Abstract
Adenovirus type 12 (Ad12) propagation in hamster BHK21 cells is blocked prior to viral DNA replication. The amounts of Ad12 DNA in the nuclei or cytoplasm of hamster cells are about 2 orders of magnitude (2 h postinfection [p.i.]) and 4 to 5 orders of magnitude (48 h p.i.) lower than in permissive human cells. Cell line BHK21-hCAR is transgenic for and expresses the human coxsackie- and adenovirus receptor (hCAR) gene. Nuclear uptake of Ad12 DNA in BHK21-hCAR cells is markedly increased compared to that in naïve BHK21 cells. Ad12 elicits a cytopathic effect in BHK21-hCAR cells but not in BHK21 cells. Quantitative PCR or [3H]thymidine labeling followed by zone velocity sedimentation fails to detect Ad12 DNA replication in BHK21 or BHK21-hCAR cells. Newly assembled Ad12 virions cannot be detected. Thus, the block in Ad12 DNA replication in hamster cells is not released by enhanced nuclear import of Ad12 DNA.
Syrian hamster cells support a replicative cycle of adenovirus type 2 (Ad2) virions; their interaction with Ad12, however, is completely abortive (6, 7, 10, 23). Studies on the interaction of Ad12 virions with nonpermissive hamster cells are of interest, because Ad12 induces tumors in newborn Syrian hamsters (Mesocricetus auratus) (9, 24). The complete abortiveness of Ad12 in hamster cells is a precondition to facilitate oncogenic transformation. By overcoming the human-hamster species barrier, Ad12 converts from a cell-killing pathogen in human cells to an oncogenic virus in newborn hamsters.
Adenoviruses enter cells by endocytosis (15, 17), in part via the coxsackie- and adenovirus receptor (CAR) (1, 18), a 46-kDa transmembrane protein with high affinity for both viruses. CAR is expressed in many cell lines. Its function in the cell involves regulation of cell proliferation or differentiation (19, 26) and cell-cell adhesion. Adenovirus-cell interactions involve additional cellular factors, among them the αvβ3 and αvβ5-based integrins (17). Adenoviruses interact with cells through the elongated fiber protein, a homotrimer consisting of an N-terminal tail, a long shaft, and a C-terminal knob region with high affinity to the receptor (14, 27).
Previous analyses of the Ad12-BHK21 cell system revealed an early block in Ad12 replication, with minimal early viral gene transcription, complete absence of viral DNA replication (6, 7, 10), and chromosomal integration of Ad12 DNA into the hamster genome (5). Ad12 DNA persists integrated in Ad12-transformed and Ad12-induced hamster tumor cells (8, 12, 21). Ad5 E1 functions in cell line BHK279-C131 (20) or overexpression of the Ad2 or Ad12 E1A or pTP gene in BHK21 cells (11) facilitate limited Ad12 DNA replication but no virion production. We have now investigated whether a critical threshold concentration of Ad12 DNA in the hamster cell nucleus might trigger Ad12 DNA replication in nonpermissive BHK21 hamster cells.
Standard techniques were described elsewhere (6, 11, 16, 20). The BHK21 cell line transgenic for the human CAR (hCAR) gene (BHK21-hCAR cells) (22) was a gift of Silvio Hemmi and Urs Greber, Zürich University, Switzerland.
Methods employed for the detection of hCAR expression by fluorescence-activated cell sorting, for immunofluorescent cell staining, for the isolation of nuclei from infected cells, for fluorescent in situ hybridization (FISH), and for the quantification of viral DNA in nuclei of infected cells are described in the supplemental material.
Metabolic labeling of newly synthesized DNA with [3H]thymidine in HeLa, BHK21, or BHK21-hCAR cells after infection with Ad12 followed by velocity sedimentation in alkaline sucrose gradients was described elsewhere (2, 6).
