Abstract
Several species of tsetse flies can be infected by the Glossina pallidipes salivary gland hypertrophy virus (GpSGHV). Infection causes salivary gland hypertrophy and also significantly reduces the fecundity of the infected flies. To better understand the molecular basis underlying the pathogenesis of this unusual virus, we sequenced and analyzed its genome. The GpSGHV genome is a double-stranded circular DNA molecule of 190,032 bp containing 160 nonoverlapping open reading frames (ORFs), which are distributed equally on both strands with a gene density of one per 1.2 kb. It has a high A+T content of 72%. About 3% of the GpSGHV genome is composed of 15 sequence repeats, distributed throughout the genome. Although sharing the same morphological features (enveloped rod-shaped nucleocapsid) as baculoviruses, nudiviruses, and nimaviruses, analysis of its genome revealed that GpSGHV differs significantly from these viruses at the level of its genes. Sequence comparisons indicated that only 23% of GpSGHV genes displayed moderate homologies to genes from other invertebrate viruses, principally baculoviruses and entomopoxviruses. Most strikingly, the GpSGHV genome encodes homologues to the four baculoviral per os infectivity factors (p74 [pif-0], pif-1, pif-2, and pif-3). The DNA polymerase encoded by GpSGHV is of type B and appears to be phylogenetically distant from all DNA polymerases encoded by large double-stranded DNA viruses. The majority of the remaining ORFs could not be assigned by sequence comparison. Furthermore, no homologues to DNA-dependent RNA polymerase subunits were detected. Taken together, these data indicate that GpSGHV is the prototype member of a novel group of insect viruses.
Tsetse flies (Diptera: Glossinidae) are responsible for the transmission of two major zoonoses in tropical Africa: human African trypanosiomiasis (sleeping sickness), which is currently estimated to infect 300,000 to 500,000 people with another 60 million at risk in 36 countries, and animal African trypanosiomiasis (nagana), which causes heavy losses of cattle, domestic pigs, and other farm animals (69, 81). Both diseases have profound impacts on agricultural development and nutritional resources in sub-Saharan Africa (37). Due to the lack of effective vaccines and efficient, inexpensive drugs for the human disease and the development of drug resistance in the animal disease (3), the control of the vector populations remains the main strategy for controlling these scourges.
The successful eradication of Glossina austeni from the island of Unguja, United Republic of Tanzania, using an integrated approach including the release of sterile male tsetse flies (77) stimulated the development of programs aimed at applying this approach on the African mainland, such as one to control Glossina pallidipes in the Southern Rift Valley in Ethiopia (22). The establishment of a laboratory colony of the target species and then its mass production in large-scale facilities are sine qua non prerequisites for the successful implementation of the sterile insect technique. In the framework of the sterile insect technique program in Ethiopia, pupae of G. pallidipes from the target area were shipped to the Entomology Unit of the FAO/IAEA Agriculture and Biotechnology Laboratory, Seibersdorf, Austria. A colony was established, which reached 15,000 females by July 2000 but experienced a steady decline in 2001 and was lost by 2002. Efforts to identify the cause(s) of the decline led to the discovery of a high proportion (over 85% in some samples) of male and female individuals with salivary gland hypertrophy (SGH), a syndrome first described in wild populations of G. pallidipes (12, 80) but later observed in many tsetse fly species from different African countries (17, 24, 38, 39, 48, 50, 54, 65). The causative agent of this syndrome was later identified as a nuclear rod-shaped, enveloped DNA virus averaging 70 by 640 nm in size (33). This virus was also found to be associated with testicular degeneration and ovarian abnormalities (38, 42, 59, 60), and its presence has been shown to affect the development, survival, fertility, and fecundity of naturally (20) or experimentally (40, 62) infected flies. Mother-to-offspring transmission, either trans-ovum or through infected milk glands (39, 59, 61), is thought to be the mode of transmission of the virus in natural tsetse fly populations. In reared colonies, however, horizontal transmission facilitated by the membrane feeding technique used for large-scale feeding of tsetse flies (21) may constitute the main route of virus transmission. The SGH syndrome was also observed in two other species of nonhematophagous diptera, the narcissus bulb fly Merodon equestris (6) and the housefly Musca domestica (15).
The correlation between the high incidence of SGH syndrome of the Ethiopian G. pallidipes colony in the Seibersdorf laboratory and its collapse prompted research to develop a sensitive assay to unequivocally identify the flies carrying the virus. Based on previous work with partial characterization of purified virions isolated from G. pallidipes showing SGH (51), a highly sensitive, nondestructive diagnostic PCR method was developed that revealed a very high (up to 100%) prevalence of the virus in asymptomatic flies (1). As a first step to obtain a better understanding of the virus-host interaction and eventually to develop ways to manage the virus in tsetse fly colonies, we isolated the virus and sequenced its complete genome. We report here the entire nucleotide sequence and annotation of the GpSGHV genome. Although sharing some properties with other large, double-stranded, circular DNA viruses of invertebrates, analysis of the 190,032-bp large genome of GpSGHV revealed that the virus cannot be assigned to any of the so far described families of DNA viruses and thus should be considered the prototype of a new type of insect virus family.
MATERIALS AND METHODS
Isolation, cloning, and sequencing of GpSGHV genomic DNA.
Virions of GpSGHV were purified by sucrose density gradient centrifugation from hypertrophied salivary glands collected from infected G. pallidipes flies that were maintained in the FAO/IAEA Agriculture & Biotechnology Laboratory, Seibersdorf, Austria (1). Intact genomic DNA was extracted from purified virions, and a partial DNA library was produced by cloning EcoRI fragments in pUC19 plasmids using Escherichia coli TG1 bacteria (1). The lengths of cloned inserts were estimated by EcoRI digestions followed by electrophoresis in a 1% agarose gel. A total of 415 recombinant plasmids with inserts ranging from approximately 1 to 7 kb were selected. DNA templates were sequenced from both ends with M13 universal sense and antisense primers (MWG-Biotech AG, Germany) using the Sanger method (63). Moreover, 3 μg of intact DNA was sent to 454 Life Sciences (Bradford, CT) for pyrophosphate-based sequencing (45). A total of 321,953 singleton reads and 402 contigs were provided by 454 Life Sciences, generated by their own assembly software (Newbler). Most of these reads (90%) averaged 80 to 139 nucleotides (nt) in length, summing to approximately 160 times the estimated length of the GpSGHV genome. Assembly of these contigs and the sequences obtained from the cloned EcoRI fragments was performed using Phrap, SeqMan (Lasergene 7.0; Dnastar Inc.), and NTI Vector 9 (Invitrogen) software packages. Additional contigs were assembled from the individual reads provided by 454 Life Sciences using simple custom-made tools (54a). Gaps and ambiguous regions were amplified by PCR using appropriate primers and viral DNA as the template and sequenced. The combination of shotgun pyrosequencing, the Sanger sequencing of EcoRI restriction fragments, and PCR gap closure reactions generated a circular DNA consensus sequence representing on average a 10-fold redundancy at each base position.
DNA sequence analysis.
DNA sequence data were analyzed using the GeneQuest (Lasergene 7.0; Dnastar Inc.), NTI Vector 9 (Invitrogen), and ORF Finder (http///www.ncbi.nlm.nih.gov/gorf/gorf.html) programs. Open reading frames (ORFs) corresponding to more than 50 amino acids with a methionine start codon and with minimal overlap (<100 nt) were evaluated for their putative protein-coding potential. All the predicted ORFs were analyzed using BLAST programs at the NCBI website (http://www.ncbi.nlm.nih.gov) against the nonredundant protein database (5), the Viral Bioinformatics Resource Center (http://athena.bioc.unvic.ca/) database, and a viral protein database that we established by downloading the Ascoviridae, Asfaviridae, Baculoviridae, Herpesviridae, Iridoviridae, Nimaviridae, Nudivirus, Polydnaviridae, and Poxviridae protein sequences of GenBank's nonredundant protein database. Similarities to viral or cellular genes were accepted on the basis of BLASTP (default parameters) scores of ≥44 and ≥50, respectively.
Protein motifs were analyzed by using the PROSITE database, release 16 (31). The sequence was analyzed for repeats using GeneQuest and Tandem Repeats finder (9). Multiple alignments were performed using BioEdit and ClustalW, and the percent amino acid identity indicated the percentage of identical residues between complete ORFs. Promoter analysis (100 and 200 nt) for motif searches was carried out using a set of computer scripts made in the computer scripting language Perl. In order to establish the relationship of GpSGHV to the large double-stranded DNA (dsDNA) virus families, phylogenetic trees were constructed using ClustalW protein alignment and MEGA 4 (70) software with neighbor joining and were confirmed by bootstrap analysis with heurisitic search and 500 replicates.
Nucleotide sequence accession number.
The complete nucleotide sequence of GpSGHV was deposited in GenBank under accession no. EF568108.
RESULTS AND DISCUSSION
General features of the GpSGHV genome.
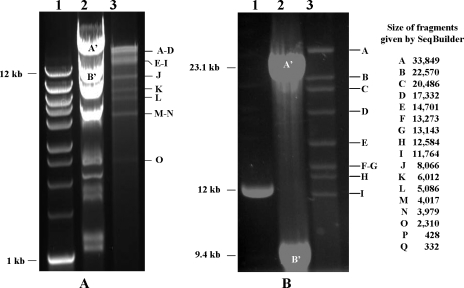
The viral DNA was prepared from purified virus particles (Fig. 1) isolated from a pool of heavily infected salivary glands as previously described (1). The sequencing of GpSGHV DNA by a combination of (i) shotgun pyrosequencing, (ii) Sanger sequencing of a partial genomic library of cloned EcoRI fragments, and (iii) sequence gap filling using PCR products, followed by sequence assembly, led us to conclude that the GpSGHV genome consists of a circular dsDNA molecule of 190,032 bp. The size is in good agreement with that of 185 to 220 kbp estimated by summing the sizes of the fragments generated by EcoRI-restricted viral DNA or measured by pulsed-field gel electrophoresis of intact DNA molecules (1). Furthermore, the sizes of the BglII restriction fragments measured from an agarose gel (Fig. 2) fit well with those predicted from the genomic sequence and no submolar bands were observed, indicating that the viral DNA was pure and that sequencing and assembly were carried out accurately. As mentioned above, the DNA used for pyrosequencing was not clonal but was extracted from a large virus population, and thus it is very likely heterogeneous. Indeed, the very large number of reads (321,953) provided by pyrophosphate sequencing allowed us to detect 101 positions with nucleotide polymorphism distributed along the whole sequence (Table 1). The most frequent nucleotide was taken into account to establish the viral sequence. The GpSGHV genome is one of the largest known genomes of insect viruses with circular dsDNA genomes, exceeded only by that of Heliothis zea nudivirus 1 (228 kbp) (14).
FIG. 1.
Virions of GpSGHV observed by electron microscopy after negative staining.
FIG. 2.
Electrophoretic profile of BglII-restricted GpSGHV DNA, showing 15 of the 17 restriction fragments generated by this enzyme. (A and B) Agarose gels after 3 h and 24 h of migration, respectively. The two small fragments P and Q (<1 kb) cannot be seen on the gels. Submolar bands were not apparent but may be present at a lower concentration. Lanes 1, 1-kb DNA ladder; lanes 2, Hind III-restricted λ DNA ladder (A′ and B] correspond to 23,130 and 9,416 bp, respectively); lanes 3, GpSGHV DNA.
TABLE 1.
