Abstract
Alveolar macrophages constitutively reside in the respiratory tracts of pigs and humans. An in vivo role of alveolar macrophages in defending against influenza viruses in mice infected with a reassorted influenza virus, 1918 HA/NA:Tx/91, was reported, but there has been no report on an in vivo role of alveolar macrophages in a natural host such as a pig using currently circulating human influenza virus. Here we show that in vivo depletion of alveolar macrophages in pigs by dichloromethylene diphosphonate (MDPCL2) treatment results in 40% mortality when pigs are infected with currently circulating human H1N1 influenza viruses, while none of the infected control pigs died. All infected pigs depleted of alveolar macrophages suffered from more severe respiratory signs than infected control pigs. Induction of tumor necrosis factor alpha in the infected pigs depleted of alveolar macrophages was significantly lower than that in the lungs of infected control pigs, and the induction of interleukin-10, an immunosuppressive cytokine, significantly increased in the lungs of infected pigs depleted of alveolar macrophages compared to infected control pigs. When we measured antibody titers and CD8+ T lymphocytes expressing gamma interferon (IFN-γ), lower antibody titers and a lower percentage of CD8+ T lymphocytes expressing IFN-γ were detectable in MDPCL2-treated infected pigs than in phosphate-buffered saline- and liposome-treated and infected pigs. Taken together, our findings suggest that alveolar macrophages are essential for controlling H1N1 influenza viruses in pigs.
Influenza viruses cause considerable morbidity and mortality in humans worldwide. Influenza viruses mainly infect epithelial cells in the respiratory tracts and macrophages in humans and animals (3, 14, 28). Each year, influenza viruses are responsible for more than 30,000 excess deaths and about 250,000 hospitalizations in the United States alone (29). Influenza viruses cause an acute infection in humans, with clinical signs of chills, fever, aches and pains in the body, fatigue, sore throat, and nasal congestion. Gastrointestinal signs such as vomiting, abdominal pain, and diarrhea sometimes occur in humans infected with influenza viruses (8, 9, 31).
Influenza-caused pathological signs might be due to the combination of direct effects of viral replication on the infected tissue and the effects of the host's response to infection by inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) produced by alveolar macrophages in the lungs in humans and animals (17). Previous studies showed that influenza A virus-infected macrophages produce proinflammatory cytokines, such as TNF-α, interleukin-1 (IL-1), IL-6, and alpha/beta interferon (IFN-α/β), and CC chemokines (14). In a study using cultured human macrophages derived from monocytes, the highly pathogenic H5N1 influenza viruses induced a larger amount of TNF-α in the cultured macrophages than human H1N1 or H3N2 influenza viruses (5). An in vivo study of pigs using human influenza viruses showed that alveolar macrophages in pigs infected with human H3N2 influenza viruses produced a larger amount of TNF-α than alveolar macrophages in pigs infected with human H1N1 influenza viruses (28).
Diphosphonates are drugs originally developed for treating diseases of bones, teeth, and calcium metabolism (10). Among various diphosphonates, dichloromethylene diphosphonate (MDPCL2) is cytotoxic to alveolar macrophages when administered as a liposome-encapsulated drug (7, 30, 32, 33, 36, 37). Liposome-encapsulated MDPCL2 specifically eliminated alveolar macrophages in lungs of mice by causing apoptosis (7, 33).
Pigs have been suggested as “mixing vessels” for the creation of pandemic influenza viruses, since they are readily infected with avian and human influenza viruses (13, 19, 27). It was suggested that two pandemic influenza viruses, H2N2 in 1957 and H3N2 in 1968, were created in pigs infected with avian and human influenza viruses (26). The molecular basis of reassortment of avian and human influenza viruses in pigs may be the presence of both avian and human influenza virus receptors in their tracheas (16), even though a recent study suggests that avian influenza viruses do not attach well to the tracheal epithelium of pigs (35).
Until now, study of the in vivo role of alveolar macrophages in controlling influenza viruses using MDPCL2 was performed only in mice (34, 38). That study, using reassorted H1N1 virus containing 1918 HA/NA:Tx/91, showed that alveolar macrophages could play a role in controlling the replication and spread of the 1918 HA/NA:Tx/91 virus in mice (34). Mice are not regarded as a natural host for influenza viruses, since influenza viruses are not circulating in mice. In this study, using pigs, an important natural host for influenza virus, and currently circulating human H1N1 influenza viruses (28), we wanted to examine the in vivo role of alveolar macrophages in controlling influenza viruses by depleting alveolar macrophages in pigs by MDPCL2 treatment and to determine whether depletion of alveolar macrophages in pigs would affect the profiles of inflammatory cytokines compared to those in control infected pigs.
MATERIALS AND METHODS
Viruses.
Currently circulating human H1N1 (A/New Caledonia/20/99) influenza viruses were propagated in the amniotic cavities of 10-day-old specific-pathogen-free eggs before used for pathogenesis study. We used human H1N1 influenza virus since we hoped to explain the role of alveolar macrophages in human influenza infections using a pig model.
Animals.
Six-week-old pigs that were serologically negative by enzyme-linked immunosorbent assay (ELISA) for exposure to porcine respiratory and reproductive syndrome viruses, Mycoplasma hyopneumoniae, Pasteurella multocida, Bordetella bronchiseptica, Actinobacillus pleuropneumoniae, and H1 and H3 influenza viruses were used in this study. Optical density values were regarded as negative when they were less than twofold higher than that of a phosphate-buffered saline (PBS) control. The animal use committee at Chungnam National University approved the experimental protocols. All animal experiments were performed at the biosafety level 2 facility at Chungnam National University.
Depletion of alveolar macrophages in the lungs of pigs.
