Abstract
Pseudovirions of human papillomavirus type 16 (HPV16), the principal etiologic agent in 50% of cervical cancers, were used as a model system to investigate the cell surface interactions involved in the exposure of the broadly cross-neutralizing papillomavirus L2 epitopes. These neutralizing epitopes were exposed only after cell surface binding and a subsequent change in capsid conformation that permitted cleavage by the cellular protease furin at a specific highly conserved site in L2 that is immediately upstream of the cross-neutralizing epitopes. Unexpectedly, binding of L2 antibodies led to the release of the capsid/antibody complexes from the cell surface and their accumulation on the extracellular matrix. Study of the dynamics of exposure of the L2 epitopes further revealed that representatives of the apparently dominant class of L1-specific neutralizing antibodies induced by virus-like particle vaccination prevent infection, not by preventing cell surface binding but rather by preventing the conformation change involved in exposure of the L2 neutralizing epitope. These findings suggest a dynamic model of virion-cell surface interactions that has implications for both evolution of viral serotypes and the efficacy of current and future HPV vaccines.
Neutralizing antibodies directed against viral structural proteins play a critical role in the control of most virus infections, particularly in limiting susceptibility to reinfection, and represent the major effector mechanism of most preventive viral vaccines. Indeed, it has been suggested that evolution into multiple serotypes is a hallmark of virus groups that are controlled by neutralizing antibodies (1).
However, the evolution of new serotypes that escape from neutralizing antibodies could in some cases be limited by the need to conserve critical domains (e.g., those needed for receptor binding) that may also contain neutralization epitopes. Some groups of viruses, such as the polyomaviruses, appear to have overcome this challenge by evolving to use alternative primary receptor/internalization pathways for different genus members (11). Other viruses, such as human immunodeficiency virus, expose critical functional domains only very transiently after cell surface attachment, thereby limiting the opportunity for inducing neutralizing antibodies directed against them (15). Such conserved epitopes can be incorporated into a vaccine, where they might induce antibodies that can neutralize in vitro under some conditions. However, the utility of these epitopes as a vaccine target could be limited because they are exposed only for a short period after virion binding or are inaccessible to antibodies at the virus-cell interface (18).
Papillomaviruses are naked icosahedral viruses that have evolved into a large number of genotypes (types). There are over 100 known types of human papillomaviruses (HPVs), a subset of which infect the mucosa of the genital tract and are the central cause of cervical cancer, with HPV type 16 (HPV16) accounting for more than 50% of the tumors. The early events of papillomavirus infection have been studied in vitro. An interesting feature is that papillomaviruses bind to the extracellular matrix (ECM) in addition to the cell surface, where heparan sulfate proteoglycans (HSPG) appear to serve as a primary attachment factor (7, 14, 19). However, the steps leading to virion internalization remain incompletely understood.
As expected for a virus group that has evolved into many types, virions and virus-like particles (VLPs) composed of L1, the major capsid protein, induce predominately type-specific neutralizing antibodies directed against divergent surface loops, with genotypes behaving for the most part as distinct serotypes (2, 24). Consistent with in vitro neutralization results, the recently licensed L1 VLP-based vaccines are highly successful at preventing type-specific infection and premalignant cervical disease in clinical efficacy trials (reviewed in reference 22). However, only limited cross-protection, against the most closely related types, has been observed (17). Our recent analysis of HPV16 VLP-induced neutralizing monoclonal antibodies (MAb) described two distinct classes: those that prevent cell surface binding but not ECM binding and those that permit cell surface binding but prevent ECM binding and capsid internalization. However, the mechanism by which the second class of neutralizing antibodies prevents capsid internalization is not understood.
In contrast to the type-specific nature of the L1 neutralization epitopes, immunogens composed of full-length versions of the minor capsid protein L2, or highly conserved N-terminal peptides of L2, induce remarkably broad cross-type neutralizing antibodies (23). In some instances, papillomavirus pseudovirions representing a diverse phylogenetic spectrum, encompassing animal and human cutaneous and genital/mucosal types, have been neutralized by the antibodies raised against a single L2 polypeptide (23). Thus, the question arises of how papillomaviruses were able to evolve into numerous distinct types when they carry a broadly cross-reactive neutralization epitope.
Using in vitro-generated pseudovirus of HPV16 as a model system, we have now investigated the exposure of the broadly cross-reactive neutralization epitopes of papillomavirus L2 in relation to cell surface interactions. Characterization of the dynamics of exposure of these epitopes has provided insight into the early events of papillomavirus infection and has also helped to elucidate how representatives of the dominant class of L1-specific neutralizing antibodies are able to prevent viral infection despite their inability to prevent cell surface binding. The results of the study have implications for the regulation of virion uptake, the evolution of HPV serotypes, and the efficacy of current and future HPV vaccines.
MATERIALS AND METHODS
Cell lines and antibodies.
HaCaT and 293TT cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. The polyclonal antiserum raised in rabbits against HPV16 capsids was previously described (28), as have the polyclonal rabbit antiserum 4724 against full-length HPV16 L2-glutathione S-transferase (23), the monoclonal antibody RG-1, and the polyclonal rabbit antiserum 17/36 against the HPV16 17-36 peptide (13). The H16.V5 and H16.E70 monoclonal antibodies were obtained from Neil Christensen (Department of Pathology, College of Medicine, Pennsylvania State University, Hershey) and have been previously described (6). The rabbit polyclonal antiserum against laminin 5 was purchased from Abcam.
Pseudovirus production.
Stocks of matured pseudovirus were produced as previously described (4). Pseudovirus was produced with the p16Llw plasmid, which encodes codon-optimized versions of HPV16 L1 and L2. These pseudovirions were utilized for all experiments except for the detection of L2 NHA exposure. For those experiments, pseudovirus produced from p16NHA and puL1B plasmids, which have wild-type infectivity, was utilized. All plasmids and production methods are fully described on the laboratory's website (http://ccr.cancer.gov/staff/staff.asp?profileid=5637).
