Abstract
Human adenovirus serotype 5 (AdH5) vector vaccines elicit strong immune responses to the encoded antigen and have been used in various disease models. We designed AdH5 vectors expressing antigen under the control of a human cytomegalovirus (HCMV) immediate-early promoter containing its intron A sequence. The transcriptional levels of antigen and immune responses to antigen for vectors with the HCMV promoter with the intron A sequence (LP) were greater than those for AdH5 vectors using the HCMV promoter sequence without intron A (SP). We compared an E1E3-deleted AdH5 adenoviral vector, which affords more space for insertion of foreign sequences, and showed it to be as immunogenic as an E1-deleted AdH5 vector. Neutralizing antibodies to AdH5 limit the efficacy of vaccines based on the AdH5 serotype, and simian adenoviral vectors offer an attractive option to overcome this problem. We constructed E1E3-deleted human and simian adenoviral vectors encoding the pre-erythrocytic-stage malarial antigen Plasmodium berghei circumsporozoite protein. We compared the immunogenicity and efficacy of AdC6, a recombinant simian adenovirus serotype 6 vector, in a murine malaria model to those of AdH5 and the poxviral vectors MVA and FP9. AdC6 induced sterile protection from a single dose in 90% of mice, in contrast to AdH5 (25%) and poxviral vectors MVA and FP9 (0%). Adenoviral vectors maintained potent CD8+ T-cell responses for a longer period after immunization than did poxviral vectors and mainly induced an effector memory phenotype of cells. Significantly, AdC6 was able to maintain protection in the presence of preexisting immunity to AdH5.
Malaria remains one the of the world's most important public health problems. It is estimated that 300 to 500 million clinical episodes occur every year, with about a million deaths, primarily in sub-Saharan Africa among children under the age of 5 years and among pregnant women (54). In spite of current control measures, prevalence as well as resurgence of malaria continues to be evident worldwide, mainly due to drug-resistant parasites and insecticide-resistant vectors. The development of a malaria vaccine could control both the transmission and burden of disease. Sterile protection against malaria parasites can be obtained in animals and also in humans by repeated immunization with radiation-attenuated sporozoites (20, 40). This protective immunity is dependent on CD8+ T cells and gamma interferon (IFN-γ) production that targets the intrahepatic schizont stage of the parasite (50). Two candidate vaccines, RTS,S/AS02 and FP9-METRAP/MVA-METRAP, that have completed phase IIb clinical trials in African children target the preerythrocytic stage of the parasite's life cycle. RTS,S/AS02 is a protein/adjuvant vaccine that encodes most of the circumsporozoite protein (CSP) of Plasmodium falciparum. CSP is the major antigen on the surface of the sporozoite and is an important target of the pre-erythrocytic-stage immune response (33). This vaccine showed 34% overall efficacy in a phase IIb field efficacy trial conducted with adults in the Gambia (13). In a trial conducted among 1- to 4-year-old children in Mozambique, the vaccine was much more immunogenic than in tropical Africa and was able to decrease clinical malaria incidence rates 28.9% in one of the two cohorts studied, maintaining this efficacy for 18 months (1, 2). The poxviral vectors MVA and FP9, encoding the P. falciparum TRAP antigen with a multiepitope string (ME-TRAP), have been used in a prime-boost regimen and tested in malaria-naive and semi-immune volunteers. Following a sporozoite challenge in naive individuals, the FP9-MVA regimen conferred sterile protection in some volunteers and delayed development of parasitemia in others (57). Trials conducted in Gambian adults (38) and Kenyan children (10) showed this vaccine regimen to be immunogenic, but it did not confer any protective efficacy in a phase IIb study in Kenyan children in an area of holoendemic transmission, where immunogenicity was unexpectedly low (9).
Adenoviral vectors of human serotype 5 (AdH5) are promising vaccine vector candidates that induce potent cellular and humoral immune responses and have demonstrated protective efficacy in different disease models (19, 47). These vaccine vectors have been tested previously and shown efficacy in mouse malaria models (28, 45). Most adenoviral vectors currently used as vaccine carriers have a deletion in the E1 region of the adenoviral genome and employ the human cytomegalovirus (HCMV) immediate-early (IE) promoter to drive antigen expression. The deletion of the E1 region of the adenoviral genome inhibits replication and increases space for foreign DNA insertion. To incorporate larger foreign DNA sequences, adenoviral vectors with the E1 and E3 regions deleted have been developed. However, few studies have compared the effects of different deletions on the immunogenicity induced by the adenoviral vaccine vector. To improve the immune response induced in response to the encoded antigen, studies have attempted to optimize the promoter used to drive antigen expression. The commonly used HCMV IE promoter is one of the strongest promoters known to date and consists of the enhancer/promoter sequence of the gene. DNA vaccines constructed with the HCMV promoter along with its intron A sequence have been shown to be immunogenic in animal and human studies (36, 48, 49), but few studies have evaluated the effect of addition of full-length intron A to the HCMV promoter sequence.
A major limitation of AdH5 use in humans is the high prevalence of neutralizing antibodies (55) to the virus caused by frequent childhood infections. To circumvent interference by preexisting immunity, rare adenovirus serotypes that do not circulate in human populations have been developed as vaccine vectors. One strategy is to use human adenovirus serotypes that have a low prevalence in human populations, such as Ad35 and Ad11 (8, 34). Unfortunately, these vaccine vectors induce weaker responses than those induced by AdH5. A different approach is the use of chimpanzee adenoviral vectors that do not circulate in human populations. Studies have demonstrated the immunogenicity and efficacy of simian adenoviral vectors in disease models of human immunodeficiency virus (HIV) infection, Ebola, and severe acute respiratory syndrome (SARS) (32, 44, 63).
Our study describes the improved design of an HCMV promoter to drive antigen expression from an adenoviral vector and further compares the immune responses induced by an E1 region-deleted vector and an E1E3 region-deleted adenoviral vector. We describe for the first time the use of a simian adenoviral vector, AdC6, as a pre-erythrocytic-stage malaria vaccine in a mouse malaria model. We show that AdC6 can induce very strong CD8+ T-cell responses compared to AdH5 and can provide high levels of sterile protection to a potent sporozoite challenge. We show that under conditions of preexisting immunity to AdH5, which abrogated protection induced by the AdH5 vector, AdC6 was still able to maintain protection.
MATERIALS AND METHODS
Cell lines.
Human embryonic kidney (HEK) 293 cells were propagated in Dulbecco's modified Eagle's medium supplemented with glutamine, antibiotic, and 10% fetal calf serum (Sigma).
Viral vectors.
The shuttle vector pENTR4.LP was constructed by inserting the enhancer/promoter sequence with exon 1 and the intron A sequence of the HCMV IE gene (nucleotides [nt] 163 to 2091; GenBank accession number M60321), a multiple cloning site, and the bovine growth hormone poly(A) sequence from the pSG2 plasmid (36) between the recombination sequences in pENTR4 (Invitrogen Life Technologies, Inc). Plasmodium yoelii merozoite surface protein 142 (MSP-142) and the Mycobacterium tuberculosis 85A gene were cloned into the multiple cloning site of either the pENTR4 or pENTR4.LP shuttle vector. This was then recombined into E1- and E3-deleted AdH5 adenoviral genome vectors from the Virapower adenoviral expression system (Invitrogen Life Technologies, Inc). pENTR4.LP was recombined with pAd/PL-DEST to generate AdH5 adenoviral genome vectors containing the HCMV long promoter (LP), antigen, and bovine growth hormone poly(A) sequence from pENTR4.LP. pENTR4 vectors containing antigen were recombined with pAd/CMV/V5/DEST, generating AdH5 adenoviral genome vectors containing antigen driven by the HCMV IE short promoter (SP) in pAd/CMV/V5/DEST. The difference in the two promoters is shown in Fig. 1a. For viral rescue, the vector was transfected into HEK 293 cells, expanded, and purified using an Adenopure purification column (Puresyn Inc.) or CsCl gradient centrifugation. The number of viral particles (vp) was determined by spectrophotometry as described previously (35). PFU were determined by plaque assay and ranged from 1 PFU per 20 to 1 PFU per 500 vp. E1-deleted AdH5 adenoviral vector and the E1E3-deleted simian adenoviral vector (AdC6) were constructed and propagated as described previously (44). Construction of the AdH5 (28), MVA, and FP9 (3) viral vectors, expressing Plasmodium berghei CSP under the control of the HCMV IE promoter containing intron A (LP) in the case of AdH5 and the vaccinia virus p7.5 promoter for MVA and FP9, has been described earlier.
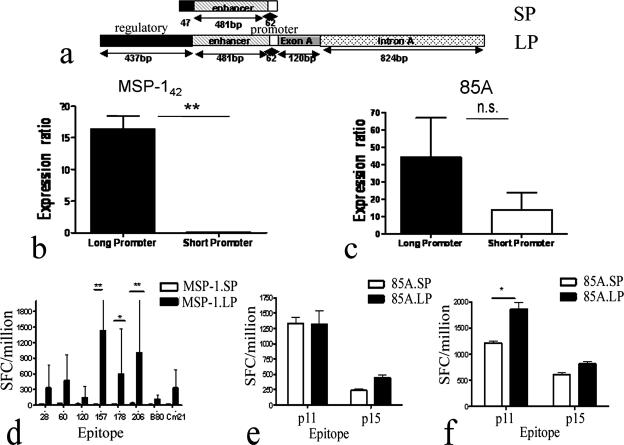
FIG. 1.
