Abstract
The vaccinia virus (VACV) complement control protein (VCP) is the major protein secreted from VACV-infected cells. It has been reported that VCP binds to the surfaces of uninfected cells by interacting with heparan sulfate proteoglycans (HSPGs). In this study, we show that VCP is also expressed on the surfaces of infected cells and demonstrate that surface localization occurs independently of HSPGs. Since VCP does not contain a transmembrane domain, we hypothesized that VCP interacts with a membrane protein that localizes to the infected-cell surface. We show that the VACV A56 membrane protein is necessary for the cell surface expression of VCP and demonstrate that VCP and A56 interact in VACV-infected cells. Since the surface expression of VCP was abrogated by reducing agents, we examined the contribution of an unpaired cysteine residue on VCP to VCP surface expression and VCP's interaction with A56. To do this, we mutated the unpaired cysteine in VCP and generated a recombinant virus expressing the altered form of VCP. Following the infection of cells with the mutant virus, VCP was neither expressed on the cell surface nor able to interact with A56. Importantly, the cell surface expression of VCP was found to protect infected cells from complement-mediated lysis. Our findings suggest a new function for VCP that may be important for poxvirus pathogenesis and impact immune responses to VACV-based vaccines.
The complement system is composed of ∼30 soluble and cell surface proteins that work in concert to protect the host from invading pathogens (reviewed in references 46 and 47). Complement can become activated by multiple pathways that converge on the formation of a C3 convertase, the proteolytic complex responsible for cleaving the central complement component, C3. Cleavage of C3 results in the production of the anaphylatoxin C3a and the opsonic fragment C3b. The generation of C3b results in the formation of a C5 convertase, the proteolytic complex responsible for cleaving C5 into the C5a anaphylatoxin and C5b. C5b, in turn, nucleates the formation of a lytic pore called the membrane attack complex.
There is growing evidence that complement plays an important role in protection against viral infection (9, 14-16, 20, 21, 23, 24, 27, 39, 43, 44). Complement activation can protect the host against viruses by several mechanisms: (i) membrane attack complex-induced lysis of virus particles or infected cells; (ii) opsonization by C3b, which neutralizes virus infectivity and enhances immune recognition; and (iii) enhancement of humoral and cellular immune responses (4). In response to the antiviral effects of complement, many viruses have developed methods to evade complement activation, primarily by producing complement-regulatory proteins or incorporating host-derived complement-regulatory proteins into the viral envelope (4, 8, 10, 11, 14, 17, 35, 42).
Orthopoxviruses encode complement control proteins that share a high degree of similarity with mammalian complement-regulatory proteins (18). The vaccinia virus (VACV) complement control protein (VCP) is the most thoroughly studied poxvirus complement-regulatory protein. It is homologous to the smallpox inhibitor of complement enzymes (SPICE) encoded by variola virus and to the monkeypox inhibitor of complement enzymes (MoPICE) (19, 33, 36, 41). VCP and SPICE inhibit the activation of the classical and alternative complement pathways by accelerating the irreversible decay of C3 and C5 convertases and by functioning as cofactors for the factor I-mediated cleavage and inactivation of C3b and C4b (3, 17, 22, 26, 32-34, 36). MoPICE has cofactor activity but differs from VCP and SPICE in that it lacks decay-accelerating activity (19). VCP, SPICE, and MoPICE also contain an unpaired cysteine residue that allows each protein to dimerize, which enhances its complement-regulatory function in vitro (19).
Although VCP has been characterized almost exclusively as a soluble protein that is secreted from infected cells (17), it has also been reported that recombinant VCP can attach to the surfaces of uninfected cells by interacting with heparan sulfate proteoglycans (HSPGs) (38). In this paper, we show that VCP is expressed on the surfaces of infected cells in an HSPG-independent fashion. This process is instead dependent on an interaction between VCP and another viral protein, A56. We show that the ability of VCP to interact with A56 and localize to the cell surface requires an unpaired cysteine residue on VCP. Importantly, the expression of VCP on the cell surface protects infected cells from complement-mediated lysis.
MATERIALS AND METHODS
Cells and infections.
BSC-1 and RK-13 cells were grown in minimal essential medium (MEM). L929, Gro2C, and STO cells were grown in high-glucose Dulbecco's modified eagle medium. Medium was supplemented with antibiotic-antimycotic (Invitrogen) and 10% fetal bovine serum (FBS). Infections were carried out in growth medium containing 2.5% FBS unless otherwise indicated.
Recombinant viruses.
Table 1 summarizes the recombinant viruses constructed for this study. VACV with wild-type VCP, mutant VCP, and VCP knocked out (vv-VCPwt, vv-VCPmut, and vv-VCPko, respectively) were generated by homologous recombination of plasmid constructs into the parental virus vSIGK-3 (17). This parental virus has a gpt selection cassette inserted within the VCP open reading frame (ORF). Recombinant viruses were then isolated by reverse gpt selection (13). After three rounds of plaque purification, isolated plaques were expanded and recombinant viruses were analyzed by PCR and Western blotting to confirm the construct. vv-VCPwt was generated using the plasmid pGK35 (13), a pUC plasmid with the ∼1-kbp HincII-HincII fragment of genomic DNA containing the VCP ORF. This plasmid also served as the template for generating the cysteine-to-threonine mutation in VCP. Mutation of VCP was achieved using the QuikChange site-directed mutagenesis kit (Stratagene) and the primers listed in Table 1 for vv-VCPmut. The mutagenized plasmid was sequenced to confirm the introduction of the N-terminal cysteine mutation and to ensure that no other mutations were introduced during PCR amplification. Complete deletion of the VCP ORF was achieved by constructing a plasmid containing only the right and left flanking regions of the VCP ORF. This was accomplished by a partial digestion of pSI89, a pUC plasmid with the ∼3-kbp HindIII-EcoRI fragment of the HindIIIC genomic DNA, with HincII to remove ∼1.5-kbp fragments containing the entire VCP ORF as well as some flanking sequence. The missing left and right flanking regions were then reintroduced into the plasmid by the ligation of PCR products generated with the primers and the common restriction site indicated in Table 1 for vv-VCPko.
TABLE 1.
