Abstract
Respiratory syncytial virus (RSV) is the major cause of infantile bronchiolitis and hospitalization. Severe RSV disease is associated with the development of wheezing in later life. In a mouse model of the delayed effects of RSV, the age at primary infection determines responses to reinfection in adulthood. During primary RSV infection, neonatal BALB/c mice developed only mild disease and recruited CD8 cells that were defective in gamma interferon production. Secondary reinfection of neonatally primed mice caused enhanced inflammation and profuse lung T-cell recruitment. CD4 cell depletion during secondary RSV challenge attenuated disease (measured by weight loss); depletion of CD8 cells also markedly attenuated disease severity but enhanced lung eosinophilia, and depletion of both CD4 and CD8 cells together completely abrogated weight loss. Depletion of CD8 (but not CD4) cells during primary neonatal infection was protective against weight loss during adult challenge. Therefore, T cells, in particular CD8 T cells, play a central role in the outcome of neonatal infection by enhancing disease during secondary challenge. These findings demonstrate a crucial role for T cells in the regulation of immune responses after neonatal infection.
Newborn children are highly susceptible to infectious diseases such as measles, mumps, pertussis, and diphtheria. In nonvaccinated infants, case fatality rates for these infections are typically 10 to 100 times greater than those in children aged 10 or above. Similarly, cytomegalovirus, Chlamydia spp., group B streptococci, and Haemophilus influenzae are important causes of neonatal pneumonia but do not normally cause pneumonia in adults. Despite the great need for vaccines that are effective in neonates, responses to vaccination are weak and usually ineffective in this age group (1, 4).
The characteristics of neonatal immune responses influence not only disease susceptibility and vaccine efficacy but also the outcomes of later infections. Childhood infection can lead to life-long protection against some viruses (e.g., measles), while giving poor protection against reinfection with other agents (e.g., many bacterial infections, helminths, and respiratory syncytial virus [RSV]). Childhood exposure to infection may also contribute nonspecifically to immune maturation. According to the hygiene hypothesis, exposure to environmental microbiota and episodes of infection help normal immune maturation and protect against the later development of allergy (23) and autoimmunity (33). However, infantile wheezy colds (21), viral bronchiolitis (31), and bacterial colonization (3) are associated with wheezing and asthma diagnosis in later childhood. This association is particularly well established for RSV (30), a pneumovirus that is the principal cause of childhood hospitalization in the developed world (29).
Although RSV infections tend to be severe in infancy, reinfections occur throughout life. The fact that severe RSV disease is associated with overexuberant immune responses (26) has held back vaccine development. We have previously shown that the age at which primary infection occurs critically influences the outcome of secondary challenge in mice. RSV infection in the first week of life causes enhanced disease during secondary challenge, characterized by increased weight loss and enhanced lung inflammation (8).
In the present studies, we found that primary RSV infection of neonatal mice caused only mild disease and led to the recruitment of RSV-specific T cells, very few of which made gamma interferon (IFN-γ). Reinfection of neonatally primed mice during adulthood led to enhanced disease characterized by lung inflammation and weight loss, and CD8 or CD4 cell depletion during secondary challenge greatly reduced disease severity. If CD8 cells (but not CD4 cells) were depleted during primary infection, no weight loss was seen during later reinfection. Therefore, CD8 cells play a key role both in programming for enhanced disease in the neonatal period and in the pathogenesis of the enhanced disease seen in adulthood.
MATERIALS AND METHODS
Mice and virus stocks.
Time-matched pregnant BALB/c mice (Harlan, Berkhamsted, United Kingdom) were purchased at <14 days of gestation, and pups were weaned when they were 3 weeks old. BALB/c mice were infected intranasally (i.n.) with 4 × 104 PFU RSV A2/g at 4 days (for neonates, ∼105 PFU) or at 4 to 6 weeks of age (for immature adults, ∼5 × 105 PFU) under isoflurane anesthesia. Secondary RSV challenge was given i.n. at week 8, with 106 PFU in 100 μl (i.e., the same number of RSV PFU/g as in the primary infection). The RSV A2 strain was grown in HEp-2 cells, and viral titers were determined by plaque assay. Following infection, sickness was monitored by measuring weight daily. Lung function was assessed using whole-body plethysmography (Buxco, United Kingdom) to record the enhanced pause (Penh), described previously (32).
For cell depletion, mice were treated with 500 μl (adults) or 50 μl (neonates) of 1 mg/ml antibody intraperitoneally (i.p.) on day −1, day +2, and day +5 postinfection (p.i.). CD4 cells were depleted using clones YTA 191 and YTA 3, CD8 cells were depleted with clone YTS 156, and the control treatment used an irrelevantly matched isotype control. All antibodies were immunoglobulin G2b (all antibodies were a kind gift of S. Cobbold, Oxford University). All work was approved and licensed by the United Kingdom Home Office. Experiments were performed at least two times with at least four mice per experiment.
Cell preparation and histology.
After infection, animals were sacrificed by i.p. pentobarbitone injection and their tissues were collected as described previously (7). Cells were processed to single-cell suspensions and live cells counted by trypan blue exclusion. Histology samples were processed from 4%-formalin-fixed lungs as previously described (32) and stained with hematoxylin and eosin (H&E), and mucus-producing goblet cells were detected using the periodic acid-Schiff method.
