Abstract
The Old World hantaviruses, members of the family Bunyaviridae, cause hemorrhagic fever with renal syndrome (HFRS). Transmission to humans occurs via inhalation of aerosols contaminated with the excreta of infected rodents. The viral antigen is detectable in dendritic cells, macrophages, lymphocytes, and, most importantly, microvascular endothelial cells. However, the site and detailed mechanism of entry of HFRS-causing hantaviruses in polarized epithelial cells have not yet been defined. Therefore, this study focused on the entry of the pathogenic hantaviruses Hantaan and Puumala into African green monkey kidney epithelial cells and primary human endothelial cells. The polarized epithelial and endothelial cells were found to be susceptible to hantavirus infection exclusively from the apical surface. Treatment with phosphatidylinositol-specific phospholipase C, which removes glycosylphosphatidylinositol (GPI)-anchored proteins from the cell surface, protects cells from infection, indicating that hantaviruses require a GPI-anchored protein as a cofactor for entry. Decay-accelerating factor (DAF)/CD55 is a GPI-anchored protein of the complement regulatory system and serves as a receptor for attachment to the apical cell surface for a number of viruses. Infection was reduced by the pretreatment of hantaviral particles with human recombinant DAF. Moreover, the treatment of permissive cells with DAF-specific antibody blocked infection. These results demonstrate that the Old World hantaviruses Hantaan and Puumala enter polarized target cells from the apical site and that DAF is a critical cofactor for infection.
Hantaviruses belong to the family Bunyaviridae (36). In contrast to other genera of the family Bunyaviridae, these viruses infect humans when hantavirus-contaminated excretions from persistently but asymptomatically infected rodents are inhaled (35). Hantaviruses are enveloped viruses with a single-stranded negative-sense RNA consisting of three segments. The small (S) segment encodes the nucleocapsid (N) protein, the medium (M) segment encodes the two envelope proteins (Gn and Gc), and the large (L) segment encodes the viral RNA polymerase (30). Within the genus Hantavirus, two groups have been identified: New World and Old World hantaviruses. They not only differ in their geographical distribution but also vary regarding the pathology of human infection. New World hantaviruses cause the hantavirus pulmonary syndrome (HPS), whereas Old World hantaviruses are the causative agent of hemorrhagic fever with renal syndrome (HFRS). Renal manifestation includes acute tubulointerstitial nephritis and interstitial hemorrhage, leading to acute renal failure. Severe forms of HFRS may even result in chronic renal failure. Despite differences concerning the main target organ, a common characteristic of both HPS and HFRS infection is capillary leakage due to infection of vascular endothelial cells (17, 46).
Endothelial cells form polarized monolayers that function as an interface between vessel lumen and vessel wall and thereby control vascular permeability. Polarized cells sort lipids and proteins to their apical and basolateral surfaces, respectively. The asymmetric segregation is maintained by tight junctions, which are specialized multiprotein-complexes at cell-to-cell contacts that inhibit the movement of components along the apical and basolateral domain and hinder passage across the endothelium (2, 9, 24, 41). Lining the blood-tissue interface, the endothelium is the site of pathogen attack. The polarized sorting of receptors renders them inaccessible to the pathogen, and many pathogens have to cross the epithelial barrier to initiate and establish infection (7, 8, 19, 27). Therefore, the polarized entry of viruses plays a pivotal role in pathogenesis. The entry of hantaviruses into polarized cells has so far been analyzed only on the basis of two New World hantaviruses. The entry of the Black Creek Canal virus into polarized Vero C1008 cells, an epithelial monkey kidney cell line, is restricted to the apical site, whereas the Andes virus can infect primary airway epithelial cells via the apical and basolateral surfaces (32, 33). Identifying the mechanisms of viral entry and spread in the renal endothelium and epithelium is an important step in understanding the clinical picture of HFRS.
In this study, we examined the entry of the pathogenic Old World hantaviruses Hantaan and Puumala into polarized epithelial and primary endothelial cells. Our results demonstrate that Hantaan and Puumala enter cells from the apical surface and require the apical glycosylphosphatidylinositol (GPI)-anchored receptor decay-accelerating factor (DAF) for infection.
MATERIALS AND METHODS
Cells and viruses.
Vero C1008 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and antibiotics. Human umbilical vein endothelial cells (HUVECs) were obtained from PromoCell (Heidelberg, Germany). Cells were grown to confluence in endothelial cell growth medium with low serum (PromoCell). Only HUVECs from passages 2 to 4 were used. To establish polarized monolayers, cells (1 × 105) were plated on 0.4-μm-pore-size 12-well Transwell culture system filters (Greiner Bio-One, Frickenhausen, Germany). The integrity of the monolayers was assessed by measuring the transepithelial electrical resistance (TER) with a Millicell-ERS voltohmmeter (Millipore, Schwalbach, Germany). Stocks of Hantaan virus strain 76-118 (HTNV) and Puumala strain CG18-20 (PUUV) were propagated on Vero E6 cells.
Immunofluorescence and flow cytometry.