To deplete alveolar macrophages in the lungs of pigs, we used liposome-encapsulated MDPCL2, which could selectively deplete alveolar macrophages in lungs (36). MDPCL2 and control (PBS)-liposomes were prepared as described earlier (37). We depleted alveolar macrophages as described previously (7) with some modifications. Six-week-old pigs were intramuscularly (i.m.) anesthetized with Zoletil (2 mg/kg) (Virbac Laboratories, Carros, France) and were intranasally (i.n.) administered 5 ml of MDPCL2 or PBS-liposome for 3 days before pigs were infected with 2 ml of 104 log10 50% egg infectious doses (EID50) of human H1N1 (A/New Caledonia/20/99) influenza virus. When we tried intratracheal administration of MDPCL2 to deplete alveolar macrophages in pigs, it was not successful. Therefore, we used the i.n. route to deplete alveolar macrophages. To deliver MDPCL2 into alveoli of pigs' lungs, we quickly closed the mouths and noses of pigs after i.n. administration of MDPCL2 to pigs.
To determine the efficiency of depletion of alveolar macrophages in lungs of pigs, bronchoalveolar lavage (BAL) and immunofluorescence assay using antibody specific for procine alveolar macrophages were performed. Pigs were euthanized with a high dose of Zoletil, and the lungs with respiratory tracts (trachea and bronchus) were collected from the pigs. The collected lungs were lavaged with 50 ml of PBS (pH 7.4). The lavaged samples were centrifuged at 800 × g for 10 min to collect cells. The collected cells were resuspended with 5 ml of PBS (pH 7.4), and 100 μl of the resuspended cells was cytospun before immunofluorescence assay was performed with mouse anti-porcine macrophage (Serotech, Oxford, United Kingdom) and fluorescein isothiocyanate (FITC)-labeled anti-mouse secondary antibody. Alveolar macrophages stained with antibody specific for porcine alveolar macrophages were counted under a fluorescence microscope (Olympus DP70) (Olympus Corporation, Tokyo, Japan).
For leukocytes, Wright staining was performed. BAL samples were cytospun on glass slides and were allowed to air dry. The dried samples were fixed by immersing them in methanol for 5 min before Wright staining solution (Muto Pure Chemicals Co., Tokyo, Japan) was dropped on the slide. The slide was incubated for 2 min, and then Wright staining buffer was dropped on it. After 5 minutes of incubation at room temperature, the glass slide was rinsed with distilled water before cells were counted under a light microscope.
Counting bacteria in BAL samples.
Pig BAL samples were tested for bacterial infection. Serial 10-fold dilutions of BAL samples from each group (days 0, 5, 9, and 14 postinfection [p.i.]) were transferred to a MacConkey agar plate, a tryptic soy agar plate, or blood agar. Two individual bacteriological loops were plated from each sample, and the number of CFU was determined at 72 h after incubation (37°C).
Observation of clinical signs in pigs.
Pigs depleted of alveolar macrophages by MDPCL2 or not depleted of alveolar macrophages by use of PBS-liposomes (10 pigs per group) were sedated with Zoletil (2 mg/kg) before they were i.n. infected with 2 ml of 104 EID50 of human H1N1 (A/New Caledonia/20/99) influenza virus. Body weight, rectal temperature, and mortality in the infected pigs were observed daily for clinical signs of infection for 14 days after infection.
Titration of virus in the lungs of pigs.
One-gram portions of the left cranial lobes of lungs of pigs infected with 2 ml of 104 EID50 of human H1N1 (A/New Caledonia/20/99) influenza virus were homogenized using a Daihan WiseStir HS-30E homogenizer (Daihan Co., Seoul, Korea) and frozen and thawed three times in 1 ml of PBS (pH 7.4) supplemented with 2× antibiotic-antimycotic solution (Sigma, St. Louis, MO). The prepared samples of lungs were serially 10-fold diluted in PBS (pH 7.4) before each diluted sample was inoculated into 10-day-old embryonated eggs. The presence of virus in the inoculated eggs was determined by hemagglutination assay using 0.5% turkey red blood cells. Viral titers were determined by log10 EID50/ml as described previously (24).
Histopathology staining.
Pigs uninfected or infected with 2 ml of 104 EID50 of human H1N1 (A/New Caledonia/20/99) influenza virus were euthanized with a high dose of Zoletil before the left cranial lobes of lungs were collected and inflated with formalin. The lung tissues were fixed by submerging them in 10% neutral buffered formalin and embedding them in paraffin. Five-micrometer sections were made before they were stained with hematoxylin and eosin (H&E) as described previously (2). The stained tissue sections were evaluated under an Olympus DP70 microscope (Olympus Corporation, Tokyo, Japan).
Immunohistochemistry staining.
Pigs uninfected or infected with 2 ml of 104 EID50 of human H1N1 (A/New Caledonia/20/99) influenza virus were euthanized with high dose of Zoletil, and then the left cranial lobes of lungs were collected. The collected lung tissues were fixed using 10% neutral-buffered formalin for 24 h and then embedded in paraffin. Five-micrometer sections were made and stained with mouse influenza A virus antinucleoprotein antibody (Serotech, United Kingdom). Tissue sections were deparaffinized and hydrated in distilled water. Sections were fixed with 100% chilled acetone for 2 h for permeabilization, and endogenous peroxidase activity was blocked by incubating tissue sections in 3% H2O2 for 15 min at 37°C before the sections were blocked with 5% bovine serum albumin in PBS (pH 7.4) for 1 h. The blocked tissue sections were labeled with mouse influenza A virus antinucleoprotein antibody (1:1,000 dilution) by incubating at room temperature for 1 h. The labeled tissue sections were stained with peroxidase-labeled goat anti-mouse immunoglobulin(KPL, Gaithersburg, MD) and with stable diaminobenzidine peroxidase substrate (KPL, Gaithersburg, MD, USA). The stained tissue sections were counterstained with hematoxylin QS (Vector Laboratories, Burlingame, CA) before the stained sections were evaluated under an Olympus DP70 microscope (Olympus Corporation, Tokyo, Japan).
