Abstract
The vaccinia virus E2L (VACWR058) gene is conserved in all sequenced chordopoxviruses and is predicted to encode an 86-kDa protein with no recognizable functional motifs or nonpoxvirus homologs. Although the region immediately upstream of the open reading frame lacked optimal consensus promoter motifs, expression of the E2 protein occurred after viral DNA replication. Transfection studies, however, indicated that the promoter was weak compared to well-characterized intermediate and late promoters. The E2 protein was present in mature virions purified from infected cells but was more abundant in extracellular enveloped forms. Despite the conservation of the E2L gene in chordopoxviruses, deletion mutants could be isolated from both the WR and IHD-J strains of vaccinia virus. These null mutants produced very small plaques in all cell lines tested, reduced amounts of mature infectious virions, and very low numbers of extracellular virions. Nevertheless, viral protein synthesis appeared qualitatively and quantitatively normal. The defect in extracellular virus formation was corroborated by electron microscopy, which also showed some aberration in the wrapping of virions by cisternal membranes. Extracellular virions that did form, however, were able to induce actin tail formation.
Poxviruses are large, enveloped, double-stranded DNA viruses that replicate entirely within the cytoplasm of vertebrate or invertebrate cells. The most intensively studied member of the family, vaccinia virus (VACV), contains approximately 200 genes, of which nearly half are conserved in all chordopoxviruses (37). The highly conserved genes encode proteins with roles in viral transcription, genome replication, and the formation of progeny virus particles (22). The viral proteins required for mRNA synthesis and modification include a multisubunit DNA-dependent RNA polymerase; early, intermediate, and late stage-specific transcription factors; elongation factors; capping and methylating enzymes; and a poly(A) polymerase. At least six viral proteins are needed for replication and processing of the viral genome (23), and many more are involved in assembly of the infectious virus particle (6). The latter include membrane proteins, components of the core, a redox system, and proteinases. Many of the less highly conserved proteins are nonessential for replication in tissue culture and have host response functions, including immune evasion (24).
Our present ignorance of the roles of many conserved genes hampers research into the reproductive cycle of poxviruses. Fortunately, experimental methods which can improve this situation are available. The purpose of the present study was to carry out the initial characterization of the product of the VACWR058 (E2L according to the Copenhagen strain nomenclature) open reading frame (ORF), which is conserved in all sequenced chordopoxviruses. We found that the encoded protein (referred to as E2) was expressed postreplicatively and was detected in purified extracellular enveloped virions (EVs) and to a lesser extent in purified intracellular mature virions (MVs). Surprisingly for such a highly conserved gene, a deletion mutant maintained an ability to replicate, although the plaques were very small, the numbers of MVs were reduced, and few EVs were detected.
MATERIALS AND METHODS
Cells and virus strains.
Monolayers of BS-C-1, RK13, and CV-1 African green monkey cells were maintained in Eagle minimum essential medium supplemented with 10% fetal bovine serum (GIBCO, Invitrogen, Carlsbad, CA), 100 units/ml of penicillin, and 100 μg/ml of streptomycin. HeLa and baby hamster kidney (BHK21) cells were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum and antibiotic as indicated above. Recombinant VACV was derived from the Western Reserve (WR) or IHD-J strain and was propagated by following standard procedures (11).
Antibodies.
Two rabbit antisera were raised at Covance Research Products (Princeton, NJ) against the peptides MISVTDIRRAFLDNEC and VETILDNNQSFKSS, derived from the predicted N-terminal and C-terminal amino acids of E2L, respectively. Antiserum 8191 was made by infecting rabbits multiple times with live VACV.
Construction of recombinant viruses with E2L deletions.
The E2L ORF was deleted from the VAC-BAC plasmid in two steps essentially as described previously (10). The first step was to replace the E2L ORF in the VAC-BAC plasmid with the ampicillin resistance gene by recombination in Escherichia coli. The ampR cassette was amplified by PCR from pBR322 using the following primers: ampR-uE2L, 5′-GATAACTCGTTTTTCTTAAGAAATATAAAACTACTGTCTCCAGAGCTCGCTCTATCGGCACTTTTCGGGGAAATGTGCGC-3′, containing 24 nucleotides (underlined) at the 5′ end of the ampR cassette and 56 nucleotides (in italics) from the 3′ side of E2L ORF (note that the last 79 nucleotides of E2L ORF, which overlap with the E1L promoter, were preserved); ampR-doE2L, 5′-GATTAAAAAATACAAATTGTAGTACTATTAACGCGACTAGTATATTCTCTAAAGTTACCAATGCTTAATCAGTGAGGCACC-3′ containing 28 nucleotides (underlined) at the 3′ end of the ampR cassette and 55 nucleotides (in italics) preceding the ATG start codon of the E2L ORF. The PCR product was used to transform E. coli harboring VAC-BAC plasmid/lambda (10), and ampicillin-resistant colonies containing VAC-BACΔE2L were isolated and sequenced to verify the deletion of the E2L ORF. To rescue the mutated VAC-BAC virus, 1 μg VAC-BACΔE2L plasmid was transfected into CV-1 cells that had been infected with 0.1 PFU per cell of helper fowlpox virus as described previously (9). After 7 days, cells were harvested, and the lysate was applied to BS-C-1 cells and covered with a solid agar overlay. Three days later, very small green plaques were detected with a fluorescent microscope, and five days later, single plaques were isolated and amplified to make a stock of viral VAC-BACΔE27 (vVAC-BACΔE2L) virus.
The E2L ORF of the IHD-J strain of VACV was replaced with the enhanced green fluorescent protein (GFP) marker gene regulated by the VACV P7.5 early/late promoter, using homologous recombination with a linear DNA segment containing the GFP sequence flanked by partial sequences of E1L and E3L ORF. As with vVAC-BAC, this construct preserved the last 79 bp of the E2L ORF. The DNA segment was generated in two steps. First, three independent PCRs were carried out to amplify the flanking regions and the GFP ORF: (i) the left flanking fragment, containing part of E1L, was amplified with the oligonucleotide primers 5′-CTCTTGCCAATTCTTCCATTGATGTTAC-3′ and 5′-GATAGAGTTTTTGATAACTCGTTTTTCTTAAGAAATATAAAACTACTGT CTCCAGAGCTCGCTCTATC-3′; (ii) the right flanking fragment, containing part of the E3L ORF, was amplified with the oligonucleotide primers 5′-CTTTAGAGAATATACTAGTCGCGTTAATA-3′ and 5′-CTGATGCTATGGCTGACGTCATAATA-3′; and (iii) the GFP sequence was amplified with the primers 5′-GAAATATAAAACTACTGTCTCCAGAGCTCGCTCTATCGCGGCCGCACTAATTCCAAAC-3′ and 5′-TGTAGTACTATTAACGCGACTAGTATATTCTCTAAAGTTACTTGTACAGCTCGTCCATGCC-3′ (VACV overlapping sequence underlined, GFP sequence in italics). Equal molar amounts of each fragment were combined by a three-way overlap PCR. The PCR product was transfected into BS-C-1 cells infected with IHD-J virus at 0.5 PFU per cell using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Recombinant viruses expressing GFP were isolated by five rounds of plaque purification using an inverted fluorescence microscope.