Summary of the recombinant viruses constructed for this study
| Name used herein | Laboratory reference | Parental virus | Description | Primer name | Primer description | Primer sequence | Additional information |
|---|---|---|---|---|---|---|---|
| vv-VCPwt | vKSNG-181 | vSIGK-3 | Wild-type VCP ORF reinserted into the gpt deletion virus | ||||
| vv-VCPmut | vKSNG-182 | vSIGK-3 | Cysteine mutation of the VCP ORF reinserted into the gpt deletion virus | olKV-160 | Forward primer for QuikChange mutagenesis of cysteine to threonine | 5′-GGGAATAGGATGCGTTCTATCAACATGTACTATTCCGTCACGACCC-3′ | Mutated codon is underlined |
| olKV-161 | Reverse primer for QuikChange mutagenesis of cysteine to threonine | 5′-GGGTCGTGACGGAATAGTACATGTTGATAGAACGCATCCTATTCCC-3′ | Mutated codon is underlined | ||||
| vv-VCPko | vYXSI-179 | vSIGK-3 | Complete deletion of the VCP ORF reinserted into the gpt deletion virus | olSI-53 | Forward primer to recreate right flank of VCP | 5′-CTTGTGTTAACGATGGAAAGTTATATGTAATAGG-3′ | HpaI site is underlined |
| olSI-54 | Reverse primer to recreate right flank of VCP | 5′-AAGGAAAAAGCGGCCGCATAAAAAGCCCCCATATATGTTCGC-5′ | NotI site is underlined | ||||
| olSI-55 | Forward primer to recreate left flank of VCP | 5′-TTTTCCTTTTGCGGCCGCATAAAACATAAAAATTATACAATGG-3′ | NotI site is underlined | ||||
| olSI-56 | Reverse primer to recreate left flank of VCP | 5′-GCCAAGTTGACCAATTCATTTCTAATAG-3′ | HincII site is underlined | ||||
| vv-VCPwt-His | vSIJC-20 | vSIGK-1 | Overexpression of VCP-His (using pSC-65) inserted into the tk locus of vSIGK-1 | olSI-59 | Forward primer to generate VCP | 5′-ACGCGTCGACATGAAGGTGGAGAGCGTGACG-3′ | SalI site is underlined; initiating ATG codon is in bold |
| olSI-60 | Reverse primer that attaches 7-His tag on the carboxy terminus of VCP | 5′-TCCCCCGGGTCTAGATTAGTGATGGTGATGGTGGTGATGCATGCGTACACATTTTGGAAGTTCCG-3′ | SmaI site is underlined; XbaI site is in italics; new stop codon is in bold | ||||
| vv-VCPmut-His | vBDSI-205 | vSIGK-1 | Overexpression of the cysteine mutation of VCP-His (using pSC-65) inserted into the tk locus of vSIGK-1 | olKV-160 | See the sequence for olKV-160 above | ||
| olKV-161 | See the sequence for olKV-161 above | ||||||
| vvA56ko | vXF186 | WR | Full deletion of the A56R ORF with insertion of the GUS cassette | olXF-156 | Forward primer of the left flank of the A56R ORF | 5′-TCAGAAGATGGATGGATGAAGCATC-3′ | PCR product was cut with a naturally occurring SalI site within the PCR product |
| olXF-157 | Reverse primer of the left flank of the A56R ORF | 5′-AACTGCAGGGTGTAGCGTATACTAATGATATT-3′ | PstI site is underlined | ||||
| olXF-158 | Forward primer of the right flank of the A56R ORF | 5′-AACTGCAGAATTATATTGTCGGCCGTGGC-3′ | PstI site is underlined | ||||
| olXF-159 | Reverse primer of the right flank of the A56R ORF | 5′-CTACAACGAAGCTTGGTCTCAACC-3′ | HindIII site is underlined | ||||
| vvK2ko | vXFAD189 | WR | Full deletion of the K2L ORF with insertion of the YFP cassette | olXF-152 | Forward primer of the left flank of the K2L ORF | 5′-CACCGGATGATGGATTAGGTCTTC-3′ | PCR product was cut with a naturally occurring EcoRI site within the PCR product |
| olXF-153 | Reverse primer of the left flank of the K2L ORF | 5′-GGGGTACCATTATTGATGTCTACACATCC-3′ | KpnI site is underlined | ||||
| olXF-154 | Forward primer of the right flank of the K2L ORF | 5′-GGGGTACCTATGGGTACGGTGTAAGGAATC-3′ | KpnI site is underlined | ||||
| olXF-155 | Reverse primer of the right flank of the K2L ORF | 5′-AACTGCAGGCATGTTACCACTATCAACCG-3′ | PstI site is underlined |
vv-VCPwt-His and vv-VCPmut-His were designed to overexpress His-tagged forms of VCPwt and VCPmut, respectively. The viruses were generated by homologous recombination of plasmid constructs into the parental virus vSIGK-1 (17). This parental virus has a gpt selection cassette replacing the entire VCP ORF (as well as a portion of the flanking regions). His-tagged forms of VCP under the control of a VACV synthetic strong early-late promoter (7) were then inserted into the thymidine kinase locus, and recombinant viruses were isolated by selection with bromodeoxyuridine and screening with β-galactosidase. After three rounds of plaque purification, isolated plaques were expanded and the sequences of the recombinant viruses were confirmed using PCR and Western blotting. The plasmids used for homologous recombination were constructed by adding a 7×-His tag to the carboxy terminus of the VCP ORF using the PCR primers listed in Table 1 for vv-VCPwt-His and by cloning this PCR product into pSC65 (7). The resulting plasmid also served as the template to generate a His-tagged VCP containing the cysteine-to-threonine mutation. This mutation was introduced using QuikChange site-directed mutagenesis (Stratagene) with the same primers used for vv-VCPmut (Table 1). Plasmids were sequenced to confirm the mutation and to ensure that no other unintended mutations were generated during PCR amplification.
Viruses with deletions in the A56R and K2L ORFs were constructed by replacing the ORFs with the genes for β-glucuronidase (GUS) or yellow fluorescent protein (YFP), respectively. Recombinant viruses were generated by homologous recombination of plasmid constructs into a wild-type VACV, strain WR. Recombinant viruses were then isolated by screening for marker expression. After three rounds of plaque purification, isolated plaques were expanded and the sequences of recombinant viruses were confirmed by PCR and Western blotting or their syncytium-inducing phenotype was confirmed in BSC cells. To generate the plasmid to delete the A56 ORF, the flanking regions of A56 were recreated using the primers listed in Table 1 and cloned into pUC19. These primers also introduce a common restriction site (PstI) between the right and left flanks. The resulting plasmid was then digested with PstI to insert a GUS expression cassette driven by a VACV promoter (6). The plasmid used to delete the K2L ORF was constructed in a similar fashion using the primers listed for vv-K2ko, except that a YFP expression cassette driven by a VACV promoter was inserted into the K2 ORF.
IF.
Confluent monolayers of BSC-1 or RK-13 cells seeded in eight-well chamber slides (Nunc) were infected overnight with 1 PFU/cell of the viruses indicated in the figures. For surface staining, infected cells were washed with phosphate-buffered saline (PBS) and fixed with 2% paraformaldehyde dissolved in PBS. For intracellular staining, cells were washed, fixed, and permeabilized with 0.1% Triton X-100. Cells were then blocked in 1% FBS in PBS and subsequently incubated for 1 h at room temperature with 10 μg/ml of a mouse anti-VCP monoclonal antibody (MAb), 3F11 (12). Cells were washed three times, and the anti-VCP antibody was detected using a secondary anti-mouse immunoglobulin G (IgG) antibody conjugated to either fluorescein isothiocyanate (FITC; Zymed) or Alexa 546 (Molecular Probes), as indicated below. Cells were incubated in secondary antibody at a dilution of 1:200 for 1 h at room temperature and then washed three times with PBS. Coverslips were mounted on slides using mounting medium containing DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories) to stain the nuclei, and the slides were examined using a Nikon Eclipse E1000 immunofluorescence (IF) microscope.
