Abstract
To evaluate the contribution of complement-mediated lysis to the in vivo activities of neutralizing antibodies, we analyzed the influence of complement activation on treatment success in a recent passive immunization trial with the neutralizing monoclonal antibodies 2G12, 2F5, and 4E10. Administration of monoclonal antibodies led to an immediate, high activation of the complement system even in the absence of viremia in the 14 participating human immunodeficiency virus-infected individuals. Lysis activity measured in patient plasma increased during passive immunization; however, the increases were modest and only partially attributable to the administration of antibodies. We found that unlike neutralization activity, lysis activity was not associated with treatment success in this trial. Compared to complement lysis mounted by the polyclonal antibody response in vivo, monoclonal antibodies were weak inducers of this activity, suggesting that polyclonal responses are more effective in reaching the required threshold of complement activation. Importantly, strong neutralization activity of the monoclonal antibodies did not predict complement lysis activity against patient and reference viruses, suggesting that these activities are not linked. In summary, our data support the notion that the in vivo activities of 2G12, 2F5, and 4E10 are likely due to direct neutralization or Fc receptor-mediated mechanisms such as phagocytosis and antibody-dependent cellular cytotoxicity.
The past decades have shaped our understanding of the role of neutralizing antibodies in inhibiting human immunodeficiency virus type 1 (HIV-1) transmission and replication in the infected host. Evidence of their impact in the course of natural infection is based on observations that antibody responses are subject to rapid viral escape (2, 3, 29, 32, 37, 42, 64, 65) and passive immunization studies with animals (19, 21, 28, 36, 38) and humans (61). Despite the knowledge gained regarding their activities, the exact modes of antibody action in vivo remain unclear. While neutralization, defined as the direct interaction of the antibody with the virion that obstructs infection of the target cells, is considered a principal mechanism in viral defense, antibodies also contribute to protection against viral pathogens by inducing phagocytosis, antibody-dependent cellular cytotoxicity, and activation of the complement system, leading to the destruction of the virus and infected cells (8, 20, 21, 26). Which of these antibody-mediated mechanisms, neutralization or effector functions, contribute most to viral containment in HIV infection and thus should be elicited by protective vaccines remains to be defined (25).
Lysis of HIV-1 upon antibody-mediated activation of the complement system has been demonstrated in vitro (51-53) and ex vivo (1, 24, 59). In vivo, antibodies inducing complement lysis were found to have an impact on viremia control during the acute phase of infection (24). These responses increase during the chronic infection phase and appear to be maintained throughout the course of the disease (1, 24, 51, 52, 59). Previous studies suggested that nonneutralizing antibodies dominate in this mechanism (1, 24). Nevertheless, complement has also been found to boost the activities of neutralizing antibodies in vitro and in vivo (17, 24, 39).
Although antibodies eliciting complement lysis can be detected at all disease stages, their activities are not high titered (24, 59), which is attributed to the ability of the virus to countermeasure complement attack by incorporating several host cell-derived complement control proteins (47, 48, 56). The latter raised concerns that substantial proportions of complement-opsonized virions not only remain intact but also may gain an increased capacity to infect complement receptor-expressing cells, as suggested by several in vitro studies (5, 6, 23, 30, 55, 58). Whether lysis or enhancement dominates the activity of antibody and complement on HIV infection in vivo and if and how the balance between beneficial and detrimental antibody function is maintained during disease progression thus remain central questions.
In the present study, we sought to define whether antibody-mediated complement lysis of HIV-1 virions contributes to the in vivo activities of neutralizing antibodies and, if so, what the relative contribution of this defense mechanism is. We addressed this question in a retrospective analysis of a passive immunization study with neutralizing antibodies 2G12, 2F5, and 4E10 in 14 HIV-infected individuals where a mix of these three monoclonal antibodies (MAbs) delayed viral rebound after cessation of therapy (61). Here, we analyzed patient plasma collected throughout the trial for complement activation, lysis activity, and neutralization capacity against the autologous isolate and the heterologous virus strain JR-FL. In addition, to verify our ex vivo findings, we investigated the complement lysis activities of MAbs 2G12, 2F5, and 4E10 individually in vitro.
MATERIALS AND METHODS
Patients, virus isolates, and plasma.
Plasma samples from six acutely and eight chronically HIV-1-infected patients derived during a passive immunization trial with neutralizing antibodies 2G12, 2F5, and 4E10 (61) were studied. Patient demographics and isolation of autologous virus were described previously (46, 61). Patient isolates used in this study were derived before the passive immunization study (pretreatment time point) (61). Virus stocks of patient isolates used in lysis assays were produced on CD8-depleted peripheral blood mononuclear cells (PBMCs) as described previously (61).
Patient blood was sampled in EDTA Vacutainers (Becton Dickinson), and plasma was collected within 6 h after the blood draw and frozen in 1-ml aliquots at −75°C. Plasma was heat inactivated (1 h at 56°C to inactivate autologous complement activity) and centrifuged at 500 × g for 10 min before use to remove cell debris and lipids. Normal human plasma used as a control was treated identically as patient plasma.
Written informed consent was obtained from all patients and HIV-1-negative blood donors according to guidelines of the local ethics committee.
Measurement of complement activation.
Plasma levels of complement components C3 (54) and terminal complement complex (TCC) (40, 66) were measured by enzyme-linked immunosorbent assay. Levels of C3a were measured by a commercial enzyme-linked immunosorbent assay kit (Quidel) as recommended by the manufacturer.
Neutralization assays.
Neutralization activity reported in this study was monitored in the frame of previous studies (61, 62). Neutralization activity mediated by the passively immunized antibodies in patient plasma has been assessed using a TZM-bl cell-based neutralization assay and 293T cell-produced reporter gene virus together with an arithmetic approach that allows the dissection of MAb mediated from autologous antibody activity as described previously by Trkola et al. (62). The MAb activity in plasma is therefore reported as the reciprocal plasma dilution causing a 70% reduction in luciferase reporter gene expression. Activities against both the autologous isolate and the heterologous virus JR-FL were measured and used for analyses in the current study.
Since lysis activity was measured against PBMC-derived virus, for direct comparison with in vitro MAb-mediated lysis activity, we utilized neutralization activities determined in a PBMC-based assay system (61). Here, the MAb concentration causing 90% inhibition (IC90) of viral replication as determined by p24 antigen production is reported. For the mix of the three MAbs, the total IC90 of an equimolar mix is reported.
HIV-1 virion complement lysis assay.
Complement-mediated lysis activity was measured by a real-time PCR-based assay as described previously (24). A mix of four to five human healthy donor sera stored at −75°C was used as the source of complement. Briefly, virus was incubated with patient plasma (final dilution, 1:5), complement (final dilution, 1:2), RNase A (Qiagen), and RPMI 1640 medium (BioWhitaker) in a total volume of 100 μl for 3 h at 37°C. The reaction mixture was then subjected to a freeze-thaw cycle and treated with RNase A (Qiagen) and DNase I (Roche) for 1 h at 37°C (14). Digestion was stopped by adding protease (Qiagen). Residual viral RNA was extracted from intact virions (MagNa Pure LC; Roche) and quantified by real-time PCR.
For each individual assay, samples were tested in triplicates. Complement-mediated lysis activity was expressed as the percentage of lysed HIV-1 RNA copies compared to control plasma treatment. A mix of five HIV-1-negative healthy donor plasmas was used as the negative control (no lysis activity, 0% value).
