Abstract
The cellular kinase Akt is a key controller of cellular metabolism, growth, and proliferation. Many viruses activate Akt due to its beneficial effects on viral replication. We previously showed that wild-type (WT) simian virus 40 (SV40) large T antigen (TAg) inhibits apoptosis via the activation of PI3K/Akt signaling. Here we show that WT TAg expressed from recombinant adenoviruses in U2OS cells induced the phosphorylation of Akt at both T308 and S473. In contrast, Akt phosphorylation was eliminated by the K1 mutation (E107K) within the retinoblastoma protein (Rb) binding motif of TAg. This suggested that Akt phosphorylation may depend on TAg binding to Rb or one of its family members. However, in Rb-negative SAOS2 cells depleted of p107 and p130 by using small hairpin RNAs (shRNAs), WT TAg still mediated Akt phosphorylation. These results suggested that the K1 mutation affects another TAg function. WT-TAg-mediated phosphorylation of Akt was inhibited by a PI3K inhibitor, suggesting that the effects of TAg originated upstream of PI3K; thus, we examined the requirement for insulin receptor substrate 1 (IRS1), which binds and activates PI3K. Depletion of IRS1 by shRNAs abolished the WT-TAg-mediated phosphorylation of Akt. Immunoprecipitation studies showed that the known interaction between TAg and IRS1 is significantly weakened by the K1 mutation. These data indicate that the K1 mutation disrupts not only Rb binding but also IRS1 binding, contributing to the loss of activation of PI3K/Akt signaling.
The serine/threonine protein kinase Akt, also known as protein kinase B, is a key kinase controlling cell growth and survival (3, 10, 14). Akt is activated by the upstream phosphatidylinositol 3-kinase (PI3K) pathway, which is activated by growth factors, such as insulin and platelet-derived growth factor. Insulin stimulation triggers the receptor's tyrosine kinase activity, leading to tyrosine phosphorylation at YXXM motifs in insulin receptor substrate (IRS) proteins (2, 27). This allows interactions between IRS proteins and the p85 regulatory subunit of PI3K, thus activating PI3K. Activated PI3K catalyzes the phosphorylation of phosphatidylinositol 4,5-bisphosphate to phosphatidylinositol 3,4,5-triphosphate. Phosphatidylinositol 3,4,5-triphosphate binds proteins with pleckstrin homology domains, such as 3-phosphoinositide-dependent protein kinase 1 (PDK1) and Akt. This localizes PDK1 and Akt at the plasma membrane and positions them such that PDK1 can phosphorylate Akt on threonine 308 (T308) (1, 25). The full activation of Akt requires not only T308 phosphorylation but also the phosphorylation of serine 473 (S473), which is mediated by mammalian target of rapamycin complex 2 (mTORC2) (23, 30).
Large T antigen (TAg), one of the early gene products of simian virus 40 (SV40), is able to transform mouse and human cells, primarily by binding to and inhibiting tumor suppressor protein p53, retinoblastoma protein (Rb), and Rb family proteins (4, 32). It has also been reported that transformation by SV40 TAg requires IRS1 (11). The coexpression of TAg and IRS1 showed that the two proteins associate and that IRS1 was translocated to the nucleus in TAg-expressing cells (11, 17, 29). Previously, we reported that SV40 TAg inhibited apoptosis through the activation of PI3K/Akt signaling (39). In this paper, we demonstrate that IRS1 is required for the TAg-mediated phosphorylation (activation) of Akt. In addition, we show that TAg with the K1 mutation (E107K), best known for disrupting the Rb binding motif, is unable to phosphorylate Akt; this correlates with the debilitated binding to IRS1. Our data indicate that the K1 mutation disrupts not only Rb binding but also IRS1 binding; the loss of IRS1 binding results in the loss of activation of PI3K/Akt signaling.
MATERIALS AND METHODS
Cell culture and transfection.
293T, U2OS, and SAOS-2 cells were propagated and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM GlutaMAX (all reagents were obtained from Invitrogen, Carlsbad, CA). Transfections were performed using FuGene6 (Roche Applied Science, Indianapolis, IN) based on the manufacturer's instructions.
Plasmids.
Plasmid pCIS-hIRS1HA, expressing hemagglutinin (HA)-tagged wild-type (WT) human IRS1 was provided by Michael Quon at NIH (16). pSG5-LT and pSG5-K1 (42) were described previously. Lentiviral plasmids expressing human IRS1 small hairpin RNA (shRNA) (catalog no. RHS3979-9607300), p107 shRNA (catalog no. RHS3979-97079661), and p130 shRNA (catalog no. RHS3979-97079668) were purchased from Open Biosystems, Huntsville, AL. Control green fluorescent protein (GFP) shRNA plasmid 12273 was purchased from Addgene, Cambridge, MA (28).