Comparison of different promoters. An AdH5 adenoviral vector was constructed to express antigens MSP-142 and 85A under the control of HCMV promoters either with or without an exon A and intron A sequence. The transcriptional activity and immunogenicity of each construct were investigated and compared. (a) Schematic diagram of the promoter elements used in the adenoviral vectors. SP is an HCMV promoter in pAd/CMV/V5/DEST containing the enhancer/promoter sequence and a short regulatory sequence, while LP is the HCMV promoter containing the enhancer/promoter sequence along with the exon A and intron A sequence. (b and c) Comparison of mRNA expression levels between promoters. 293 cells were infected at an MOI of 50 with AdH5.85A.SP, AdH5.85A.LP, AdH5.MSP-142.SP or AdH5.MSP-142.LP, and RNAs were extracted from the cells 48 h after infection. Real-time RT-PCR was undertaken to compare mRNA levels of antigen transcribed by the LP or SP. Antigen mRNA levels were normalized to AdE4orf1 levels and expressed as ratios of antigen levels to AdE4orf1 levels. Panels b and c show the data for the MSP-142 and 85A antigens, respectively. Statistical analysis was performed using an unpaired t test (GraphPad Prism). **, P < 0.01. The results shown are representative of two independent experiments. (d to f) Effect of promoter on immunogenicity of adenoviral vectors. Female BALB/c mice (three/group) were immunized with 1010 vp of AdH5.85A.SP or AdH5.85A.LP (e), 1010 vp of AdH5.MSP-142.SP or AdH5.MSP-142.LP (d), or 109 vp of AdH5.85A.SP or AdH5.85A.LP (f) intradermally (i.d.) in the ear. Fourteen days later, splenocytes were assayed for IFN-γ secretion by ex vivo ELISPOT assay. Columns represent mean numbers of IFN-γ-producing SFCs per million splenocytes plus standard errors of the means (SEM). Statistical analysis was performed using a nonparametric two-way ANOVA (GraphPad Prism). *, P < 0.05; **, P < 0.01 (compared to mice immunized with vectors containing SP).
Quantitative real-time RT-PCR.
293A cells were infected at a multiplicity of infection (MOI) of 50 with adenoviral vectors expressing antigen 85A or MSP-142 under the control of either the SP or the LP. Cells were harvested at 48 h postinfection, centrifuged, and used to extract mRNA. mRNA was extracted using a QIAamp RNA blood mini kit (Qiagen) following the manufacturer's instructions. RNA was then reverse transcribed into cDNA using an Omniscript reverse transcriptase (RT) kit (Qiagen) (containing Omniscript RT with its buffer and a deoxynucleoside triphosphate mix) and an oligo(dT) primer. Real-time PCR on cDNA was performed on a Rotorgene 3000 (Corbett Research, Sydney, Australia) machine, using a Quantitect SYBR green PCR kit (Qiagen). The SYBR green mix contained HotStar Taq DNA polymerase, SYBR green I dye, optimized SYBR green buffer, 5 mM MgCl2, and a deoxynucleoside triphosphate mix including dUTP. The forward primer MSP-142 SS2 (5′-CATGGATGGTATGGATTTATTAGGTG-3′) and reverse primer MSP-142 SS2 (5′-TCCGTACTTTCCGCATTTTGA-3′) were used to amplify a fragment of 237 base pairs from the MSP-142 antigen. The forward primer 85AU SS (5′-GCG CCG TCA CGG GTA TGT-3′) and reverse primer 85AL SS (5′-GCA CCA CCA CTT TGGAAT TG-3′) were used to amplify a fragment of 196 base pairs from the 85A antigen. A 243-bp fragment from the region of the adenoviral gene AdE4orf1 was also amplified from each sample, using the forward primer AdE4orf F1 (5′-GGTAGCGCGGGTCTCTGTCTCA-3′) and reverse primer AdE4orf R1 (5′-GGGGGCGCCTGGATGCT-3′). Real-time PCR amplification was performed in a 25-μl volume containing Quantitect master mix (Qiagen), 100 nM of each primer, and 1 μl cDNA. The thermal cycling conditions were 95°C for 15 min and 40 cycles of 95°C for 10 seconds, annealing at 55°C for 15 seconds, and extension at 72°C. At least two no-template controls and a plasmid encoding the antigen as a positive control were used in every PCR run. Standard curves of quantified and diluted PCR product for each primer pair were generated and used in different experiments to calculate the cDNA amount from each sample. A standard curve was rejected if the correlation coefficient of the trend line was <0.95. The numbers of copies of the RT-PCR standards were calculated and used to compare antigen mRNA expression levels. To normalize differences in RNA quality between the individual samples and in RNA loading for the RT-PCR procedure, the antigen mRNA expression level for a particular sample was defined as its antigen gene expression level divided by its Ad.E4orf1 gene expression level.
Mice and immunizations.
Female BALB/c mice of 4 to 6 weeks of age were purchased from the Biomedical Services Unit at the John Radcliffe Hospital, Oxford, United Kingdom, with all animals used in accordance with the terms of a UK Home Office Animals Act project license. Immunizations were performed intradermally, which has previously been shown to elicit better immunogenicity than other routes, e.g., the subcutaneous or intramuscular route (28). MVA.PbCSP or FP9.PbCSP was administered at a dose of 1 × 107 PFU, and all adenoviruses were given at a dose of 1 × 1010 or 1 × 109 vp. All vectors were resuspended in endotoxin-free phosphate-buffered saline prior to immunization.
Peptides.
The Pb9 (SYIPSAEKI) peptide (28), which carries the immunodominant H-2Kd-restricted epitope of P. berghei CSP, and the p11 (EWYDQSGLSVVMPVGGQSSF) and p15 (TFLTSELPGWLQANRHVKPT) peptides of 85A, carrying CD8 and CD4 epitopes, respectively (22), have been described previously. T-cell epitopes in MSP-142 are mainly contained in the MSP-133 region. CD8+ T-cell epitopes within MSP-133 were predicted from known H-2 class I binding motifs, using the epitope prediction section of the SYFPEITHI website (http://www.syfpeithi.de/Scripts/MHCServer.dll/EpitopePrediction.htm) developed at the University of Tübingen. The six strongest candidates for BALB/c (H-2d) mice (157, 206, 178, 28, 60, and 120) were selected. Epitope B80 was the strongest predicted epitope for C57BL/6 mice but was also a weaker candidate for BALB/c mice. Cm15 and Cm21 are known T-cell 20-mer “protective” epitopes within MSP-133 from BALB/c mice and are recognized by CD4+ effector T-cell lines capable of delaying growth of blood-stage P. yoelii YM (59). Table 1 gives the sequences and predicted strengths of MSP-142 epitopes.
TABLE 1.
Sequences of MSP-142 epitopesa
| Peptide | Amino acid position in MSP-133 | Sequence |
|---|---|---|
| 157 | 1550-1558 | KYIPILEDL |
| 206 | 1599-1607 | KYIQIDEKL |
| 178 | 1571-1580 | EYSEELQNRL |
| 28 | 1421-1429 | TYKSIKKHML |
| 60 | 1453-1462 | DFLEVLSHEL |
| 120 | 1513-1521 | KFFNKMVEL |
| Cm15 | 1528-1547 | AVKEQIATIEAETNDTNKEE |
| Cm21 | 1588-1607 | AEFEILTKNLEKYIQIDEKL |
| B80 | 1473-1481 | YVIRNPYQL |
The amino acid sequences of predicted and published epitopes from P. yoelii strain YM MSP-142 are listed. Epitopes were predicted using the epitope prediction section of the SYFPEITHI website (http://www.syfpeithi.de/Scripts/MHCServer.dll/EpitopePrediction.htm) developed at the University of Tübingen.
Ex vivo IFN-γ ELISPOT assay.
Ammonium chloride-potassium-treated peripheral blood mononuclear cells or spleens were cultured for 18 to 20 h on IPVH membrane plates (Millipore) with the peptide at a final concentration of 1 μg/ml. Enzyme-linked immunospot (ELISPOT) assay was performed as previously described (37).
Intracellular cytokine staining.
Ammonium chloride-potassium-treated splenocytes were incubated for 5 h in the presence of 1 μg/ml Pb9 and 2 μl/ml Golgi-Plug (BD). Intracellular cytokine staining (ICS) was performed with a BD Cytofix/Cytoperm Plus kit according to the manufacturer's instructions. Splenocytes were stained with a suitable combination of fluorochrome-conjugated antibodies specific for CD8 (clone 53-6.7; eBioscience), IFN-γ (clone XMG1.2; eBioscience), CD27 (clone LG.7F9; eBioscience), CD43 (clone 1B11; BD/Pharmingen), CD127 (clone A7R34; eBioscience), interleukin-2 (IL-2) (clone JES6-5H4; eBioscience), tumor necrosis factor alpha (TNF-α) (clone MP6-XT22; eBioscience), mouse isotype control IgG2a (eBR2a; eBioscience), CD16/CD32 Fcγ III/II receptor (2.4G2; BD/Pharmingen), granzyme B (GrB) (clone GB12; Caltag), and an IgG1 isotype control (Caltag). When CD62 ligand (CD62L) (clone MEL-14; eBioscience) was used, stimulated cells were incubated with TAPI-2 peptide (Peptides International) at a final concentration of 250 μM to prevent CD62L shedding from the cell surface. Flow cytometric analyses were performed using a FACSCanto flow cytometer (BD Biosciences), and data were analyzed with FlowJo (Tree Star) software.