Flow cytometry.
Cell staining was performed as described previously (7). For surface staining, antibodies against the surface markers CD4, CD8, CD44, CD62L, MHCII, and CD11c (BD) were used or the RSV M2 major histocompatibility complex class I pentamer (SYIGSINNI; Proimmune) was added at a 1:100 dilution for 30 min on ice. Gating for lymphocytes was determined by back gating on CD3+ CD8+ cells. Stimulation for intracellular staining was performed using the SYIGSINNI peptide (RSV M282-90; 1 μg ml−1 for 4 h in the presence of interleukin 2 [IL-2] at 50 U ml−1) and brefeldin as described previously (7). Vβ screening was performed by flow cytometry (Pharmingen). Samples were run on an LSR (BD) and analyzed using Winlist (Verity).
Cytokine ELISA.
Cytokine levels were assessed in bronchoalveolar lavage (BAL) fluid or lung mash supernatants by enzyme-linked immunosorbent assay (ELISA) using a pair of capture and biotinylated detection antibodies (cytokines were from BD; chemokines were from R&D Systems) by following the manufacturer's instructions. Mediator concentrations were quantified by comparison to recombinant cytokine standards. For neonatal BAL fluid for which sample volume was low, cytokines were quantified using Luminex (Applied Cytometry Systems) by following the manufacturer's instructions.
Quantification of viral RNA.
Total RNA was extracted from snap-frozen lungs using RNA STAT-60 (AMS Biotech Ltd.), and cDNA was generated with random hexamers using Omniscript reverse transcriptase (Qiagen). Real-time PCR was carried out for the RSV L gene using a 900 nM concentration of the forward primer (5′-GAACTCAGTGTAGGTAGAATGTTTGCA-3′), a 300 nM concentration of the reverse primer (5′-TTCAGCTATCATTTTCTCTGCCAAT-3′), and a 100 nM concentration of the probe (5′-FAM-TTTGAACCTGTCTGAACAT-TAMRA-3′, where FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine) on an ABI Prism 7000 sequence detection system as described previously (8).
Statistical analysis.
Results are expressed as means ± standard errors of the means (SEM); statistical significance was calculated by analysis of variance, followed by Tukey tests when there were more than three groups and t tests for the comparison of two groups with GraphPad Prism software.
RESULTS
Primary RSV infection in neonatal and adult mice.
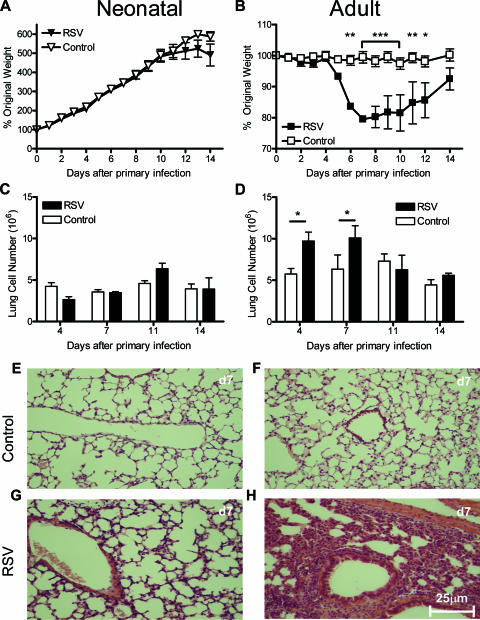
In neonatal mice, RSV infection had no effect on normal growth (Fig. 1A) (the slowdown in growth seen in neonates on days 12 to 14 p.i. is normal just before weaning). In adult mice, illness and weight loss appeared from day 5 of RSV infection (Fig. 1B). There was a small and nonsignificant increase in the cellularity of the lungs of neonates (Fig. 1C), less marked than that seen in adult mice after infection (Fig. 1D). There was no inflammation seen in control, treated lungs of either age (Fig. 1E and F). Neonatal infection resulted in a minor perivascular and peribronchiolar infiltration of mononuclear cells (Fig. 1G), in contrast to the marked histopathological effects seen after adult infection (Fig. 1H). Adult mice had significantly greater viral loads (3.1 × 105 ± 105 copies of the L gene per μg lung RNA) than neonatal mice (7.5 × 104 ± 104 copies of the L gene per μg lung RNA) on day 4 p.i. (P < 0.001). This difference in viral load was seen when adult and neonatal mice were infected with the same dose per gram of body weight or identical doses.
FIG. 1.
Primary RSV infection is more severe in adult mice than in neonates. Mice were i.n. infected neonatally (age, 4 days) or as immature adults (age, 4 to 6 weeks) with 4 × 104 PFU RSV per gram body weight. (A and B) Graphs show changes in body weight after the primary RSV or mock infection (Control) in neonatal (A) and adult (B) mice. (C and D) Lung cell counts p.i. in neonatal (C) and adult (D) mice after RSV infection or mock infection. (E to H) H&E-stained, formalin-fixed-lung histology sections at day 7 (d7) posttreatment in neonatal or adult mice (controls, E and F; RSV-infected mice, G and H). There were at least four mice per group. Data are means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In addition to the significant differences in disease, weight loss, cell recovery, and histological change, adults showed an enhanced recruitment of lymphocytes to the lungs. In control mice undergoing mock infection, there was no time-dependent increase in activated CD4 or CD8 T cells and no accumulation of pentamer-positive or IFN-γ-producing cells (data not depicted). However, after live RSV infection of adults, the number of activated CD4 T cells increased rapidly to a peak on day 11, declining thereafter (Fig. 2A). A similar pattern of accumulation of activated CD8 T cells was seen after a slight delay (Fig. 2B).