Recombinant adenoviruses.
Recombinant adenoviruses expressing WT TAg (Ad-LT) and the K1 mutant (Ad-K1) have been described previously (40). Cells to be infected with the adenoviruses were pretreated for 1 h with either 50 μM of the PI3K inhibitor LY294002 (LY) or the same volume of dimethyl sulfoxide (the solvent) at 37°C and then mock infected or infected with recombinant adenoviruses (multiplicity of infection [MOI] of 5) in the presence or absence of 50 μM of LY. At 24 h postinfection, whole-cell lysates were prepared and analyzed as described below. In experiments where LY was not used, the cells were infected with the recombinant adenoviruses and harvested 24 h postinfection. When shRNA expression lentiviruses (see below) were used, the cells were infected with the lentiviral vectors 3 days prior to infection with the recombinant adenoviruses; cell lysates were prepared 24 h after the adenovirus infection.
shRNA lentiviruses.
One day before transfection, 3 × 106 293T cells were seeded in a 100-mm dish and cultured overnight at 37°C. Amounts of 10.0 μg of shRNA lentiviral transfer vector, 6.5 μg of the Gag/Pol-expressing plasmid pMDL, 3.5 μg of the envelope protein-expressing plasmid pVSVG, and 2.5 μg of the Rev-expressing plasmid pREV (35) were mixed with 45 μl of FuGene6 in 400 μl of Dulbecco's modified Eagle's medium and transfected into 293T cells as described in the manufacturer's instructions. Six hours after the DNA was added, 293T cells were refed with 5 ml of fresh medium. The conditioned culture medium was collected every 24 h for 3 days. The pooled media were cleared by centrifugation at 5,000 rpm in a Sorvall RT7 centrifuge. The cleared supernatant was used to infect cells by adding Polybrene to a final concentration of 8.0 μg/ml.
Immunoprecipitation.
Whole-cell lysates were prepared in HNIG buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EDTA, 10 mM sodium pyrophosphate, 10 mM NaF, 200 μM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 1.5 μg/ml aprotinin, and 1.0 μg/ml leupeptin). Amounts of 400 to 600 μg of total extract protein were incubated with anti-HA affinity matrix (Roche Diagnostics, Mannheim, Germany) for 4 h at 4°C. The beads were washed three times with cold HNIG buffer and boiled in 25 μl of 1× sodium dodecyl sulfate loading buffer (62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 150 mM 2-mercaptoethanol, 0.01% bromophenol blue). The eluted proteins were separated by 9% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subjected to Western analysis.
Western analysis.
The procedures for Western analysis and the preparation of cellular extracts for Western analysis have been previously described (40). The following primary antibodies, with the vendor catalog numbers in parentheses, were used for analyses. Anti-phospho-Akt T308 (9275) and anti-phospho-Akt S473 (9271) were purchased from Cell Signaling Technology, Beverly, MA. Anti-p107 (sc-318), antiactin (sc-1615), anti-Gab2 (sc-9313), anti-mTOR (sc-8319), anti-PTEN (sc-7974), and anti-Rb (sc-102) were purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, CA. Anti-p130 (610261), anti-Akt (610861), anti-p85 (610045), anti-p110α (611398), and anti-PDK1 (611070) were purchased from BD Biosciences Pharmingen, San Diego, CA. Anti-Gab1 (06-579) and anti-IRS1 (06-248) were purchased from Upstate, Charlottesville, VA. Anti-Rictor (A300-458) was purchased from Bethyl, Inc., Montgomery, TX. Anti-HA/12CA5 was purchased from Roche Applied Science, Indianapolis, IN. The monoclonal antibody PAb419 (9), which reacts with both SV40 TAg and SV40 small t antigen, was produced and purified in this lab.
RESULTS
The K1 mutant of TAg fails to activate Akt.