For immunofluorescence, cells on microporous filters were fixed with 3% paraformaldehyde-phosphate-buffered saline (PBS) or acetone and incubated with primary antibodies and appropriate fluorescently labeled secondary antibodies. The former were the monoclonal anti-Hantaan nucleocapsid protein B5D9, monoclonal anti-Puumala nucleocapsid protein A1C5 (Progen, Heidelberg, Germany), monoclonal anti-CD59 (BD Pharmingen, Heidelberg, Germany), and monoclonal anti-ZO-1 (Invitrogen, Karlsruhe, Germany), whereas the latter came from Dianova (Hamburg, Germany). Cell nuclei were stained with Hoechst 33342 (Invitrogen). Images were taken using a Nikon DS-Qi1Mc quantitative black-and-white charge-coupled device camera attached to a Nikon Eclipse 80i upright microscope (Nikon, Düsseldorf, Germany). Series of optical sections distanced 0.5 μm on the z axis were taken with a confocal scanning laser microscope (Nikon C1Si spectral imaging confocal laser scanning system on a Nikon TE2000-E inverted microscope). The same contrast and intensity settings were applied to samples stained with identical antibodies.
For flow cytometry, Vero C1008 cells were washed, scraped, and stained with rabbit polyclonal anti-DAF H319 antibody (Santa Cruz, Heidelberg, Germany). After 1 h, cells were washed again and incubated with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit antibody. After 30 min of incubation, the cells were washed a third time and then analyzed by flow cytometry with FACSCalibur (BD Pharmingen).
Depolarization with EDTA.
Vero C1008 cell monolayers were grown to confluence until the maximum TER was reached. The disruption of tight junctions was induced by 2.5 mM EDTA in PBS without Ca2+ or Mg2+. Control cells were incubated in PBS containing CaCl2 and MgCl2. TER measurements were taken at various time points. For the imaging of depolarized monolayers, cells were fixed and processed for immunofluorescence as described above.
Infection.
Virus inocula were added to the apical or basolateral site of polarized monolayer surfaces in a serum-free medium. After incubation for 1 h at 37°C, unbound virus was removed by a triple washing, and cells were incubated for 48 h at 37°C. The infection was monitored by the immunofluorescence of hantaviral N protein or by the Western blot analysis of N-protein expression. For immunofluorescence, acetone-fixed cells were stained with mouse monoclonal antinucleocapsid protein and a secondary Cy3-conjugated anti-mouse antibody. For Western blot analysis, cells were lysed and, after being boiled in sodium dodecyl sulfate sample buffer and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane. The infection was monitored by the detection of hantaviral N protein using rabbit polyclonal anti-Hantaan or anti-Puumala nucleocapsid protein antibody (26). Equal loading was verified by the detection of tubulin on the same membrane with the anti-α-tubulin monoclonal antibody DM 1A (Sigma, Deisenhofen, Germany). Protein detection was performed after the incubation with primary and peroxidase-conjugated secondary antibodies using a Supersignal Pico detection kit (Pierce, Bonn, Germany) according to the manufacturer's instructions. The quantitative Western blot analysis was performed by using Alexa 680-conjugated secondary antibody (Invitrogen) and an Odyssey infrared imaging system (Li-Cor Biosciences, Bad Homburg, Germany).
PI-PLC and MCD treatment.
Monolayers were washed with serum-free medium and treated with 1.0 unit of phosphatidylinositol-specific phospholipase C (PI-PLC) from Bacillus cereus (Invitrogen) in serum-free medium or, for control purposes, with the corresponding dilution of the storage buffer (20 mM Tris-HCl [pH 7.5], 1 mM EDTA, 0.01% sodium azide, 50% glycerol) of PI-PLC. After incubation at 37°C for 30 min, cells were washed and infected as described above. For disruption of lipid rafts, cells were washed twice and incubated for 1 h at 37°C in serum-free medium containing various concentrations of the raft-disrupting agent methyl-β-cyclodextrin (MCD) (Sigma). Cell viability was assessed by trypan blue staining.
Blocking with antibodies and recombinant human proteins.
Antibodies specific for DAF (CD55) (rabbit polyclonal antibody H319; Santa Cruz) or integrin αvβ3 (mouse monoclonal antibody 1976; Millipore) were added to polarized Vero C1008 cells. Cells were treated with increasing concentrations of antibodies for 1 h at 4°C. Then the hantavirus inocula were added to the monolayer. After incubation for 1 h at 37°C, the cells were washed again and incubated for 48 h prior to N-protein expression analysis. For blocking assays with DAF or urokinase plasminogen activator receptor (uPAR) protein, virus was pretreated with carrier-free recombinant glycosylated human DAF (rhDAF) or uPAR (rhuPAR) (R&D Systems, Wiesbaden-Nordenstadt, Germany) in serum-free medium or with serum-free medium alone and then allowed to complex on ice for 1 h, and infection was performed as described above.
RESULTS
Vero C1008 cells form polarized monolayers.
