Abstract
Replication of picornaviral genomes requires recognition of at least three cis-acting replication elements: oriL, oriI, and oriR. Although these elements lack an obvious consensus sequence or structure, they are all recognized by the virus-encoded 3C protein. We have studied the poliovirus 3C-oriI interaction in order to begin to decipher the code of RNA recognition by picornaviral 3C proteins. oriI is a stem-loop structure that serves as the template for uridylylation of the peptide primer VPg by the viral RNA-dependent RNA polymerase. In this report, we have used nuclear magnetic resonance (NMR) techniques to study 3C alone and in complex with two single-stranded RNA oligonucleotides derived from the oriI stem. The 1H-15N spectra of 3C recorded in the presence of these RNAs revealed site-specific chemical shift perturbations. Residues that exhibit significant perturbations are primarily localized in the amino terminus and in a highly conserved loop between residues 81 and 89. In general, the RNA-binding site defined in this study is consistent with predictions based on biochemical and mutagenesis studies. Although some residues implicated in RNA binding by previous studies are perturbed in the 3C-RNA complex reported here, many are unique. These studies provide unique site-specific insight into residues of 3C that interact with RNA and set the stage for detailed structural investigation of the 3C-RNA complex by NMR. Interpretation of our results in the context of an intact oriI provides insight into the architecture of the picornavirus VPg uridylylation complex.
Poliovirus (PV) is a small, nonenveloped virus with a single-stranded RNA (ssRNA) genome. PV is the best-studied member of the picornavirus family of viruses, which includes numerous viruses of medical importance, for example coxsackieviruses, human rhinoviruses, and hepatitis A virus. The genome contains a single open reading frame that encodes a large polyprotein that undergoes proteolytic cleavage to produce mature viral gene products. Polyprotein cleavage events are performed by one of two viral proteases: 2A and 3C. In addition to its major role as a proteinase, 3C and/or precursors thereof bind to cis-acting replication elements (CREs) located in the RNA genome (1, 12, 13, 19, 30-33, 44).
Three CREs are known. One CRE, located at the 5′ end of the genome in the nontranslated region, adopts a cloverleaf-like secondary structure (Fig. 1a and b). This element is often referred to as the cloverleaf (CL) or oriL (origin of replication left). Another CRE is located at the extreme 3′ end, again in the nontranslated region, and consists of two stem-loop structures capable of forming a pseudoknot (Fig. 1a and b). This element is often referred to as oriR (origin of replication right). Finally, a CRE that consists of a single stem-loop exists within the 2C protein-coding sequence (Fig. 1a and b); an analogous CRE exists in all picornaviruses. While the exact position of this CRE varies, it is always located at an internal position of the genome. We will refer to this element as oriI (origin of replication internal).
FIG. 1.
(a) Structure of PV genome. The locations of the three CREs are shown. (b) cis-Acting RNA elements of PV. oriL, located at the 5′ end of the genome in the nontranslated region, adopts a cloverleaf-like secondary structure. oriI is found in the 2C-coding region of the genome. oriR, located at the extreme 3′ end, consists of two stem-loops. There is no recognizable sequence similarity between these CREs, which are specifically recognized by the 3C protein.
For all picornaviruses examined, the 3C domain confers specificity to the binding of 3C or its precursors to all three RNA elements (1, 3, 18, 31), despite the fact that no recognizable sequence or structural consensus exists between the PV CREs (Fig. 1b). Importantly, these 3C-CRE interactions are absolutely required for picornavirus genome replication. Molecular genetic and biochemical studies have identified residues of 3C required for RNA binding (1, 3, 18), and structural information for 3C has shown that residues required for RNA binding cluster on the same surface of 3C (27). Although structural information exists for some CRE subdomains, this information has not clarified the molecular basis for RNA recognition by 3C (8, 9, 20, 22, 26, 29, 40).
Recent studies of PV 3C binding to oriI have suggested a two-step binding mechanism in which oriI binds to a 3C dimer, followed by unwinding of the stem and binding of the two single-stranded regions corresponding to the 5′ and 3′ halves of the stem (stmL and stmR) to the two 3C-RNA-binding sites (31). These RNAs bind specifically and with high affinity to 3C relative to uridine oligonucleotides of similar lengths. Therefore, the PV 3C-stmL and 3C-stmR interactions represent useful model systems for beginning to decipher the code of RNA recognition by picornavirus 3C proteins.
In this study, we report the nuclear magnetic resonance (NMR) assignments of PV 3C protein and residue-specific interactions. In the presence of either stmL or stmR RNAs, 1H-15N NMR spectra revealed site-specific chemical shift perturbations. These changes allowed us to identify the RNA-binding region of the PV 3C protein and indicate that ssRNAs representing both halves of the oriI can be recognized by the protein in a similar manner. Interestingly, many 3C residues identified in this study have not been identified in the past as participating in RNA binding, although they are located on the expected RNA-binding surface. The implication of this study on our understanding of the biology of the 3C-oriI interaction will be discussed.
MATERIALS AND METHODS
Expression and purification of PV 3C.