FIG. 2.
Dynamic changes in lungs after primary RSV infection. Neonatal and adult mice were infected as described in the legend for Fig. 1, and lungs were harvested at days 4, 7, 11, and 14 after the primary infection. (A and B) Cell types in the lung (CD4 T-cell effector memory types [CD44 hi and CD62L lo] [A] and CD8 T-cell effector memory types [CD44 hi and CD62L lo] [B]) were assessed by flow cytometry. (C) CCL5 in lung digests was measured by ELISA. (D to F) RSV (M2) pentamer-positive CD8 T cells, sample plots from neonatal (D) and adult (E) mice on day 11 and time course (F). (G to I) RSV (M2) peptide-specific IFN-γ-producing CD8 T cells measured by intracellular staining, sample plots from neonatal (G) and adult (H) mice on day 11 and time course (I). There were at least four mice per group. Data are means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As previously demonstrated, the infection of adult mice results in CCL5 (RANTES) release (7). Measurements of CCL5 levels in the supernatants of lung homogenates showed a rapid increase between days 4 and 11, peaking at approximately 8,000 pg/ml (Fig. 2C). The pattern of CCL5 levels mirrored CD4 T-cell recruitment. A small increase in CCL5 was also detected in neonatal mice (reaching only 2,000 pg/ml). Similar profiles were observed in BAL supernatants, but levels were lower. On day 9 p.i., neonatal mice had reduced TNF responses compared with those of adult mice (Table 1). There were no differences in the levels of IL-4 and IL-15 between adult and neonatal mice. There was more IL-5 detected in the BAL samples of neonatal mice (not significant) and significantly more IL-9 (P < 0.001) than in adult mice.
TABLE 1.
Lung inflammatory mediators following primary and secondary infections
| Mediatora | Level (ng/ml) on day 9 after primary infection
|
Significancee | Level (ng/ml) on day 7 after secondary infection
|
Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adult miceb
|
Neonatal micec
|
Adult miced
|
Neonatal miced
|
|||||||
| Mean | ±SEM | Mean | ±SEM | Mean | ±SEM | Mean | ±SEM | |||
| IL-4 | 0.66 | 0.22 | 0.26 | 0.12 | NS | 0.08 | 0.02 | 0.58 | 0.41 | NS |
| IL-5 | 4.05 | 1.13 | 10.91 | 5.14 | NS | 1.28 | 0.23 | 9.39 | 6.24 | NS |
| IL-9 | 65.36 | 33.0 | 244.73 | 12.6 | *** | 5.76 | 0.00 | 27.15 | 4.57 | NS |
| IL-15 | 29.53 | 24.9 | 55.87 | 32.9 | NS | 0.40 | 0.24 | 0.99 | 0.00 | NS |
| CCL5 | 232.4 | 8.15 | 5.60 | 1.57 | *** | 9.81 | 1.68 | 20.90 | 6.16 | NS |
| TNF | 11.94 | 2.16 | 1.21 | 0.15 | NS | 5.17 | 2.30 | 3.22 | 0.51 | NS |
Inflammatory mediators were measured in BAL fluid. Shown are means from experiments using three or more mice.
Primary infection occurred at 4 weeks of age.
Primary infection occurred at 4 days of age.
Secondary infection occurred 8 weeks after the primary infection.
NS, not significant; ***, P < 0.001.
We were interested in the source of these cytokines and whether production was RSV specific. CD8 T cells specific to the immunodominant, H-2Kd-restricted peptide epitope of the RSV M2 protein (amino acids 82 to 90) were measured in the lung. The proportion of RSV-specific CD8 T cells increased rapidly to a peak on day 11, declining thereafter (sample plots in Fig. 2D and E are from day 11; quantitative data are shown in Fig. 2F). The proportion of cells responding to this peptide by IFN-γ production peaked on day 7 (sample plots in Fig. 2G and H are from day 11; quantitative data are shown in Fig. 2I). There was no significant difference in levels of B-cell recruitment to the lungs of infected animals (data not depicted).
The response of neonates was similar to that seen in adults, but it was significantly reduced in magnitude. Interestingly, at day 11 there were similar proportions of RSV-specific CD8 T cells in adults and neonates (Fig. 2F), but only the adult cells responded to peptide stimulation by abundant IFN-γ production (Fig. 2I). Peptide-specific tumor necrosis factor (TNF) production by CD8 T cells followed the same pattern in adults and neonates, as did IFN-γ production following phorbol myristate acetate and ionomycin stimulation of T cells (data not depicted).
Secondary infection of adult mice or neonatally primed mice.