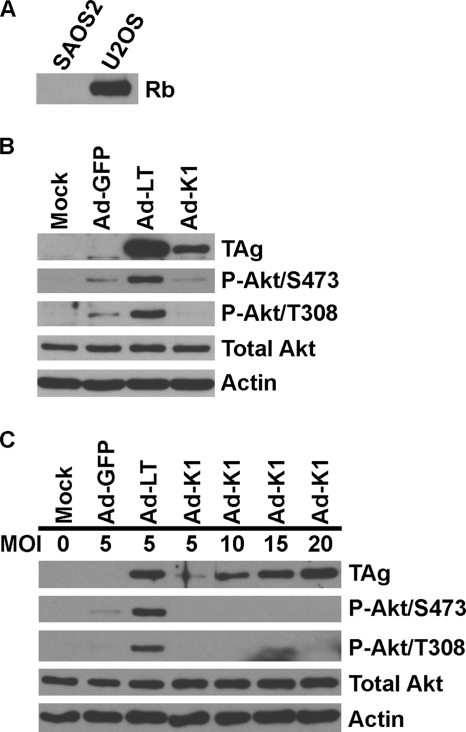
The K1 mutant of TAg has a single amino acid change from glutamic acid to lysine at residue 107 within the Rb binding motif (7, 24). The mutation abolishes binding to Rb and its family members (12). Ad-LT, Ad-K1, and the control adenovirus expressing GFP (Ad-GFP) were used to infect U2OS cells in the presence or absence of LY. In the absence of LY, the Ad-GFP control slightly increased the phosphorylation of Akt (Fig. 1A); however, Ad-LT induced much higher phosphorylation levels at both T308 and S473. In contrast, the ability of Ad-K1 to activate Akt phosphorylation was less than that of the Ad-GFP control. The total Akt protein levels remained constant in infected and mock-infected cells. In all cases, the presence of LY completely inhibited the phosphorylation of Akt at both S473 and T308, indicating that the activation originated upstream of PI3K (Fig. 1A).
FIG. 1.
WT TAg, but not the K1 mutant, induces phosphorylation of Akt in U2OS cells. (A) U2OS cells were pretreated with either 50 μM of LY (+) or the same volume of dimethyl sulfoxide (the LY solvent; −) for 1 h at 37°C and then mock infected or infected with recombinant adenoviruses (MOI of 5) in the presence or absence of 50 μM of LY. At 24 h postinfection, whole-cell lysates were prepared and evaluated by Western analysis using antibodies described in Materials and Methods. (B) The activation of Akt by TAg is not due to alterations in key protein levels. The lysates from mock-infected or recombinant-adenovirus-infected U2OS cells were analyzed by Western analysis for the proteins indicated (see text for details). P-Akt, phosphorylated Akt.
TAg does not increase the levels of key cellular proteins which affect the phosphorylation of Akt.
There are a significant number of proteins that could affect the phosphorylation of Akt due to changes in their expression. In this regard, it is known that TAg is an efficient transcriptional activator which can transcriptionally activate simple promoter structures (18). To determine if the expression of key proteins was altered by TAg, we analyzed their expression in U2OS cells infected with Ad-GFP, Ad-LT, and Ad-K1. The proteins examined included the PI3K subunits, p85 and p110α; the PI3K/Akt negative regulator PTEN; adaptor proteins IRS1 and Grb2-associated binder 1 and 2 (Gab1 and Gab2); the T308-kinase PDK1; and mTORC2 components mTOR kinase and Rictor. As shown in Fig. 1B, none of these proteins showed a significant change in level in comparisons of mock-infected cells with cells infected with any of the adenovirus vectors.
The lack of induction of Gab1 and -2 is notable. The Gab proteins are adaptor proteins which play a role in transducing signals from activated receptors to PI3K/Akt, similar to the function of IRS proteins (19, 20, 22, 26). It has been reported that the Gab2 gene is transcriptionally activated by E2F, resulting in increased levels of Gab2 and, consequently, the phosphorylation of Akt (5). By this mechanism, the interaction between TAg and Rb, which releases E2F, could result in transcriptional activation of the Gab2 genes, increase the levels of the Gab2 protein, and activate Akt (4, 6). However, as indicated by the results shown in Fig. 1B, neither the Gab1 nor the Gab2 level increases in the presence of WT TAg, suggesting that the above mechanism is not functioning in WT-TAg-expressing cells.
WT TAg induces phosphorylation of Akt in Rb-negative (Rb−) SAOS2 cells.