Sequence polymorphisms in the GpSGHV genome
| Position | Variation
|
|
|---|---|---|
| Nucleotides | Abundance | |
| 13312 | A/T | 53/4 |
| 13322 | C/T | 53/4 |
| 13337 | C/T | 53/4 |
| 13346 | G/T | 53/4 |
| 14631 | C/T | 54/4 |
| 16740 | G/A | 18/2 |
| 16870 | A/G | 29/2 |
| 16894 | A/G | 28/3 |
| 20645 | T/A | 47/3 |
| 20692 | A/G | 48/2 |
| 22920 | C/T | 45/4 |
| 23169 | C/T | 40/1 |
| 53914 | C/G | 23/3 |
| 66279 | A/G | 61/1 |
| 66341 | T/A | 55/2 |
| 66449 | C/T | 69/3 |
| 81140 | G/A | 62/17 |
| 81362 | A/C | 81/1 |
| 81411 | T/C | 48/2 |
| 81413 | A/G | 48/2 |
| 81427 | T/A | 48/2 |
| 81434-81435 | GT/AC | 48/2 |
| 81440 | A/C | 82/5 |
| 81456 | C/A | 83/4 |
| 81470 | G/A | 84/3 |
| 81480 | G/C | 84/3 |
| 81510 | G/C | 69/2 |
| 81514 | G/T | 68/3 |
| 81532 | G/A | 69/2 |
| 81556 | A/G | 80/7 |
| 81561 | A/C | 80/7 |
| 81572 | A/T | 80/7 |
| 81638 | G/A | 37/3 |
| 81664-81665 | AG/GA | 81/3 |
| 81680 | G/A | 81/3 |
| 81684 | T/C | 81/3 |
| 81687 | C/T | 81/3 |
| 81699 | G/C | 64/3 |
| 81711-81712 | TT/CA | 64/3 |
| 81716 | G/C | 64/3 |
| 81719 | A/C | 64/3 |
| 81741 | T/C | 63/1 |
| 81743 | C/A | 63/1 |
| 81762 | C/T | 63/1 |
| 81768 | G/A | 62/2 |
| 82518 | A/G | 68/1 |
| 82529 | T/A | 68/1 |
| 82531 | A/G | 68/1 |
| 82549 | G/A | 44/1 |
| 82941 | G/A | 62/1 |
| 83042 | A/G | 58/3 |
| 83062 | T/A | 58/3 |
| 83064 | T/G | 58/3 |
| 83066 | G/A | 58/3 |
| 83069 | A/G | 58/3 |
| 83071 | A/G | 58/3 |
| 83073 | A/T | 58/3 |
| 83125-83126 | CG/TA | 41/1 |
| 83137 | T/C | 41/1 |
| 83158 | C/T | 36/2 |
| 83204 | G/A | 59/1 |
| 83223 | C/T | 58/2 |
| 83237 | A/G | 59/1 |
| 83277 | G/A | 43/3 |
| 83289 | G/A | 52/2 |
| 83302 | A/G | 52/2 |
| 83411 | A/C | 61/4 |
| 83448 | G/A | 53/2 |
| 83644 | T/C | 78/2 |
| 83665 | T/C | 78/2 |
| 83674 | A/G | 78/2 |
| 83683 | A/G | 78/2 |
| 83711 | T/C | 49/2 |
| 86544 | T/C | 69/4 |
| 86577 | T/C | 69/4 |
| 86582 | A/G | 69/4 |
| 86584-86585 | AT/CA | 69/4 |
| 86592 | A/G | 61/2 |
| 86594 | A/G | 61/2 |
| 86596 | T/C | 61/2 |
| 90097 | A/G | 110/4 |
| 106126 | C/T | 88/4 |
| 120777 | T/A | 45/3 |
| 123761 | T/A | 81/1 |
| 123783 | A/G | 77/3 |
| 123997 | C/T | 56/3 |
| 124547 | T/C | 59/1 |
| 124566 | T/C | 59/1 |
| 134386 | G/A | 62/5 |
| 134401 | G/A | 62/5 |
| 144551 | A/G | 72/2 |
| 151745 | C/T | 56/3 |
| 151778 | T/G | 56/3 |
| 159185 | T/C | 35/2 |
| 159213 | T/T | 35/2 |
| 159215 | G/A | 35/2 |
The very low G+C content of 28% of the GpSGHV genome significantly differs from those reported for most invertebrate viruses with large circular dsDNA genomes, including baculoviruses (33 to 58% G+C) (36), nudiviruses (42% G+C), (14, 79) (with the exception of the cricket nudivirus GbNV [28% G+C] [79]), nimaviruses (41% G+C) (75, 84), and ascoviruses (35.5 to 49% G+C) (7, 10, 78). It also differs from those of the large linear dsDNA genomes of herpesviruses (35 to 75% G+C) (74) and of most iridoviruses (49% G+C) (73), except Chilo iridescent virus and lymphocystis disease virus (both 29% G+C) (34, 72), but is closer to the low (18 to 27%) G+C content of entomopoxviruses (2, 8).
Gene content analysis.
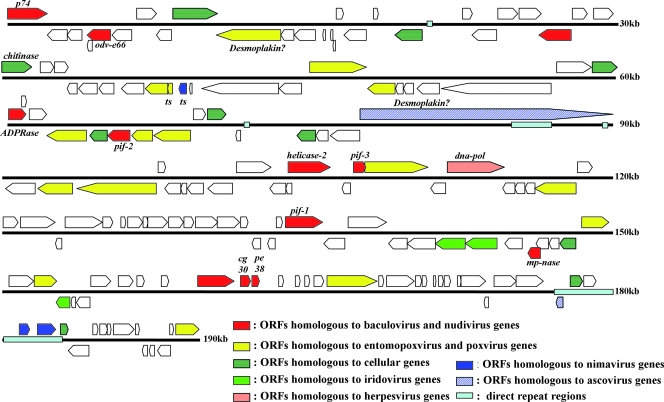
A total of 322 potential ORFs with a methionine start codon and corresponding to a minimum protein size of 50 amino acids were detected in the GpSGHV genome. Among these, 160 ORFs with no or minimal overlap (≤82 nt) were assumed to encode putative proteins (Table 2). The predicted ORFs are evenly distributed on both strands (51% forward and 49% reverse); many of them are arranged in unidirectional gene clusters (Fig. 3). They represent 86% of the genome, with an average density of one gene per 1.2 kb. The A of the ATG start codon of ORF SGHV001, encoding a homologue of a baculovirus p74 occlusion-derived virion (odv) envelope protein, was arbitrarily assigned as position 1 of the GpSGHV genome sequence.
TABLE 2.
Potentially expressed ORFs in GpSGHV
| ORF | Position | Length (amino acids) | Intergenic distance (bp)a | Best BLASTP match
|
Conserved domain(s) or signature(s)c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, source | Accession no. | Score | E value | Identity (%) | Length (amino acids) | MegAlign identity (%)b | |||||
| SGHV001 | 1>2091 | 696 | 7 | p74 protein, Spodoptera litura NPV | CAA09849 | 90 | 6.E−16 | 131/656 (19) | 657 | 20.8 | TM, baculo-p74 |
| SGHV002 | 3071<2088 | 327 | −4 | ||||||||
| SGHV003 | 3785<3234 | 183 | 162 | ||||||||
| SGHV004 | 4448<4185 | 87 | 399 | TM | |||||||
| SGHV005 | 5439<4378 | 353 | −71 | ODV-E66, Epiphyas postvittana nucleopolyhedrovirus | NP_203211 | 62 | 2.E−08 | 46/192 (23) | 680 | 15.3 | TM, SP, lyase, Baculo-E66 |
| SGHV006 | 6576<5491 | 361 | 51 | DUF676 hydrolase, lipase | |||||||
| SGHV007 | 6714>7778 | 354 | 137 | ||||||||
| SGHV008 | 8618<7803 | 271 | 24 | Y045_METJA 13-219 | |||||||
| SGHV009 | 8631>10871 | 746 | 12 | Hypothetical protein MAL7P1.132, Plasmodium falciparum 3D7 | XP_001349148 | 50 | 6.E−04 | 60/225 (26) | 2,041 | 22.4 | |
| SGHV010 | 14205<10891 | 1,104 | 19 | ORF MSV156, Melanoplus sanguinipes EPV | NP_048227 | 67 | 9.E−09 | 98/449 (21) | 1,127 | 15.4 | Coiled-coil region, kinetochore Spc7 |
| SGHV011 | 14780<14202 | 192 | −4 | ||||||||
| SGHV012 | 15897<14836 | 353 | 55 | ||||||||
| SGHV013 | 16552<16376 | 58 | 478 | ||||||||
| SGHV014 | 16895<16719 | 58 | 166 | TM, SP | |||||||
| SGHV015 | 17011<16853 | 52 | −43 | TM | |||||||
| SGHV016 | 17068>17436 | 122 | 56 | ||||||||
| SGHV017 | 18627<17449 | 392 | 12 | ZFC3HC4 ring | |||||||
| SGHV018 | 18941>19168 | 75 | 313 | ||||||||
| SGHV019 | 21510<20179 | 443 | 1,010 | Hypothetical protein TA04315, Theileria annulata strain Ankara | XP_954808 | 55 | 9.E−06 | 32/131 (24) | 791 | 18.7 | |
| SGHV020 | 23524>24054 | 176 | 2,013 | ||||||||
| SGHV021 | 25329<24049 | 426 | −6 | ||||||||
| SGHV022 | 25337>26368 | 343 | 7 | ||||||||
| SGHV023 | 26422>27552 | 376 | 53 | AARP2CN (NUC121) domain SM00785 | |||||||
| SGHV024 | 29113<27557 | 518 | 4 | Mv-ORF74 peptide, Maruca vitrata MNPV | YP_950804 | 49 | 1.E−03 | 31/103 (30) | 224 | 24.6 | TM |
| SGHV025 | 29205>29957 | 250 | 91 | ||||||||
| SGHV026 | 30329>31375 | 348 | 371 | ||||||||
| SGHV027 | 31398>32774 | 458 | 22 | Chitinase Chit1 precursor, Glossina morsitans morsitans | AAL65401 | 226 | 3.E−57 | 129/365 (35) | 460 | 33 | TM, Glycoside hydrolase family 18 catalytic domain |
| SGHV028 | 33269>33862 | 197 | 494 | ||||||||
| SGHV029 | 33890>34573 | 227 | 27 | Staphylococcal AgrD protein SM00794 | |||||||
| SGHV030 | 34978<34577 | 133 | 3 | ||||||||
| SGHV031 | 35917<35060 | 285 | 81 | HDAC interaction domain, histone deacetylase | |||||||
| SGHV032 | 36744<35965 | 259 | 47 | ||||||||
| SGHV033 | 38089<37043 | 348 | 298 | ||||||||
| SGHV034 | 39187<38138 | 349 | 48 | ORF AMV 260, Amsacta moorei EPV | NP_065042 | 44 | 2.E−02 | 45/173 (26) | 504 | 19.2 | |
| SGHV035 | 39460<39197 | 87 | 9 | ORF MSV 238, Melanoplus sanguinipes EPV | NP_048309 | 57 | 3.E−07 | 28/80 (35) | 292 | 33.3 | |
| SGHV036 | 40088<39741 | 115 | 280 | ORF 67, shrimp white spot syndrome virus thymidylate synthase | NP_477589 | 62 | 1.E−08 | 28/78 (35) | 289 | 37.4 | |
| SGHV037 | 40351<40187 | 54 | 98 | ||||||||
| SGHV038 | 44374<40850 | 1,174 | 498 | SP | |||||||
| SGHV039 | 45446<44439 | 335 | 64 | TM, SP | |||||||
| SGHV040 | 45768>48473 | 901 | −1 | ORF AMV 130, Amsacta moorei EPV | NP_064912 | 44 | 5.E−02 | 44/141 (31) | 1,384 | 19.1 | |
| SGHV041 | 49765<48524 | 413 | 50 | ORF MSV 214, Melanoplus sanguinipes EPV, SCG gene family protein | NP_048285 | 44 | 2.E−02 | 61/319 (21) | 386 | 18.2 | |
| SGHV042 | 50183<49809 | 124 | 43 | TM | |||||||
| SGHV043 | 50652<50218 | 144 | 34 | TM | |||||||
| SGHV044 | 51903<50824 | 359 | 171 | SP | |||||||
| SGHV045 | 57046<51860 | 1,728 | −44 | ||||||||
| SGHV046 | 57316>58917 | 533 | 269 | PPASE, inorganic pyrophosphatase signature | |||||||
| SGHV047 | 58933>60147 | 404 | 15 | Hypothetical protein CBG22662, Caenorhabditis briggsae | CAE74824 | 56 | 4.E−06 | 57/311 (18) | 743 | 16.6 | |
| SGHV048 | 60195>60986 | 263 | 47 | ORF 033, Heliocoverpa armigera nucleopolyhedrovirus G4 ADP-pyrophosphatase | NP_075102 | 59 | 4.E−07 | 56/231 (24) | 238 | 18.8 | |
| SGHV049 | 60935>61207 | 90 | −52 | ||||||||
| SGHV050 | 61314>62189 | 291 | 106 | ||||||||
| SGHV051 | 64184<62202 | 660 | 12 | ORF MSV 152, Melanoplus sanguinipes EPV putative core protein | NP_048223 | 47 | 3.E−03 | 52/218 (25) | 1,306 | 20 | TM |
| SGHV052 | 65228<64308 | 306 | 123 | Hypothetical protein, Plasmodium falciparum | XP_001351434 | 53 | 2.E−05 | 69/251 (27) | 540 | 23.9 | |
| SGHV053 | 66323<65241 | 360 | 12 | Per os infectivity factor 2, Gryllus bimaculatus nudivirus | YP_001111333 | 66 | 3.E−09 | 68/274 (24) | 378 | 21.2 | Baculo-44 |
| SGHV054 | 67507<66473 | 344 | 149 | ORF AMV 054, Amsacta moorei EPV (putative RNA polymerase-associated transcriptional specificity factor) | NP_064836 | 45 | 5.E−03 | 90/379 (23) | 822 | 29.9 | |