For confocal microscopy, cells were infected and processed as described above. The anti-VCP MAb (3F11) was detected using a goat-anti mouse IgG secondary antibody conjugated to allophycocyanin (Biolegend). A56 was detected using polyclonal antisera raised against recombinant A56 (generously provided by Bernard Moss) and a goat anti-rabbit IgG secondary antibody conjugated to FITC (Zymed). Slides were examined using a Nikon TE2000-U inverted microscope coupled to a PerkinElmer confocal imaging system.
Flow cytometry.
BSC-1, L929, and Gro2C cells were infected overnight at a multiplicity of infection (MOI) of 1. The next day, the medium was removed and cells were washed with PBS and lifted with PBS containing 0.5 mM EDTA. For surface staining, cells were washed once in FACS buffer (PBS with 2% bovine serum albumin and 0.04% NaN3). For intracellular staining, cells were permeabilized using Cytofix/CytoPerm (BD) as per the manufacturer's instructions and washed twice with PermWash buffer (BD). Cells were then incubated with 0.4 μg/sample of the anti-VCP MAb 3F11 (12) for 30 min at room temperature. After being washed, cells were incubated with 1 μl of a phycoerythrin-conjugated anti-mouse IgG antibody (Biolegend)/sample. Cells were washed, fixed in 2% paraformaldehyde, and collected using a FACSCalibur (BD). Data were analyzed using FlowJo software (Treestar). Dead cells were excluded from the analysis gate based on forward-scatter and side-scatter profiles.
Heparin competition.
BSC-1 cells were infected at an MOI of 1 overnight in 2.5% growth medium. Medium was aspirated and cells were washed once with fresh medium. Soluble heparin (Sigma) was then added to the cells at the concentrations indicated in Fig. 1 and incubated for 2 h at 37°C; afterwards, the cells were washed two times and lifted with 0.5 mM EDTA in PBS. VCP expression was analyzed using flow cytometry as described above.
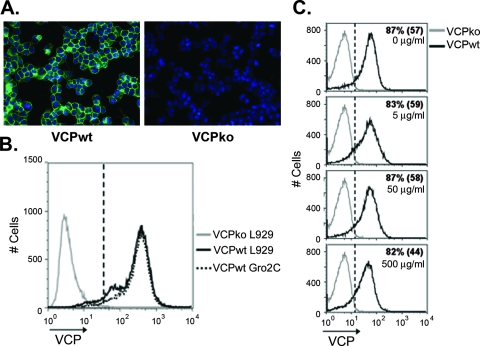
FIG. 1.
Expression of VCP on the surfaces of infected cells occurs independently of HSPGs. (A) BSC-1 cells were infected overnight with vv-VCPwt (VCPwt) or vv-VCPko (VCPko), and the surface expression of VCP (green) was examined using IF by staining nonpermeabilized cells with MAb 3F11 (10 μg/ml). Nuclei were counterstained with DAPI (blue). (B) L929 (HSPG-positive) or Gro2C (HSPG-negative) cells were infected overnight with vv-VCPko or vv-VCPwt, and the surface expression of VCP was examined on nonpermeabilized cells by staining them with MAb 3F11 using flow cytometry. Gates (dashed line) were drawn such that 1% of cells in the VCPko population are positive (MFI = 66). Ninety-three percent of L929 cells were VCP positive, with an MFI of 370; 96% of Gro2C cells were positive, with an MFI of 400. (C) BSC-1 cells were infected overnight at an MOI of 1 with vv-VCPko and vv-VCPwt and then treated with soluble heparin at the indicated concentrations for 2 h prior to being stained for VCP. Numbers indicate the percentages of VCP-positive cells. The numbers in parentheses are MFI. Results are representative of several independent experiments.
Western blotting.
BSC-1 cells were infected overnight at an MOI of 1 in serum-free Opti-MEM. Supernatant from infected cells was collected and centrifuged to remove cells and cellular debris. The supernatant was then concentrated using a Centricon centrifugal filter (Millipore) with a 30-kDa-protein cutoff. The remaining infected cells were lysed using cell lysis buffer (Promega) containing a 1× protease inhibitor cocktail (Sigma). Proteins were boiled in sample buffer containing 1% sodium dodecyl sulfate (SDS) and 2-mercaptoethanol, resolved on a 10% precast Tris-glycine polyacrylamide gel (Invitrogen), transferred to nitrocellulose, and incubated overnight in blocking buffer (PBS, 5% milk, and 0.02% Tween 20). For nonreducing, nondenaturing (native) gels, cells were infected in 2.5% MEM and cell lysates were harvested as described above and lysed in RIPA buffer (1% Triton X-100, 150 mM NaCl, 50 mM Tris) containing 1× protease inhibitor (Sigma). Native samples were diluted in sample buffer containing 0.1% SDS, and proteins were resolved on a 10% Tris-glycine gel and Western blotted for VCP.
For reducing and denaturing gels, membranes were probed for VCP using the mouse anti-VCP MAb 5F1 (12) at a concentration of 5 μg/ml in blocking buffer for 2 h at room temperature. Native VCP was detected using the mouse anti-VCP MAb 3F11 (12) at a concentration of 5 μg/ml. After being washed with PBS containing 0.02% Tween 20, horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Santa Cruz) was diluted 1:2,000 in blocking buffer and incubated with the membranes for 2 h. The membranes were then washed, and proteins were detected by chemiluminescence using the enhanced-chemiluminescence Western blotting detection kit (GE Healthcare Biosciences). Blots were imaged using an LAS-1000 Pus gel documentation system (Fujifilm).
A56 pulldown.
BSC-1 cells were infected in 2.5% MEM at an MOI of 1 for 48 h. Cells were harvested, washed once in ice-cold buffer (50 mM Tris-HCl [pH 7.4] and 150 mM NaCl), and lysed in lysis buffer (50 mM Tris-HCl [pH 7.4], 1% Triton X-100, 150 mM NaCl, and 1× protease inhibitor cocktail [Sigma]). Cells lysates were clarified by centrifugation and incubated with nickel-agarose beads for 2 h at 4°C. Samples were loaded onto a column, washed three times with lysis buffer containing 20 mM imidazole, and eluted with lysis buffer containing 500 mM imidazole. Proteins eluted from the column were separated by SDS-polyacrylamide gel electrophoresis (PAGE) under reducing and denaturing conditions and transferred to nitrocellulose. Membranes were probed for His-tagged VCP and for A56. To probe for His-tagged VCP, membranes were incubated with a primary anti-His tag MAb (Qiagen) diluted 1:200. The membrane was then washed and incubated with a horseradish peroxidase-conjugated secondary goat anti-mouse IgG antibody diluted 1:3,000 (Santa Cruz). Membranes were also probed for A56 using polyclonal rabbit anti-A56 peptide antiserum (generous gift from Bernard Moss) diluted 1:1,250. The membranes were then washed and incubated with a secondary donkey anti-rabbit antibody conjugated to horseradish peroxidase (GE Healthcare Biosciences). Proteins were detected with SuperSignal West Pico chemiluminescence reagent and imaged using an LAS-1000 Pus gel documentation system (Fujifilm).
Complement lysis assay.