To determine whether the Rb protein was needed for the TAg-induced phosphorylation of Akt, we used the adenovirus expression vectors to infect SAOS2 cells, an osteosarcoma cell line which does not express the Rb protein, as shown in Fig. 2A. Figure 2B shows that the TAg phosphorylation of Akt in SAOS2 cells was similar to that in U2OS cells. Specifically, Akt S473 and T308 phosphorylation was modestly increased by Ad-GFP but significantly increased at both sites by Ad-LT. However, Ad-K1 was unable to activate the phosphorylation of Akt even to the level seen with the Ad-GFP control. It is notable that the expression of K1 TAg in SAOS2 cells was significantly reduced compared to the expression of WT TAg. It is possible that the reduced K1-TAg levels contributed to the loss of Akt phosphorylation. To eliminate this possibility, we infected SAOS2 cells with increasing MOIs (5, 10, 15, and 20) of Ad-K1 to increase the K1-TAg levels. The results in Fig. 2C show that cells infected with Ad-K1 at MOIs of 15 and 20 produce K1 TAg at levels equivalent to the levels of WT TAg capable of Akt phosphorylation (Ad-LT; MOI of 5). Even at equivalent levels, K1 TAg showed no significant phosphorylation of Akt at either S473 or T308. Thus, the defect in Akt activation is not due to a problem in K1-TAg expression.
FIG. 2.
Rb is not required for WT-TAg-mediated phosphorylation of Akt. (A) Western analysis confirmation that SAOS2 cells express no Rb protein. (B) Western analysis of WT- and K1-TAg phosphorylation of Akt in SAOS2 cells. Experiments were similar to those described for Fig. 1A. (C) Increasing the level of the K1 TAg does not induce Akt phosphorylation. Experiments were similar to those described for Fig. 1A except that the recombinant adenoviruses were used at higher MOIs. P-Akt, phosphorylated Akt.
WT TAg can induce Akt phosphorylation in the absence of Rb, p107, and p130.
The above data suggest that the inability of K1 TAg to induce Akt phosphorylation is not due to its defect in Rb binding. However, WT TAg also binds Rb family members p107 and p130 (15, 38, 42). To test the possibility that defective binding to p107 and/or p130 is responsible for the inability to phosphorylate Akt, we introduced lentiviral vectors expressing shRNAs to deplete p107 and p130 individually and together in Rb− SAOS2 cells. A lentiviral vector expressing an shRNA against GFP was used as the control. Three days after the introduction of the lentiviral vectors, the cells were infected with Ad-GFP, Ad-LT, and Ad-K1 for 24 h. As shown in Fig. 3, shRNA targeting p107 specifically depleted the p107 protein to undetectable levels compared to the levels in the control and had little effect on p130 protein levels. Similarly, p130 shRNA reduced the p130 protein to undetectable levels and had no effect on p107. Figure 3 also shows the results of a short and a long exposure of the Western analysis of TAg under the various depletion conditions. Compared to the results for the GFP control (Rb−, p107+, p130+; lanes 1 to 4), the depletion of p107 (Rb−, p107−, p130+; lanes 5 to 8) decreased the levels of WT TAg and, especially, K1 TAg. In contrast, the depletion of p130 (Rb−, p107+, p130−; lanes 9 to 12) had little effect on the levels of WT TAg and appeared to allow increased accumulation of the K1 TAg. Depletion of both p107 and p130 (Rb−, p107−, p130−; lanes 13 to 16) did not decrease the levels of WT or K1 TAg as much as the depletion of p107 alone. Taken together, the data suggest that p107 increases the steady-state levels of both WT and K1 TAg, since the levels of each go down when p107 is depleted and the levels are increased when only p107 is present (i.e., when p130 is depleted). In contrast, p130 may have the opposite effect; specifically, the loss of WT and K1 TAg due to p107 depletion is not as great when p130 is also depleted.
FIG. 3.
WT TAg induces phosphorylation of Akt in SAOS2 cells in which p107 and p130 have been depleted. SAOS2 cells were infected with lentiviruses expressing shRNA against GFP and p107 and/or p130. Three days after lentiviral infection, the cells were mock infected or infected with the recombinant adenoviruses. At 24 h after adenoviral infection, cell lysates were harvested for Western analysis of the proteins indicated. The results of short and long exposures of TAg in Western analysis are shown. P-Akt, phosphorylated Akt.
Examination of Akt phosphorylation by WT TAg showed the expected phosphorylation of S473 and T308 in the GFP controls (Fig. 3, lanes 1 to 4). In the p107-depleted cells (lanes 5 to 8), significant levels of phosphorylation of both Akt sites remained despite the lowered amounts of WT TAg; however, the phosphorylation of T308 appeared to be disproportionately reduced. Depletion of p130 (Fig. 3, lanes 9 to 12) had little effect on WT-TAg-induced phosphorylation of S473 but, again, there was a reduction in T308 phosphorylation. Depletion of both p107 and p130 (Fig. 3, lanes 13 to 16) had an effect on S473 phosphorylation similar to that of depletion of only p107; however, again a significantly greater effect on T308 phosphorylation is seen. These data suggest that WT TAg retains the ability to mediate Akt phosphorylation in the absence of Rb, p107, and p130; however the presence of p107 and p130 enhanced Akt phosphorylation, with the greatest effect on T308 phosphorylation. In summary, the data show that depletion of Rb, p107, and p130 does not abolish Akt phosphorylation, whereas the K1 mutation in the Rb binding site does. This suggests that the K1 mutation affects another function of TAg which mediates Akt phosphorylation.