Immunofluorescent staining.
Cells were seeded onto glass number 01 coverslips in a 24-well plate at a density of 1 × 105/well and cultured overnight. For studies to evaluate the capsid binding pattern, 50 ng of pseudovirus was incubated with a given antibody or antiserum at a neutralizing dose, or without treatment, and then added to the cells as indicated below. Cells were fixed in ice-cold ethanol containing 15 mM glycine. For detection of antibody-bound particles, the cells were stained with Alexa Fluor 488-conjugated donkey anti-mouse immunoglobulin G (IgG; Molecular Probes). When indicated, rhodamine-conjugated phalloidin (Molecular Probes) was included in the secondary antibody stain at a dilution of 1/1,000. For determination of L2 N-terminal hemagglutinin (HA) exposure, following incubation with virus, cells were fixed with 2% paraformaldehyde. The HA tag was detected with mouse anti-HA (clone C-5; Santa Cruz Biotechnology). For determination of RG-1 or 17-36 epitope exposure on the cell surface, cells were incubated with virus for the indicated times. Cells were washed and incubated with antibody diluted in phosphate-buffered saline supplemented with 2% fetal bovine serum and 0.01% NaN3 in the cold for 1 h. Cells were washed and incubated in donkey anti-mouse Alexa Fluor 488 in phosphate-buffered saline-fetal bovine serum-NaN3 for an additional hour. Cells were washed again and fixed in 2% paraformaldehyde for 20 min. When indicated, pseudovirus was preincubated with H16.V5 or H16.E70 prior to addition to cells. In all cases, coverslips were inverted onto 4′,6′-diamidino-2-phenylindole-containing mounting solution (Prolong Gold; Molecular Probes). All images were acquired with a Zeiss LSM 510 confocal system interfaced with a Zeiss Axiovert 100 M microscope. Images were collated with Adobe Photoshop software.
Immunoprecipitation.
To compare the immunoprecipitation profiles of H16.V5 and RG-1, 293TT cells were transfected with plasmids to express both HPV16 L1 and L2 or only HPV16 L1. After 24 h, cells were lysed in immunoprecipitation buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP-40 containing Complete protease inhibitor cocktail [Roche]) for 20 min on ice. Cellular debris was removed by centrifugation. Lysates were precleared by incubation in the cold with rocking, in the presence of protein G-Sepharose (Pierce), for 60 min. Protein G was pelleted, and the remaining lysates were incubated with either H16.V5 ascites fluid or purified RG-1 in the cold with rocking for 60 min. Following this incubation, donkey anti-mouse IgG (Jackson Immunoresearch) was added to the lysate for 60 min. Then, protein G-Sepharose was added for a final 60 min. Immunoprecipitated complexes were collected by centrifugation and washed four times in immunoprecipitation buffer. The remaining complexes were boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer, resolved on a 10% NuPage gel (Invitrogen), and transferred to an Immobilon membrane (Millipore). L1 species were detected using an anti-L1 monoclonal antibody (Camvir1; Abcam) that was conjugated to biotin using the EZ-Link NHS-PEO solid-phase biotinylation kit (Pierce) according to the manufacturer's directions. Streptavidin-linked horseradish peroxidase (Invitrogen) was utilized for detection.
RESULTS
L2 neutralization sequesters virions on the ECM.
The RG-1 MAb, which recognizes a highly conserved HPV16 L2 peptide sequence, amino acids 17 to 36, has been shown to neutralize both HPV16 and HPV18 infection (13). A polyclonal antibody, 17/36, raised against this peptide has a broader cross-neutralizing profile, effectively preventing infection by the additional types HPV5, HPV6, HPV11, HPV31, HPV45, HPV52, HPV58, and bovine papillomavirus type 1 (13). We wished to determine the mechanism of anti-L2 neutralization by these two reagents, as this region includes major cross-neutralization epitopes for PVs (23). We also investigated the neutralizing phenotype of a polyclonal antiserum, 4724, that was raised against a fusion protein composed of the full-length HPV16 L2 protein linked to glutathione S-transferase. This serum has previously been demonstrated to neutralize HPV18 and HPV31, in addition to its homologous neutralization of HPV16 (23).
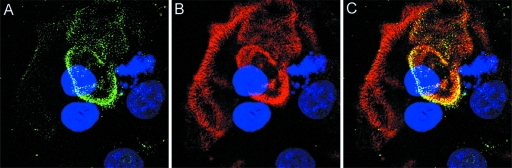
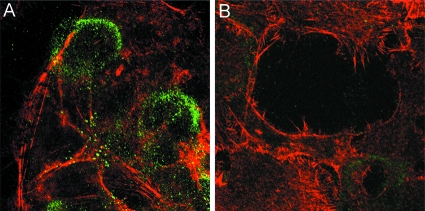
Infectious papillomavirus virions, containing both L1 and L2 capsid proteins, and L1-only VLP utilize indistinguishable cell entry pathways and can compete for cell surface receptor binding (9, 29, 36). However, encapsidated DNA does not escape from the endosomal compartment in the absence of L2 (20). Therefore, we considered it likely that anti-L2 neutralizing antibodies would cause the accumulation of pseudovirions in late endosomes. Surprisingly, overnight incubation of HPV16 pseudovirions with the human keratinocyte line HaCaT in the presence of the anti-L2 neutralizing MAb resulted in the sequestration of pseudovirions on the ECM, demonstrated by colocalization of L2 antibody-bound capsids with laminin 5 (Fig. 1A to C). ECM binding was confirmed to occur similarly with the other neutralizing anti-L2 reagents (data not shown). In the absence of antibody, HPV16 capsids are typically distributed between the ECM and cell surface (7, 10). The fact that the polyclonal serum raised against the full-length protein results in the same pattern of neutralization suggests that sequestration of the virions on the ECM may be related to the major mechanism of anti-L2 neutralization.
