Abstract
Human immunodeficiency virus type 1 (HIV-1) infection has been associated with perturbations of plasmacytoid dendritic cells (PDC), including diminished frequencies in the peripheral blood and reduced production of type I interferons (IFNs) in response to in vitro stimulation. However, recent data suggest a paradoxical increase in production of type 1 interferons in vivo in HIV-infected patients compared to uninfected controls. Using a flow cytometric assay to detect IFN-α-producing cells within unseparated peripheral blood mononuclear cells, we observed that short-term interruptions of antiretroviral therapy are sufficient to result in significantly reduced IFN-α production by PDC in vitro in response to CpG A ligands or inactivated HIV particles. The primary cause of diminished IFN-α production was reduced responsiveness of PDC to de novo stimulation, not diminished per cell IFN-α production or migration of cells to lymphoid organs. Real-time PCR analysis of purified PDC from patients prior to and during treatment interruptions revealed that active HIV-1 replication is associated with upregulation of type I IFN-stimulated gene expression. Treatment of hepatitis C virus-infected patients with IFN-α2b and ribavirin for hepatitis C virus infection resulted in a profound suppression of de novo IFN-α production in response to CpG A or inactivated HIV particles, similar to the response observed in HIV-infected patients. Together, these results suggest that diminished production of type I interferons in vitro by PDC from HIV-1-infected patients may not represent diminished interferon production in vivo. Rather, diminished function in vitro is likely a consequence of prior activation via type I interferons or HIV virions in vivo.
Immature plasmacytoid dendritic cells (PDC), also known as precursors of type 2 dendritic cells or natural interferon (IFN)-producing cells, are cells of the innate immune system that recognize pathogens via interactions between toll-like receptors (TLRs) and evolutionarily conserved bacterial and viral motifs (recently reviewed in references 2 and 49). Human PDC are defined as CD11c− CD123+ HLA-DR+ cells and, along with CD11c+ CD123− myeloid dendritic cells, represent the two major dendritic cell subsets present in the peripheral blood. PDC express TLR7, which recognizes single-stranded RNA, and TLR9, which recognizes unmethylated DNA, and respond to these stimuli by producing type I interferons and other proinflammatory cytokines and maturing into dendritic cells capable of stimulating adaptive immune responses (reviewed in reference 28). The endosomal localization of TLR7 and TLR9 in PDC is hypothesized to enable specific recognition of RNA and DNA motifs from endocytosed pathogens while avoiding triggering by cellular mRNA or DNA.
Type I interferon production by PDC has been demonstrated in response to a wide variety of viral stimuli. These include stimulation with herpes simplex virus types 1 and 2 (30, 38, 42), influenza A virus (12, 43), Sendai virus (23, 26), vesicular stomatitis virus (3, 43), and human immunodeficiency virus type 1 (HIV-1) (50, 65) in vitro. Following exposure to HIV-1 particles in vitro, PDC respond by producing type I interferons, upregulating expression of the costimulatory molecules CD80 and CD86 and the maturation marker CD83, and triggering the maturation of myeloid dendritic cells in a cytokine-dependent manner (18, 65). A recent study suggested that, in the case of HIV-1, PDC recognition is mediated by the binding of viral RNA by the TLR7 or -9 receptors (6).
HIV infection is known to have effects upon the frequency and function of peripheral blood PDC (recently reviewed in reference 39). A number of studies have reported that the frequency of PDC declines with continuing HIV-1 infection (4, 9, 13, 15, 16, 21, 44, 61, 63). Additionally, several reports suggest that the frequency of PDC correlates inversely with viral load and is at least partially restored with antiretroviral therapy (4, 15). Some changes in peripheral blood PDC frequencies have also been ascribed to trafficking to lymph nodes during ongoing HIV replication. PDC isolated from HIV-1-infected patients also demonstrate functional abnormalities, including diminished production of IFN-α when exposed to viruses, including HIV-1 and herpes simplex virus in vitro (17, 41), and these effects have since been ascribed to the functional defects of PDC (15, 16, 27). Control of HIV viral replication with effective antiretroviral therapy during early or chronic infection has been reported to partially restore the ability of PDC to respond to viruses by producing IFN-α (33, 34, 59). Together these results suggest that, during HIV infection, there are diminished frequencies and functions of PDC observed upon restimulation in vitro that are only partially recovered during antiretroviral therapy.
Although there is evidence for diminished PDC frequencies and functions during HIV infection, there are several very recent lines of evidence suggesting increased production of IFN-α in vivo. Patients with early infection and advanced HIV disease have been reported to have elevations in serum IFN-α levels despite the diminished ability of PDC to produce IFN-α following stimulation (reviewed in reference 19). IFN-α transcripts have been shown to be elevated in the lymphoid tissue of progressor patients compared to nonprogressor patients (24). In addition, IFN-α-induced genes have been found to be upregulated in cells from HIV-infected patients in peripheral blood mononuclear cells (PBMC), B cells, NK cells, monocytes, and CD4+ and CD8+ T cells (36, 51, 53; R. Lempicki and S. Migueles, personal communication). Each of these observations suggests that PDC function, at least with regard to IFN-α production, is not diminished but rather is very active during HIV infection. Distinguishing between these possibilities is important, given some recent data suggesting IFN-α may play a central role in the aberrant immune activation and progression of disease (25).
In the present study, we examined the effect of HIV replication and exogenous IFN-α stimulation on the ability of PDC to produce IFN-α in response to TLR ligands and viral particles. In this study, we used a flow cytometric assay to measure IFN-α production by PDC in PBMC cultures. Using this technique, reduced PDC production of IFN-α in response to CpG A and HIV-1ADA in patients off antiretroviral therapy was found to be due primarily to reduced responsiveness of PDC rather than due to changes in the frequency of PDC in the peripheral blood. A similar decrease in ex vivo IFN-α production was found in lymph node-derived cells from viremic patients, suggesting the effect was not attributable to migration of PDC to lymph nodes during periods of active HIV replication. The decreased responsiveness of peripheral blood PDC to TLR ligands and viral particles suggested TLR stimulation in vivo due to unrestricted HIV replication may directly or indirectly affect PDC function measured in vitro. Consistent with these data, real-time PCR analysis of gene transcripts in purified PDC demonstrated that unrestricted HIV replication was associated with elevated transcription of type I interferon-stimulated genes. Furthermore, patients with hepatitis C virus (HCV)-HIV coinfection who were treated with exogenous IFN-α2b and ribavirin demonstrated significantly diminished IFN-α production by PDC in response to CpG A and HIV-1ADA. Together, these results suggest that diminished production of IFN-α in vitro, during the early stages of HIV infection, is not due to diminished PDC numbers or trafficking to lymph nodes, but rather due to feedback inhibition or prior stimulation through TLRs in vivo. These results likely reconcile the seemingly conflicting evidence of IFN-α effects in vivo with diminished PDC function in vitro and provide a mechanism for these changes in function.
MATERIALS AND METHODS
Study population.