IRS1 is required for TAg-induced phosphorylation of Akt.
IRS1 is a major IRS which plays an important role in insulin signaling. The protein undergoes rapid tyrosine phosphorylation by the receptor kinase and forms a stable complex with the p85 subunit of PI3K, thereby activating it and its downstream pathways (2, 27). It has been reported that TAg cannot transform cells that lack IRS1 expression (11). The transforming activity can be restored by the expression of a WT IRS1 but not by an IRS1 mutated at the PI3K binding sites (11). For this reason, the role of IRS1 in the TAg-mediated phosphorylation of Akt was tested. In depletion experiments similar to those whose results are shown in Fig. 3, we introduced a lentiviral vector expressing an shRNA that would deplete human IRS1. Two days after lentiviral infection, the cells were infected with Ad-GFP and Ad-LT. Figure 4 shows that the human IRS1 depletion reduced IRS1 protein levels by more than 95% compared to the levels with the controls, which were either no shRNA or the shRNA targeting GFP. In IRS1-depleted cells, the phosphorylation of Akt at both T308 and S473 induced by Ad-GFP was not changed, as shown by comparing the nontreated and the GFP controls. However, the induced phosphorylation of Akt S473 and T308 seen in Ad-LT infection was abolished in the IRS1-depleted cells, suggesting that an IRS1/TAg interaction results in Akt phosphorylation.
FIG. 4.
Depletion of IRS1 abolished TAg-mediated phosphorylation of Akt. U2OS cells were mock infected or infected with lentiviruses expressing shRNA against GFP or IRS1. Six hours after lentiviral infection, cells were fed with fresh medium and cultured for another 42 h at 37°C. shRNA- or mock-treated cells were then mock infected or infected with the recombinant adenoviruses. At 24 h after adenoviral infection, cell lysates were harvested for Western analysis. hIRS1, human IRS1; P-Akt, phosphorylated Akt.
The K1 mutation significantly weakens IRS1 binding to TAg.
It has been previously reported that TAg binds IRS1 and that this requires the N-terminal 250 amino acids of TAg (17). The K1 mutation at amino acid 107 is within this region; thus, we asked whether a deficiency in IRS1 binding may correlate with the inability of the K1 mutant to induce Akt phosphorylation. U2OS cells were transfected with a plasmid expressing HA-tagged human IRS1 or a control vector plasmid and cotransfected with plasmids expressing either WT TAg or the K1 mutant TAg. Cell lysates were prepared 24 h posttransfection and immunoprecipitated with anti-HA antibody. The precipitates or samples of the whole-cell extracts were subjected to Western analysis probing for HA or TAg. The results show that the binding between IRS1 and the K1 mutant is significantly impaired compared to the binding of the WT TAg (Fig. 5). Thus, a direct association between TAg and IRS1 correlates with the requirement for IRS1 in the TAg-mediated phosphorylation of Akt.
FIG. 5.
The K1 mutant of TAg cannot efficiently bind IRS1. U2OS cells were cotransfected with 1.0 μg of plasmid expressing HA-tagged WT human IRS1 (hIRS1) or the vector plasmid plus 1.0 μg of plasmid expressing either WT TAg (LT) or the K1 mutant; the vector plasmid (pSG5) was used as the control. At 24 h posttransfection, cell lysates were prepared and immunoprecipitated (IP) with anti-HA antibody. Immunoprecipitates and whole-cell lysates (WCE) were analyzed by Western blotting.
DISCUSSION
The activation of the cellular kinase Akt is pivotal to the successful replication of many DNA viruses (reviewed in reference 8). In large part this is due to the central role that activated Akt plays in inhibiting apoptosis while increasing cell growth, metabolism, and synthesis rates (10). We have previously shown that TAg can activate Akt (39); additionally, SV40 small t antigen has also been reported to induce the phosphorylation of Akt (37, 41). Akt is fully activated when phosphorylated on both T308 and S473. We show that WT TAg induces phosphorylation at both sites and that this is abolished by the K1 mutation in the Rb binding site which renders TAg unable to bind Rb and its family members. This suggested that binding to Rb, p107, and/or p130 may be needed for Akt phosphorylation. However, the WT-TAg-mediated phosphorylation of Akt was not abolished in Rb− SAOS2 cells which were also depleted of both p107 and p130 using shRNAs.