FIG. 1.
L2 neutralization sequesters virions on the ECM. HPV16 pseudovirus was added to HaCaT cells in the presence of RG-1 and incubated overnight. Antibody/virus complexes were detected with Alexa 488-conjugated donkey anti-mouse IgG (A). Laminin 5, a marker of the ECM, was detected with a polyclonal antiserum and Alexa 594-conjugated donkey anti-rabbit IgG (B). The merged image is shown in panel C.
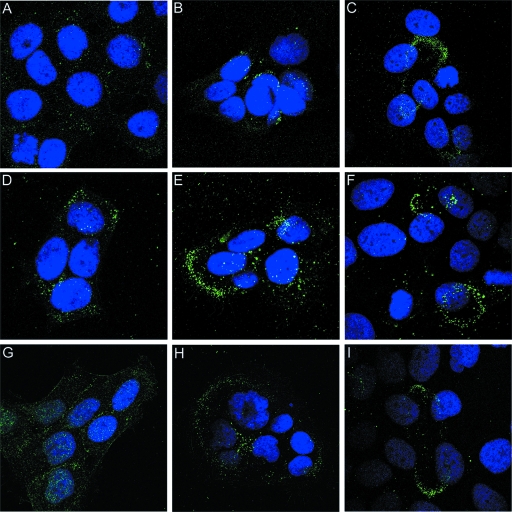
To examine the process of neutralization over time, pseudovirions and RG-1 MAb were incubated with HaCaT cells for 2 hours. Unbound virions were then removed by washing and either immediately fixed or chased in the presence of additional RG-1 MAb for either 4 h or 22 h. After 2 hours, negligible binding of RG-1 was detected (Fig. 2A). By contrast, binding was evident by 6 h, although the signal was quite weak (Fig. 2B) and was associated with the cell surface. By 24 h the signal had increased substantially, and the majority of the virus-antibody complexes was localized to the ECM (Fig. 2C), although some punctate cell surface complexes were evident. A parallel experiment was performed with the 4724 polyclonal antiserum. The general pattern of binding was similar to that of the RG-1 MAb (Fig. 2D to F). However, more antiserum bound to the pseudovirions at the earliest time point (Fig. 2D), indicating that some L2 epitopes are exposed on the virions at this time, and more ECM localization was evident at the intermediate time point (Fig. 2E). The initial pattern was predominantly cell associated, but the ECM staining pattern became increasingly more prominent with longer incubation times. These results support three conclusions: the cross-neutralization epitope 17-36 is not well exposed initially on cell-bound pseudovirions; this epitope becomes progressively exposed on the cell surface during incubation; and engagement of this epitope with L2 antibody results in the relocalization of virions to the ECM.
FIG. 2.
Antibody-bound virus moves from the cell surface to the ECM. HaCaT cells were incubated with HPV16 pseudovirus and either RG-1 (A to C) or the 4724 serum (D to F) for 3 h. Unbound virus and antibody were removed and either stained immediately (A and D) or chased in the presence of additional antibody (RG-1 [B and C] or 17/36 [E and F]) for an additional 4 h (B and E) or 22 h (C and F). The antibody-bound virus was detected with Alexa Fluor 488-coupled donkey anti-mouse IgG. To follow a cohort of antibody-neutralized virus move from the cell body to the ECM, we incubated HaCaT cells with HPV16 pseudovirus and the 4724 serum for 3 h (G). Unbound virus and antibody were removed, and the initial cohort was chased for either 5 h (H) or 23 h (I). The antibody-bound virus was detected with Alexa Fluor 488-coupled donkey anti-mouse IgG.
Neutralized virus moves from the cell surface to the ECM.
The finding that the 4724 polyclonal serum could bind after a relatively short exposure of pseudovirions to the cells allowed us to directly determine if virus-antibody complexes translocate from the cell surface to the ECM. First, HaCaT cells were incubated for 3 hours with the 4724 serum in conjunction with the pseudovirions. After washing, antibody-virion complexes were then chased in the absence of any additional virus or antibody, allowing us to follow a cohort of antibody-bound virus. The results (Fig. 2G to I) confirmed that the complexes could be initially detected on the cell surface (Fig. 2G). The pattern appeared more coalesced on the surface at the next time point, after a 5-h chase (Fig. 2H). By the last time point, after a 24-h total incubation, essentially all the virion-antibody complexes had relocalized to the ECM (Fig. 2I).
Exposure of the 17-36 epitope on the cell surface.
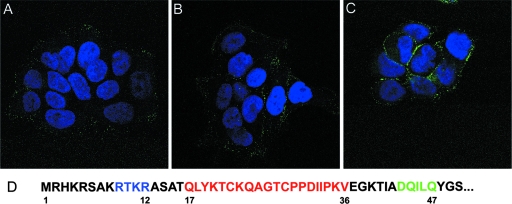
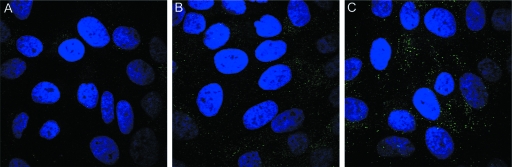
Progressive exposure of the 17-36 epitope on capsids associated with the cell surface was then examined using the RG-1 MAb. Pseudovirions (without antibody) were incubated with HaCaT cells for 1 h, unbound virus was removed, and the cultures were incubated for an additional 2 h or 4 h. At each time point, RG-1 MAb was added to evaluate its ability to bind surface-associated virus. Incubation with the anti-L2 antibody was performed in the cold prior to fixation, to allow binding of the antibody to the surface-bound pseudovirions without the possibility of fixation artifacts. By this assay, the L2 neutralization epitope was well exposed by the 4-h time point (Fig. 3C), with binding of the antibody at earlier time points being significantly weaker (Fig. 3A and B). These results are consistent with the L2 neutralization epitope being buried on pseudovirions in their mature conformation, although the possibility that binding of the primary cell surface receptor occludes the L2 neutralizing epitope was not formally excluded in this experiment. The time-dependent exposure of this epitope was confirmed with the 17/36 polyclonal serum (data not shown).