| SGHV055 | 69398<67500 | 632 | −8 | ORF AMV253, Amsacta moorei EPV (possible surface protein) | NP_065035 | 55 | 1.E−05 | 100/456 (21) | 485 | 20.6 | Internal repeat |
| SGHV056 | 69464>70111 | 215 | 65 | ||||||||
| SGHV057 | 70161>71117 | 318 | 49 | Rhoptry protein, Plasmodium yoelli yoelli strain 17XNL | XP_725453 | 57 | 2.E−06 | 51/167 (30) | 2,664 | 30.9 | |
| SGHV058 | 71829<71593 | 78 | 475 | TM, SP | |||||||
| SGHV059 | 75616<74639 | 325 | 2809 | RpoD, Plasmodium falciparum | CAA64574 | 55 | 5.E−06 | 74/308 (24) | 960 | 28.9 | |
| SGHV060 | 76249<75623 | 208 | 6 | ||||||||
| SGHV061 | 77789<76305 | 494 | 55 | ||||||||
| SGHV062 | 77753>90874 | 4,373 | −37 | ORF 147, Tricoplusia ni ascovirus 2c | YP_803369 | 123 | 3.E−25 | 271/1374 (19) | 1,481 | 22.5 | |
| SGHV063 | 92270<90876 | 464 | 1 | TM, Pfam:CBF, SP | |||||||
| SGHV064 | 94234<92447 | 595 | 176 | ORF AMV130, Amsacta moorei EPV (putative ATP-binding cassette transporter) | NP_064912 | 44 | 4.E−02 | 81/354 (22) | 1,384 | 22.4 | |
| SGHV065 | 98511<94246 | 1,421 | 11 | ORF AMV039, Amsacta moorei EPV (putative ATPase/DNA helicase) | NP_064821 | 46 | 2.E−02 | 72/302 (23) | 532 | 22.8 | |
| SGHV066 | 98557>98877 | 106 | 45 | ||||||||
| SGHV067 | 99700<98921 | 259 | 43 | ||||||||
| SGHV068 | 100046<99720 | 108 | 19 | ||||||||
| SGHV069 | 100917<100105 | 270 | 58 | TM, SP | |||||||
| SGHV070 | 102415<101105 | 436 | 187 | ||||||||
| SGHV071 | 102502>104328 | 608 | 86 | ||||||||
| SGHV072 | 105120<104311 | 269 | 1,808 | TM | |||||||
| SGHV073 | 105372<105133 | 79 | 12 | ||||||||
| SGHV074 | 105419>107557 | 712 | 46 | ORF 105, Choristoneura occidentalis GV (helicase-2) | YP_654526 | 45 | 1.E−02 | 25/75 (33) | 461 | 20.6 | ATPases |
| SGHV075 | 108415<107600 | 271 | −1 | ||||||||
| SGHV076 | 108439>109074 | 211 | 23 | ORF101, Helicoverpa zea single nucleocapsid NPV per os infectivity factor 3 | NP_542724 | 64 | 4.E−09 | 38/154 (24) | 199 | 21.9 | TM Pfam:DUF666, SP |
| SGHV077 | 109141>112320 | 1,059 | 66 | ORF AMV, 130 Amsacta moorei EPV (putative ATP-binding cassette transporter) | NP_064912 | 45 | 4.E−02 | 80/368 (21) | 1,384 | 17.8 | |
| SGHV078 | 113291<112581 | 236 | 260 | TM | |||||||
| SGHV079 | 113342>116203 | 953 | 50 | Alcelaphine herpesvirus 1 DNA polymerase | NP_065512 | 240 | 3.E−61 | 208/740 (28) | 1,026 | 24.9 | |
| SGHV080 | 116825<116220 | 201 | 16 | Coiled-coil region | |||||||
| SGHV081 | 117346<116831 | 171 | 5 | TM | |||||||
| SGHV082 | 117839<117360 | 159 | 13 | ||||||||
| SGHV083 | 119926<117842 | 694 | 2 | ORF AMV 214, Amsacta moorei EPV | NP_064996 | 46 | 7.E−03 | 69/271 (25) | 404 | 20.8 | |
| SGHV084 | 120018>120677 | 219 | 2,175 | ||||||||
| SGHV085 | 120898>121665 | 255 | 220 | ||||||||
| SGHV086 | 121756>123534 | 592 | 90 | ||||||||
| SGHV087 | 123845<123555 | 96 | 20 | C2C2 zinc finger | |||||||
| SGHV088 | 123967>125925 | 652 | 121 | ||||||||
| SGHV089 | 125940>126482 | 180 | 14 | Coiled-coil region | |||||||
| SGHV090 | 126855>127091 | 78 | 372 | TM | |||||||
| SGHV091 | 127188>127994 | 268 | 96 | TM, SP | |||||||
| SGHV092 | 128014>128265 | 83 | 19 | ||||||||
| SGHV093 | 128273>129262 | 329 | 7 | TM | |||||||
| SGHV094 | 129284>130105 | 273 | 21 | ||||||||
| SGHV095 | 130116>130589 | 157 | 10 | ||||||||
| SGHV096 | 130626>131771 | 381 | 36 | TM, SP | |||||||
| SGHV097 | 131758>132942 | 394 | −14 | TM | |||||||
| SGHV098 | 132962>133309 | 115 | 19 | ||||||||
| SGHV099 | 134004<133522 | 160 | 212 | ||||||||
| SGHV100 | 134769<134335 | 144 | 330 | TM, SP | |||||||
| SGHV101 | 134768>135088 | 106 | −2 | TM, SP | |||||||
| SGHV102 | 135141>137099 | 652 | 52 | Per os infectivity factor 1, Neodiprion abietis nucleopolyhedrovirus | YP_667927 | 79 | 1.E−12 | 92/355 (25) | 537 | 19.4 | EGF-like domain, TM, SP |
| SGHV103 | 138292<137162 | 376 | 62 | TM, SP | |||||||
| SGHV104 | 138341>140323 | 660 | 48 | TM, coiled-coil region | |||||||
| SGHV105 | 141381<140503 | 292 | 179 | Coiled-coil region | |||||||
| SGHV106 | 142793<141384 | 469 | 2 | Coiled-coil region, Fib-alpha, fibrinogen alpha chain | |||||||
| SGHV107 | 144369<142804 | 521 | 10 | Lymphocystis disease virus isolate China cell division protein 48 | YP_073712 | 67 | 2.E−09 | 47/156 (30) | 690 | 20.7 | |
| SGHV108 | 145984<144347 | 545 | −23 | Lymphocystis disease virus isolate China cell division protein 48 | YP_073712 | 53 | 5.E−05 | 41/162 (25) | 690 | 13.8 | |
| SGHV109 | 147225<146368 | 285 | 383 | TM, SP | |||||||
| SGHV110 | 147867<147262 | 201 | 36 | mp-nase, Spodoptera litura granulovirus | YP_001256988 | 60 | 5.E−08 | 37/104 (35) | 464 | 28.9 | TM, zinc-dependent metalloprotease, matrixin signature peptidase_M10 |
| SGHV111 | 148623<147964 | 219 | 96 | TM, SP, zinc protease | |||||||
| SGHV112 | 149132<148629 | 167 | 5 | TM, SP | |||||||
| SGHV113 | 149968<149123 | 281 | −10 | Hypothetical protein PY00593, Plasmodium yoelli yoelli strain 17XNL | XP_725532 | 50 | 2.E−04 | 58/231 (25) | 1647 | 23.8 | |
| SGHV114 | 150262>151584 | 440 | 293 | ORF MSV016, Melanoplus sanguinipes EPV (leucine-rich repeat gene family protein) | NP_048087 | 55 | 7.E−06 | 88/377 (23) | 572 | 20.5 | |
| SGHV115 | 151638>152870 | 410 | 53 | ||||||||
| SGHV116 | 152888>153967 | 359 | 17 | ORF AMV134, Amsacta moorei EPV (leucine-rich repeat gene family protein) | NP_064916 | 52 | 6.E−05 | 55/236 (23) | 535 | 18.4 | |
| SGHV117 | 154687<153968 | 239 | 0 | ORF 099L, infectious spleen and kidney necrosis virus | NP_612321 | 47 | 1.E−03 | 19/52 (36) | 107 | 29 | |
| SGHV118 | 154959<154711 | 82 | 23 | ZF C3HC4 type, ring finger | |||||||
| SGHV119 | 155648<154995 | 217 | 35 | ||||||||
| SGHV120 | 155692>156747 | 351 | 43 | ||||||||
| SGHV121 | 157037>157798 | 253 | 289 | TM | |||||||
| SGHV122 | 157992>158342 | 116 | 193 | TM, SP | |||||||
| SGHV123 | 159162>159632 | 156 | 819 | ZnF_C2H2 domain, coiled-coil region | |||||||
| SGHV124 | 160975>162828 | 617 | 1,342 | ORF 168, Xestia c-nigrum GV | NP_059316 | 46 | 6.E−03 | 20/63 (31) | 198 | 22.2 | |
| SGHV125 | 163098>163592 | 164 | 269 | ORF 067, Ecotropis obliqua NPV Cg30 | YP_874260 | 50 | 6.E−05 | 35/131 (26) | 269 | 25.9 | |
| SGHV126 | 163629>163937 | 102 | 36 | ORF 149, Anticarsia gemmatalis NPV pe38-like | YP_803543 | 44 | 3.E−03 | 25/66 v(37) | 209 | 29.4 | |
| SGHV127 | 164989>165237 | 82 | 1,051 | ZnF_C2H2 domain | |||||||
| SGHV128 | 165802>166026 | 74 | 564 | ||||||||
| SGHV129 | 166271>166507 | 78 | 244 | Coiled-coil region | |||||||
| SGHV130 | 166702>167232 | 176 | 194 | ||||||||
| SGHV131 | 167329>169824 | 831 | 96 | ORF MSV156, Melanoplus sanguinipes EPV | NP_048227 | 51 | 4.E−04 | 114/463 (24) | 1,127 | 19.1 | |
| SGHV132 | 169957>170157 | 66 | 132 | ||||||||
| SGHV133 | 170364>171623 | 419 | 206 | EGF-like domain signature 1 | |||||||
| SGHV134 | 171764>172063 | 99 | 140 | ||||||||
| SGHV135 | 172083>172313 | 76 | 19 | ||||||||
| SGHV136 | 172640>172804 | 54 | 326 | Coiled-coil region, | |||||||
| SGHV137 | 172868>173140 | 90 | 63 | ||||||||
| SGHV138 | 173153>173515 | 120 | 12 | Coiled-coil region, | |||||||
| SGHV139 | 173797>173994 | 65 | 281 | Coiled-coil region, | |||||||
| SGHV140 | 174002>175228 | 408 | 7 | Coiled-coil region, | |||||||
| SGHV141 | 175407<175141 | 88 | −88 | TM | |||||||
| SGHV142 | 175631>176704 | 357 | 223 | ||||||||
| SGHV143 | 176745>178037 | 430 | 40 | Coiled-coil region, DUF572, family of unknown function | |||||||
| SGHV144 | 179093<178719 | 124 | 681 | ORF 086, Trichoplusia ni ascovirus 2c | YP_803309 | 55 | 1.E−06 | 25/63 (39) | 116 | 33.6 | |
| SGHV145 | 179383>180018 | 211 | 289 | Similar to Plasmodium falciparum trophozoite antigen r45-like protein, Danio rerio | XP_001343112 | 61 | 4.E−08 | 64/174 (36) | 334 | 38.5 | |
| SGHV146 | 180034>180669 | 211 | 15 | ||||||||
| SGHV147 | 181201>181731 | 176 | 531 | ORF 179, shrimp white spot syndrome virus | NP_477701 | 45 | 2.E−03 | 25/116 (21) | 221 | 19.3 | |
| SGHV148 | 182102>182929 | 275 | 370 | ORF 179, shrimp white spot syndrome virus | NP_477701 | 55 | 3.E−06 | 41/190 (21) | 221 | 19.9 | |
| SGHV149 | 183239>183601 | 120 | 309 | Sensory appendage protein 5, Manduca sexta | AAF16716 | 53 | 6.E−06 | 38/113 (33) | 231 | 20.8 | Internal repeat |
| SGHV150 | 184602<183613 | 329 | 11 | ||||||||
| SGHV151 | 184795>185043 | 82 | 192 | ||||||||
| SGHV152 | 185143>185514 | 123 | 99 | ||||||||
| SGHV153 | 185514>185747 | 77 | −1 | ||||||||
| SGHV154 | 185801>186817 | 338 | 53 | ||||||||
| SGHV155 | 186879>187079 | 66 | 61 | ||||||||
| SGHV156 | 187447<187274 | 57 | 194 | ||||||||
| SGHV157 | 187856<187626 | 76 | 178 | ||||||||
| SGHV158 | 188655<187951 | 234 | 94 | TM | |||||||
| SGHV159 | 188579>188788 | 69 | −77 | ||||||||
| SGHV160 | 188838>190025 | 395 | 49 | Possible surface protein AMV253, Amsacta moorei entomopoxvirus | NP_065035 | 50 | 2.E−04 | 68/274 (24) | 485 | 21.5 | TM |
−, overlap between adjacent ORFs.