HIV-1 infection in study participants was documented by HIV-1/2 immunoassay. All subjects signed informed consent and participated in protocols approved by a National Institutes of Allergy and Infectious Diseases (NIAID) investigational review board. The treatment interruption cohort included 14 patients on antiretroviral therapy (minimum three-drug regimen) with CD4+ T-cell counts of >350 cells/μl within the 3 months prior to study, asymptomatic for significant HIV-related illness, and viral load of <50 copies/ml plasma for greater than 1 year who consented to a therapy interruption and leukopheresis at both on-therapy and off-therapy time points. The lymph node cohort included 14 additional patients with active HIV replication off of antiretroviral therapy who consented to simultaneous lymph node biopsy and peripheral blood draws. The HCV/HIV cohort included 26 coinfected patients who underwent blood draws prior to and 8 weeks into treatment with pegylated IFN-α2b (1.5 μg/kg of body weight/week) and ribavirin (1 to 1.2 g/day). Patients were eligible for the study if they had CD4+ T-cell counts of >100 cells/mm3, absolute neutrophil counts of >1,000 cells/mm3, an HCV viral load of >2,000 copies/ml, histologic evidence of chronic hepatitis C, and stable HIV disease with or without antiretroviral therapy. Patients with other causes of liver disease, advanced cirrhosis, or severe liver decompensation, severe cardiopulmonary or renal disorder, severe retinopathies, or who were receiving steroids or other immunosuppressive drugs were excluded. At initiation of treatment, 18 patients had undetectable HIV viral loads (<50 copies/ml plasma), while the remaining 8 patients had viral loads of 71, 125, 487, 699, 1,484, 5,459, 5,760, and 33,242 copies/ml. Median HCV viral load at initiation of therapy was 3,215,000 copies/ml (range, 2,111 to 22,300,000 copies/ml). The cohort used in the PDC sorting experiments consisted of 10 HIV-negative controls, 10 HIV-infected patients on antiretroviral therapy with a viral load of <50 copies HIV RNA/ml plasma, and 10 HIV-infected patients off antiretroviral therapy with a median viral load of 36,660 copies/ml plasma (range, 2,138 to 74,940 copies/ml). Exclusion criteria for each of the protocols included psychiatric illness, substance abuse, pregnancy, creatinine levels of >2, thrombocytopenia or coagulopathy, history of opportunistic infection or HIV-associated malignancy, or prior CD4 count of ≤150 cells/mm3. Serum type I interferons were measured using the high-sensitivity Biotrak assay (GE Healthcare, Piscataway, NJ) according to the manufacturer's protocols. Detailed information on the Biotrak assay can be found at http://www.gelifesciences.co.jp/tech_support/manual/pdf/cellasy/RPN2789.pdf.
Intracellular IFN-α assays.
PBMC were freshly isolated from peripheral blood or apheresis donor packs by sodium diatrizoate-Ficoll density centrifugation (ICN Biomedicals, Aurora, OH). PBMC were cryopreserved in cell culture freezing medium with dimethyl sulfoxide (Gibco, Grand Island, NY). Cryopreserved samples were maintained at −140°C. Cryopreserved PBMC or lymph node samples were thawed, washed twice with RPMI culture medium containing 10% human AB serum and (0.5 mg/ml) gentamicin, and aliquoted at 2 × 106 to 4 × 106 cells per stimulation tube. To determine the frequency of IFN-α-producing PDC, one of the following TLR ligands or viral particles was added: 5 μg/ml Staphylococcus aureus peptidoglycan, 25 μg/ml of a synthetic double-stranded RNA analog [poly(I·C)], 0.5 μg/ml lipopolysaccharide, 5 μg/ml flagellin, 0.5 μg/ml mycoplasma lipoprotein 2, 0.5 μg/ml single-stranded polyuridine (ssPolyU/LyoVec), 250 μM loxoribine, 2.5 μg/ml CpG containing ODN type A/2216 (ggG GGA CGA TCG Tcg ggg gg, where CpG motifs are underlined and phosphorothioate bases are shown in lowercase letters), 2.5 μg/ml control CpG ODN type A (ggG GGA GCA TGC Tgg ggg gc), 2.5 CpG ODN type B/2206 (tcg tcg ttt tgt cgt ttt gtc gtt), and 2.5 μg/ml control CpG ODN type B (tgc tgc ttt tgt gct ttt gtg ctt) (all from Invivogen, San Diego, CA), 1 μg/ml R848 (gift from K. Loré, Vaccine Research Center, NIAID, Bethesda, MD), 50 hemagglutinin units/ml of Sendai virus Cantell strain (Charles River Laboratories, North Franklin, CT), 250 ng p24CA equivalents/ml HIV-1ADA or HIV-1MN, or control microvesicles from SUPT1 or CEMX174(T1) cells at a similar total protein concentration (5 to 10 μg/ml; J. Lifson, SAIC-Frederick, Frederick, MD). Samples were placed in an incubator at 37°C and 5% CO2, and after 4 h of incubation, brefeldin A (Sigma Aldrich, St. Louis, MO) was added to the medium at a final concentration of 10 μg/ml to inhibit cytokine secretion. After four additional hours of incubation, the cells were washed twice and stained with anti-CD3, anti-CD16, anti-CD19, and anti-CD56 conjugated with Alexa 700, anti-CD14 with allophycocyanin-Cy7, and anti-HLA-DR with Pacific Blue or phycoerythrin (PE)-Cy7 (Becton Dickinson, San Jose, CA). Cells were washed twice and fixed in 2% paraformaldehyde (Sigma Aldrich). The cells were washed, permeabilized, and blocked overnight in a buffer containing saponin (Sigma Aldrich) and 5% milk. Following permeabilization, cells were stained with anti-IFN-α antibody (PBL Biomedical Labs, Piscataway, NJ) labeled with the Zenon Alexa 488 anti-human immunoglobulin G1 labeling kit (Invitrogen, Carlsbad, CA). Cells were washed and stained with anti-CD123-PE and anti-CD11c-PE-Cy5 (Becton Dickinson) prior to data collection. Data were collected with a FACSAria three-laser cytometer (Becton Dickinson). Between 70,000 and 1,000,000 CD3+ CD4+ events were collected and analyzed using FlowJo software (TreeStar, San Carlos, CA).
Sorting and real-time PCR of PDC.
Purified PDC were isolated from PBMC samples by thawing cells, staining with anti-CD304 beads (Miltenyi Biotech, Bergish Gladbach, Germany), and sorting on an AutoMACS cell separator (Miltenyi Biotech) using the Possel S setting. Cells were then washed, stained with anti-CD123-PE, anti-CD11c-PE-Cy5, and allophycocyanin-Cy7-HLA-DR (Beckton Dickinson), washed again, and sorted for lineage-negative (lin−) CD123+ CD11c− cells on a FACSAria cell sorter. Sort purities were typically >90 to 95% lin− CD123+ CD11c− HLA-DR+ cells. Sorted PDC were washed, resuspended in RNAlater (Ambion, Austin, TX), and frozen at −80°C. Measurements of changes in gene transcription of PDC were performed using TLR and IFN-α and -β signaling real-time PCR arrays according to the manufacturer's protocols (SuperArray Biosciences, Frederick, MD).