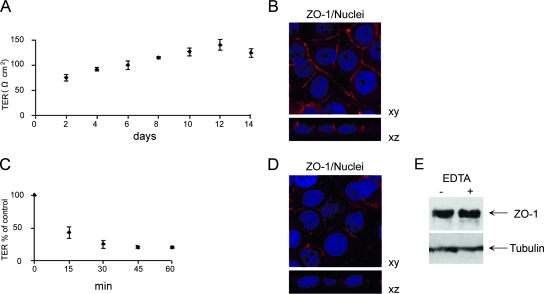
In order to examine the entry of HTNV into polarized cells, we used the African green monkey kidney epithelial cell line Vero C1008. To confirm confluence of monolayers grown on permeable filter supports, we monitored the TER and the formation of tight junctions. The development of TER was measured for 14 days after seeding. TER increased continuously to a maximum on day 12 and declined afterwards (Fig. 1A). Confocal immunofluorescence analysis of xy sections revealed expression of the tight junction protein ZO-1 (zonula occludens 1) on cell-to-cell contacts, whereas the vertical xz section displayed the distribution of ZO-1 exclusively along lateral plasma membranes of adjacent cells (Fig. 1B). The integrity and the barrier function of tight junctions strictly depend on extracellular calcium concentration (22). Depolarization of Vero C1008 monolayers by chelation of extracellular calcium with EDTA resulted in a continuously decreasing TER (Fig. 1C). The disruption of tight junctions is also demonstrated by the redistribution of ZO-1. Cells treated with EDTA exhibited a discontinuous and weaker staining of ZO-1 at their margins (Fig. 1D). To ensure that EDTA initiates a redistribution and not reduction of ZO-1 protein, equal amounts of total cell protein were analyzed by Western blotting (Fig. 1E). The results revealed a characteristic 225-kDa band representing ZO-1 and demonstrated that expression levels of ZO-1 were essentially the same in monolayers incubated with and without EDTA. These results show that Vero C1008 cells develop confluent, polarized monolayers with functional tight junctions.
FIG. 1.
Vero C1008 cells form polarized monolayers. (A) Cells were seeded on microporous filter inserts, and the TER was measured at the indicated time points (values are means ± standard deviations for three monolayers). (B) Expression of the marker protein for tight junctions, ZO-1, in polarized Vero C1008 cells. Monolayers were stained for ZO-1 (red) and analyzed by confocal microscopy. Nuclei (blue) were stained with Hoechst 33342. (C) Monolayers on Transwell filters were exposed to EDTA, and changes in TER were monitored at the indicated time points (values are means ± standard deviations for three monolayers). (D) Polarized Vero C1008 cells were depolarized with EDTA for 15 min and immunostained for ZO-1 (red). Nuclei were stained with Hoechst 33342. (E) Polarized Vero C1008 monolayers were depolarized with EDTA for 15 min, and equal amounts of total protein were analyzed for ZO-1 content.
Hantavirus enters Vero C1008 cells from the apical site.
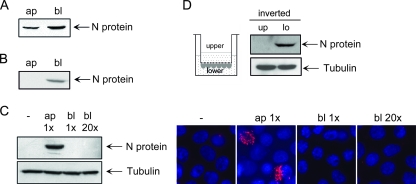
In the Transwell system, viral particles have to overcome the filter of the permeable support to enter cells from the basolateral surface. Therefore, we examined whether the Transwell filter per se represents a barrier for the virions. We placed HTNV inocula into the lower chamber, and after shaking the Transwell system for 1 h, we compared the amount of viral particles in both chambers. The quantification of the hantaviral N-protein content by quantitative Western blot analysis using Li-Cor infrared immunofluorescence technology showed that about 30% of the HTNV particles had reached the upper chamber (Fig. 2A).
FIG. 2.
HTNV enters from the apical surface of Vero C1008 cells. (A) Quantification of HTNV particles traversing the filter of the Transwell system. Apical (ap) and basolateral (bl) medium was analyzed for N protein 1 h after addition of viral inocula to the basolateral chamber of the Transwell system. Shown is a representative Western blot of three independent experiments. (B) Western blot analysis of HTNV particles crossing the polarized Vero C1008 monolayer grown on the Transwell filter. The apical and basolateral medium was analyzed for the presence of viral N protein 1 h after addition of viral inocula to the basolateral chamber of the Transwell system. (C) Western blot and immunofluorescence analysis of polarity of HTNV infection. Polarized Vero C1008 cells grown on filter inserts were inoculated with HTNV through either the apical or basolateral chamber. At 48 h postinfection, cells were lysed and assessed for expression of N protein and tubulin or fixed and immunostained for N protein with anti-N protein and a Cy3-conjugated anti-mouse immunoglobulin secondary antibody. Nuclei were stained with Hoechst 33342. (D) HTNV infection of inverse growing polarized Vero C1008 monolayers. Cells were inoculated with HTNV either through the upper (up) or lower (lo) chamber. The addition of virus to the upper chamber corresponds to basolateral application due to the inverted growing of the cells on the bottom side of the filter.
To confirm that the confluent polarized Vero C1008 monolayer is a valid in vitro cell culture model for the epithelial barrier function, we placed virus in the basolateral chamber of the Transwell filter system. After 1 h, we analyzed whether viral particles crossed the monolayer by analyzing the hantaviral N-protein content in the lower and upper chambers by Western blot analysis. In the basolateral chamber, the viral inocula were detectable. In contrast, no N protein of HTNV was visible in the upper chamber (Fig. 2B).
We performed studies on the entry of HTNV into polarized Vero C1008 cells by applying virus to the upper and lower chambers of the Transwell system. To solve the problem that the majority of viruses had been trapped in the filter, we placed a 20-fold excess of virus inoculum in the lower chamber. The analysis of hantaviral N-protein expression by Western blot revealed that HTNV entered from the apical surface. In contrast, polarized Vero C1008 cells were not susceptible to viral infection through the basolateral membrane, independent of the number of virus particles added to the lower compartment. The entry of hantavirus via the apical surface was also confirmed by the staining of N protein with mouse monoclonal anti-N protein antibody. No single cell of the monolayer inoculated with virus via the basolateral chamber was positive for N-protein expression (Fig. 2C).