Quantification of inflammatory cytokines in lungs by ELISA.
Pigs uninfected or infected with 2 ml of 104 EID50 of human H1N1 (A/New Caledonia/20/99) influenza virus were euthanized with high dose of Zoletil, and then the left cranial lobes of lungs were collected. One gram of left cranial lobes of lungs was suspended in 1 ml of PBS (pH 7.4) before being disrupted by homogenizing using a Daihan WiseStir HS-30E homogenizer (Daihan Co., Seoul, Korea) and freezing and thawing three times. The disrupted cells were centrifuged at 14,000 × g for 10 min. The supernatants were collected and stored at −70°C before analysis of cytokines using porcine cytokine ELISA kits. The ELISA kits specific for porcine IL-4, IL-10, and IFN-γ were purchased from Biosource Inc., and that used for porcine TNF-α was purchased from R&D Systems. The cytokine detections were performed according to the manufacturers' instructions. The quantity of the cytokines was calculated and plotted based on a standard curve for each cytokine.
Determination of antibody titers by HI assay.
Sera from MDPCL2-treated or untreated infected pigs were collected until 14 days p.i. Sera were treated with receptor-destroying enzyme (Denka Seiken, Tokyo, Japan) before serial dilution was performed in PBS (pH 7.4). The serially diluted sera were reacted with 8 HA units of A/New Caledonia/20/99 (H1N1) for 15 min before turkey red blood cells (0.5%) were added. Hemagglutination inhibition (HI) titers were determined at 30 min after incubation.
Intracellular staining of CD8+ T lymphocytes expressing IFN-γ.
MDPCL2-treated or untreated pigs were infected with 2 ml of 104 EID50 of human H1N1 (A/New Caledonia/20/99) influenza virus, and BAL samples were collected until 14 days p.i. Lymphocytes were isolated by centrifuging (800 × g, 30 min) with Histopaque (Sigma, MO). Alveolar macrophages were depleted by incubating cells in a flask for 2 h with RPMI medium supplemented with 10% fetal bovine serum and penicillin-streptomycin. The purified lymphocytes (106) were stimulated with human H1N1 influenza virus (A/New Caledonia/20/99) (multiplicity of infection, 0.1) at 37°C for 4 h, and then brefeldin A (Sigma, MO) was added at a final concentration of 1 μl/ml. After overnight incubation, the cells were washed twice with cold PBS (pH 7.4) by spinning at 800 × g for 10 min. The washed cells were fixed with 0.5 ml of cold 4% paraformaldehyde by incubating at room temperature for 10 min. The fixed cells were incubated for 10 min at room temperature in 2 ml of SAP buffer containing PBS with 0.5% bovine serum albumin and 0.2% saponin (Sigma, MO) for permeabilization. Permeabilized cells were incubated with 200 μl of SAP buffer containing 10 μl of phycoerythrin-labeled rabbit anti-porcine IFN-γ antibody (R&D Systems, MN) and FITC-labeled mouse anti-porcine CD8 antibody (Seotech, NC) for 30 min at room temperature. Stained lymphocytes were measured by flow cytometry (FACSCalibur; Becton Dickinson).
Statistical analysis.
The statistical significances of macrophage depletion, viral titers, and cytokines were determined by a two-tailed, paired Student t test. A P value of less than 0.05 was regarded as significant. We compared data for pigs depleted of alveolar macrophages by MDPCL2 with those of pigs not depleted of alveolar macrophages and given PBS-liposomes.
RESULTS
Efficiency of depletion of alveolar macrophages by MDPCL2 treatment.
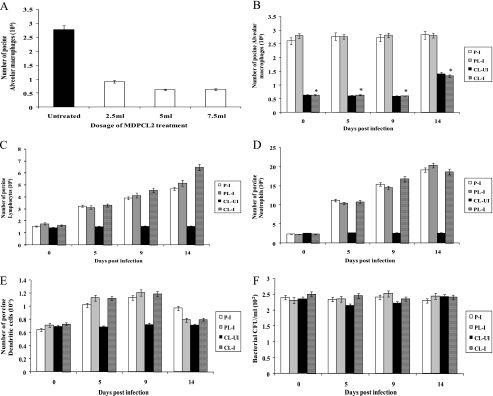
We were interested in finding out the role of alveolar macrophages in controlling influenza virus infection by depleting alveolar macrophages in the lungs of pigs by MDPCL2 treatment. The efficiency of depletion of alveolar macrophages in the lungs of pigs by MDPCL2 was determined before the in vivo role of alveolar macrophages in controlling human H1N1 influenza virus infection was studied (Fig. 1A). Pigs were treated daily with different doses of MDPCL2 for 3 days before alveolar macrophages were collected. The average number of alveolar macrophages in lungs of untreated pigs was 2.78 × 106, while the average number of alveolar macrophages in lungs of pigs treated with 2.5 ml, 5 ml, and 7.5 ml of MDPCL2 was 0.91 × 106, 0.62 × 106, and 0.63 × 106, respectively. The results suggest that the efficiencies of depletion of alveolar macrophages by 5 ml or 7.5 ml of MDPCL2 are similar. Therefore, we used 5 ml of MDPCL2 throughout this study.
FIG. 1.