In vitro lysis activities of MAbs were measured analogously using 25 μg/ml of each antibody in RPMI medium instead of patient plasma, and medium alone was used as the negative control.
Real-time PCR.
HIV-1 virions were quantified using primers to the 5′ end of HIV RNA as described previously (mf86, cr1, and cr2) (24). For detection, a dual-labeled fluorescent probe with a 6-carboxyfluorescein moiety at its 5′ end and a 6-carboxytetramethylrhodamine moiety at its 3′ end was used (mf74tq) (24). PCR was performed as described previously (13, 43) using a single-tube system (Qiagen one-step reverse transcription-PCR) and an additional “hot start” using Paraplast (Fluka) to separate cDNA synthesis and PCR amplification. cDNA synthesis and subsequent amplification were performed in duplicates in a real-time thermocycler (i-Cycler; Bio-Rad) as described previously (13, 43).
Statistical analysis.
Data analyses were performed using Prism, version 4.03, for Windows (GraphPad Software) and Stata SE/10 for Windows (Stata Corp.). Because of the longitudinal nature of our study, repeated measurements with more than two data points per patient were performed. Therefore, we could not use standard statistical techniques such as t tests. Instead, we used linear regression models with robust standard errors, which allow the modeling of longitudinal data with more than two measurements per patient. Analogous to t tests, specific subgroup comparisons were then performed based on the coefficients and standard errors from the regression models with the Wald test (41).
Correlation analysis was performed using Spearman's rank correlation. Tests of significance were two tailed unless indicated otherwise.
RESULTS
Passively administered MAbs activate the complement system.
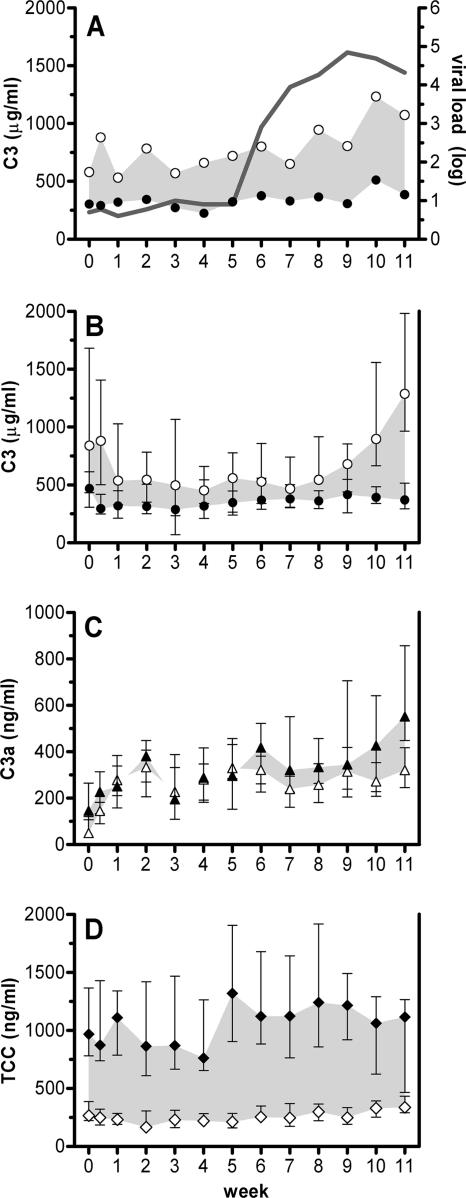
To obtain insights into whether neutralizing antibody function in vivo includes the activation of defense mechanisms mediated by the complement system, we quantified plasma levels of the complement components C3, C3a, and TCC during 12 weeks of passive immunization with neutralizing MAbs 2G12, 2F5, and 4E10. Blood for these analyses was collected immediately before and after infusion of the three MAbs. Each infusion cycle consisted of a consecutive infusion of the three individual MAbs. The time window between initiation of the first infusion and completion of the last was usually 90 min (30 min per antibody), with the exception of the first infusion, where each antibody was infused over a 60-min period. As activation of the complement system is known to occur within minutes (35, 68), this allowed us to monitor complement activation in the investigated time frame. We found that the administration of the antibodies induced a decrease in plasma levels of complement component C3 (mean change, −293 μg/ml [95% confidence interval, −471 to −115 μg/ml] [P = 0.003]), while those of the cleavage product C3a (mean change, 57 ng/ml [95% confidence interval, −51 to 88 ng/ml] [P = 0.57]) and the TCC (mean change, 865 ng/ml [95% confidence interval, 660 to 1,069 ng/ml] [P < 0.001]) increased, indicating that the three MAbs induced strong complement activation (Fig. 1). Notably, however, activation had already occurred at the first time point (week 0) in the absence of any viremia when patients were still on antiretroviral therapy (ART) as exemplified for the changes in C3 levels during the course of treatment in patient NAB11 (Fig. 1A). This was substantiated when we analyzed all three parameters across all patients (Fig. 1B to D). Activation of complement, indicated by the magnitude of change between pre- and postinfusion measurements, did not appear to increase over time (C3 change per week of −7.0 μg/ml [−95% confidence interval, 35.2 to 21.2 μg/ml] [P = 0.60]; C3a change per week, 23.6 ng/ml [95% confidence interval, 1.1 to 46.1 ng/ml] [P = 0.04]; TCC change per week, 3.1 ng/ml [95% confidence interval, −14.44 to 20.65 ng/ml] [P = 0.71]), nor was complement activation associated with the in vivo plasma levels of the MAbs with the exception of 2G12 and the increase of C3a (r = 0.32; P < 0.0001). In summary, the quantification of complement components indicated that the complement system was strongly activated in vivo by MAbs 2G12, 2F5, and 4E10 independently of viral replication but did not provide insight into a potential contribution to virus lysis. Whether lysis did not occur or whether the relative contribution of lysis-associated complement activation (TCC) was too low to be detected could not be verified in this analysis.
FIG. 1.
Complement is activated during passive immunization independent of viremia. Plasma levels of complement components were measured before and after the infusion of a mix of MAbs 2G12, 2F5, and 4E10. Levels of C3 before infusion (open circles) and after infusion (closed circles) are shown for patient NAB11 (A) together with the viral load (solid line) for weeks 1 to 11. Shaded areas indicate complement activation. Median levels of C3 (B), C3a (C), and TCC (D) before (open symbols) and after (closed symbols) infusion are shown for all 14 patients. Error bars show 95% confidence intervals.
High level of complement activation by the MAbs did not trigger infection enhancement in vivo.