L929 cells infected overnight at an MOI of 1 were washed twice with PBS containing 1% FBS, lifted with 0.5 mM EDTA in PBS, and counted. To activate the classical pathway, 5 × 105 infected cells were incubated for 1 h at room temperature with 50 μg/ml of a polyclonal rabbit anti-VACV antibody (Virostat) that binds to infected cells (our unpublished observation). Cells were washed once and incubated with 25% guinea pig complement serum (Sigma) diluted in gelatin barbital (Veronal)-buffered saline containing Ca2+ and Mg2+ (Sigma) for 45 min at 37°C. Cells were then washed twice, and viability was assessed using a fixable amine-reactive viability dye (Invitrogen). This dye penetrates damaged cell membranes and reacts with amine groups in the cytoplasm, resulting in an increase in the fluorescence intensity of damaged cells (31). The percentage of cells with increased fluorescence was determined by using Overton subtraction (28) to compare populations treated with antibody and complement to those treated with antibody alone. Statistical significance was determined using Student's t test.
RESULTS
VCP is expressed on the surfaces of infected cells.
Purified recombinant VCP has been reported to attach to the surfaces of uninfected cells by interacting with HSPGs (38), but the binding of VCP to cells during VACV infection has not been examined. To determine if VCP attaches to the surfaces of infected cells, we infected BSC-1 cells and examined the cell surface expression of VCP using IF. Staining of nonpermeabilized cells infected with the wild-type virus, vv-VCPwt, revealed that VCP is expressed on the surfaces of infected cells (Fig. 1A). In contrast, no staining was observed following infection with a recombinant virus with a deletion in the gene encoding VCP, vv-VCPko (Fig. 1A). Cell surface staining of VCP was confirmed by confocal microscopy (data not shown).
Since the binding of recombinant VCP to the surfaces of uninfected cells has been attributed to its interaction with HSPGs (38), we asked whether the surface expression of VCP during infection was also dependent on HSPGs. To address this question, we infected wild-type L929 cells and an L929-derived heparan-deficient cell line, Gro2C (1). Ninety-three percent of L929 cells and 96% of Gro2C cells expressed VCP with mean fluorescence intensities (MFI) of 370 and 400, respectively (Fig. 1B). These results indicate that VCP expression on the surfaces of infected cells was independent of heparan sulfate (Fig. 1B). To further validate that the cell surface expression of VCP on infected cells was not dependent on HSPGs, we performed a competition experiment to determine if soluble heparin, an analog of HSPG, could remove VCP from the surfaces of infected cells, as has been demonstrated for other heparin-binding proteins (2). BSC-1 cells were infected overnight and then incubated for 2 hours in the presence of soluble heparin prior to being stained for VCP. We found that concentrations of heparin up to 500 μg/ml affected neither the percentage of cells expressing VCP on the surface nor the MFI of VCP staining (Fig. 1C). Even at 500 μg/ml of heparin, 82% of cells were positive for VCP, with an MFI of 44, while 87% of untreated cells were positive, with an MFI of 57 (Fig. 1C). These results suggest that the majority of VCP expressed on the cell surface during infection attaches independently of HSPGs.
The expression of VCP on the cell surface requires an unpaired cysteine residue.
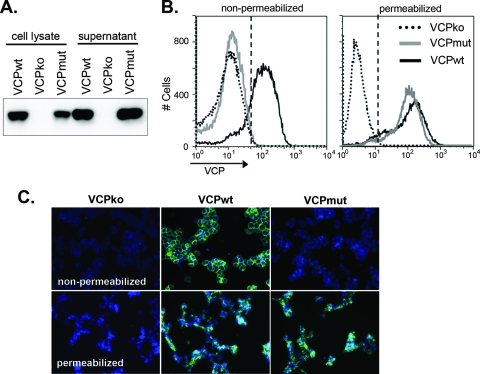
To understand how VCP is expressed on the cell surface during infection, we treated infected cells with various buffers to see if we could dissociate VCP from the cell surface. Interestingly, buffers containing the reducing agents 2-mercaptoethanol or dithiothreitol abrogated the cell surface staining of VCP but did not affect the surface staining of a virus-encoded transmembrane protein, B5 (data not shown). Based on this finding, we hypothesized that disulfide bond formation is important for localizing VCP to the cell surface during infection. To address this hypothesis, we mutated the N-terminal unpaired cysteine on VCP to a threonine residue using site-directed mutagenesis and generated a recombinant virus expressing this altered form of VCP (vv-VCPmut). Western blot analysis of lysates and supernatants from BSC-1 cells infected with vv-VCPwt and vv-VCPmut confirmed that VCPmut is expressed in and secreted from infected cells, indicating that mutating the unpaired cysteine residue did not affect protein expression (Fig. 2A). However, when cells infected with vv-VCPmut were surface stained for VCP, VCP was not detected on the surfaces of nonpermeabilized cells, as measured by flow cytometry and IF (Fig. 2B and C). However, VCP was still detected in permeabilized cells (Fig. 2B and C), confirming that mutating the cysteine residue did not affect protein expression. Taken together, these results show that mutation of the unpaired cysteine residue on VCP abrogates surface expression, without affecting the production or secretion of this protein.
FIG. 2.
Cell surface expression of VCP requires an unpaired cysteine residue. (A) BSC-1 cells were infected overnight with vv-VCPko, vv-VCPwt, or vv-VCPmut, a virus that expresses VCP with its N-terminal unpaired cysteine residue mutated to a threonine. Cell lysate and supernatant from infected cells were resolved using SDS-PAGE and Western blotted for VCP using MAb 5F1 at a concentration of 5 μg/ml. (B) BSC-1 cells were infected overnight with the indicated viruses, and the expression of VCP was examined on the cell surfaces of nonpermeabilized cells and within permeabilized cells using flow cytometry. Infected cells were washed and lifted with PBS-0.5 mM EDTA. For surface staining, cells were washed with FACS buffer and stained for VCP. For intracellular staining, cells were permeabilized using Cytofix/Cytoperm (BD) prior to being stained for VCP. Gates (dashed lines) were drawn such that 1% of cells in the VCPko population are positive. For nonpermeabilized cells, 78% of cells infected with vv-VCPwt are positive for VCP, with an MFI of 141, while 1.5% of cells infected with vv-VCPmut are positive, with an MFI of 16. For permeabilized cells, 91% of cells infected with vv-VCPwt are positive for VCP, with an MFI of 160, while 94% of cells infected with vv-VCPmut are positive, with an MFI of 125. (C) BSC-1 cells were infected overnight at an MOI of 1 with the indicated viruses. Cells were treated with (permeabilized) or without (nonpermeabilized) 0.1% Triton X-100 and stained for VCP with MAb 3F11 (10 μg/ml). The expression of VCP was examined using IF.
A56, but not K2, is required for the cell surface expression of VCP.