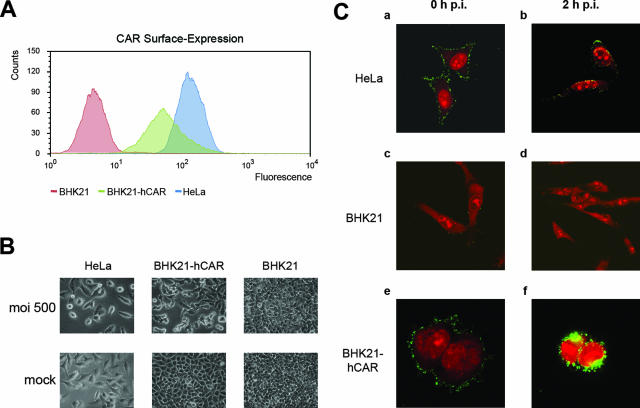
Continued expression of the hCAR gene in BHK21-hCAR cells was documented by fluorescence-activated cell sorting (Fig. 1A). Human HeLa and BHK21-hCAR cells, the latter cultured in the presence of 1 mg/ml G418, expressed the hCAR protein; BHK21 cells did not.
FIG. 1.
(A) Surface expression of hCAR in human HeLa cells, in hamster BHK21 cells, or in BHK21-hCAR cells. Continued expression of the hCAR protein was determined by fluorescence-activated cell sorting as described in the supplemental material. Fluorescence was measured in arbitrary units and plotted against the number of cells. The BHK21-hCAR cells were screened periodically for stable hCAR expression. (B) Ad12 infection elicits progressive CPE in BHK21-hCAR cells. Ad12-infected HeLa cells served as positive controls and nontransgenic BHK21 cells as negative controls. Mock-infected cells were treated with phosphate-buffered saline devoid of virus. Photographs were taken at 30 h p.i. with 500 PFU of Ad12 per cell in a Zeiss Axiovert 10 microscope. (C) Adsorption and penetration of Ad12 in different cell systems as determined by immunofluorescence. Ad12 virions were adsorbed at 4°C to human HeLa (a and b), hamster BHK21 (c and d), or BHK21-hCAR (e and f) cells. The cells either were fixed directly after 30 min of incubation with Ad12 at 4°C (a, c, and e) or were shifted to 37°C for 2 h (b, d, and f). Ad12 virions were detected by immunofluorescence staining with rabbit polyclonal antiserum against Ad12 protein IX (green channel), and nucleic acids were counterstained with 20 nM propidium iodide (red channel).
The inoculation of BHK21-hCAR cells with Ad12 (multiplicity of infection [MOI], 500 PFU/cell) led to a distinct and progressive cytopathic effect (CPE) starting at 24 h postinfection (p.i.). CPE in productively Ad12-infected HeLa cells (MOI of 500 PFU/cell) developed earlier and with a different morphology. Ad12-infected BHK21 cells continued to grow without CPE (Fig. 1B).
The adsorption and entry of Ad12 virions into HeLa, BHK21, or BHK21-hCAR cells were compared by immunofluorescence with an antiserum against Ad12 protein IX (Fig. 1C). Adsorption of Ad12 to human HeLa cells was by far more efficient than that to uncomplemented BHK21 hamster cells (Fig. 1C, panels a and c). At 2 h after inoculation, intracellular Ad12 virions were hardly detectable in nontransgenic BHK21 cells (Fig. 1C, panel d), whereas in human cells penetration had proceeded effectively (Fig. 1C, panel b). Ad12 virions adsorbed to BHK21-hCAR cells (Fig. 1C, panel e), and at 2 h p.i., most cells contained Ad12 in distinct perinuclear areas of the cytoplasm (Fig. 1C, panel f), a distribution pattern not observed in permissive human HeLa cells. Thus, the expression of the hCAR in BHK21-hCAR cells enables Ad12 virions to enter the cytoplasm.