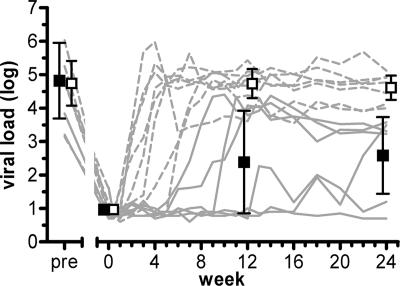
A major concern with respect to antibody-mediated effector mechanisms in vivo remains the possibility that Fc receptor (FcR) and complement receptor interactions may lead to the enhancement of infection, as demonstrated under certain in vitro conditions (5, 6, 31, 33, 57). During the investigated passive immunization trial, patient virus rebounded upon escape to antibody 2G12 (27, 61). Antibody treatment was continued for the entire 12-week study period, irrespective of when the antibodies had lost the capacity to control viremia and rebound had occurred. Notably, while rebounding virus had lost sensitivity to 2G12, virus isolates from all patients retained their sensitivities to 2F5 and 4E10. Theoretically, in a setting where antibodies are present in abundance but neutralization activity is too low to show effect, enhancing mechanisms may be particularly prone to occur. To assess whether antibodies prompted infection enhancement in vivo, we compared viral set points reached in each individual in presence of MAbs to those in the absence of MAbs. Viral load before the last instance of ART was comparable with the viral set point during passive immunization among patients who lost control of viremia while still receiving MAb treatment, indicating that MAbs did not enhance infectivity in the absence of inhibitory activity (mean change for noncontrollers, −0.01 log viral load [95% confidence interval, −0.61 to 0.59] [P = 0.98]) (Fig. 2) (see Table S1 in the supplemental material). When we analyzed the effects on viral load upon washout of the MAbs in noncontrollers, no differences in virus load in the presence (week 12) or absence (week 24) of antibodies were observed (mean change, −0.12 log viral load [95% confidence interval, −0.42 to 0.18] [P = 0.40]), indicating that despite the high level of complement activation, the MAbs had no enhancing effect on viral replication. Although it cannot be ruled out that in other settings during natural HIV infection, antibody-mediated enhancement through complement or FcR-dependent mechanisms may occur, our analysis failed to substantiate a prominent role of this mechanism in vivo.
FIG. 2.
Complement activation by 2G12, 2F5, and 4E10 did not trigger infection enhancement. Activation of complement by MAbs has neither a decreasing nor an enhancing effect on viral set point in noncontrolling patients. Dashed lines represent noncontrollers, and solid lines represent controllers. Means for controllers (closed squares) and noncontrollers (open squares) and 95% confidence intervals (error bars) from a multivariable linear regression model with a robust variance estimator are shown.
Longitudinal analysis of autologous complement-mediated lysis activity.
To assess if the passively administered antibodies elicited complement-mediated lysis of the virions, lysis activity against the autologous virus isolate derived before the passive immunization was measured in patient plasma before antibody infusion (week 0), before the last infusion (week 12), when most patients had the highest antibody trough levels, and after washout of the antibodies (week 24). We observed virolysis activity in 12 out of 14 individuals before passive immunization and antibody infusion led to a notable increase in this activity (Table 1 and Fig. 3A and B). Virus lysis activity increased in both chronic patients (mean change in lysis activity, 18.8% [95% confidence interval, 3.0% to 34.5%] [P = 0.02]) and acute patients (32.9% [95% confidence interval, 14.9% to 50.9%] [P = 0.002]) (see Table S3 in the supplemental material) between week 0 and week 12. This elevation could be due to both the administered MAbs present at week 12 and a boosted autologous antibody response. The latter is known to occur upon resuming viral replication following treatment interruption (60).
TABLE 1.
Autologous and heterologous complement-mediated lysis activities during passive immunization against autologous pretreatment virus isolatesa
| Patient | Infection phase | Viremia control | % Autologous virolysis [%]
|
% Heterologous JR-FL virolysis
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preb | Wk 0c | Wk 12d | Wk 24e | Preb | Wk 0c | Wk 12d | Wk 24e | |||
| NAB 01 | Chronic | No | 71.2 | 83.8 | 89.6 | 87.6 | 6.5 | 9.8 | 43.3 | 35.2 |
| NAB 02 | Chronic | No | 54.3 | 40.3 | 52.9 | 24.9 | 18.2 | −7.9 | 49.7 | 36.3 |
| NAB 03 | Chronic | Yes | 36.5 | 31.8 | 77.3 | 56.8 | 40.7 | 40.7 | 48.1 | 65.0 |
| NAB 04 | Chronic | Yes | 16.1 | 18.3 | 44.7 | 27.6 | 68.6 | 63.7 | 73.3 | 72.4 |
| NAB 05 | Chronic | No | 65.6 | 59.8 | 71.7 | 67.0 | 61.9 | 42.3 | 56.4 | 50.5 |
| NAB 06f | Chronic | No | 87.4 | 83.6 | ND | NA | ND | ND | ND | NA |
| NAB 07 | Chronic | No | 34.9 | 35.8 | 78.9 | 45.4 | 10.2 | −0.3 | 47.3 | 25.4 |
| NAB 08 | Acute | Yes | 29.3 | 18.1 | 75.7 | 52.7 | 38.2 | 17.2 | 44.3 | 54.8 |
| NAB 09 | Chronic | No | 49.7 | 52.6 | 71.6 | 40.6 | 41.8 | 38.9 | 68.4 | 48.8 |
| NAB 10 | Acute | No | 5.8 | 5.3 | 45.1 | 46.2 | −0.6 | −27.4 | 56.6 | 36.6 |
| NAB 11 | Acute | No | 9.9 | 18.7 | 59.5 | 58.6 | 51.7 | 49.3 | 50.7 | 68.9 |
| NAB 12 | Acute | Yes | −3.1 | 19.6 | 60.9 | 56.8 | 4.3 | 25.0 | 39.9 | 47.2 |
| NAB 13 | Acute | Yes | 10.1 | 30.1 | 46.1 | 50.7 | 0.0 | −4.9 | 13.7 | 21.6 |
| NAB 14 | Acute | Yes | 48.1 | 53.4 | 55.3 | 37.1 | 56.4 | 38.2 | 18.7 | 26.4 |
Means (95% confidence intervals) from the multivariable linear regression model were 39.4% (24.9% to 53.8%) at week 0, 63.8% (54.8% to 72.8%) at week 12, and 50.2% (40.0% to 60.3%) for autologous virolysis and 21.9% (5.3% to 38.4%) at week 0, 47.0% (36.6% to 57.3%) at week 12, and 45.3% (34.9% to 55.7%) at week 24 for heterologous virolysis (see Table S2 in the supplemental material). NA, not applicable; ND, not done.
Before last ART.
Before antibody infusion.
At highest antibody trough level.
After wash-out of the antibodies.
Patient NAB 06 stopped the passive immunization study after week 12 and went back on ART.
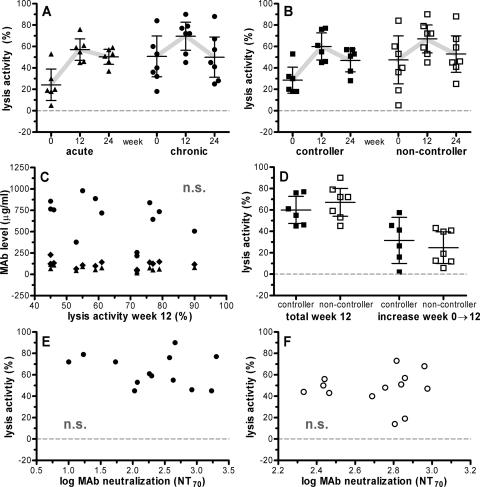
FIG. 3.