FIG. 3.
The RG-1 epitope is initially poorly exposed on the cell surface. HaCaT cells were incubated with HPV16 pseudovirions for 1 h at 37°C. Unbound virus was removed by washing and either processed immediately (A) or chased for 2 h (B) or 4 h (C) without antibodies. Cells were subsequently stained with RG-1 at 4°C prior to fixation. (D) The N terminus of HPV16 L2, with locations for the furin cleavage site depicted in blue, the RG-1 epitope in red, and the syntaxin 18 binding site in green.
It is known that other regions of L2 that are inaccessible on mature particles become exposed during the entry and uncoating processes (8, 26). Notably, the extreme amino terminus of L2 becomes accessible and is cleaved by the furin proprotein convertase after cell surface binding. The furin cleavage site, which is immediately upstream of the RG-1 neutralizing epitope (Fig. 3D), is not exposed on mature virions but is accessible in immature virions.
A recent publication found that RG-1 reacted with mature HPV16 pseudovirions in an enzyme-linked immunosorbent assay (13). As a positive result with this assay is usually considered to be indicative of a surface-exposed epitope, we wondered whether this apparent discrepancy with our cell surface binding results indicated that the interaction of the particle with the plastic in the plate-based assay had caused a conformational change that allows exposure of the epitope, thereby falsely giving the impression that the epitope is generally exposed on mature capsids. It has been previously demonstrated that passive adsorption of proteins to polystyrene can result in the alteration of antigenic epitopes because of partial denaturation (5, 32).
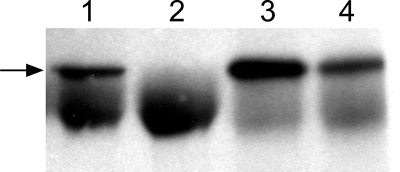
To address this possibility, the ability of RG-1 to interact with intact pseudovirions in solution was examined (Fig. 4). The RG-1 antibody was unable to immunoprecipitate purified mature pseudovirions (lane 2), unlike the neutralizing anti-L1 MAb H16.V5 (lane 1). To rule out the possibility that the L2 MAb had too low an avidity to immunoprecipitate L1/L2 complexes, we also examined the ability of these two MAb to immunoprecipitate pseudovirion assembly intermediates and/or immature capsids that are present in crude cell lysates. Lysates of 293TT cells that were transfected with both L1- and L2-encoding plasmids were prepared. Both H16.V5 and RG-1 could effectively precipitate complexes from these lysates (Fig. 4, lanes 3 and 4). When we performed immunoprecipitations from cell lysates that contained only the L1 protein, H16.V5 was able to precipitate L1, as expected, and RG-1 did not (data not shown). The immunoprecipitation was performed with or without a donkey anti-mouse “bridging antibody,” to evaluate if the RG-1 antibody was able to bind the mature particle, but in a manner that was inaccessible to the bulky protein G-Sepharose, and the results were identical (data not shown). These findings demonstrate that RG-1 is able to recognize L2 in the context of an incompletely assembled or immature particle, but not in the context of intact mature particles in solution. The results also indicate that the initial lack of RG-1 binding to cell-associated pseudovirions is unlikely to be attributable to occlusion of the epitope by receptor engagement.
FIG. 4.
Immunoprecipitation of L1/L2 complexes. The anti-L1 MAb H16.V5 (lanes 1 and 3) or anti-L2 MAb RG-1 (lanes 2 and 4) was used to immunoprecipitate either L1- and L2-containing mature pseudovirions (lanes 1 and 2) or crude lysates that contained a mixture of L1 and L2 assembly intermediates and immature pseudovirions (lanes 3 and 4). The immunoblot was reacted with a biotinylated anti-L1 monoclonal antibody. The arrow indicates the migration of L1. The IgG heavy chain is the lower heavy band.
Furin cleavage of L2 is necessary for exposure of the 17-36 epitope.
Furin cleavage of the N terminus of L2 is required for infection of the host cell (26). Because of the proximity of the furin cleavage site to the cross-neutralization epitopes (Fig. 3D), we speculated that L2 cleavage by furin and the ability of the L2 neutralizing antibody to bind might be mechanistically linked. To address this possibility, RG-1 binding to cell surface-bound HPV16 pseudovirions was evaluated in the presence of a furin inhibitor, decanoyl-RVKR-cmk. RG-1 binding was effectively prevented by treatment with the furin inhibitor (Fig. 5, compare A and B). Thus, we infer that after cell surface binding, mature capsids must undergo a conformational change that exposes the furin cleavage site; the subsequent step of furin cleavage of L2 leads to exposure of the L2 17-36 polypeptide, which can then be bound by neutralizing antibodies. The furin-dependent exposure of this epitope was confirmed with the 17/36 polyclonal antiserum (data not shown).
FIG. 5.
Exposure of the 17-36 epitope requires furin cleavage. HaCaT cells were exposed to pseudovirus for 3 h at 37°C either with no additives (A) or in the presence of a furin inhibitor (B). Unbound virus was removed, and cells were incubated overnight with RG-1 and the furin inhibitor when indicated. Cells were ethanol fixed, and antibody-bound virus was visualized with Alexa Fluor 488-conjugated donkey anti-mouse IgG.
Exposure of the amino terminus of L2 on the cell surface.