To study the effect of a primary RSV infection of neonates on the outcome of a secondary infection, mice were challenged at 8 weeks of age with 106 PFU of RSV, the same number of PFU/g body weight as was used in the primary infection. Neonatally primed mice showed significant weight loss during secondary RSV challenge, while mice first infected at 4 weeks lost no weight (Fig. 3A). Weight loss in neonatally primed mice peaked on day 6 and was preceded by a decrease in baseline lung function, inferred from Penhs upon whole-body plethysmography (Buxco Technologies, United Kingdom) (Fig. 3B), that peaked on days 3 and 4. Compared to what occurred with a primary infection of adults, the onset of illness (measured by weight loss) in neonatally primed mice was accelerated. The weight loss during adult challenge following neonatal priming was not dependent upon the priming dose; the weight loss in neonatal mice was also seen when both adult and neonatal mice were primed with identical doses (5 × 105 PFU RSV [data not depicted]).
FIG. 3.
Timing of primary RSV infection determines the effect of adult rechallenge. Mice were infected with RSV at the age of 4 days (Neonatal) or as adults (age, 4 weeks) and challenged with 5 × 105 PFU RSV at 8 weeks old. (A and B) Changes in body weight (A) and baseline Penh (B) after secondary infection; (C) lung cell counts; (D) BAL granulocyte counts; (E) chemokine levels; (F) lung CD4 and CD8 T cell counts; (G and H) percentages of CD4 (G) and CD8 (H) cells positive for IFN-γ, TNF, and IL-4 at day 7 p.i. (d7). There were at least four mice per group. Data are means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In addition to this enhanced disease, neonatally primed mice showed significantly increased lung cell numbers on day 7 p.i. compared to numbers in adults (P < 0.001) (Fig. 3C), and this trend was reflected in BAL samples (data not depicted). Significantly more eosinophilia and neutrophilia were seen in the BAL fluid of neonatally primed mice (P < 0.001) (Fig. 3D). This was associated with significantly enhanced levels of CCL11 (eosinophil chemoattractant) and KC (a functional homologue of IL-8 in mice and a neutrophil chemoattractant) in the BAL fluid (P < 0.01) (Fig. 3E). More CD8 than CD4 T cells were recruited to the lungs of neonatally primed mice (Fig. 3F). In contrast, mice first infected during adulthood and then rechallenged had equal numbers of CD4 and CD8 T cells at day 7 p.i. (Fig. 3F). During rechallenge, CD8 T cells recovered from the lungs of adult mice or neonatally primed mice were approximately 70% RSV pentamer positive (data not depicted). There was no significant difference in T-cell receptor Vβ usage between adults and neonates, and the response was polyclonal (data not depicted).
As previously demonstrated (8), the CD4 T-cell response was Th2 skewed, with significantly more IL-4-secreting Th2 CD4 T cells in neonatally primed mice than in adults (P < 0.05) (Fig. 3G). There were more TNF- and IFN-γ-positive CD8 T cells (Fig. 3H) in neonatally primed mice, indicating a stronger inflammatory response to secondary infection. Inflammatory mediators in neonatally primed mice were also greater during secondary challenge, as there was significantly more lung IL-2 and CCL3 (P < 0.05) (data not depicted). Similar patterns (although with lower absolute levels) were seen in the BAL fluid.
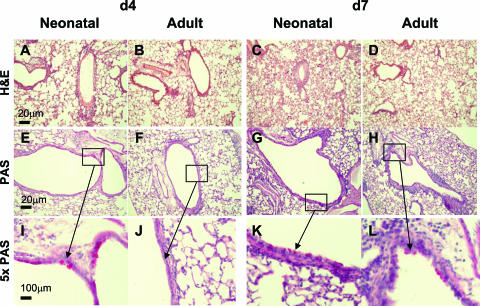
Neonatally primed mice also showed an increase in lung inflammation during adult rechallenge, as judged by histology results (Fig. 4). At day 4 p.i., this was characterized by peribronchiolar and perivascular infiltrates similar to those seen in adult mice (Fig. 4A and B), but at day 7 p.i., there was more evidence of alveolar infiltrate than in adult mice (Fig. 4C and D). At day 4 p.i., there were more goblet cells in the neonatally primed mice (Fig. 4E and I), which was not seen in primed adult mice (Fig. 4F and J). At day 7 p.i., there were detectable goblet cells in both adult and neonatally primed mice (Fig. 4G and H).
FIG. 4.
Neonatal RSV infection induces inflammation and goblet cell hyperplasia following secondary RSV challenge. Mice were infected as adults or neonates and rechallenged 8 weeks later as described for Fig. 3. Representative H&E-stained (A to D) and periodic acid-Schiff-stained (PAS) (E to H), formalin-fixed lung sections taken on day 4 (d4) and day 7 after adult challenge. The scale bar represents 20 μm. (I to L) Fivefold enlargements of regions with goblet cells are shown.
T-cell depletion.
