Abstract
Macrophages are abundant in the lower respiratory tract. They play a central role in the innate response to infection but may also modulate excessive inflammation. Both macrophages and ciliated epithelial cells respond to infection by releasing soluble mediators, leading to the recruitment of innate and adaptive effector cells. To study the role of lung macrophages in acute respiratory viral infection, we depleted them by the inhalation of clodronate liposomes in an established mouse model of respiratory syncytial virus (RSV) disease. Infection caused an immediate local release of inflammatory cytokines and chemokines, peaking on day 1, which was virtually abolished by clodronate liposome treatment. Macrophage depletion inhibited the activation (days 1 to 2) and recruitment (day 4) of natural killer (NK) cells and enhanced peak viral load in the lung (day 4). However, macrophage depletion did not affect the recruitment of activated CD4 or CD8 T cells, weight loss, or virus-induced changes in lung function. Therefore, lung macrophages play a central role in the early responses to viral infection but have remarkably little effect on the adaptive response occurring at the time of peak disease severity.
Macrophages are key effector cells of the innate immune response to pathogen invasion but are also thought to have an immune-suppressive effect in the lung, limiting excess inflammation (12). Normal resting alveolar macrophages (AM) produce low levels of inflammatory cytokines and are less actively phagocytic than their counterparts in other tissues, possibly due to lower levels of the phagocytic receptor CD11b (12). AM activation causes increased phagocytosis and production of numerous proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-8 (2). They are critical in determining the outcome of a number of respiratory infections, playing a role in controlling the replication and spread of both viruses, e.g., influenza virus (36), and bacteria, e.g., Mycobacterium tuberculosis (21, 22).
Respiratory syncytial virus (RSV) is a nonsegmented, negative-strand RNA virus of the family Paramyxoviridae. It is the leading cause of infant hospital admissions, causing 70% of bronchiolitis hospitalizations in the developed world (10). The relative contribution of the adaptive and innate responses to the pathogenesis of RSV disease is unclear. The importance of T cells in RSV disease has been extensively studied in the mouse model, where skewed Th1/Th2 responses are associated with different forms of lung disease (30). In humans, severe infantile bronchiolitis is associated with markers of Th2 immunity (1, 23), and RSV-specific T cells producing gamma interferon (IFN-γ), IL-4, and IL-5 have been detected in children with RSV, with IL-4 and IL-5 being detected only in those children with severe bronchiolitis (31, 20). In the 1960s, the vaccination of children with formalin-inactivated RSV caused exacerbated illness when the children subsequently became infected with RSV (18, 24). A recent study of fatal cases of RSV bronchiolitis in infants has shown that death is associated with a lack of evidence of adaptive immune responses to infection, such as an absence of recruitment of cytotoxic T cells and lymphocyte-derived cytokines, robust viral replication, and increased apoptosis within bronchiolar epithelial cells (39). In addition, single-nucleotide polymorphism screening of infants hospitalized for severe RSV bronchiolitis shows that polymorphisms in genes associated with innate immunity, such as IFNA2 and NOS2, as well as the known polymorphisms in the adaptive pathways have strong associations with RSV disease in childhood (17).
Liposome-encapsulated dichloro-methylene-diphosphonate (clodronate) is taken up by phagocytic cells in vitro and in vivo. The liposome bilayers are disrupted in the lysosome by phospholipases, allowing the escape of clodronate into the cell; once the clodronate has accumulated sufficiently, the macrophage is irreversibly damaged and dies by apoptosis (38). In mice, the administration of clodronate liposomes (CL) can therefore be used to selectively deplete macrophages (37). The depletion of macrophages from the lungs is associated with an increased pulmonary immune response characterized by dendritic cell (DC) trafficking (16, 35). Macrophage depletion of mice followed by RSV infection has been shown to result in increased viral titers (3); however, the role that macrophages play in initiating and modulating the immune responses and disease after RSV infection has not been fully elucidated.
To delineate the role of macrophages in the immune response to RSV infection, we depleted macrophages by the intranasal administration of CL prior to infection and characterized both the innate and adaptive immune responses and their effects on viral replication and disease. While macrophage depletion strongly inhibited the immediate release of inflammatory mediators and the activation and recruitment of natural killer (NK) cells after viral infection, it had little effect on the adaptive response or overt disease.
MATERIALS AND METHODS
Mice and virus stocks.
Eight-week-old female BALB/c mice (Harlan Ltd., United Kingdom) were maintained under pathogen-free conditions in accordance with institutional and United Kingdom Home Office guidelines. In order to obtain a pathogenic stock of RSV, human RSV (type A2 strain from the ATCC) was plaque purified and grown in HEp-2 cell culture in RPMI 1640 medium supplemented with 2% fetal bovine serum, 2 mM l-glutamine, 100-U/ml penicillin, and 100-μg/ml streptomycin.