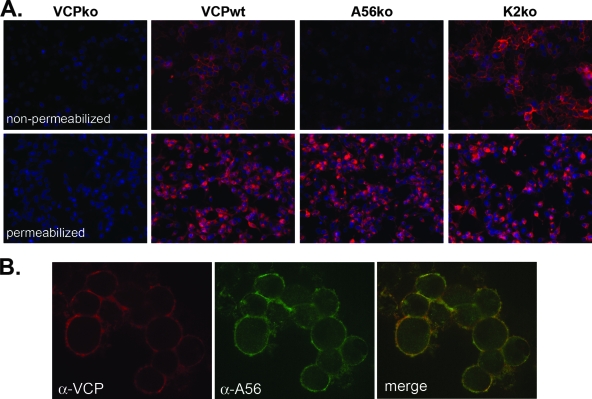
After finding that VCP is expressed on the surfaces of infected cells independently of HSPGs, we were interested in identifying potential binding partners of VCP. When a plasmid encoding the VCP gene under a constitutive promoter was transfected into uninfected cells, VCP was expressed but could not be detected on the cell surface (data not shown), suggesting that the surface expression of VCP requires an additional viral protein(s). To find the binding partner of VCP, we screened a panel of VACVs with deletions in genes that encode various viral transmembrane proteins. We found that VCP does not localize to the surfaces of cells infected with a virus that lacks the gene encoding the VACV protein A56 (vv-A56ko) (Fig. 3A). Using confocal microscopy, we also found that A56 and VCP colocalize on the surfaces of infected cells (Fig. 3B). Importantly, all of the VCP on the cell surface colocalizes with A56 (Fig. 3B, merged image). However, the presence of some green staining in the merged image indicates that not all of the A56 is bound to VCP. A56 forms a complex with another VACV protein, K2, and this complex localizes to the surfaces of infected cells and inhibits infected-cell-cell fusion (5, 40). A56 is necessary for the surface localization of K2 (5), raising the possibility that the surface expression of VCP is dependent on its association with K2 and not A56. To address this possibility, we used IF to examine the localization of VCP in the absence of K2. When cells were infected with vv-VCPwt or vv-K2ko, VCP was expressed on the surface, as shown by the staining of nonpermeabilized cells (Fig. 3A). This indicates that the expression of VCP on the cell surface is dependent on A56 but not K2.
FIG. 3.
The cell surface expression of VCP requires A56 but not K2. (A) RK-13 cells were infected with the indicated viruses overnight at an MOI of 1, and VCP expression (red) on the surfaces of nonpermeabilized cells and within permeabilized cells was analyzed using IF as described in the text. Nuclei were counterstained with DAPI (blue). (B) RK-13 cells were infected with vv-VCPwt and surface stained for VCP (red) and A56 (green). Images were merged to examine the colocalization of A56 and VCP (yellow). α, anti.
VCP interacts directly with A56.
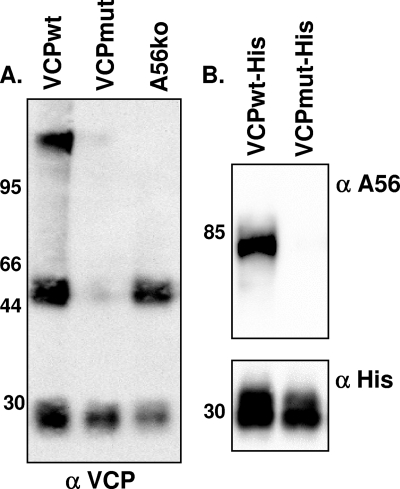
Although the preceding data suggest that A56 is necessary for the localization of VCP to the cell surface, it does not provide evidence of a specific interaction between VCP and A56. When lysates from cells infected with vv-VCPwt were resolved under nonreducing, nondenaturing (native) conditions and probed for VCP, three distinct bands were present (Fig. 4A). These bands include an ∼30-kDa band representing the VCP monomer, an ∼50-kDa band representing the VCP dimer, and a third high-molecular-mass band. The presence of the high-molecular-mass band suggests that VCP interacts with another protein. To determine if A56 was required for the formation of the high-molecular-mass complex, lysate from cells infected with vv-A56ko was resolved under native conditions and probed for VCP (Fig. 4A). In the absence of A56, we found the ∼30-kDa and ∼50-kDa bands, representing monomeric VCP and dimeric VCP, but the high-molecular-mass band was no longer present (Fig. 4A). This suggests that the high-molecular-mass complex does not form in the absence of A56. Furthermore, when lysates from cells infected with vv-VCPmut were probed for VCP, only monomeric VCP was detected, demonstrating the importance of the unpaired cysteine residue on VCP for dimer formation, as well as the formation of the higher-molecular-mass complex (Fig. 4A).
FIG. 4.
VCP and A56 interact under native conditions. (A) BSC-1 cells were infected overnight with the indicated virus at an MOI of 5. Infected cells were then harvested and washed with and lysed in RIPA buffer. Lysates were run on a 10% precast gel under native (nonreducing, nondenaturing) conditions. Membranes were transferred to nitrocellulose and probed for VCP using MAb 5F1 (5 μg/ml). (B) BSC-1 cells were infected for 48 h with the indicated viruses expressing His-tagged versions of VCP at an MOI of 5. Lysates were run through a nickel-agarose column. Proteins were eluted with 500 mM imidazole; separated on a 10% precast gel under reducing, denaturing conditions; and probed for the presence of A56 using a rabbit polyclonal antibody (top) or an anti-His antibody (bottom). Molecular mass markers (in kDa) are indicated along the left-hand side of the gels. α, anti.
To determine if VCP and A56 interact in infected cells, we used viruses encoding His-tagged forms of VCP (vv-VCPwt-His) or VCPmut (vv-VCPmut-His) to attempt to pull down A56. Lysates from cells infected with vv-VCPwt-His or vv-VCPmut-His were run over nickel-agarose columns. Following extensive washing, proteins were eluted with imidazole, separated using SDS-PAGE, and probed for A56 (Fig. 4B). We found that A56 was present only in eluates from vv-VCPwt-His-infected cells, not vv-VCPmut-His-infected cells (Fig. 4B), indicating that A56 interacts with vv-VCPwt-His but not vv-VCPmut-His. Taken together, these results indicate that VCP and A56 interact in infected cells. Of note, Wagenaar and Moss recently used tandem affinity purification and mass spectrometry to identify proteins that interact with A56 (45). Their study identified VCP as one of many proteins that copurifies with A56, lending support to our finding that these two proteins interact.
The expression of VCP on the cell surface protects infected cells from complement-mediated lysis.
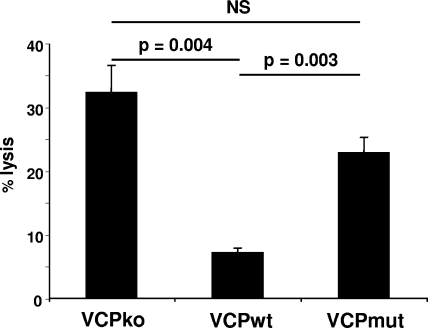
Activation of complement can result in the lysis of virus-infected cells (4, 8, 10). Furthermore, it has been shown that VCP engineered to contain a transmembrane domain is capable of protecting uninfected cells from complement-mediated lysis (32). Thus, we sought to determine if VCP that is naturally expressed on the surface protects infected cells from complement (Fig. 5). For these experiments, we used L929 cells since they are known to be susceptible to lysis by guinea pig complement serum. Prior to the treatment of infected cells with a polyclonal rabbit anti-VACV antibody and 25% guinea pig complement serum, cells were washed to remove secreted VCP in order to examine only the effect of surface-bound VCP. Following treatment with complement, the viability of infected cells was assessed using an amine-reactive viability dye (31). This dye binds to amine groups on the surfaces of cells but also penetrates damaged cell membranes and reacts with amine groups in the cytoplasm, resulting in an increase in the fluorescence intensity of damaged cells (31). We found that treatment of vv-VCPwt infected cells with antibody and complement resulted in 7% ± 0.7% more positive cells than treatment with antibody alone. However, treatment of vv-VCPko- and vv-VCPmut-infected cells resulted in 32% ± 4% and 23% ± 4%, respectively, more positive cells than treatment with antibody alone (Fig. 5). Thus, the presence of VCP on the surfaces of infected cells decreased cell lysis approximately fourfold. This level of protection is similar to the level of protection provided by VCP that has been engineered for membrane expression (32). Complement-mediated lysis of infected cells was prevented when complement activation was inhibited with EDTA or when classical pathway activation was inhibited with EGTA containing Mg2+, indicating that cell lysis was due to classical-pathway activation (data not shown).