Significantly, p107 and p130 were found to have modulating effects on both the steady-state TAg levels and the levels of Akt phosphorylation. Specifically, the p107 and p130 depletion experiments showed that the levels of both WT and K1 TAg are positively affected by p107, while p130 appears to have a negative effect. At this point, the mechanisms of these controls are not known. The TAg-mediated phosphorylation of Akt, although functional in the absence of all Rb family members, is enhanced by p107 and p130. In cells where p107 was depleted, phosphorylation at both T308 and S473 was reduced. Interestingly, this reduction was greater for phosphorylation at T308 than at S473. In this regard, the depletion of p130 resulted in only a reduction in T308 phosphorylation. Since PDK1 mediates Akt T308 phosphorylation, these data suggest that p107 and p130 affect PDK1 activity much more than the activity of mTORC2, which phosphorylates Akt at S473. It has been speculated that the phosphorylation of T308 by PDK1 and PI3K is a prerequisite for the phosphorylation at S473 by mTORC2 (30). Our data do not agree with this but support other evidence suggesting that S473 phosphorylation may precede T308 phosphorylation or may be independent of it (30, 31).
Thus, we have observed that depletion of all the Rb family proteins does not abolish the TAg-mediated phosphorylation of Akt; however, mutation of their binding site in TAg does. This suggested that the K1 mutation affects another function of TAg which does not involve the Rb family proteins. The observation that the TAg-mediated phosphorylation of Akt is inhibited by a PI3K inhibitor suggested that this function of TAg is targeted upstream of PI3K. Therefore, we considered adaptor proteins, specifically the IRS proteins for which a functionally significant interaction with TAg has been suggested. The transformation of mouse embryo fibroblasts by TAg requires tyrosine-phosphorylated IRS1 (11), and cells lacking IRS1 or containing serine-phosphorylated IRS1 cannot be transformed by TAg (11). In addition, an association between the two proteins has been shown (11, 17, 29). The results of our studies show that by depleting IRS1, the ability of TAg to mediate Akt phosphorylation is abolished. Further, the inability of K1 TAg to mediate Akt phosphorylation correlates with inefficient binding to IRS1.
We can only speculate on how TAg, primarily a nuclear protein, may interact with IRS1, which is primarily located near the plasma membrane and the insulin receptor. It has been reported that a small fraction of TAg stays in the cytoplasm and associates with the cellular membrane (13, 21, 33); this fraction may be in a position to functionally interact with IRS1, leading to IRS1 activation of PI3K. Alternatively, IRS1 has been reported in the nucleus of TAg-expressing cells, suggesting that its interaction with TAg may relocate it to the nucleus (29, 34, 36). Thus, it is possible that nuclear IRS1 mediates novel signaling mechanisms resulting in Akt phosphorylation.
Regardless of the mechanism, our data suggest that the interaction between TAg and IRS1 is needed to induce the phosphorylation of Akt on both S473 and T308. The K1 mutation in the Rb binding domain of TAg inhibits Akt phosphorylation and also disrupts the TAg-IRS1 interaction. This single amino acid change from glutamic acid to lysine changes the residual charge from negative to positive, which may not only affect the Rb binding domain but also alter the N-terminal structure of TAg, resulting in weakened binding to IRS1. Our data suggest that some of the phenotypes of the K1 mutation attributed to loss of binding to Rb family proteins may, in fact, be due to the loss of IRS1 binding.
Acknowledgments
We thank Alan Diehl for valuable discussions of the data and the members of the Alwine laboratory for critical evaluation of the data and reading of the manuscript. Cheers to all.
This work was supported by the National Institutes of Health grants R01CA28379 and R01GM45773 awarded to J.C.A.
Footnotes
Published ahead of print on 27 February 2008.