Revertant viruses.
To reinsert the E2L ORF into the endogenous locus of ΔE2L viruses, we took advantage of the small-plaque phenotype of both vVAC-BACΔE2L and vIHD-JΔE2L. A PCR fragment containing the whole E2L gene as well as about 450 bp on each side of the E2L deletion site was used to transfect BS-C-1 cells infected with vVAC-BACΔE2L or vIHD-JΔE2L virus at 0.5 PFU per cell using Lipofectamine 2000 (Invitrogen). Recombinant viruses forming plaques of wild-type size were picked and isolated by five rounds of plaque purification.
Detergent extraction of purified virions.
Purified virions (3 μg of total protein) were extracted with 50 mM Tris-HCl (pH 7.4) containing 1% Nonidet-40 detergent in the presence or absence of 50 mM dithiothreitol (DTT). The mixture was incubated for 1 h at 37°C, and the soluble and insoluble materials were separated by centrifugation at 12,000 × g for 30 min.
Western blot analysis.
Virions or whole-cell lysates were solubilized in NuPage reduced loading sample buffer (Invitrogen) and heat denatured. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 4 to 12% bis-Tris gels (Invitrogen) and transferred to a polyvinylidene fluoride membrane. Membranes were blocked in Tris-buffered saline with 5% nonfat dry milk and 0.05% Tween 20 and incubated with antibodies for 1 h at room temperate or overnight at 4°C. Protein bands were visualized by chemiluminescence using West-Duro or Femto kits (Pierce, Appleton, WI).
Purification of virions by sucrose gradient centrifugation.
Monolayers of RK13 cells (12 T150 dishes, equivalent to 3.6 × 108 cells) were infected at a multiplicity of 2 PFU per cell, with either VACV IHD-J or vIHD-JΔE2L. At 48 h after infection, the medium was harvested, and cells and large debris were removed by low-speed centrifugation. The cells were scraped, collected by low-speed centrifugation, resuspended in swelling buffer (10 mM Tris-HCl, pH 9.0), incubated on ice for 10 min, disrupted by Dounce homogenization, and clarified by low-speed centrifugation. Virions from the cell lysates was centrifuged twice through a 36% sucrose cushion at 13,000 rpm for 1 h at 4°C, further purified by sedimentation through two continuous 25-to-40% sucrose gradients, and resuspended in 1 mM Tris-HCl, pH 9.0 (12). The culture medium was clarified by low-speed centrifugation at 800 rpm for 10 min, and the EVs were pelleted by centrifugation at 10,000 × g for 1 h at 4°C. The EVs were further purified by two continuous 25-to-40% sucrose density gradients, as for MVs.
CsCl gradient analysis of virions.
A monolayer of RK13 cells (one T150 dish) was infected at a multiplicity of 10 PFU per cell, with either VACV IHD-J or vIHD-JΔE2L. After 2 h, the inoculum was replaced with methionine- and cysteine-free growth medium that contained 2% dialyzed fetal bovine serum (Gibco/Invitrogen) and 500 μCi of a mixture of [35S]methionine and [35S]cysteine (Easy Tag; New England Nuclear/Perkin Elmer, Waltham, MA). After 18 h of infection, the medium was collected, and cells and large debris were removed by low-speed centrifugation. The cells were scraped, collected by low-speed centrifugation, resuspended in swelling buffer (10 mM Tris-HCl, pH 9.0), incubated on ice for 10 min, disrupted by Dounce homogenization, and clarified by low-speed centrifugation. The virus from the cell lysates or the medium was centrifuged through a 36% sucrose cushion at 13,000 rpm for 1 h at 4°C. The virus pellets were resuspended in swelling buffer and layered over a preformed CsCl step gradient as described previously (40). After centrifugation at 32,000 rpm for 4 h at 20°C, fractions were collected from the bottom of the tube, and the radioactive emission in each fraction was determined by scintillation counting.
Electron microscopy.
BS-C-1 cells were grown in dishes with 60-mm-diameter wells and infected with 10 PFU per cell of purified virus. At 18 h after infection, cells were fixed with 2% glutaraldehyde, embedded in Epson resin, and viewed with a FEI-CM100 transmission electron microscope (FEI Company, Hillsboro, OR).
RESULTS
E2L is conserved in all chordopoxviruses.
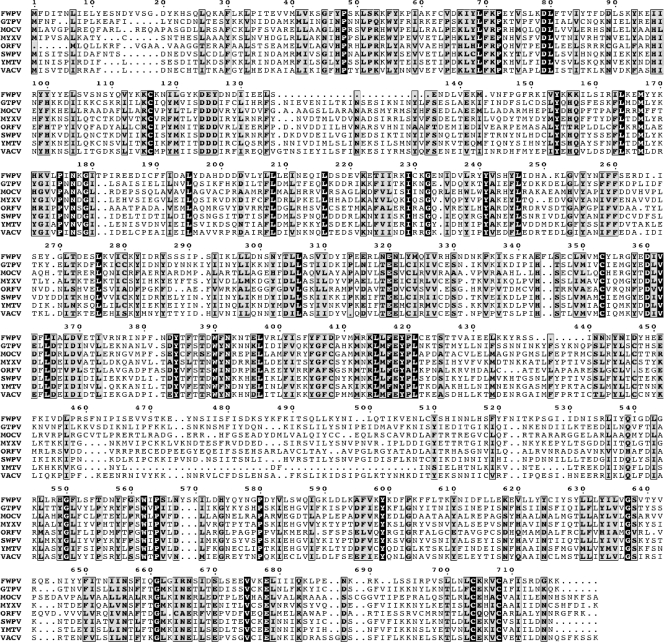
The VACV E2L ORF is 2,214 bp in length and is predicted to encode a protein of 737 amino acids with a mass of 86 kDa. There is a 97% or greater amino acid sequence identity between E2L and homologs in other members of the orthopoxvirus genus (not shown) and 27 to 40% with homologs in more distantly related chordopoxviruses (Fig. 1). Even modified VACV Ankara, a highly passaged, host range-restricted, and greatly attenuated virus, has retained the complete E2L ORF. However, no nonpoxvirus homologs were found, nor were there recognizable signal peptide, transmembrane domain, or functional motifs (except for putative late domains, discussed below). Thus, the conservation of the gene suggested an essential function, but the sequence provided no clue as to what that might be.
FIG. 1.