Efficiency of depletion of alveolar macrophages in the lungs of pigs by MDPCL2 treatment. (A) Determination of MDPCL2 dosage. Six-week-old pigs were i.m. anesthetized with Zoletil (2 mg/kg) and were daily i.n. administered 2.5 ml, 5 ml, or 7.5 ml of MDPCL2. Three pigs per time point were euthanized, and BAL was performed with 50 ml of PBS (pH 7.4). The lavaged samples were cytospun and stained with mouse anti-porcine macrophage antibody and FITC-labeled goat anti-mouse secondary antibody. Alveolar macrophages stained with antibody specific for porcine alveolar macrophages were counted under a fluorescence microscope. Data are the means for three pigs ± standard errors per time point. (B) Number of alveolar macrophages in MDPCL2-treated infected pigs. Six-week-old pigs were i.m. anesthetized with Zoletil (2 mg/kg) and were daily i.n. administered 5 ml of MDPCL2 or PBS-liposomes for 3 days before being infected with 2 ml of 104 EID50 of human H1N1 (A/New Caledonia/20/99) influenza virus. Three pigs per time point were euthanized, and BAL was performed with 50 ml of PBS (pH 7.4). The lavaged samples were cytospun and stained with mouse anti-porcine macrophage antibody and FITC-labeled goat anti-mouse secondary antibody. Alveolar macrophages stained with antibody specific for porcine alveolar macrophages were counted under a fluorescence microscope. Data are the means for three pigs ± standard errors per time point. We statistically compared group 1-4 with group 1-2. *, P < 0.05. P-I (group 1-1), PBS-treated infected pigs; PL-I (group 1-2), PBS-liposome-treated infected pigs; CL-UI (group 1-3), MDPCL2-treated uninfected pigs; CL-I (group 1-4), MDPCL2-treated infected pigs. (C) Number of porcine lymphocytes in MDPCL2-treated infected pigs. Lymphocytes were counted by Wright staining using the same samples as for panel B. Data are the means for three pigs ± standard errors per time point. (D) Number of neutrophils in MDPCL2-treated infected pigs. Neutrophils were counted by Wright staining using the same samples as for panel B. Data are the means for three pigs ± standard errors per time point. (E) Number of dendritic cells in MDPCL2-treated infected pigs. Dendritic cells were counted by Wright staining using the same samples as for panel B. Data are the means for three pigs ± standard errors per time point. (F) Number of bacteria in MDPCL2-treated infected pigs. Bacteria were counted using a MacConkey agar plate, a tryptic soy agar plate, or blood agar and the same samples as for panel B. Data are the means for three pigs ± standard errors per time point.
Six-week-old pigs (12 pigs per group) were i.m. anesthetized with Zoletil (2 mg/kg) and were i.n. inoculated daily with 5 ml of MDPCL2 or PBS-liposomes for 3 days before being infected with 2 ml of 104 EID50/ml of human H1N1 (A/New Caledonia/20/99) influenza virus. The alveolar macrophages in the lungs of infected pigs treated with MDPCL2 for 3 days prior to infection (group 1-4) were depleted up to 77% (0.63 × 106) (P < 0.05) compared to those in the lungs of PBS-liposome-treated and infected pigs (group 1-2) (2.8 × 106). The levels of alveolar macrophages in the lungs of MDPCL2-treated uninfected pigs (group 1-3) were 0.62 × 106, and those in the lungs of PBS-treated infected pigs (group 1-1) were 2.61 × 106 (Fig. 1B). When pigs were infected with human H1N1 influenza virus, the number of depleted alveolar macrophages in group 1-3 and group 1-4 did not return to that of alveolar macrophages in group 1-1 or group 1-2 (P < 0.05) until 9 days or 14 days p.i. The results suggest that MDPCL2 treatment could efficiently deplete alveolar macrophages in the lungs of pigs in vivo.
When we compared lymphocytes, neutrophils, and dendritic cells in BAL samples of pigs, the numbers of lymphocytes and neutrophils among group 1-1, group 1-2, group 1-3, and group 1-4 were similar before infection (Fig. 1C, D, and E). The numbers of lymphocytes, neutrophils, and dendritic cells after infection with human H1N1 influenza virus increased in BAL samples of pigs in group 1-1, group 1-2, and group 1-4 until 14 days p.i. The results suggest that MDPCL2 treatment does not deplete lymphocytes, neutrophils, and dendritic cells in the lungs of pigs.
We also counted bacteria in BAL samples. The numbers of bacteria in lungs of pigs in group 1-1, group 1-2, group 1-3, and group 1-4 were similar until 14 days p.i. (Fig. 1F).
Clinical signs in alveolar macrophage-depleted and infected pigs.
To investigate the role of alveolar macrophages in the lungs, pigs (10 pigs per group) depleted of alveolar macrophages by MDPCL2 treatment or pigs not depleted of alveolar macrophages were i.n. infected with 2 ml of 104 EID50 of human H1N1 (A/New Caledonia/20/99) influenza virus. Clinical signs, survival rates, body temperatures, body weights, and lung images were observed until 14 days p.i., the last day that we monitored. Infected pigs in group 2-1 and group 2-2 showed minor clinical signs such as sneezing, nasal rattling, and lethargy until 6 days p.i., while infected pigs in group 2-4 showed severe clinical signs such as difficulty breathing (dyspnea), emaciation showing spinal cords, and ruffled fur together with minor clinical signs such as sneezing, nasal rattling, and lethargy (Table 1). On day 14 p.i., all six surviving infected pigs in group 2-4 showed the clinical signs of sneezing, nasal rattling, dyspnea, lethargy, emaciation, and ruffled fur, but pigs in group 2-1, group 2-2, group 2-3, and group 2-5 (MDPCL2-treated and uninfected) did not show any clinical signs.