Amino acid identity based on MegAlign ClustalW analysis of entire ORFs.
SP, signal peptides; TM, transmembrane domains.
FIG. 3.
Linearized representation of the GpSGHV genome. The genome was linearized at the ATG start codon of p74 (SGHV001). Arrows indicate the positions and directions of transcription for potential ORFs, which are colored according to suggested homology to those of other viruses. Light blue boxes indicate positions of repeat regions.
BLAST searches revealed that 47 of the 160 predicted GpSGHV ORFs had putative homologues in the NCBI protein database. These include 28 putative homologues to putative or known genes from other large dsDNA viruses, essentially baculoviruses (12 ORFs), entomopoxviruses (16 ORFs), and 10 ORFs homologous to putative or known genes from cellular organisms (Table 2). The level of amino acid identity between the SGHV putative ORFs and those of other viruses was usually low, ranging from 13.8% to 37.4% with an average of around 22%. The remaining 113 predicted ORFs produced no significant BLASTP hits.
We also studied the relative abundance of putative regulatory elements in the promoters of the ORFs by computational analysis of the SGHV genome. This enumeration analysis was carried out using the program Perl for all 4- and 5-nt motifs in the 100- and 200-nt upstream sequences (Table 3). The method was calibrated on extensively studied large DNA viruses such as herpesviruses, baculoviruses, and vaccinia virus (46). Analysis of SGHV revealed that the 4-nt motif TAAG has the highest relative enrichment (3 times; P ≤ 0.05) in the 100-nt promoter compared to the whole SGHV genome (Table 3). For the 5-nt motif, ATAAG and TAAGA were highly enriched (4.9 and 3.7 times, respectively; P ≤ 0.05). This suggests that the TAAG motif may have a regulatory function in transcription. The same motif is part of the major late transcription initiation site of baculoviruses, and this may suggest an ancient regulatory relationship between GpSGHV and baculoviruses. However, no transcriptional analysis has been done to support the hypothesis that TAAG plays a role in SGHV transcription.
TABLE 3.
Frequencies of 4- or 5-nt motifs in the 5′ upstream regions of the SGHV ORFs compared to the complete genome for the baculovirus AcMNPVa
| Virus and motif length | 100 nt upstream
|
200 nt upstream
|
||||||
|---|---|---|---|---|---|---|---|---|
| Motif | Occurrence in genomeb (% of expected occurrencec) | Occurrence in upstream regions (% of expected occurrence) | Relative enrichment in upstream regions | Motif | Occurrence in genome (% of expected occurrence) | Occurrence in upstream regions (% of expected occurrence) | Relative enrichment in upstream regions | |
| AcMNPV | TAAGd | 393 (29) | 90 (114) | 4 | TAAGd | 393 (29) | 137 (87) | 3 |
| TATA | 1,314 (66) | 172 (149) | 2.3 | TATA | 1,314 (66) | 255 (111) | 1.7 | |
| ATAA | 1,973 (101) | 222 (198) | 1.9 | ATAA | 1,973 (101) | 363 (162) | 1.6 | |
| ATAT | 1,616 (81) | 170 (147) | 1.8 | ATAT | 1,616 (81) | 268 (116) | 1.4 | |
| AGTA | 671 (49) | 70 (89) | 1.8 | AGTA | 671 (49) | 109 (69) | 1.4 | |
| AAGG | 473 (51) | 41 (76) | 1.5 | GATAe | 867 (63) | 137 (87) | 1.4 | |
| GATAe | 867 (63) | 74 (94) | 1.5 | AATA | 2,230 (115) | 346 (154) | 1.3 | |
| CACTf | 612 (64) | 52 (95) | 1.5 | CAGTg | 698 (73) | 106 (96) | 1.3 | |
| AATA | 2,230 (115) | 186 (166) | 1.4 | CTTA | 393 (28) | 59 (37) | 1.3 | |
| ATTA | 1,957 (98) | 163 (141) | 1.4 | TCACf | 669 (70) | 99 (90) | 1.3 | |
| GTAT | 949 (68) | 79 (98) | 1.4 | GCTA | 541 (57) | 77 (70) | 1.2 | |
| CAGTg | 698 (73) | 58 (105) | 1.4 | CCCC | 190 (42) | 27 (52) | 1.2 | |
| TAAA | 2,716 (140) | 222 (198) | 1.4 | TACC | 444 (47) | 63 (57) | 1.2 | |
| AGGG | 233 (35) | 19 (50) | 1.4 | TAGT | 737 (53) | 104 (64) | 1.2 | |
| TAGT | 737 (53) | 58 (72) | 1.4 | CACTf | 612 (64) | 86 (78) | 1.2 | |
| SGHV | ||||||||
| 4-mer | TAAG | 848 (32) | 106 (96) | 3.0 | TAAG | 848 (32) | 154 (69) | 2.1 |
| AGTC | 506 (53) | 36 (88) | 1.7 | AGTC | 506 (53) | 67 (82) | 1.6 | |
| AGGT | 536 (52) | 38 (87) | 1.7 | GTAG | 676 (66) | 88 (101) | 1.5 | |
| TAGG | 458 (44) | 32 (73) | 1.6 | AGGG | 245 (58) | 30 (84) | 1.4 | |
| AGTA | 1,515 (58) | 103 (93) | 1.6 | AAGT | 1,703 (65) | 208 (94) | 1.4 | |
| CAGT | 814 (85) | 55 (135) | 1.6 | AGTA | 1,515 (58) | 183 (83) | 1.4 | |
| GCGC | 178 (123) | 12 (195) | 1.6 | GCAG | 418 (106) | 50 (149) | 1.4 | |
| AAGT | 1,703 (65) | 113 (102) | 1.6 | AGCC | 347 (94) | 41 (131) | 1.4 | |
| TAGT | 1,451 (58) | 95 (89) | 1.5 | TAGG | 458 (44) | 53 (61) | 1.4 | |
| GTAG | 676 (66) | 44 (101) | 1.5 | AAGC | 631 (63) | 71 (84) | 1.3 | |
| GTAA | 1,989 (76) | 128 (115) | 1.5 | ATAG | 1,655 (63) | 186 (84) | 1.3 | |
| CTTA | 848 (36) | 54 (54) | 1.5 | TAGT | 1,451 (58) | 163 (77) | 1.3 | |
| ATAA | 6,696 (101) | 420 (149) | 1.5 | CTTA | 848 (36) | 95 (48) | 1.3 | |
| AAGA | 2,215 (81) | 135 (117) | 1.4 | AGGT | 536 (52) | 60 (69) | 1.3 | |
| AGCC | 347 (94) | 21 (135) | 1.4 | CAGT | 814 (85) | 91 (112) | 1.3 | |
| AGTT | 1,764 (70) | 106 (100) | 1.4 | AAGA | 2,215 (81) | 243 (105) | 1.3 | |
| 5-mer | ATAAG | 338 (35) | 70 (172) | 4.9 | ATAAG | 338 (35) | 89 (109) | 3.1 |
| TAAGA | 297 (31) | 46 (113) | 3.7 | TAAGA | 297 (31) | 64 (79) | 2.5 | |
| GTCAG | 80 (57) | 11 (187) | 3.2 | CAGTC | 93 (72) | 20 (182) | 2.5 | |
| GTAAG | 128 (34) | 16 (100) | 3.0 | AGGGC | 57 (100) | 12 (248) | 2.5 | |
| AGGGC | 57 (100) | 7 (289) | 2.9 | TCCGC | 52 (109) | 10 (248) | 2.3 | |
| TCCGC | 52 (109) | 6 (297) | 2.7 | GTAAG | 128 (34) | 24 (75) | 2.2 | |
| CCTTA | 83 (26) | 9 (67) | 2.6 | CTGGG | 67 (122) | 12 (258) | 2.1 | |
| CGCGC | 28 (143) | 3 (362) | 2.5 | CGCGC | 28 (143) | 5 (301) | 2.1 | |
| GCGCA | 79 (148) | 8 (354) | 2.4 | TAGCC | 96 (74) | 17 (155) | 2.1 | |
| TAAGC | 99 (28) | 10 (67) | 2.4 | GGGCG | 30 (133) | 5 (262) | 2.0 | |
| TAAGT | 369 (40) | 37 (95) | 2.4 | CCTAA | 139 (42) | 23 (82) | 2.0 | |
| CGCCC | 30 (164) | 3 (388) | 2.4 | TAAGT | 369 (40) | 61 (78) | 2.0 | |
| TAAGG | 83 (22) | 8 (50) | 2.3 | GTCAG | 80 (57) | 13 (110) | 1.9 | |
| AAGGG | 94 (60) | 9 (136) | 2.3 | AGTAG | 200 (53) | 32 (100) | 1.9 | |
| AGCGC | 42 (79) | 4 (177) | 2.2 | CCCTG | 25 (52) | 4 (99) | 1.9 | |
| AGGTC | 42 (30) | 4 (68) | 2.2 | CAGGG | 25 (44) | 4 (83) | 1.9 | |
Only the 15 motifs with the highest relative enrichment are shown for each virus. For AcMNPV, sequences that are part of the consensus TATA box [TATA(a/t)A] are underlined. Bold indicates a P value of ≤0.05
Both strands, excluding homologous repeats (present for AcMNPV and SHGV).