Multiple-sequence alignment of E2L orthologs. A multiple-sequence alignment was constructed with the T-Coffee algorithm (25). The alignment includes one representative amino acid sequence from each genus of Chordopoxvirus. Amino acids at invariant positions are shown as white letters on a black background; moderately conserved amino acids are shown as black letters against a gray background. Abbreviations: FWPV, fowlpox virus (Avipoxvirus); GTPV, goatpox virus (Capripoxvirus); MOCV, molluscum contagiosum (Molluscipoxvirus); MYXV, myxoma virus (Leporipoxvirus); ORFV, orf virus (Parapoxvirus); SWPV, swinepox virus (Suipoxvirus); YMTV, yaba monkey tumor virus (i); VACV, Orthopoxvirus.
E2 is synthesized late in infection.
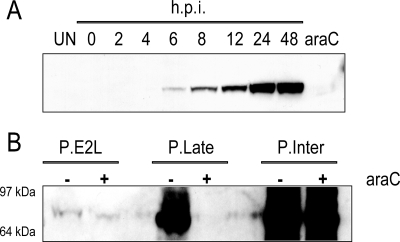
Inspection of the DNA sequence upstream of the E2L ORF did not reveal a previously described optimal consensus poxvirus promoter motif nearby (1, 7, 8). Although there were a few potential intermediate-stage TAAA transcription initiation sites, these did not contain a core sequence with critical A residues 10 to 11 bp upstream (1, 19). To determine whether the E2L ORF is expressed in infected cells, we produced a rabbit polyclonal antibody to a predicted N-terminal peptide. A reactive band, migrating slightly faster than predicted, was resolved by Western blotting of VACV-infected-cell lysates but not of lysates of uninfected cells (Fig. 2A) or cells infected with an E2L deletion mutant (see below). The band was detected at 6 h after infection and increased up to 24 h, whereas the band was faint even after 24 h in the presence of 1-β-D-arabinofuranosylcytosine (AraC), an inhibitor of DNA replication (Fig. 2A). The timing of E2 synthesis and the effect of AraC suggested that the E2L ORF has an intermediate- or late-stage promoter.
FIG. 2.
E2 synthesis. (A) BS-C-1 cells were infected with 10 PFU per cell of VACV strain WR, and whole-cell extracts were prepared at the indicated times. Extracts from mock-infected cells and cells infected in the presence of cytosine arabinoside (AraC) were also prepared, and all extracts were analyzed by SDS-PAGE and Western blotting with rabbit antiserum to E2 followed by anti-rabbit immunoglobulin G conjugated to horseradish peroxidase. h.p.i., hours postinfection. (B) Comparison of E2L with intermediate and late promoters. Plasmids containing the E2L ORF preceded by the E2L promoter, the G8R intermediate promoter (P.Inter), or the F17R late promoter (P.Late) were transfected into cells that had been infected with VACV in the presence (+) or absence (−) of AraC. After 24 h, the cells were harvested, and lysates were analyzed by Western blotting as for panel A. The positions and masses of marker proteins are indicated on the left.
The transcription factors that recognize intermediate promoters are synthesized prior to viral DNA replication, whereas the factors that recognize late promoters are synthesized after viral DNA replication. On this basis, intermediate and late promoters can be distinguished by transfecting expression plasmids into infected cells that have been treated with AraC (1, 39). Three plasmids were constructed, each containing the full-length E2L ORF in case any novel regulatory sequences were present downstream of the start codon. One plasmid contained the natural E2L promoter extending 450 bp upstream of the start codon; the second plasmid contained the F17R late promoter and the third the G8R intermediate promoter. Plasmids were transfected at 1 h after infection in the presence or absence of AraC, and E2 synthesis was measured by Western blotting after an additional 24 h. Overexposure of the blot was necessary to detect E2 synthesized from its natural promoter (Fig. 2B). As expected, the intermediate promoter induced abundant synthesis of E2 in the presence and absence of AraC, whereas the late promoter allowed synthesis only in the absence of the drug (Fig. 2B). Most strikingly, the natural E2L promoter was much weaker than either the G8R or F17R promoter (Fig. 2B). Although an E2 band was detected in the presence of AraC, it was less intense than in the absence of the drug (Fig. 2B). A similar pattern of expression was obtained when the three promoters were used to express firefly luciferase (data not shown). We tentatively concluded that the E2L gene has a very weak, probably atypical intermediate promoter. Further studies are needed to determine the transcription initiation site(s) and to assess the effects of mutations.
E2 is present in purified virions.
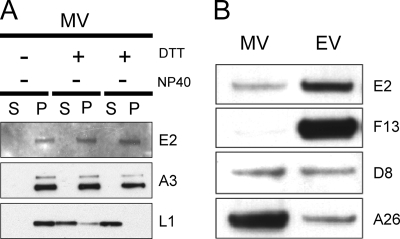
The E2 protein was not previously detected by mass-spectroscopic analyses of purified MVs (5, 29, 45), suggesting that it is not present or is present in small amounts. Nevertheless, we could detect E2 by sensitive chemiluminescence after Western blotting of proteins from MVs purified by sedimentation through a cushion of sucrose followed by two successive sucrose gradients. The E2 protein, like the core component A3, was not solubilized by NP-40 detergent alone or in combination with DTT (Fig. 3A). In contrast, the membrane protein L1 was largely extracted by these procedures (Fig. 3A). These results were consistent with an internal location of E2, although even some membrane-associated proteins are insoluble under these conditions (26).
FIG. 3.
E2 is a virion protein. (A) Resistance to detergent extraction. Purified MVs (WR strain) were extracted (+) with NP-40 or with NP-40 and DTT or mock treated (−) and separated into soluble (S) and pellet (P) fractions. Proteins in both fractions were resolved by SDS-PAGE and subjected to Western blotting with rabbit antibody to E2, A3, or L1 followed by anti-rabbit immunoglobulin G conjugated to horseradish peroxidase. (B) Distribution of E2 in MVs and EVs. RK13 cells were infected with vIHD-J at 2 PFU per cell, and 48 h later MVs and EVs were purified from the cell lysate or medium, respectively, by sedimentation through two continuous 25-to-40% sucrose gradients, as described in Materials and Methods. The same amounts of total protein from both MV and EV samples were adjusted to a final volume of 15 μl with 1 mM Tris (pH 7.4). The proteins were separated by SDS-PAGE, followed by Western blotting with antibody to E2, F13, D8, or A26, as for panel A.
Equal amounts of MVs and EVs (determined by optical density and confirmed by silver staining of an SDS-polyacrylamide gel) were analyzed by Western blotting using antibodies to proteins that are present equally in MVs and EVs or predominantly in one or the other. We noted relatively more E2 in EVs purified from the medium than in MVs isolated from cell lysates (Fig. 3B). The distinction was less complete than for F13 (Fig. 3B), which is an EV-specific protein (16). In contrast, the MV membrane protein D8 was present equally in MVs and EVs, and the membrane protein A26 was greatly enriched in MVs (Fig. 3B), as previously described (36). E2 was also detected in EVs purified from the medium by cesium chloride gradient sedimentation (data not shown). The preferential accumulation of E2 in EVs relative to MVs was unexpected for a protein without a signal peptide or transmembrane domain.