Evaluation of antigen-specific CD8+ T-cell response by flow cytometry.
The frequency of IFN-γ+ CD8+ T cells was calculated by subtracting the value from the unstimulated control value, which never exceeded 0.1% in any of the experiments. For the phenotypic makers investigated, each marker was compared to an isotype control.
Parasite challenge.
Plasmodium berghei (ANKA strain clone 234) sporozoites were isolated from salivary glands of female Anopheles stephensi mosquitoes. Parasites were resuspended in RPMI 1640 medium, with each mouse receiving a total of 1,000 sporozoites via the intravenous route. Blood samples were taken daily from days 5 to 20; smears were stained with Giemsa stain and screened for the presence of blood-stage parasites. Survival was defined as the complete absence of parasites in blood.
Statistical analysis.
The statistical significance of differences in flow cytometry samples was analyzed with either one- or two-way analysis of variance (ANOVA) and a Bonferroni posttest. All statistical tests were performed using GraphPad Prism, version 4.03, for Windows (GraphPad Software, San Diego, CA).
RESULTS
Effect of intron A on the strength of the HCMV IE promoter.
We compared antigen transcript levels driven by the HCMV IE promoter with intron A (LP) and the promoter without intron A (SP) (Fig. 1a) by RT-PCR. Cell cultures of HEK 293 cells were infected at an MOI of 50 with AdH5 expressing either MSP-142 antigen or 85A antigen under the control of the HCMV IE promoter containing intron A (Ad.H5.MSP-142.LP and Ad.H5.85A.LP) or the HCMV IE promoter without intron A (Ad.H5.MSP-142.SP and Ad.H5.85A.SP). Cells were harvested 24 h later, and quantitative real-time RT-PCR was undertaken for the specific antigen and AdE4orf1 gene to quantify gene mRNA levels. Antigen mRNA levels were normalized to Ad.E4orf1 mRNA and compared. For both antigens, the HCMV IE promoter with intron A (LP) directed a higher level of transcription than did the promoter without intron A. Ad.MSP-142.LP had 15-fold higher levels of gene expression than did Ad.MSP-142.SP (Fig. 1B), while Ad.85ALP showed 2-fold higher expression than did Ad.85ASP (Fig. 1C). We further tested whether this difference in transcriptional activity of the antigen resulted in a more immunogenic vaccine. BALB/c mice were immunized with 1010 vp of AdH5.MSP-142.SP, AdH5.MSP-142.LP, AdH5.85A.LP, or AdH5.85A.SP. Immune responses in the spleen were measured by ex vivo IFN-γ ELISPOT assay 14 days after immunization. The immune responses were stronger for all defined epitopes in MSP-142 and were significantly stronger for defined CD8 epitopes in MSP-142 (Fig. 1d) for vaccine constructs driving antigen expression from the HCMV promoter with intron A than for constructs using the HCMV promoter without intron A to drive antigen expression. Although no difference in the immune response to epitopes in 85A was seen with AdH5.85A.LP compared to AdH5.85A.SP (Fig. 1e) when mice were immunized with 1010 vp, lowering the immunizing dose to 109 vp showed a significant difference in the immune response to the CD8 epitope in 85A (Fig. 1f). The data suggest that antigen under the control of the HCMV promoter with intron A (LP) is expressed at higher levels and results in stronger immune responses than those with the HCMV promoter without intron A.
Comparison of E1 region-deleted or E1E3 region-deleted adenoviral vectors.
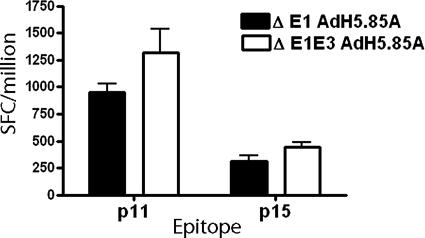
Most adenoviral vectors currently in use have the E1 region of adenovirus deleted, which makes the virus replication incompetent in mammalian cells. The E3 region of the adenovirus expresses a number of proteins that are involved in a complex interplay functioning mainly to subvert the host immune system. We compared the immunogenicity of an AdH5 vector with only the E1 region deleted to that of a vector that had the E1 and E3 regions deleted. BALB/c mice were immunized with 1010 vp of E1-deleted AdH5.85A.LP and E1E3-deleted AdH5.85A.LP, and immune responses were measured in the spleen 2 weeks after immunization. While there was a trend toward the E1E3-deleted vector inducing stronger immune responses to antigen 85A epitopes, these responses were not significantly different from that induced by E1-deleted AdH5.85A.LP (Fig. 2). The advantages of using E1E3-deleted vectors are the ability to incorporate larger sequences of foreign DNA and potentially diminished antivector immunity (52). While E1-deleted vectors can accommodate a 5-kb foreign sequence, E1E3-deleted adenoviral vectors allow for the insertion of up to 7.5 kb of foreign DNA, and therefore we used E1E3-deleted adenoviral vectors for further studies.
FIG. 2.
Comparison of E1-deleted and E1E3-deleted adenoviral vectors. BALB/c mice (three/group) were immunized with 1010 vp of E1-deleted AdH5.85A.LP or E1E3-deleted AdH5.85A.LP i.d. in the ear. Fourteen days later, splenocytes were assayed for IFN-γ secretion by ex vivo ELISPOT assay. Columns represent mean numbers of IFN-γ-producing SFCs per million splenocytes plus SEM.
Evaluation of antigen-specific CD8+ T-cell responses.
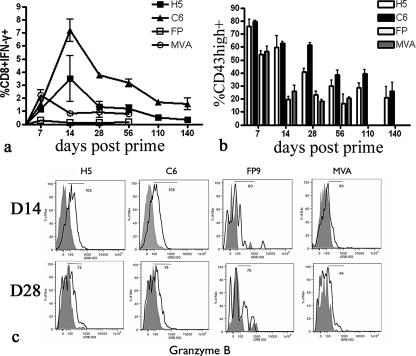
We constructed E1E3-deleted adenoviral vectors expressing PbCSP under the control of the HCMV promoter with intron A. We evaluated the immunogenicity and immune kinetics of recombinant AdH5, simian adenovirus serotype 6 (AdC6), and poxviral vectors MVA and FP9 expressing PbCSP by measuring the IFN-γ response to the previously defined CD8+-specific T-cell epitope Pb9. Of all the vectors, AdC6 was the most immunogenic vaccine and FP9 was the least immunogenic (Fig. 3a). The adenoviral vectors were more immunogenic than the poxviral vectors at all time points measured except for 1 week after immunization, when the immune response induced by MVA.PbCSP was the strongest. The kinetics of the immune response varied between the poxviral and adenoviral vectors. While poxviral vectors MVA and FP produced an immune response which peaked 1 week after vaccination and contracted rapidly by 14 days, the immune response induced by the adenoviral vectors peaked 2 weeks after immunization and contracted more slowly (Fig. 3A). Between the adenoviral vectors, AdC6 was significantly more immunogenic and was able to maintain a higher frequency of CD8+ IFN-γ+ cells for up to at least 140 days after immunization. Observing the difference in the immune kinetics induced by different viral vectors, we further extended our study to the difference between viral vectors in the development of effector and memory phenotypes of CD8+ IFN-γ+ cell populations.
FIG. 3.
Immunogenicity and effector T-cell development following immunization with viral vectors expressing PbCSP. (a) Immunogenicity and immune kinetics. Female BALB/c mice (three mice/group) were immunized with AdH5.PbCSP (1010 vp), AdC6.PbCSP (1010 vp), MVA.PbCSP (107 PFU), or FP9.PbCSP (107 PFU) i.d. Spleens were harvested at different time points postimmunization, and antigen-specific responses were assayed by intracellular cytokine staining for IFN-γ (CD8+ T cells) following stimulation of splenocytes with Pb9. Data are means plus SEM for each group. (b) Phenotyping of effector cell population. Data show the percentages of CD8+ IFN-γ+ CD43high cells. Columns represent means plus SEM for each group. (c) Cytolytic function of antigen-specific cells. CD8+ IFN-γ+ GrB+ coexpression is shown for representative mice at the indicated days postprime. The histograms show GrB expression (white background) after staining with anti-human GrB compared to staining with an isotype control (gray background). The number corresponds to the mean fluorescence intensity of the positive sample (black solid line).
Development of effector CD8+ T-cell response.
The acquisition of effector functions was determined by the expression of the phenotypic markers CD43 and GrB. CD43 expression has previously been shown to be upregulated during the effector phase of the CD8 response. Effector cells undertake their cytolytic functions through effector molecules such as GrB and TNF-α, which are involved in direct CD8+ T-cell-mediated killing of infected cells. In general, adenoviral vectors induced a significantly higher percentage of CD8+ IFN-γ+ CD43high cells over the entire course of the immune response than did the poxviral vectors. Irrespective of the vector used, the percentage of CD8+ IFN-γ+ CD43high cells decreased with time, indicating a transition to the memory phase of the immune response, but the kinetics of this transition varied between poxviral and adenoviral vectors. At the peak of the immune response induced by poxviral vectors (day 7), 50% of CD8+ IFN-γ+ cells were CD43high, but as early as 2 weeks postvaccination, this frequency dropped to 20%, suggesting a rapid transition toward the memory phase (Fig. 3b). In comparison, mice immunized with the adenoviral vectors still retained a high percentage of CD8+ IFN-γ+ CD43high cells even until 56 days postprime (40% for AdC6 and 35% for AdH5) compared to the poxviral vectors (Fig. 3b), and it took 140 days after immunization for the levels of CD8+ IFN-γ+ CD43high cells to drop to about 20%. CD8 T cells induced by both adenoviral vectors expressed greater levels of GrB than did those induced by the poxviral vectors at day 14, indicating a rapid loss of cytolytic molecules following poxviral vaccination. However, by day 28, the adenoviral vector-induced cells also had low levels of GrB expression (Fig. 3c). These results demonstrate that the adenoviruses are able to maintain an effector response with preservation of cytolytic molecules for longer periods of time than are poxviral vectors.