Mouse treatment and infection.
For macrophage depletion, mice were treated intranasally (i.n.) with 100 μl of CL suspension (a gift from Nico van Rooijen, Boehringer GmbH, Germany), or with the control, while under light anesthesia with isoflurane. Mice were infected i.n. with 100 μl of the chosen dose of RSV strain A2 while under light anesthesia with isoflurane. The weight and baseline lung function (assessed by noninvasive, barometric, whole-body plethysmography [Buxco, United Kingdom] as described in reference 8) of mice were monitored daily until they were sacrificed by pentobarbitone injection. Bronchoalveolar lavage (BAL) was performed by inflating the lungs via the trachea with 1 ml of 12 mM lidocaine in Eagle's minimum essential medium. The BAL cells were prepared for flow cytometry, and the BAL cell-free supernatant was retained for enzyme-linked immunosorbent assay (ELISA). Collagenase-digested lung tissue was prepared for flow cytometry analysis, and the supernatant from a homogenized lung was used in an RSV infectious-focus assay to measure the viral load. The supernatants from the lung tissue, strained through 100-μm filters, were tested by ELISA.
Flow cytometric analysis.
The following antibodies were used: Pacific Blue-anti-CD11c (eBioscience, United Kingdom), Pacific Blue-anti-CD4, fluorescein isothiocyanate conjugated with anti-major histocompatibility complex class II antigen (FITC-anti-MHC-II), FITC-anti-CD44, FITC-anti-CD69, phycoerythrin (PE)-anti-CD86, PE-anti-CD80, PE-anti-DX5, PE-anti-tumor necrosis factor (TNF), antigen-presenting cell (APC)-anti-F4/80, APC-anti-CD8, PE-Cy7-anti-CD11b, PE-Cy7-anti-CD3, and PE-anti-RSV M2 pentamer (Proimmune, United Kingdom), plus isotype controls (antibodies from BD Biosciences, United Kingdom, unless otherwise stated). Cells were blocked with Fc block (anti-CD16/32; BD), and surface staining was carried out on live cells resuspended in phosphate-buffered saline (PBS)-1% bovine serum albumin-0.1% azide-5 μM EDTA (fluorescence-activated cell sorter buffer). For intracellular TNF analysis, cells were cultured for 4 h with GolgiStop (BD). Cells were run on a cyan ADP LX 9 color flow cytometer (Dako, United Kingdom) with data analyzed using the Dako Summit analysis program.
Cytokine and chemokine analysis.
Cytokines and chemokines were quantified by ELISA performed according to the manufacturers' instructions. TNF was assessed using a Biosource Cytoset kit (Biosource, United Kingdom), IFN-α was assessed with capture antibody (Hycult Biotechnology, United Kingdom) and a two-step detection (rabbit anti-IFN-α from PBL Biomedical Laboratories, United Kingdom, and mouse anti-rabbit serum from Jackson ImmunoResearch, United Kingdom), and IFN-γ, IL-6, IL-12 (p70), IL-15, CCL3 (MIP-1α), CCL5 (RANTES), and keratinocyte chemokine were assessed using R+D Systems Duoset antibody pairs (R+D Systems, United Kingdom). Briefly, Immunosorb ELISA plates (Nunc, United Kingdom) were coated with capture antibody and blocked either with 1% bovine serum albumin in PBS or, in the case of IFN-α, with 2.5% fetal calf serum in PBS with 0.02% NaN3. Sample or standard was added, and bound cytokines were detected by biotinylated anticytokine antibody, avidin horseradish peroxidase, and tetramethylbenzidine. Color development was blocked with 2 M H2SO4, and optical densities were read at 450 nm. The concentration of cytokines in each sample was determined from the standard curve.
Infectious-focus assay.
RSV titer was assessed in lung homogenate by titration on HEp-2 cell monolayers in 96-well, flat-bottom plates. Twenty-four hours after infection, monolayers were washed, fixed with methanol, and incubated with peroxidase-conjugated goat anti-RSV antibody (Biogenesis, United Kingdom). Infected cells were detected using 3-amino-9-ethylcarbazole and infectious units enumerated by light microscopy.
Statistical analysis.
Statistical analysis was performed using a two-way analysis of variance (ANOVA) statistic program with GraphPad Prism. Significance was noted when P was <0.05.
RESULTS
Titration of infectious RSV dose.