Passively administered antibodies 2G12, 2F5, and 4E10 can contribute to in vivo complement lysis, but activity is low. Autologous lysis activities in patients with acute (triangles) and chronic (circles) infection (A) as well as in controller (closed squares) and noncontroller patients (open squares) (B). Means and 95% confidence intervals (error bars) from a multivariable linear regression model with robust variance estimator are shown (see Table S3 in the supplemental material). (C) Correlation analysis of MAb concentration in vivo and complement lysis activity. Week 12 plasma levels of MAb 2G12 (dots), 2F5 (triangles), and 4E10 (diamonds) do not correlate with matched plasma complement lysis activity against autologous virus. (D) Neither total lysis activity at week 12 nor the absolute increase in lysis activity from weeks 0 to 12 differs between controller and noncontroller patients. Means and 95% confidence intervals (error bars) are shown. (E and F) MAb-mediated neutralization in patient plasma at week 12 and complement lysis activity from the same time point against autologous (E) and heterologous (F) virus is not correlated. MAb-mediated 70% neutralization (NT70) was determined previously using a TZM-bl cell-based neutralization assay (62).
Notably, however, among most chronically HIV-infected individuals, lysis activity dropped to the preimmunization level after washout of the antibodies by week 24, indicating that the passively administered MAbs contributed to the elevated lysis activity observed at week 12 (Fig. 3A) (see Table S3 in the supplemental material). Interestingly, patients with acute HIV infection preserved heightened lysis activity even after washout of the MAbs (Fig. 3A). In this group of individuals, viral rebound occurred later than in chronically infected patients (weeks 5 to >24 for patients with acute infection and weeks 2 to 18 for patients with chronic infection) (61), and consequently, the induction of autologous antibody responses following viral rebound will have peaked in most patients with acute infection after week 12. To what extent the increases in lysis activity at week 12 were mediated by autologous antibody or MAb is thus difficult to assess in the setting of acute infection but likely is associated to some extent with MAb activity as observed in chronic infection (Table 1 and Fig. 3A). When we stratified patients into responders and nonresponders based on whether or not MAb treatment led to a delay in viral rebound, we observed a similar pattern of reactivities for responders and patients with acute infection and nonresponders and chronically infected individuals (Fig. 3B). The latter is not unexpected, as the majority of nonresponders were individuals with chronic infection (six out of eight patients), and responders were found mostly among patients with acute infection (four out of six patients) (Table 1). Since MAb levels and lysis activities varied substantially among individuals, we investigated to what extent the plasma antibody levels reached among chronically infected individuals reflected the increases in virolysis activity measured ex vivo. A correlation analysis revealed no association (r value of −0.42 and P value of 0.16 for 2G12, r value of −0.33 and P value of 0.27 for 2F5, and r value of −0.04 and P value of 0.91 for 4E10) (Fig. 3C), suggesting that the concentration of the MAbs was not a limiting factor and/or that the overall contribution of the MAbs to lysis activity must be low.
The mean increase in lysis activity attributable to the MAbs in chronic infection was 23.5%. However, neither total lysis activity measured at week 12 nor the increase over baseline levels at this time point was associated with response to the passive immunization treatment (Fig. 3D).
Neutralization and lysis activities of passively administered antibodies 2G12, 2F5, and 4E10 are not linked.
In order to investigate if the requirements for high efficiency in neutralization and complement lysis activity are related, we analyzed the effects of the three neutralizing MAbs in both activities. In a longitudinal assessment of the neutralization activities against the heterologous reference strain JR-FL throughout the passive immunization trial, we previously established that all three MAbs were active in vivo and that neutralization activity against JR-FL was mediated predominantly by the administered MAbs (61, 62). However, here, we found no correlation between lysis activity against JR-FL and patient virus isolates measured in plasma during the trial (week 12) (Table 1) and the MAb-mediated neutralization activity against these viruses at the same time point (62), indicating that there is no direct relationship between the two antibody functions (r value of −0.11 and P value of 0.72 for autologous activity and r value of 0.20 and P value of 0.50 for heterologous lysis activity) (Fig. 3E and F).
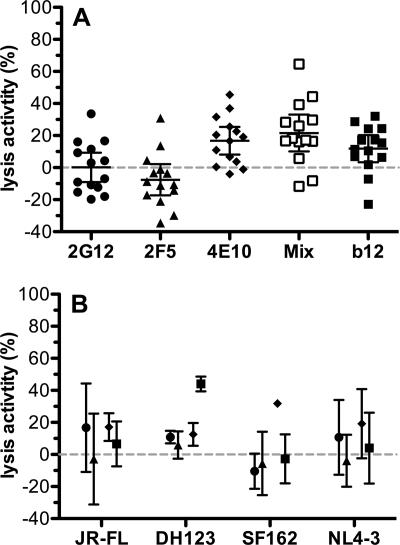
To verify our ex vivo analysis, we performed in vitro lysis assays with antibodies 2G12, 2F5, and 4E10 and with a mix of these three MAbs. Lysis activity against the virus isolates from the 14 patients that participated in the passive immunization trial and four reference strains varied broadly (Fig. 4A and B). Despite the relatively high concentrations probed, none of the MAbs by themselves reached lysis activity above 50% against any of the 18 virus strains tested. Antibodies 2G12 and 2F5 elicited lysis activity that was above 30% against only a single isolate (NAB03). In contrast, 4E10 by itself as well as the mix of the three antibodies showed evidence for lysis activity that, although still low, was significantly above background (0.2% [95% confidence interval, −9.0 to 9.3] [P = 0.97] for 2G12, −7.6% [95% confidence interval, −17.4% to 2.2%] [P = 0.12] for 2F5, 16.8% [95% confidence interval, 8.2% to 25.4%] [P = 0.001] for 4E10, and 21.6% [95% confidence interval, 10.1% to 33.1%] [P = 0.001] for the mix by one-sample t test) (Fig. 4). This relatively weak capacity of monoclonal neutralizing antibodies to elicit virus lysis appears to be common, as MAb b12 also has only low lysis activity (11.8% [95% confidence interval, 3.4% to 20.3%] [P = 0.01]). Interestingly, in passive immunization experiments in the macaque model, it was recently shown that complement activity does not contribute to b12 protection against strain SHIVSF162P3 in vivo (21). In agreement with this, we found that b12 by itself harbors no/low activity against the parental HIV strain SF162 (Fig. 4).
FIG. 4.
MAbs have weak lysis activity in vitro. (A) Lysis activities of neutralizing MAbs 2G12, 2F5, 4E10, and b12 were tested against the 14 patient virus isolates. Only 4E10, the mix of 2G12/2F5/4E10, and b12 show moderate lysis activity. Error bars show 95% confidence intervals (one-sample t test). (B) Lysis activities of 2G12 (circles), 2F5 (triangles), 4E10 (diamonds), and b12 (squares) against reference viruses. Error bars show standard deviations of triplicate measurements.
Overall, while the three MAbs potently neutralized most of the patient viruses, they failed to effectively lyse these isolates (Fig. 5). When the MAbs were probed in vitro in the absence of patient antibodies, no or only marginal lysis activity was detectable in most cases. Notably, however, the mean lysis activities of 4E10 and the triple combination reached in vitro against the viruses derived from trial participants were in the ranges of increases measured in patient plasma ex vivo (16.8% for 4E10 and 21.6% for the triple mix) (Fig. 4).