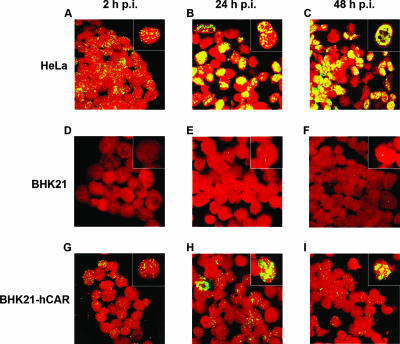
Nuclear uptake of Ad12 DNA was next determined by FISH in Ad12-infected HeLa, BHK21, or BHK21-hCAR cells. Nuclei of Ad12-infected cells were isolated 2, 24, or 48 h p.i., and Ad12 DNA was visualized by FISH (Fig. 2). At 2 h p.i., most Ad12-infected HeLa nuclei carried viral DNA, and at 24 or 48 h p.i., intranuclear centers of Ad12 DNA replication were apparent (Fig. 2A to C). Only very few nuclei from uncomplemented BHK21 cells contained minute amounts of Ad12 DNA (Fig. 2D to F). In contrast, nuclear uptake of Ad12 DNA in BHK21-hCAR cells was enhanced; about half of the nuclei showed multiple Ad12 DNA signals (Fig. 2G to I). There were occasional nuclei (1 in ∼200) with very high Ad12 signal intensities (Fig. 2G to I, insets), perhaps due to high Ad12 DNA uptake by individual BHK21-hCAR cells. We conclude that BHK21-hCAR cells are capable of Ad12 DNA nuclear uptake which is far above that in uncomplemented BHK21 cells.
FIG. 2.
Analyses of the nuclei of Ad12-infected cells by FISH. HeLa, BHK21, or BHK21-hCAR cells were infected with Ad12 at an MOI of 500 PFU/cell. At the indicated times, nuclei were prepared and Ad12 genomes were visualized by FISH as described in the supplemental material (green channel). Photographs were taken with a Zeiss LSM4 confocal laser scan microscope. Nuclei were counterstained with 20 nM propidium iodide (red channel). The insets show magnifications of selected cells.
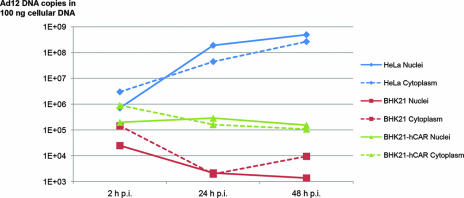
We next quantified the amounts of viral DNA present in the nuclei or cytoplasm of Ad12-infected human HeLa, hamster BHK21, or BHK21-hCAR cells at 2, 24, and 48 h p.i. by using quantitative PCR (Fig. 3). Nuclear uptake of Ad12 DNA at 2 h p.i. in permissive HeLa or hCAR-complemented BHK21 cells exceeded that in nonpermissive BHK21 cells by about 1.5 or about 1 log unit, respectively (Fig. 3). At 24 and 48 h p.i., Ad12 DNA had replicated to high copy numbers in human HeLa cells, whereas in uncomplemented as well as in hCAR-expressing BHK21 cells, the amounts of Ad12 DNA failed to increase. In BHK21 cells, the quantity of Ad12 DNA at 48 h p.i. had decreased to levels >5 log units lower than in human cells. Similarly, in BHK21-hCAR cells a gradual loss of Ad12 DNA was observed (Fig. 3). In spite of the enhanced nuclear uptake of Ad12 DNA in BHK21-hCAR cells, Ad12 DNA did not replicate in this cell system. As both BHK21 and BHK21-hCAR cells continue to divide vigorously after Ad12 infection, low levels of Ad12 replication in a few BHK21-hCAR cells might have been masked by a higher rate of DNA degradation.
FIG. 3.