Deletion of the E2L gene results in a small-plaque phenotype.
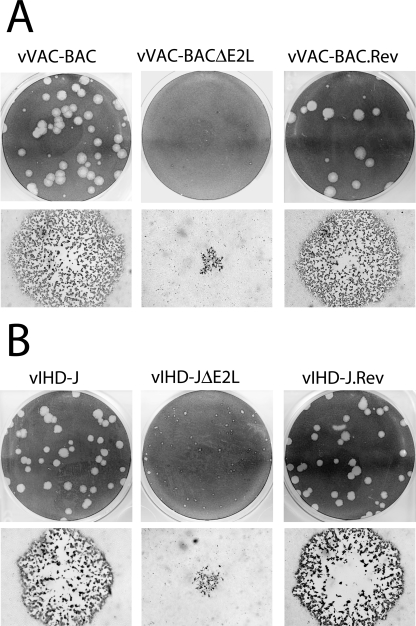
To determine if the E2 protein is essential for virus propagation in cell culture, we deleted the entire gene except for the C-terminal 79 bp, which overlaps the promoter of the adjacent gene. This was accomplished using a novel application of the VAC-BAC system in which the complete VACV genome is cloned in a bacterial artificial chromosome and rescued by fowlpox virus, a distantly related avian poxvirus (9). A two-step procedure was used to replace the E2L ORF of the VAC-BAC plasmid with the ampicillin resistance gene by homologous recombination in E. coli (10). Ampicillin-resistant colonies were isolated and shown to contain a plasmid with a full-length VACV genome lacking E2L. The resulting VAC-BACΔE2L plasmid was then transfected into fowlpox virus-infected CV-1 cells, which are nonpermissive for fowlpox virus. After 7 days, the cells were harvested, and a plaque assay was performed in BS-C-1 cells. Very small plaques were detected by fluorescence microscopy, enabled by the presence of the gene encoding GFP in the TK locus of the parental VAC-BAC plasmid, and were amplified by propagation in BS-C-1 cells. A comparison of the plaques formed by vVAC-BACΔE2L and the vVAC-BAC is shown in Fig. 4A. To confirm that the small-plaque phenotype was due to the E2L deletion and not to an adventitious mutation elsewhere in the VACV genome, DNA containing the intact E2L gene and flanking region was transfected into cells infected with vVAC-BACΔE2L. A recombinant virus forming normal-size plaques, vVAC-BAC.Rev, was isolated (Fig. 4A).
FIG. 4.
Plaque sizes of ΔE2L viruses. (A) Comparison of ΔE2L viruses in the VACV WR background. vVAC-BAC, vVAC-BACΔE2L, and vVAC-BAC.Rev were plated on BS-C-1 cells under semisolid medium. After 4 days, plates were stained with crystal violet to visualize a field of plaques (top row) or fixed with acetone-methanol (1:1) and stained with anti-VACV antibody 8191 followed by anti-rabbit immunoglobulin G conjugated to peroxidase to visualize individual plaques by microscopy (bottom row). (B) Comparison of ΔE2L viruses in the VACV IHD-J background. vIHD-J, vIHD-JΔE2L, and vIHD-J.Rev were analyzed as described for panel A.
We also constructed an E2L deletion mutant in a different VACV background using an alternative method. The VACV IHD-J strain was selected because it produces large amounts of EV, and our data suggested preferential accumulation of E2 in EVs. BS-C-1 cells were infected with the IHD-J strain of VACV and transfected with a plasmid containing GFP flanked by DNA upstream and downstream of E2L, including the C-terminal 79 bp. Green plaques were identified by fluorescence microscopy, and the virus was clonally purified and propagated. A comparison of plaques formed by the parental strain VACV IHD-J and vIHD-JΔE2L (Fig. 4B) indicated a small-plaque phenotype for the mutant. We also made a revertant virus, vIHD-J.REV, by replacing the GFP gene with the E2L gene. The normal plaque size of the revertant is shown in Fig. 4B. Thus, the small-plaque phenotype was independent of the VACV strain background and the method of deletion.
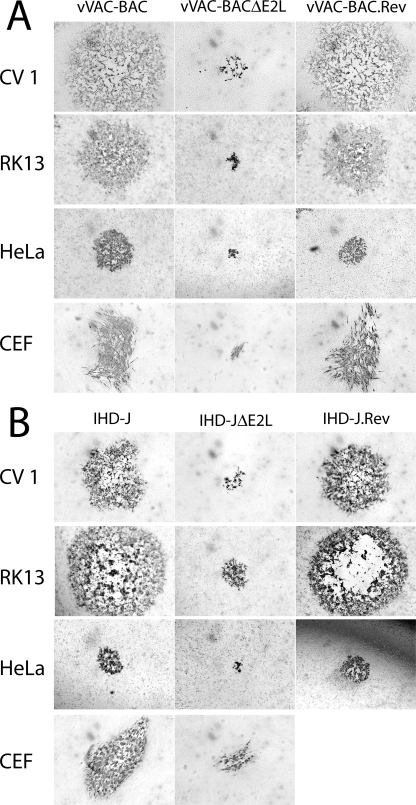
The above-described plaque assay was carried out with BS-C-1 cells. We tested a variety of other cell lines to determine whether the small size plaque phenotype was host related. Both vVAC-BACΔE2L and vIHD-JΔE2L produced small plaques in monkey CV-1 cells, rabbit RK13 cells, human HeLa cells, and chicken embryo fibroblasts (Fig. 5). Thus, the small-plaque phenotype of viruses lacking E2L gene was not cell line specific.
FIG. 5.
Plaque morphology of ΔE2L viruses in multiple cell lines. Viruses were plated on CV1, RK13, HeLa, or CEF cells under semisolid medium and immunostained as for Fig. 4. ΔE2L viruses were compared in the VACV WR (A) and VACV IHD-J (B) backgrounds.
Formation of intracellular and extracellular virus.
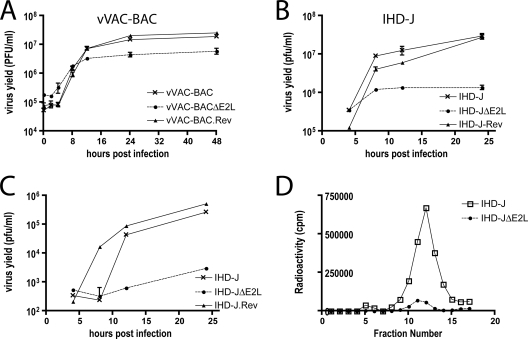
A small-plaque phenotype can arise from either decreased virus production or decreased virus spread. To determine the basis for the small-plaque phenotype of E2L deletion mutants, we carried out one-step growth experiments. The yield of vVAC-BACΔE2L was about 1 log lower than that of either vVAC-BAC or vVAC-BAC.Rev (Fig. 6A), although in other experiments the difference was only three- to fivefold. A reduction of 1 log or less was also obtained for IHD-JΔE2L compared to VACV IHD-J and vIHD-J.REV (Fig. 6B). Additional experiments showed that deletion of the E2L gene did not appreciably affect the infectivity of purified MVs, which was 8 × 107 and 1 × 108 PFU/unit of optical density at 260 nm for vVAC-BAC and vVAC-BACΔE2L, respectively.