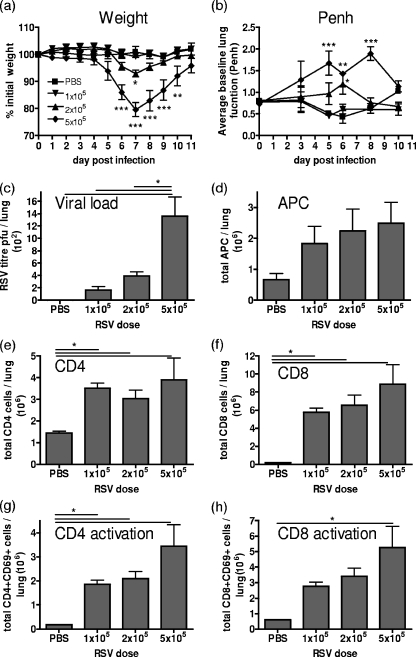
RSV was titrated in naïve 8-week-old BALB/c mice at doses of 5 × 105, 2 × 105, and 1 × 105 PFU per mouse. Doses of 5 × 105 or 2 × 105 PFU per mouse resulted in significant weight loss on day 7 postinfection compared to that of the naïve animals (Fig. 1a) and increased the baseline enhanced pause (Penh), representing increased respiratory effort, on days 5 to 8 (Fig. 1b). The peak viral titer measured on day 4 by an infectious-focus assay confirmed that the RSV load in the lung was proportional to the dose administered (Fig. 1c). The dose-dependent recruitment of CD11c+ MHC-II+ APC and CD8 T cells (CD3+ CD8+) was also evident on day 7 postinfection, while the peak recruitment of CD4 T cells (CD3+ CD4+) correlates with the highest viral dose (Fig. 1d to f). The activation of T cells was also related to the dose of RSV administered, with the expression of the early activation marker CD69 being greatest for mice which received 5 × 105 PFU and lowest for those which received 1 × 105 PFU of RSV (Fig. 1g and h).
FIG. 1.
Immune response to RSV titration in a BALB/c mouse model. Mice were infected i.n. with 100 μl containing 5 × 105, 2 × 105, or 1 × 105 PFU of RSV or PBS (control). Illness was assessed by weight loss (a) and whole-body plethysmography (b). Viral titer was measured in the lung on day 4 by infectious-focus assay (c). On day 7, APC (CD11c+ MHC-II+) numbers (d) and CD4+ (e) and CD8+ (f) T-cell numbers plus their activation states (g and h) were analyzed. Bars/data points represent the means ± SEM of the results from four animals and are representative of two separate experiments. ***, P < 0.001; **, P < 0.01; *, P < 0.05, for test versus control values (a and b), and where indicated by solid horizontal lines (c, e, f, g, and h), determined by ANOVA.
Intranasal administration of CL.
In order to determine the correct concentration needed to deplete local macrophages without initiating excess inflammation, we titrated the stock concentration of CL. Naïve 8-week-old BALB/c mice were given i.n. 100% or 30% CL, diluted in PBS to a total volume of 100 μl. Control mice received either PBS or empty liposomes (PL). Flow cytometric analysis of the BAL cells for CD11c+ AM with a low level of MHC-II (MHC-IIlo) (19) showed that both the 100% and 30% CL treatments resulted in an ∼80% reduction in the AM number on days 1 and 3 after treatment (Fig. 2a). Macrophage depletion in the remaining lung tissue was less marked, with a 50% reduction after either dose (Fig. 2b). Interestingly, treatment with empty liposomes caused a significant increase in the number of recovered macrophages on day 1 postdepletion compared to the number in PBS-treated animals, probably due to macrophage activation caused by the phagocytosis of liposomes.
FIG. 2.
Macrophage depletion by CL treatment. Mice were given i.n. 100 μl of liposomes, containing undiluted clodronate (100% CL), 30% CL in PBS, or undiluted empty liposomes (PL). AM in BAL cells (a), macrophages (Mφ) in lung cells (b), total BAL cells (c), and total lung cells (d) were counted on days 1 and 3 posttreatment. Values from naïve animals are displayed as broken lines on each plot. Bars represent means ± SEM of the results from five animals, and data are representative of three separate experiments. *, P < 0.05, where indicated by solid horizontal lines (determined by ANOVA); ns, not significant.
Total BAL and lung cell numbers were increased after treatment with 100% CL but not with 30% CL (Fig. 2c and d); hematoxylin and eosin staining revealed that the additional cells were polymorphonuclear in appearance (data not shown). No treatment resulted in weight loss or altered lung function as measured by baseline Penh (data not shown). The dose of 30% CL was chosen, as this gave the maximal depletion of macrophages and minimal inflammation.
CL treatment depletes macrophages during infection.
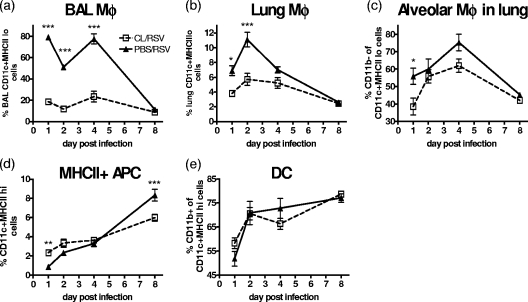
On day 0 of infection (day 3 after CL treatment), there was a marked depletion of CD11c+ MHC-IIlo cells. CD11c+ MHC-IIlo AM constituted approximately 80% of the BAL cells of healthy mice on day 1 postinfection compared to 20% of the BAL cells of mice which received CL (Fig. 3a). At the same time point in the lungs, macrophages represented 4% of the total cell population after CL treatment compared to 7% without this treatment (Fig. 3b). RSV infection caused the percentage of AM in the BAL cells to drop to a level similar to that of the CL group by day 8 postinfection. The percentage of lung macrophages also dropped as the disease progressed.