FIG. 5.
Surface-bound VCP protects infected cells from complement-mediated lysis. L929 cells infected overnight with the indicated viruses were washed, lifted with PBS-0.5 mM EDTA, and counted. Cells (5 × 105) were incubated with 50 μg/ml of the IgG fraction of a polyclonal rabbit anti-VACV antibody (Virostat), washed, and incubated in the presence or absence of 25% guinea pig complement serum (Sigma) for 45 min at 37°C. Cell lysis was assessed using flow cytometry by measuring the uptake of an amine-reactive viability dye by damaged cells. The percentage of positive (lysed) cells was determined using Overton subtraction. Results are representative of several independent experiments. Error bars are ± the standard error of the mean. Statistical significance was determined using Student's t test. NS, not significant.
DISCUSSION
Prior studies have characterized VCP as the major protein secreted from VACV-infected cells (17, 18). In addition, studies with recombinant VCP have demonstrated that VCP is able to associate with the surfaces of uninfected cells (38). However, the ability of VCP to bind to the surfaces of VACV-infected cells has not been examined. In this study, we show that VCP is expressed on the surfaces of infected cells and examine the mechanism by which surface localization occurs.
The ability of recombinant VCP to associate with the cell surface has been attributed to the presence of four putative heparin binding sites that could allow VCP to interact with heparin or heparin-like molecules on the cell surface (38). Thus, when we initially observed VCP staining on the surfaces of infected cells using IF (Fig. 1A), we assumed that the surface staining was due to secreted VCP binding back to the cell surface. Several observations led us to question this assumption. First, we noted that when monolayers of cells were infected at an MOI low enough to create plaques but to leave most cells of the monolayer uninfected, VCP was detected only on the surfaces of infected cells within the plaques and not on adjacent uninfected cells. This led us to examine the surface staining of VCP on uninfected cells after adding recombinant VCP or concentrated supernatant containing VCP harvested from infected cells. Neither of these approaches resulted in detectable surface staining using IF. In order to rule out the possibility that our observation was due to soluble VCP binding back to the surfaces of infected cells, we first infected cells with a VCPko virus and then added recombinant VCP or concentrated supernatant containing VCP to these cells. Again, we were unable to detect VCP on the surfaces of these cells. These findings led us to compare levels of expression of VCP on the surfaces of wild-type (L929) and HSPG-deficient (Gro2C) cell lines. We found that the levels of surface expression of VCP were similar on both cell lines (Fig. 1B). Furthermore, incubating infected cells with soluble heparin did not diminish the surface staining of VCP. Taken together, these results indicate that the localization of VCP to the cell surface during infection can occur independently of HSPGs. The apparent discrepancy between our findings and that of Smith et al. (38) may be due the fact that we studied VACV-infected cells and they used an uninfected human umbilical cord vascular endothelial cell line. In addition, they employed FITC-labeled recombinant VCP to detect surface staining, and this may have allowed them to detect a lower level of binding than we were able to detect using antibodies.
Since the attachment of VCP to the infected cell surface occurred independently of HSPGs and was also not diminished following the incubation of cells with high-salt buffers, we speculated that the expression of VCP on the infected-cell surface was mediated by a covalent interaction or a very high affinity noncovalent interaction. We hypothesized that the unpaired N-terminal cysteine residue in VCP might allow this protein to form a heterodimer with another protein that is expressed on the cell surface. We found that the expression of VCP on the cell surface is indeed dependent on this unpaired cysteine residue (Fig. 2). While this N-terminal cysteine residue is conserved in the VCP ortholog found in variola virus, SPICE, the virulent central African strain of monkeypox that expresses a VCP ortholog, MoPICE, does not contain an unpaired N-terminal cysteine but instead has an unpaired C-terminal cysteine residue (19). The unpaired cysteines on VCP, SPICE, and MoPICE have been shown to mediate the homodimerization of these proteins (19). Given our finding that an unpaired cysteine residue is necessary for the cell surface localization of VCP, we suspect that the unpaired cysteines on SPICE and MoPICE may also mediate the expression of these proteins on the infected-cell surface.
Our initial hypothesis that the expression of VCP on the cell surface was mediated by a covalent interaction also led us to search for a potential binding partner for VCP. VCP was not expressed on the surfaces of cells transfected with a plasmid bearing the VCP ORF, suggesting that another viral protein is necessary for VCP surface expression. By screening a panel of recombinant VACVs that have various transmembrane proteins genetically deleted, we discovered that the surface expression of VCP is dependent on the presence of another viral protein, A56 (Fig. 3A). This finding is supported by a recent publication by Wagenaar and Moss (45), in which they demonstrated that tandem affinity purification of A56 pulled down VCP. Although the interaction between the A56-K2 complex and the VACV entry-fusion complex seems to be dependent on both A56 and K2 (45), we found that K2 is not required for the cell surface expression of VCP (Fig. 3A). It is interesting to note that the ectodomain of A56 contains three cysteine residues. Although the disulfide bonding pattern of A56 is not known, the presence of an uneven number of cysteine residues in the ectodomain and the importance of an unpaired cysteine residue for the cell surface localization of VCP make it possible that these proteins form a disulfide-bonded heterodimer. Analysis of the A56 amino acid sequences from variola virus and monkeypox virus revealed 92% and 96% identity, respectively, with the VACV A56 protein and conservation of three cysteine residues in the A56 ectodomain. The high degree of sequence conservation and the preservation of an uneven number of cysteine residues in A56 and its orthologs make it interesting to speculate that SPICE and MoPICE may interact with their cognate A56 proteins using the same mechanism as VCP's.
VACV infection results in the production of two morphologically and biology distinct infectious forms (25, 37), mature virus (MV) and extracellular virus (EV), which differ in their susceptibilities to complement-mediated neutralization. While MV is susceptible to neutralization by complement (14, 42), EV is relatively resistant due to the incorporation of host cell-derived complement-regulatory proteins into the EV outer envelope (42). Since A56 is incorporated into the EV envelope (29, 30) and is not found on MV, it raises the possibility that VCP is also displayed on EV. If VCP is present on EV, this may provide additional protection against complement-mediated neutralization. Vanderplasschen et al. examined the ability of several EV-specific proteins, including A56, to confer resistance to complement neutralization and found that none of the proteins examined affected the resistance of EV to complement (42). However, perhaps in the setting of host cell-derived complement-regulatory proteins incorporated into the EV outer envelope, the assays used were not sensitive enough to measure the contribution of VCP attached to A56. Given our finding that VCP and A56 interact in infected cells, it is possible that VCP is also displayed on EV and provides additional protection against complement-mediated neutralization. We are currently investigating this possibility.