Occurrence of a 4-mer or 5-mer based on random distribution of nucleotides in the complete genome.
Part of the AcMNPV late initiator sequence (a/g/t)TAAG.
Part of the AcMNPV upstream activating element with sequence (a/t)GATA(a/t).
Part of the AcMNPV downstream activating element with sequence (a/t)CACNG.
Sequence of the AcMNPV early initiator sequence CAGT.
Repetitive regions.
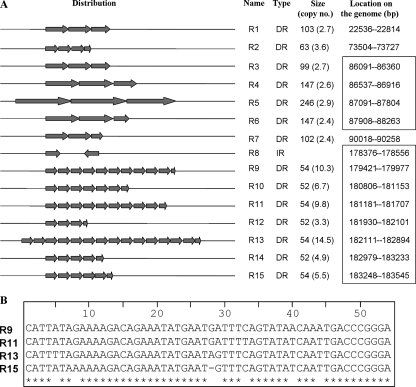
There are 14 repetitive regions (RS) with head-to-tail tandem repeat sequences (TRS) and one inverted repeat sequence distributed throughout the GpSGHV genome, representing about 3% of the genome sequence (Fig. 4). The RS are 171 to 556 nt in length and consist of 78 TRS minifragments. Most of these TRS are highly homologous to each other and are clustered in two genome areas located in the 86- to 88-kb and 179- to 183-kb regions. The sizes of the TRS varied from 52 to 246 nt, and the number of TRS per RS varied from 2.7 to 14.5. Within the same RS, the identity of TRS was over 80%, but the sequence identity among the different RS varied from 21.2 to 96.2%. TRS 9, 11, 13, and 15 showed 90.7% identity. No significant homology was found among the other RS.
FIG. 4.
(A) Loci of the GpSGHV genome with repeated elements. The repeat core elements and their direction are represented by arrows. The name, type, size, copy number, and genome location of the repeat are indicated on the right. DR, direct repeat; IR, inverted repeat. Length, >50 bp; number, >2). (B) Alignment of nucleotide sequences of R9, R11, R13, and R15 direct repeats.
Repeat sequences have been observed in all large dsDNA viruses, e.g., baculoviruses, entomopoxviruses, insect iridoviruses, ascoviruses, herpesviruses, and adenoviruses (35). They occur at multiple locations along the genome and may serve as origins of DNA replication (43) and/or as enhancers of transcription (25, 26).
Proteins similar to baculovirus-nudivirus structural envelope proteins.
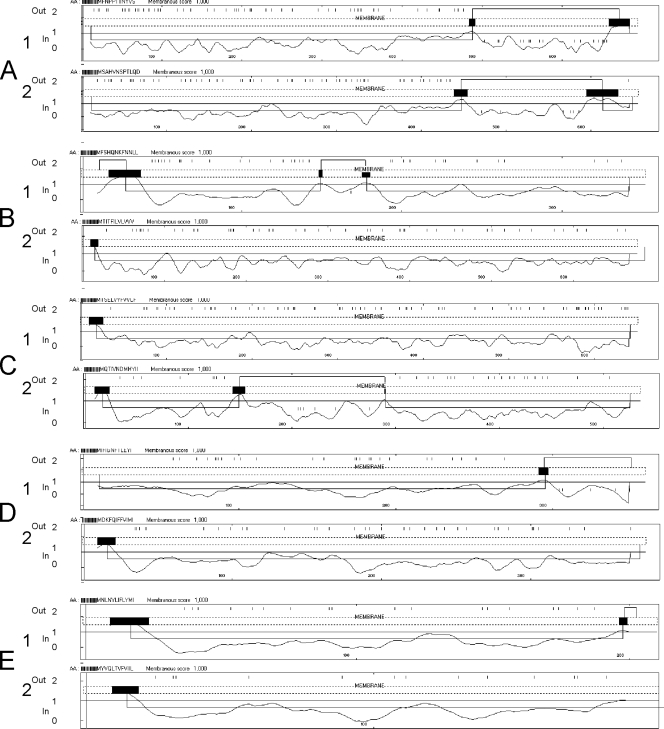
Among the 12 ORFs in the GpSGHV sequence showing similarities to baculovirus and nudivirus genes, five of these, SGHV001, SGHV102, SGHV053, SGHV076, and SGHV005, were found to be putative orthologues of the p74 (pif-0), pif-1, pif-2 and pif-3 per os infectivity factors (pifs) and to ODV-e66 genes, respectively. The pif genes are structural genes common to all nudiviruses and baculoviruses, whereas odv-e66 is specific to lepidopteran baculoviruses. They are known or presumed to encode envelope proteins typical of the odv phenotype that are defined as important proteins for oral infectivity of baculoviruses released from occlusion bodies (polyhedra or granules) in the midgut lumen. The four PIF proteins were shown to be essential for oral infectivity (41, 44, 52, 58). PIF-0, PIF-1, and PIF-2 are thought to be external proteins with a C-terminal (PIF-0) or an N-terminal (PIF-1 and PIF-2) hydrophobic transmembrane domain assumed to anchor these proteins in the ODV envelope (19, 67), whereas the highly hydrophobic N-terminal sequences of ODV-e66 and PIF-3 would anchor these proteins to the inner side of the ODV envelope (11, 32, 66). Interestingly, the SGHV p74-like, ODV-e66-like, PIF-1-like, and PIF-3-like proteins display predicted transmembrane domains very similar to those of their baculovirus homologues (Fig. 5). In contrast, the predicted transmembrane domain of SGHV PIF-2-like protein is clearly located in the C-terminal region, whereas it is N terminal in the baculovirus PIF-2 sequence. Whereas PIF-0 and PIF-1 have been identified as attachment proteins that mediate specific binding of ODVs to midgut target cells (44, 52), the role of PIF-2 and PIF-3 in the early events of midgut cell infection is not yet elucidated.
FIG. 5.
Hydrophobicity profiles of GpSGHV PIF- and ODV-E66-like proteins compared to their insect virus homologues. (A) SGHV001 (1) and p74 of Spodoptera litura NPV (2); (B) SGHV005 (odv-e66) (1) and odv-e66 of Epiphyas postvittana NPV (2); (C) SGHV102 (1) and pif-1 of Neodiprion abietis NPV (2); (D) SGHV053 (1) and pif-2 of Gryllus bimaculatus nudivirus (2); (E) SGHV076 (1) and pif-3 of Helicoverpa zea SNPV (2).
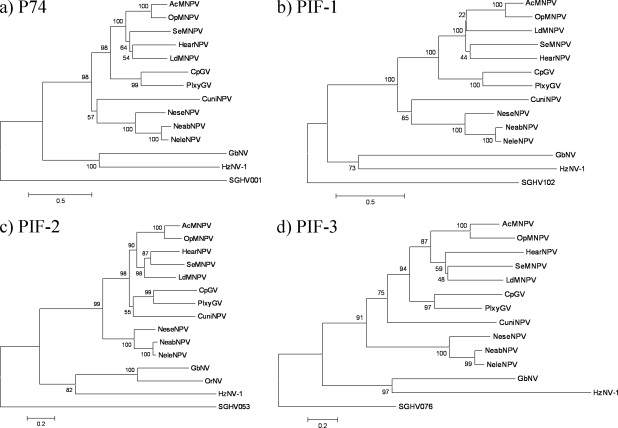
Taken together, these comparisons suggest that the four PIF-like and the ODV-e66-like GpSGHV proteins could be putative orthologues to baculovirus ODV envelope proteins. The pif-0 to -3 genes have been identified so far in all sequenced baculoviruses and nudiviruses and are considered core structural genes of these viruses (78). The phylogenetic analyses shown in Fig. 6 did not provide any evidence that any of these pif genes was horizontally transferred from a baculovirus or a nudivirus to GpSGHV. Considering the very low sequence similarities, and the consistent phylogenetic trees for the different pif genes and assuming that these viruses share a common ancestor, it is suggested that SGHV, nudiviruses, and baculoviruses diverged a long time ago. Nevertheless, the effective presence of these proteins and their localization in the GpSGHV virion first need to be demonstrated before assigning them a putative function.
FIG. 6.
Neighbor-joining phylogenetic trees of SGHV001 (p74) (a), SGHV102 (pif-1) (b), SGHV053 (pif-2) (c), and SGHV076 (pif-3) (d) and their homologues in baculoviruses and nimaviruses. The following viruses (GenBank accession numbers are in parentheses) were included: lepidopteran-specific NPVs AcMNPV (NC_001623), O. pseudotsugata (Op) MNPV (NC_001875), L. dispar (Ld) MNPV (NC_001973), S. exigua (Se) MNPV (NC_002169), and H. armigera (Hear) NPV (NC_002654); granuloviruses C. pomonella (Cp) GV (NC_002816) and P. xylostella (Plxy) GV (NC_002593); hymenopteran-specific NPVs N. sertifer (Nese) NPV (NC_005905), N. lecontei (Nele) NPV (NC_005906), and N. abietes (Neab) NPV (NC_008252); dipteran-specific NPV C. nigripalpus (Cuni) NPV (NC_003084); and nudiviruses H. zea (Hz) NV-1 (NC_004156), G. bimaculatus (Gb) NV (NC_009240), and O. rhinoceros (Or) NV (DQ665871 and DQ665870). Distances were calculated using Poisson correction. The homogeneous substitution pattern among lineages with gamma distributed rate among sites (gamma parameter 2.25) was employed for reconstruction of the trees. The robustness of the tree was tested using bootstrap analysis (500 replicates). Numbers on the nodes indicate bootstrap values.
Putative orthologues to baculovirus-nudivirus auxiliary and unknown proteins.
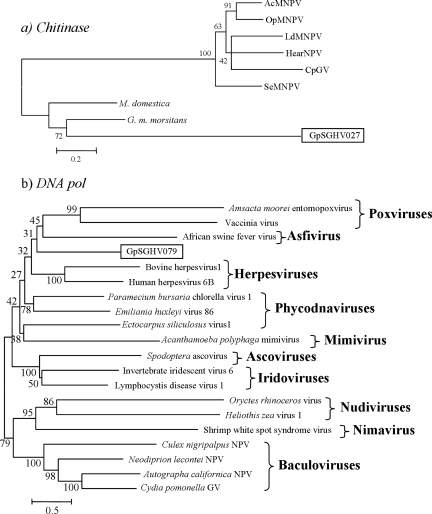
Baculovirus auxiliary genes are nonessential for replication and virus production but most likely provide a selective advantages to the host-virus relationships under natural conditions (53). Most baculoviruses isolated from lepidoptera have a late-transcribed chitinase that, in association with a cysteine cathepsin, promotes liquefaction of larval tissue and release of occlusion bodies into the environment, thus favoring horizontal transmission (29, 68). We have identified a chitinase gene (SGHV027) showing high BLASTP similarities and significant amino acid identity (33%) to the Glossina morsitans midgut chitinase gene. The best BLAST hit to a viral gene was to Chrysodeixis chalcites nucleo polyhedro virus (NPV) chitinase (76), with only 14.7% amino acid identity. Unlike baculovirus chitinases, SGHV027 protein lacks the C-terminal KDEL endoplasmic reticulum retention motif as well as the highly conserved family 18 glycosyl hydrolase motif SGGWT. Furthermore, phylogenetic analysis confirmed that it is distantly related to its baculovirus homologues but is closer to dipteran chitinases (Fig. 7). These data suggest (i) that GpSGHV probably acquired its chitinase from its host rather than from a prokaryote gene as has been suggested for baculovirus chitinases (28) and (ii) that its role is probably different from that of baculovirus chitinases involved, in association with viral cathepsins, in the liquefaction of larval tissues. It is noteworthy that flies dying from GpSGHV infection do not show symptoms of internal lysis. The role played by the predicted GpSGHV chitinase, assuming that this gene is functional, remains to be elucidated.