FIG. 3.
Clodronate treatment during RSV infection depletes macrophages. Mice were treated with 30% CL 3 days prior to i.n. infection with 2 × 105 PFU of RSV. Using fluorescence-activated cell sorter analysis, macrophages were defined as CD11c+ MHC-IIlo and DC were defined as CD11c+ MHC-IIhi. The percentages of BAL cells (a) and lung macrophages (Mφ) (b and c) and the percentages of DC (d and e) were plotted. Data points represent means ± SEM of the results from five animals, and data are representative of three separate experiments. ***, P < 0.001; **, P < 0.01; *, P < 0.05, for test versus control values determined by ANOVA.
The AM of naïve mice are typically CD11c+ CD11blo, while the DC of naïve mice are CD11c+ CD11b+ (16). The BAL cells of a naïve BALB/c mouse are entirely CD11c+ MHC-IIlo CD11b−. CL treatment preferentially depleted CD11b− AM (Fig. 3c), and the remaining CD11c+ MHC-IIlo cells were predominantly CD11b+, suggestive of immature DC. CL treatment led to an early increase in CD11c+ cells with a high level of MHC-II (MHC-IIhi) cells compared to that of the untreated mice on day 0; however, the expansion of MHC-IIhi APC in the lungs on day 8 postinfection was actually reduced by macrophage depletion (Fig. 3d). The majority of these APC were CD11b+ (Fig. 3e), suggesting that they were DC.
Mediator release by macrophages.
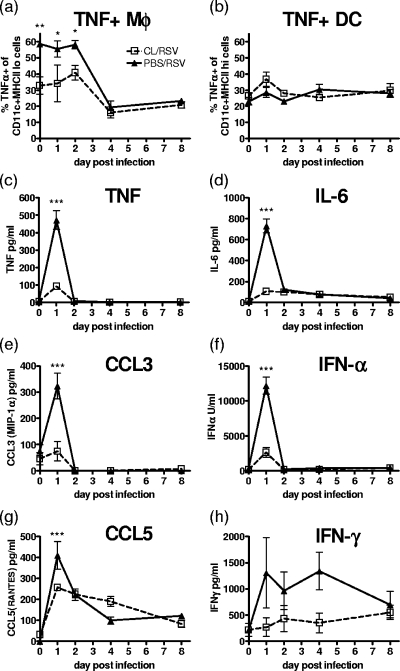
Macrophages are widely thought to be a significant source of TNF, which is readily available for release following infection. Intracellular staining for TNF in lung macrophages and DC showed that prior to RSV infection, 60% of the macrophages, but only 20% of the DC, stained positive for TNF (Fig. 4a and b). After day 2 of the RSV infection, there was a rapid drop in the number of TNF+ macrophages; CL treatment appeared to selectively deplete TNF+ macrophages but had no effect on TNF+ DC.
FIG. 4.
Macrophage depletion reduces early cytokine and chemokine production. Mice were treated with 30% CL 3 days prior to i.n. infection with 2 × 105 PFU of RSV. Intracellular TNF in lung macrophages (Mφ) (a) and DC (b) was measured by flow cytometry. TNF (c), IL-6 (d), CCL3 (e), IFN-α (f), CCL5 (g), and IFN-γ (h) levels in BAL cells were measured by ELISA. Data points represent means ± SEM of the results from five animals; data are representative of three separate experiments. ***, P < 0.001; **, P < 0.01; *, P < 0.05, for test versus control values determined by ANOVA.
On day 1 after RSV infection, there was a peak of inflammatory mediators in the airways, including TNF, IL-6, CCL3, IFN-α, CCL5, and IFN-γ. All were significantly reduced by the CL treatment of mice prior to RSV infection, with the exception of IFN-γ (Fig. 4c to h). RSV infection resulted in a less pronounced peak of chemokine and cytokine release in the surrounding lung tissue; however, macrophage depletion did not affect the concentrations detected (data not shown).
Effects of macrophage depletion on viral load and subsequent disease.