Our data support a model in which the unpaired cysteine residue in VCP mediates the cell surface localization of VCP by allowing VCP and A56 to form a covalently bonded heterodimer. However, because mutating the cysteine residue on VCP disrupts both dimer formation and cell surface attachment, it is possible that the binding of VCP to the cell surface is dependent on VCP dimer formation. Thus, there are two possible mechanisms by which VCP could attach to the cell surface in an HSPG-independent fashion: (i) dimeric VCP could interact with A56 via a high-affinity interaction or (ii) monomeric VCP could form a disulfide-linked heterodimer with A56. We favor a model where VCP interacts with A56 via a disulfide bond, rather than by dimerizing and noncovalently associating with A56. This is based on our observation that the high-molecular-mass band indicative of VCP bound to A56 is still present when cell lysates are run under nonreducing, but denaturing, conditions. Further mutagenesis studies of VCP and A56 will be necessary to better define the interaction between these two proteins.
The secretion of large amounts of VCP by infected cells has led to the assumption that soluble VCP protects the site of infection, including infected cells, from attack by complement. The present study shows that the expression of VCP on the surfaces of infected cells could provide infected cells with additional protection against complement. Of note, Rosengard et al. have shown that when VCP is engineered to contain a transmembrane domain that allows it to be expressed on the cell surface, it is capable of protecting cells from complement-mediated lysis (32). They demonstrate a threefold decrease in lysis in the presence of VCP, which is similar to what we observed using infected cells.
The ability of VCP to bind to the surfaces of infected cells and protect them from complement-mediated lysis represents an important additional function of this protein. By preventing or delaying the lysis of infected cells, surface-bound VCP could prolong the production of virus and increase viral titers. Furthermore, limiting complement activation on the surfaces of cells could also reduce the production of the proinflammatory peptides C3a and C5a, thereby diminishing local inflammation and immune system activation. Future studies will determine the role of surface-bound VCP in poxvirus pathogenesis and immune responses to VACV-based vaccines. Examining the role of surface-bound VCP in animal models is complicated by the fact that the unpaired cysteine residue on VCP mediates both dimerization and cell surface attachment. In order to fully define the roles of surface-bound and dimeric VCP in poxvirus pathogenesis, it will be necessary to separate these two functions. Detailed molecular studies of the interaction between VCP and A56 may define mutations in A56 that abrogate the surface expression of VCP without affecting the other functions of A56 and thus allow us to delineate the roles of dimeric and surface-bound VCP in poxvirus pathogenesis.
Acknowledgments
We thank Bernard Moss for the anti-A56 antibody, Paul Bates for the plasmid encoding the YFP gene, Yuhong Xiao for the isolation of the VCPko virus, Abigail Druck Shudofsky for assisting with the isolation of the K2ko virus, Jeannie Chu for assisting with the isolation of vv-VCPwt-His, and Edward Alexander for technical assistance. We also thank Katie Stiles, Roselyn Eisenberg, and Gary Cohen for assistance with confocal microscopy.
This work was supported by NIH grants AI-066333 and AI-047237 to S.N.I. and grant T32 AI-055400 to N.M.G.
Footnotes
Published ahead of print on 20 February 2008.
REFERENCES
- 1.Banfield, B. W., Y. Leduc, L. Esford, K. Schubert, and F. Tufaro. 1995. Sequential isolation of proteoglycan synthesis mutants by using herpes simplex virus as a selective agent: evidence for a proteoglycan-independent virus entry pathway. J. Virol. 693290-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender, F. C., J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J. Virol. 7911588-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernet, J., J. Mullick, Y. Panse, P. B. Parab, and A. Sahu. 2004. Kinetic analysis of the interactions between vaccinia virus complement control protein and human complement proteins C3b and C4b. J. Virol. 789446-9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blue, C. E., O. B. Spiller, and D. J. Blackbourn. 2004. The relevance of complement to virus biology. Virology 319176-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brum, L. M., P. C. Turner, H. Devick, M. T. Baquero, and R. W. Moyer. 2003. Plasma membrane localization and fusion inhibitory activity of the cowpox virus serpin SPI-3 require a functional signal sequence and the virus encoded hemagglutinin. Virology 306289-302. [DOI] [PubMed] [Google Scholar]
- 6.Carroll, M. W., and B. Moss. 1995. E. coli beta-glucuronidase (GUS) as a marker for recombinant vaccinia viruses. BioTechniques 19352-356. [PubMed] [Google Scholar]
- 7.Chakrabarti, S., J. R. Sisler, and B. Moss. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques 231094-1097. [DOI] [PubMed] [Google Scholar]
- 8.Chung, K. M., M. K. Liszewski, G. Nybakken, A. E. Davis, R. R. Townsend, D. H. Fremont, J. P. Atkinson, and M. S. Diamond. 2006. West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H. Proc. Natl. Acad. Sci. USA 10319111-19116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Da Costa, X. J., M. A. Brockman, E. Alicot, M. Ma, M. B. Fischer, X. Zhou, D. M. Knipe, and M. C. Carroll. 1999. Humoral response to herpes simplex virus is complement-dependent. Proc. Natl. Acad. Sci. USA 9612708-12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris, S. L., I. Frank, A. Yee, G. H. Cohen, R. J. Eisenberg, and H. M. Friedman. 1990. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J. Infect. Dis. 162331-337. [DOI] [PubMed] [Google Scholar]
- 11.Hook, L. M., J. M. Lubinski, M. Jiang, M. K. Pangburn, and H. M. Friedman. 2006. Herpes simplex virus type 1 and 2 glycoprotein C prevents complement-mediated neutralization induced by natural immunoglobulin M antibody. J. Virol. 804038-4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaacs, S. N., E. Argyropoulos, G. Sfyroera, S. Mohammad, and J. D. Lambris. 2003. Restoration of complement-enhanced neutralization of vaccinia virus virions by novel monoclonal antibodies raised against the vaccinia virus complement control protein. J. Virol. 778256-8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaacs, S. N., G. J. Kotwal, and B. Moss. 1990. Reverse guanine phosphoribosyltransferase selection of recombinant vaccinia viruses. Virology 178626-630. [DOI] [PubMed] [Google Scholar]
- 14.Isaacs, S. N., G. J. Kotwal, and B. Moss. 1992. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc. Natl. Acad. Sci. USA 89628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, A. H., I. D. Dimitriou, M. C. Holland, D. Mastellos, Y. M. Mueller, J. D. Altman, J. D. Lambris, and P. D. Katsikis. 2004. Complement C5a receptor is essential for the optimal generation of antiviral CD8+ T cell responses. J. Immunol. 1732524-2529. [DOI] [PubMed] [Google Scholar]