Quantitative time course analyses of Ad12 DNA in the nuclei or cytoplasm of Ad12-infected HeLa, BHK21, or BHK21-hCAR cells. Cells were infected with Ad12 at an MOI of 500 PFU/cell for time periods of 2, 24, and 48 h. Nuclear or cytoplasmic DNA was then isolated, and 100 ng was used in quantitative PCR. PCR primers were selected for a 78-bp fragment within the MLP region of Ad12 DNA.
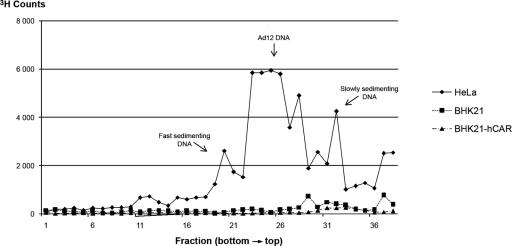
Viral DNA replication in Ad12-infected BHK21-hCAR cells as well as in naïve BHK21 and HeLa cells was also investigated by metabolic labeling of the newly synthesized DNA with [3H]thymidine (2). At 80 h p.i., the cells were lysed on top of an alkaline sucrose density gradient, and the DNA was analyzed by zone velocity sedimentation (Fig. 4). Human HeLa cells were infected and analyzed in a similar experiment. In naïve BHK21 cells or in BHK21-hCAR cells, there was no evidence for the de novo synthesis of Ad12 DNA. This finding agreed with the quantitative PCR data (Fig. 3). Hence, improved intranuclear uptake, even at levels close to those in permissive human HeLa cells (Fig. 3), did not suffice to elicit viral DNA replication in BHK21-hCAR cells. Ad12 DNA replicated in human HeLa cells, and the data in Fig. 4 revealed the main peak of Ad12 DNA, fast-sedimenting DNA that had previously been characterized as viral DNA linked to cellular DNA (2-4), and fragments of Ad12 DNA.
FIG. 4.
[3H]thymidine labeling of newly synthesized DNA in Ad12-infected HeLa, BHK21, or BHK21-hCAR cells. Analysis of newly synthesized DNA at 80 h p.i. from cells infected with Ad12 (200 PFU/cell) by velocity sedimentation in alkaline sucrose density gradients is shown. Experimental details were as described previously (2).
At 96 h p.i., the maintenance medium and extracts of Ad12-infected BHK21-hCAR cells were assayed for the presence of infectious Ad12 virions by plaque assay or by inoculating susceptible human HeLa cells. Medium or extracts from Ad12-infected BHK21-hCAR cells showed a decrease of Ad12 titers by plaque assay on human A549 cells. In controls with medium or extracts from Ad12-infected HeLa cells, Ad12 virion titers increased as expected. Moreover, when the infectivity of medium and extracts from Ad12-infected BHK21-hCAR cells was assessed by inoculating monolayers of HeLa cells, CPE did not develop within 10 days p.i. Medium or extracts from Ad12-infected HeLa cells elicited CPE at 3 days p.i. in susceptible HeLa cells. Hence, Ad12 virions were not produced in BHK21-hCAR cells.
There are multiple blocks for Ad12 replication in nonpermissive BHK21 cells (6, 7, 10, 11), and we have tried to overcome them by individually supplying the viral E1 and/or pTP functions of Ad12 or Ad2 (11) or the cellular hCAR function in hCAR-transgenic cells (this study). In the Ad5-transformed BHK297-C131 hamster cell line, which carries the left terminal 18.7% and the 32.4- to 42.4-map-unit fragment of the Ad5 genome chromosomally integrated and constitutively expresses it (25), Ad12 DNA and late Ad12 RNAs are synthesized in limited amounts, but virion proteins are not made (11, 20). None of these gene products suffices to allow Ad12 virion production in BHK21 hamster cells. This tight block renders each Ad12-infected hamster cell susceptible to oncogenic transformation, as Ad12 virions cannot replicate and destroy the infected hamster cells. In the present study, adsorption, import, and nuclear entry of Ad12 DNA were enhanced in BHK21-hCAR cells (Fig. 2 and 3). However, markedly increased levels of intranuclear Ad12 DNA templates did not lead to Ad12 DNA replication (Fig. 3 and 4). The FISH data (Fig. 2H and I), show isolated BHK21-hCAR cells with distinct centers of Ad12 DNA; however, there is no evidence for Ad12 DNA replication.