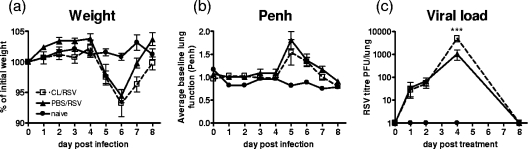
Despite the elimination of early inflammatory signals after macrophage depletion, weight loss was not affected (Fig. 5a); neither were increases in the baseline Penh upon infection (Fig. 5b). There was, however, a significant increase in RSV viral titer in the lungs on day 4 postinfection in mice that were pretreated with CL (means ± standard errors of the means [SEM] of 1,044 ± 497.2 PFU/lung for PBS/RSV versus 5,028 ± 1,575 PFU/lung for CL/RSV; P < 0.05), but levels of viral clearance were similar (Fig. 5c).
FIG. 5.
Macrophage depletion during RSV infection does not affect weight loss but increases viral load. Mice were treated with 30% CL 3 days prior to i.n. infection with 2 × 105 PFU of RSV. Weight (a) and body plethysmography (b) were recorded throughout. (c) Viral titer was measured on harvested lung tissue supernatants by infectious-focus assay. Data points represent means ± SEM of the results from five animals; data are representative of three separate experiments. ***, P < 0.001; **, P < 0.01; *, P < 0.05, for test versus control values determined by ANOVA.
Immune responses to infection.
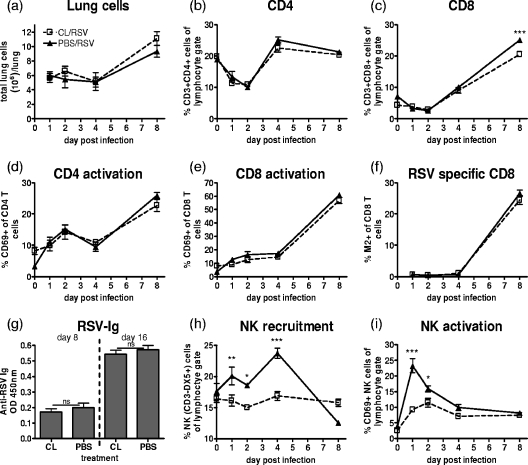
Macrophage depletion during RSV infection did not cause significant changes in the total cell recruitment to the lungs compared to that in the nondepleted mice (Fig. 6a). Furthermore, the proportion of CD4 and CD8 T-cell recruitment was unaffected (Fig. 6b and c), and their activation, as measured by CD69 expression, was also unaltered (Fig. 6d and e). The majority of the RSV-specific CD8 T cells in the BALB/c mice respond to a peptide within the RSV M2 protein. Use of an M2-specific pentamer to stain for RSV-specific CD8 T cells revealed no changes following CL treatment, with ∼24% of the CD8 T cells being RSV M2 positive on day 8 irrespective of treatment (Fig. 6f). Additionally, the humoral immune response was unaffected by depletion. There was no change in the serum levels of RSV-specific immunoglobulin on either day 8 or day 16 postinfection (Fig. 6g).
FIG. 6.
Macrophage depletion reduces NK cell recruitment and activation but does not alter the adaptive immune responses. Mice were treated with 30% CL 3 days prior to i.n. infection with 2 × 105 PFU of RSV. Total lung cell counts (a) and percentages of CD4 (b), CD8 (c), activated CD4 (d), activated CD8 (e), and RSV-specific CD8 T cells (f) in the lungs of CL- or PBS-treated mice at different time points after RSV infection are shown. (g) Anti-RSV serum antibody was detected by RSV-specific immunoglobulin ELISA, and values from 1/400 serum dilutions are shown. NK cell number (h) and activation (i) in lung postinfection are shown. Data points/bars represent means ± SEM of the results from five animals, and data are representative of three separate experiments. ***, P < 0.001; **, P < 0.01; *, P < 0.05, for test versus control values determined by ANOVA; ns, not significant; Ig, immunoglobulin; OD, optical density.
During RSV infection, in the presence of macrophages, NK cells (CD3− DX5+) were recruited to the lungs and their numbers peaked on day 4. Interestingly, CL treatment prevented this NK cell recruitment (Fig. 6 h). Further, CD69, an activation marker, was rapidly upregulated on the surfaces of NK cells after RSV infection, peaking on day 1. The depletion of macrophages also reduced CD69 expression (Fig. 6i).
DISCUSSION
Depleting macrophages by the inhalation of CL caused a profound inhibition of the early release of inflammatory cytokines into the airways after RSV infection and lessened the activation and recruitment of NK cells. Despite the virtual abolition of early inflammatory mediator release and a rise in viral load at day 4, there was no change in the weight loss, lung function deterioration, or T-cell recruitment that characterizes the later stages of RSV infection. In view of the known viral sensing, proinflammatory, and immunomodulatory effects of AM, depletion seemed to have remarkably little effect on these responses.
A number of studies have observed a very early release of cytokines and chemokines after RSV infection similar to that seen here (6). Our data suggest that this release is AM dependent. This is supported by other studies that show that the activation of NF-κB signaling pathways, which are key in initiating many proinflammatory responses, in the lungs of mice infected with RSV was entirely dependent on the presence of AM (7).