FIG. 7.
Neighbor-joining phylogenetic trees of SGHV027 (chitinase) (a) and SGHV079 (DNA polymerase) (b) proteins. The phylogenetic tree of SGHV027 chitinase was constructed by using the following lepidopteran baculovirus chitinases (GenBank accession number are in parentheses): AcMNPV (NC_001623), O. pseudotsugata (Op) MNPV (NC_001875), L. dispar (Ld) MNPV (NC_001973), H. armigera (Hear) NPV (NC_002654), C. pomonella (Cp) GV (NC_002816), S. exigua (Se) MNPV (NC_002169), and two putative chitinase proteins from Musca domestica (ABI29879) and Glossina morsitans morsitans (AF337908). Distances were calculated as for Fig. 6. The phylogenetic tree of SGHV079 DNA polymerase and its homologues is based on 2,374 sites of DNA polymerases of 20 viruses from various families. Distances were calculated using Poisson correction. The robustness of the tree was tested using bootstrap analysis (1,000 replicates). Numbers on the nodes indicate bootstrap values. The names of the selected virus families are indicated on the tree. The GenBank accession numbers of the viral DNA polymerases (from top to bottom with the exception of SGHV079) are NP_064832, NP_063712, NP_042783, NP_045328, NP_050219, NP_048532, YP_293784, NP_077578, YP_142676, AAC54632, NP_149500, NP_078724, ABF93350, NP_690550, NP_478036, NP_203396, YP_025217, NP_054095, and NP_148895.
The GpSGHV genome has an additional baculovirus-like auxiliary gene, SGHV110, a putative orthologue of mp-nnase. This gene, which belongs to the Zn-dependent metalloproteases of the matrix metalloproteinase superfamily, is present in most granulovirus genomes. It is considered to be involved in the breakdown of the peritrophic membrane in the late stage of infection (27, 30, 82). Since no homologues to baculovirus cathepsin or viral enhancing factor genes were detected in the GpSGHV sequence, we hypothesize that the GpSGHV mp-nase-like protein might be involved in the disruption of the peritrophic membrane.
Proteins involved in nucleotide metabolism.
Two consecutive genes, the SGHV035 and SGHV036 ORFs, show homology to two putative viral thymidylate synthase genes from Melanoplus sanguinipes entomopoxvirus (MSV238, 33.3% identity) and from white spot syndrome virus of shrimp (ORF 67, 37.4% identity), respectively. Thymidylate synthase catalyzes the metabolism of dUTP to yield the nucleotide precursor of dTMP and promotes an important step in the de novo pathway for the biosynthesis of pyrimidines (13). An orthologue of SGHV035 and SGHV036 is also present in H. zea nudivirus-1 (14), in Chilo iridescent virus (49), and in several herpesviruses (4).
Proteins involved in DNA replication and transcription.
The SGHV079 ORF encodes a putative 108-kDa protein homologous to the delta catalytic subunit of DNA polymerases from several eukaryotic organisms (5). It contains LDFASLYPS, KX3NSXYG, and YGDTS, highly conserved motifs of DNA polymerase of the B family (64). The BLAST best match to a viral gene was to acelaphine herpesvirus DNA polymerase, with 24.9% identity (Table 2), whereas the best hit to a baculovirus had a score of 96 and only 15.9% identity to Choristoneura fumiferana MNPV DNA polymerase (76). Alignment and phylogenetic analysis of GpSGHV DNA polymerase with 19 DNA polymerase sequences from different families of large dsDNA viruses provided evidence that GpSGHV is not closely related to any of the previously known families of large dsDNA viruses.
Four GpSGHV ORFs showed weak similarities with four nonconserved baculovirus genes involved in DNA replication. These are SGHV048 with 18.8% identity to ADP pyrophosphatase (ADPRase) of Helicoverpa armigera NPV, SGHV074 with 20.6% identity to helicase-2 of Choristoneura occidentalis granulovirus, SGHV125 with 25.9% identity to cg30 of Ectotropis obliqua NPV, and SGHV126 with 29.4% identity to pe38 of Anticarsia gemmatalis NPV (Table 2).
An ADPRase gene has been found only in lepidopteran NPVs and granuloviruses and not in hymenopteran and dipteran specific baculoviruses (36). ADPRases belong to a subfamily of Nudix proteins that catalyze the breakdown of ADP-ribose to AMP and ribose-5 phosphate (47). Sequence alignment of SGHV048 with the cellular ADPRases clearly shows that it contains the consensus Nudix motif, G/X5/E/X7/REU/X/EE/X2/U. Deletion of this gene from the Autographa californica MNPV (AcMNPV) sequence resulted in a significant increase in DNA replication in the early stage of infection, but its precise function in virus replication is unclear (23). This type of enzyme is also found in poxviruses (D10 gene of vaccinia virus), where it is considered to be involved in the down-regulation of viral gene expression (55). The helicase-2 gene (homologous to SGHV052) (data not shown in Table 2) is specific to granulovirus and nudivirus genomes (79). It is distantly related to the conserved helicase core gene of baculoviruses and is probably involved in DNA repair and recombination (57). pe38 is an immediate-early baculoviral transactivator gene stimulating early promoter activity and DNA replication (83), whereas cg-30 has characteristics of eukaryotic transcriptional activator (71). Finally, SGHV065 has 22.8% identity with the AMV039 ORF, encoding a putative ATPase-DNA helicase of Amsacta moorei EPV.
Like those of most large DNA viruses, the GpSGHV genome is assumed to encode a DNA-dependent RNA polymerase necessary for the transcription of late genes. This function is normally fulfilled by a complex of proteins including at least two RNA polymerase subunits and initiation, elongation, and termination factors. Surprisingly, none of the 160 putative ORFs of the GpSGHV sequence showed significant hits to DNA-dependent RNA polymerase subunits from large circular or linear dsDNA invertebrate viruses. The only similarities found concerned SGHV059, which had a BLASTP hit to a DNA-dependent RNA polymerase of Plasmodium falciparum with 28.9% identity, and SGHV054 showing 29.9% identity to the AMV054 ORF, a putative RNA polymerase-associated transcriptional specificity factor of Amsacta moorei EPV (Table 2). The apparent lack of a DNA-dependent RNA polymerase orthologue in the GpSGHV genome may reflect differences in the modalities of the expression of its late genes compared to other large DNA viruses.
Similarities of two SGHV ORFs to A-type inclusion poxvirus genes.
Sixteen (34%) of the 47 SGHV genes showing similarities to other viral genes had best BLASTP scores to entomopoxvirus genes (Table 2). Two of these, SGHV010 and SGHV131, were homologous to a family of proteins originally described in poxviruses as viral A-type (for acidophilic type) inclusion proteins (ATIs) but later found in many unicellular organisms. This name refers originally to large proteinaceous bodies observed in cells infected with cowpox virus (16). ATIs are made up primarily of intracellular mature virions embedded in a large (160-kDa) late protein, named A-type inclusion protein (56). The gene encoding this protein (ati) is highly conserved via a truncated version in many orthopoxviruses that do not make inclusions. It is not essential in tissue culture, and its function as a truncated protein is not known (B. Moss, personal communication). SGHV010 had a low (15.8%) identity with cowpox ATI protein (GenBank accession no. BAA00222). However, alignment of the two proteins revealed a regular distribution of the 194 identical amino acid residues along the homologous region of 1,227 amino acid residues (data not shown). This suggests that the two proteins very likely belong to the same gene family. In addition to poxvirus A-type inclusion proteins, SGHV010 and SGHV131 also shared significant similarities with putative desmoplakins of Gryllus bimaculatus nudivirus (ORF77, GenBank accession no. YP_654512) and Choristoneura occidentalis granulovirus (ORF 91 GenBank accession no. YP_006242). Desmoplakins are products of cellular genes involved in intracellular junctions, but no function has been assigned to date to their viral homologues. Given these homologies, we suggest that the SGHV010 and SGHV131 proteins could play a role similar to that of poxvirus A-type proteins in embedding virus particles. This could favor cell-to-cell transportation of virions or their protection and dissemination during the extracellular phase of their infection cycle. A similar function could be assigned to desmoplakins of rod-shaped, enveloped, large DNA viruses devoid of an occlusion body (18, 79).
GpSGHV transcripts.
We looked for putative GpSGHV mRNAs in the G. morsitans morsitans expressed sequence tag (EST) database (http://www.genedb.org/genedb/glossina/BLAST.jsp). The tissues used to prepare cDNA came from flies known to be chronically infected with GpSGHV (S. Aksoy, personal communication). The search (TBLASTX) indeed revealed significant identities (64 to 100%) between 12 GpSGHV ORFs and G. morsitans morsitans ESTs, mainly and not surprisingly from the salivary gland cDNA library (data not shown). The homologies concerned SGHV010, SGHV044, SGHV045, SGHV060, SGHV061, SGHV062, SGHV064, SGHV074, SGHV107, SGHV108, SGHV111, and SGHV112. Surprisingly, only six of these ORFs (SGHV010, SGHV062, SGHV064, SGHV074, SGHV107, and SGHV108) had hits to viral genes, whereas six (SGHV044, SGHV045, SGHV060, SGHV061, SGHV111, and SGHV112) had no hit with any of the NCBI protein databases. Hence, these 12 ORFs can be considered to encode bona fide functional SGHV proteins.
GpSGHV shares several important features with baculoviruses, nudiviruses, and nimaviruses, such as rod-shaped enveloped virions (Fig. 1), a nuclear site of replication (33), and a large circular dsDNA genome with multiple dispersed repeat regions. However, its genome content and gene arrangement differ in many aspects, and phylogenetic analyses of selected genes did not provide evidence of a close relationship to any of these viruses. In addition, there are many unique biological features of this virus. One important difference concerns the pathology and tissue tropism of GpSGHV. GpSGHV induces a characteristic syndrome of SGH in its host, a property shared with two other viruses of Diptera described in Musca domestica (15) and Merodon equestris (6). It also induces lesions in gonads and accessory glands of male and female tsetse flies (38, 39, 59, 60) and in the milk glands of females (61), which may be responsible for mother-to-offspring transmission. This specific tissue tropism for some target organs of adult flies reflects a close and very likely ancient adaptation of the virus to the biology of its host. In this respect, the pathology induced by GpSGHV differs profoundly from those described for other large dsDNA viruses of insects.
The analysis of the GpSGHV genome reported here provides a second set of evidence that this virus not only differs from baculoviruses, nudiviruses, and nimaviruses but in fact cannot be assigned to any of the large dsDNA virus families so far known to infect invertebrates or vertebrates. The similarity between the GpSGHV genome and those of other large dsDNA viruses appears to be limited to a very small number of genes with well-known functions, including, surprisingly, four putative orthologues to baculovirus envelope genes p74, pif-1, pif-2, and pif-3 and a DNA polymerase gene similar to those of herpesviruses. However, the phylogenic trees of these five GpSGHV genes clearly established their ancient divergence from their putative orthologues. The similarity to about 40 additional viral genes from different virus families and whose function is mostly unknown further underlines the uniqueness of GpSGHV. Taken together these data justify, in our opinion, the proposal that GpSGHV represents a new type of insect virus for which a taxonomic position has to be defined.