To exclude any gravitational effects as a reason for apical infection, the Transwell insert was turned upside down. The bottom side of the filter was reversed, and cells were seeded on it. After the attachment of the cells, the filter was brought back to its former position. In this system, the basolateral cell surface faced upwards. Polarized monolayers of this inverted system were exposed to HTNV, and infection was monitored by N-protein expression (Fig. 2D). The inverted growing monolayers were not susceptible to infection via the upper chamber. On the other hand, the addition of virus to the medium of the lower chamber surrounding the apical cell surface resulted in infection.
These data indicate that the entry of HTNV into polarized cells is restricted to the apical surfaces of polarized monolayers.
Treatment with PI-PLC and MCD abrogates Hantavirus infection.
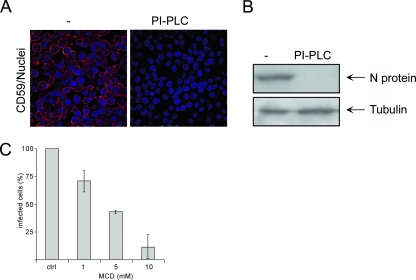
To determine whether HTNV requires an apical attachment factor, we investigated the infection of polarized Vero C1008 cells treated with PI-PLC. This enzyme releases proteins with a GPI anchor from the cell surface. The glycolipid anchor of proteins serves as an apical sorting signal in polarized cells (5). To demonstrate that PI-PLC removed GPI-anchored proteins efficiently from the cell surface of Vero C1008 monolayers, we performed immunofluorescence studies to visualize the presence of the GPI-anchored protein CD59 on the cell surface. Figure 3A demonstrates that PI-PLC sequestered GPI-anchored proteins completely from the cell surface by the fact that no staining of CD59 was observed in the treated monolayers. The absence of N protein in lysates from cells treated with PI-PLC revealed that the removal of GPI-anchored proteins inhibited infection (Fig. 3B). Since GPI-anchored proteins are clustered in lipid raft microdomains (10, 14), we investigated the effect of the lipid raft-disrupting agent MCD on hantavirus infection. MCD removes cholesterol from the plasma membrane, thereby destabilizing lipid rafts (34). Pretreatment of monolayers with increasing concentrations of MCD rendered cells less susceptible to infection, as shown by the reduced number of infected cells (Fig. 3C). These results suggest that infection by HTNV depends on the presence of a specific GPI-anchored protein on the cell surface and the integrity of lipid raft microdomains.
FIG. 3.
PI-PLC and MCD treatment inhibit HTNV infection. (A) Vero C1008 cells were treated with 1.0 U PI-PLC or with the corresponding dilution of the PI-PLC storage buffer and stained with anti-CD59 and a Cy3-conjugated anti-mouse immunoglobulin secondary antibody. Nuclei were stained with Hoechst 33342. (B) Monolayers of Vero C1008 cells were left untreated (−) or treated with 1.0 U PI-PLC and infected with HTNV from the apical site. At 48 h postinfection, cell lysates were analyzed for expression of N protein and tubulin. The data are representative of three independent experiments. (C) Cells were treated with increasing concentrations of MCD and subsequently subjected to HTNV infection. Two days postinfection, cells were fixed and analyzed for expression of N protein by immunofluorescence with anti-N protein antibody and Cy3-labeled anti-mouse immunoglobulin secondary antibody. The data are representative of three independent experiments. Error bars represent the standard deviation.
Anti-DAF antibodies and recombinant DAF inhibit hantavirus entry.
Since the GPI-anchored protein DAF, a 70-kDa member of the regulator proteins of the complement system, serves as an attachment factor for a number of echovirus and coxsackie B virus serotypes of enteroviruses (3, 4, 18, 38, 39, 45), we analyzed whether this protein is also involved in the entry process of hantaviruses. First, we assessed the expression of DAF on Vero C1008 cells by Western blot analysis in total lysate. DAF was detectable as a double band of approximately 70 kDa in the lysate of Vero C1008 cells (Fig. 4A). The surface expression of DAF on Vero C1008 cells was demonstrated by flow cytometry (Fig. 4B).
FIG. 4.
DAF-specific antibodies and soluble DAF block entry of HTNV. (A) Lysates of Vero C1008 cells were analyzed by Western blotting with polyclonal anti-DAF antibody H319. (B) Surface expression of DAF was assessed by flow cytometry. Vero C1008 cells were stained with anti-DAF antibody and FITC-conjugated secondary antibody and analyzed by flow cytometry. The open histogram represents the secondary-antibody control, and the shaded histogram represents cells stained with DAF-specific primary and appropriate fluorescently labeled secondary antibody. (C) Cells were incubated with increasing concentrations of DAF-specific antibody (anti-DAF H319) prior to infection. At 48 h postinfection, infected cells were quantified by the immunofluorescence analysis of N-protein expression. The data are representative of three independent experiments. Error bars represent standard deviations. (D) HTNV particles were preincubated with increasing concentrations of rhDAF. Confluent monolayers of Vero C1008 cells were infected with untreated particles or particles that had been pretreated with rhDAF. At 48 h postinfection, cells were lysed and analyzed for expression of N protein and tubulin. Similar results were obtained in three independent experiments; results of a representative one are shown here. (E) Vero C1008 cells were infected with HTNV particles pretreated with rhDAF. At 48 h postinfection, cells were stained for N protein, and infected cells were quantified by counting N-protein-expressing cells.