In contrast with our findings that the marked reduction of proinflammatory mediators in the airways did not affect the overall disease, the targeted removal of individual mediators does lead to reduced disease. CCL3−/− mice have reduced RSV inflammation (6), the use of depleting antibody to remove TNF reduces weight loss associated with RSV infection (15), and neutralizing the function of CCL5 by the administration of Met-RANTES (a competitive inhibitor) reduces both the CD4 and CD8 responses to RSV (4). In addition, non-IFN-responsive, STAT-1 knockout mice show increased illness and Th2-skewed disease (5). These studies describe the effects of systemic and presumably complete removal of either the factor or the signaling, whereas we specifically depleted AM and found altered cytokine levels in the BAL cells but not the lungs. This reflects a greater (80%) reduction in the number of AM in the BAL cells than the 50% reduction in the number of macrophages in the lung tissue. Therefore, it is possible that the inflammatory mediators in the surrounding lung tissue and alternative sites, such as the lymph nodes and spleen, contribute to the disease seen in the current study.
DC are thought to “license” NK cells, potentiating their activation and cytotoxicity (25, 26). Here we show in naïve lungs, where DC are scarce, that AM are required to both recruit and activate NK cells in response to RSV infection. Recently, human macrophages have been shown to be able to activate NK cells by a mechanism that involves contact-mediated signaling through the immune synapse (28). The loss of this signaling may explain the loss of NK cell activation that we observed. It has been shown that IFN-α production by resident cells in the liver promotes MIP-1α production and subsequent NK cell migration (32, 33). Therefore, it seems likely that the loss of chemokine production, such as with MIP-1α, may also be critical in determining NK cell recruitment to the lungs.
We have previously shown that NK cells are a major source of early IFN-γ during viral infection (13). In addition, they play an essential role in RSV immunity along with specific T cells, as the depletion of both NK cells and CD8 T cells led to the dissemination of virus from the lungs to the lymph nodes (14). This suggests that the loss of early (days 1 to 4) IFN-γ seen after depletion is most likely due to reduced NK cell recruitment; furthermore, the loss of NK cells may also explain the increased viral titer on day 4. AM could also potentially be directly antiviral; they are the first cells to encounter pathogens in the airways, acquiring the vast majority of inhaled particles by efficient phagocytosis (16). Although viral titers in the lung on days 1 and 2 were unaffected by macrophage depletion, suggesting that initial RSV replication takes place mainly in epithelial cells, at the peak of replication on day 4, depletion led to a higher viral load. This points to a role for AM in controlling antiviral activity against RSV infection. Virus was cleared by day 8 in both normal and macrophage-depleted mice; later clearance of virus is associated with effective CD8 and antibody responses, both of which were unaffected by CL treatment.
In addition to their direct cytotoxic role, NK cells also have been shown to strongly influence the subsequent CD8 T-cell response via their cytokine secretion (13). Although IFN-γ and NK cells were reduced up to day 4 in our study, subsequent T-cell numbers were not altered with the CL treatment. In addition to the normal T-cell response to RSV, weight loss and lung function were unaffected by AM depletion. T-cell infiltration correlated well with these indicators of disease after RSV infection (Fig. 1), and exacerbated CD8 T-cell responses have been strongly associated with both measures of disease, in both RSV (9) and influenza A virus (27) infection.
The dual role of macrophages in both regulation and inflammation may explain why, despite decreasing inflammatory mediator release, AM depletion has no effect on the adaptive immune response to infection and therefore disease. AM suppress the migration (16) and antigen presentation capacity (11) of DC. AM removal has been shown to increase the trafficking of antigen toward the lymph nodes (16); this may be the case in RSV infection. Such increased antigen transport may be due to the presence of enhanced DC numbers in the lungs during the early stages of infection in macrophage-depleted mice. Our results support those of Wijburg et al., showing that DC are the main APC for the induction of virus-specific T-cell responses, since these responses were still effectively induced in the absence of AM (40). The increased viral burden, and antigen load, on day 4 postinfection may also promote enhanced T-cell recruitment, compensating for the loss of inflammatory signals. NK cells may also play a role in the suppression of T-cell responses (29, 34), and therefore, the decrease in NK cell recruitment following AM depletion may allow an increase in the T-cell response.
In conclusion, the depletion of lung macrophages dampens the innate response to RSV infection and increases the peak viral load but does not change weight loss or lung function, as parameters of disease. Therefore, our findings support a role for T-cell-mediated factors in RSV disease. However, in infants with diminished adaptive responses to viral infection, macrophages and the innate responses that they control could be critical in controlling viral load in the lung.
Footnotes
Published ahead of print on 20 February 2008.