Statistical analysis.
The Wilcoxon signed rank test was used to compare paired data. Independent groups were compared by the Wilcoxon two-sample test. Correlation was determined by the Spearman rank method. The Bonferroni method was used to adjust P values for multiple testing. Medians are reported.
RESULTS
Flow cytometric detection of plasmacytoid dendritic cell IFN-α production in response to TLR ligands and viruses.
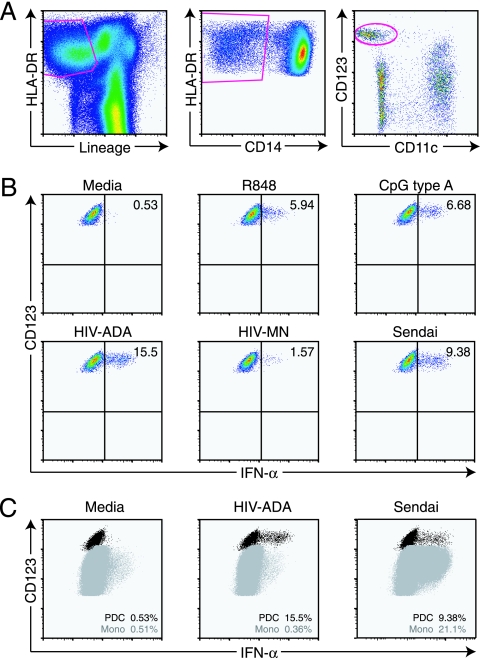
In most untreated patients, the effects of HIV infection on peripheral blood PDC have been characterized by indirect evidence of IFN-α production in vivo but diminished frequency and function in vitro. Most prior work in this area was performed using sorting techniques and an IFN-α enzyme-linked immunosorbent assay to measure PDC function. Although these studies have given considerable insight regarding PDC function during HIV infection, they were limited by the difficulty in measuring IFN-α production on a per cell basis, sorting of extremely low numbers of these cells during HIV infection and correcting for cell number, and the possibility that IFN-α was produced by contaminating subsets (54). In order to overcome these limitations, we have adapted a flow cytometric technique to identify PDC and their production of IFN-α in response to TLR ligands and viruses without separating them from PBMC (10). Using this assay, PDC are readily identifiable as lineage− (CD3− CD8− CD16 CD19− CD56−), HLA-DR+ CD14− CD11c− CD123+ cells (Fig. 1A). IFN-α production by PDC was observed in response to the TLR7/8 ligands loxoribine and R848 as well as to the TLR9 ligands CpG A (ODN 2216) and, to a lesser extent, CpG B (ODN 2006), consistent with previous reports that PDC express TLR7 and TLR9 (Fig. 1B) (reviewed in reference 28). IFN-α was not produced by PDC in response to stimulation with ligands that are recognized by other TLRs (data not shown). Stimulation of PDC with viral particles, including 2-aldithriol-inactivated HIV particles (HIV-1ADA-M and HIV-1MN) and Sendai virus also led to detectable IFN-α production (Fig. 1B). These data suggest that the flow cytometric assay is capable of specifically detecting IFN-α production by PDC within PBMC cultures, enabling direct analysis of the frequency of IFN-α-producing PDC and providing a measure of their relative production of IFN-α through mean fluorescent intensity. An additional advantage of this assay is the ability to detect IFN-α production by multiple cell types, for instance, the simultaneous production of IFN-α by PDC and monocytes in response to Sendai virus (Fig. 1C).
FIG. 1.
Flow cytometric assay for production of IFN-α in unfractionated peripheral blood mononuclear cells. A) The gating strategy for PDC consisted of HLA-DR+ cells that were lineage− (CD3− CD8− CD16− CD19− CD56−), CD14− CD11c− CD123+. B) Production of IFN-α by PDC in response to medium or stimulation with TLR agonists (R848 and CpG) or viral particles (HIVADA, HIVMN, and Sendai virus). C) Detection of IFN-α produced by multiple cell types in response to medium, HIVADA, or Sendai virus.
HIV-infected patients undergoing treatment interruption demonstrate rapid loss of ex vivo PDC production of IFN-α in response to viruses and TLR ligands.
Unrestricted HIV replication has been associated with diminished frequencies of PDC in the peripheral blood (63) and with diminished ex vivo production of IFN-α in response to viruses, including HIV-1 and herpes simplex virus (17). IFN-α production by PBMC in response to viral antigens partially recovers in patients receiving effective antiretroviral therapy (61). Although diminished PDC production of IFN-α in response to viral particles has been described in patients with HIV infection, it remained unclear whether this is predominantly due to reduced total numbers of PDC in the peripheral blood, reduced responsiveness of PDC to viral stimulation, or diminished IFN-α production by stimulated PDC on a per cell basis or some combination.
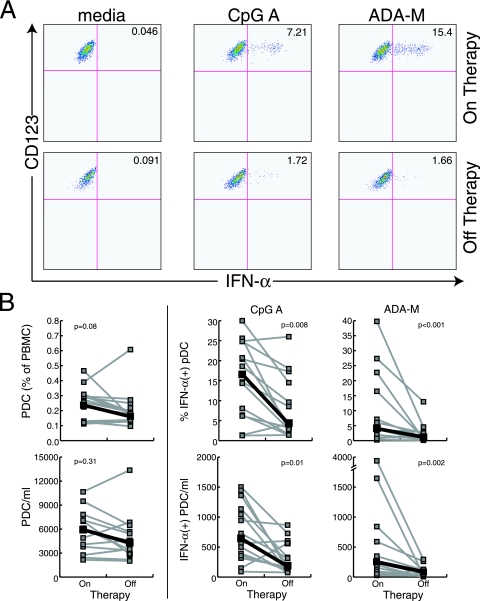
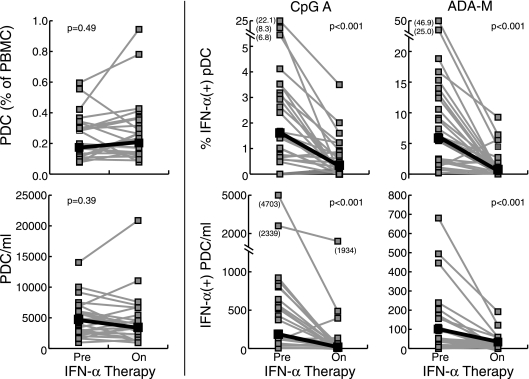
In a cohort of 14 patients undergoing treatment interruption, we measured the frequencies and production of IFN-α by PDC utilizing the flow cytometric assay described above. In patients on therapy, 0.26% of peripheral blood cells were lin− CD14− HLA-DR+ CD11c− CD123+ PDC, while in patients off therapy 0.18% of PBMC were PDC (P = 0.16) (Fig. 2). Similarly, there was no statistically significant change in the absolute numbers of PDC in the peripheral blood during an interruption of therapy. Previous studies had demonstrated an inverse correlation between viral load and the frequency of PDC in the peripheral blood, but the short duration of treatment interruption in this cohort (median time off therapy, 28 days) may explain why no significant differences in PDC frequencies were observed. In contrast, patients off antiretroviral therapy had significantly reduced percentages of PDC that responded to CpG A (16.2% of PDC produced IFN-α on therapy, versus 4.0% off therapy; P = 0.008) and to HIV-1ADA (5.2% versus 1.5%; P < 0.001) (Fig. 2). Finally, the average PDC production of IFN-α per cell, as measured by mean fluorescence intensity, was higher in patients on antiretroviral therapy, although this did not reach statistical significance (CpG A mean fluorescence intensity, 5,112 on therapy versus 3,635 off therapy [P = 0.14]; HIV-1ADA mean fluorescence intensity, 3,087 versus 2,133 [P = 0.07]). These data suggest that HIV viremia alone has a very dramatic effect on in vitro production of IFN-α by PDC. Although diminished frequencies of PDC may play a role in reduction of IFN-α production in late-stage disease, the observed decrease in ex vivo PDC production of IFN-α in response to CpG A and HIV-1ADA in HIV-infected patients with even brief periods of viremia is primarily the result of a decrease in the percentages of PDC producing IFN-α in response to stimulation.