Since enhanced disease was associated with a rapid recruitment of activated CD4 and CD8 T cells during secondary challenge, we wished to determine the role of T cells by selectively depleting CD4 cells, CD8 cells, or both on days −1, +2, and + 5 of infection. It has been previously demonstrated that T-cell depletion during primary infection reduces illness (14), and we wished to confirm that this was the case in our model. Depletion of T cells during primary RSV infection reduced weight loss (Fig. 5A). While CD4 cell depletion reduced the severity of weight loss, CD8 cell depletion reduced weight loss significantly more than CD4 cell depletion (P < 0.01 on days 6 and 7). Reduced disease severity was accompanied by a significant reduction in lung cellularity (Fig. 5B). Antibody treatment was highly effective at depleting T cells in the lung (Fig. 5C). The reduction in weight loss was accompanied by a reduction in neutrophilia (Fig. 5D) and KC (Fig. 5E); there was minimal eosinophilia detected in all groups (data not depicted). Levels of the inflammatory mediators CCL5, IL-2, and TNF followed the same pattern as that of KC in the BAL samples, with higher levels in mice with greater weight loss (data not depicted). There were significantly more CD8 cells producing IFN-γ and TNF after RSV peptide stimulation in mice with weight loss than in those without weight loss (P was <0.01 in a comparison of control antibody- and anti-CD4-treated mice and naïve mice [data not depicted]).
FIG. 5.
Depletion of T lymphocytes during primary RSV infection reduces lung inflammation. During primary RSV infection, adult mice were treated i.p. with T-cell-depleting antibodies on days −1, +2, and +5 of infection. (A) Weight change after RSV infection. (B and C) Lung cell numbers (B) and T-cell numbers (C) on day 7 after RSV infection. (D and E) Neutrophilia (D) and KC level (E) measured in BAL fluid 7 days after RSV infection. There were four mice per group. Data are means ± SEM. ***, P < 0.001.
In secondary infections, T cells were depleted according to the schedule in Fig. 6A. As seen in adults after primary infection, CD4 cell depletion reduced the severity of weight loss and CD8 cell depletion reduced weight loss significantly more than CD4 cell depletion (P was <0.05 for data taken on days 4 and 5) (Fig. 6B). Combined CD4/8 cell depletion virtually abolished disease in neonatally primed mice during adult reinfection (Fig. 6B). Both CD4 cell depletion and CD8 cell depletion increased the viral load, and combined depletion significantly increased it further (P < 0.05) (Fig. 6C). The reduction in disease severity was accompanied by a significant reduction in lung cellularity (P < 0.01) (Fig. 6D). Antibody treatment was highly effective at depleting T cells in the lung, reducing each respective cell type to ≤1% of the lung lymphocytes (Fig. 6E). While CD4 depletion reduced eosinophilia, CD8 cell depletion during secondary challenge dramatically increased airway eosinophilia during challenge (Fig. 6F). This was associated with significantly enhanced CCL11 (P < 0.001) (Fig. 6G) and IL-5 (Table 2) levels in the BAL samples. CD8 depletion also increased BAL neutrophilia (Fig. 6H) and KC levels (Fig. 6I). The pattern of reduced disease following T-cell depletion (Fig. 6B) was reflected in inflammatory mediator production. IL-2, IL-4, IL-13, and CCL5 levels all decreased in proportion to the decrease in inflammation (Table 2).
FIG. 6.
Depletion of T lymphocytes during secondary RSV challenge reduces lung inflammation. Mice were infected at 4 days of age (primary infection [1o]) and rechallenged at 8 weeks (secondary infection [2o]). (A) During secondary RSV challenge, mice were treated i.p. with T-cell-depleting antibodies (Ab) according to the treatment schedule. d, day. (B) Weight change after secondary RSV challenge. NN, neonates. (C) Lung viral load 4 days after secondary challenge. (D and E) Lung cell numbers (D) and T-cell numbers (E) on day 7 after RSV challenge. (E to I) Eosinophilia (F), CCL11 (G), neutrophilia (H), and KC (I) levels were measured in BAL fluid 7 days after the secondary RSV challenge. There were at least four mice per group. Data are means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
TABLE 2.
Lung inflammatory mediators following depletion during secondary challenge
| Mediatora | Level (ng/ml)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Neonatal mice
|
Anti-CD4-treated mice
|
Anti-CD8-treated mice
|
Anti-CD4/8-treated mice
|
|||||
| Mean | ±SEM | Mean | ±SEM | Mean | ±SEM | Mean | ±SEM | |
| IFN-γ | 2.10 | 0.39 | 0.99 | 0.40 | 0.49 | 0.24 | 0.90 | 0.34 |
| IL-2 | 7.35 | 0.75 | 5.12 | 0.94 | 5.16 | 0.98 | 3.41 | 1.07 |
| IL-4 | 0.34 | 0.01 | 0.27 | 0.02 | 0.26 | 0.04 | 0.19 | 0.04 |
| IL-5 | 13.72 | 9.82 | 1.41 | 0.13 | 34.22 | 9.45 | ||
| IL-13 | 2.44 | 0.32 | 1.64 | 0.47 | 1.14 | 0.45 | 1.01 | 0.33 |
| CCL5 | 29.67 | 7.38 | 8.21 | 1.84 | 3.54 | 0.87 | ||
| TNF | 4.14 | 0.35 | 1.94 | 0.18 | 3.53 | 0.50 | ||
Inflammatory mediators were measured in BAL fluid 7 days after the secondary challenge. Shown are means ± SEM from experiments using at least four mice.