PV 3C-coding DNA encoding the C147A and C153S substitutions was cloned between the SacII and BamHI sites of the pET26Ub-CHIS plasmid (21) to produce the pET26Ub-PV-3C-C147A-C153S-CHIS plasmid. The C147A mutation was introduced to inactivate the protease active site and prevent proteolytic degradation of 3C during data acquisition. The C153S mutation was introduced to prevent oxidation of the remaining cysteine residue. Mutations were introduced by using overlap extension mutagenesis as previously described (32). The oligonucleotides employed were as follows: 3C-C147A-for (5′-CCA ACC AGA GCA GGA CAG GCT GGT GGA GTC ATC ACA-3′), 3C-C147A-rev (5′-TGT GAT GAC TCC ACC AGC CTG TCC TGC TCT GGT TGG-3′), 3C-C153S-for (5′-GGT GGA GTC ATC ACA TCG ACT GGG AAA GTC ATC-3′), and 3C-C153S-rev (5′-GAT GAC TTT CCC AGT CGA TGT GAT GAC TCC ACC-3′). The 3C protein was expressed in BL21(DE3)pCG1 by using the ubiquitin fusion system as described previously for PV polymerase (16). The protein is expressed as an ubiquitin fusion that is subsequently processed by an ubiquitin-specific carboxy-terminal hydrolase to liberate the PV 3C protein having an authentic amino terminus and bearing a C-terminal His6 tag fused to the protein via a short linker. For simplicity, this variant of the protein will be referred to simply as “3C”.
BL21(DE3)pCG1 cells were transformed with the pET26Ub-PV-3C-C147A-C153S-CHIS plasmid, plated onto NZCYM (4) plates containing kanamycin at 25 μg/ml (K25), chloramphenicol at 20 μg/ml (C20), and dextrose at 0.4% and grown overnight at 30°C. Multiple colonies were then used to seed two 100-ml batches of NZCYM media supplemented with K25 and C20. The culture was grown at 37°C to an optical density at 600 nm of ∼1.0. This culture was then used to inoculate eight 500-ml cultures of NZCYM media supplemented with K25 and C20. The cultures were grown at 37°C to an optical density at 600 nm of 0.8. Cells were harvested by centrifugation at 6,000 × g at 25°C in 1-liter centrifuge bottles by using a Sorvall SLC-6000 rotor. Cells were then washed with 1 liter of M9 media (42 mM Na2HPO4, 22 mM KH2PO4, 8.6 mM NaCl, 0.027 mM CaCl2H2O, 1 mM MgSO4, 5 × 10−5% thiamine). Cells were again centrifuged at 6,000 × g at 25°C in 1-liter centrifuge bottles by using a Sorvall SLC-6000 rotor. Cells were then suspended in 1 liter of M9 media containing 0.1% 15N-labeled ammonium chloride (Isotec) and 0.4% [13C]glucose (Isotec). Deuterated samples were obtained by using 99.9% 2H2O in place of water. After being exchanged into minimal media, the cultures were grown at 37°C for 1 to 2 h, after which the cultures were chilled to 25°C and induced by addition of isopropyl-d-thiogalactopyranoside (IPTG) to give a final concentration of 0.8 mM. Cultures were grown for 16 to 20 h at 25°C. The cells were harvested by centrifugation at 6,000 × g for 10 min, washed once in 200 ml of T10E1 (10 mM Tris, 1 mM EDTA, pH 8.0), and centrifuged again, and the cell paste was frozen and stored at −80°C until used.
Frozen cell pellets were thawed on ice and suspended in lysis buffer (50 mM HEPES, pH 7.5, 50 mM NaCl, 10 mM 2-mercaptoethanol, 20% glycerol, 1.4 μg/ml pepstatin A, and 1 μg/ml leupeptin) at a concentration of 5 ml lysis buffer per 1 g cell pellet. Cells were lysed by passing them through a French pressure cell at 20,000 lb/in2. Phenylmethylsulfonyl fluoride and Nonidet P-40 (NP-40) were added after lysis to give final concentrations of 1 mM and 0.1% (vol/vol), respectively. Polyethylenimine (PEI) was slowly added to give 0.25% (vol/vol) while stirring at 4°C in order to precipitate nucleic acids. The extract was stirred for an additional 15 to 30 min at 4°C after addition of PEI and then centrifuged for 30 min at 25,000 rpm (75,000 × g) at 4°C, using a Beckman JA 30.50 rotor. The PEI supernatant was then passed through a 10-ml Q-Sepharose column (GE Healthcare) connected in tandem to a 5-ml Ni-nitrilotriacetic acid column (Qiagen). After the clarified lysate was loaded, the columns were washed with 50 ml of buffer B (50 mM HEPES, pH 7.5, 10 mM 2-mercaptoethanol, 20% glycerol) containing 50 mM NaCl and 5 mM imidazole. After being washed, the Ni-nitrilotriacetic acid column was disconnected from the Q-Sepharose column and subsequently washed with 10 ml of buffer B containing 500 mM NaCl and 5 mM imidazole and with 10 ml of buffer B containing 500 mM NaCl and 50 mM imidazole. Protein was then eluted with buffer B containing 50 mM NaCl and 500 mM imidazole. The eluted protein (3 to 5 ml) was dialyzed overnight against 1 liter of buffer B containing 50 mM NaCl, using a 6- to 8-kDa molecular mass cutoff membrane. The dialyzed sample was then loaded onto a 2-ml SP-Sepharose column (GE Healthcare) equilibrated with buffer B containing 50 mM NaCl. After the loading, the column was washed with 10 ml of buffer B containing 50 mM NaCl and the protein was eluted with 10 ml of buffer B containing 150 mM NaCl. The eluted protein was then loaded onto a second 1-ml SP-Sepharose column equilibrated with buffer B containing 50 mM NaCl. After the loading, the column was washed with 50 ml of 50 mM HEPES, pH 8.0, and 50 mM NaCl. The protein was eluted with 5 ml of 50 mM HEPES, pH 8.0, and 2 M NaCl; a protein purity of >95% was estimated from overloaded sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels visualized by Coomassie blue staining. Purified protein samples were dialyzed into 10 mM HEPES (pH 8 to 8.5), 50 mM NaCl. Protein concentration was determined by using a NanoDrop spectrophotometer and the calculated extinction coefficient at 280 nm of 0.00768 μM−1·cm−1 (15).