Development of memory CD8+ T cells.
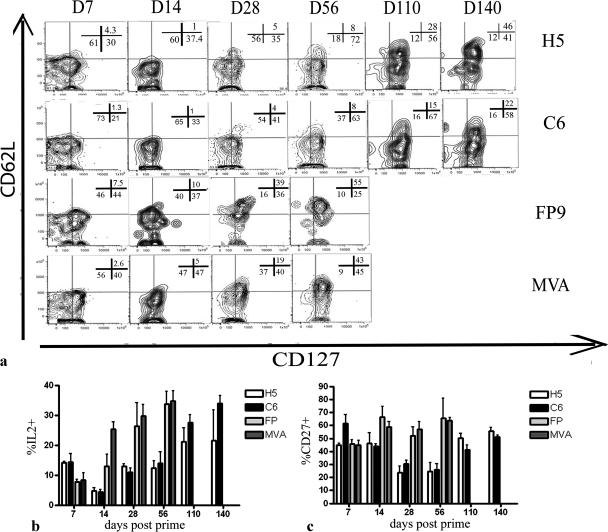
One of the main objectives of any vaccination regimen is the generation of memory CD8+ T cells that are capable of persisting in vivo and expanding rapidly upon encounter with pathogens, thus affording protection. It has been proposed that the development of the intrahepatic effector and central memory phenotype of cells is crucial to protection against malaria sporozoite challenge (11). Different molecules have been suggested to correspond to different subtypes of memory cells (7). In this study, we chose to differentiate effector (TE), effector memory (TEM), and central memory (TCM) cells on the basis of the phenotypic markers CD127, CD62L, IL-2, and CD27 to determine whether individual vectors induced different memory populations.
During the first 14 days postimmunization, CD8+ IFN-γ+ cells generated by either FP9 or MVA displayed a CD62L− CD127+ or CD62L− CD127− phenotype, indicating mainly a TEM or TE phenotype, respectively. During the same period, the majority of cells generated by adenoviral vectors were TE cells with a CD127− CD62L− phenotype (Fig. 4a). By 56 days postimmunization, the majority of CD8+ IFN-γ+ cells induced by poxviral vectors were of a CD62L+ CD127+ phenotype, and 40% of antigen-specific cells were producing IL-2, indicating the development of TCM cells. On the other hand, by day 56 postprime, the majority of CD8+ IFN-γ+ cells induced by adenoviral vectors displayed a TEM phenotype (CD62L− CD127+; low percentage of IL-2-producing cells), and only 8% of CD8+ IFN-γ+ cells showed a TCM phenotype (Fig. 4a and b). Interestingly, while AdH5 was able to generate 46% of CD8+ IFN-γ+ cells with a TCM phenotype by day 140, the majority of cells induced by AdC6 still remained of a TEM phenotype, suggesting the delayed development of central memory cells following AdC6 immunization (Fig. 4a).
FIG. 4.
Phenotyping of memory CD8+ T cells following viral vector immunization. Mice were immunized as described in the legend to Fig. 2. (a) Long-term evolution of CD62L and CD127 on antigen-specific cells following immunization. Splenocytes were costained for CD8, IFN-γ, CD62L, and CD127. Representative data are shown for three mice per group. (b) Development of IL-2-producing cells. Data show the percentages of CD8+ IFN-γ+ IL-2+ cells. Columns represent means plus SEM for each group. (c) CD27 expression. Data show the percentages of CD8+ IFN-γ+ CD27+ cells. Columns represent means plus SEM for each group.
CD27 is a surface marker that is down regulated following repeated antigenic stimulation by CD70. Initially, the levels of CD8+ IFN-γ+ CD27+ cells were close to 40% in mice vaccinated with adenoviral vectors, but by day 28 postprime, this frequency dropped to 20% and only began to return to its original levels by day 110. This is in stark contrast to poxviral vectors, which showed a steady increase in the percentage of CD8+ CD27+ cells over time. Since CD8+ CD27− cells are maintained in response to persistent antigenic stimulation (4), this may suggest that prolonged antigen stimulation was occurring in response to adenoviral immunization. Antigen stimulation is thought to hinder the development of central memory cells while maintaining cells in an effector state, and interestingly, this rise in CD27+ cells corresponds to the beginning of development of cells with a TCM phenotype. In summary, these results demonstrate that vaccination with adenoviral vectors using the PbCSP insert maintains a predominantly TEM response (as evidenced by a CD62L− CD127+ IL-2low phenotype) for up to 8 weeks after immunization and delays the development of central memory cells (as evidenced by a CD62L+ CD127+ IL-2high phenotype) compared to vaccination with poxviral vectors.
Efficacy of adenoviral vectors against sporozoite challenge.
To test the efficacy of the adenoviral vectors, mice immunized with viral vectors were challenged with 1,000 sporozoites of P. berghei. Mice were challenged 7 days, 14 days, and 56 days after immunization to investigate efficacy data at the peak of the effector response and during the memory phase. Both AdC6 and AdH5 afforded significant protection (P = 0.0001 and P = 0.01) when mice were challenged 14 days after immunization. AdC6 protected 90% of mice, compared to only 25% protection by AdH5, while both poxviral vectors failed to offer any sterile protection (Table 2). Mice were also challenged 7 days after immunization, corresponding to the peak of the immune response for poxviral vectors, but neither MVA nor FP9 conferred protection at this time point. To investigate the durability of protection, mice were challenged 8 weeks after immunization, and only AdC6 afforded a statistically significant degree of protection (P = 0.015) and was able to protect 36% of the challenged mice.
TABLE 2.
Efficacy of viral vector vaccines in response to sporozoite challengea
| Vaccine | Time of challenge (days postimmunization) | No. of protected mice/no. of challenged mice (prepatency period [days]) | % Protection (P value) |
|---|---|---|---|
| H5.PbCSP | 7 | 0/8 (6, 7) | 0 (0.14) |
| C6.PbCSP | 7 | 2/8 (6) | 25 (0.06) |
| FP.PbCSP | 7 | 0/8 (6) | 0 |
| MVA.PbCSP | 7 | 0/8 (6, 8) | 0 (0.06) |
| Naive | 0/8 (6) | ||
| H5.PbCSP | 14 | 5/20 (6) | 25 (0.01)* |
| C6.PbCSP | 14 | 18/20 (6) | 90 (0.0001)* |
| FP.PbCSP | 14 | 0/8 (6) | 0 |
| MVA.PbCSP | 14 | 0/8 (6) | 0 |
| Naive | 0/20 (6) | ||
| H5.PbCSP | 56 | 2/14 (6) | 14 (0.15) |
| C6.PbCSP | 56 | 5/14 (6) | 36 (0.015)* |
| FP.PbCSP | 56 | 0/14 (6) | 0 |
| MVA.PbCSP | 56 | 0/14 (6) | 0 |
| Naive | 0/14 (6) |
BALB/c mice were immunized with adenoviral (1010 vp) or poxviral (107 PFU) vectors and challenged intravenously with 1,000 P. berghei sporozoites at different time points. The prepatency period is the time until the appearance of blood-stage parasites in Giemsa-stained blood smears. Statistical analysis was performed using Kaplan-Meyer survival analysis to calculate the P value (GraphPad Prism).
Effect of preexisting immunity on immunogenicity and efficacy of adenoviral vectors.
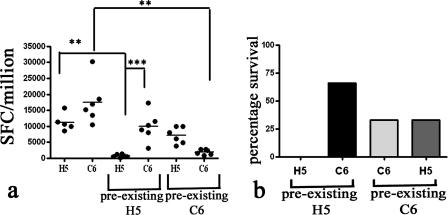
Forty-five percent of humans have neutralizing antibodies to AdH5 (55), which limits the efficacy of AdH5 vaccine vectors (63). Simian adenoviruses offer a promising strategy to circumvent this problem. To test the efficacy of AdH5 and AdC6 vectors in the presence of preexisting immunity, we immunized mice with AdH5 or AdC6 expressing an unrelated antigen (85A) and, 6 weeks later, immunized them with vectors containing PbCSP. Titers of neutralizing antibodies to the AdHu5 and AdC6 vectors in the sera of mice were tested before immunization with vectors containing PbCSP. The titers were found to be ∼1:320 to 1:400, which is higher than titers commonly found in human adults. In the presence of preexisting immunity to AdH5, immunization with AdH5.PbCSP led to a significant decrease in the immune response to Pb9 (P < 0.01) (Fig. 5a) and in abrogation of protection (Fig. 5b) compared to AdH5.PbCSP in the absence of preexisting immunity, while AdC6.PbCSP afforded 66% protection and significantly better immune responses than did AdH5.PbCSP (P < 0.001) in the presence of preexisting immunity to AdH5. In mice with preexisting immunity to AdC6, the immunogenicity of AdC6.PbCSP was significantly reduced (P < 0.01), while the immune response induced by AdH5.PbCSP was not reduced compared to the immune response induced by the respective vaccines in the absence of preexisting immunity to AdC6. This decrease in immunogenicity led to a decrease in protective efficacy, as AdC6 was able to protect only 33% of mice in the presence of preexisting immunity to AdC6, while AdH5.PbCSP provided 33% protection (Fig. 5a and b). These results further emphasize the problem of preexisting immunity in the context of adenoviral vaccines and demonstrate the efficacy of the AdC6 vaccine in the presence of preexisting immunity to AdH5.