FIG. 2.
Plasmacytoid dendritic cell production of IFN-α in response to CpG A and HIVADA particles is diminished in patients with active HIV replication. A) Representative flow cytometric data from a patient undergoing treatment interruption. B) Summary data of PDC frequency and absolute numbers (left panel) or responsiveness to CpG type A and HIVADA (right panels) in 14 patients undergoing structured treatment interruptions. PDC production of IFN-α in response to stimulation with TLR ligands or viral particles was significantly decreased in patients with active HIV replication.
Diminished ex vivo PDC production of IFN-α is not explained by migration of cells to lymph nodes.
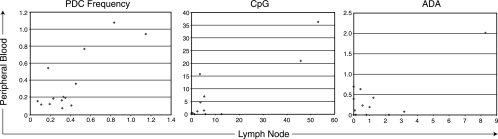
Although no significant difference in the frequency of PDC in the peripheral blood of patients on or off antiretroviral therapy was observed, it remained possible that a small subset of activated IFN-α-producing PDC might be migrating to secondary lymphoid tissues during active HIV replication and that this migration could underlie the diminished IFN-α production in peripheral blood PDC restimulated ex vivo. This possibility was investigated in 14 HIV-infected patients with active viral replication (median viral load, 20,645 copies/ml of plasma; range, 7,511 to 170,300) who underwent simultaneous peripheral blood draws and lymph node biopsies. The frequencies of PDC in the peripheral blood and lymph nodes were similar in this cohort (ranges, 0.07 to 1.08% and 0.08 to 1.15%, respectively; P > 0.5). There was a trend toward a positive correlation between the frequency of PDC in the peripheral blood and lymph nodes; however, this was only of borderline significance (r = 0.58; P = 0.09). The lack of an inverse association suggested that the decreased frequency of PDC in the peripheral blood was not due to accumulation in lymph nodes. Further, the percentages of PDC producing IFN-α in response to CpG A or HIV-1ADA were also not correlated between lymph node and peripheral blood samples (Fig. 3) (P = 0.28 and P > 0.5, respectively). These data are indirect in that they are cross-sectional for a separate group of patients, and we did not observe enrichment of a large population of IFN-α-producing cells in the lymph nodes of viremic patients. Rather, the frequency of PDC producing IFN-α upon ex vivo stimulation was similar between blood and lymph nodes. These data suggest that selective migration of IFN-α-producing PDC from peripheral blood to lymph nodes does not appear to be a primary mechanism for the diminished ex vivo production of IFN-α in the peripheral blood in patients off antiretroviral therapy.
FIG. 3.
Correlation between PDC frequency and IFN-α production in response to CpG type A and HIVADA in matched peripheral blood and lymph node samples. A positive association between the total frequency of PDC in PBMC or lymph node samples was observed, although this did not reach statistical significance (r = 0.58; P = 0.09). The percentages of PDC producing IFN-α in response to CpG type A and HIVADA were not correlated between lymph node and peripheral blood samples.
PDC from patients off antiretroviral therapy have increased transcription of interferon-stimulated genes.
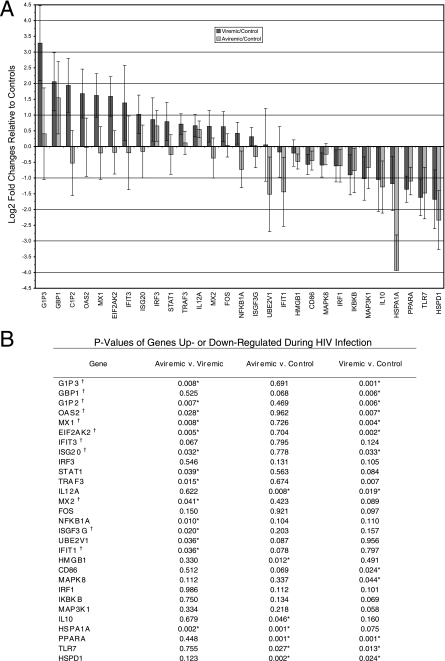
The observation that HIV-infected patients off therapy have diminished responsiveness of PDC to CpG A and HIV-1ADA stimulation suggested that unrestricted HIV-1 replication might alter the function of PDC observed upon restimulation. This might occur through prior activation through TLRs or feedback inhibition by IFN-α produced in vivo. To test this hypothesis, PDC from HIV-infected patients on effective antiretroviral therapy (n = 10), off antiretroviral therapy (n = 10), and from HIV-negative controls (n = 10) were purified based on CD304 (BDCA-4) magnetic bead enrichment followed by sorting of CD123+ CD11c− cells. Purity of the isolated PDC populations was determined by flow cytometry to be >90 to 95%. Purified PDC populations were analyzed by real-time reverse transcription-PCR assay for expression of interferon-stimulated genes as well as genes involved in the type I interferon and TLR pathways. PDC isolated from patients with active HIV replication had significantly increased expression of approximately 25 genes compared with PDC from patients on effective antiretroviral therapy or normal controls. A large number of these genes are well-characterized interferon-stimulated genes, including MX1, MX2, OAS2, IFIT3, GBP1, G1P2, G1P3, ISGF3G, and ISG20 (Fig. 4). The five genes with the largest increases in expression in patients off therapy were all interferon-stimulated genes. Additionally, changes in genes involved in TLR pathways and interferon signaling were also identified, including upregulation of STAT1 and STAT2 in patients off antiretroviral therapy. Expression of TLR7, a receptor believed to be involved in type I IFN production by PDC in response to HIV-1 particles, was diminished in patients off therapy compared to normal controls but was similarly down-regulated in HIV-infected patients on antiretroviral therapy. No significant effects on levels of transcripts of TLR9 or IRF7 were observed.