Preparation of RNA oligonucleotides.
ssRNA 11-mer regions corresponding to the sequence of the left (stmL) and right (stmR) halves of the oriI upper stem (stmL, 5′-AGUUCAAGAGC-3′; and stmR, 5′-GUAUUGAACCA-3′) and the 29-nucleotide (nt) oriI RNA (Fig. 2c) were chemically synthesized by Dharmacon, Inc., and deprotected prior to use. Deprotection was performed by suspension of the RNA in 500 mM acetic acid and incubation at 65°C for 15 min followed by addition of an equal volume of 660 mM Tris and further incubation at 65°C for 15 min. RNA was then passed over two consecutive G25 spin columns equilibrated with deionized, distilled H2O. The RNA solutions were lyophilized and suspended in 10 mM HEPES (pH 8 to 8.5), 50 mM NaCl, 90% H2O-10% D2O to give a final concentration of 5 mM. The calculated extinction coefficients at 260 nm for the stmL, stmR, and 29-nt oriI RNAs are 0.1163 μM−1·cm−1 (stmL), 0.1164 μM−1·cm−1 (stmR), and 0.3785 M−1·cm−1 (29-nt oriI). Protein-RNA complexes were assembled by adding protein solution (∼0.5 mM) to a highly concentrated ssRNA (∼5 mM), mixed by pipetting, and transferred to an NMR tube. The typical final concentrations were ∼0.5 mM (protein and RNA).
FIG. 2.
Protein and RNA sequences. (a) PV 3C protein sequence; plasmid-encoded residues are indicated by the gray box. Secondary structure elements observed in the crystal structure (Protein Data Bank accession no. 1L1N) are indicated schematically. Red indicates residues whose resonances could be assigned. The Cys/Ala and Cys/Ser mutations are boxed. (b) PV 29-nt oriI RNA. The rectangles on the left and right define the RNA sequences of stmL and stmR ssRNAs, respectively, used in the NMR experiments. The red adenine nucleotide is the uridylylation template.
DLS.
Dynamic light scattering (DLS) was used to obtain molecular mass estimates through measurement of the decay rates of scattered light and calculation of translational diffusion coefficients (5). The hydrodynamic radii (RH) were obtained from the diffusion coefficients (D) via the Stokes-Einstein equation:
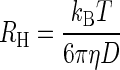 |
where kB is Boltzmann's constant, T is the temperature, and η is the viscosity of the solution. With possible differences in temperature and viscosity neglected, the molecular mass (Mr) was then estimated from RH, using a standard of known diffusion coefficient and molecular mass:
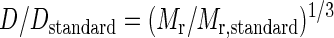 |
DLS experiments were performed at 25°C, using a Viscotek 802 DLS instrument. Samples were analyzed at the same concentrations used for NMR experiments (∼0.5 mM). Typically, 10 scans of 10 s were obtained for each sample. Data were analyzed by using the protein mass model in Omnisize 2.0 software (Viscotek Europe Ltd.).
NMR spectroscopy.
NMR spectra were recorded at 25°C on 600-MHz and 800-MHz Bruker Avance DMX and DRX spectrometers equipped with cryogenically cooled triple-resonance pulsed-field gradient probes. NMR spectra were recorded on proteins uniformly labeled with 15N, 15N/13C, or 2H/13C/15N. Backbone assignments were made using transverse relaxation optimized spectroscopy (TROSY)-based triple-resonance spectra [TROSY-HNCA, TROSY-HNCACB, CBCA(CO)NH, and TROSY-HNCO] (10, 37) recorded at 600 MHz and three-dimensional 15N-edited NOESY-TROSY (τm, 150 ms) recorded at 800 MHz. Backbone resonance assignments of the PV 3C-RNA complexes were confirmed using triple-resonance spectra (TROSY-HNCA and TROSY-HNCO at 600 MHZ). All NMR data were processed and analyzed using NMRPipe (7) and CARA (23). NMR samples were 0.5 mM in 10 mM HEPES, pH 8 to 8.5, 90% H2O-10% D2O, and 50 mM NaCl.
Interactions between PV 3C and stmL or stmR RNAs were examined by comparing peak positions in two- and three-dimensional NMR spectra. RNA binding to 15N-13C-enriched 3C resulted in site-specific chemical shift perturbations in 3C. Perturbations were quantitated and analyzed using the weighted average chemical shift perturbations calculated as follows:
 |
where a is 8 for Gly and 6 otherwise (36).
Diffusion coefficients for 3C and the 3C-RNA complexes were measured using pulsed-field-gradient-stimulated echo experiments (35). A series of 50 spectra were collected, with the z-axis gradient strength varied in linear steps from 5% to 95%. Tetramethylsilane (molecular mass, 88.2 Da) was used as an internal reference.
Protein structure accession number.
Chemical shifts of backbone resonances have been deposited in the BioMagResBank database (http://www.bmrb.wisc.edu/) under BMRB accession number 15222.
RESULTS AND DISCUSSION
RNA-induced changes in the oligomeric state of PV 3C protein.