FIG. 5.
Immunogenicity and efficacy of adenoviral vectors in the presence of preexisting immunity. Preexisting immunity was induced by immunizing groups of six BALB/c mice with 5 × 109 vp of AdH5.85A or C6.85A. Six weeks later, mice were immunized with adenoviral vectors expressing PbCSP at a dose of 1010 vp per mouse. (a) Effect of preexisting immunity on immunogenicity of viral vectors. Twelve days after immunization, blood was collected to analyze IFN-γ secretion by ex vivo ELISPOT assay. Data shown represent individual mouse responses, expressed in IFN-γ-producing SFCs per million peripheral blood mononuclear cells, and the line represents the mean response per group. Statistical analysis was performed using nonparametric two-way ANOVA, with adjustment for multiple comparisons (GraphPad Prism). Statistical differences are indicated as follows: **, P < 0.05; ***, P < 0.001. (b) Protective efficacy in the presence of preexisting immunity. Fourteen days after immunization with adenoviral vectors coding for PbCSP, mice were challenged intravenously with 1,000 P. berghei sporozoites. The bars represent the percentages of mice that were protected in each group. All naïve mice were infected by day 6.
DISCUSSION
Replication-deficient adenoviral vectors have been used as a vaccine strategy to deliver antigen in different disease models. A variable influencing the capacity for adenoviral vectors to be successful as vaccines could be an ability to generate high-level expression of the antigen for induction of a strong immune response. The gene encoding the IE 72-kDa protein of HCMV is transcribed by one of the strongest enhancers/promoters currently known (14) and is able to induce high levels of transgene expression in a wide variety of cell types after both transient and stable transfections (14, 26). Therefore, it is currently the most widely used viral promoter in vaccine and gene therapy applications. However, the expression levels induced in vivo are often suboptimal for therapeutic applications. While the strength of the HCMV IE promoter lies in its enhancer region, which contains binding sites for transcription factors of the NF-κB, cyclic AMP-responsive binding protein, and nuclear factor 1 families, the intron A region of the HCMV promoter has been investigated for its regulatory role on the enhancer region and has been shown to enhance gene expression in a transient transfection system (18). The enhancing effect of intron A on HCMV promoter-directed transgene expression led to the addition of the intron A sequence to the HCMV enhancer/promoter sequence for controlling antigen expression in DNA vaccines (42, 53) and viral vectors (28). The intron A sequence used in the DNA vaccine constructs was a partial intron sequence. Here we demonstrate that addition instead of the full intron A sequence to the HCMV promoter to drive antigen expression in adenoviral vectors can significantly improve immunogenicity to the antigen compared to that induced with a similar HCMV promoter without the intron A sequence. Adenoviral vectors expressing Plasmodium yoelii MSP-142 antigen and Mycobacterium tuberculosis 85A antigen from HCMV promoters, either with or without the intron A sequence, were compared. These antigens, containing defined murine CD4 and CD8 epitopes, were chosen for vaccine constructs to allow the measurement of CD4- and CD8-specific immune responses. The HCMV promoter with the intron A sequence showed higher levels of mRNA expression in vitro for both antigens than did the HCMV promoter without intron A. Furthermore, AdH5 vectors with the HCMV promoter with intron A were capable of inducing higher levels of IFN-γ-producing antigen-specific CD8 and CD4 T cells than those obtained with vectors with the HCMV promoter without the intron A sequence. Immune responses to epitopes of MSP-142 antigen were significantly improved by the addition of intron A to the promoter, while peptide-specific responses to antigen 85A did not showed any difference between vaccine constructs containing promoters with and without intron A. However, lowering the immunizing dose of the Ad.85A vaccines unmasked the enhanced immunogenicity of the vaccine with the HCMV promoter containing intron A compared to that of the vaccine without intron A (Fig. 1F). The difference in mRNA transcription levels between AdH5.85A.LP and AdH5.85A.SP was not as significant as that with AdH5.MSP-142 vectors, and this may explain the lack of difference in immune responses between the two AdH5.85A vaccines. In addition, we also suggest that a high immunizing dose of AdH5.85A provides a level of immunogenicity that cannot be enhanced significantly by the addition of intron A to the promoter. Our findings suggest that the HCMV promoter with intron A may be preferentially beneficial in vaccines or systems with low levels of antigen expression, such as MSP-142, for which even a high immunizing dose is unable to compensate for low expression levels of antigen from the HCMV promoter without intron A. While the differences between the two promoter constructs (Fig. 1A) include a larger regulatory sequence, an exon sequence, and the full-length intron A sequence, we suggest that the differences in levels of expression and immunogenicity are most likely linked to the effect of intron A on enhancing antigen expression. Similar use of introns in plasmids was shown to increase levels of expression in various systems (16, 31). Intron A of the HCMV promoter contains a nuclear factor 1 binding site that may act as a downstream transcriptional activator, but it is more likely that the positive effects of intron A arise from a combination of mRNA stabilization and increased transcriptional activity of intron A (18).
Packaging capability of the adenoviral capsid is an important consideration in constructing adenoviral vectors (12, 56). Foreign sequences of up to 1.8 kb can be inserted into the adenoviral genome without necessitating deletions. The deletion of the E1 region renders the viral construct replication incompetent and allows an extra 3.5 kb of foreign DNA to be added. The E3 region of the adenovirus genome, while dispensable for growth of the virus, encodes a series of proteins that function as immunomodulatory agents (23) and has been deleted in vaccine vectors (62) to increase the cloning capacity of the adenoviral vector to 7.5 kb. We demonstrated that there is no significant difference in the immune responses to antigen delivered by an E1-deleted and an E1E3-deleted adenoviral vector (Fig. 2) after a single dose of vaccine. Our promoter of choice, the HCMV promoter with intron A (LP), is 1.9 kb long, and its use in an E1-deleted adenoviral vector would permit the insertion of an antigen of no greater than 3.4 kb in size. Our results demonstrate that an E1E3-deleted vector is as potent as an E1-deleted vector, and the E1E3-deleted vector with the LP to drive antigen would allow an antigen of up to 5.6 kb to be inserted. The use of an E1E3-deleted adenoviral vector with the HCMV promoter containing intron A combines a potent promoter and enough free space in the adenoviral genome to insert large antigens. Further studies comparing the HCMV promoter containing full-length intron A with HCMV promoters with shortened versions of intron A need to be undertaken.
A malaria vaccine against the preerythrocytic stage of the parasite would be able to control infection, thereby preventing any symptoms of the disease. While sterile protection induced by strong IFN-γ+ CD8+ T cells has been achieved using γ-irradiated sporozoites, it would be very difficult to deploy such a vaccine system, which requires liquid nitrogen cryopreservation, in a worldwide immunization program. Thus, the search for an efficacious and immunogenic vaccine delivery system that can be deployed in humans continues. CSP is the most abundant antigen expressed on the surfaces of sporozoites and has been the main target antigen in the development of pre-erythrocytic-stage malaria vaccines. Immunization of mice with DNA vaccines (27) or recombinant viruses (45, 57) has previously been shown to elicit CD8+ T-cell responses against epitopes in CSP.
Adenoviral vectors are potent inducers of a cellular immune response and have conferred protection against a malaria sporozoite challenge when used either as a single dose (P. yoelii) (45) or in prime-boost regimens in combination with poxviral vectors (P. berghei) (28). However, in the only previous study of adenoviral vectors with the P. berghei model of malaria, protection by a homologous prime-boost regimen using AdH5 was minimal (28). AdH5 is a ubiquitous virus causing recurrent asymptomatic infection and subsequently resulting in over one-third of the adult human population carrying high levels of neutralizing antibodies to AdH5. This antibody-mediated preexisting immunity has been shown to reduce the efficacy of AdH5 vaccine vectors in animal models (17). To circumvent this issue, chimpanzee adenovirus serotypes which do not circulate in the human population have been developed as vaccine vectors. Previous studies have shown the ability of such recombinant simian adenoviral vectors to elicit potent B- and CD8+ T-cell-mediated immune responses in mouse and primate models of rabies (60), SARS (63), and HIV infection (44). This paper reports the use of a simian adenoviral vector using the HCMV promoter with intron A (LP) to drive expression of P. berghei CSP and compares the immunogenicity and efficacy of this novel simian adenoviral vector (AdC6) and the human adenoviral vector AdH5 to those of MVA and FP9, poxviral vectors that have been used widely in clinical trials.