To address the question of whether the GPI-anchored protein DAF plays a role in hantaviral entry, we investigated the effect of anti-DAF antibody. The incubation of the monolayers with anti-DAF antibody resulted in a substantial loss of infection (Fig. 4C). These results indicate that the binding to DAF is required for infection.
To verify that DAF is required for the infection of Vero C1008 cells by Hantaan virus, we preincubated viral particles with soluble rhDAF. The preincubation of viral particles with increasing concentrations of rhDAF inhibited infection in a concentration-dependent manner, as revealed by the analysis of N-protein expression in cell lysates and the quantification of N-protein-expressing cells by immunofluorescence (Fig. 4D and E). These results strongly suggest that DAF is a cofactor for the infection of polarized epithelial cells by HTNV.
Both integrin αvβ3 and DAF are required for HTNV infection.
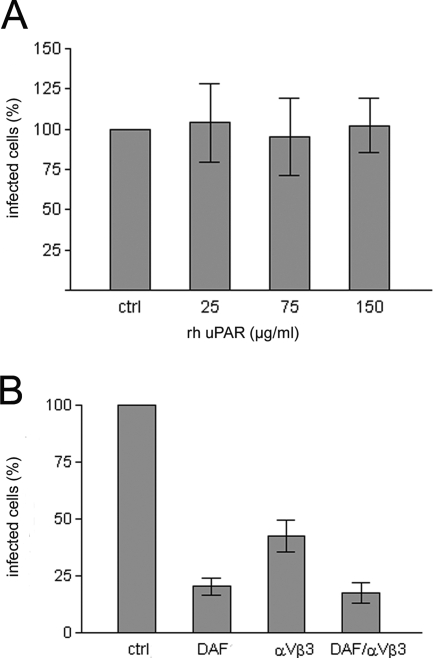
To confirm the specificity of HTNV for the GPI-anchored receptor DAF, we pretreated viral particles with increasing concentrations of the raft-resident GPI-linked protein uPAR. In contrast to preincubation with rhDAF (Fig. 4E), the pretreatment of viral particles with rhuPAR did not decrease viral infectivity (Fig. 5A). Integrins of the β3 family have been found to serve as receptors for hantaviruses causing HFRS in unpolarized cells. To determine whether both DAF and integrins play a role in entry in polarized monolayers, we examined the inhibitory effect of anti-DAF, anti-integrin αvβ3, and also their combination on hantaviral infectivity (Fig. 5B). Antibodies to DAF were added to the polarized Vero C1008 monolayer via the apical chamber and antibodies to integrin αvβ3 via the basal chamber. Pretreatment with anti-DAF or anti-integrin αvβ3 inhibited hantaviral infection. The combination of antibodies did not lead to an additive inhibitory effect, in comparison to the pretreatment with the DAF antibody alone. These results suggest that both DAF and integrins participate in the entry of hantaviruses into polarized cells.
FIG. 5.
Inhibition of HTNV infectivity is receptor and antibody specific. (A) HTNV particles were pretreated with rhuPAR and added via the apical chamber to Vero C1008 cells grown on Transwell filters. At 48 h postinfection, cells were analyzed for N-protein expression. Data are representative of two independent experiments. Error bars represent standard deviations. (B) Triplicate wells of polarized Vero C1008 cells were pretreated with 40 μg/ml anti-DAF, anti-integrin αvβ3, or both (in combination). At 48 h postinfection, infected cells were quantified by immunofluorescence analysis of N-protein expression. Data are representative of three independent experiments. Error bars represent standard deviations.
Hantaan virus enters endothelial cells from the apical site.
To examine whether HTNV applies the same mechanism of infection to human endothelial cells, we performed entry studies with primary HUVECs. HUVECs were grown to confluence on Transwell membranes. Confluent HUVEC monolayers were subjected to HTNV infection via the apical or basolateral chamber of the Transwell system. The addition of virus to the apical surfaces of the monolayers resulted in infection, as demonstrated by the analysis of N-protein expression, whereas basolateral inoculation did not allow infection of HUVECs (Fig. 6A). The removal of GPI-anchored proteins by pretreatment with PI-PLC abolished susceptibility of polarized endothelial cells to infection with Hantaan virus (Fig. 6B). Furthermore, infection was completely blocked when viral particles were preincubated with rhDAF (Fig. 6C). Therefore, as in polarized epithelial Vero C1008 cells, the infection of HUVECs by HTNV occurs from the apical site and requires the interaction of virus particles with the GPI-anchored receptor DAF.
FIG. 6.
HTNV enters HUVECs from the apical surface and requires DAF for infection. (A) HUVECs grown on Transwell filters were inoculated with HTNV via the apical or basolateral chamber. At 48 h postinfection, cells were harvested, and expression of N protein and tubulin was analyzed by Western blotting. (B) Monolayers of HUVECs were left untreated (−) or were treated with PI-PLC and infected with HTNV. Expression of N protein and tubulin was detected by Western blot analysis. (C) HTNV particles were pretreated with rhDAF. Following incubation, particles were added to HUVECs grown on Transwell filters. At 48 h postinfection, cells were harvested, and infection was monitored by N-protein expression.
Puumala virus enters from the apical surface.