Structural and biochemical studies of PV 3C have suggested that this protein has the ability to form dimers (27, 31), and recent molecular genetic and biochemical experiments support the proposal that that the PV 3C dimer observed crystallographically functions in VPg uridylylation (38). We investigated the oligomeric state of PV 3C (20 kDa) and PV 3C-RNA complexes by DLS (5) and NMR diffusion experiments (35). Although DLS is often not an accurate method for determining the absolute molecular mass of a species in solution, it can be used reliably to reveal a change in molecular mass, for example due to a monomer-to-dimer transition, and to distinguish homogeneous from polydisperse samples. Using coordinates derived from the crystal structure of the 3C dimer (27), the translational diffusion coefficient predicted by hydrodynamic calculations (14) for a 3C monomer is 0.99 × 10−6 cm2/s, while for a dimer, a diffusion coefficient of 0.75 × 10−6 cm2/s is predicted. DLS measurements of solutions containing 3C protein were monodisperse, with an apparent diffusion coefficient of 0.81 × 10−6 cm2/s, while the translational diffusion coefficient measured by NMR pulsed-field gradient experiments was 1.06 × 10−6 cm2/s. Although there is not perfect agreement between the experimental and predicted values, based on these analyses and the quality of the NMR spectra, we conclude that under these conditions this 3C protein variant (C147A/C153S) is principally monomeric in solution.
DLS measurements of 3C in the presence of the 29-nt oriI RNA (9.3 kDa) yielded an apparent diffusion coefficient of 0.53 × 10−6 cm2/s, consistent with multimerization of 3C in the presence of this RNA hairpin. Because NMR spectroscopy of such an asymmetric multimeric 3C-RNA complex is complicated by the presence of multiple sets of signals for the protein as well as the associated broad lines, we sought to evaluate complexes with other RNAs that would not lead to protein multimerization. We have shown that the 11-nt ssRNAs stmL and stmR, which are derived from the upper stem of oriI, bind to 3C more tightly than intact oriI (31). DLS experiments with 3C and equimolar concentrations of these RNAs yielded apparent diffusion coefficients of 0.87 × 10−6 cm2/s. Translational diffusion coefficients obtained from NMR of 3C bound to the stmR (0.91 × 10−6 cm2/s) or stmL (0.92 × 10−6 cm2/s) RNAs were consistent with monomeric 3C-RNA complexes with a 1:1 stoichiometry.
Characterization of PV 3C protein by NMR spectroscopy.
Two-dimensional 1H-15N correlation spectra of PV 3C showed well-dispersed resonances (Fig. 3a). However, not all of the expected amide signals were observed, as the spectrum contained only 150 of the 185 expected resonances (excluding the 8 prolines but including 10 residues from the C-terminal His tag and linker). The absence of these signals was most likely due to intermediate exchange broadening, indicating that regions of the protein are solvent exposed and not well structured.
FIG. 3.
(a) Residue-specific backbone assignments of the PV 3C protease indicated on a two-dimensional 1H-15N heteronuclear single quantum correlation spectroscopy spectrum. (b) Representative strip plot from the three-dimensional TROSY-HNCACB spectrum of PV 3C, showing sequential connectivities for residues 82 to 86.
In order to study RNA recognition by PV 3C, we obtained backbone resonance assignments for the free protein (Fig. 3a and b). Although amide resonances were generally well resolved, broad lines and limited solubility yielded triple-resonance spectra of moderate quality. Backbone resonance assignments could be obtained for 146 (81%) of the 193 residues. These assignments were achieved by a combination of gradient-enhanced triple-resonance spectra and 1H nuclear Overhauser effects (NOEs) from a three-dimensional 15N-edited NOESY spectrum (6). In addition to sequential NOE patterns, several long-range NOEs between neighboring β strands could be identified, facilitating these resonance assignments. Chemical shift index analysis (42) and NOE patterns confirmed that residues 5 to 14 and 83 to 87 formed alpha helices and the rest of the free protein was primarily composed of beta strands, in agreement with the X-ray crystal structure of the 3C dimer (27).
Chemical shift perturbations reveal the oriI-binding site of PV 3C protein.
Changes in the chemical environments of the atoms in the interface of two interacting biomolecules result in chemical shift changes in NMR spectra. Therefore, spectral perturbation analysis can be used to detect ligand-binding sites (11). Comparison of the 1H and 15N chemical shifts of 3C in the absence and presence of stmL and stmR RNAs showed that both RNAs bound to the protein in similar manners (Fig. 4a-d). Consistent with the modest affinity of the protein for these ssRNAs (31), NMR spectra revealed modest but site-specific chemical shift perturbations. Residues that exhibited significant shift perturbations were primarily localized in two regions: the N terminus and a long loop that connects domains I and II of the protein (residues 82 to 89). Normalized weighted average 1H and 15N shift differences were color mapped onto the crystal structure (27) to identify the RNA-binding regions of the protein (Fig. 4b). Notably, these two regions cluster together and form a positively charged patch on the side of the protein opposite from the proteolytic active site. Overall, the same residues of 3C were perturbed by binding of stmL or stmR RNAs to the protein (Fig. 4c and d).
FIG. 4.