FIG. 6.
Intracellular and extracellular virus production. (A and B) Infectious-MV formation. BS-C-1 cells were infected with 5 PFU per cell of vVAC-BAC, vVAC-BACΔE2L, or vVAC-BAC.Rev or with the same amount of vIHD-J, vIHD-JΔE2L, or vIHD-J.rev. At the indicated times after infection, cells from individual triplicate wells were harvested and disrupted, and the viral yields were measured by plaque assays on BS-C-1 cells. Standard error bars are shown. (C) Infectious-EV formation. The media from triplicate wells were individually harvested at the indicated times and clarified by low-speed centrifugation. Contaminating MVs were neutralized with MAb 7D11 to the L1 protein (43) prior to plaque assay, as for panels A and B. (D) Analysis of metabolically labeled EV by CsCl gradient sedimentation. RK13 cells were infected with vIHD-J or vIHD-JΔE2L and labeled with [35S]cysteine and [35S]methionine. The medium was collected at 18 h and clarified by low-speed centrifugation. The supernatant material was purified by sedimentation through a 36% sucrose cushion followed by CsCl gradient centrifugation. Fractions were collected from the bottom of the tube, and radioactivity was determined by scintillation counting.
IHD-J is known to produce large amounts of extracellular virus. The yield of infectious extracellular virus from the medium of cells infected with vIHD-JΔE2L was consistently more than 2 logs lower than that of vIHD-J or vIHD-J.REV (Fig. 6C). Decreased EV formation was demonstrated by CsCl gradient sedimentation of extracellular virus from the medium of cells infected with VACV IHD-J or vIHD-JΔE2L and labeled with [35S]methionine and [35S]cysteine (Fig. 6D). The decrease was approximately 90% and therefore did not entirely account for the decrease in infectious virus. However, as shown below, a greater reduction in EV was found by electron microscopy.
Synthesis and processing of viral proteins.
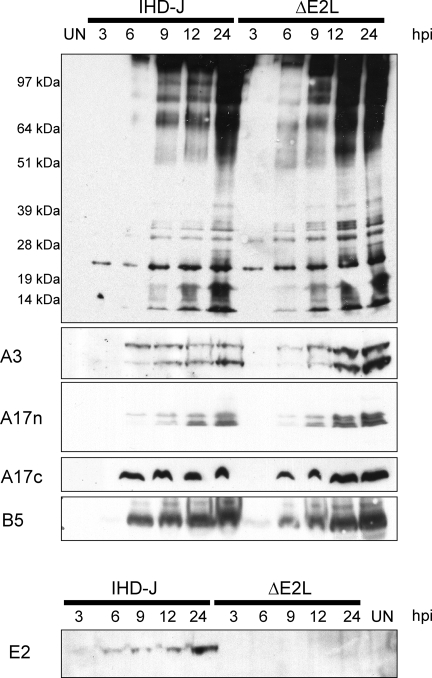
The synthesis of viral proteins was analyzed by SDS-PAGE and Western blotting of infected cell lysates. Figure 7 (top) shows a blot probed with polyclonal antiserum generated by live VACV infection of rabbits. The temporal pattern and intensity of protein bands induced by VACV IHD-J and the vIHD-JΔE2L deletion mutant were similar. Additional blots were also probed with antisera specific to the core protein A3, the MV membrane protein A17, the EV membrane protein B5, and the deleted protein E2. Except for the absence of the E2 band in lysates from cells infected with vIHD-JΔE2L, the patterns appeared virtually identical (Fig. 7). Furthermore, since proteolytic processing of VACV core proteins is coupled with morphogenesis (18), the presence of both precursor and product A3 proteins in lysates of cell infected with IHD-JΔE2L suggested the absence of a major block at this step. Similarly, the peptide antibody A17n detected both processed and unprocessed forms of the A17 MV membrane protein (Fig. 7). Only the precursor form of A17 was detected with the peptide antibody A17c (Fig. 7), since the C-terminal epitope is lost after processing (2). Processing of the core proteins was also examined by pulse-labeling with radioactive amino acids and then chasing with unlabeled amino acids. Proteolytic cleavages occurred, consistent with the above results, but processing appeared to be less complete in cells infected with the E2L deletion mutant (data not shown).
FIG. 7.
Synthesis and processing of viral proteins. The synthesis of viral proteins was determined by Western blotting of lysates from cells harvested at the indicated hours postinfection (hpi) with vIHD-J or vIHD-JΔE2L. (Top) Blot probed with antibodies induced by infection of rabbits with live VACV. Positions of marker proteins are shown on the left. (Bottom) Blots probed with antibodies to peptides derived from the sequence of the A3 (core), A17 (MV membrane), B5 (EV membrane), and E2 proteins. The antibodies A17n and A17c target the N terminus of the mature A17 protein and the C terminus of the A17 precursor protein, respectively.
Virus assembly.
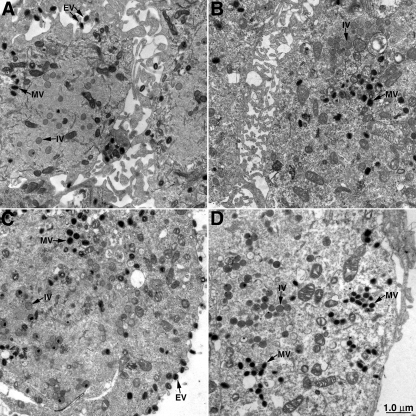
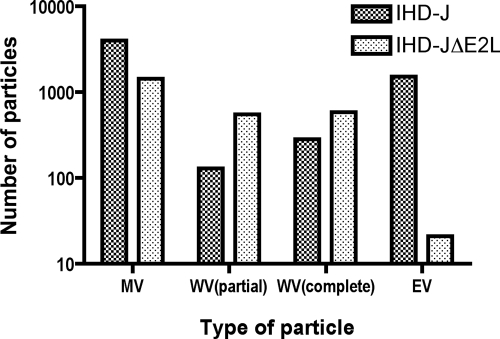
Electron microscopy of cells infected with control and E2L deletion mutants was carried out to further analyze the possibility of a defect in morphogenesis. At 18 h after infection, cells infected with vVAC-BACΔE2L (Fig. 8B) or vIHD-JΔE2L (Fig. 8D) contained immature virions, numerous MVs, and wrapped virions (WVs) and in this regard were not distinguishable from cells infected with vVAC-BAC (Fig. 8A) or VACV IHD-J (Fig. 8C). However, there was a marked reduction in EVs on the surface of the cells infected with the E2L deletion mutants compared to the controls. To substantiate this impression, the intra- and extracellular particles were counted. Of the total particles (MVs plus WVs plus EVs) in cells infected with vIHD-J and vIHD-JΔE2L, 35% and 0.82%, respectively, were EVs (Fig. 9). Similarly, 48% and 0.01% of the total particles were EV in cells infected with vVAC-BAC and vVAC-BACΔE2L, respectively (data not shown). In contrast, the smaller differences between the numbers of MVs and WVs (Fig. 9) were not consistent when vVAC-BAC and vVAC-BACΔE2L were analyzed (data not shown) and therefore may not be significant. Also, the distinction between partially wrapped and fully wrapped virions should be interpreted cautiously, as partially wrapped virions could appear fully wrapped depending on the section. Therefore, our main conclusion is that the number of EVs is greatly reduced, consistent with the 2-log decrease in infectious particles in the medium.