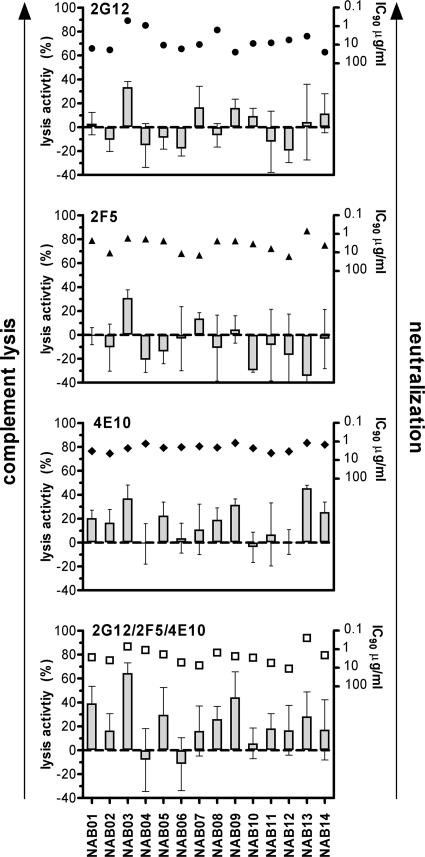
FIG. 5.
In vitro neutralization activity does not predict complement lysis activity. Lysis activities (shown as bars) of MAbs 2G12, 2F5, and 4E10 and the mix of these three antibodies against the 14 patient virus isolates do not show a common pattern. Neutralization activities (IC90 values, inverse logarithmic scale) of MAbs 2G12 (circles), 2F5 (triangle), and 4E10 (diamonds) and the mix of the three MAbs (open squares) in a PBMC-based neutralization assay against each virus is depicted above the corresponding bars. The IC90s of the individual MAbs and the equimolar mix of the MAbs were determined using a PBMC-based neutralization assay (61). Error bars show standard deviations of triplicate measurements.
In sum, these analyses revealed that compared to the lysis activities mounted by the polyclonal antibody response in vivo, MAbs by themselves are weak inducers of this activity. Although the MAbs tested clearly had the capacity to bind to virions, as they were able to neutralize, we found that the ability to bind and neutralize provided no indication of the efficiency of a given antibody to induce complement lysis. We found neither a common pattern of antibody lysis and neutralization activity against the tested virus isolates nor interdependencies between these activities (r value of 0.08 and P value of 0.78 for 2G12, r value of 0.14 and P value of 0.64 for 2F5, r value of −0.33 and P value of 0.25 for 4E10, and r value of −0.48 and P value of 0.08 for the equimolar mix of the three MAbs). Our analysis thus strongly suggests that complement lysis activity cannot be predicted by neutralization activity.
DISCUSSION
To our current understanding, neutralizing antibodies are a key immune defense mechanism against HIV (10, 11). Although their activities have been ascertained in a compelling number of in vitro and in vivo studies (4, 17, 19, 22, 28, 32, 36, 38, 42, 45, 50, 61, 65), we still lack information on whether antibodies act solely by directly binding and neutralizing the virus particle or by eliciting effector functions of the immune system (20, 21, 24, 25). In the present study, we addressed this question by assessing if complement-mediated lysis contributes to the in vivo effect of neutralizing antibodies. The complement system can be activated by direct binding of complement components to HIV or more potently via antibodies bound to the particle (49, 53, 55), which can result in complement attack and lysis of the particle (1, 24, 59). In the current study, our primary interest was to define whether neutralizing antibodies, upon binding to viral particles, induce the activation of the complement cascade in vivo and trigger lysis of the virus. Utilizing specimens collected during a passive immunization trial with the three neutralizing MAbs 2G12, 2F5, and 4E10, we were able to assess the influence of MAb administration on neutralization, complement activation, and lysis activities in patients who responded and in those that did not respond to passive immunization treatment by controlling viremia rebound (61).
The complement cascade was highly activated in all study participants upon passive immunization. However, this was not a direct indication of ongoing virus lysis, as activation also occurred in the absence of detectable viremia as an immediate consequence of immunoglobulin administration. Antibody preparations used for passive immunization were controlled to ensure that no or only low amounts of aggregates were contained to limit immune activation (data not shown). Nevertheless, as evident from our study and from previous reports in the literature, the high concentration of antibodies administered remains by itself conceivably sufficient to induce complement activation in vivo (12, 63). Activation of the complement system due to the binding of MAbs to virions (which consequently leads to their destruction) appears to be minute compared to this high “background” activation upon the administration of the MAbs, as we did not observe an increase in complement activation when virus became detectable in patients. Complement activation levels in our study therefore did not provide insight into a potential contribution toward virus lysis.
In all patients, complement-mediated lysis activity increased during the trial, which can be attributed to both the activities of the MAbs and a boost of the autologous response known to occur upon viral rebound (60). The latter may explain the observation that among acutely HIV-infected patients, lysis activity levels remained elevated compared to pretreatment levels even after washout of the MAbs (Fig. 3A). Early antiretroviral treatment prevents a full maturation of humoral immune responses, which has been found to fully develop and profoundly increase only upon resuming viral replication after cessation of ART (7). This also appears to be the case in our cohort: while pretreatment lysis activity was somewhat lower among individuals with acute infection (mean difference in lysis activity, −26.6% [95% confidence interval, −50.5% to −2.6%] [P = 0.03]), no difference between acute and chronically infected individuals was observed upon completion of the trial (mean difference in lysis activity, −0.37% [95% confidence interval, −20.4% to 19.7%] [P = 0.97]). For chronic patients, whose antibody responses have already matured and are expected to reach a steady-state level more rapidly upon rebound, our data suggest that the MAbs had a modest impact on the overall lysis activity. Although the induction of autologous responses overlapped with MAb administration in these individuals and the effects of passively administered and autologous antibody responses cannot be dissected apart, it is feasible to assume that the MAbs had a similar impact on lysis activity, as seen in chronically infected individuals. Overall, increases in lysis activity were moderate and not paralleled by an increase in complement activation, which is probably due to the fact that the complement activation leading to virolysis, induced by autologous antibodies and MAbs, is low compared to the strong background activation induced by the passively transferred MAbs themselves.
The rise and fall in lysis activity that parallel the administration and washout of MAbs in chronically infected patients in our analysis provide the best evidence that the MAbs are capable of inducing modest complement lysis in vivo either by themselves or in combination with the autologous response (Fig. 3A and B). However, in general, the lysis activity of MAbs was low compared to the activity observed in the autologous, polyclonal antibody response, suggesting that polyclonal responses are more effective in inducing the threshold for complement cascade activation. Our study further substantiates the evidence that antibodies mediating complement lysis of HIV are common at all stages of HIV infection, that the mediated activity is comparatively low titered, and that polyclonal responses appear to be more effective in mediating this activity.
While overall, a modest increase in lysis activity occurred upon passive immunization, we did not find evidence that this was linked to the protective effect of the MAbs, as increases in controlling and noncontrolling patients were comparable (Fig. 3D). We therefore conclude that the in vivo activities of the administered neutralizing antibodies 2G12, 2F5, and 4E10 likely were dominated by the direct neutralization of free virus or FcR-mediated mechanisms such as phagocytosis and antibody-dependent cellular cytotoxicity. This supports observations made by Hessell and colleagues using wild-type and effector function-defective variants of neutralizing antibody b12 (21), where they reported that MAb b12 displays no complement-mediated antiviral activity in protecting rhesus macaques against infection with SHIVSF162P3. Based on our data, this is not unexpected but does not allow us to infer with certainty that, in general, complement-dependent mechanisms do not contribute to neutralizing antibody activity in vivo, as b12 by itself fails to induce complement lysis of SF162 (Fig. 4B).