NMR spectral perturbations reveal the interaction between 3C and oriI. (a) Overlay of a region of 1H-15N correlation spectra of free 3C (black) and 3C in complex with stmL RNA (red), illustrating the site-specific chemical shift perturbations that reveal which residues interact with the RNA ligand. (b) RNA-binding residues of 3C protease. RNA-induced shift perturbations are mapped onto a cartoon diagram of the crystal structure of the protease (Protein Data Bank accession no. 1L1N), color coded according to the chemical shift perturbations via a linear ramp from cyan (no change) to magenta (maximal perturbation). Residues for which the effect of RNA binding could not be determined are shown in light gray. (c, d) Chemical shift perturbations induced by binding of ssRNAs corresponding to the left (stmL) (c) and right (stmR) (d) sides of the oriI stem loop. Perturbations were quantitated by normalizing for variation in the 1H and 15N chemical shift ranges and are shown on a per-residue basis. In panels c and d, residues previously identified to interact with RNA are highlighted by an asterisk (1, 3, 18, 25). The dotted line represents the standard deviation of the shifts. Generally, the same residues as those for Fig. 3C are perturbed in both complexes, indicating that the RNAs bind the same site on the protein.
The chemical shift perturbation data complement existing molecular genetic, biochemical, and structural studies of the RNA-binding domain of picornaviral 3C proteins (Table 1). In some instances, the NMR data provide confirmation of the interaction of 3C residues predicted by these studies to contribute to RNA binding (Fig. 4c and d). For example, mutations D32, R84, D85, T154, G155, K156, and R176 were shown to affect formation of the ribonucleoprotein complex at oriL based on a mobility shift assay, without interfering with proteolytic activity (1). However, we did not detect significant NMR perturbations for T154, G155, or R176, suggesting that these residues are not intimately involved in binding ssRNA. Another study, using a cell-based RNA-binding assay to characterize RNA-protein interactions in vivo, showed that residues Y6, K12, R13, K82, and R84 are required for 3C binding to RNA (3). Interestingly, this study excluded participation of H89 in RNA binding by 3C; this residue is present on the same surface of the protein and was clearly perturbed upon binding of the oriI-derived ssRNAs. Finally, perturbations observed for 3C complexed with an RNA element derived from oriL only partially overlap those reported here, revealing many unique interactions with this RNA (unpublished results).
TABLE 1.
Chemical shift perturbations and comparison with previous RNA binding studies
| Residue | Resulta for:
|
Reference(s)b | |
|---|---|---|---|
| NMR | Biochemistry | ||
| 6 | ++ | + | 3e |
| 7 | + | NA | |
| 8 | ++ | NA | |
| 9 | + | NA | |
| 10 | + | NA | |
| 11 | ++ | NA | |
| 12 | + | + | 3e |
| 13 | ++ | + | 3e |
| 14 | ++ | NA | |
| 15 | + | NA | |
| 30 | + | NA | |
| 32 | ++ | + | 1 |
| 40 | + | + | 41b |
| 79 | − | + | 28c |
| 81 | ++ | NA | |
| 82 | + | + | 3,e18, 28,c39,d41b |
| 83 | ++ | + | 39d |
| 84 | ++ | + | 1, 3,e18, 28,c39,d41b |
| 85 | ++ | + | 1, 24,b39,d41b |
| 86 | + | + | 18, 39d |
| 87 | ++ | NA | |
| 89 | ++ | − | 3e |
| 90 | + | NA | |
| 101 | + | NA | |
| 102 | + | NA | |
| 104 | + | NA | |
| 105 | + | NA | |
| 142 | + | + | 2 |
| 150 | + | NA | |
| 154 | − | + | 1, 39,d41b |
| 155 | − | + | 1, 39,d41b |
| 156 | ++ | + | 1, 39,d41b |
| 157 | + | NA | |
| 162 | ++ | NA | |
| 165 | + | NA | |
| 168 | + | NA | |
| 172 | − | + | 2 |
| 176 | − | +/− | 1, 3e |
| 180 | + | NA | |
NMR chemical shift perturbations: −, <1 standard deviation; +, >1 standard deviation; ++, >2 standard deviations. RNA binding: NA, not assayed; −, mutation had no effect on RNA binding; +, mutation affected RNA binding activity.
Human rhinovirus type 14.
Foot-and-mouth disease virus.
Enterovirus 71.
Tested in vivo.
Together, these studies are consistent with the same general surface and structural elements of 3C being used to bind a variety of RNAs. However, it is likely that different RNAs induce a different network of interactions with residues of 3C, consistent with the broad specificity observed for this protein. It is important to note that a change in chemical shift does not necessarily equate to a direct interaction with RNA, as only complete structure determination will reveal the details of the interaction of 3C with the various RNAs. The findings reported here will facilitate structure determination.
The amino acid residues comprising the RNA-binding site cluster on the side of the protein opposite from the proteolytic active site, indicating that the two functions are spatially separate. However, comparison of the two molecules in the asymmetric unit of the crystallographic structure of PV 3C reveals significant structural variability in the RNA-binding residues, particularly in helix A. Such variability raises the interesting possibility that conformational flexibility in the RNA-binding region of the protein could be used for allosteric regulation of protease activity and/or substrate specificity. Some evidence for this possibility has been published (17, 34, 39).
Model for intact oriI binding to PV 3C protein.
The crystal structure of PV 3C revealed the ability of this protein to form a dimer. Some early biochemical and yeast two-hybrid data suggested that this dimer might be an artifact of the high protein concentration employed for crystallography (43). However, recent cross-linking (31), stoichiometry (31), and molecular genetic and biochemical (38) studies and the DLS data presented herein are consistent with the ability of 3C to dimerize in solution, particularly when bound by oriI, and that the dimer is involved in oriI-templated VPg uridylylation (31, 38). An unsolved mystery regarding the use of oriI as a template by polymerase is that the templating adenylate residue is located in a loop and inaccessible to the template-binding site of polymerase, at least in a conformation resembling a traditional template (40). Therefore, it has been suggested that oriI might be melted prior to use (31, 40).