Acknowledgments
We thank Christine Glazer from the Veterinary University, Vienna, Austria, for her help with electron microscopy and Maria Bernery at the Austrian Research Centre for her help with ultracentrifugation. We thank Rudi Boigner for his technical assistance.
Footnotes
Published ahead of print on 13 February 2008.
REFERENCES
- 1.Abd-Alla, A., H. Bossin, F. Cousserans, A. Parker, M. Bergoin, and A. Robinson. 2007. Development of a non-destructive PCR method for detection of the salivary gland hypertrophy virus (SGHV) in tsetse flies. J. Virol. Methods 139143-149. [DOI] [PubMed] [Google Scholar]
- 2.Afonso, C. L., E. R. Tulman, Z. Lu, E. Oma, G. F. Kutish, and D. L. Rock. 1999. The genome of Melanoplus sanguinipes entomopoxvirus. J. Virol. 73533-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aksoy, S., and R. V. M. Rio. 2005. Interactions among multiple genomes: Tsetse, its symbionts and trypanosomes. Insect Biochem. Mol. Biol. 35691-698. [DOI] [PubMed] [Google Scholar]
- 4.Albrecht, J. C., J. Nicholas, D. Biller, K. R. Cameron, B. Biesinger, C. Newman, S. Wittmann, M. A. Craxton, H. Coleman, B. Fleckenstein, and R. W. Honess. 1992. Primary structure of the herpesvirus saimiri genome. J. Virol. 665047-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amargier, A., J. P. Lyon, C. Vago, G. Meynadier, and J. C. Veyrunes. 1979. Discovery and purification of a virus in gland hyperplasia of insects. Study of Merodon equestris F. (Diptera, Syrphidae). C. R. Acad. Sci. D 289481-484. [PubMed] [Google Scholar]
- 7.Asgari, S., J. Davis, D. Wood, P. Wilson, and A. McGrath. 2007. Sequence and organization of the Heliothis virescens ascovirus genome. J. Gen. Virol. 881120-1132. [DOI] [PubMed] [Google Scholar]
- 8.Bawden, A. L., K. J. Glassberg, J. Diggans, R. Shaw, W. Farmerie, and R. W. Moyer. 2000. Complete genomic sequence of the Amsacta moorei entomopoxvirus: analysis and comparison with other poxviruses. Virology 274120-139. [DOI] [PubMed] [Google Scholar]
- 9.Benson, G. 1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27573-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bideshi, D. K., M. V. Demattei, F. Rouleux-Bonnin, K. Stasiak, Y. Tan, S. Bigot, Y. Bigot, and B. A. Federici. 2006. Genomic sequence of Spodoptera frugiperda ascovirus 1a, an enveloped, double-stranded DNA insect virus that manipulates apoptosis for viral reproduction. J. Virol. 8011791-11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braunagel, S. C., S. T. Williamson, S. Saksena, Z. Zhong, W. K. Russell, D. H. Russell, and M. D. Summers. 2004. Trafficking of ODV-E66 is mediated via a sorting motif and other viral proteins: facilitated trafficking to the inner nuclear membrane. Proc. Natl. Acad. Sci. USA 1018372-8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burtt, E. 1945. Hypertrophied salivary glands in Glossina: evidence that G. pallidipes with this abnormality is particularly suited to trypanosome infection. Ann. Trop. Med. Parasitol. 3911-13. [Google Scholar]
- 13.Carreras, C. W., and D. V. Santi. 1995. The catalytic mechanism and structure of thymidylate synthase. Annu. Rev. Biochem. 64721-762. [DOI] [PubMed] [Google Scholar]
- 14.Cheng, C. H., S. M. Liu, T. Y. Chow, Y. Y. Hsiao, D. P. Wang, J. J. Huang, and H. H. Chen. 2002. Analysis of the complete genome sequence of the Hz-1 virus suggests that it is related to members of the Baculoviridae. J. Virol. 769024-9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coler, R. R., D. G. Boucias, J. H. Frank, J. E. Maruniak, A. Garcia-Canedo, and J. C. Pendland. 1993. Characterization and description of a virus causing salivary gland hyperplasia in the housefly, Musca domestica. Med. Vet. Entomol. 7275-282. [DOI] [PubMed] [Google Scholar]
- 16.Downie, A. W. A. 1939. Study of the lesions produced experimentally by cowpox virus. J. Pathol. Bacteriol. 48361-379. [Google Scholar]
- 17.Ellis, D. S., and I. Maudlin. 1987. Salivary gland hyperplasia in wild caught tsetse from Zimbabwe. Entomol. Exp. Appl. 45167-173. [Google Scholar]
- 18.Escasa, S. R., H. A. Lauzon, A. C. Mathur, P. J. Krell, and B. M. Arif. 2006. Sequence analysis of the Choristoneura occidentalis granulovirus genome. J. Gen. Virol. 871917-1933. [DOI] [PubMed] [Google Scholar]
- 19.Faulkner, P., J. Kuzio, G. V. Williams, and J. A. Wilson. 1997. Analysis of p74, a PDV envelope protein of Autographa californica nucleopolyhedrovirus required for occlusion body infectivity in vivo. J. Gen. Virol. 783091-3100. [DOI] [PubMed] [Google Scholar]
- 20.Feldmann, H. U., H. F. Barnor, and R. Acs. 1992. Abweichungen in der Reproduktion von Glossina morsitans submorsitans Newstead (Diptera: Glossinidae): Untersuchungen zur Behebung eines gestörten Geschlechterverhältnisses und zum Übertragungsweg von Fertilitäts-reduzierenden Viren an die Nachkommen. Mitt. Dtsch. Ges. Allg. Angew. Ent. 8248-251. [Google Scholar]
- 21.Feldmann, U. 1994. Guidelines for the rearing of tsetse flies using the membrane feeding technique, p. 449-471. In J. P. R. Ochieng'-Odero (ed.), Techniques of insect rearing for the development of integrated pest and vector management strategies. ICIPE Science Press, Nairobi, Kenya.
- 22.Feldmann, U., V. A. Dyck, R. C. Mattioli, and J. Jannin. 2005. Potential impact of tsetse fly control involving the sterile insect technique, p. 701-723. In V. A. Dyck, J. Hendrichs, and A. S. Robinson (ed.), Sterile insect technique. Principles and practice in area-wide integrated pest management. Springer, Dordrecht, The Netherlands.
- 23.Ge, J., Z. Wei, Y. Huang, J. Yin, Z. Zhou, and J. Zhong. 2007. AcMNPV ORF38 protein has the activity of ADP-ribose pyrophosphatase and is important for virus replication. Virology 361204-211. [DOI] [PubMed] [Google Scholar]
- 24.Gouteux, J. P. 1987. Prevalence of enlarged salivary glands in Glossina palpalis, G. pallicera, and G. nigrofusca (Diptera: Glossinidae) from the Vavoua area, Ivory Coast. J. Med. Entomol. 24268. [DOI] [PubMed] [Google Scholar]
- 25.Guarino, L. A., M. A. Gonzalez, and M. D. Summers. 1986. Complete sequence and enhancer function of the homologous DNA regions of Autographa californica nuclear polyhedrosis virus. J. Virol. 60224-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guarino, L. A., and M. D. Summers. 1986. Interspersed homologous DNA of Autographa californica nuclear polyhedrosis virus enhances delayed-early gene expression. J. Virol. 60215-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto, Y., T. Hayakawa, Y. Ueno, T. Fujita, Y. Sano, and T. Matsumoto. 2000. Sequence analysis of the Plutella xylostella granulovirus genome. Virology 275358-372. [DOI] [PubMed] [Google Scholar]
- 28.Hawtin, R. E., K. Arnold, M. D. Ayres, P. M. Zanotto, S. C. Howard, G. W. Gooday, L. H. Chappell, P. A. Kitts, L. A. King, and R. D. Possee. 1995. Identification and preliminary characterization of a chitinase gene in the Autographa californica nuclear polyhedrosis virus genome. Virology 212673-685. [DOI] [PubMed] [Google Scholar]
- 29.Hawtin, R. E., T. Zarkowska, K. Arnold, C. J. Thomas, G. W. Gooday, L. A. King, J. A. Kuzio, and R. D. Possee. 1997. Liquefaction of Autographa californica nucleopolyhedrovirus-infected insects is dependent on the integrity of virus-encoded chitinase and cathepsin genes. Virology 238243-253. [DOI] [PubMed] [Google Scholar]
- 30.Hayakawa, T., R. Ko, K. Okano, S. I. Seong, C. Goto, and S. Maeda. 1999. Sequence analysis of the Xestia c-nigrum granulovirus genome. Virology 262277-297. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann, K., P. Bucher, L. Falquet, and A. Bairoch. 1999. The PROSITE database, its status in 1999. Nucleic Acids Res. 27215-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong, T., M. D. Summers, and S. C. Braunagel. 1997. N-terminal sequences from Autographa californica nuclear polyhedrosis virus envelope proteins ODV-E66 and ODV-E25 are sufficient to direct reporter proteins to the nuclear envelope, intranuclear microvesicles and the envelope of occlusion derived virus. Proc. Natl. Acad. Sci. USA 944050-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaenson, T. G. T. 1978. Virus-like rods associated with salivary gland hyperplasia in tsetse, Glossina pallidipes. Trans. R. Soc. Trop. Med. Hyg. 72234-238. [DOI] [PubMed] [Google Scholar]
- 34.Jakob, N. J., K. Müller, U. Bahr, and G. Darai. 2001. Analysis of the first complete DNA sequence of an invertebrate iridovirus: coding strategy of the genome of Chilo iridescent virus. Virology 286182-196. [DOI] [PubMed] [Google Scholar]
- 35.Jancovich, J. K., J. Mao, V. G. Chinchar, C. Wyatt, S. T. Case, S. Kumar, G. Valente, S. Subramanian, E. W. Davidson, J. P. Collins, and B. L. Jacobs. 2003. Genomic sequence of a ranavirus (family Iridoviridae) associated with salamander mortalities in North America. Virology 31690-103. [DOI] [PubMed] [Google Scholar]
- 36.Jehle, J. A., M. Lange, H. Wang, Z. Hu, Y. Wang, and R. Hauschild. 2006. Molecular identification and phylogenetic analysis of baculoviruses from Lepidoptera. Virology 346180-193. [DOI] [PubMed] [Google Scholar]
- 37.Jordan, A. M. 1986. Trypanosomiasis control and African rural development. Longman, London, United Kingdom.