Furin is known to be present and active on the cell surface. For instance, cleavage of the furin substrates anthrax PA toxin, proaerolysin toxin, and Clostridium alpha-toxin occurs prior to endocytosis (16, 21). It has not been determined at which point in the infectious process that furin cleavage of L2 normally occurs. To examine whether furin cleavage of the HPV16 L2 can occur on the cell surface in our cell culture system, exposure of the amino terminus of L2 was monitored by specific antibody detection of an HA tag inserted at the extreme N terminus of the L2 protein. This tag does not interfere with the uptake or infectivity of the pseudovirus (26). Detection of the HA tag on the cell surface (i.e., in nonpermeabilized cells) increased in a time-dependent manner (data not shown). However, efficient detection of the amino-terminal tag required inhibition of furin (Fig. 6, compare B and C). The retention of the HA tag in the presence of the furin inhibitor, which, as shown above, prevents access of the RG-1 epitope at the cell surface as well as furin cleavage, supports the conclusion that furin cleavage of the L2 amino terminus can occur on the cell surface and provides further confirmation of a conformational change in the virion occurring prior to furin cleavage.
FIG. 6.
Exposure of the N terminus of L2 occurs on the cell surface. For these experiments, pseudovirus containing the amino-terminal HA-tagged L2 was incubated with HaCaT cells for 12 h. The cells were paraformaldehyde fixed and stained with an anti-HA MAb and Alexa Fluor 488-conjugated donkey anti-mouse IgG. (A) Background staining with cells only. (B) Staining of untreated cells that were incubated with the virus. (C) Staining of cells incubated with the virus in the presence of a furin inhibitor.
H16.V5 and H16.E70 prevent exposure of the 17-36 epitope.
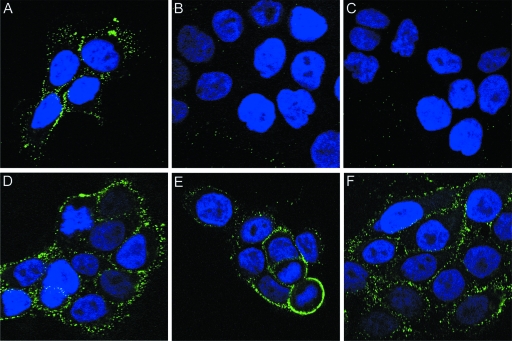
H16.V5 and H16.E70 are two well-characterized HPV16-specific neutralizing MAb, induced by HPV16 L1 VLPs, that recognize conformation-dependent L1 epitopes (6). We recently reported that these MAbs, which bind mature virions in contrast to L2-neutralizing antibodies, prevent endocytosis of virions without affecting the ability of the virions to bind to the cell surface (10). As the mechanism of the block to virion internalization is unknown, we examined if neutralization with these antibodies prevented the exposure of the L2 neutralization epitope 17-36. In the first set of experiments, the virus was preincubated with an anti-L1 antibody, and the virus-MAb complex was then added to cells. Exposure of the 17-36 epitope on the cell surface was evaluated using the 17/36 polyclonal antiserum in the same manner as shown for RG-1 in Fig. 3. We observed a dramatic inhibition of anti-L2 binding to the cell-bound capsids after prior interaction of the capsids with the anti-L1 antibodies (Fig. 7, compare untreated virus in A with V5-neutralized virus in B and E70-neutralized virus in C), with the signal being reduced to approximately background levels. However, due to the relatively high background staining seen with the 17/36 serum, it is impossible to conclude that all the specific 17-36 binding is prevented by the anti-L1 MAb neutralization.
FIG. 7.
Neutralization with anti-L1 MAb can prevent exposure of the neutralizing L2 epitope. To examine the mechanism of neutralization of H16.V5 and H16.E70, pseudovirus was incubated with these antibodies prior to addition to HaCaT cells for 3 h. The exposure of the 17-36 epitope was monitored by binding of the 17/36 polyclonal antiserum as described for RG-1 in the legend for Fig. 3. (A) 17-36 staining of cells that were incubated with nonneutralized virus. (B and C) Staining of cells neutralized with H16.V5 (B) or H16.E70 (C). (D to F) To ensure that the 17-36 was not simply sterically obstructed by the MAb binding, cells were incubated with pseudovirions for 3 h. Unbound virus was removed, and cell-associated virus was incubated with no antibody (D), H16.V5 (E), or H16.E70 (F). Following this incubation, cells were processed for 17-36 binding as in the previous panels.
To evaluate whether the lack of 17-36 detection simply resulted from steric hindrance of L2 antibody binding by the L1 MAb, we performed a second set of experiments. In this case, the virus was first bound to cells for 4 h (to allow the initial conformational changes in the virion to occur), and cell surface capsids were then exposed to saturating amounts of the anti-L1 MAb. Both H16.V5 and H16.E70 bind strongly to cell-bound capsids and neutralize under these conditions (data not shown). Subsequent to the anti-L1 MAb binding, the 17/36 antiserum was added. The strong anti-L2 antibody staining using the second protocol (Fig. 7D to F) made it clear that the diminution of signal seen in Fig. 7B and C did not result from steric effects. Therefore, we conclude that both H16.V5 and H16.E70 prevent the cell surface conformational change in the capsids needed for anti-L2 antibody binding and/or furin cleavage.
DISCUSSION
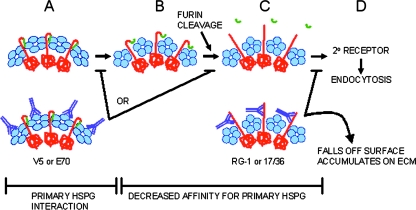
The findings of this study, coupled with previously published results, support a dynamic model of the initial events during papillomavirus infection (illustrated in Fig. 8). Binding of the primary cell surface receptor (presumably heparan sulfate proteoglycans) results in a conformational change in the capsid that exposes the furin cleavage site at the amino terminus of L2. Several recent reports have also provided less direct evidence that conformational changes in the papillomavirus capsid occur on the cell surface (34, 38). ECM binding is also able to induce this change (unpublished data), possibly through the newly described HSPG interaction at this site (33). However, infection can occur in the absence of ECM binding, suggesting that ECM binding is not required for these changes to occur (unpublished data). The ECM might function as the in vitro equivalent of the epithelial basement membrane, which we have recently identified as the primary site of virus binding during genital tract infection in vivo (27).