The data presented here permit us to model a melted oriI RNA on the crystallographically observed PV 3C dimer such that each half of the melted RNA stem binds the sites identified by chemical shift and ask whether the intervening loop is sufficiently long to accommodate the overall organization. We note that although it is 3CD that is involved in the uridylylation complex, the recent crystal structure of the 3CD complex (25) was obtained with a 3CD variant designed to disrupt the observed 3C-3C contacts, and the orientation between the 3C and 3D domains occludes the experimentally determined RNA-binding site. However, given the flexibility of the linker between the domains and the paucity of contacts between them, we expect that the domains can readily reorient, allowing the organization observed in the 3C crystal structure, with an extended RNA-binding surface that would further contribute to binding affinity. For these reasons, we use that model to examine how the RNA might bind the 3C domain. As shown in Fig. 5, oriI can be modeled to interact with the PV 3C dimer in a manner that extends the loop and maintains the interaction of the melted stem RNAs with the RNA-binding site implicated by the NMR data. Interestingly, the polymerase can be docked to this 3C2-oriI complex such that the templating adenylate residue is located in the template-binding site in a typical conformation (Fig. 5b). Sequence alignments suggest that all entero- and rhinovirus 3C proteins and likely similar 3C2-oriI complexes can form this dimer (38). An implication of this model is that with this organization of the 3C2-oriI complex, the VPg (3B) primer could not originate from a precursor that binds directly to oriI, as the 3C amino terminus would place VPg too far from the polymerase catalytic site.
FIG. 5.
Model of the picornavirus uridylylation complex. (a) The molecular surface of the PV 3C dimer, colored in gray, is displayed with the key residues involved in RNA binding, revealed from the NMR, mapped onto the surface and colored in black. The melted oriI ssRNA is modeled as tracking around the 3C dimer such that the stmL and stmR regions bind their corresponding sites on the 3C dimer. The templating adenylate residues are shown as sticks. (b) The same view as that in panel a, but with the thumb subdomain (residues 381 to 461) of 3Dpol modeled onto the surface of the 3C dimer (see Shen et al. [38]) and depicted as a red cartoon. The rest of the polymerase in the complex model is removed from the figure for clarity.
Conclusion.
All picornaviruses utilize their 3C protein alone or in the context of precursors for specific recognition of three CREs. Structural studies of 3C and CREs alone have yielded limited information on 3C-CRE interactions. NMR backbone resonance assignments of the PV 3C protein have allowed us to identify the binding sites for stmL and stmR based on residue-specific chemical shift perturbations, providing residue-specific insights into the interactions between 3C and the oriI RNA. Because residues of 3C implicated in binding oriL and oriR only partially overlap those perturbed upon stmR or stmL binding, it follows that RNA recognition by 3C involves unique as well as common sets of interactions. The site-specific interactions with the individual single strands of the oriI stem reveal that 3C can bind to ssRNAs corresponding to stmL and stmR. These observations are consistent with a two-step mechanism for oriI binding to 3C that involves unwinding of the stem and formation of a complex in which the oriI loop is made available for use as a template by the viral RNA-dependent RNA polymerase. This study represents an important step toward development of high-resolution structural models for all 3C-CRE complexes and an understanding of the mechanism employed by 3C to utilize these RNAs to support viral genome replication.
Acknowledgments
This work was supported by a grant from NIH/NIAID (AI053531) to C.E.C.
We thank Christopher J. Falzone (Pennsylvania State University) for helping to initiate this work and Charles Cottrell and Chunhua Yuan (Ohio State University CCIC) for instrument support.
Footnotes
Published ahead of print on 27 February 2008.
REFERENCES
- 1.Andino, R., G. E. Rieckhof, P. L. Achacoso, and D. Baltimore. 1993. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5′-end of viral RNA. EMBO J. 123587-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blair, W. S., J. H. Nguyen, T. B. Parsley, and B. L. Semler. 1996. Mutations in the poliovirus 3CD proteinase S1-specificity pocket affect substrate recognition and RNA binding. Virology 2181-13. [DOI] [PubMed] [Google Scholar]
- 3.Blair, W. S., T. B. Parsley, H. P. Bogerd, J. S. Towner, B. L. Semler, and B. R. Cullen. 1998. Utilization of a mammalian cell-based RNA binding assay to characterize the RNA binding properties of picornavirus 3C proteinases. RNA 4215-225. [PMC free article] [PubMed] [Google Scholar]
- 4.Blattner, F. R., B. G. Williams, A. E. Blechl, K. Denniston-Thompson, H. E. Faber, L. Furlong, D. J. Grunwald, D. O. Kiefer, D. D. Moore, J. W. Schumm, E. L. Sheldon, and O. Smithies. 1977. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science 196161-169. [DOI] [PubMed] [Google Scholar]
- 5.Brown, W. 1993. Dynamic light scattering: the method and some applications. Clarendon Press, Oxford, United Kingdom.
- 6.Cavanagh, J., W. J. Fairbrother, A. G. Palmer III, N. J. Skelton, and M. Rance. 2006. Protein NMR spectroscopy: principles and practice, 2nd ed. Academic Press, San Diego, CA.