First, we investigated the immunogenicity and kinetics of the antigen-specific CD8+ response following single immunization with the viral vectors. The simian adenovirus vector AdC6 was the most immunogenic vaccine, eliciting the strongest peptide-specific CD8+ IFN-γ+ T-cell response. The responses elicited by the adenoviral vectors peaked at around 2 weeks, in contrast to those to the poxviral vectors, which peaked by day 7. While the response to poxviral vectors contracted rapidly after day 7, the Pb9-specific response by adenoviral vectors contracted more slowly, with a high frequency of CD8+ IFN-γ+ cells remaining even 56 days after immunization with AdC6 (Fig. 3A). Characterization of the immune response using phenotypic markers for effector and memory CD8 T cells showed that adenoviral vectors were able to elicit a much more potent effector response, as measured by the levels of CD43 and GrB expression, than that seen with the poxviral vectors. The effector response induced by adenoviral vectors was also maintained for a longer period of time than was that induced by the poxviral vectors (Fig. 3B and C). We further analyzed the phenotype of memory T cells generated in response to the different viral vectors. Based on the expression of CD127 and CD62L, antigen-specific CD8+ T cells can be classified into TE (CD127− CD62L−), TEM (CD127+ CD62L−), and TCM (CD127+ CD62L+) cells, with each phenotype having different functional characteristics (5). At the peak of the immune response with adenoviral vectors (day 14 postprime), about 60% of cells were CD8+ CD127− CD62L− TE cells. By 56 days postprime, the majority of antigen-specific CD8+ cells in mice immunized with poxviral vectors were of the CD127+ CD62L+ TCM phenotype, while adenoviral vectors mainly induced CD8+ CD127+ CD62L− TEM cells at the same time point. TCM cells constituted the majority of antigen-specific CD8+ T cells only 140 days after immunization with AdH5 and constituted only 22% of cells following AdC6 immunization. The development of memory T cells is thought to depend on antigen exposure, the type of antigen, and the persistence of antigen (6). To further understand the delayed development of TCM cells following adenoviral immunization, we analyzed the expression of CD27 on antigen-specific cells. CD27 is a member of the TNF receptor family, and in mice, repeated antigenic stimulation leads to the loss of CD27 from the CD8+ T-cell surface and these CD8+ CD27− cells express high levels of cytotoxic effector molecules, such as GrB and perforin (4). Following immunization with adenoviral vectors, 20% of antigen-specific CD8+ IFN-γ+ CD27+ cells are maintained until 56 days postprime. This is different from the case for poxviral vectors, which induce a high percentage of CD27+ cells as early as 14 days after immunization. Our results demonstrating the delayed development of TCM cells and the maintenance of CD43high and TEM cells in combination with the low frequency of CD27+ cells suggest that adenoviral vectors may cause prolonged expression of antigen, thereby delaying contraction of the immune response and subsequently maintaining an effector response for an extended time. This feature of adenoviral vectors makes them especially attractive as vaccine vectors for disease states that require high levels of effector response for protection.
Studies conducted with various disease models have underlined the importance of TEM and TCM cells in conferring protection (58). It has also been shown that sporozoite-induced protection against P. berghei sporozoite challenge was effected mainly by intrahepatic TEM cells, not TCM cells (11). In this study, both AdH5 and AdC6 conferred protection (25% and 90%, respectively) against a stringent P. berghei challenge 2 weeks after immunization. It is interesting that there was a striking difference in protective efficacy between the two adenoviral vectors when mice were challenged 14 days after immunization. At this time point, there was no difference in the percentages of antigen-specific TE and TEM cells (Fig. 3 and 4) or the percentage of CD8+ IFN-γ+ cells secreting effector cytokines, such as GrB and TNF-α (unpublished data). It is therefore likely that this difference in protective efficacy is a result of the enhanced immunogenicity of AdC6 compared to that of AdH5. However, pre-erythrocytic-stage protection is also mediated through factors other than IFN-γ, such as nitric oxide, IL-12, and natural killer cells (29), and it is probable that AdC6 and AdH5 differ in their induction of these factors as well. When the challenge was performed 8 weeks after immunization, AdC6 was able to protect 36% of the mice, while AdH5 showed only 14% protection. Neither poxviral vector was able to protect against sporozoite challenge, even when the challenge was undertaken at the peak of the effector response (day 7) for these vectors. It is encouraging that AdC6 was able to protect 36% of mice challenged 8 weeks after immunization. Long-lasting sterile protection with a single vaccine dose has been achieved only with whole-parasite vaccine strategies, either by irradiation or by genetic attenuation. The deployment of these whole-parasite vaccine strategies worldwide would be logistically very problematic, so a subunit vaccine that confers durable protection after a single dose is a promising finding. Sterile protection against rodent malaria by use of adenoviruses has been achieved with prime-boost regimens involving AdH5-vaccinia virus (P. yoelii) (15) or AdH5-MVA (P. berghei) (28). It was previously shown that a single dose of AdH5 encoding P. yoelii CSP resulted in a 93% reduction in liver-stage development of the parasite (45), but the dose used in our study is 10 times lower than the dose of adenovirus used in that particular study. Similar efficacy with a single dose of adenovirus has not been demonstrated for the P. berghei model of malaria. In humans, the liver stage of P. falciparum lasts for 6 to 7 days, in contrast to that of P. berghei (2 to 3 days). While effector and TEM cells that respond rapidly to infection may provide sterile protection following P. berghei infection, the longer hepatic stage of P. falciparum may allow time for TCM cells that can proliferate and divide into effector cells to play a role in affording protection. The protective efficacy of adenoviral vectors against the liver stage of rodent malaria may be ascribed to their ability to induce high frequencies of effector and TEM cells, which provide immediate protection. The drop in protection over time with adenoviral vectors parallels a drop in the levels of effector and TEM cells. This supports the notion that immediate protection (conferred by effector cells) requires persisting antigen (64). On the other hand, in P. falciparum infection, the ability of adenoviral vectors to delay the development of potentially protective TCM cells might make such vaccines provide long-lasting protection, especially if adenoviral vaccines are used in prime-boost regimens that induce a protective threshold level of TCM cells.
Our results demonstrating AdC6 to be more immunogenic and efficacious than AdH5 are consistent with an earlier report evaluating AdC6 as an HIV vaccine in a vaccinia virus challenge model (43). It is as yet unclear why simian adenoviruses are more immunogenic than AdH5. The cell entry receptor for AdC6 has yet to be identified, but its hexon and fiber sequences share 90% homology with AdC68 (46), which uses coxsackie adenovirus receptor (CAR) to enter cells (21). It is likely that AdC6 uses CAR to gain entry into cells, although other, non-CAR-mediated entry into cells cannot be ruled out. This mechanism of cellular entry could impact the manner of activation of the innate immune system and downstream activation of the adaptive cellular response. Type I IFN can induce IFN-γ secretion from CD8 T cells (39), and the activation of naïve CD8 T cells is dependent on costimulatory signals expressed from mature dendritic cells (DCs). It has been reported that simian adenoviruses induce more type I IFN and also effect a more rapid and effective maturation of DCs (30). We speculate that one of the reasons for the difference in immunogenicity between AdC6 and AdH5 might be related to their differential induction of type I IFN and activation of DCs.
A significant percentage of humans carry neutralizing antibodies to AdH5, and preexisting immunity to AdH5 has been shown to reduce the efficacy of AdH5 viral vectors (63). To avoid interference by preexisting antibodies to AdH5, alternative human serotypes (41) and simian adenoviral vectors have been developed, and testing their efficacy in the presence of preexisting immunity is important to establish the potential of vaccines for use in humans. We induced the development of strong preexisting immunity by immunizing mice with AdH5.85A 6 weeks before immunization with a malaria vaccine. We show that preexisting immunity to AdH5 decreased immune responses and completely abrogated protection induced by AdH5.PbCSP vaccination. In contrast, there was no significant drop in immunogenicity of the AdC6.PbCSP vaccine, which afforded 66% protection in the setting of preexisting immunity to AdH5. These results are consistent with similar reports demonstrating the abrogation of protective efficacy of AdH5 vaccines in the presence of preexisting immunity to AdH5 (44, 63). Our result showing a drop in protection afforded by the AdC6 vaccine in the presence of preexisting immunity to AdH5 is similar to findings of studies using AdC7 (63) and AdC68 (44) vaccines with SARS and HIV models, respectively, and it is suggested that this phenomenon might be explained by cross-reactive CD8+ T cells that kill transduced antigen-presenting cells. A recent study demonstrated between 8 and 18% prevalence of neutralizing antibodies to AdC6 in parts of sub-Saharan Africa (61). A malaria vaccine is most required in this region, and despite this low prevalence of neutralizing antibodies to AdC6, we were interested in assessing the effect of our adenoviral vaccines in the presence of preexisting immunity to AdC6. We induced the development of preexisting immunity to AdC6 by immunizing mice with AdC6.85A 6 weeks before immunization with a malaria vaccine. In the presence of preexisting immunity to AdC6, the immunogenicity of AdC6.PbCSP was significantly decreased, and consequently, protection afforded by AdC6.PbCSP dropped to 33%. Immunogenicity of AdH5.PbCSP did not decrease significantly in the presence of preexisting immunity to AdC6, and 33% of mice were protected. We observed that similar levels of protection were offered by AdC6 and AdH5, despite differences in the levels of peptide-specific IFN-γ in the blood, as measured by ELISPOT assay. This lack of correlation between IFN-γ spot-forming cells (SFCs) and protection has been observed previously in humans (24, 25) and in mice (51) and may be indicative of other correlates of protection. While this is the first study to report the effect of preexisting immunity to simian adenovirus on the efficacy of a simian adenoviral vaccine, further work is needed to confirm whether this reduction of efficacy is also caused by neutralizing antibodies.