Ad2 infection of hamster BHK21 cells leads to a productive cycle that is less efficient than in human HeLa cells (6, 23). Ad2 seems to be capable of utilizing the hamster CAR or, perhaps more likely, might enter hamster cells primarily through a CAR-independent pathway. In contrast, Ad12 enters BHK21 cells extremely inefficiently and fails to replicate. Ad12 adsorption, entry, and nuclear import are markedly improved in BHK21-hCAR cells. The majority of amino acid residues necessary for the interaction between fiber and human CAR are conserved between Ad2, Ad5, and Ad12 (13). However, there must still be essential differences in the ways that the Ad12 and Ad2 fiber structures interact with proteins on the hamster cell surface. The replication machinery of Ad2 has been able to exploit cellular mechanisms for transcription, translation, and replication in hamster cells, whereas Ad12, due to inefficient entry, has not. Thus, even when Ad12 virion entry and nuclear Ad12 DNA import are assisted by artificially supplying the hCAR product, essential hamster host factors cannot be utilized by the Ad12 replication machinery even in the presence of above-threshold amounts of imported Ad12 DNA. We have not yet investigated whether BHK21-hCAR cells could be more efficiently transformed by Ad12.
Supplementary Material
Acknowledgments
Silvio Hemmi and Urs Greber, Zurich University, Switzerland, kindly provided the hCAR-transgenic BHK21 cell line.
D.W. and N.H. received stipends from Amaxa GmbH, Cologne, Germany. This research was supported by Amaxa GmbH and by the Institute for Virology, Erlangen University Medical School.
Footnotes
Published ahead of print on 6 February 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 2751320-1323. [DOI] [PubMed] [Google Scholar]
- 2.Burger, H., and W. Doerfler. 1974. Intracellular forms of adenovirus DNA. 3. Integration of the DNA of adenovirus type 2 into host DNA in productively infected cells. J. Virol. 13975-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deuring, R., and W. Doerfler. 1983. Proof of recombination between viral and cellular genomes in human KB cells productively infected by adenovirus type 12: structure of the junction site in a symmetric recombinant (SYREC). Gene 26283-289. [DOI] [PubMed] [Google Scholar]
- 4.Deuring, R., G. Klotz, and W. Doerfler. 1981. An unusual symmetric recombinant between adenovirus type 12 DNA and human cell DNA. Proc. Natl. Acad. Sci. USA 783142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doerfler, W. 1968. 1968. The fate of the DNA of adenovirus type 12 in baby hamster kidney cells. Proc. Natl. Acad. Sci. USA 60636-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doerfler, W. 1969. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology 38587-606. [DOI] [PubMed] [Google Scholar]
- 7.Doerfler, W. 2007. Human adenovirus type 12: crossing species barriers to immortalize the viral genome. Methods Mol. Med. 131197-211. [DOI] [PubMed] [Google Scholar]
- 8.Hochstein, N., I. Muiznieks, L. Mangel, H. Brondke, and W. Doerfler. 2007. The epigenetic status of an adenovirus transgenome upon long-term cultivation in hamster cells. J. Virol. 815349-5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohlweg, U., A. Dorn, M. Hosel, D. Webb, R. Buettner, and W. Doerfler. 2004. Tumorigenesis by adenovirus type 12 in newborn Syrian hamsters. Curr. Top. Microbiol. Immunol. 273215-244. [DOI] [PubMed] [Google Scholar]
- 10.Hösel, M., D. Webb, J. Schroer, and W. Doerfler. 2003. The abortive infection of Syrian hamster cells with human adenovirus type 12. Curr. Top. Microbiol. Immunol. 272415-440. [DOI] [PubMed] [Google Scholar]