- 38.Jura, W. G. Z. O., T. R. Odhiambo, L. H. Otieno, and N. O. Tabu. 1988. Gonadal lesions in virus-infected male and female tsetse, Glossina pallidipes (Diptera: Glossinidae). J. Invertebr. Pathol. 521-8. [DOI] [PubMed] [Google Scholar]
- 39.Jura, W. G. Z. O., L. H. Otieno, and M. M. B. Chimtawi. 1989. Ultrastructural evidence for trans-ovum transmission of the DNA virus of tsetse, Glossina pallidipes (Diptera: Glossinidae). Curr. Microbiol. 181-4. [Google Scholar]
- 40.Jura, W. G. Z. O., J. Zdarek, and L. H. Otieno. 1993. A simple method for artificial infection of tsetse, Glossina morsitans morsitans larvae with the DNA virus of G. pallidipes. Insect Sci. Appl. 14383-387. [Google Scholar]
- 41.Kikhno, I., S. Gutierrez, L. Croizier, G. Croizier, and M. L. Ferber. 2002. Characterization of pif, a gene required for the per os infectivity of Spodoptera littoralis nucleopolyhedrovirus. J. Gen. Virol. 833013-3022. [DOI] [PubMed] [Google Scholar]
- 42.Kokwaro, E. D., M. Nyindo, and M. Chimtawi. 1990. Ultrastructural changes in salivary glands of tsetse, Glossina morsitans morsitans, infected with virus and rickettsia-like organisms. J. Invertebr. Pathol. 56337-346. [DOI] [PubMed] [Google Scholar]
- 43.Kool, M., C. H. Ahrens, J. M. Vlak, and G. F. Rohrmann. 1995. Replication of baculovirus DNA. J. Gen. Virol. 762103-2118. [DOI] [PubMed] [Google Scholar]
- 44.Kuzio, J., R. Jaques, and P. Faulkner. 1989. Identification of p74, a gene essential for virulence of baculovirus occlusion bodies. Virology 173759-763. [DOI] [PubMed] [Google Scholar]
- 45.Margulies, M., M. Egholm, W. E. Altman, S. Attiya, J. S. Bader, L. A. Bemben, J. Berka, M. S. Braverman, Y. J. Chen, Z. Chen, S. B. Dewell, L. Du, J. M. Fierro, X. V. Gomes, B. C. Godwin, W. He, S. Helgesen, C. H. Ho, G. P. Irzyk, S. C. Jando, M. L. Alenquer, T. P. Jarvie, K. B. Jirage, J. B. Kim, J. R. Knight, J. R. Lanza, J. H. Leamon, S. M. Lefkowitz, M. Lei, J. Li, K. L. Lohman, H. Lu, V. B. Makhijani, K. E. McDade, M. P. McKenna, E. W. Myers, E. Nickerson, J. R. Nobile, R. Plant, B. P. Puc, M. T. Ronan, G. T. Roth, G. J. Sarkis, J. F. Simons, J. W. Simpson, M. Srinivasan, K. R. Tartaro, A. Tomasz, K. A. Vogt, G. A. Volkmer, S. H. Wang, Y. Wang, M. P. Weiner, P. Yu, R. F. Begley, and J. M. Rothberg. 2005. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437376-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marks, H., X. Y. Ren, H. Sandbrink, M. C. Van Hulten, and J. M. Vlak. 2006. In silico identification of putative promoter motifs of white spot syndrome virus. BMC Bioinformatics 7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLennan, A. G. 2006. The Nudix hydrolase superfamily. Cell Mol. Life Sci. 63123-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minter-Goedbloed, E., and D. M. Minter. 1989. Salivary gland hyperplasia and trypanosome infection of Glossina in two areas of Kenya. Trans. R. Soc. Trop. Med. Hyg. 83640-641. [DOI] [PubMed] [Google Scholar]
- 49.Müller, K., C. A. Tidona, U. Bahr, and G. Darai. 1998. Identification of a thymidylate synthase gene within the genome of Chilo iridescent virus. Virus Genes 17243-258. [DOI] [PubMed] [Google Scholar]
- 50.Odindo, M. O., P. A. Amutalla, D. A. Turner, E. D. Kokwaro, W. A. Otieno, and D. M. Sabwa. 1982. Morphological variation and incidence of cuticular lesions in the tsetse Glossina pallidipes Austen, G. brevipalpis Newstead and G. austeni Newstead (Diptera: Glossinidae) on the Kenyan coast. Insect Sci. Appl. 365-71. [Google Scholar]
- 51.Odindo, M. O., C. C. Payne, N. E. Crook, and P. Jarret. 1986. Properties of a novel DNA virus from the tsetse fly, Glossina pallidipes. J. Gen. Virol. 67527-536. [DOI] [PubMed] [Google Scholar]
- 52.Ohkawa, T., J. O. Washburn, R. Sitapara, E. Sid, and L. E. Volkman. 2005. Specific binding of Autographa californica M nucleopolyhedrovirus occlusion-derived virus to midgut cells of Heliothis virescens larvae is mediated by products of pif genes Ac119 and Ac022 but not by Ac115. J. Virol. 7915258-15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Reilly, D. R. 1997. Auxiliary genes of baculoviruses, p. 266-300. In L. K. Miller (ed.), The baculoviruses. Plenum, New York, NY.
- 54.Otieno, L. H., E. D. Kokwaro, M. Chimtawi, and P. Onyango. 1980. Prevalence of enlarged salivary glands in wild populations of Glossina pallidipes in Kenya, with a note on the ultrastructure of the affected organ. J. Invertebr. Pathol. 36113-118. [Google Scholar]
- 54a.Parker, N. J., and A. G. Parker. Simple tools for assembling and searching high-density picolitre pyrophosphate sequence data. Source Code Biol. Med., in press. [DOI] [PMC free article] [PubMed]
- 55.Parrish, S., and B. Moss. 2006. Characterization of a vaccinia virus mutant with a deletion of the D10R gene encoding a putative negative regulator of gene expression. J. Virol. 80553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel, D. D., D. J. Pickup, and W. K. Joklik. 1986. Isolation of cowpox virus A-type inclusions and characterization of their major protein component. Virology 149174-189. [DOI] [PubMed] [Google Scholar]
- 57.Pearson, M. N., and G. F. Rohrmann. 1998. Characterization of a baculovirus-encoded ATP-dependent DNA ligase. J. Virol. 729142-9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pijlman, G. P., A. J. Pruijssers, and J. M. Vlak. 2003. Identification of pif-2, a third conserved baculovirus gene required for per os infection of insects. J. Gen. Virol. 842041-2049. [DOI] [PubMed] [Google Scholar]
- 59.Sang, R. C., W. G. Z. O. Jura, L. H. Otieno, and R. W. Mwangi. 1998. The effects of a DNA virus infection on the reproductive potential of female tsetse flies, Glossina morsitans centralis and Glossina morsitans morsitans (Diptera: Glossinidae). Mem. Inst. Oswaldo Cruz 93861-864. [DOI] [PubMed] [Google Scholar]
- 60.Sang, R. C., W. G. Z. O. Jura, L. H. Otieno, R. W. Mwangi, and P. Ogaja. 1999. The effects of a tsetse DNA virus infection on the functions of the male accessory reproductive gland in the host fly Glossina morsitans centralis (Diptera; Glossinidae). Curr. Microbiol. 38349-354. [DOI] [PubMed] [Google Scholar]
- 61.Sang, R. C., W. G. Z. O. Jura, L. H. Otieno, and P. Ogaja. 1996. Ultrastructural changes in the milk gland of tsetse Glissina morsitans centralis (Diptera; Glissinidae) female infected by a DNA virus. J. Invertebr. Pathol. 68253-259. [DOI] [PubMed] [Google Scholar]
- 62.Sang, R. C., W. G. Z. O. Jura, L. H. Otieno, P. M. Tukei, and R. W. Mwangi. 1997. Effects of tsetse DNA virus infection on the survival of a host fly Glossina morsitans centralis (Diptera: Glossinidae). J. Invertebr. Pathol. 69253-260. [Google Scholar]
- 63.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 745463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shamoo, Y., and T. A. Steitz. 1999. Building a replisome from interacting pieces: sliding clamp complexed to a peptide from DNA polymerase and a polymerase editing complex. Cell 99155-166. [DOI] [PubMed] [Google Scholar]
- 65.Shaw, M. K., and S. K. Moloo. 1993. Virus-like particles in Rickettsia within the midgut epithelial cells of Glossina morsitans centralis and Glossina brevipalpis. J. Invertebr. Pathol. 61162-166. [Google Scholar]
- 66.Slack, J., and B. M. Arif. 2007. The baculoviruses occlusion-derived virus: virion structure and function. Adv. Virus Res. 6999-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slack, J. M., E. M. Dougherty, and S. D. Lawrence. 2001. A study of the Autographa californica multiple nucleopolyhedrovirus ODV envelope protein p74 using a GFP tag. J. Gen. Virol. 822279-2287. [DOI] [PubMed] [Google Scholar]
- 68.Slack, J. M., J. Kuzio, and P. Faulkner. 1995. Characterization of v-cath, a cathepsin L-like proteinase expressed by the baculovirus Autographa californica multiple nuclear polyhedrosis virus. J. Gen. Virol. 761091-1098. [DOI] [PubMed] [Google Scholar]
- 69.Steelman, C. D. 1976. Effects of external and internal arthropod parasites on domestic livestock production. Annu. Rev. Entomol. 21155-178. [DOI] [PubMed] [Google Scholar]
- 70.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 241596-1599. [DOI] [PubMed] [Google Scholar]
- 71.Thiem, S. M., and L. K. Miller. 1989. A baculovirus gene with a novel transcription pattern encodes a polypeptide with a zinc finger and a leucine zipper. J. Virol. 634489-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tidona, C. A., and G. Darai. 2000. Iridovirus homologues of cellular genes—implications for the molecular evolution of large DNA viruses. Virus Genes 2177-81. [PubMed] [Google Scholar]
- 73.Tsai, C. T., J. W. Ting, M. H. Wu, M. F. Wu, I. C. Guo, and C. Y. Chang. 2005. Complete genome sequence of the grouper iridovirus and comparison of genomic organization with those of other iridoviruses. J. Virol. 792010-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tyler, S. D., and A. Severini. 2006. The complete genome sequence of herpesvirus papio 2 (Cercopithecine herpesvirus 16) shows evidence of recombination events among various progenitor herpesviruses. J. Virol. 801214-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Hulten, M. C., J. Witteveldt, S. Peters, N. Kloosterboer, R. Tarchini, M. Fiers, H. Sandbrink, R. K. Lankhorst, and J. M. Vlak. 2001. The white spot syndrome virus DNA genome sequence. Virology 2867-22. [DOI] [PubMed] [Google Scholar]
- 76.van Oers, M. M., M. H. C. Abma-Henkens, E. A. Herniou, J. C. de Groot, S. Peters, and J. M. Vlak. 2005. Genome sequence of Chrysodeixis chalcites nucleopolyhedrovirus, a baculovirus with two DNA photolyase genes. J. Gen. Virol. 862069-2080. [DOI] [PubMed] [Google Scholar]
- 77.Vreysen, M. J. B., K. M. Saleh, M. Y. Ali, A. M. Abdulla, Z.-R. Zhu, K. G. Juma, A. Dyck, A. R. Msangi, P. A. Mkonyi, and H. U. Feldmann. 2000. Glossina austeni (Diptera: Glossinidae) eradicated on the island of Unguja, Zanzibar, using the sterile insect technique. J. Econ. Entomol. 93123-135. [DOI] [PubMed] [Google Scholar]
- 78.Wang, L., J. Xue, C. P. Seaborn, B. M. Arif, and X. W. Cheng. 2006. Sequence and organization of the Trichoplusia ni ascovirus 2c (Ascoviridae) genome. Virology 354167-177. [DOI] [PubMed] [Google Scholar]
- 79.Wang, Y., R. G. Kleespies, A. M. Huger, and J. A. Jehle. 2007. The genome of Gryllus bimaculatus nudivirus indicates an ancient diversification of baculovirus-related nonoccluded nudiviruses of insects. J. Virol. 815395-5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whitnall, A. B. M. 1934. The Trypanosome infections of Glossina pallidipes in the Umfolosi Game Reserve, Zululand. Onderstepoort J. Vet. Sci. Anim. Ind. 27-21. [Google Scholar]
- 81.WHO. 2001. Scientific working group on African trypanosomiasis (sleeping sickness), WHO/TDR Committee Report. World Health Organization, Geneva, Switzerland.
- 82.Wormleaton, S., J. Kuzio, and D. Winstanley. 2003. The complete sequence of the Adoxophyes orana granulovirus genome. Virology 311350-365. [DOI] [PubMed] [Google Scholar]
- 83.Wu, X., S. Stewart, and D. A. Theilmann. 1993. Characterization of an early gene coding for a highly basic 8.9K protein from the Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus. J. Gen. Virol. 741591-1598. [DOI] [PubMed] [Google Scholar]
- 84.Yang, F., J. He, X. Lin, Q. Li, D. Pan, X. Zhang, and X. Xu. 2001. Complete genome sequence of the shrimp white spot bacilliform virus. J. Virol. 7511811-11820. [DOI] [PMC free article] [PubMed] [Google Scholar]