To determine the role of T cells recruited during primary infection on the outcome of secondary infection, we selectively depleted CD4 cells, CD8 cells, or both on days −1, +2, and +5 of primary infection (Fig. 7A). Depletion in the neonatal period was very effective, reducing the respective cell type to ≤2% of the lung lymphocytes, but had no apparent effect on the course of primary infection. However, the effect on weight loss during secondary rechallenge was striking. While CD4 depletion had no effect, CD8 depletion or CD4/8 dual depletion inhibited weight loss (Fig. 7B). T-cell depletion had no significant effect on viral load at day 4 p.i. of secondary infection (Fig. 7C) or in the primary infection (data not shown). CD8 cell depletion during primary infection significantly reduced the cellularity of the BAL fluid during secondary challenge (P < 0.05) (Fig. 7D) but did not significantly alter the lung cell number (data not depicted). Neonatal CD8 depletion increased the number of CD4 T cells and decreased the number of CD8 T cells in the lung during secondary challenge (Fig. 7E). This change was RSV specific because mice treated with both anti-CD4 and anti-CD8 as neonates (in the absence of RSV infection) had normal T-cell numbers and responded normally to a primary RSV infection as adults (data not depicted). CD8 depletion increased BAL fluid eosinophilia (Fig. 7F) and neutrophilia (Fig. 7H), with changes seen in the related chemokines CCL11 (Fig. 7G) and KC (Fig. 7I). No distinct pattern of other cytokines was seen following primary neonatal depletion; CD8 depletion increased IFN-γ production and IL-2 production but reduced CCL3 production; however, CD4/8 dual depletion, which caused a similar phenotype of protection against rechallenge, led to no changes to these mediators (data not depicted).
FIG. 7.
Depletion of CD8 cells during primary neonatal RSV infection (1o) affects the outcome of adult rechallenge. Mice were infected with RSV as neonates (NN) and challenged 8 weeks later with RSV (secondary infection [2o]). (A) During primary neonatal RSV infection, mice were treated i.p. with T-cell-depleting antibodies (Ab) according to the treatment schedule. d, day. (B) Weight change after secondary RSV challenge. (C) Lung viral loads at day 4 p.i. (D to I) BAL cell numbers (D), lung T-cell numbers (E), eosinophilia (F), CCL11 levels (G), neutrophilia (H), and KC levels (I) measured in BAL fluid 7 days after secondary RSV challenge. There were at least four mice per group. Data are means ± SEM. *, P < 0.05.
DISCUSSION
Age at primary infection is critical to the outcome of secondary RSV infection, apparently driven by CD4 T cells that give rise to Th2 cytokines (8). The current study shows that neonatal CD8 T-cell responses to primary infection also have a profound influence on the outcome of secondary infection during adulthood. During primary infection, both adult and neonatal mice recruited RSV-specific T cells, but neonatal CD8 cells produced less IFN-γ. These CD8 T cells were of critical importance to the disease seen upon adult rechallenge, because the detrimental effects of neonatal priming were largely prevented by their depletion during the first infection. In addition to playing a role in priming for disease enhancement, T cells act as effectors during secondary challenge when the depletion of either CD4 or CD8 reduces weight loss and cell recruitment.
During the primary infection of mice, it has previously been shown that RSV replication, disease, and pathology increase with age (15). This seems paradoxical, in that natural RSV disease is most severe in very young infants (aged <6 months) and rarely leads to hospitalization in older children. However, it is notable that elderly persons are highly susceptible to reinfection and can experience life-threatening effects of RSV disease (13). In the current study, neonatal RSV infection was associated with less inflammation and disease than in adults, possibly because of different cytokine production profiles of the cells recruited to the site of infection. Neonatal immune responses have been characterized as “Th2” skewed (1); we found that following primary RSV infection, neonates did indeed make higher levels of IL-5 and IL-9 than adults, indicating Th2 skewing. IL-9 has previously been shown to be elevated in babies with severe bronchiolitis (25) and is associated with asthma (10). IL-9 promotes IL-5 secretion in naïve T cells (27). We suggest that in our studies, IL-9 produced during early life infection changed the response to future infections by committing naïve T cells to Th2 patterns.
The difference in IFN-γ production by RSV-specific CD8 T cells recruited during primary infection is intriguing. Our results compare with those of Chang et al. (5), who showed that pulmonary RSV-specific CD8 cells are deficient in IFN-γ production and that this defect is reversed by the addition of IL-2 to ex vivo-cultured cells. During stimulation with peptide for intracellular staining, we routinely add IL-2. This explains the high levels of peptide-specific IFN-γ production that we observed in adult cells but suggests that neonatal cells are either refractory to IL-2 or defective in IFN-γ production.
CD8 T cells recruited during primary infection are of critical importance in causing weight loss during secondary challenge, because their depletion during primary infection reduced weight loss. Addition of recombinant IFN-γ during neonatal RSV infection leads to reduced disease during adult rechallenge (22). We now show that neonatal CD8 T cells make less IFN-γ; CD8 T cells have been shown to sometimes produce Th2 cytokines, leading to eosinophilia (28), and IL-13-producing CD8 cells have been reported during secondary infection of neonatal mice (9). We have previously shown that cytokine delivery using recombinant RSV can shape future infections, with recombinant-RSV/IL-4 priming leading to lung eosinophilia during secondary challenge with wild-type RSV or influenza A virus (16). The increased levels of IL-9 and IL-5 and decreased IFN-γ that we observed during primary infection suggest that it is this cytokine environment influencing the functional phenotype of CD8 T cells, which is a critical factor in causing enhanced disease.