FIG. 4.
Quantitative PCR of purified PDC demonstrates an increase in type I interferon-stimulated gene transcription in patients with active HIV replication. A) Changes in genes up- or down-regulated in HIV-positive patients compared with normal controls. Data from patients with active HIV replication are represented by dark gray bars; data from patients with control of HIV replication due to antiretroviral therapy are shown by light gray bars. B) P values of genes up- or down-regulated during HIV infection and with active HIV replication. †, gene known to be stimulated by type I interferons; *, statistically significant at the 0.05 level.
Serum samples from patients on and off therapy were analyzed for levels of type I IFN. Levels of serum IFN were detectable in both groups of patients and were not significantly different. However, it should be noted there are 13 known subtypes of IFN-α, as well as IFN-β and several type I IFN-like cytokines, that can stimulate interferon-responsive genes. The currently available serum assays only measure a subset of these cytokines. As a result, it is likely that expression of type I interferon-stimulated genes, rather than the level of plasma interferons, remains the best indicator of the level of activation of the type I interferon pathway in vivo.
Ex vivo PDC production of IFN-α is diminished by long-term IFN-α treatment in vivo.
The observation that PDC from patients off antiretroviral therapy had increased transcription of type I interferon-stimulated genes suggested that signaling through the IFN-α/β pathway might affect the ability of PDC to respond to subsequent stimulation with TLR ligands. The effects of short-term and long-term treatment with IFN-α on the ability of PDC to produce new IFN-α in response to CpG A and HIV-1ADA were examined. A 2-h preincubation of PBMC with recombinant IFN-α2A was found to augment the ability of PDC to produce IFN-α in response to CpG A and HIV-1ADA (data not shown). Although short-term treatment with IFN-α2A increased IFN-α production upon restimulation, PDC in HIV-infected patients are likely chronically exposed to high levels of IFN-α. To test the effects of long-term IFN-α stimulation in vivo, we examined IFN-α production upon restimulation in 26 patients receiving pegylated IFN-α2b and ribavirin for the treatment of chronic HCV infection. These patients, who had been receiving 1.5 μg/kg pegylated IFN-α2b per week and 1 to 1.2 g ribavirin per day for 8 weeks, demonstrated a significant reduction in the frequency of PDC producing IFN-α in response to CpG A and HIV-1ADA stimulation while on IFN-α and ribavirin therapy (CpG A, 6.12% IFN-α+ PDC off IFN-α and ribavirin therapy versus 0.54% on IFN-α and ribavirin therapy [P < 0.001]; HIV-1ADA, 1.63% off therapy versus 0.32% on therapy [P < 0.001]) (Fig. 5). No statistically significant changes in PDC frequency or absolute number were observed. Given IFN-α and ribavirin are standard care for such patients, we were unable to perform studies in patients receiving IFN-α alone. Thus, we are unable to formally exclude a possible effect of ribavirin in these experiments. However, these data do suggest that long-term stimulation of the type I interferon pathway, as is seen in PDC of HIV-infected patients with unrestricted viral replication, may diminish the ability of PDC to produce new IFN-α upon subsequent stimulation.
FIG. 5.
PDC production of IFN-α in response to HIVADA or CpG type A stimulation is significantly reduced during long-term treatment with exogenous IFN-α2A. PDC frequency and absolute numbers (left panel) or response to CpG or ADA (right panel) are shown. Data are from patients with hepatitis C virus infection that were treated with IFN-α2A and ribavirin. PBMC samples were collected prior to the initiation of therapy and at week 8 of therapy, and PDC production of IFN-α was measured with the flow cytometric assay described in the legend for Fig. 1.
DISCUSSION
The data from the present study, taken in the context of previous reports, provide considerable additional insight into the function of PDC during HIV infection. HIV infection and disease progression have been associated with a decrease in the frequency and function of PDC in the peripheral blood (4, 9, 14, 63) Consistent with these observations, diminished IFN-α-dependent maturation of myeloid dendritic cells has been observed in viremic patients (31). This maturation can be reconstituted by the addition of exogenous IFN-α in vitro. In addition, asymptomatic long-term nonprogressors maintain high frequencies of circulating PDC and produce large amounts of type I interferons upon stimulation ex vivo (63). These data have suggested that diminished PDC function represents a defective function that is preserved in long-term nonprogressors. Somewhat paradoxically, more recent expression array and in situ hybridization data have suggested that IFN-α production, most likely from PDC, is high in vivo in HIV-infected progressors, suggesting this PDC function is intact (24, 64). However, the results of the present study may help to reconcile these seemingly contradictory data. The flow cytometric assay utilized in this study allowed the visualization of IFN-α production by PDC or other cells in PBMC samples in response to CpG A ligands and HIV particles without the need to isolate or culture PDC in vitro. Utilizing this assay system, we found that a significantly lower percentage of PDC respond to CpG A ligands and HIV particles in patients with active HIV replication than in uninfected controls. Although HIV disease has been well documented to result in lower circulating levels of PDC, the short duration of treatment interruption in this study did not reveal significant differences in PDC frequency in patients off therapy. These data suggest that active HIV replication rapidly reduces the responsiveness of PDC to subsequent stimulation by CpG A and HIV particles. Analysis of PDC frequencies and functions within lymph nodes from patients off antiretroviral therapy were not inversely correlated with matched peripheral blood samples, suggesting that selective trafficking of functional PDC to lymph nodes does not explain the reduced responsiveness observed in the peripheral blood. Real-time PCR analysis of purified PDC revealed that interferon-stimulated gene expression was significantly higher in patients off antiretroviral therapy. To determine the effects of interferon stimulation upon PDC function, we investigated the effects of short-term and long-term exposure to interferons. Short-term exposure to exogenous IFN-α2A significantly enhanced the expression of type I interferons by PDC in response to CpG and HIV particles. In contrast, the long-term exposure to pegylated IFN-α2b in patients receiving treatment for chronic hepatitis C virus infection resulted in significant reductions in the ability of PDC to respond to these ligands without changing the circulating frequency of PDC, a finding similar to that seen in patients with active HIV replication. It should be noted that we were unable to exclude a potential role of ribavirin in the effects observed in PDC function in hepatitis C virus-infected patients. However, taken together, these data suggest that chronic stimulation of PDC with IFN-α in vivo may lead to hyporesponsiveness of these cells upon exposure to CpG A or HIV particles. These data also suggest that although advanced disease may be associated with declines in PDC frequencies, many of the differences in function observed between infected patients with high-level viral replication and nonprogressors or treated patients are likely a consequence of viremia and not a defect in IFN-α production in vivo.