REFERENCES
- 1.Aberle, J. H., S. W. Aberle, W. Rebhandl, E. Pracher, M. Kundi, and T. Popow-Kraupp. 2004. Decreased interferon-gamma response in respiratory syncytial virus compared to other respiratory viral infections in infants. Clin. Exp. Immunol. 137146-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker, S., J. Quay, and J. Soukup. 1991. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J. Immunol. 1474307-4312. [PubMed] [Google Scholar]
- 3.Benoit, A., Y. Huang, J. Proctor, G. Rowden, and R. Anderson. 2006. Effects of alveolar macrophage depletion on liposomal vaccine protection against respiratory syncytial virus (RSV). Clin. Exp. Immunol. 145147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Culley, F. J., A. M. J. Pennycook, J. S. Tregoning, J. S. Dodd, G. Walzl, T. N. Wells, T. Hussell, and P. J. M. Openshaw. 2006. Role of CCL5 (RANTES) in viral lung disease. J. Virol. 808151-8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durbin, J. E., T. R. Johnson, R. K. Durbin, S. E. Mertz, R. A. Morotti, R. S. Peebles, and B. S. Graham. 2002. The role of IFN in respiratory syncytial virus pathogenesis. J. Immunol. 1682944-2952. [DOI] [PubMed] [Google Scholar]
- 6.Haeberle, H. A., W. A. Kuziel, H.-J. Dieterich, A. Casola, Z. Gatalica, and R. P. Garofalo. 2001. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1α in lung pathology. J. Virol. 75878-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haeberle, H. A., R. Takizawa, A. Casola, A. R. Brasier, H. J. Dieterich, N. van Rooijen, Z. Gatalica, and R. P. Garofalo. 2002. Respiratory syncytial virus-induced activation of nuclear factor-kappaB in the lung involves alveolar macrophages and toll-like receptor 4-dependent pathways. J. Infect. Dis. 1861199-1206. [DOI] [PubMed] [Google Scholar]
- 8.Hamelmann, E., J. Schwarze, K. Takeda, A. Oshiba, G. L. Larsen, C. G. Irvin, and E. W. Gelfand. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156766-775. [DOI] [PubMed] [Google Scholar]
- 9.Harker, J., A. Bukreyev, P. L. Collins, B. Wang, P. J. M. Openshaw, and J. S. Tregoning. 2007. Virally delivered cytokines alter the immune response to future lung infections. J. Virol. 8113105-13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henrickson, K. J., S. Hoover, K. S. Kehl, and W. Hua. 2004. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatr. Infect. Dis. J. 23S11-S18. [DOI] [PubMed] [Google Scholar]
- 11.Holt, P. G. 2000. Antigen presentation in the lung. Am. J. Respir. Crit. Care Med. 162S151-S156. [DOI] [PubMed] [Google Scholar]
- 12.Holt, P. G. 1978. Inhibitory activity of unstimulated alveolar macrophages on T-lymphocyte blastogenic response. Am. Rev. Respir. Dis. 118791-793. [DOI] [PubMed] [Google Scholar]
- 13.Hussell, T., and P. J. Openshaw. 1998. Intracellular IFN-gamma expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J. Gen. Virol. 792593-2601. [DOI] [PubMed] [Google Scholar]
- 14.Hussell, T., and P. J. M. Openshaw. 2000. IL-12 activated NK cells reduce lung eosinophilia to the attachment protein of respiratory syncytial virus but do not enhance the severity of illness after lung challenge in CD8 cell-immunodeficient conditions. J. Immunol. 1657109-7115. [DOI] [PubMed] [Google Scholar]
- 15.Hussell, T., A. Pennycook, and P. J. Openshaw. 2001. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 312566-2573. [DOI] [PubMed] [Google Scholar]
- 16.Jakubzick, C., F. Tacke, J. Llodra, N. van Rooijen, and G. J. Randolph. 2006. Modulation of dendritic cell trafficking to and from the airways. J. Immunol. 1763578-3584. [DOI] [PubMed] [Google Scholar]
- 17.Janssen, R., L. Bont, C. L. Siezen, H. M. Hodemaekers, M. J. Ermers, G. Doornbos, R. Slot, C. Wijmenga, J. J. Goeman, J. L. Kimpen, H. C. van Houwelingen, T. G. Kimman, and B. Hoebee. 2007. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J. Infect. Dis. 196826-834. [DOI] [PubMed] [Google Scholar]
- 18.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89422-434. [DOI] [PubMed] [Google Scholar]
- 19.Kumagai, Y., O. Takeuchi, H. Kato, H. Kumar, K. Matsui, E. Morii, K. Aozasa, T. Kawai, and S. Akira. 2007. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity 27240-252. [DOI] [PubMed] [Google Scholar]
- 20.Lee, F. E., E. E. Walsh, A. R. Falsey, M. E. Lumb, N. V. Okam, N. Liu, A. A. Divekar, C. B. Hall, and T. R. Mosmann. 2007. Human infant respiratory syncytial virus (RSV)-specific type 1 and 2 cytokine responses ex vivo during primary RSV infection. J. Infect. Dis. 1951779-1788. [DOI] [PubMed] [Google Scholar]