TABLE 1.
Clinical signs in pigs infected with human H1N1 (A/New Caledonia/20/99) influenza virus
| Clinical sign | No. of pigs showing sign/total at day p.i.a:
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3
|
6
|
9
|
14
|
|||||||||||||||||
| UI | CL-UI | P-I | PL-I | CL-I | UI | CL-UI | P-I | PL-I | CL-I | UI | CL-UI | P-I | PL-I | CL-I | UI | CL-UI | P-I | PL-I | CL-I | |
| Sneezing | 0/10 | 0/10 | 3/10 | 4/10 | 9/10 | 0/10 | 0/10 | 3/10 | 4/10 | 10/10 | 0/10 | 0/10 | 2/10 | 2/10 | 10/10 | 0/10 | 0/10 | 0/10 | 0/10 | 6/6 |
| Nasal rattling | 0/10 | 0/10 | 3/10 | 3/10 | 7/10 | 0/10 | 0/10 | 3/10 | 4/10 | 9/10 | 0/10 | 0/10 | 1/10 | 2/10 | 10/10 | 0/10 | 0/10 | 0/10 | 0/10 | 6/6 |
| Dyspnea | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 3/10 | 0/10 | 0/10 | 0/10 | 0/10 | 4/10 | 0/10 | 0/10 | 0/10 | 0/10 | 6/6 |
| Lethargy | 0/10 | 0/10 | 0/10 | 0/10 | 2/10 | 0/10 | 0/10 | 1/10 | 2/10 | 4/10 | 0/10 | 0/10 | 0/10 | 0/10 | 5/10 | 0/10 | 0/10 | 0/10 | 0/10 | 6/6 |
| Emaciation | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 5/10 | 0/10 | 0/10 | 0/10 | 0/10 | 10/10 | 0/10 | 0/10 | 0/10 | 0/10 | 6/6 |
| Ruffled fur | 0/10 | 0/10 | 0/10 | 0/10 | 2/10 | 0/10 | 0/10 | 0/10 | 0/10 | 7/10 | 0/10 | 0/10 | 0/10 | 0/10 | 10/10 | 0/10 | 0/10 | 0/10 | 0/10 | 6/6 |
P-I (group 2-1), PBS-treated infected pigs; PL-I (group 2-2), PBS-liposome-treated infected pigs; CL-UI (group 2-3), MDPCL2-treated uninfected pigs; CL-I (group 2-4), MDPCL2-treated infected pigs; UI (group 2-5), untreated and uninfected pigs.
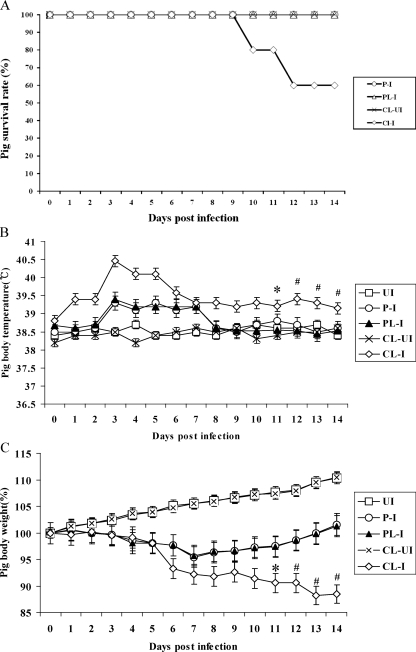
When we monitored the survival rates of pigs (Fig. 2A), 2 out of 10 pigs in group 3-4 died at 10 days p.i., and an additional 2 pigs died at 12 days p.i., while none of the pigs in group 3-1, group 3-2, and group 3-3 died. We also rectally measured body temperatures until 14 days p.i. (Fig. 2B). Pigs in group 3-4 had fevers over 39°C until 14 days p.i., whereas pigs in group 3-1 and group 3-2 had fevers over 39°C only until 7 days p.i., and then their body temperatures returned to normal (under 39°C) like for the uninfected pigs in group 3-3 and group 3-5. We measured the pigs' body weights until 14 days p.i. (Fig. 2C). Pigs in group 3-4 lost up to 11% of their original weights until 14 days p.i., but pigs in group 3-1 and group 3-2 lost up to 4.5% of their original weights until 7 days p.i. and then started to gain weight until 14 days p.i. The uninfected pigs in group 3-3 and group 3-5 did not lose initial weight and gained weights until 14 days p.i.
FIG. 2.
Clinical signs in pigs depleted of alveolar macrophages by MDPCL2 treatment. MDPCL2-treated pigs or PBS-liposome-treated pigs were i.n. infected with 2 ml of 104 EID50 of human H1N1 (A/New Caledonia/20/99) influenza virus. Clinical signs such as survival rates, change of body temperatures, change of pig body weights, and change of lung images were observed daily for 14 days after infection. This experiment was independent from the one described for Fig. 1. (A) Survival rates of pigs. Two MDPCL2-treated and infected pigs died at 10 days p.i., and an additional two MDPCL2-treated and infected pigs died at 12 days p.i. P-I (group 3-1), PBS-treated infected pigs; PL-I (group 3-2), PBS-liposome-treated infected pigs; CL-UI (group 3-3), MDPCL2-treated uninfected pigs; CL-I (group 3-4), MDPCL2-treated infected pigs. (B) Change of body temperature in pigs. Pig body temperatures were measured rectally. Data are the means for 10 pigs ± standard errors per time point, except at 10 and 12 days p.i., when two pigs at each time point died. *, mean for eight pigs ± standard errors; #, mean for six pigs ± standard errors. UI (group 3-5), untreated and uninfected pigs. (C) Change of body weight in pigs. Data are the means for 10 pigs ± standard errors per time point, except at 10 and 12 days p.i., when two pigs at each time point died. *, mean for eight pigs ± standard errors; #, mean for six pigs ± standard errors.