These data also provide some insight regarding the mechanism of the diminished ex vivo production of IFN-α observed during HIV infection. A number of IFN-α-inducible genes were observed to be upregulated during high-level viremia, suggesting that diminished ex vivo IFN-α production was a consequence of feedback inhibition. Consistent with this hypothesis, in hepatitis C virus-infected patients, exogenous IFN-α and ribavirin in vivo were sufficient to cause diminished production of IFN-α upon stimulation in vitro under these experimental conditions. As outlined above, and consistent with the array data presented here, several lines of evidence indicate that there are increases in IFN-α production in vivo during active HIV replication. Thus, the diminished IFN-α production observed upon ex vivo restimulation might potentially be caused by stimulation through TLRs of a small subpopulation of PDC, resulting in paracrine feedback inhibition. However, the real-time PCR data also suggest that direct, ongoing stimulation through TLRs in vivo may contribute to diminished IFN-α production. Increased expression levels of Stat1, IFIT3, and NFKB1A in the off-therapy group compared to on-therapy time points suggest ongoing stimulation through TLRs by HIV RNA. The pattern of expression of these genes is similar to those observed within splenocytes in vitro or in animal models of chronic stimulation through TLR9 by CPG (35). Some of these, such as NFKB1A, have counter-regulatory activity that may contribute to diminished production of IFN-α upon restimulation. Together, these results suggest that the refractory state of PDC from viremic patients may be due to a combination of feedback inhibition and prior direct activation through TLRs by HIV RNA.
It is becoming increasingly clear that immune activation plays a central role in the pathogenesis of HIV disease and is the sum total of many direct and indirect effects of ongoing viral replication. Viral replication has been demonstrated to lead to a rapid turnover of CD4+ and CD8+ T-cell populations (37, 52) and with diminished interleukin-2 (IL-2) production by HIV-specific CD4+ T cells in vitro (22, 29, 55, 66). Additionally, B-cell and NK-cell subsets show signs of activation with abnormal skewing of their respective subsets (1, 45, 46, 48). Many subsets of immune cells in the peripheral blood and lymph nodes of HIV-infected patients demonstrate increased transcription of interferon-stimulated genes, suggesting activation by type I interferons is occurring in vivo. In addition to PDC, unrestricted HIV-1 replication has profound IFN-α-mediated effects on the functions of monocytes, NK cells, B cells, CD4+ T cells, and CD8+ T cells (36, 40, 48, 53, 64; S. Migueles, personal communication). Our laboratory has recently demonstrated that spontaneous monocyte production of the proinflammatory cytokines IL-1β, IL-6, and tumor necrosis factor alpha is reduced by active HIV-1 replication, and this effect correlated with elevated transcripts of type I interferon-stimulated genes in monocytes and was recapitulated by the addition of exogenous IFN-α2A.
There is some recent evidence that suggests these effects of IFN-α on other immune system cells may be deleterious. In many viral infections, type I interferons are thought to be beneficial to the host in that they exhibit antiviral properties both by activating cellular intrinsic defense mechanisms and by stimulating the immune system (reviewed in references 47 and 58). The latter mechanisms include upregulation of class I major histocompatibility complex molecules, maturation of monocytes and immature myeloid dendritic cells into mature, functional dendritic cells, increasing NK cell cytotoxicity, stimulation of antibody responses, and enhanced survival and proliferation of CD4+ and CD8+ cell responses (7, 8, 11, 32, 56). Type I interferons also have well-characterized antiviral effects on HIV replication in vitro (5, 20, 57, 60). However, in vivo they are increasingly thought to play a central role in the aberrant immune system activation that may drive HIV disease progression. This widespread immune activation may favor viral replication by a variety of mechanisms, including providing large numbers of activated target cells and by dampening the immune response. Consistent with this hypothesis, sooty mangabeys, which have been demonstrated to avoid progression to AIDS despite high viral loads, also have very low levels of systemic immune activation despite high-level viral replication (reviewed in reference 62). In contrast to rhesus macaques infected with simian immunodeficiency virus or humans infected with HIV, these natural hosts do not demonstrate IFN-α production to HIV particles in vitro, nor do they show signs of type I interferon-stimulated gene stimulation in vivo (35a). These findings raise the possibility that type I interferons play a central role in immune activation and disease progression in susceptible hosts. If this is found to be the case in humans, this may have important therapeutic implications for agents that block the effects of type I interferons.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIAID.
We thank Kristina Abel and Christopher J. Miller for their assistance with flow cytometry techniques and scientific conversations.
Footnotes
Published ahead of print on 6 February 2008.
REFERENCES
- 1.Alter, G., N. Teigen, B. T. Davis, M. M. Addo, T. J. Suscovich, M. T. Waring, H. Streeck, M. N. Johnston, K. D. Staller, M. T. Zaman, X. G. Yu, M. Lichterfeld, N. Basgoz, E. S. Rosenberg, and M. Altfeld. 2005. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 1063366-3369. [DOI] [PubMed] [Google Scholar]
- 2.Barchet, W., M. Cella, and M. Colonna. 2005. Plasmacytoid dendritic cells—virus experts of innate immunity. Semin. Immunol. 17253-261. [DOI] [PubMed] [Google Scholar]
- 3.Barchet, W., M. Cella, B. Odermatt, C. Asselin-Paturel, M. Colonna, and U. Kalinke. 2002. Virus-induced interferon alpha production by a dendritic cell subset in the absence of feedback signaling in vivo. J. Exp. Med. 195507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barron, M. A., N. Blyveis, B. E. Palmer, S. MaWhinney, and C. C. Wilson. 2003. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J. Infect. Dis. 18726-37. [DOI] [PubMed] [Google Scholar]
- 5.Bednarik, D. P., J. D. Mosca, N. B. Raj, and P. M. Pitha. 1989. Inhibition of human immunodeficiency virus (HIV) replication by HIV-trans-activated alpha 2-interferon. Proc. Natl. Acad. Sci. USA 864958-4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beignon, A. S., K. McKenna, M. Skoberne, O. Manches, I. DaSilva, D. G. Kavanagh, M. Larsson, R. J. Gorelick, J. D. Lifson, and N. Bhardwaj. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Investig. 1153265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biron, C. A. 2001. Interferons alpha and beta as immune regulators—a new look. Immunity 14661-664. [DOI] [PubMed] [Google Scholar]