- 11.Hösel, M., D. Webb, J. Schröer, B. Schmitz, and W. Doerfler. 2001. Overexpression of the adenovirus type 12 (Ad12) pTP or E1A gene facilitates Ad12 DNA replication in nonpermissive BHK21 hamster cells. J. Virol. 7510041-10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knoblauch, M., J. Schröer, B. Schmitz, and W. Doerfler. 1996. The structure of adenovirus type 12 DNA integration sites in the hamster cell genome. J. Virol. 703788-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Law, L. K., and B. L. Davidson. 2005. What does it take to bind CAR? Mol. Ther. 12599-609. [DOI] [PubMed] [Google Scholar]
- 14.Li, J., S. Lad, G. Yang, Y. Luo, M. Iacobelli-Martinez, F. J. Primus, R. A. Reisfeld, and E. Li. 2006. Adenovirus fiber shaft contains a trimerization element that supports peptide fusion for targeted gene delivery. J. Virol. 8012324-12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lonberg-Holm, K., and L. Philipson. 1969. Early events of virus-cell interaction in an adenovirus system. J. Virol. 4323-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundholm, U., and W. Doerfler. 1971. Temperature-sensitive mutants of human adenovirus type 12. Virology 45827-829. [DOI] [PubMed] [Google Scholar]
- 17.Nemerow, G. R., and P. L. Stewart. 1999. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol. Mol. Biol. Rev. 63725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philipson, L., and R. F. Pettersson. 2004. The coxsackie-adenovirus receptor—a new receptor in the immunoglobulin family involved in cell adhesion. Curr. Top. Microbiol. Immunol. 27387-111. [DOI] [PubMed] [Google Scholar]
- 19.Pickles, R. J., D. McCarty, H. Matsui, P. J. Hart, S. H. Randell, and R. C. Boucher. 1998. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J. Virol. 726014-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiedner, G., B. Schmitz, and W. Doerfler. 1994. Late transcripts of adenovirus type 12 DNA are not translated in hamster cells expressing the E1 region of adenovirus type 5. J. Virol. 685476-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schröer, J., I. Hölker, and W. Doerfler. 1997. Adenovirus type 12 DNA firmly associates with mammalian chromosomes early after virus infection or after DNA transfer by the addition of DNA to the cell culture medium. J. Virol. 717923-7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirena, D., B. Lilienfeld, M. Eisenhut, S. Kalin, K. Boucke, R. R. Beerli, L. Vogt, C. Ruedl, M. F. Bachmann, U. F. Greber, and S. Hemmi. 2004. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 784454-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strohl, W. A. 1969. The response of BHK21 cells to infection with type 12 adenovirus. II. Relationship of virus-stimulated DNA synthesis to other viral functions. Virology 39653-665. [DOI] [PubMed] [Google Scholar]
- 24.Trentin, J. J., Y. Yabe, and G. Taylor. 1962. The quest for human cancer viruses. Science 137835-841. [DOI] [PubMed] [Google Scholar]
- 25.Visser, L., M. W. van Maarschalkerweerd, T. H. Rozijn, A. D. Wassenaar, A. M. Reemst, and J. S. Sussenbach. 1980. Viral DNA sequences in adenovirus-transformed cells. Cold Spring Harbor Symp. Quant. Biol. 44541-550. [DOI] [PubMed] [Google Scholar]
- 26.Walters, R. W., W. van't Hof, S. M. Yi, M. K. Schroth, J. Zabner, R. G. Crystal, and M. J. Welsh. 2001. Apical localization of the coxsackie-adenovirus receptor by glycosyl-phosphatidylinositol modification is sufficient for adenovirus-mediated gene transfer through the apical surface of human airway epithelia. J. Virol. 757703-7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia, D., L. J. Henry, R. D. Gerard, and J. Deisenhofer. 1994. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure 21259-1270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.