To determine if Old World hantaviruses other than Hantaan virus utilize the apical surface to gain entry into their host cells, we studied the entry of the pathogenic hantaviral strain PUUV. As shown in Fig. 7A and B, Puumala virus enters polarized Vero C1008 cells and HUVECs via the apical surface. We examined whether the entry of Puumala virus is mediated by DAF. Pretreatment of polarized monolayers of Vero C1008 cells with increasing concentrations of anti-DAF antibodies inhibited infection (Fig. 7C). Apical entry and the requirement of DAF appear to be common mechanisms of Old World hantavirus infection.
FIG. 7.
PUUV enters Vero C1800 cells and HUVECs from the apical surface and requires DAF for infection. (A) Vero C1008 cells grown on Transwell filters were inoculated with PUUV via the apical or basolateral chamber. At 48 h postinfection, cells were harvested, and infection was monitored by Western blot analysis of N-protein expression. (B) Monolayers of HUVECs were inoculated with PUUV via the apical or basolateral chamber. At 48 h postinfection, cells were harvested and expression of N protein, and tubulin was analyzed by Western blotting. (C) Monolayers of Vero C1008 cells were incubated with increasing concentrations of DAF-specific antibody (anti-DAF H319) prior to infection with PUUV. At 48 h postinfection, infected cells were quantified by immunofluorescence analysis of N-protein expression. Data are representative of three independent experiments. Error bars represent standard deviations.
DISCUSSION
In this study, we demonstrate that the entry of the pathogenic hantaviruses Hantaan and Puumala in polarized epithelial and endothelial cells is restricted to the apical surface and depends on the apical receptor DAF. Hantaviruses causing HFRS or HPS primarily infect epithelial cells. In renal tissue of HTNV-infected patients, virus was found in endothelial and tubular cells and in urinary sediment containing tubular cells (25). Since the epithelium represents a polarized monolayer, the distribution of proteins is segregated into apical and basolateral localization. This segregation affects the accessibility of viral receptors and the mechanism of viral entry. Integrins of the β3 family have been identified as receptors on endothelial cells that are required for cellular entry by pathogenic hantaviruses (12, 13). Integrins, a large family of heterodimeric transmembrane glycoproteins, mediate cell adhesion and binding to the extracellular matrix. Given the fact that the expression of β3 integrins is restricted to the basolateral surfaces of polarized cells (37, 42), the viral receptor β3 integrin is inaccessible to the virus and does not allow initial attachment. Many viruses have to overcome the epithelial barrier to initiate and establish infection and have evolved different strategies to get access to polarized host cells. Some viruses, such as herpes simplex virus (11), adenoviruses (16), and Epstein-Barr virus (42), interact with alternative receptors to infect epithelia. Coxsackievirus uses a basolateral entry receptor and interacts with a second receptor that is localized apically and mediates attachment. One prominent apical attachment factor for pathogens of the endothelium is the complement factor DAF (3, 45). DAF is an inhibitory regulator protein of the complement system and is expressed on a wide variety of cells. In normal kidney, DAF has been detected in tubules, cells of the juxtaglomerular apparatus, and glomerular cells (6, 15, 23, 31, 40). The localization in polarized cells is exclusively apical, as shown for rat podocytes and mucosal epithelial cell lines (1, 7, 20, 21). The concerted mechanism of initial binding to apical DAF and virus entry via the entry receptor CAR, which is normally hidden beneath the tight junctions, was recently elucidated for coxsackievirus (7). The attachment to DAF activates a signaling cascade, leading to cytoskeletal rearrangements and opening of the tight junctions that facilitate the transport of the viral particle to CAR. The entry process of hantaviruses has not been well understood so far. Hitherto, β3 integrins have been identified as receptors mediating solely the entry of pathogenic hantaviruses into unpolarized cells. We demonstrate that integrin αvβ3 is also required for the infection of polarized cells. This observation suggests that both integrin αvβ3 and DAF participate in hantaviral entry. Our results raise the possibility that, in analogy to the coxsackievirus mechanism, hantaviruses overcome the tight junction barrier by DAF-induced signaling.
The entry of viruses into the epithelium is often intimately connected with the pathogenesis of the viral disease. The entry of rotavirus induces increased permeability by disruption of the integrity of tight junctions (8, 27, 29), and Ebola virus glycoprotein in virus-like particles affects the endothelial barrier function (44). The possible causal relationship between hantaviral entry and disturbance of the epithelial barrier function has so far not been elucidated. The differences in the polarized susceptibility of two New World hantaviruses—bidirectional for Andes virus and apical for Black Creek Canal virus—demonstrate that the mechanism of hantaviral entry into polarized epithelial cells depends on the virus strain and cell type-specific determinants (32, 33). In the case of the Old World hantaviruses, the endothelial and epithelial cells of the kidney are the main but not the primary target. The route of infection is through inhalation. The DAF coreceptor is abundantly expressed in the normal human respiratory tract and in cells of the hematopoietic lineage (28, 43). To what extent the mechanism of DAF receptor usage demonstrated for the kidney epithelial cell line and primary endothelial cells is applicable to target cells in the lung remains to be investigated.
Histopathological findings in HFRS patients document a generalized capillary damage by congestion of intertubular capillaries and interstitial hemorrhage. The extent of pathological alterations in tubular, interstitial, and glomerular histology correlates with the severity of renal failure. However, the mechanism underlying hantavirus-induced endothelial and epithelial dysfunction is still unclear. Although the exact mechanism of viral pathogenesis is not clear, the polarized entry of hantavirus may have an essential impact on the clinical picture of HFRS. Therefore, further investigations are necessary to elucidate the steps of viral entry and replication in the mechanism of pathogenesis.