- 8.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1305-310. [DOI] [PubMed] [Google Scholar]
- 9.Chehimi, J., D. E. Campbell, L. Azzoni, D. Bacheller, E. Papasavvas, G. Jerandi, K. Mounzer, J. Kostman, G. Trinchieri, and L. J. Montaner. 2002. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J. Immunol. 1684796-4801. [DOI] [PubMed] [Google Scholar]
- 10.Chung, E., S. B. Amrute, K. Abel, G. Gupta, Y. Wang, C. J. Miller, and P. Fitzgerald-Bocarsly. 2005. Characterization of virus-responsive plasmacytoid dendritic cells in the rhesus macaque. Clin. Diagn. Lab. Immunol. 12426-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalod, M., T. Hamilton, R. Salomon, T. P. Salazar-Mather, S. C. Henry, J. D. Hamilton, and C. A. Biron. 2003. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J. Exp. Med. 197885-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 3031529-1531. [DOI] [PubMed] [Google Scholar]
- 13.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 1014505-4511. [DOI] [PubMed] [Google Scholar]
- 14.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c+ myeloid and CD11c− plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 982574-2576. [DOI] [PubMed] [Google Scholar]
- 15.Feldman, S., D. Stein, S. Amrute, T. Denny, Z. Garcia, P. Kloser, Y. Sun, N. Megjugorac, and P. Fitzgerald-Bocarsly. 2001. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 101201-210. [DOI] [PubMed] [Google Scholar]
- 16.Feldman, S. B., M. C. Milone, P. Kloser, and P. Fitzgerald-Bocarsly. 1995. Functional deficiencies in two distinct interferon alpha-producing cell populations in peripheral blood mononuclear cells from human immunodeficiency virus seropositive patients. J. Leukoc. Biol. 57214-220. [DOI] [PubMed] [Google Scholar]
- 17.Ferbas, J., J. Navratil, A. Logar, and C. Rinaldo. 1995. Selective decrease in human immunodeficiency virus type 1 (HIV-1)-induced alpha interferon production by peripheral blood mononuclear cells during HIV-1 infection. Clin. Diagn. Lab. Immunol. 2138-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonteneau, J. F., M. Larsson, A. S. Beignon, K. McKenna, I. Dasilva, A. Amara, Y. J. Liu, J. D. Lifson, D. R. Littman, and N. Bhardwaj. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 785223-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis, M. L., M. S. Meltzer, and H. E. Gendelman. 1992. Interferons in the persistence, pathogenesis, and treatment of HIV infection. AIDS Res. Hum. Retrovir. 8199-207. [DOI] [PubMed] [Google Scholar]
- 20.Gendelman, H. E., L. M. Baca, J. Turpin, D. C. Kalter, B. Hansen, J. M. Orenstein, C. W. Dieffenbach, R. M. Friedman, and M. S. Meltzer. 1990. Regulation of HIV replication in infected monocytes by IFN-alpha. Mechanisms for viral restriction. J. Immunol. 1452669-2676. [PubMed] [Google Scholar]
- 21.Grassi, F., A. Hosmalin, D. McIlroy, V. Calvez, P. Debre, and B. Autran. 1999. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS 13759-766. [DOI] [PubMed] [Google Scholar]
- 22.Harari, A., F. Vallelian, P. R. Meylan, and G. Pantaleo. 2005. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J. Immunol. 1741037-1045. [DOI] [PubMed] [Google Scholar]
- 23.Her, L. S., E. Lund, and J. E. Dahlberg. 1997. Inhibition of Ran guanosine triphosphatase-dependent nuclear transport by the matrix protein of vesicular stomatitis virus. Science 2761845-1848. [DOI] [PubMed] [Google Scholar]
- 24.Herbeuval, J. P., J. Nilsson, A. Boasso, A. W. Hardy, M. J. Kruhlak, S. A. Anderson, M. J. Dolan, M. Dy, J. Andersson, and G. M. Shearer. 2006. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc. Natl. Acad. Sci. USA 1037000-7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbeuval, J. P., and G. M. Shearer. 2007. HIV-1 immunopathogenesis: how good interferon turns bad. Clin. Immunol. 123121-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornung, V., J. Schlender, M. Guenthner-Biller, S. Rothenfusser, S. Endres, K. K. Conzelmann, and G. Hartmann. 2004. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 1735935-5943. [DOI] [PubMed] [Google Scholar]
- 27.Howell, D. M., S. B. Feldman, P. Kloser, and P. Fitzgerald-Bocarsly. 1994. Decreased frequency of functional natural interferon-producing cells in peripheral blood of patients with the acquired immune deficiency syndrome. Clin. Immunol. Immunopathol. 71223-230. [DOI] [PubMed] [Google Scholar]
- 28.Ito, T., Y. H. Wang, and Y. J. Liu. 2005. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin. Immunopathol. 26221-229. [DOI] [PubMed] [Google Scholar]
- 29.Iyasere, C., J. C. Tilton, A. J. Johnson, S. Younes, B. Yassine-Diab, R. P. Sekaly, W. W. Kwok, S. A. Migueles, A. C. Laborico, W. L. Shupert, C. W. Hallahan, R. T. Davey, Jr., M. Dybul, S. Vogel, J. Metcalf, and M. Connors. 2003. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 7710900-10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izaguirre, A., B. J. Barnes, S. Amrute, W. S. Yeow, N. Megjugorac, J. Dai, D. Feng, E. Chung, P. M. Pitha, and P. Fitzgerald-Bocarsly. 2003. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 741125-1138. [DOI] [PubMed] [Google Scholar]
- 31.Jiang, W., M. M. Lederman, J. R. Salkowitz, B. Rodriguez, C. V. Harding, and S. F. Sieg. 2005. Impaired monocyte maturation in response to CpG oligodeoxynucleotide is related to viral RNA levels in human immunodeficiency virus disease and is at least partially mediated by deficiencies in alpha/beta interferon responsiveness and production. J. Virol. 794109-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadowaki, N., S. Antonenko, J. Y. Lau, and Y. J. Liu. 2000. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 192219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamga, I., S. Kahi, L. Develioglu, M. Lichtner, C. Maranon, C. Deveau, L. Meyer, C. Goujard, P. Lebon, M. Sinet, and A. Hosmalin. 2005. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J. Infect. Dis. 192303-310. [DOI] [PubMed] [Google Scholar]
- 34.Killian, M. S., S. H. Fujimura, F. M. Hecht, and J. A. Levy. 2006. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. AIDS 201247-1252. [DOI] [PubMed] [Google Scholar]
- 35.Klaschik, S., I. Gursel, and D. M. Klinman. 2007. CpG-mediated changes in gene expression in murine spleen cells identified by microarray analysis. Mol. Immunol. 441095-1104. [DOI] [PubMed] [Google Scholar]
- 35a.Klucking, S., A. P. Barry, R. Chavan, K. A. Dalbey, G. Silvestri, S. Staprans, and M. Feinberg. 2005. Keystone Symposia on HIV Pathogenesis, abstr. 244.
