC, present in the outer leaflet, not in the inner leaflet, of plasma membrane has been shown to be selectively extracted by incubation with excess BSA (referred to as back exchange) (27, 28). Therefore, to assess the rate of translocation of the PS or PC analog from the outer leaflet of the outer mitochondrial membrane to other mitochondrial compartments including the inner leaflet of the outer membrane and the inner membrane mitochondria isolated from CHO-K1 and R-41 mutant, cells were incubated with C6-NBD-PS or C6-NBD-PC at 30°C and then subjected to back exchange to remove the fluorescence-labeled lipid from the outer leaflet of the outer mitochondrial membrane. As shown in Fig. 6A, there is no significant difference between CHO-K1 and the mutant mitochondria in the total association of the PS analog. However, after back exchange, the amount of the residual PS analog in the mutant mitochondria was about half of that in CHO-K1 mitochondria (Fig. 6B). In contrast, there is no significant difference between CHO-K1 and the mutant mitochondria in both the total association and the residual PC analog (Fig. 6 C and D). These results indicate that R-41 mutant is defective in intramitochondrial transport of PS and provide genetic evidence for the presence of a specific machinery for intramitochondrial transport of PS, which is distinct from that used for PC transport.
Figure 6.
Association of C6-NBD-lipids with isolated mitochondria from CHO-K1 and R-41 mutant cells. Mitochondria from CHO-K1 or R-41 mutant cells were incubated with 10 μM C6-NBD-PS (A and B) or C6-NBD-PC (C and D) at 30°C, and then total C6-NBD-lipids associated with mitochondria were extracted and quantified (A and C). (B and D) Mitochondria incubated with C6-NBD-lipids were subsequently subject to back exchange to remove the fluorescent lipids from the outer leaflet of the outer mitochondrial membrane, and then C6-NBD-lipids remaining in mitochondria were extracted and quantified. The amount of C6-NBD-lipids was normalized to total mitochondrial proteins. ○, CHO-K1; ●, R-41.
Discussion
Although intracellular transport of membrane phospholipids is indispensable for cell growth and organelle assembly, the transport processes are largely unknown with respect to the specific mechanisms, genes, and proteins involved. For studying intracellular transport of phospholipids, cultured mammalian cell mutants with specific defects in the phospholipids transport must be useful. In this study, we have developed a method for isolation of mutants with a specific decrease in cellular PE level by use of an antibiotic peptide, Ro, that binds PE on plasma membrane and subsequently induces cytolysis (19–23) (Table 1). The method is potentially useful for isolation of mutant strains defective in PS transport from ER/MAM to mitochondria and PE export from mitochondria or ER to other organelles, as well as transport of PS synthase and PSD. The following results indicate that CHO mutant strain R-41 is defective in intramitochondrial PS transport: (i) the mutant was defective in PE formation through the PS decarboxylation pathway (Fig. 2), in which PS synthesized in ER/MAM is imported into the inner mitochondrial membrane and then converted to PE by PS decarboxylase; (ii) the level of PS biosynthetic activity of the intact mutant cells and PSD activity in a homogenate of the mutant cells were normal, similar to those of CHO-K1 cells (Fig. 3); (iii) the intact mitochondria isolated from R-41 mutant cells were defective in the translocation-dependent decarboxylation of a fluorescence-labeled PS analog, C6-NBD-PS, whereas mechanically disrupted mitochondria of the mutant had a normal activity of the decarboxylation of the PS analog (Fig. 5); and (iv) the amount of the residual PS analog after back exchange in the mutant mitochondria was about half that in CHO-K1 mitochondria (Fig. 6).