FIG. 8.
Model of conformational changes in the HPV virion on the cell surface and mechanisms of antibody neutralization. (A) Conformation of the mature particle (L1 is indicated in blue and L2 is in red) in which the N terminus of L2 is inaccessible to L2-neutralizing antibody and furin. (B) A conformational change reduces the affinity for HSPG and increases accessibility of the L2 N terminus to furin. The N-terminal 12 amino acids of L2, indicated in green, are released following furin cleavage. This cleavage of L2 exposes the portion of L2 that encompasses the neutralizing epitope defined by amino acids 17 to 36 (C). The virus associates with a second receptor and is internalized (D). As shown in the lower panels, neutralizing antibodies affect virus entry at different points in this schema. Engagement with either of the anti-L1 MAb, H16.V5 or H16.E70, prevents the conformational change required for reducing the affinity for HSPG and exposure of the neutralizing L2 epitope. Virus neutralized with either of these MAbs remains associated with the cell surface and is not endocytosed. These antibodies could act either prior to furin cleavage or by preventing a conformational change subsequent to this event. Neutralization with the anti-L2 reagents RG-1 or 17/36 sterically inhibits the binding of the viral particle to the putative secondary receptor. However, the decreased affinity for the primary receptor results in virus relocation to the ECM, an alternate binding site.
Furin cleavage of L2 on the cell surface was shown to expose the broadly cross-neutralizing epitopes on L2 that are likely to be critical for binding an L2-specific receptor. Interestingly, the binding site of one putative intracellular protein receptor, syntaxin 18, is immediately downstream of the 17-36 region (3). The initial conformational change also apparently exposes the binding site of a secondary cell surface receptor, or it lowers the affinity for the primary receptor which effects the hand-off. This idea was supported by a recent publication suggesting that a conformational change in the PV capsid precedes the binding to a non-HSPG receptor (33). We favor the hypothesis that this unidentified receptor is L1 specific, because we have found that trafficking of L1 VLPs is indistinguishable from that of infectious virions through this stage (9). Additionally, L1/L2 capsids are internalized normally under conditions of furin inhibition (26). The L2 in these particles is not cleaved by furin, which implies that the RG-1 epitope is not exposed. If exposure of this L2 epitope were critical for a cell surface receptor interaction that allowed endocytosis, we would expect to see these particles withheld on the cell surface.
Surprisingly, binding of neutralizing antibodies to the exposed N terminus of L2 led to the release of the capsids from the cell surface. This finding implies that the L2 antibody sterically hinders engagement of the secondary receptor. It also implies that the initial conformational change leads to release from the primary receptor; otherwise, the capsid/L2 antibody complex would probably remain attached to the cell surface, as seen for H16.V5- and H16.E70-bound capsids. L2 antibody binding clearly did not block the binding to the ECM, and hence the released HPV16 capsids accumulated at this site. Interestingly, we previously demonstrated that the HPV16 L1 neutralizing MAb H16.U4 also induces an ECM-binding-only neutralization pattern (10). However, in the case of H16.U4 the capsid/antibody complexes are never detected on the cell surface, unlike what we have reported in this study.
It is interesting that although furin cleavage exposes the putative conserved L2 receptor binding site on the cell surface, our model suggests that this site is not functionally required for papillomavirus infection until later, perhaps not until the stage of L2-mediated endosome escape. This is suggested by the finding that in the absence of furin cleavage, pseudovirions are withheld in the late endosome (26).
A consensus furin cleavage site at the N terminus of L2 is remarkably conserved in all papillomaviruses that we have examined. It is unlikely that this specific proteolytic process evolved to ensure exposure of the putative L2 receptor binding site at a specific point during the entry process, since immature capsids can be treated with furin in solution and subsequently infect cells in a furin-independent manner (26). Therefore, we hypothesize that this elaborate process evolved early in the speciation of papillomaviruses to prevent B-cell exposure of conserved virion epitopes critical for infection.
Exposure of neutralizing epitopes after cell surface binding has also been documented for other viruses, including parvoviruses and flaviviruses, although the mechanisms differ. Critical neutralizing regions of the VP1u protein are not exposed in human parvovirus capsids in solution. However, they become accessible following capsid rearrangement induced by cell receptor attachment (31). For flaviviruses, a cluster of antigenic determinants within a conserved domain of the E protein can elicit cross-neutralizing antibodies. These sequences are inaccessible at the surface of intact virions but become significantly more exposed after disintegration of the viral envelope (35).
The requirement for L2 cleavage on the cell surface for the exposure of L2 cross-neutralization epitopes provides a mechanistic explanation for the previous finding that vaccination with L1/L2 VLPs induced no greater cross-neutralizing responses than did vaccination with L1-only VLPs (30). Furthermore, the exposure of L2 neutralization epitopes during natural infection is not accompanied by a strong immune response. When removed from the context of the viral particle, however, immunization with L2 N-terminal peptides can induce broadly cross-neutralizing antibodies (23).
In principle, monovalent L2 vaccines might overcome some of the inherent production cost and type-specific limitations of HPV VLP vaccines, two factors that are expected to limit the impact of these vaccines in developing countries, where 80% of cervical cancers occur. However, some skepticism has been raised concerning the potential efficacy of vaccines that are based on the induction of antibodies against intermediate virion structures that only form during certain stages of infection. It is our opinion, however, that the results of the current study encourage the further development of L2-based vaccines, by providing evidence that the intermediate structure targeted by these neutralizing antibodies remains on the cell surface for an extended time, which could afford ample opportunity for engagement by neutralizing antibodies. This situation is in marked contrast to human immunodeficiency virus vaccines that are designed to target fusion intermediates, which are very transiently exposed structures (reviewed in reference 25). In support of our hypothesis, vaccination of rabbits with L2 peptides from heterologous papillomaviruses has been shown to induce protection against experimental viral challenge in two rabbit papillomavirus models (12).