Determination of viral titers and tissue staining of alveolar macrophage-depleted and infected pigs.
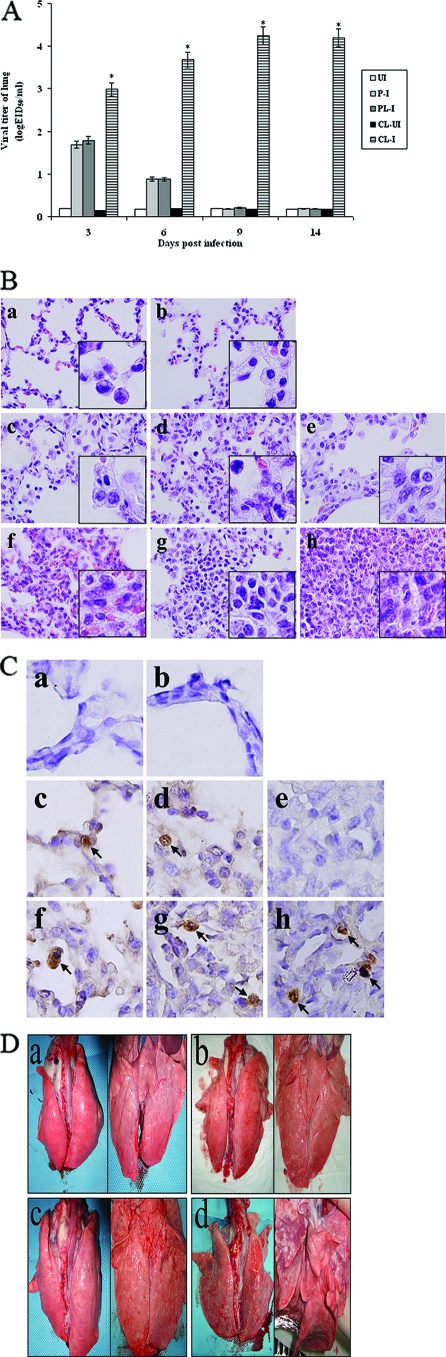
We performed another independent experiment to measure viral titers in lungs, to stain lung tissues using H&E, and to stain lung tissues using antibody specific for influenza A virus nucleoprotein. Twenty pigs per group were infected with 2 ml of 104 EID50 of human H1N1 (A/New Caledonia/20/99) influenza virus, and lung tissues were taken from three surviving pigs at each time point after pigs were euthanized with high doses of Zoletil. Eight pigs died until 14 days p.i. When we measured viral titers in lungs (Fig. 3A), influenza viruses were detected in the lungs of pigs in group 4-2 at 3 days p.i. (1.79 log10 EID50/ml) and 6 days p.i. (0.88 log10 EID50/ml), but viruses were not detected at either 9 days p.i or 14 days p.i. (<0.5 log10 EID50/ml). In the lungs of pigs in group 4-4, considerable viral titers (P < 0.05) were detected until 14 days p.i. (4.195 log10 EID50/ml), when all surviving pigs were euthanized with high doses of Zoletil because of severe illness. No virus was detectable in the control pigs of group 4-3 and group 4-5.
FIG. 3.
Viral titers, cytokine responses, and histopathological signs in the lungs of pigs. Pigs (20 pigs per group) were i.n. infected with 2 ml of 104 EID50 of human H1N1 (A/New Caledonia/20/99) influenza virus. Three pigs per time point were euthanized before the left cranial lobes of lungs were collected for determining viral titers, cytokine responses, and histopathology. This experiment was independent from those described for Fig. 1 and Fig. 2. (A) Viral titers in the lungs of pigs. Viral titers were determined by log10 EID50/ml. Data are the means for three pigs ± standard errors per time point. We statistically compared group 4-4 with group 4-2. *, P < 0.05. P-I (group 4-1), PBS-treated infected pigs; PL-I (group 4-2), PBS-liposome-treated infected pigs; CL-UI (group 4-3), MDPCL2-treated uninfected pigs; CL-I (group 4-4), MDPCL2-treated infected pigs; UI (group 4-5), untreated and uninfected pigs. (B) Staining of lungs of pigs with H&E (magnification, ×400). The left cranial lobes of lungs were cut by 5 micrometers and were stained with H&E. a, lung of an untreated and uninfected pig (group 4-5); b, lung of an MDPCL2-treated and uninfected pig (group 4-3); c to e, lungs of PBS-liposome-treated and infected pigs (group 4-2) at 3, 6, and 9 days p.i., respectively; f to h, lungs of MDPCL2-treated and infected pigs (group 4-4) at 3, 6, and 9 days p.i., respectively. The insets show magnifications of ×1,000. (C) Immunohistochemical staining of lungs of pigs using influenza A virus antinucleoprotein antibody (magnification, ×1,000). The left cranial lobes of lungs were cut by five micrometers and were stained with mouse influenza A virus antinucleoprotein, peroxidase-labeled goat anti-mouse immunoglobulin, and with stable diaminobenzidine peroxidase substrate. The stained tissue sections were counterstained with hematoxylin before they were evaluated under the microscope. a, lung of an untreated and uninfected pig (group 4-5); b, lung of an MDPCL2-treated and uninfected pig (group 4-3); c to e, lungs of PBS-liposome-treated and infected pigs (group 4-2) at 3, 6, and 9 days p.i.; f to h, lungs of MDPCL2-treated and infected pigs (group 4-4) at 3, 6, and 9 days p.i. Arrows, cells positive for influenza A virus nucleoprotein protein. (D) Photographs of lungs of pigs. Lungs of pigs were photographed at 9 days p.i. after surviving pigs were euthanized. a, lung of an untreated and uninfected pig (group 4-5); b, lung of an MDPCL2-treated and uninfected pig (group 4-3); c, lung of a PBS-liposome-treated and infected pig (group 4-2); d, lung of an MDPCL2-treated and infected pig (group 4-4). Left, dorsal position; right, ventral position.