Secondary infection after neonatal priming showed some characteristics of “Th2” disease, e.g., increased IL-4-producing CD4 T cells and eosinophilia. However, the enhanced disease is not solely accounted for by Th2 CD4 cells, since CD4 depletion only partially prevented these effects and CD8 depletion had more impact than CD4 depletion. Furthermore, depletion of CD8 cells during challenge increased eosinophilia without increasing weight loss.
We have previously shown the inhibitory effect of CD8 cells on eosinophilia. Mice primed with recombinant vaccinia virus expressing the RSV F protein develop eosinophilia only after CD8 cell depletion (19), and passive transfer of CD8 T cells attenuates eosinophilia caused by the passive transfer of Th2 CD4 T cells (2). Enhanced eosinophilia following RSV infection is not always associated with weight loss (16). In the present studies, CD8 depletion boosted lung neutrophilia, suggesting that CD8 cells can exert an inhibitory effect on neutrophil recruitment and survival under some circumstances. It has recently been shown that adaptive immune cells can dampen innate responses (11), possibly by delivering an inhibitory signal to infected cells or by taking up cytokines released by infected cells that would otherwise activate noninfected cells and sustain a cycle of enhanced inflammation. It is possible that T cells have inhibitory effects on innate responses in our studies and that effects on neutrophil recruitment reflect this inhibition.
We show that T cells are critical as the effector cells for weight loss in adults after both primary infection and secondary infection. The key role that T cells play in causing disease in primary (14) or secondary (6) disease has been well demonstrated. We found that production of inflammatory mediators is associated with weight loss and have previously demonstrated the importance of CCL5 (7), CCL11 (24), and TNF (20) in this regard. During secondary infection, T-cell depletion had an effect as early as day 2 p.i. (Fig. 6B), prior to detectable T-cell recruitment. Hikono et al. (17) and Hogan et al. (18) have shown that resident airway cells can lead to the specific control of initial viral infections, probably by releasing IFN-γ, which recruits/activates other cells in the airway (particularly macrophages and NK cells), which in turn can recruit more T cells. There is also evidence of nonspecific protection against heterologous infection (12). We suggest that the production of proinflammatory mediators by resident T cells may lead to enhanced cell recruitment and delayed resolution of inflammation, weight loss, and disease after RSV infection.
In conclusion, we find that T cells, particularly CD8 T cells, play a pivotal role in controlling disease enhancement following neonatal RSV infection. However, T cells seem to play different roles in primary and secondary infections; in primary infection, only CD8 T cells play a role, while in secondary adult disease, both CD4 and CD8 T cells contribute to weight loss. These are important and novel findings, because CD4 T cells were previously thought to be the driving factor of RSV disease enhancement. The discovery that CD8 cells are critical in neonatal responses has important implications for the development of early-life vaccines against RSV.
Acknowledgments
This work was funded by program grant number 071381/Z/03/Z from the Wellcome Trust, United Kingdom.
We thank Ita Askonas for advice and encouragement and Steve Cobbold (University of Oxford) for depleting antibodies.
We have no conflicting financial interests.
Footnotes
Published ahead of print on 13 February 2008.
REFERENCES
- 1.Adkins, B., C. LeClerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4553-564. [DOI] [PubMed] [Google Scholar]
- 2.Alwan, W. H., F. M. Record, and P. J. M. Openshaw. 1992. CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus: comparison with the effects of CD8+ cells. Clin. Exp. Immunol. 88527-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisgaard, H., M. N. Hermansen, F. Buchvald, L. Loland, L. B. Halkjaer, K. Bonnelykke, M. Brasholt, A. Heltberg, N. H. Vissing, S. V. Thorsen, M. Stage, and C. B. Pipper. 2007. Childhood asthma after bacterial colonization of the airway in neonates. N. Engl. J. Med. 3571487-1495. [DOI] [PubMed] [Google Scholar]
- 4.Bonhoeffer, J., C. A. Siegrist, and P. T. Heath. 2006. Immunisation of premature infants. Arch. Dis. Child. 91929-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, J., and T. J. Braciale. 2002. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat. Med. 854-60. [DOI] [PubMed] [Google Scholar]
- 6.Connors, M., A. B. Kulkarni, C. Y. Firestone, K. L. Holmes, H. C. Morse, A. V. Sotnikov, and B. R. Murphy. 1992. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J. Virol. 667444-7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culley, F. J., A. M. Pennycook, J. S. Tregoning, J. S. Dodd, G. Walzl, T. N. Wells, T. Hussell, and P. J. Openshaw. 2006. Role of CCL5 (RANTES) in viral lung disease. J. Virol. 808151-8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culley, F. J., J. Pollott, and P. J. Openshaw. 2002. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J. Exp. Med. 1961381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dakhama, A., J. W. Park, C. Taube, A. Joetham, A. Balhorn, N. Miyahara, K. Takeda, and E. W. Gelfand. 2005. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J. Immunol. 1751876-1883. [DOI] [PubMed] [Google Scholar]