PE in mammalian cells can be produced by three distinct pathways: the PS decarboxylation pathway, the CDP-ethanolamine pathway, and the phospholipid base-exchange pathway (31–33). The cellular content of PE in R-41 mutant cells was specifically reduced to about half that in CHO-K1 cells (Table 1). PE formation via the PS decarboxylation pathway in R-41 mutant cells was reduced to about 40% that of CHO-K1 cells when determined by the metabolic labeling with [14C]serine, whereas the mutant cells were normal in their incorporation of [14C]ethanolamine into PE (Fig. 2). These results are consistent with the previous finding that the decarboxylation of PS is a major pathway for cellular PE formation in CHO-K1 cells (7–10). The rate of translocation-dependent decarboxylation of C6-NBD-PS in R-41 mutant mitochondria decreased to about 40% of that in CHO-K1 mitochondria (Fig. 5), proportional to the decrease in PE formation via the PS decarboxylation in intact cells. In addition, overexpression of PSD gene in R-41 mutant cells caused no significant increase in the synthetic rate of PE via the PS decarboxylation pathway, whereas PSD activity in a homogenate increased about 20-fold (Fig. 3B). It is therefore likely that the translocation of PS from the outer to inner mitochondrial membranes is a crucial step in determining the overall rate of PS decarboxylation pathway for PE formation.
Translocation-dependent PS decarboxylation in mitochondria is inhibited by 1,4-dinitrophenol (14), which diminishes the number of the contact sites between the outer and inner mitochondrial membranes. It has been also reported that radiolabeled PS, introduced into isolated mitochondria by coincubating with microsomal donor vesicles, is accumulated in a contact site-enriched subfraction of mitochondria when PSD activity is inhibited by hydroxylamine (16). These previous observations suggest that PS import into the inner mitochondrial membrane is mediated by the contact sites between the outer and inner mitochondrial membranes, which are also well known to be the zones of protein import into mitochondria (35, 36). Although R-41 mutant is defective in PS transport to the inner mitochondrial membrane, protein import is normal in the mutant, as examined by monitoring the posttranslational processing of PSD gene product (Fig. 4). This result suggest that the defect of R-41 mutant is not due to a mutation inducing general inactivation of mitochondria or a large structural alteration in mitochondrial membrane such as loss of the contact sites. In addition, there was no significant difference between CHO-K1 and the mutant in the amount of C6-NBD-PC associated with mitochondria after back exchange (Fig. 6), suggesting that intramitochondrial transport of PC is normal in the mutant. This result is consistent with the recent report that PS and PC import into mitochondria occur by distinct mechanisms (37). It is therefore likely that R-41 mutant has a mutation in its PS transport machinery or its regulatory factor that is distinct from that involved in PC or protein transport. Isolation of the gene that complements the defect in PS transport of R-41 mutant may provide additional information about this mechanism.
Abbreviations
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- PC
phosphatidylcholine
- Ro
Ro09–0198
- PSD
phosphatidylserine decarboxylase
- C6-NBD
1-palmitoyl-2-{N-[6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]caproyl}
- MAM
mitochondria-associated membrane
- NCS
newborn calf serum
References
- 1.Schatz G. J Biol Chem. 1996;271:31763–31766. doi: 10.1074/jbc.271.50.31763. [DOI] [PubMed] [Google Scholar]
- 2.Neupert W. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 3.Daum G. Biochim Biophys Acta. 1985;822:1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- 4.Vance J E. J Biol Chem. 1991;266:89–97. [PubMed] [Google Scholar]