When we analyzed lung tissues by H&E staining, the lung tissues of pigs in group 4-2 showed alveolitis with inflammation (Fig. 3B, panels c and d) until 6 days p.i., and lung tissues (Fig. 3B, panel e) returned to the status of lung of control pigs in group 4-5 and group 4-3 (Fig. 3B, panels a and b) by 9 days p.i., while the lung tissues of pigs in group 4-4 had severe interstitial pneumonia with infiltration of inflammatory cells and atelectasis in alveoli (Fig. 3B, panels f, g, and h).
To detect antigens of influenza viruses in alveoli in lungs of pigs, immunohistochemical staining was performed using antibody specific for influenza A virus nucleoprotein. In the lungs of pigs in group 4-2, positive staining of influenza virus was observed in alveoli until 6 days p.i., (Fig. 3C, panels c and d), and no positive staining of influenza viruses in alveoli was detected at 9 days p.i. (Fig. 3C, panel e). In alveoli in the lungs of pigs in group 4-4, positive staining was observed until 9 days p.i. (Fig. 3C, panels f, g, and h). No positive staining was observed in alveoli in the lungs of control pigs in group 4-5 and group 4-3 (Fig. 3C, panels a and b).
When we grossly observed the lungs of pigs at 9 days p.i., the lungs of pigs in group 4-2 (Fig. 3D, panel c) appeared normal like those of control pigs in group 4-5 and group 4-3 (Fig. 3D, panels a and b), but the lungs of pigs in group 4-4 showed signs of severe pneumonia (Fig. 3D, panel d). At 3 and 6 days p.i., the lungs of pigs in group 4-4 showed mild pneumonia and at 12 and 14 days p.i., the lungs of pigs in group 4-4 showed severe pneumonia (data not shown).
Modulation of inflammatory cytokines in alveolar macrophage-depleted and infected pigs.
It has been suggested that inflammatory responses are responsible for the lung pathology elicited by influenza viruses (5, 28). To determine whether depletion of alveolar macrophages by MDPCL2 treatment would affect the induction of inflammatory cytokines in lungs of pigs infected with human H1N1 influenza virus, we measured TNF-α, IFN-γ, IL-10, and IL-4 in the left cranial lobes of lungs from the same pigs used for Fig. 3. We measured these cytokines since they are involved in inflammatory responses in lungs (28). The Th1 cytokines TNF-α and IFN-γ were induced to lower levels in the lungs of pigs in group 4-4 than in the lungs of pigs in group 4-2 (Fig. 4A and B), and Th2 cytokine such as IL-10 (23) were induced to higher levels in the lungs of pigs in group 4-4 than in the lungs of pigs in group 4-2 (Fig. 4C). In the lungs of pigs in group 4-2, the induction of TNF-α peaked at 6 days p.i. (1,519.7 pg/ml) and then declined until 14 days p.i. (588.9 pg/ml), while in the lungs of pigs in group 4-4, the amount of induced TNF-α was 369.1 pg/ml (P < 0.001) at 6 days p.i. and was similar until 14 days p.i. (347 pg/ml). The amount of TNF-α in the lungs of control pigs in group 4-3 and group 4-5 was less than 90 pg/ml (Fig. 4A). When we measured the amount of induced IFN-γ (Fig. 4B), it was less than 10 pg/ml in the lungs of pigs in group 4-4 (P < 0.001). However, the amount of induced IFN-γ in the lungs of pigs in group 4-2 peaked at 3 days p.i. (312.2 pg/ml) and then declined until 14 days p.i. (85.5 pg/ml). The immune suppressive cytokines such as IL-10 and IL-4 were also measured in the lungs of pigs (Fig. 4C and D). IL-10 induction peaked in the lungs of pigs in group 4-4 at 9 days p.i. (200.36 pg/ml), while its induction peaked in the lungs of pigs in group 4-2 at 6 days p.i (64.88 pg/ml). IL-10 induction in the lungs of control pigs in group 4-3 and group 4-5 was less than 10 pg/ml (Fig. 4C). The amount of induced IL-4 in the lungs of pigs in group 4-1, group 4-2, and group 4-4 was very low (less than 8 pg/ml), like that in pigs in group 4-3 and group 4-5 (less than 9 pg/ml) (Fig. 4D). The results suggest that alveolar macrophages play an important role in cytokine production in influenza virus-infected porcine lungs and that the increased induction of IL-10 in lungs is correlated with severe alveolitis in lungs of pigs.
FIG. 4.
Quantification of inflammatory cytokines in lungs. One-gram portions of left cranial lobes of lungs from the same pigs per time point described in Fig. 3 were suspended in 1 ml of PBS (pH 7.4) before being disrupted by homogenizing and then freezing and thawing three times. The supernatants from the disrupted tissues were collected to analyze the porcine inflammatory cytokines TNF-α (A), IFN-γ (B), IL-10 (C), and IL-4 (D) using porcine cytokine-specific ELISA kits. The quantity of cytokines was calculated and plotted based on a standard curve for each cytokine. Data are the mean for three pigs ± standard errors per time point. We statistically compared group 4-4 with group 4-2. **, P < 0.001; *, P < 0.05. P-I (group 4-1), PBS-treated infected pigs; PL-I (group 4-2), PBS-liposome-treated infected pigs; CL-UI (group 4-3), MDPCL2-treated uninfected pigs; CL-I (group 4-4), MDPCL2-treated infected pigs; UI (group 4-5), untreated and uninfected pigs.