- 10.Devos, S., F. Cormont, S. Vrtala, E. Hooghe-Peters, F. Pirson, and J. Snick. 2006. Allergen-induced interleukin-9 production in vitro: correlation with atopy in human adults and comparison with interleukin-5 and interleukin-13. Clin. Exp. Allergy 36174-182. [DOI] [PubMed] [Google Scholar]
- 11.Dong, K. K., J. Zhao, S. Auh, X. Yang, P. Du, H. Tang, and Y. X. Fu. 2007. Adaptive immune cells temper initial innate responses. Nat. Med. 131248-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ely, K. H., L. S. Cauley, A. D. Roberts, J. W. Brennan, T. Cookenham, and D. L. Woodland. 2003. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J. Immunol. 1701423-1429. [DOI] [PubMed] [Google Scholar]
- 13.Falsey, A. R., P. A. Hennessey, M. A. Formica, C. Cox, and E. E. Walsh. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 3521749-1759. [DOI] [PubMed] [Google Scholar]
- 14.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Investig. 881026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham, B. S., M. D. Perkins, P. F. Wright, and D. T. Karzon. 1988. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 26153-162. [DOI] [PubMed] [Google Scholar]
- 16.Harker, J., A. Bukreyev, P. L. Collins, B. Wang, P. J. Openshaw, and J. S. Tregoning. 2007. Virally delivered cytokines alter the immune response to future lung infections. J. Virol. 8113105-13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hikono, H., J. E. Kohlmeier, K. H. Ely, I. Scott, A. D. Roberts, M. A. Blackman, and D. L. Woodland. 2006. T-cell memory and recall responses to respiratory virus infections. Immunol. Rev. 211119-132. [DOI] [PubMed] [Google Scholar]
- 18.Hogan, R. J., W. Zhong, E. J. Usherwood, T. Cookenham, A. D. Roberts, and D. L. Woodland. 2001. Protection from respiratory virus infections can be mediated by antigen-specific CD4+ T cells that persist in the lungs. J. Exp. Med. 193981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussell, T., C. J. Baldwin, A. O'Garra, and P. J. M. Openshaw. 1997. CD8+ T-cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur. J. Immunol. 273341-3349. [DOI] [PubMed] [Google Scholar]
- 20.Hussell, T., A. Pennycook, and P. J. M. Openshaw. 2001. Inhibition of tumour necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 312566-2573. [DOI] [PubMed] [Google Scholar]
- 21.Illi, S., E. von Mutius, S. Lau, R. Bergmann, B. Niggemann, C. Sommerfeld, and U. Wahn. 2001. Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. Br. Med. J. 322390-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, Y. M., N. Miyahara, K. Takeda, J. Prpich, A. Oh, A. Balhorn, A. Joetham, E. W. Gelfand, and A. Dakhama. 2008. IFN-gamma production during initial infection determines the outcome of reinfection with respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 177208-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, A. H., and D. Y. Leung. 2006. Renaissance of the hygiene hypothesis. J. Allergy Clin. Immunol. 1171063-1066. [DOI] [PubMed] [Google Scholar]
- 24.Matthews, S. P., J. S. Tregoning, A. J. Coyle, T. Hussell, and P. J. Openshaw. 2005. Role of CCL11 in eosinophilic lung disease during respiratory syncytial virus infection. J. Virol. 792050-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNamara, P. S., B. F. Flanagan, L. M. Baldwin, P. Newland, C. A. Hart, and R. L. Smyth. 2004. Interleukin 9 production in the lungs of infants with severe respiratory syncytial virus bronchiolitis. Lancet 3631031-1037. [DOI] [PubMed] [Google Scholar]
- 26.Moghaddam, A., W. Olszewska, B. Wang, J. S. Tregoning, R. Helson, Q. J. Sattentau, and P. J. Openshaw. 2006. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat. Med. 12905-907. [DOI] [PubMed] [Google Scholar]
- 27.Poulin, L. F., C. Habran, P. Stordeur, M. Goldman, A. McKenzie, S. J. Van, J. C. Renauld, and M. Y. Braun. 2005. Interleukin-9 stimulates the production of interleukin-5 in CD4+ T cells. Eur. Cytokine Netw. 16233-239. [PubMed] [Google Scholar]
- 28.Schwarze, J., G. Cieslewicz, A. Joetham, T. Ikemura, E. Hamelmann, and E. W. Gelfand. 1999. CD8 T cells are essential in the development of respiratory syncytial virus-induced lung eosinophilia and airway hyperresponsiveness. J. Immunol. 1624207-4211. [PubMed] [Google Scholar]
- 29.Shay, D. K., R. C. Holman, R. D. Newman, L. L. Liu, J. W. Stout, and L. J. Anderson. 1999. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 2821440-1446. [DOI] [PubMed] [Google Scholar]
- 30.Sigurs, N., P. M. Gustafsson, R. Bjarnason, F. Lundberg, S. Schmidt, F. Sigurbergsson, and B. Kjellman. 2005. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med. 171137-141. [DOI] [PubMed] [Google Scholar]
- 31.Smyth, R. L., and P. J. Openshaw. 2006. Bronchiolitis. Lancet 368312-322. [DOI] [PubMed] [Google Scholar]
- 32.Wang, H., N. Peters, and J. Schwarze. 2006. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J. Immunol. 1776263-6270. [DOI] [PubMed] [Google Scholar]
- 33.Zaccone, P., Z. Fehervari, J. M. Phillips, D. W. Dunne, and A. Cooke. 2006. Parasitic worms and inflammatory diseases. Parasite Immunol. 28515-523. [DOI] [PMC free article] [PubMed] [Google Scholar]