Acknowledgments
We thank Ulrike Engel and Christian Ackermann from the Nikon Imaging Center at the University of Heidelberg for access to and training on the confocal microscope. We thank Claudia Tolliver for technical assistance and Gabi Lux for her kind help with the preparation of the virus. We thank Walter Nickel for access to the Li-Cor system.
Footnotes
Published ahead of print on 27 February 2008.
REFERENCES
- 1.Bao, L., O. B. Spiller, P. L. St John, M. Haas, B. K. Hack, G. Ren, P. N. Cunningham, M. Doshi, D. R. Abrahamson, B. P. Morgan, and R. J. Quigg. 2002. Decay-accelerating factor expression in the rat kidney is restricted to the apical surface of podocytes. Kidney Int. 622010-2021. [DOI] [PubMed] [Google Scholar]
- 2.Bazzoni, G., and E. Dejana. 2004. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol. Rev. 84869-901. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., M. Chan, K. R. Solomon, N. F. St. John, H. Lin, and R. W. Finberg. 1994. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc. Natl. Acad. Sci. USA 916245-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. G. Mohanty, R. L. Crowell, N. F. St John, D. M. Lublin, and R. W. Finberg. 1995. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55). J. Virol. 691903-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, D. A., B. Crise, and J. K. Rose. 1989. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science 2451499-1501. [DOI] [PubMed] [Google Scholar]
- 6.Cosio, F. G., D. D. Sedmak, J. D. Mahan, and N. S. Nahman, Jr. 1989. Localization of decay accelerating factor in normal and diseased kidneys. Kidney Int. 36100-107. [DOI] [PubMed] [Google Scholar]
- 7.Coyne, C. B., and J. M. Bergelson. 2006. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124119-131. [DOI] [PubMed] [Google Scholar]
- 8.Dickman, K. G., S. J. Hempson, J. Anderson, S. Lippe, L. Zhao, R. Burakoff, and R. D. Shaw. 2000. Rotavirus alters paracellular permeability and energy metabolism in Caco-2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 279G757-G766. [DOI] [PubMed] [Google Scholar]
- 9.Eaton, S., and K. Simons. 1995. Apical, basal, and lateral cues for epithelial polarization. Cell 825-8. [DOI] [PubMed] [Google Scholar]
- 10.Elortza, F., T. S. Nuhse, L. J. Foster, A. Stensballe, S. C. Peck, and O. N. Jensen. 2003. Proteomic analysis of glycosylphosphatidylinositol-anchored membrane proteins. Mol. Cell. Proteomics 21261-1270. [DOI] [PubMed] [Google Scholar]
- 11.Galen, B., N. Cheshenko, A. Tuyama, B. Ramratnam, and B. C. Herold. 2006. Access to nectin favors herpes simplex virus infection at the apical surface of polarized human epithelial cells. J. Virol. 8012209-12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavrilovskaya, I. N., E. J. Brown, M. H. Ginsberg, and E. R. Mackow. 1999. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by β3 integrins. J. Virol. 733951-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavrilovskaya, I. N., M. Shepley, R. Shaw, M. H. Ginsberg, and E. R. Mackow. 1998. β3 integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. USA 957074-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harder, T., P. Scheiffele, P. Verkade, and K. Simons. 1998. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141929-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichida, S., Y. Yuzawa, H. Okada, K. Yoshioka, and S. Matsuo. 1994. Localization of the complement regulatory proteins in the normal human kidney. Kidney Int. 4689-96. [DOI] [PubMed] [Google Scholar]
- 16.Johansson, C., M. Jonsson, M. Marttila, D. Persson, X. L. Fan, J. Skog, L. Frangsmyr, G. Wadell, and N. Arnberg. 2007. Adenoviruses use lactoferrin as a bridge for CAR-independent binding to and infection of epithelial cells. J. Virol. 81954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanerva, M., J. Mustonen, and A. Vaheri. 1998. Pathogenesis of puumala and other hantavirus infections. Rev. Med. Virol. 867-86. [DOI] [PubMed] [Google Scholar]
- 18.Karnauchow, T. M., D. L. Tolson, B. A. Harrison, E. Altman, D. M. Lublin, and K. Dimock. 1996. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J. Virol. 705143-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller, T. T., A. T. Mairuhu, M. D. de Kruif, S. K. Klein, V. E. Gerdes, H. ten Cate, D. P. Brandjes, M. Levi, and E. C. van Gorp. 2003. Infections and endothelial cells. Cardiovasc. Res. 60:40-48. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence, D. W., W. J. Bruyninckx, N. A. Louis, D. M. Lublin, G. L. Stahl, C. A. Parkos, and S. P. Colgan. 2003. Antiadhesive role of apical decay-accelerating factor (CD55) in human neutrophil transmigration across mucosal epithelia. J. Exp. Med. 198999-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis, N. A., K. E. Hamilton, T. Kong, and S. P. Colgan. 2005. HIF-dependent induction of apical CD55 coordinates epithelial clearance of neutrophils. FASEB J. 19950-959. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Palomo, A., I. Meza, G. Beaty, and M. Cereijido. 1980. Experimental modulation of occluding junctions in a cultured transporting epithelium. J. Cell Biol. 87736-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medof, M. E., E. I. Walter, J. L. Rutgers, D. M. Knowles, and V. Nussenzweig. 1987. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J. Exp. Med. 165848-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitic, L. L., and J. M. Anderson. 1998. Molecular architecture of tight junctions. Annu. Rev. Physiol. 60121-142. [DOI] [PubMed] [Google Scholar]