- 5.Dennis E A, Kennedy E P. J Lipid Res. 1972;13:263–267. [PubMed] [Google Scholar]
- 6.Voelker D R. Experientia. 1990;46:569–579. doi: 10.1007/BF01939695. [DOI] [PubMed] [Google Scholar]
- 7.Voelker D R. Proc Natl Acad Sci USA. 1984;81:2669–2673. doi: 10.1073/pnas.81.9.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuge O, Nishijima M, Akamatsu Y. Proc Natl Acad Sci USA. 1985;82:1926–1930. doi: 10.1073/pnas.82.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuge O, Nishijima M, Akamatsu Y. J Biol Chem. 1986;261:5790–5794. [PubMed] [Google Scholar]
- 10.Voelker D R, Frazier J L. J Biol Chem. 1986;261:1002–1008. [PubMed] [Google Scholar]
- 11.Voelker D R. Proc Natl Acad Sci USA. 1989;86:9921–9925. doi: 10.1073/pnas.86.24.9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voelker D R. J Biol Chem. 1993;268:7069–7074. [PubMed] [Google Scholar]
- 13.Shiao Y-J, Lupo G, Vance J E. J Biol Chem. 1995;270:11190–11198. doi: 10.1074/jbc.270.19.11190. [DOI] [PubMed] [Google Scholar]
- 14.Hovius R, Faber B, Brigot B, Nicolay K, Kruijff B D. J Biol Chem. 1992;267:16790–16795. [PubMed] [Google Scholar]
- 15.Voelker D R. J Biol Chem. 1991;266:12185–12188. [PubMed] [Google Scholar]
- 16.Ardail D, Lerme F, Louisot P. J Biol Chem. 1991;266:7978–7981. [PubMed] [Google Scholar]
- 17.Simbeni R, Paltauf F, Daum G. J Biol Chem. 1990;265:281–285. [PubMed] [Google Scholar]
- 18.Trotter P J, Wu W-I, Pedretti J, Yates R, Voelker D R. J Biol Chem. 1998;273:13189–13196. doi: 10.1074/jbc.273.21.13189. [DOI] [PubMed] [Google Scholar]
- 19.Choung S-Y, Kobayashi T, Takemoto K, Ishituka H, Inoue K. Biochim Biophys Acta. 1988;940:171–179. doi: 10.1016/0005-2736(88)90192-7. [DOI] [PubMed] [Google Scholar]
- 20.Wakamatsu K, Choung S-Y, Kobayashi T, Inoue K, Higashijima T, Miyazawa T. Biochemistry. 1990;29:113–118. doi: 10.1021/bi00453a013. [DOI] [PubMed] [Google Scholar]
- 21.Emoto K, Kobayashi T, Yamaji A, Aizawa H, Yahara I, Inoue K, Umeda M. Proc Natl Acad Sci USA. 1996;93:12867–12872. doi: 10.1073/pnas.93.23.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emoto K, Toyama-Sorimachi N, Karasuyama H, Inoue K, Umeda M. Exp Cell Res. 1997;232:430–434. doi: 10.1006/excr.1997.3521. [DOI] [PubMed] [Google Scholar]
- 23.Aoki Y, Uenaka T, Aoki J, Umeda M, Inoue K. J Biochem. 1994;116:291–297. doi: 10.1093/oxfordjournals.jbchem.a124522. [DOI] [PubMed] [Google Scholar]
- 24.Nishijima M, Kuge O, Akamatsu Y. J Biol Chem. 1986;261:5784–5789. [PubMed] [Google Scholar]
- 25.Kuge O, Saito K, Kojima M, Akamatsu Y, Nishijima M. Biochem J. 1996;319:33–38. doi: 10.1042/bj3190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 27.Matin O C, Pagano R E. J Biol Chem. 1987;262:5890–5898. [PubMed] [Google Scholar]
- 28.Zegers M M, Hoekstra D. J Cell Biol. 1997;138:307–321. doi: 10.1083/jcb.138.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiao Y-J, Vance J E. Biochem J. 1995;310:673–679. doi: 10.1042/bj3100673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Voelker D R. Biochim Biophys Acta. 1997;1348:236–244. doi: 10.1016/s0005-2760(97)00101-x. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy E P, Weiss S B. J Biol Chem. 1956;222:193–214. [PubMed] [Google Scholar]
- 33.Bjerve K S. Biochim Biophys Acta. 1973;296:549–562. [PubMed] [Google Scholar]
- 34.Zborowski J, Dygas A, Wojtczak L. FEBS Lett. 1983;157:179–182. doi: 10.1016/0014-5793(83)81141-7. [DOI] [PubMed] [Google Scholar]
- 35.Schleyer M, Neuper W. Cell. 1985;43:339–350. doi: 10.1016/0092-8674(85)90039-x. [DOI] [PubMed] [Google Scholar]
- 36.Vestweber D, Schatz G. J Cell Biol. 1988;107:2037–2043. doi: 10.1083/jcb.107.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiao Y-J, Balcerzak, Vance J E. Biochem J. 1998;331:217–223. doi: 10.1042/bj3310217. [DOI] [PMC free article] [PubMed] [Google Scholar]