Notably, all four MAbs tested displayed hardly any lysis activity. The detected activities were restricted mostly to MAbs 4E10 and b12. However, even there, the activities were very low and clearly beyond the activity of the polyclonal autologous antibody response. The finding that antibodies can harbor in vitro neutralization but no measurable lysis activity against specific isolates (Fig. 5) is striking and asks for further assessment of the underlying mechanisms. The incapacity to induce lysis activity could be due to a lack of binding sites on functional envelope spikes for these neutralizing antibodies, as a certain density of antibody binding sites will be required to prompt the activation of the classical complement pathway (9, 44). On the other hand, the threshold in antibody binding to induce neutralization could be relatively low and might require only a single bound antibody according to a model described previously by Yang et al. (67). In support of this, we found that while neutralization activity of the MAbs against the tested virus strains was generally high, lysis activity was rarely mediated and, if so, at comparatively low levels. This is in contrast to patient sera, which mediate significant lysis activity (24), indicating that polyclonal responses may be needed to reach the threshold of effective complement lysis, as previously suggested (53). We and others observed previously that lysis activity against HIV plateaus at about 90% lysis of virus irrespective of the concentration of MAbs or patient sera used (24, 59). The remaining 10% of virions remain protected from virolysis, which could be similarly caused by a lack of binding sites on these virions or, alternatively, by a higher incorporation of complement control proteins in these virions.
While neutralizing antibodies need to bind to functional trimers in order to inhibit virus infectivity (16), lysis-inducing antibodies could theoretically bind anywhere on the virion, i.e., to nonfunctional trimers, monomers, and uncleaved gp160 and gp41 stumps (34). However, a lack of density of the appropriate epitopes alone does not explain the differential performance of the neutralizing antibodies probed in our study in eliciting virus lysis. While 2G12 and b12 neutralized the majority of virus isolates tested (61) (data not shown), and both are able to bind gp120 in the context of the monomer and trimer and should consequently have comparable numbers of binding sites on the virion available, only b12 showed measurable lysis activity. The same was true for the membrane-proximal external region-specific MAbs, where 4E10 but not 2F5 induced the lysis of the majority of isolates. This is particularly surprising, as the MAbs are directed to adjacent domains in the viral envelope and, like 2G12, are engineered proteins that share an identical Fc region. The latter suggests that additional factors such as accessibility, spatial arrangement, and membrane distance of the epitopes shape the lysis activities of individual antibodies. To what extent neutralizing and/or nonneutralizing antibodies elicited by the autologous immune response in vivo trigger complement defense mechanisms, how the activities of neutralizing and lysis-inducing antibodies overlap, and which epitopes are recognized by these antibodies are currently unknown and will require further investigations to define the mechanisms of their action and in vivo relevance. Notably, the efficacy of complement-mediated lysis of HIV-infected cells deserves to be explored in this respect, as effector mechanisms targeting infected cells may be of particular importance in controlling infection. Equally, phagocytosis of antibody-opsonized virus may be a relevant yet not-quantified factor in eliminating infectious virus. Research efforts in recent years have highlighted the potency of the humoral immune response (1, 15, 18, 21, 24) but have also made it evident that a detailed assessment of the relative contribution of direct and effector-mediated antibody functions to virus containment in vivo is a necessity to define relevant immune responses and direct future vaccine design.
Supplementary Material
Acknowledgments
We thank the participating patients for their commitment and Leonardo Aceto, Roland Hafner, Mike Winniger, Ursi Berberat, Christina Grube, and Christine Schneider for excellent patient care. Antibody b12 was provided by Dennis R. Burton.
Support was provided by the Swiss National Science Foundation (grant PP00B-102647 to A.T. and grant 3100A0-103748 to H.F.G. and A.T.), the Janggen-Pöhn Foundation (grant to M.H.), the Gebert Rüf Foundation, and the UBS Foundation. A.T. is an Elizabeth Glaser Scientist supported by the Elizabeth Glaser Pediatric AIDS Foundation.
Footnotes
Published ahead of print on 30 January 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Aasa-Chapman, M. M., S. Holuigue, K. Aubin, M. Wong, N. A. Jones, D. Cornforth, P. Pellegrino, P. Newton, I. Williams, P. Borrow, and A. McKnight. 2005. Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection. J. Virol. 792823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert, J., B. Abrahamsson, K. Nagy, E. Aurelius, H. Gaines, G. Nystrom, and E. M. Fenyo. 1990. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS 4107-112. [DOI] [PubMed] [Google Scholar]
- 3.Arendrup, M., C. Nielsen, J. E. Hansen, C. Pedersen, L. Mathiesen, and J. O. Nielsen. 1992. Autologous HIV-1 neutralizing antibodies: emergence of neutralization-resistant escape virus and subsequent development of escape virus neutralizing antibodies. J. Acquir. Immune Defic. Syndr. 5303-307. [PubMed] [Google Scholar]
- 4.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6200-206. [DOI] [PubMed] [Google Scholar]
- 5.Bajtay, Z., C. Speth, A. Erdei, and M. P. Dierich. 2004. Cutting edge: productive HIV-1 infection of dendritic cells via complement receptor type 3 (CR3, CD11b/CD18). J. Immunol. 1734775-4778. [DOI] [PubMed] [Google Scholar]
- 6.Banki, Z., H. Stoiber, and M. P. Dierich. 2005. HIV and human complement: inefficient virolysis and effective adherence. Immunol. Lett. 97209-214. [DOI] [PubMed] [Google Scholar]
- 7.Binley, J. M., A. Trkola, T. Ketas, D. Schiller, B. Clas, S. Little, D. Richman, A. Hurley, M. Markowitz, and J. P. Moore. 2000. The effect of highly active antiretroviral therapy on binding and neutralizing antibody responses to human immunodeficiency virus type 1 infection. J. Infect. Dis. 182945-949. [DOI] [PubMed] [Google Scholar]
- 8.Blue, C. E., O. B. Spiller, and D. J. Blackbourn. 2004. The relevance of complement to virus biology. Virology 319176-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borsos, T., and H. J. Rapp. 1965. Complement fixation on cell surfaces by 19S and 7S antibodies. Science 150505-506. [DOI] [PubMed] [Google Scholar]
- 10.Burton, D. R. 2002. Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2706-713. [DOI] [PubMed] [Google Scholar]