- 25.Muranyi, W., U. Bahr, M. Zeier, and F. J. van der Woude. 2005. Hantavirus infection. J. Am. Soc. Nephrol. 163669-3679. [DOI] [PubMed] [Google Scholar]
- 26.Muranyi, W., R. Kehm, U. Bahr, S. Muller, M. Handermann, G. Darai, and M. Zeier. 2004. Bovine aortic endothelial cells are susceptible to hantavirus infection; a new aspect in hantavirus ecology. Virology 318112-122. [DOI] [PubMed] [Google Scholar]
- 27.Nava, P., S. Lopez, C. F. Arias, S. Islas, and L. Gonzalez-Mariscal. 2004. The rotavirus surface protein VP8 modulates the gate and fence function of tight junctions in epithelial cells. J. Cell Sci. 1175509-5519. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson-Weller, A., J. P. March, C. E. Rosen, D. B. Spicer, and K. F. Austen. 1985. Surface membrane expression by human blood leukocytes and platelets of decay-accelerating factor, a regulatory protein of the complement system. Blood 651237-1244. [PubMed] [Google Scholar]
- 29.Obert, G., I. Peiffer, and A. L. Servin. 2000. Rotavirus-induced structural and functional alterations in tight junctions of polarized intestinal Caco-2 cell monolayers. J. Virol. 744645-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plyusnin, A., O. Vapalahti, and A. Vaheri. 1996. Hantaviruses: genome structure, expression and evolution. J. Gen. Virol. 772677-2687. [DOI] [PubMed] [Google Scholar]
- 31.Quigg, R. J., A. Nicholson-Weller, A. V. Cybulsky, J. Badalamenti, and D. J. Salant. 1989. Decay accelerating factor regulates complement activation on glomerular epithelial cells. J. Immunol. 142877-882. [PubMed] [Google Scholar]
- 32.Ravkov, E. V., S. T. Nichol, and R. W. Compans. 1997. Polarized entry and release in epithelial cells of Black Creek Canal virus, a New World hantavirus. J. Virol. 711147-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowe, R. K., and A. Pekosz. 2006. Bidirectional virus secretion and nonciliated cell tropism following Andes virus infection of primary airway epithelial cell cultures. J. Virol. 801087-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheiffele, P., M. G. Roth, and K. Simons. 1997. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 165501-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmaljohn, C., and B. Hjelle. 1997. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 395-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmaljohn, C. S., and J. M. Dalrymple. 1983. Analysis of Hantaan virus RNA: evidence for a new genus of bunyaviridae. Virology 131482-491. [DOI] [PubMed] [Google Scholar]
- 37.Schoenenberger, C. A., A. Zuk, G. M. Zinkl, D. Kendall, and K. S. Matlin. 1994. Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. J. Cell Sci. 107 (Pt 2):527-541. [DOI] [PubMed] [Google Scholar]
- 38.Shafren, D. R., R. C. Bates, M. V. Agrez, R. L. Herd, G. F. Burns, and R. D. Barry. 1995. Coxsackieviruses B1, B3, and B5 use decay accelerating factor as a receptor for cell attachment. J. Virol. 693873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafren, D. R., D. J. Dorahy, R. A. Ingham, G. F. Burns, and R. D. Barry. 1997. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J. Virol. 714736-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata, T., F. G. Cosio, and D. J. Birmingham. 1991. Complement activation induces the expression of decay-accelerating factor on human mesangial cells. J. Immunol. 1473901-3908. [PubMed] [Google Scholar]
- 41.Shin, K., V. C. Fogg, and B. Margolis. 2006. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 22207-235. [DOI] [PubMed] [Google Scholar]
- 42.Tugizov, S. M., J. W. Berline, and J. M. Palefsky. 2003. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat. Med. 9307-314. [DOI] [PubMed] [Google Scholar]
- 43.Varsano, S., I. Frolkis, and D. Ophir. 1995. Expression and distribution of cell-membrane complement regulatory glycoproteins along the human respiratory tract. Am. J. Respir. Crit. Care Med. 1521087-1093. [DOI] [PubMed] [Google Scholar]
- 44.Wahl-Jensen, V. M., T. A. Afanasieva, J. Seebach, U. Stroher, H. Feldmann, and H. J. Schnittler. 2005. Effects of Ebola virus glycoproteins on endothelial cell activation and barrier function. J. Virol. 7910442-10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward, T., P. A. Pipkin, N. A. Clarkson, D. M. Stone, P. D. Minor, and J. W. Almond. 1994. Decay-accelerating factor CD55 is identified as the receptor for echovirus 7 using CELICS, a rapid immuno-focal cloning method. EMBO J. 135070-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaki, S. R., P. W. Greer, L. M. Coffield, C. S. Goldsmith, K. B. Nolte, K. Foucar, R. M. Feddersen, R. E. Zumwalt, G. L. Miller, A. S. Khan, et al. 1995. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am. J. Pathol. 146552-579. [PMC free article] [PubMed] [Google Scholar]