The current study also provides insight into a mechanism by which L1 neutralizing antibodies may interfere with infection. We found that the L1 MAb H16.V5 and H16.E70, which bind to mature virus but do not interfere with its ability to bind the cell surface, prevented the initial conformational change(s) after cell surface binding, as indicated by the failure to expose the L2 17-36 polypeptide. However, in marked contrast to immune complexes with L2-neutralizing antibodies, these L1 antibody complexes remain cell surface associated. The simplest mechanistic explanation for this finding is that the documented prevention of the conformational change that exposes the L2 epitope also prevents release of the capsid from the primary L1 receptor. The finding that these antibodies prevent a conformational change in the capsid structure could explain why they neutralize so efficiently. H16.V5 has a very low 50% inhibitory concentration (IC50) of 1.9 pM, and H16.E70 has an IC50 of 39 pM (10). In contrast, H16.U4, which neutralizes by preventing cell surface binding, has an IC50 of 5.4 nM. A lower antibody occupancy may be needed to block a concerted shift in capsid conformation than that needed to block cell surface binding. H16.V5 and, to a lesser extent, H16.E70, have been shown to competitively inhibit the binding of most L1-specific antibodies induced by natural infection and by VLP vaccination (37). Therefore, they likely represent the dominant class of L1-specific neutralizing antibodies for HPV16. Consistent with this conjecture, polyclonal sera from HPV16 L1 VLP-vaccinated women also induce retention of the capsids on the cell surface (unpublished results). The remarkably high efficacy of the current VLP vaccines might be attributable, in part, to their ability to induce neutralizing antibodies that are active even at low virion occupancy.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. R.B.S.R. received grants from the PHS, grant P50 CA098252 from the National Cancer Institute, SPORE in Cervical Cancer, and grant CA118790. R.B.S.R. is a paid consultant of Knobbe, Martens, Olson and Bear LLC. Under a licensing agreement among PaxVax, Inc., the National Cancer Institute, and Johns Hopkins University, R.G. and R.B.S.R. are entitled to a share of the royalties received from sales of products described in this article. Under a licensing agreement among Acambis, the National Cancer Institute, and Johns Hopkins University, R.G. and R.B.S.R. are entitled to a share of the royalties received from sales of products described in this article. The terms of these arrangement are being managed by Johns Hopkins University in accordance with its conflict of interest policies.
We thank Neil Christensen (Department of Pathology, College of Medicine, Pennsylvania State University, Hershey) for the antibodies H16.V5 and H16.E70.
Footnotes
Published ahead of print on 27 February 2008.
REFERENCES
- 1.Bachmann, M. F., and R. M. Zinkernagel. 1996. The influence of virus structure on antibody responses and virus serotype formation. Immunol. Today 17553-558. [DOI] [PubMed] [Google Scholar]
- 2.Bishop, B., J. Dasgupta, M. Klein, R. L. Garcea, N. D. Christensen, R. Zhao, and X. S. Chen. 2007. Crystal structures of four types of human papillomavirus L1 capsid proteins: understanding the specificity of neutralizing monoclonal antibodies. J. Biol. Chem. 28231803-31811. [DOI] [PubMed] [Google Scholar]
- 3.Bossis, I., R. B. Roden, R. Gambhira, R. Yang, M. Tagaya, P. M. Howley, and P. I. Meneses. 2005. Interaction of tSNARE syntaxin 18 with the papillomavirus minor capsid protein mediates infection. J. Virol. 796723-6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buck, C. B., C. D. Thompson, Y.-Y. S. Pang, D. R. Lowy, and J. T. Schiller. 2005. Maturation of papillomavirus capsids. J. Virol. 792839-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler, J. E. 2000. Solid supports in enzyme-linked immunosorbent assay and other solid-phase immunoassays. Methods 224-23. [DOI] [PubMed] [Google Scholar]
- 6.Christensen, N. D., J. Dillner, C. Eklund, J. J. Carter, G. C. Wipf, C. A. Reed, N. M. Cladel, and D. A. Galloway. 1996. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 223174-184. [DOI] [PubMed] [Google Scholar]
- 7.Culp, T. D., L. R. Budgeon, and N. D. Christensen. 2006. Human papillomaviruses bind a basal extracellular matrix component secreted by keratinocytes which is distinct from a membrane-associated receptor. Virology 347147-159. [DOI] [PubMed] [Google Scholar]
- 8.Day, P. M., C. C. Baker, D. R. Lowy, and J. T. Schiller. 2004. Establishment of papillomavirus infection is enhanced by promyelocytic leukemia protein (PML) expression. Proc. Natl. Acad. Sci. USA 10114252-14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day, P. M., D. R. Lowy, and J. T. Schiller. 2003. Papillomaviruses infect cells via a clathrin-dependent pathway. Virology 3071-11. [DOI] [PubMed] [Google Scholar]
- 10.Day, P. M., C. D. Thompson, C. B. Buck, Y. Y. Pang, D. R. Lowy, and J. T. Schiller. 2007. Neutralization of human papillomavirus with monoclonal antibodies reveals different mechanisms of inhibition. J. Virol. 818784-8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dugan, A. S., S. Eash, and W. J. Atwood. 2006. Update on BK virus entry and intracellular trafficking. Transpl. Infect. Dis. 862-67. [DOI] [PubMed] [Google Scholar]