In conclusion, we describe an optimized adenoviral vaccine vector system that amalgamates a novel improved HCMV promoter in an E1E3-deleted adenoviral vector to increase antigen transcription and subsequently enhance the immune response. We transferred this optimized adenoviral vector design to the simian adenoviral vector AdC6 to elicit potent CD8+ T-cell responses against a pre-erythrocytic-stage antigen of rodent malaria. In contrast to rare human adenovirus serotypes, such as AdHu35 (34), the immunogenicity and efficacy of the AdC6 simian vector were greater than those of AdH5 and two poxviral vectors, FP9 and MVA. We also demonstrated that the adenoviruses were able to induce and maintain a high frequency of effector CD8+ T cells for a longer period of time than were poxviral vectors. Unlike the poxviral vectors, which induced TCM cells early after immunization, the adenoviral vectors delayed the development of TCM cells and maintained a TEM type of memory response for a prolonged duration after immunization. In addition, AdC6.PbCSP induced significant levels of sterile and long-lasting protection in the absence and presence of preexisting immunity to AdH5. Our data demonstrate for the first time that a single dose of a subunit vaccine is able to elicit protection against P. berghei and highlight the potential of AdC6 as a viral vector for further clinical consideration.
Footnotes
Published ahead of print on 6 February 2008.
REFERENCES
- 1.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, P. Aide, B. Sigauque, J. Milman, I. Mandomando, Q. Bassat, C. Guinovart, M. Espasa, S. Corachan, M. Lievens, M. M. Navia, M. C. Dubois, C. Menendez, F. Dubovsky, J. Cohen, R. Thompson, and W. R. Ballou. 2005. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 3662012-2018. [DOI] [PubMed] [Google Scholar]
- 2.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, J. Milman, I. Mandomando, B. Spiessens, C. Guinovart, M. Espasa, Q. Bassat, P. Aide, O. Ofori-Anyinam, M. M. Navia, S. Corachan, M. Ceuppens, M. C. Dubois, M. A. Demoitie, F. Dubovsky, C. Menendez, N. Tornieporth, W. R. Ballou, R. Thompson, and J. Cohen. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 3641411-1420. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, R. J., C. M. Hannan, S. C. Gilbert, S. M. Laidlaw, E. G. Sheu, S. Korten, R. Sinden, G. A. Butcher, M. A. Skinner, and A. V. Hill. 2004. Enhanced CD8+ T cell immune responses and protection elicited against Plasmodium berghei malaria by prime boost immunization regimens using a novel attenuated fowlpox virus. J. Immunol. 1723094-3100. [DOI] [PubMed] [Google Scholar]
- 4.Baars, P. A., S. Sierro, R. Arens, K. Tesselaar, B. Hooibrink, P. Klenerman, and R. A. van Lier. 2005. Properties of murine (CD8+)CD27− T cells. Eur. J. Immunol. 353131-3141. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann, M. F., P. Wolint, K. Schwarz, P. Jager, and A. Oxenius. 2005. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J. Immunol. 1754686-4696. [DOI] [PubMed] [Google Scholar]
- 6.Badovinac, V. P., and J. T. Harty. 2006. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol. Rev. 21167-80. [DOI] [PubMed] [Google Scholar]
- 7.Badovinac, V. P., K. A. Messingham, A. Jabbari, J. S. Haring, and J. T. Harty. 2005. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat. Med. 11748-756. [DOI] [PubMed] [Google Scholar]
- 8.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 1726290-6297. [DOI] [PubMed] [Google Scholar]
- 9.Bejon, P., J. Mwacharo, O. Kai, T. Mwangi, P. Milligan, S. Todryk, S. Keating, T. Lang, B. Lowe, C. Gikonyo, C. Molyneux, G. Fegan, S. C. Gilbert, N. Peshu, K. Marsh, and A. V. Hill. 2006. A phase 2b randomised trial of the candidate malaria vaccines FP9 ME-TRAP and MVA ME-TRAP among children in Kenya. PLoS Clin. Trials 1e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bejon, P., J. Mwacharo, O. K. Kai, S. Todryk, S. Keating, T. Lang, S. C. Gilbert, N. Peshu, K. Marsh, and A. V. Hill. 2006. Immunogenicity of the candidate malaria vaccines FP9 and modified vaccinia virus Ankara encoding the pre-erythrocytic antigen ME-TRAP in 1-6 year old children in a malaria endemic area. Vaccine 244709-4715. [DOI] [PubMed] [Google Scholar]
- 11.Berenzon, D., R. J. Schwenk, L. Letellier, M. Guebre-Xabier, J. Williams, and U. Krzych. 2003. Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells. J. Immunol. 1712024-2034. [DOI] [PubMed] [Google Scholar]
- 12.Bett, A. J., L. Prevec, and F. L. Graham. 1993. Packaging capacity and stability of human adenovirus type 5 vectors. J. Virol. 675911-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bojang, K. A., P. J. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J. Conway, W. H. Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A. Hill, K. P. McAdam, N. Tornieporth, J. D. Cohen, and T. Doherty. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 3581927-1934. [DOI] [PubMed] [Google Scholar]
- 14.Boshart, M., F. Weber, G. Jahn, K. Dorsch-Hasler, B. Fleckenstein, and W. Schaffner. 1985. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell 41521-530. [DOI] [PubMed] [Google Scholar]
- 15.Bruna-Romero, O., G. Gonzalez-Aseguinolaza, J. C. Hafalla, M. Tsuji, and R. S. Nussenzweig. 2001. Complete, long-lasting protection against malaria of mice primed and boosted with two distinct viral vectors expressing the same plasmodial antigen. Proc. Natl. Acad. Sci. USA 9811491-11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchman, A. R., and P. Berg. 1988. Comparison of intron-dependent and intron-independent gene expression. Mol. Cell. Biol. 84395-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casimiro, D. R., A. Tang, L. Chen, T. M. Fu, R. K. Evans, M. E. Davies, D. C. Freed, W. Hurni, J. M. Aste-Amezaga, L. Guan, R. Long, L. Huang, V. Harris, D. K. Nawrocki, H. Mach, R. D. Troutman, L. A. Isopi, K. K. Murthy, K. Rice, K. A. Wilson, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Vaccine-induced immunity in baboons by using DNA and replication-incompetent adenovirus type 5 vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 777663-7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman, B. S., R. M. Thayer, K. A. Vincent, and N. L. Haigwood. 1991. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 193979-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiuchiolo, M. J., J. L. Boyer, A. Krause, S. Senina, N. R. Hackett, and R. G. Crystal. 2006. Protective immunity against respiratory tract challenge with Yersinia pestis in mice immunized with an adenovirus-based vaccine vector expressing V antigen. J. Infect. Dis. 1941249-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clyde, D. F., H. Most, V. C. McCarthy, and J. P. Vanderberg. 1973. Immunization of man against sporozite-induced falciparum malaria. Am. J. Med. Sci. 266169-177. [DOI] [PubMed] [Google Scholar]
- 21.Cohen, C. J., Z. Q. Xiang, G. P. Gao, H. C. Ertl, J. M. Wilson, and J. M. Bergelson. 2002. Chimpanzee adenovirus CV-68 adapted as a gene delivery vector interacts with the coxsackievirus and adenovirus receptor. J. Gen. Virol. 83151-155. [DOI] [PubMed] [Google Scholar]
- 22.Denis, O., A. Tanghe, K. Palfliet, F. Jurion, T. P. van den Berg, A. Vanonckelen, J. Ooms, E. Saman, J. B. Ulmer, J. Content, and K. Huygen. 1998. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect. Immun. 661527-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fessler, S. P., F. Delgado-Lopez, and M. S. Horwitz. 2004. Mechanisms of E3 modulation of immune and inflammatory responses. Curr. Top. Microbiol. Immunol. 273113-135. [DOI] [PubMed] [Google Scholar]
- 24.Flanagan, K. L., E. A. Lee, M. B. Gravenor, W. H. Reece, B. C. Urban, T. Doherty, K. A. Bojang, M. Pinder, A. V. Hill, and M. Plebanski. 2001. Unique T cell effector functions elicited by Plasmodium falciparum epitopes in malaria-exposed Africans tested by three T cell assays. J. Immunol. 1674729-4737. [DOI] [PubMed] [Google Scholar]
- 25.Flanagan, K. L., T. Mwangi, M. Plebanski, K. Odhiambo, A. Ross, E. Sheu, M. Kortok, B. Lowe, K. Marsh, and A. V. Hill. 2003. Ex vivo interferon-gamma immune response to thrombospondin-related adhesive protein in coastal Kenyans: longevity and risk of Plasmodium falciparum infection. Am. J. Trop. Med. Hyg. 68421-430. [PubMed] [Google Scholar]
- 26.Foecking, M. K., and H. Hofstetter. 1986. Powerful and versatile enhancer-promoter unit for mammalian expression vectors. Gene 45101-105. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert, S. C., M. Plebanski, S. J. Harris, C. E. Allsopp, R. Thomas, G. T. Layton, and A. V. Hill. 1997. A protein particle vaccine containing multiple malaria epitopes. Nat. Biotechnol. 151280-1284. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert, S. C., J. Schneider, C. M. Hannan, J. T. Hu, M. Plebanski, R. Sinden, and A. V. Hill. 2002. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine 201039-1045. [DOI] [PubMed] [Google Scholar]