- 7.Delaglio, F., S. Grzesiek, G. W. Vuister, G. Zhu, J. Pfeifer, and A. Bax. 1995. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6277-293. [DOI] [PubMed] [Google Scholar]
- 8.Du, Z., J. Yu, N. B. Ulyanov, R. Andino, and T. L. James. 2004. Solution structure of a consensus stem-loop D RNA domain that plays important roles in regulating translation and replication in enteroviruses and rhinoviruses. Biochemistry 4311959-11972. [DOI] [PubMed] [Google Scholar]
- 9.Du, Z., J. Yu, R. Andino, and T. L. James. 2003. Extending the family of UNCG-like tetraloop motifs: NMR structure of a CACG tetraloop from coxsackievirus B3. Biochemistry 424373-4383. [DOI] [PubMed] [Google Scholar]
- 10.Eletsky, A., A. Kienhöfer, and K. Pervushin. 2001. TROSY NMR with partially deuterated proteins. J. Biomol. NMR 20177-180. [DOI] [PubMed] [Google Scholar]
- 11.Foster, M. P., D. S. Wuttke, K. R. Clemens, W. Jahnke, I. Radhakrishnan, L. Tennant, M. Reymond, J. Chung, and P. E. Wright. 1998. Chemical shift as a probe of molecular interfaces: NMR studies of DNA binding by the three amino-terminal zinc finger domains from transcription factor IIIA. J. Biomol. NMR 1251-71. [DOI] [PubMed] [Google Scholar]
- 12.Franco, D., H. B. Pathak, C. E. Cameron, B. Rombaut, E. Wimmer, and A. V. Paul. 2005. Stimulation of poliovirus RNA synthesis and virus maturation in a HeLa cell-free in vitro translation-RNA replication system by viral protein 3CDpro. Virol. J. 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamarnik, A. V., and R. Andino. 2000. Interactions of viral protein 3CD and poly(rC) binding protein with the 5′ untranslated region of the poliovirus genome. J. Virol. 742219-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García de la Torre, J., M. L. Huertas, and B. Carrasco. 2000. HYDRONMR: prediction of NMR relaxation of globular proteins from atomic-level structures and hydrodynamic calculations. J. Magn. Reson. 147138-146. [DOI] [PubMed] [Google Scholar]
- 15.Gill, S. C., and P. H. von Hippel. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182319-326. [DOI] [PubMed] [Google Scholar]
- 16.Gohara, D. W., C. S. Ha, S. Kumar, B. Ghosh, J. J. Arnold, T. J. Wisniewski, and C. E. Cameron. 1999. Production of “authentic” poliovirus RNA-dependent RNA polymerase (3D(pol)) by ubiquitin-protease-mediated cleavage in Escherichia coli. Protein Expr. Purif. 17128-138. [DOI] [PubMed] [Google Scholar]
- 17.Gouvea, I. E., W. A. S. Judice, M. H. S. Cezari, M. A. Juliano, T. Juhász, Z. Szeltner, L. Polgár, and L. Juliano. 2006. Kosmotropic salt activation and substrate specificity of poliovirus protease 3C. Biochemistry 4512083-12089. [DOI] [PubMed] [Google Scholar]
- 18.Hämmerle, T., A. Molla, and E. Wimmer. 1992. Mutational analysis of the proposed FG loop of poliovirus proteinase 3C identifies amino acids that are necessary for 3CD cleavage and might be determinants of a function distinct from proteolytic activity. J. Virol. 666028-6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris, K. S., W. Xiang, L. Alexander, W. S. Lane, A. V. Paul, and E. Wimmer. 1994. Interaction of poliovirus polypeptide 3CDpro with the 5′ and 3′ termini of the poliovirus genome. Identification of viral and cellular cofactors needed for efficient binding. J. Biol. Chem. 26927004-27014. [PubMed] [Google Scholar]
- 20.Headey, S. J., H. Huang, J. K. Claridge, G. A. Soares, K. Dutta, M. Schwalbe, D. Yang, and S. M. Pascal. 2007. NMR structure of stem-loop D from human rhinovirus-14. RNA 13351-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, L., E. V. Sineva, M. R. S. Hargittai, S. D. Sharma, M. Suthar, K. D. Raney, and C. E. Cameron. 2004. Purification and characterization of hepatitis C virus non-structural protein 5A expressed in Escherichia coli. Protein Expr. Purif. 37144-153. [DOI] [PubMed] [Google Scholar]
- 22.Ihle, Y., O. Ohlenschläger, S. Häfner, E. Duchardt, M. Zacharias, S. Seitz, R. Zell, R. Ramachandran, and M. Görlach. 2005. A novel cGUUAg tetraloop structure with a conserved yYNMGg-type backbone conformation from cloverleaf 1 of bovine enterovirus 1 RNA. Nucleic Acids Res. 332003-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller, R. 2004. The Computer Aided Resonance Assignment tutorial. Cantina Verlag, Goldau, Switzerland.