REFERENCES
- 1.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 7261-269. [DOI] [PubMed] [Google Scholar]
- 2.Backer, J. M., M. G. Myers, Jr., S. E. Shoelson, D. J. Chin, X. J. Sun, M. Miralpeix, P. Hu, B. Margolis, E. Y. Skolnik, J. Schlessinger, et al. 1992. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 113469-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brazil, D. P., Z. Z. Yang, and B. A. Hemmings. 2004. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem. Sci. 29233-242. [DOI] [PubMed] [Google Scholar]
- 4.Butel, J. S., and J. A. Lednicky. 1999. Cell and molecular biology of simian virus 40: implications for human infections and disease. J. Natl. Cancer Inst. 91119-134. [DOI] [PubMed] [Google Scholar]
- 5.Chaussepied, M., and D. Ginsberg. 2004. Transcriptional regulation of AKT activation by E2F. Mol. Cell 16831-837. [DOI] [PubMed] [Google Scholar]
- 6.Chellappan, S., V. B. Kraus, B. Kroger, K. Munger, P. M. Howley, W. C. Phelps, and J. R. Nevins. 1992. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc. Natl. Acad. Sci. USA 894549-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, S., and E. Paucha. 1990. Identification of a region of simian virus 40 large T antigen required for cell transformation. J. Virol. 643350-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooray, S. 2004. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J. Gen. Virol. 851065-1076. [DOI] [PubMed] [Google Scholar]
- 9.Crawford, L., K. Leppard, D. Lane, and E. Harlow. 1982. Cellular proteins reactive with monoclonal antibodies directed against simian virus 40 T-antigen. J. Virol. 42612-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 132905-2927. [DOI] [PubMed] [Google Scholar]
- 11.DeAngelis, T., J. Chen, A. Wu, M. Prisco, and R. Baserga. 2006. Transformation by the simian virus 40 T antigen is regulated by IGF-I receptor and IRS-1 signaling. Oncogene 2532-42. [DOI] [PubMed] [Google Scholar]
- 12.DeCaprio, J. A., J. W. Ludlow, J. Figge, J. Y. Shew, C. M. Huang, W. H. Lee, E. Marsilio, E. Paucha, and D. M. Livingston. 1988. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell 54275-283. [DOI] [PubMed] [Google Scholar]
- 13.Deppert, W., K. Hanke, and R. Henning. 1980. Simian virus 40 T-antigen-related cell surface antigen: serological demonstration on simian virus 40-transformed monolayer cells in situ. J. Virol. 35505-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downward, J. 2004. PI 3-kinase, Akt and cell survival. Semin. Cell Dev. Biol. 15177-182. [DOI] [PubMed] [Google Scholar]
- 15.Dyson, N., K. Buchkovich, P. Whyte, and E. Harlow. 1989. The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell 58249-255. [DOI] [PubMed] [Google Scholar]
- 16.Esposito, D. L., Y. Li, A. Cama, and M. J. Quon. 2001. Tyr(612) and Tyr(632) in human insulin receptor substrate-1 are important for full activation of insulin-stimulated phosphatidylinositol 3-kinase activity and translocation of GLUT4 in adipose cells. Endocrinology 1422833-2840. [DOI] [PubMed] [Google Scholar]
- 17.Fei, Z. L., C. D'Ambrosio, S. Li, E. Surmacz, and R. Baserga. 1995. Association of insulin receptor substrate 1 with simian virus 40 large T antigen. Mol. Cell. Biol. 154232-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilinger, G., and J. C. Alwine. 1993. Transcriptional activation by simian virus 40 large T antigen: requirements for simple promoter structures containing either TATA or initiator elements with variable upstream factor binding sites. J. Virol. 676682-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu, H., R. J. Botelho, M. Yu, S. Grinstein, and B. G. Neel. 2003. Critical role for scaffolding adapter Gab2 in Fc gamma R-mediated phagocytosis. J. Cell Biol. 1611151-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu, H., H. Maeda, J. J. Moon, J. D. Lord, M. Yoakim, B. H. Nelson, and B. G. Neel. 2000. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol. Cell. Biol. 207109-7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henning, R., J. Lange-Mutschler, and W. Deppert. 1981. SV40-transformed cells express SV40 T antigen-related antigens on the cell surface. Virology 108325-337. [DOI] [PubMed] [Google Scholar]
- 22.Holgado-Madruga, M., D. K. Moscatello, D. R. Emlet, R. Dieterich, and A. J. Wong. 1997. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc. Natl. Acad. Sci. USA 9412419-12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hresko, R. C., and M. Mueckler. 2005. mTOR·RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J. Biol. Chem. 28040406-40416. [DOI] [PubMed] [Google Scholar]