- 16.Kopf, M., B. Abel, A. Gallimore, M. Carroll, and M. F. Bachmann. 2002. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat. Med. 8373-378. [DOI] [PubMed] [Google Scholar]
- 17.Kotwal, G. J., S. N. Isaacs, R. McKenzie, M. M. Frank, and B. Moss. 1990. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250827-830. [DOI] [PubMed] [Google Scholar]
- 18.Kotwal, G. J., and B. Moss. 1988. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature 335176-178. [DOI] [PubMed] [Google Scholar]
- 19.Liszewski, M. K., M. K. Leung, R. Hauhart, R. M. Buller, P. Bertram, X. Wang, A. M. Rosengard, G. J. Kotwal, and J. P. Atkinson. 2006. Structure and regulatory profile of the monkeypox inhibitor of complement: comparison to homologs in vaccinia and variola and evidence for dimer formation. J. Immunol. 1763725-3734. [DOI] [PubMed] [Google Scholar]
- 20.Lubinski, J. M., M. Jiang, L. Hook, Y. Chang, C. Sarver, D. Mastellos, J. D. Lambris, G. H. Cohen, R. J. Eisenberg, and H. M. Friedman. 2002. Herpes simplex virus type 1 evades the effects of antibody and complement in vivo. J. Virol. 769232-9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubinski, J. M., L. Wang, A. M. Soulika, R. Burger, R. A. Wetsel, H. Colten, G. H. Cohen, R. J. Eisenberg, J. D. Lambris, and H. M. Friedman. 1998. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J. Virol. 728257-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenzie, R., G. J. Kotwal, B. Moss, C. H. Hammer, and M. M. Frank. 1992. Regulation of complement activity by vaccinia virus complement-control protein. J. Infect. Dis. 1661245-1250. [DOI] [PubMed] [Google Scholar]
- 23.Mehlhop, E., and M. S. Diamond. 2006. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J. Exp. Med. 2031371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehlhop, E., K. Whitby, T. Oliphant, A. Marri, M. Engle, and M. S. Diamond. 2005. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J. Virol. 797466-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moss, B. 2006. Poxvirus entry and membrane fusion. Virology 34448-54. [DOI] [PubMed] [Google Scholar]
- 26.Mullick, J., J. Bernet, Y. Panse, S. Hallihosur, A. K. Singh, and A. Sahu. 2005. Identification of complement regulatory domains in vaccinia virus complement control protein. J. Virol. 7912382-12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochsenbein, A. F., D. D. Pinschewer, B. Odermatt, M. C. Carroll, H. Hengartner, and R. M. Zinkernagel. 1999. Protective T cell-independent antiviral antibody responses are dependent on complement. J. Exp. Med. 1901165-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overton, W. R. 1988. Modified histogram subtraction technique for analysis of flow cytometry data. Cytometry 9619-626. [DOI] [PubMed] [Google Scholar]
- 29.Payne, L. G. 1992. Characterization of vaccinia virus glycoproteins by monoclonal antibody preparations. Virology 187251-260. [DOI] [PubMed] [Google Scholar]
- 30.Payne, L. G. 1979. Identification of the vaccinia hemagglutinin polypeptide from a cell system yielding large amounts of extracellular enveloped virus. J. Virol. 31147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perfetto, S. P., P. K. Chattopadhyay, L. Lamoreaux, R. Nguyen, D. Ambrozak, R. A. Koup, and M. Roederer. 2006. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J. Immunol. Methods 313199-208. [DOI] [PubMed] [Google Scholar]
- 32.Rosengard, A. M., L. C. Alonso, L. C. Korb, W. M. Baldwin III, F. Sanfilippo, L. A. Turka, and J. M. Ahearn. 1999. Functional characterization of soluble and membrane-bound forms of vaccinia virus complement control protein (VCP). Mol. Immunol. 36685-697. [DOI] [PubMed] [Google Scholar]
- 33.Rosengard, A. M., Y. Liu, Z. Nie, and R. Jimenez. 2002. Variola virus immune evasion design: expression of a highly efficient inhibitor of human complement. Proc. Natl. Acad. Sci. USA 998808-8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahu, A., S. N. Isaacs, A. M. Soulika, and J. D. Lambris. 1998. Interaction of vaccinia virus complement control protein with human complement proteins: factor I-mediated degradation of C3b to iC3b1 inactivates the alternative complement pathway. J. Immunol. 1605596-5604. [PubMed] [Google Scholar]
- 35.Saifuddin, M., T. Hedayati, J. P. Atkinson, M. H. Holguin, C. J. Parker, and G. T. Spear. 1997. Human immunodeficiency virus type 1 incorporates both glycosyl phosphatidylinositol-anchored CD55 and CD59 and integral membrane CD46 at levels that protect from complement-mediated destruction. J. Gen. Virol. 781907-1911. [DOI] [PubMed] [Google Scholar]
- 36.Sfyroera, G., M. Katragadda, D. Morikis, S. N. Isaacs, and J. D. Lambris. 2005. Electrostatic modeling predicts the activities of orthopoxvirus complement control proteins. J. Immunol. 1742143-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 832915-2931. [DOI] [PubMed] [Google Scholar]
- 38.Smith, S. A., N. P. Mullin, J. Parkinson, S. N. Shchelkunov, A. V. Totmenin, V. N. Loparev, R. Srisatjaluk, D. N. Reynolds, K. L. Keeling, D. E. Justus, P. N. Barlow, and G. J. Kotwal. 2000. Conserved surface-exposed K/R-X-K/R motifs and net positive charge on poxvirus complement control proteins serve as putative heparin binding sites and contribute to inhibition of molecular interactions with human endothelial cells: a novel mechanism for evasion of host defense. J. Virol. 745659-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suresh, M., H. Molina, M. S. Salvato, D. Mastellos, J. D. Lambris, and M. Sandor. 2003. Complement component 3 is required for optimal expansion of CD8 T cells during a systemic viral infection. J. Immunol. 170788-794. [DOI] [PubMed] [Google Scholar]
- 40.Turner, P. C., and R. W. Moyer. 2006. The cowpox virus fusion regulator proteins SPI-3 and hemagglutinin interact in infected and uninfected cells. Virology 34788-99. [DOI] [PubMed] [Google Scholar]
- 41.Uvarova, E. A., and S. N. Shchelkunov. 2001. Species-specific differences in the structure of orthopoxvirus complement-binding protein. Virus Res. 8139-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanderplasschen, A., E. Mathew, M. Hollinshead, R. B. Sim, and G. L. Smith. 1998. Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope. Proc. Natl. Acad. Sci. USA 957544-7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verschoor, A., M. A. Brockman, D. M. Knipe, and M. C. Carroll. 2001. Cutting edge: myeloid complement C3 enhances the humoral response to peripheral viral infection. J. Immunol. 1672446-2451. [DOI] [PubMed] [Google Scholar]
- 44.Volanakis, J. E. 2002. The role of complement in innate and adaptive immunity. Curr. Top. Microbiol. Immunol. 26641-56. [DOI] [PubMed] [Google Scholar]
- 45.Wagenaar, T. R., and B. Moss. 2007. Association of vaccinia virus fusion regulatory proteins with the multicomponent entry/fusion complex. J. Virol. 816286-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walport, M. J. 2001. Complement. First of two parts. N. Engl. J. Med. 3441058-1066. [DOI] [PubMed] [Google Scholar]
- 47.Walport, M. J. 2001. Complement. Second of two parts. N. Engl. J. Med. 3441140-1144. [DOI] [PubMed] [Google Scholar]