- 29.Good, M. F., and D. L. Doolan. 1999. Immune effector mechanisms in malaria. Curr. Opin. Immunol. 11412-419. [DOI] [PubMed] [Google Scholar]
- 30.Hensley, S. E., W. Giles-Davis, K. C. McCoy, W. Weninger, and H. C. Ertl. 2005. Dendritic cell maturation, but not CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J. Immunol. 1756032-6041. [DOI] [PubMed] [Google Scholar]
- 31.Huang, M. T., and C. M. Gorman. 1990. Intervening sequences increase efficiency of RNA 3′ processing and accumulation of cytoplasmic RNA. Nucleic Acids Res. 18937-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobinger, G. P., H. Feldmann, Y. Zhi, G. Schumer, G. Gao, F. Feldmann, S. Jones, and J. M. Wilson. 2006. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology 346394-401. [DOI] [PubMed] [Google Scholar]
- 33.Kumar, K. A., G. Sano, S. Boscardin, R. S. Nussenzweig, M. C. Nussenzweig, F. Zavala, and V. Nussenzweig. 2006. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature 444937-940. [DOI] [PubMed] [Google Scholar]
- 34.Lemckert, A. A., S. M. Sumida, L. Holterman, R. Vogels, D. M. Truitt, D. M. Lynch, A. Nanda, B. A. Ewald, D. A. Gorgone, M. A. Lifton, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2005. Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-Ad5 immunity. J. Virol. 799694-9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maizel, J. V., Jr., D. O. White, and M. D. Scharff. 1968. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 36115-125. [DOI] [PubMed] [Google Scholar]
- 36.McConkey, S. J., W. H. Reece, V. S. Moorthy, D. Webster, S. Dunachie, G. Butcher, J. M. Vuola, T. J. Blanchard, P. Gothard, K. Watkins, C. M. Hannan, S. Everaere, K. Brown, K. E. Kester, J. Cummings, J. Williams, D. G. Heppner, A. Pathan, K. Flanagan, N. Arulanantham, M. T. Roberts, M. Roy, G. L. Smith, J. Schneider, T. Peto, R. E. Sinden, S. C. Gilbert, and A. V. Hill. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 9729-735. [DOI] [PubMed] [Google Scholar]
- 37.Moore, A. C., A. Gallimore, S. J. Draper, K. R. Watkins, S. C. Gilbert, and A. V. Hill. 2005. Anti-CD25 antibody enhancement of vaccine-induced immunogenicity: increased durable cellular immunity with reduced immunodominance. J. Immunol. 1757264-7273. [DOI] [PubMed] [Google Scholar]
- 38.Moorthy, V. S., E. B. Imoukhuede, S. Keating, M. Pinder, D. Webster, M. A. Skinner, S. C. Gilbert, G. Walraven, and A. V. Hill. 2004. Phase 1 evaluation of 3 highly immunogenic prime-boost regimens, including a 12-month reboosting vaccination, for malaria vaccination in Gambian men. J. Infect. Dis. 1892213-2219. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen, K. B., W. T. Watford, R. Salomon, S. R. Hofmann, G. C. Pien, A. Morinobu, M. Gadina, J. J. O'Shea, and C. A. Biron. 2002. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science 2972063-2066. [DOI] [PubMed] [Google Scholar]
- 40.Nussenzweig, R. S., J. Vanderberg, H. Most, and C. Orton. 1967. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature 216160-162. [DOI] [PubMed] [Google Scholar]
- 41.Ophorst, O. J., K. Radosevic, M. J. Havenga, M. G. Pau, L. Holterman, B. Berkhout, J. Goudsmit, and M. Tsuji. 2006. Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice. Infect. Immun. 74313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otten, G. R., M. Schaefer, B. Doe, H. Liu, I. Srivastava, J. Megede, J. Kazzaz, Y. Lian, M. Singh, M. Ugozzoli, D. Montefiori, M. Lewis, D. A. Driver, T. Dubensky, J. M. Polo, J. Donnelly, D. T. O'Hagan, S. Barnett, and J. B. Ulmer. 2005. Enhanced potency of plasmid DNA microparticle human immunodeficiency virus vaccines in rhesus macaques by using a priming-boosting regimen with recombinant proteins. J. Virol. 798189-8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto, A. R., J. C. Fitzgerald, G. P. Gao, J. M. Wilson, and H. C. Ertl. 2004. Induction of CD8+ T cells to an HIV-1 antigen upon oral immunization of mice with a simian E1-deleted adenoviral vector. Vaccine 22697-703. [DOI] [PubMed] [Google Scholar]
- 44.Reyes-Sandoval, A., J. C. Fitzgerald, R. Grant, S. Roy, Z. Q. Xiang, Y. Li, G. P. Gao, J. M. Wilson, and H. C. Ertl. 2004. Human immunodeficiency virus type 1-specific immune responses in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes. J. Virol. 787392-7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodrigues, E. G., F. Zavala, D. Eichinger, J. M. Wilson, and M. Tsuji. 1997. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J. Immunol. 1581268-1274. [PubMed] [Google Scholar]
- 46.Roy, S., G. Gao, D. S. Clawson, L. H. Vandenberghe, S. F. Farina, and J. M. Wilson. 2004. Complete nucleotide sequences and genome organization of four chimpanzee adenoviruses. Virology 324361-372. [DOI] [PubMed] [Google Scholar]
- 47.Santra, S., M. S. Seaman, L. Xu, D. H. Barouch, C. I. Lord, M. A. Lifton, D. A. Gorgone, K. R. Beaudry, K. Svehla, B. Welcher, B. K. Chakrabarti, Y. Huang, Z. Y. Yang, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2005. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J. Virol. 796516-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4397-402. [DOI] [PubMed] [Google Scholar]
- 49.Schneider, J., J. A. Langermans, S. C. Gilbert, T. J. Blanchard, S. Twigg, S. Naitza, C. M. Hannan, M. Aidoo, A. Crisanti, K. J. Robson, G. L. Smith, A. V. Hill, and A. W. Thomas. 2001. A prime-boost immunisation regimen using DNA followed by recombinant modified vaccinia virus Ankara induces strong cellular immune responses against the Plasmodium falciparum TRAP antigen in chimpanzees. Vaccine 194595-4602. [DOI] [PubMed] [Google Scholar]
- 50.Schofield, L., J. Villaquiran, A. Ferreira, H. Schellekens, R. Nussenzweig, and V. Nussenzweig. 1987. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330664-666. [DOI] [PubMed] [Google Scholar]
- 51.Sedegah, M., T. R. Jones, M. Kaur, R. Hedstrom, P. Hobart, J. A. Tine, and S. L. Hoffman. 1998. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 957648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sekaly, R. P. 2008. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 2057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skinner, M. A., B. M. Buddle, D. N. Wedlock, D. Keen, G. W. de Lisle, R. E. Tascon, J. C. Ferraz, D. B. Lowrie, P. J. Cockle, H. M. Vordermeier, and R. G. Hewinson. 2003. A DNA prime-Mycobacterium bovis BCG boost vaccination strategy for cattle induces protection against bovine tuberculosis. Infect. Immun. 714901-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tatsis, N., and H. C. Ertl. 2004. Adenoviruses as vaccine vectors. Mol. Ther. 10616-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tatsis, N., L. Tesema, E. R. Robinson, W. Giles-Davis, K. McCoy, G. P. Gao, J. M. Wilson, and H. C. Ertl. 2006. Chimpanzee-origin adenovirus vectors as vaccine carriers. Gene Ther. 13421-429. [DOI] [PubMed] [Google Scholar]
- 57.Webster, D. P., S. Dunachie, J. M. Vuola, T. Berthoud, S. Keating, S. M. Laidlaw, S. J. McConkey, I. Poulton, L. Andrews, R. F. Andersen, P. Bejon, G. Butcher, R. Sinden, M. A. Skinner, S. C. Gilbert, and A. V. Hill. 2005. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc. Natl. Acad. Sci. USA 1024836-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4225-234. [DOI] [PubMed] [Google Scholar]
- 59.Wipasa, J., C. Hirunpetcharat, Y. Mahakunkijcharoen, H. Xu, S. Elliott, and M. F. Good. 2002. Identification of T cell epitopes on the 33-kDa fragment of Plasmodium yoelii merozoite surface protein 1 and their antibody-independent protective role in immunity to blood stage malaria. J. Immunol. 169944-951. [DOI] [PubMed] [Google Scholar]
- 60.Xiang, Z., G. Gao, A. Reyes-Sandoval, C. J. Cohen, Y. Li, J. M. Bergelson, J. M. Wilson, and H. C. Ertl. 2002. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 762667-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiang, Z., Y. Li, A. Cun, W. Yang, S. Ellenberg, W. M. Switzer, M. L. Kalish, and H. C. Ertl. 2006. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 121596-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiang, Z. Q., Y. Yang, J. M. Wilson, and H. C. Ertl. 1996. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 219220-227. [DOI] [PubMed] [Google Scholar]
- 63.Zhi, Y., J. Figueredo, G. P. Kobinger, H. Hagan, R. Calcedo, J. R. Miller, G. Gao, and J. M. Wilson. 2006. Efficacy of severe acute respiratory syndrome vaccine based on a nonhuman primate adenovirus in the presence of immunity against human adenovirus. Hum. Gene Ther. 17500-506. [DOI] [PubMed] [Google Scholar]
- 64.Zinkernagel, R. M., M. F. Bachmann, T. M. Kundig, S. Oehen, H. Pirchet, and H. Hengartner. 1996. On immunological memory. Annu. Rev. Immunol. 14333-367. [DOI] [PubMed] [Google Scholar]