- 24.Leong, L. E., P. A. Walker, and A. G. Porter. 1993. Human rhinovirus-14 protease 3C (3Cpro) binds specifically to the 5′-noncoding region of the viral RNA. Evidence that 3Cpro has different domains for the RNA binding and proteolytic activities. J. Biol. Chem. 26825735-25739. [PubMed] [Google Scholar]
- 25.Marcotte, L. L., A. B. Wass, D. W. Gohara, H. B. Pathak, J. J. Arnold, D. J. Filman, C. E. Cameron, and J. M. Hogle. 2007. Crystal structure of poliovirus 3CD protein: virally encoded protease and precursor to the RNA-dependent RNA polymerase. J. Virol. 813583-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melchers, W. J. G., J. Zoll, M. Tessari, D. V. Bakhmutov, A. P. Gmyl, V. I. Agol, and H. A. Heus. 2006. A GCUA tetranucleotide loop found in the poliovirus oriL by in vivo SELEX (un)expectedly forms a YNMG-like structure: extending the YNMG family with GYYA. RNA 121671-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosimann, S. C., M. M. Cherney, S. Sia, S. Plotch, and M. N. James. 1997. Refined X-ray crystallographic structure of the poliovirus 3C gene product. J. Mol. Biol. 2731032-1047. [DOI] [PubMed] [Google Scholar]
- 28.Nayak, A., I. G. Goodfellow, K. E. Woolaway, J. Birtley, S. Curry, and G. J. Belsham. 2006. Role of RNA structure and RNA binding activity of foot-and-mouth disease virus 3C protein in VPg uridylylation and virus replication. J. Virol. 809865-9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohlenschläger, O., J. Wöhnert, E. Bucci, S. Seitz, S. Häfner, R. Ramachandran, R. Zell, and M. Görlach. 2004. The structure of the stemloop D subdomain of coxsackievirus B3 cloverleaf RNA and its interaction with the proteinase 3C. Structure 12237-248. [DOI] [PubMed] [Google Scholar]
- 30.Parsley, T. B., J. S. Towner, L. B. Blyn, E. Ehrenfeld, and B. L. Semler. 1997. Poly (rC) binding protein 2 forms a ternary complex with the 5′-terminal sequences of poliovirus RNA and the viral 3CD proteinase. RNA 31124-1134. [PMC free article] [PubMed] [Google Scholar]
- 31.Pathak, H. B., J. J. Arnold, P. N. Wiegand, M. R. S. Hargittai, and C. E. Cameron. 2007. Picornavirus genome replication: assembly and organization of the VPg uridylylation ribonucleoprotein (initiation) complex. J. Biol. Chem. 28216202-16213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathak, H. B., S. K. B. Ghosh, A. W. Roberts, S. D. Sharma, J. D. Yoder, J. J. Arnold, D. W. Gohara, D. J. Barton, A. V. Paul, and C. E. Cameron. 2002. Structure-function relationships of the RNA-dependent RNA polymerase from poliovirus (3Dpol). A surface of the primary oligomerization domain functions in capsid precursor processing and VPg uridylylation. J. Biol. Chem. 27731551-31562. [DOI] [PubMed] [Google Scholar]
- 33.Paul, A. V., E. Rieder, D. W. Kim, J. H. van Boom, and E. Wimmer. 2000. Identification of an RNA hairpin in poliovirus RNA that serves as the primary template in the in vitro uridylylation of VPg. J. Virol. 7410359-10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters, H., Y. Y. Kusov, S. Meyer, A. J. Benie, E. Bäuml, M. Wolff, C. Rademacher, T. Peters, and V. Gauss-Müller. 2005. Hepatitis A virus proteinase 3C binding to viral RNA: correlation with substrate binding and enzyme dimerization. Biochem. J. 385363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price, W. S. 1997. Pulsed-field gradient nuclear magnetic resonance as a tool for studying translational diffusion. Part 1. Basic theory. Concepts Magn. Reson. 9299-336. [Google Scholar]
- 36.Rooney, L. M., Sachchidanand, and J. M. Werner. 2004. Characterizing domain interfaces by NMR. Methods Mol. Biol. 278123-138. [DOI] [PubMed] [Google Scholar]
- 37.Salzmann, M., K. Pervushin, G. Wider, H. Senn, and K. Wüthrich. 1998. TROSY in triple-resonance experiments: new perspectives for sequential NMR assignment of large proteins. Proc. Natl. Acad. Sci. USA 9513585-13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen, M., Z. J. Reitman, Y. Zhao, I. Moustafa, Q. Wang, J. J. Arnold, H. B. Pathak, and C. E. Cameron. 2008. Picornavirus genome replication: identification of the surface of the poliovirus (PV) 3C dimer that interacts with PV 3Dpol during VPg uridylylation and construction of a structural model for the PV 3C2-3Dpol complex. J. Biol. Chem. 283875-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shih, S., C. Chiang, T. Chen, C. Wu, J. T. Hsu, J. Lee, M. Hwang, M. Li, G. Chen, and M. Ho. 2004. Mutations at KFRDI and VGK domains of enterovirus 71 3C protease affect its RNA binding and proteolytic activities. J. Biomed. Sci. 11239-248. [DOI] [PubMed] [Google Scholar]
- 40.Thiviyanathan, V., Y. Yang, K. Kaluarachchi, R. Rijnbrand, D. G. Gorenstein, and S. M. Lemon. 2004. High-resolution structure of a picornaviral internal cis-acting RNA replication element (cre). Proc. Natl. Acad. Sci. USA 10112688-12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker, P. A., L. E. Leong, and A. G. Porter. 1995. Sequence and structural determinants of the interaction between the 5′-noncoding region of picornavirus RNA and rhinovirus protease 3C. J. Biol. Chem. 27014510-14516. [DOI] [PubMed] [Google Scholar]
- 42.Wishart, D. S., and B. D. Sykes. 1994. The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J. Biomol. NMR 4171-180. [DOI] [PubMed] [Google Scholar]
- 43.Xiang, W., A. Cuconati, D. Hope, K. Kirkegaard, and E. Wimmer. 1998. Complete protein linkage map of poliovirus P3 proteins: interaction of polymerase 3Dpol with VPg and with genetic variants of 3AB. J. Virol. 726732-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin, J., A. V. Paul, E. Wimmer, and E. Rieder. 2003. Functional dissection of a poliovirus cis-acting replication element [PV-cre(2C)]: analysis of single- and dual-cre viral genomes and proteins that bind specifically to PV-cre RNA. J. Virol. 775152-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]