- 11.Burton, D. R., R. L. Stanfield, and I. A. Wilson. 2005. Antibody vs. HIV in a clash of evolutionary titans. Proc. Natl. Acad. Sci. USA 10214943-14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doekes, G., L. A. Vanes, and M. R. Daha. 1982. Influence of aggregate size on the binding and activation of the first component of human complement by soluble IgG aggregates. Immunology 45705-713. [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer, M., B. Joos, B. Hirschel, G. Bleiber, R. Weber, and H. F. Gunthard. 2004. Cellular viral rebound after cessation of potent antiretroviral therapy predicted by levels of multiply spliced HIV-1 RNA encoding nef. J. Infect. Dis. 1901979-1988. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, M., J. K. Wong, D. Russenberger, B. Joos, M. Opravil, B. Hirschel, A. Trkola, H. Kuster, R. Weber, and H. F. Gunthard. 2002. Residual cell-associated unspliced HIV-1 RNA in peripheral blood of patients on potent antiretroviral therapy represents intracellular transcripts. Antivir. Ther. 791-103. [PubMed] [Google Scholar]
- 15.Florese, R. H., K. K. Van Rompay, K. Aldrich, D. N. Forthal, G. Landucci, M. Mahalanabis, N. Haigwood, D. Venzon, V. S. Kalyanaraman, M. L. Marthas, and M. Robert-Guroff. 2006. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J. Immunol. 1774028-4036. [DOI] [PubMed] [Google Scholar]
- 16.Fouts, T. R., J. M. Binley, A. Trkola, J. E. Robinson, and J. P. Moore. 1997. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J. Virol. 712779-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauduin, M. C., P. W. Parren, R. Weir, C. F. Barbas, D. R. Burton, and R. A. Koup. 1997. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 31389-1393. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Roman, V. R., L. J. Patterson, D. Venzon, D. Liewehr, K. Aldrich, R. Florese, and M. Robert-Guroff. 2005. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J. Immunol. 1742185-2189. [DOI] [PubMed] [Google Scholar]
- 19.Haigwood, N. L., D. C. Montefiori, W. F. Sutton, J. McClure, A. J. Watson, G. Voss, V. M. Hirsch, B. A. Richardson, N. L. Letvin, S. L. Hu, and P. R. Johnson. 2004. Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. J. Virol. 785983-5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hangartner, L., R. M. Zellweger, M. Giobbi, J. Weber, B. Eschli, K. D. McCoy, N. Harris, M. Recher, R. M. Zinkernagel, and H. Hengartner. 2006. Nonneutralizing antibodies binding to the surface glycoprotein of lymphocytic choriomeningitis virus reduce early virus spread. J. Exp. Med. 2032033-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hessell, A. J., L. Hangartner, M. Hunter, C. E. Havenith, F. J. Beurskens, J. M. Bakker, C. M. Lanigan, G. Landucci, D. N. Forthal, P. W. Parren, P. A. Marx, and D. R. Burton. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449101-104. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann-Lehmann, R., J. Vlasak, R. A. Rasmussen, B. A. Smith, T. W. Baba, V. Liska, F. Ferrantelli, D. C. Montefiori, H. M. McClure, D. C. Anderson, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, H. Katinger, G. Stiegler, L. A. Cavacini, M. R. Posner, T. C. Chou, J. Andersen, and R. M. Ruprecht. 2001. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J. Virol. 757470-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horakova, E., O. Gasser, S. Sadallah, J. M. Inal, G. Bourgeois, I. Ziekau, T. Klimkait, and J. A. Schifferli. 2004. Complement mediates the binding of HIV to erythrocytes. J. Immunol. 1734236-4241. [DOI] [PubMed] [Google Scholar]
- 24.Huber, M., M. Fischer, B. Misselwitz, A. Manrique, H. Kuster, B. Niederost, R. Weber, V. von Wyl, H. F. Gunthard, and A. Trkola. 2006. Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection. PLoS Med. 3e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber, M., and A. Trkola. 2007. Humoral immunity to HIV-1: neutralization and beyond. J. Intern. Med. 2625-25. [DOI] [PubMed] [Google Scholar]
- 26.Kohl, S., M. S. West, C. G. Prober, W. M. Sullender, L. S. Loo, and A. M. Arvin. 1989. Neonatal antibody-dependent cellular cytotoxic antibody levels are associated with the clinical presentation of neonatal herpes simplex virus infection. J. Infect. Dis. 160770-776. [DOI] [PubMed] [Google Scholar]
- 27.Manrique, A., P. Rusert, B. Joos, M. Fischer, H. Kuster, C. Leemann, B. Niederost, R. Weber, G. Stiegler, H. Katinger, H. F. Gunthard, and A. Trkola. 2007. In vivo and in vitro escape from neutralizing antibodies 2G12, 2F5, and 4E10. J. Virol. 818793-8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6207. [DOI] [PubMed] [Google Scholar]
- 29.McKeating, J. A., J. Gow, J. Goudsmit, L. H. Pearl, C. Mulder, and R. A. Weiss. 1989. Characterization of HIV-1 neutralization escape mutants. AIDS 3777-784. [DOI] [PubMed] [Google Scholar]
- 30.Moir, S., A. Malaspina, Y. Li, T. W. Chun, T. Lowe, J. Adelsberger, M. Baseler, L. A. Ehler, S. Liu, R. T. Davey, Jr., J. A. Mican, and A. S. Fauci. 2000. B cells of HIV-1-infected patients bind virions through CD21-complement interactions and transmit infectious virus to activated T cells. J. Exp. Med. 192637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montefiori, D. C. 1997. Role of complement and Fc receptors in the pathogenesis of HIV-1 infection. Springer Semin. Immunopathol. 18371-390. [DOI] [PubMed] [Google Scholar]
- 32.Montefiori, D. C., T. S. Hill, H. T. T. Vo, B. D. Walker, and E. S. Rosenberg. 2001. Neutralizing antibodies associated with viremia control in a subset of individuals after treatment of acute human immunodeficiency virus type 1 infection. J. Virol. 7510200-10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montefiori, D. C., W. E. Robinson, Jr., V. M. Hirsch, A. Modliszewski, W. M. Mitchell, and P. R. Johnson. 1990. Antibody-dependent enhancement of simian immunodeficiency virus (SIV) infection in vitro by plasma from SIV-infected rhesus macaques. J. Virol. 64113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore, P. L., E. T. Crooks, L. Porter, P. Zhu, C. S. Cayanan, H. Grise, P. Corcoran, M. B. Zwick, M. Franti, L. Morris, K. H. Roux, D. R. Burton, and J. M. Binley. 2006. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J. Virol. 802515-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ollert, M. W., J. V. Kadlec, K. David, E. C. Petrella, R. Bredehorst, and C. W. Vogel. 1994. Antibody-mediated complement activation on nucleated cells. A quantitative analysis of the individual reaction steps. J. Immunol. 1532213-2221. [PubMed] [Google Scholar]
- 36.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 758340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parren, P. W., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13(Suppl A)S137-S162. [PubMed] [Google Scholar]
- 38.Poignard, P., R. Sabbe, G. R. Picchio, M. Wang, R. J. Gulizia, H. Katinger, P. W. Parren, D. E. Mosier, and D. R. Burton. 1999. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity 10431-438. [DOI] [PubMed] [Google Scholar]
- 39.Posner, M. R., H. S. Elboim, T. Cannon, L. Cavacini, and T. Hideshima. 1992. Functional activity of an HIV-1 neutralizing IgG human monoclonal antibody: ADCC and complement-mediated lysis. AIDS Res. Hum. Retrovir. 8553-558. [DOI] [PubMed] [Google Scholar]
- 40.Prufer, F., J. Scheiring, S. Sautter, D. B. Jensen, R. Treichl, R. Wurzner, and L. B. Zimmerhackl. 2006. Terminal complement complex (C5b-9) in children with recurrent hemolytic uremic syndrome. Semin. Thromb. Hemost. 32121-127. [DOI] [PubMed] [Google Scholar]
- 41.Ramanathan, R. 1997. Introductory econometrics with applications, 4th ed. Dryden Press, Ft. Worth, TX.