- 36.Kottilil, S., K. Shin, J. O. Jackson, K. N. Reitano, M. A. O'Shea, J. Yang, C. W. Hallahan, R. Lempicki, J. Arthos, and A. S. Fauci. 2006. Innate immune dysfunction in HIV infection: effect of HIV envelope-NK cell interactions. J. Immunol. 1761107-1114. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs, J. A., R. A. Lempicki, I. A. Sidorov, J. W. Adelsberger, B. Herpin, J. A. Metcalf, I. Sereti, M. A. Polis, R. T. Davey, J. Tavel, J. Falloon, R. Stevens, L. Lambert, R. Dewar, D. J. Schwartzentruber, M. R. Anver, M. W. Baseler, H. Masur, D. S. Dimitrov, and H. C. Lane. 2001. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J. Exp. Med. 1941731-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 1031433-1437. [DOI] [PubMed] [Google Scholar]
- 39.Lakshmanan, V., G. Alter, M. Altfeld, and N. Bhardwaj. 2007. Biology of plasmacytoid dendritic cells and natural killer cells in HIV-1 infection. Curr. Opin. HIV AIDS 2189-200. [DOI] [PubMed] [Google Scholar]
- 40.Li, Q., T. Schacker, J. Carlis, G. Beilman, P. Nguyen, and A. T. Haase. 2004. Functional genomic analysis of the response of HIV-1-infected lymphatic tissue to antiretroviral therapy. J. Infect. Dis. 189572-582. [DOI] [PubMed] [Google Scholar]
- 41.Lopez, C., P. A. Fitzgerald, and F. P. Siegal. 1983. Severe acquired immune deficiency syndrome in male homosexuals: diminished capacity to make interferon-alpha in vitro associated with severe opportunistic infections. J. Infect. Dis. 148962-966. [DOI] [PubMed] [Google Scholar]
- 42.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 1015598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macatonia, S. E., R. Lau, S. Patterson, A. J. Pinching, and S. C. Knight. 1990. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology 7138-45. [PMC free article] [PubMed] [Google Scholar]
- 45.Malaspina, A., S. Moir, S. Kottilil, C. W. Hallahan, L. A. Ehler, S. Liu, M. A. Planta, T. W. Chun, and A. S. Fauci. 2003. Deleterious effect of HIV-1 plasma viremia on B cell costimulatory function. J. Immunol. 1705965-5972. [DOI] [PubMed] [Google Scholar]
- 46.Malaspina, A., S. Moir, S. M. Orsega, J. Vasquez, N. J. Miller, E. T. Donoghue, S. Kottilil, M. Gezmu, D. Follmann, G. M. Vodeiko, R. A. Levandowski, J. M. Mican, and A. S. Fauci. 2005. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J. Infect. Dis. 1911442-1450. [DOI] [PubMed] [Google Scholar]
- 47.Malmgaard, L. 2004. Induction and regulation of IFNs during viral infections. J. Interferon Cytokine Res. 24439-454. [DOI] [PubMed] [Google Scholar]
- 48.Mavilio, D., G. Lombardo, J. Benjamin, D. Kim, D. Follman, E. Marcenaro, M. A. O'Shea, A. Kinter, C. Kovacs, A. Moretta, and A. S. Fauci. 2005. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc. Natl. Acad. Sci. USA 1022886-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKenna, K., A. S. Beignon, and N. Bhardwaj. 2005. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J. Virol. 7917-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meier, A., G. Alter, N. Frahm, H. Sidhu, B. Li, A. Bagchi, N. Teigen, H. Streeck, H. J. Stellbrink, J. Hellman, J. van Lunzen, and M. Altfeld. 2007. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-Like receptor ligands. J. Virol. 818180-8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyers, J. H., J. S. Justement, C. W. Hallahan, E. T. Blair, Y. A. Sun, M. A. O'Shea, G. Roby, S. Kottilil, S. Moir, C. M. Kovacs, T. W. Chun, and A. S. Fauci. 2007. Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS ONE 2e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohri, H., A. S. Perelson, K. Tung, R. M. Ribeiro, B. Ramratnam, M. Markowitz, R. Kost, A. Hurley, L. Weinberger, D. Cesar, M. K. Hellerstein, and D. D. Ho. 2001. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J. Exp. Med. 1941277-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moir, S., A. Malaspina, O. K. Pickeral, E. T. Donoghue, J. Vasquez, N. J. Miller, S. R. Krishnan, M. A. Planta, J. F. Turney, J. S. Justement, S. Kottilil, M. Dybul, J. M. Mican, C. Kovacs, T. W. Chun, C. E. Birse, and A. S. Fauci. 2004. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J. Exp. Med. 200587-599. [PubMed] [Google Scholar]
- 54.Olshalsky, S. L., and P. Fitzgerald-Bocarsly. 2005. Flow cytometric techniques for studying plasmacytoid dendritic cells in mixed populations. Methods Mol. Med. 116183-194. [DOI] [PubMed] [Google Scholar]
- 55.Palmer, B. E., E. Boritz, and C. C. Wilson. 2004. Effects of sustained HIV-1 plasma viremia on HIV-1 Gag-specific CD4+ T cell maturation and function. J. Immunol. 1723337-3347. [DOI] [PubMed] [Google Scholar]
- 56.Poeck, H., M. Wagner, J. Battiany, S. Rothenfusser, D. Wellisch, V. Hornung, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2004. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood 1033058-3064. [DOI] [PubMed] [Google Scholar]
- 57.Poli, G., J. M. Orenstein, A. Kinter, T. M. Folks, and A. S. Fauci. 1989. Interferon-alpha but not AZT suppresses HIV expression in chronically infected cell lines. Science 244575-577. [DOI] [PubMed] [Google Scholar]
- 58.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt, B., S. H. Fujimura, J. N. Martin, and J. A. Levy. 2006. Variations in plasmacytoid dendritic cell (PDC) and myeloid dendritic cell (MDC) levels in HIV-infected subjects on and off antiretroviral therapy. J. Clin. Immunol. 2655-64. [DOI] [PubMed] [Google Scholar]
- 60.Shirazi, Y., and P. M. Pitha. 1992. Alpha interferon inhibits early stages of the human immunodeficiency virus type 1 replication cycle. J. Virol. 661321-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siegal, F. P., P. Fitzgerald-Bocarsly, B. K. Holland, and M. Shodell. 2001. Interferon-alpha generation and immune reconstitution during antiretroviral therapy for human immunodeficiency virus infection. AIDS 151603-1612. [DOI] [PubMed] [Google Scholar]
- 62.Silvestri, G. 2005. Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS? J. Med. Primatol. 34243-252. [DOI] [PubMed] [Google Scholar]
- 63.Soumelis, V., I. Scott, F. Gheyas, D. Bouhour, G. Cozon, L. Cotte, L. Huang, J. A. Levy, and Y. J. Liu. 2001. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood 98906-912. [DOI] [PubMed] [Google Scholar]
- 64.Tilton, J. C., A. J. Johnson, M. R. Luskin, M. M. Manion, J. Yang, J. W. Adelsberger, R. A. Lempicki, C. W. Hallahan, M. McLaughlin, J. M. Mican, J. A. Metcalf, C. Iyasere, and M. Connors. 2006. Diminished production of monocyte proinflammatory cytokines during human immunodeficiency virus viremia is mediated by type I interferons. J. Virol. 8011486-11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yonezawa, A., R. Morita, A. Takaori-Kondo, N. Kadowaki, T. Kitawaki, T. Hori, and T. Uchiyama. 2003. Natural alpha interferon-producing cells respond to human immunodeficiency virus type 1 with alpha interferon production and maturation into dendritic cells. J. Virol. 773777-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Younes, S. A., B. Yassine-Diab, A. R. Dumont, M. R. Boulassel, Z. Grossman, J. P. Routy, and R. P. Sekaly. 2003. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 1981909-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]