- 21.Leemans, J. C., N. P. Juffermans, S. Florquin, N. van Rooijen, M. J. Vervoordeldonk, A. Verbon, S. J. van Deventer, and T. van der Poll. 2001. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J. Immunol. 1664604-4611. [DOI] [PubMed] [Google Scholar]
- 22.Leemans, J. C., T. Thepen, S. Weijer, S. Florquin, N. van Rooijen, J. G. van de Winkel, and T. van der Poll. 2005. Macrophages play a dual role during pulmonary tuberculosis in mice. J. Infect. Dis. 19165-74. [DOI] [PubMed] [Google Scholar]
- 23.Legg, J. P., I. R. Hussain, J. A. Warner, S. L. Johnston, and J. O. Warner. 2003. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 168633-639. [DOI] [PubMed] [Google Scholar]
- 24.Moghaddam, A., W. Olszewska, B. Wang, J. S. Tregoning, R. Helson, Q. J. Sattentau, and P. J. Openshaw. 2006. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat. Med. 12905-907. [DOI] [PubMed] [Google Scholar]
- 25.Moretta, A., E. Marcenaro, S. Parolini, G. Ferlazzo, and L. Moretta. 2008. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 15226-233. [DOI] [PubMed] [Google Scholar]
- 26.Moretta, L., G. Ferlazzo, C. Bottino, M. Vitale, D. Pende, M. C. Mingari, and A. Moretta. 2006. Effector and regulatory events during natural killer-dendritic cell interactions. Immunol. Rev. 214219-228. [DOI] [PubMed] [Google Scholar]
- 27.Moskophidis, D., and D. Kioussis. 1998. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J. Exp. Med. 188223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nedvetzki, S., S. Sowinski, R. A. Eagle, J. Harris, F. Vely, D. Pende, J. Trowsdale, E. Vivier, S. Gordon, and D. M. Davis. 2007. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood 1093776-3785. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen, K. B., T. P. Salazar-Mather, M. Y. Dalod, J. B. Van Deusen, X. Q. Wei, F. Y. Liew, M. A. Caligiuri, J. E. Durbin, and C. A. Biron. 2002. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 1694279-4287. [DOI] [PubMed] [Google Scholar]
- 30.Openshaw, P. J. M., and J. S. Tregoning. 2005. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin. Microbiol. Rev. 18541-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pala, P., R. Bjarnason, F. Sigurbergsson, C. Metcalfe, N. Sigurs, and P. J. Openshaw. 2002. Enhanced IL-4 responses in children with a history of respiratory syncytial virus bronchiolitis in infancy. Eur. Respir. J. 20376-382. [DOI] [PubMed] [Google Scholar]
- 32.Salazar-Mather, T. P., T. A. Hamilton, and C. A. Biron. 2000. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J. Clin. Investig. 105985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salazar-Mather, T. P., C. A. Lewis, and C. A. Biron. 2002. Type I interferons regulate inflammatory cell trafficking and macrophage inflammatory protein 1alpha delivery to the liver. J. Clin. Investig. 110321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su, H. C., K. B. Nguyen, T. P. Salazar-Mather, M. C. Ruzek, M. Y. Dalod, and C. A. Biron. 2001. NK cell functions restrain T cell responses during viral infections. Eur. J. Immunol. 313048-3055. [DOI] [PubMed] [Google Scholar]
- 35.Thepen, T., N. van Rooijen, and G. Kraal. 1989. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J. Exp. Med. 170499-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tumpey, T. M., A. García-Sastre, J. K. Taubenberger, P. Palese, D. E. Swayne, M. J. Pantin-Jackwood, S. Schultz-Cherry, A. Solórzano, N. Van Rooijen, J. M. Katz, and C. F. Basler. 2005. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J. Virol. 7914933-14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 17483-93. [DOI] [PubMed] [Google Scholar]
- 38.van Rooijen, N., A. Sanders, and T. K. van den Berg. 1996. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J. Immunol. Methods 19393-99. [DOI] [PubMed] [Google Scholar]
- 39.Welliver, T. P., R. P. Garofalo, Y. Hosakote, K. H. Hintz, L. Avendano, K. Sanchez, L. Velozo, H. Jafri, S. Chavez-Bueno, P. L. Ogra, L. McKinney, J. L. Reed, and R. C. Welliver, Sr. 2007. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 1951126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wijburg, O. L., G. P. van den Dobbelsteen, J. Vadolas, A. Sanders, R. A. Strugnell, and N. van Rooijen. 1998. The role of macrophages in the induction and regulation of immunity elicited by exogenous antigens. Eur. J. Immunol. 28479-487. [DOI] [PubMed] [Google Scholar]