FIG. 8.
Electron-microscopic images of cells infected with viruses containing or lacking the E2L ORF. Thin sections of cells infected with vVAC-BAC (A), vVAC-BACΔE2L (B), vIHD-J (C), or vIHD-JΔE2L (D) are shown. Representative immature virions (IV), MVs, and EVs are indicated with arrows. The junction between two cells appears in panels A and B.
FIG. 9.
Reduced number of EVs made by an E2L deletion mutant, as determined by electron microscopy. Thin sections of cells infected with vIHD-J and vIHD-JΔE2L were examined by electron microscopy. The total numbers of MVs, partially wrapped WVs, completely wrapped WVs, and EVs on the cell surface were determined by counting 90 cell sections. The designation “complete WV” was based on thin-section images, and therefore it is possible that the wrapping membranes were not fully closed.
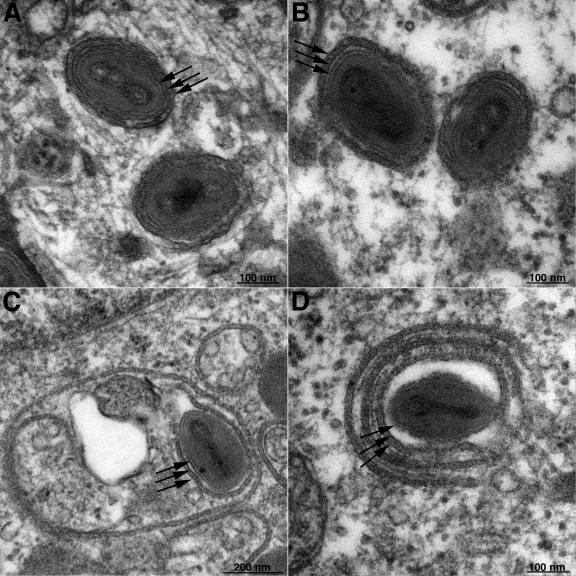
The majority of WVs in the cells infected with wild-type and mutant virus appeared normal (Fig. 10A and B). Curiously, however, there were examples of multilayered wrapping of MVs in cells infected with VAC-BACΔE2L (Fig. 10C and D), which was not seen in the VAC-BAC control or in our previous experience with other VACV mutants. There also were more particles in vesicles in the cells infected with VAC-BACΔE2L and vIHD-JΔE2L than in the viruses with wild-type E2L.
FIG. 10.
Electron-microscopic images of WVs in cells infected with vVAC-BAC (A) and vVAC-BACΔE2L (B, C, and D). The three arrows in each image indicate the single MV membrane and two wrapping membranes. Note that in panels C and D there are additional layers of wrapping membranes.
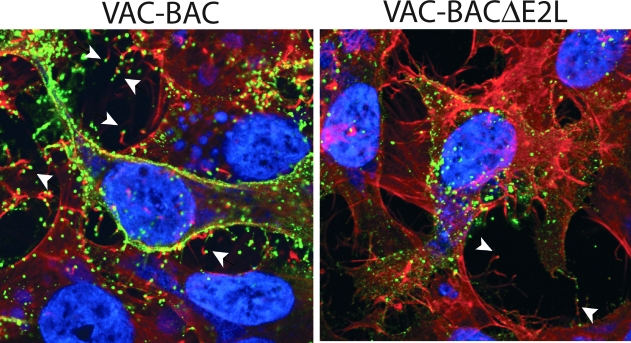
Confocal microscopy was used to determine whether the infrequent EVs on the surface of cells infected with E2 deletion mutants were capable of inducing actin tail formation, which is important for virus spread. HeLa cells were infected with VAC-BAC control or VAC-BACΔE2L and stained with a MAb to the B5 protein, an outer membrane component of the EV. The cells were then permeabilized and stained with phalloidin to detect actin. Numerous EVs with actin tails were detected in the cells infected with the control virus and a much smaller number in cells infected with the E2 deletion mutant (Fig. 11). Accordingly, E2 was not required for actin tail formation.
FIG. 11.
Visualization of EVs with actin tails. Cells infected with VAC-BAC or VAC-BACΔE2L were fixed with 3% paraformaldehyde and quenched with 2% glycine. EVs were labeled with MAb to the B5 protein and Cy5-conjugated goat anti-rat immunoglobulin G secondary antibody. Following this, cells were permeabilized by the addition of 0.1% Triton X-100 and stained with 4′,6-diamidino-2-phenylindole to visualize DNA and with Alexa Fluor 568 phalloidin to visualize filamentous actin. Arrowheads indicate representative EVs at the tips of actin tails.
DISCUSSION
Most highly conserved genes are essential for poxvirus replication, and studies of conditionally lethal mutants have contributed greatly to our understanding of the reproductive cycle. The E2L ORF is one of nearly 100 genes that are conserved in all chordopoxviruses (37). Sequence analysis did not suggest the presence of a signal peptide or transmembrane domain, and the absence of an identifiable functional motif or homolog outside of the poxvirus family did not allow us to predict a role for E2. In addition, the sequence upstream of the E2L ORF did not contain an optimal consensus poxvirus promoter, although there were potential TAAA intermediate-stage transcription initiation sites (1). Western blotting demonstrated that the protein was made with the kinetics and DNA synthesis requirement expected of the product of an intermediate- or late-stage gene. To assess the relative strength of the E2L promoter, we constructed plasmids with the E2L ORF or firefly luciferase gene regulated by previously defined strong intermediate- and late-stage promoters as well as the natural E2L promoter and compared their expression following transfection of infected cells. This comparison indicated that the E2L promoter is very weak. Although some expression occurred in the presence of a DNA synthesis inhibitor, the level was less than that achieved in the absence of inhibitor. By contrast, a previously defined intermediate promoter had equivalent or even higher expression in the absence of viral DNA replication. Pending detailed transcriptional analysis, we tentatively concluded that the E2L promoter is an atypical weak intermediate promoter. Poxviruses appear to regulate expression by promoter sequence instead of using enhancer elements. It would be interesting to boost the promoter strength by genetic engineering to see whether high expression is deleterious and whether down regulation would occur by spontaneous mutation.