- 42.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 1004144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roscic-Mrkic, B., M. Fischer, C. Leemann, A. Manrique, C. J. Gordon, J. P. Moore, A. E. Proudfoot, and A. Trkola. 2003. RANTES (CCL5) uses the proteoglycan CD44 as an auxiliary receptor to mediate cellular activation signals and HIV-1 enhancement. Blood 1021169-1177. [DOI] [PubMed] [Google Scholar]
- 44.Rosse, W. F. 1969. Fixation of the first component of complement (C'la) by human antibodies. J. Clin. Investig. 472430-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruprecht, R. M., R. Hofmann-Lehmann, B. A. Smith-Franklin, R. A. Rasmussen, V. Liska, J. Vlasak, W. Xu, T. W. Baba, A. L. Chenine, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, D. C. Montefiori, and H. M. McClure. 2001. Protection of neonatal macaques against experimental SHIV infection by human neutralizing monoclonal antibodies. Transfus. Clin. Biol. 8350-358. [DOI] [PubMed] [Google Scholar]
- 46.Rusert, P., H. Kuster, B. Joos, B. Misselwitz, C. Gujer, C. Leemann, M. Fischer, G. Stiegler, H. Katinger, W. C. Olson, R. Weber, L. Aceto, H. F. Gunthard, and A. Trkola. 2005. Virus isolates during acute and chronic human immunodeficiency virus type 1 infection show distinct patterns of sensitivity to entry inhibitors. J. Virol. 798454-8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saifuddin, M., M. Ghassemi, C. Patki, C. J. Parker, and G. T. Spear. 1994. Host cell components affect the sensitivity of HIV type 1 to complement-mediated virolysis. AIDS Res. Hum. Retrovir. 10829-837. [DOI] [PubMed] [Google Scholar]
- 48.Saifuddin, M., C. J. Parker, M. E. Peeples, M. K. Gorny, S. Zolla-Pazner, M. Ghassemi, I. A. Rooney, J. P. Atkinson, and G. T. Spear. 1995. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J. Exp. Med. 182501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Senaldi, G., M. Peakman, T. McManus, E. T. Davies, D. E. Tee, and D. Vergani. 1990. Activation of the complement system in human immunodeficiency virus infection: relevance of the classical pathway to pathogenesis and disease severity. J. Infect. Dis. 1621227-1232. [DOI] [PubMed] [Google Scholar]
- 50.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5204-210. [DOI] [PubMed] [Google Scholar]
- 51.Spear, G. T., G. G. Olinger, M. Saifuddin, and H. M. Gebel. 2001. Human antibodies to major histocompatibility complex alloantigens mediate lysis and neutralization of HIV-1 primary isolate virions in the presence of complement. J. Acquir. Immune Defic. Syndr. 26103-110. [DOI] [PubMed] [Google Scholar]
- 52.Spear, G. T., B. L. Sullivan, A. L. Landay, and T. F. Lint. 1990. Neutralization of human immunodeficiency virus type 1 by complement occurs by viral lysis. J. Virol. 645869-5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spear, G. T., D. M. Takefman, B. L. Sullivan, A. L. Landay, and S. Zolla-Pazner. 1993. Complement activation by human monoclonal antibodies to human immunodeficiency virus. J. Virol. 6753-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stiegler, G., C. Armbruster, B. Vcelar, H. Stoiber, R. Kunert, N. L. Michael, L. L. Jagodzinski, C. Ammann, W. Jager, J. Jacobson, N. Vetter, and H. Katinger. 2002. Antiviral activity of the neutralizing antibodies 2F5 and 2G12 in asymptomatic HIV-1-infected humans: a phase I evaluation. AIDS 162019-2025. [DOI] [PubMed] [Google Scholar]
- 55.Stoiber, H., L. Kacani, C. Speth, R. Wurzner, and M. P. Dierich. 2001. The supportive role of complement in HIV pathogenesis. Immunol. Rev. 180168-176. [DOI] [PubMed] [Google Scholar]
- 56.Stoiber, H., C. Pinter, A. G. Siccardi, A. Clivio, and M. P. Dierich. 1996. Efficient destruction of human immunodeficiency virus in human serum by inhibiting the protective action of complement factor H and decay accelerating factor (DAF, CD55). J. Exp. Med. 183307-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoiber, H., M. Pruenster, C. G. Ammann, and M. P. Dierich. 2005. Complement-opsonized HIV: the free rider on its way to infection. Mol. Immunol. 42153-160. [DOI] [PubMed] [Google Scholar]
- 58.Stoiber, H., C. Speth, and M. P. Dierich. 2003. Role of complement in the control of HIV dynamics and pathogenesis. Vaccine 21(Suppl 2)S77-S82. [DOI] [PubMed] [Google Scholar]
- 59.Sullivan, B. L., E. J. Knopoff, M. Saifuddin, D. M. Takefman, M. N. Saarloos, B. E. Sha, and G. T. Spear. 1996. Susceptibility of HIV-1 plasma virus to complement-mediated lysis. Evidence for a role in clearance of virus in vivo. J. Immunol. 1571791-1798. [PubMed] [Google Scholar]
- 60.Trkola, A., H. Kuster, C. Leemann, A. Oxenius, C. Fagard, H. Furrer, M. Battegay, P. Vernazza, E. Bernasconi, R. Weber, B. Hirschel, S. Bonhoeffer, and H. F. Gunthard. 2004. Humoral immunity to HIV-1: kinetics of antibody responses in chronic infection reflects capacity of immune system to improve viral set point. Blood 1041784-1792. [DOI] [PubMed] [Google Scholar]
- 61.Trkola, A., H. Kuster, P. Rusert, B. Joos, M. Fischer, C. Leemann, A. Manrique, M. Huber, M. Rehr, A. Oxenius, R. Weber, G. Stiegler, B. Vcelar, H. Katinger, L. Aceto, and H. F. Gunthard. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11615-622. [DOI] [PubMed] [Google Scholar]
- 62.Trkola, A., H. Kuster, P. Rusert, V. von Wyl, C. Leemann, R. Weber, G. Stiegler, H. Katinger, B. Joos, and H. F. Günthard. 2007. In vivo efficacy of HIV neutralizing antibodies: estimates for protective titers. J. Virol. 821591-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tschopp, J., T. Schulthess, J. Engel, and J. C. Jaton. 1980. Antigen-independent activation of the first component of complement C1 by chemically crosslinked rabbit IgG-oligomers. FEBS Lett. 112152-154. [DOI] [PubMed] [Google Scholar]
- 64.Watkins, B. A., M. S. Reitz, Jr., C. A. Wilson, K. Aldrich, A. E. Davis, and M. Robert-Guroff. 1993. Immune escape by human immunodeficiency virus type 1 from neutralizing antibodies: evidence for multiple pathways. J. Virol. 677493-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 66.Wurzner, R., M. Schulze, L. Happe, A. Franzke, F. A. Bieber, M. Oppermann, and O. Gotze. 1991. Inhibition of terminal complement complex formation and cell lysis by monoclonal antibodies. Complement Inflamm. 8328-340. [DOI] [PubMed] [Google Scholar]
- 67.Yang, X., S. Kurteva, S. Lee, and J. Sodroski. 2005. Stoichiometry of antibody neutralization of human immunodeficiency virus type 1. J. Virol. 793500-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ziccardi, R. J. 1981. Activation of the early components of the classical complement pathway under physiologic conditions. J. Immunol. 1261769-1773. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.