- 12.Gambhira, R., S. Jagu, B. Karanam, P. E. Gravitt, T. D. Culp, N. D. Christensen, and R. B. Roden. 2007. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J. Virol. 8111585-11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambhira, R., B. Karanam, S. Jagu, J. N. Roberts, C. B. Buck, I. Bossis, H. Alphs, T. Culp, N. D. Christensen, and R. B. Roden. 2007. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J. Virol. 8113927-13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giroglou, T., L. Florin, F. Schafer, R. E. Streeck, and M. Sapp. 2001. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 751565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golding, H., M. Zaitseva, E. de Rosny, L. R. King, J. Manischewitz, I. Sidorov, M. K. Gorny, S. Zolla-Pazner, D. S. Dimitrov, and C. D. Weiss. 2002. Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates. J. Virol. 766780-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon, V. M., R. Benz, K. Fujii, S. H. Leppla, and R. K. Tweten. 1997. Clostridium septicum alpha-toxin is proteolytically activated by furin. Infect. Immun. 654130-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harper, D. M., E. L. Franco, C. M. Wheeler, A. B. Moscicki, B. Romanowski, C. M. Roteli-Martins, D. Jenkins, A. Schuind, S. A. Costa Clemens, and G. Dubin. 2006. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 3671247-1255. [DOI] [PubMed] [Google Scholar]
- 18.Haynes, B. F., and D. C. Montefiori. 2006. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev. Vaccines 5579-595. [DOI] [PubMed] [Google Scholar]
- 19.Joyce, J. G., J. S. Tung, C. T. Przysiecki, J. C. Cook, E. D. Lehman, J. A. Sands, K. U. Jansen, and P. M. Keller. 1999. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 2745810-5822. [DOI] [PubMed] [Google Scholar]
- 20.Kamper, N., P. M. Day, T. Nowak, H. C. Selinka, L. Florin, J. Bolscher, L. Hilbig, J. T. Schiller, and M. Sapp. 2006. A membrane-destabilizing peptide in capsid protein L2 is required for egress of papillomavirus genomes from endosomes. J. Virol. 80759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klimpel, K. R., S. S. Molloy, G. Thomas, and S. H. Leppla. 1992. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc. Natl. Acad. Sci. USA 8910277-10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowy, D. R., and J. T. Schiller. 2006. Prophylactic human papillomavirus vaccines. J. Clin. Investig. 1161167-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastrana, D. V., R. Gambhira, C. B. Buck, Y. Y. Pang, C. D. Thompson, T. D. Culp, N. D. Christensen, D. R. Lowy, J. T. Schiller, and R. B. Roden. 2005. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology 337365-372. [DOI] [PubMed] [Google Scholar]
- 24.Pastrana, D. V., W. C. Vass, D. R. Lowy, and J. T. Schiller. 2001. NHPV16 VLP vaccine induces human antibodies that neutralize divergent variants of HPV16. Virology 279361-369. [DOI] [PubMed] [Google Scholar]
- 25.Phogat, S., R. T. Wyatt, and G. B. Karlsson Hedestam. 2007. Inhibition of HIV-1 entry by antibodies: potential viral and cellular targets. J. Intern. Med. 26226-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards, R. M., D. R. Lowy, J. T. Schiller, and P. M. Day. 2006. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc. Natl. Acad. Sci. USA 1031522-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts, J. N., C. B. Buck, C. D. Thompson, R. Kines, M. Bernardo, P. L. Choyke, D. R. Lowy, and J. T. Schiller. 2007. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 13857-861. [DOI] [PubMed] [Google Scholar]
- 28.Roden, R. B., H. L. Greenstone, R. Kirnbauer, F. P. Booy, J. Jessie, D. R. Lowy, and J. T. Schiller. 1996. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J. Virol. 705875-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roden, R. B., R. Kirnbauer, A. B. Jenson, D. R. Lowy, and J. T. Schiller. 1994. Interaction of papillomaviruses with the cell surface. J. Virol. 687260-7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roden, R. B., W. H. T. Yutzy, R. Fallon, S. Inglis, D. R. Lowy, and J. T. Schiller. 2000. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology 270254-257. [DOI] [PubMed] [Google Scholar]
- 31.Ros, C., M. Gerber, and C. Kempf. 2006. Conformational changes in the VP1-unique region of native human parvovirus B19 lead to exposure of internal sequences that play a role in virus neutralization and infectivity. J. Virol. 8012017-12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwab, C., and H. R. Bosshard. 1992. Caveats for the use of surface-adsorbed protein antigen to test the specificity of antibodies. J. Immunol. Methods 147125-134. [DOI] [PubMed] [Google Scholar]
- 33.Selinka, H. C., L. Florin, H. D. Patel, K. Freitag, M. Schmidtke, V. A. Makarov, and M. Sapp. 2007. Inhibition of transfer to secondary receptors by heparan sulfate-binding drug or antibody induces noninfectious uptake of human papillomavirus. J. Virol. 8110970-10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selinka, H. C., T. Giroglou, T. Nowak, N. D. Christensen, and M. Sapp. 2003. Further evidence that papillomavirus capsids exist in two distinct conformations. J. Virol. 7712961-12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stiasny, K., S. Kiermayr, H. Holzmann, and F. X. Heinz. 2006. Cryptic properties of a cluster of dominant flavivirus cross-reactive antigenic sites. J. Virol. 809557-9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volpers, C., F. Unckell, P. Schirmacher, R. E. Streeck, and M. Sapp. 1995. Binding and internalization of human papillomavirus type 33 virus-like particles by eukaryotic cells. J. Virol. 693258-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, Z., N. Christensen, J. T. Schiller, and J. Dillner. 1997. A monoclonal antibody against intact human papillomavirus type 16 capsids blocks the serological reactivity of most human sera. J. Gen. Virol. 782209-2215. [DOI] [PubMed] [Google Scholar]
- 38.Yang, R., P. M. Day, W. H. T. Yutzy, K. Y. Lin, C. F. Hung, and R. B. Roden. 2003. Cell surface-binding motifs of L2 that facilitate papillomavirus infection. J. Virol. 773531-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]