The E2 protein was detected by SDS-PAGE and Western blotting of purified MVs and was not extracted under conditions that are effective with most membrane proteins. Surprisingly, there was relatively more E2 in EVs than in MVs, possibly contributing to the previous failures to detect E2 in MVs by mass spectroscopy (5, 29, 45). We suspect that E2 is associated with one of the EV membrane proteins, but its low abundance has made it difficult for us to confirm this by coimmunoprecipitation or to localize epitope-tagged E2 by confocal or electron microscopy. When high expression was achieved by transfection of a plasmid with E2 regulated by a strong late promoter, the epitope-tagged protein was found throughout the cytoplasm.
Because the E2L gene is highly conserved, we anticipated that it would be essential for virus replication. This supposition was wrong, as viable deletion mutants were isolated in both the WR and IHD-J strains of VACV. The mutants were greatly impaired, however, as they formed very tiny plaques in all cell lines tested. The small-plaque phenotype is frequently associated with a reduction in the number of EVs on the outer surface of the plasma membrane or the actin tails needed for their efficient cell-to-cell spread (4, 31-33, 42, 44, 46). One-step growth experiments consistently revealed a greater effect of the E2L deletion on EV formation than MV formation, although both were reduced. Furthermore, electron-microscopic images showed very low numbers of EVs on the surfaces of cells infected with E2L deletion mutants, whereas MVs and WVs were numerous. We did find actin tails on some of the infrequent EVs, indicating that E2 was not required for the nucleation of actin polymerization. Based on previous studies with other VACV mutants that exhibit defects in virus spread (13, 20, 27, 41, 46), we can confidently predict reduced virulence for E2L deletion mutants.
There are notable similarities between the previously described F12 protein and E2L. F12 is also conserved in all chordopoxviruses, has no predicted transmembrane domain, and is nonessential, and deletion mutants form small plaques and have greatly diminished EVs (46). Initially, F12 was thought to be a component of the EV; however, follow-up electron microscopy studies indicated that the protein was associated with the WV but not the EV (15, 38). The defect of F12 null mutants is thought to reside in the failure of WVs to be transported on microtubules to the periphery of the cell.
The EV membrane is derived from trans-Golgi or endosomal cisternae (34, 35), which initially enclose MVs to form WVs with a double wrapper. A27 (30) and F13 (4), components of the MV and EV membranes, respectively, are required to initiate the wrapping step. Although WVs were numerous in cells infected with the E2 deletion mutants, it is difficult to gauge the completeness of wrapping when cells are viewed in thin sections. The possibility of a defect in closure of the wrapping membrane was suggested by the presence of spiral, multilayered wrapping membranes around some particles in cells infected with vVAC-BACΔE2L. In certain ways, the wrapping of MVs resembles the formation of multivesicular bodies (28). We can consider the MV the cargo, with the wrapping membrane budding into itself and closing around the MV to form the WV. Other viruses, notably retroviruses, have been shown to interact through so-called late motifs in Gag proteins with the endosomal budding machinery for virus release (3, 21). Honeychurch et al. (17) recently suggested the importance of a YXXL late motif in the VACV F13 protein and a role for the cellular endosomal sorting complex required for transport proteins. The E2 protein has three YXXL motifs as well as a YPLT late motif. Nevertheless, the presence of such sequences does not necessarily correlate with use of the endosomal sorting complex required for transport machinery for envelopment as shown for human cytomegalovirus (14). Further studies are needed to determine the role of the E2 protein in EV formation.
Acknowledgments
We thank Norman Cooper for providing cells and members of our laboratory for discussions.
Funding was from the NIAID, NIH intramural program.
Footnotes
Published ahead of print on 20 February 2008.
REFERENCES
- 1.Baldick, C. J., J. G. Keck, and B. Moss. 1992. Mutational analysis of the core, spacer and initiator regions of vaccinia virus intermediate class promoters. J. Virol. 664710-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betakova, T., E. J. Wolffe, and B. Moss. 1999. Regulation of vaccinia virus morphogenesis: phosphorylation of the A14L and A17L membrane proteins and C-terminal truncation of the A17L protein are dependent on the F10L protein kinase. J. Virol. 733534-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieniasz, P. D. 2006. Late budding domains and host proteins in enveloped virus release. Virology 34455-63. [DOI] [PubMed] [Google Scholar]
- 4.Blasco, R., and B. Moss. 1991. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J. Virol. 655910-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung, C. S., C. H. Chen, M. Y. Ho, C. Y. Huang, C. L. Liao, and W. Chang. 2006. Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles. J. Virol. 802127-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Condit, R. C., N. Moussatche, and P. Traktman. 2006. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 6631-124. [DOI] [PubMed] [Google Scholar]
- 7.Davison, A. J., and B. Moss. 1989. The structure of vaccinia virus early promoters. J. Mol. Biol. 210749-769. [DOI] [PubMed] [Google Scholar]
- 8.Davison, A. J., and B. Moss. 1989. The structure of vaccinia virus late promoters. J. Mol. Biol. 210771-784. [DOI] [PubMed] [Google Scholar]
- 9.Domi, A., and B. Moss. 2002. Cloning the vaccinia virus genome as a bacterial artificial chromosome in Escherichia coli and recovery of infectious virus in mammalian cells. Proc. Natl. Acad. Sci. USA 9912415-12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domi, A., and B. Moss. 2005. Engineering of a vaccinia virus bacterial artificial chromosome in Escherichia coli by bacteriophage lambda-based recombination. Nat. Methods 295-97. [DOI] [PubMed] [Google Scholar]
- 11.Earl, P. L., N. Cooper, S. Wyatt, B. Moss, and M. W. Carroll. 1998. Preparation of cell cultures and vaccinia virus stocks, p. 16.16.1-16.16.3. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. John Wiley and Sons, New York, NY. [Google Scholar]
- 12.Earl, P. L., B. Moss, L. S. Wyatt, and M. W. Carroll. 1998. Generation of recombinant vaccinia viruses, p. 16.17.1-16.17.19. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Greene Publishing Associates & Wiley Interscience, New York, NY. [Google Scholar]
- 13.Engelstad, M., and G. L. Smith. 1993. The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology 194627-637. [DOI] [PubMed] [Google Scholar]