- 24.Kaelin, W. G., Jr., M. E. Ewen, and D. M. Livingston. 1990. Definition of the minimal simian virus 40 large T antigen- and adenovirus E1A-binding domain in the retinoblastoma gene product. Mol. Cell. Biol. 103761-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lietzke, S. E., S. Bose, T. Cronin, J. Klarlund, A. Chawla, M. P. Czech, and D. G. Lambright. 2000. Structural basis of 3-phosphoinositide recognition by pleckstrin homology domains. Mol. Cell 6385-394. [DOI] [PubMed] [Google Scholar]
- 26.Maeda, K., H. Murakami, R. Yoshida, M. Ichihara, A. Abe, M. Hirai, T. Murohara, and M. Takahashi. 2004. Biochemical and biological responses induced by coupling of Gab1 to phosphatidylinositol 3-kinase in RET-expressing cells. Biochem. Biophys. Res. Commun. 323345-354. [DOI] [PubMed] [Google Scholar]
- 27.Myers, M. G., Jr., J. M. Backer, X. J. Sun, S. Shoelson, P. Hu, J. Schlessinger, M. Yoakim, B. Schaffhausen, and M. F. White. 1992. IRS-1 activates phosphatidylinositol 3′-kinase by associating with src homology 2 domains of p85. Proc. Natl. Acad. Sci. USA 8910350-10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orimo, A., P. B. Gupta, D. C. Sgroi, F. Arenzana-Seisdedos, T. Delaunay, R. Naeem, V. J. Carey, A. L. Richardson, and R. A. Weinberg. 2005. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121335-348. [DOI] [PubMed] [Google Scholar]
- 29.Prisco, M., F. Santini, R. Baffa, M. Liu, R. Drakas, A. Wu, and R. Baserga. 2002. Nuclear translocation of insulin receptor substrate-1 by the simian virus 40 T antigen and the activated type 1 insulin-like growth factor receptor. J. Biol. Chem. 27732078-32085. [DOI] [PubMed] [Google Scholar]
- 30.Sarbassov, D. D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 3071098-1101. [DOI] [PubMed] [Google Scholar]
- 31.Scheid, M. P., P. A. Marignani, and J. R. Woodgett. 2002. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol. Cell. Biol. 226247-6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons, D. T. 2000. SV40 large T antigen functions in DNA replication and transformation. Adv. Virus Res. 5575-134. [DOI] [PubMed] [Google Scholar]
- 33.Soule, H. R., R. E. Lanford, and J. S. Butel. 1980. Antigenic and immunogenic characteristics of nuclear and membrane-associated simian virus 40 tumor antigen. J. Virol. 33887-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan, C. S., and J. M. Pipas. 2002. T antigens of simian virus 40: molecular chaperones for viral replication and tumorigenesis. Microbiol. Mol. Biol. Rev. 66179-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiscornia, G., O. Singer, and I. M. Verma. 2006. Production and purification of lentiviral vectors. Nat. Protoc. 1241-245. [DOI] [PubMed] [Google Scholar]
- 36.Tu, X., P. Batta, N. Innocent, M. Prisco, I. Casaburi, B. Belletti, and R. Baserga. 2002. Nuclear translocation of insulin receptor substrate-1 by oncogenes and Igf-I. Effect on ribosomal RNA synthesis. J. Biol. Chem. 27744357-44365. [DOI] [PubMed] [Google Scholar]
- 37.Ugi, S., T. Imamura, H. Maegawa, K. Egawa, T. Yoshizaki, K. Shi, T. Obata, Y. Ebina, A. Kashiwagi, and J. M. Olefsky. 2004. Protein phosphatase 2A negatively regulates insulin's metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol. Cell. Biol. 248778-8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf, D. A., H. Hermeking, T. Albert, T. Herzinger, P. Kind, and D. Eick. 1995. A complex between E2F and the pRb-related protein p130 is specifically targeted by the simian virus 40 large T antigen during cell transformation. Oncogene 102067-2078. [PubMed] [Google Scholar]
- 39.Yu, Y., and J. C. Alwine. 2002. Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3′-OH kinase pathway and the cellular kinase Akt. J. Virol. 763731-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu, Y., S. B. Kudchodkar, and J. C. Alwine. 2005. Effects of simian virus 40 large and small tumor antigens on mammalian target of rapamycin signaling: small tumor antigen mediates hypophosphorylation of eIF4E-binding protein 1 late in infection. J. Virol. 796882-6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan, H., T. Veldman, K. Rundell, and R. Schlegel. 2002. Simian virus 40 small tumor antigen activates AKT and telomerase and induces anchorage-independent growth of human epithelial cells. J. Virol. 7610685-10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zalvide, J., and J. A. DeCaprio. 1995. Role of pRb-related proteins in simian virus 40 large-T-antigen-mediated transformation. Mol. Cell. Biol. 155800-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]