- 14.Fraile-Ramos, A., A. Pelchen-Matthews, C. Risco, M. T. Rejas, V. C. Emery, A. F. Hassan-Walker, M. Esteban, and M. Marsh. 2007. The ESCRT machinery is not required for human cytomegalovirus envelopment. Cell. Microbiol. 92955-2967. [DOI] [PubMed] [Google Scholar]
- 15.Herrero-Martinez, E., K. L. Roberts, M. Hollinshead, and G. L. Smith. 2005. Vaccinia virus intracellular enveloped virions move to the cell periphery on microtubules in the absence of the A36R protein. J. Gen. Virol. 862961-2968. [DOI] [PubMed] [Google Scholar]
- 16.Hirt, P., G. Hiller, and R. Wittek. 1986. Localization and fine structure of a vaccinia virus gene encoding an envelope antigen. J. Virol. 58757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honeychurch, K. M., G. Yang, R. Jordan, and D. E. Hruby. 2007. The vaccinia virus F13L YPPL motif is required for efficient release of extracellular enveloped virus. J. Virol. 817310-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz, E., and B. Moss. 1970. Formation of a vaccinia virus structural polypeptide from a higher molecular weight precursor: inhibition by rifampicin. Proc. Natl. Acad. Sci. USA 6677-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knutson, B. A., X. Liu, J. Oh, and S. S. Broyles. 2006. Vaccinia virus intermediate and late promoter elements are targeted by the TATA-binding protein. J. Virol. 806784-6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntosh, A. A., and G. L. Smith. 1996. Vaccinia virus glycoprotein A34R is required for infectivity of extracellular enveloped virus. J. Virol. 70272-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita, E., and W. I. Sundquist. 2004. Retrovirus budding. Annu. Rev. Cell Dev. Biol. 20395-425. [DOI] [PubMed] [Google Scholar]
- 22.Moss, B. 2007. Poxviridae: the viruses and their replication, p. 2905-2946. In D. M. Knipe (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 23.Moss, B., and F. De Silva. 2006. Poxvirus DNA replication and human disease, p. 707-727. In M. L. DePamphilis (ed.), DNA replication and human disease. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 24.Nazarian, S. H., and G. McFadden. 2006. Immune evasion by poxviruses. Future Virol. 1123-132. [Google Scholar]
- 25.Notredame, C., D. G. Higgins, and J. Heringa. 2000. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302205-217. [DOI] [PubMed] [Google Scholar]
- 26.Ojeda, S., T. G. Senkevich, and B. Moss. 2006. Entry of vaccinia virus and cell-cell fusion require a highly conserved cysteine-rich membrane protein encoded by the A16L gene. J. Virol. 8051-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parkinson, J. E., and G. L. Smith. 1994. Vaccinia virus gene A36R encodes a Mr 43-50 K protein on the surface of extracellular enveloped virus. Virology 204376-390. [DOI] [PubMed] [Google Scholar]
- 28.Piper, R. C., and D. J. Katzmann. 2007. Biogenesis and function of multivesicular bodies. Annu. Rev. Cell Dev. Biol. 23519-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resch, W., K. K. Hixson, R. J. Moore, M. S. Lipton, and B. Moss. 2007. Protein composition of the vaccinia virus mature virion. Virology 358233-247. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez, J. F., and G. L. Smith. 1990. IPTG-dependent vaccinia virus: identification of a virus protein enabling virion envelopment by Golgi membrane and egress. Nucleic Acids Res. 185347-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roper, R., E. J. Wolffe, A. Weisberg, and B. Moss. 1998. The envelope protein encoded by the A33R gene is required for formation of actin-containing microvilli and efficient cell-to-cell spread of vaccinia virus. J. Virol. 724192-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rottger, S., F. Frischknecht, I. Reckmann, G. L. Smith, and M. Way. 1999. Interactions between vaccinia virus IEV membrane proteins and their roles in IEV assembly and actin tail formation. J. Virol. 732863-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanderson, C. M., F. Frischknecht, M. Way, M. Hollinshead, and G. L. Smith. 1998. Roles of vaccinia virus EEV-specific proteins in intracellular actin tail formation and low pH-induced cell-cell fusion. J. Gen. Virol. 791415-1425. [DOI] [PubMed] [Google Scholar]
- 34.Schmelz, M., B. Sodeik, M. Ericsson, E. J. Wolffe, H. Shida, G. Hiller, and G. Griffiths. 1994. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans-Golgi network. J. Virol. 68130-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tooze, J., M. Hollinshead, B. Reis, K. Radsak, and H. Kern. 1993. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur. J. Cell Biol. 60163-178. [PubMed] [Google Scholar]
- 36.Ulaeto, D., D. Grosenbach, and D. E. Hruby. 1996. The vaccinia virus 4c and A-type inclusion proteins are specific markers for the intracellular mature virus particle. J. Virol. 703372-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Upton, C., S. Slack, A. L. Hunter, A. Ehlers, and R. L. Roper. 2003. Poxvirus orthologous clusters: toward defining the minimum essential poxvirus genome. J. Virol. 777590-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Eijl, H., M. Hollinshead, G. Rodger, W. H. Zhang, and G. L. Smith. 2002. The vaccinia virus F12L protein is associated with intracellular enveloped virus particles and is required for their egress to the cell surface. J. Gen. Virol. 83195-207. [DOI] [PubMed] [Google Scholar]
- 39.Vos, J. C., and H. G. Stunnenberg. 1988. Derepression of a novel class of vaccinia virus genes upon DNA replication. EMBO J. 73487-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward, B. M., and B. Moss. 2001. Vaccinia virus intracellular movement is associated with microtubules and independent of actin tails. J. Virol. 7511651-11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolffe, E. J., S. N. Isaacs, and B. Moss. 1993. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J. Virol. 674732-4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolffe, E. J., E. Katz, A. Weisberg, and B. Moss. 1997. The A34R glycoprotein gene is required for induction of specialized actin-containing microvilli and efficient cell-to-cell transmission of vaccinia virus. J. Virol. 713904-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolffe, E. J., S. Vijaya, and B. Moss. 1995. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology 21153-63. [DOI] [PubMed] [Google Scholar]
- 44.Wolffe, E. J., A. S. Weisberg, and B. Moss. 1998. Role for the vaccinia virus A36R outer envelope protein in the formation of virus-tipped actin-containing microvilli and cell-to-cell virus spread. Virology 24420-26. [DOI] [PubMed] [Google Scholar]
- 45.Yoder, J. D., T. S. Chen, C. R. Gagnier, S. Vemulapalli, C. S. Maier, and D. E. Hruby. 2006. Pox proteomics: mass spectrometry analysis and identification of vaccinia virion proteins. Virol. J. 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, W. H., D. Wilcock, and G. L. Smith. 2000. Vaccinia virus F12L protein is required for actin tail formation, normal plaque size, and virulence. J. Virol. 7411654-11662. [DOI] [PMC free article] [PubMed] [Google Scholar]