Modulation of antibody production and IFN-γ-expressing T cells in pigs depleted of alveolar macrophages.
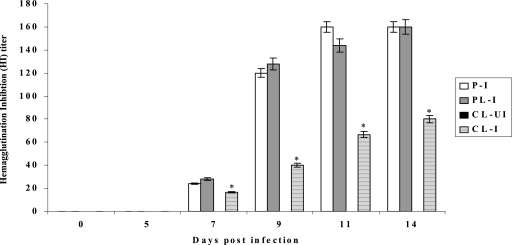
It is known that macrophages can perform immunoregulatory functions such as cytokine production and antigen presentation (15). We wanted to know whether depletion of alveolar macrophages in pigs would affect the production of antibody and the induction of CD8+ T cells expressing IFN-γ in pigs infected with human H1N1. Sera were collected from MDPCL2-treated or untreated infected pigs, and antibody titers were determined by HI assay using human H1N1 (A/New Caledonia/20/99) influenza virus. Lower HI titers were detected in pigs in group 5-4 than in pigs in group 5-2 (Fig. 5). HI titers were detectable at 7 days p.i. and increased until 14 days p.i. in pigs of both group 5-4 and group 5-2. At 14 days p.i., the HI titer in pigs in group 5-4 was 80, while that in group 5-1 and group 5-2 was 160. No antibody for specific human H1N1 viruses was detectable in control pigs in group 5-3.
FIG. 5.
Determination of antibody titers in pigs. Sera were collected from MDPCL2-treated or untreated pigs infected with 2 ml of 104 EID50 of human H1N1 (A/New Caledonia/20/99) influenza virus until 14 days p.i. Antibody titers were determined by HI assay using A/New Caledonia/20/99 (H1N1) and turkey red blood cells (0.5%). Data are the means for three pigs ± standard errors per time point. P-I (group 5-1), PBS-treated infected pigs; PL-I (group 5-2), PBS-liposome-treated and infected pigs; CL-UI (group 5-3), MDPCL2-treated uninfected pigs; CL-I (group 5-4), MDPCL2-treated infected pigs. We statistically compared group 5-4 with group 5-2. *, P < 0.05.
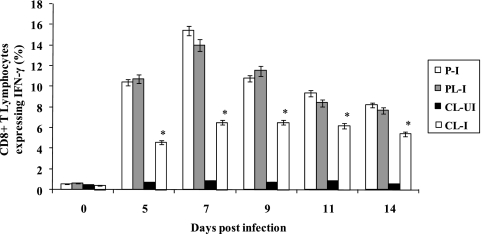
It has been known that CD8+ T cells play an important role in recovery from infection with viruses (18). When we measured CD8+ T lymphocytes expressing IFN-γ in lymphocytes from BAL samples from the same pigs used for HI assay by using flow cytometry analysis, a lower percentage of CD8+ T lymphocytes expressing IFN-γ was detected in pigs in group 5-4 than in pigs in group 5-2 (Fig. 6). CD8+ T cells expressing IFN-γ and specific for human H1N1 influenza viruses were detectable at 5 days p.i. and peaked at 7 days p.i. At 7 days p.i., the percentage of CD8+ T cells expressing IFN-γ in pigs in group 5-4 was 6.5%, while that in pigs in group 5-2 was 14%. The results suggest that depletion of alveolar macrophages may affect the functions of B and T lymphocytes.
FIG. 6.
Determination of CD8+ T lymphocytes expressing IFN-γ. Lymphocytes were isolated from BAL samples from MDPCL2-treated or untreated pigs infected with 2 ml of 104 EID50 of human H1N1 (A/New Caledonia/20/99) influenza virus. The percentage of CD8+ T cells expressing IFN-γ was determined by flow cytometry analysis using phycoerythrin-labeled rabbit anti-porcine IFN-γ antibody and FITC-labeled mouse anti-porcine CD8 antibody. Data are the means for three pigs ± standard errors per time point. We statistically compared group 5-4 with group 5-2. *, P < 0.05. P-I (group 5-1), PBS-treated infected pigs; PL-I (group 5-2), PBS-liposome-treated infected pigs; CL-UI (group 5-3), MDPCL2-treated uninfected pigs; CL-I (group 5-4), MDPCL2-treated infected pigs.
DISCUSSION
Our study showed that when pigs depleted of alveolar macrophages by MDPCL2 treatment were infected with human H1N1 influenza virus, 40% mortality was observed, with severe clinical signs of dyspnea, emaciation, ruffled fur, prolonged higher body temperatures, and severe loss of body weight, while none of the pigs not depleted of alveolar macrophages and infected with human H1N1 influenza virus died and these pigs showed minor clinical signs of sneezing, nasal rattling, lethargy, mild increase of body temperature, and mild loss of body weight. This result is quite different from those of previous studies that suggested inflammatory cytokines produced by macrophages as a cause for influenza virus-induced immunopathology in the lungs of humans and animals (5, 28). In our current study, the induction of Th1 cytokines TNF-α and IFN-γ in pigs depleted of alveolar macrophages by MDPCL2 treatment and infected with human H1N1 influenza virus was significantly reduced compared to that in infected pigs not depleted of alveolar macrophages. However, the clinical signs in infected pigs depleted of alveolar macrophages worsened, with 40% mortality, in contrast to the case for infected pigs not depleted of alveolar macrophages. The increase in pig mortality after depletion of alveolar macrophages is similar to what was seen in a study of mice infected with a sublethal dose of 1918 HA/NA:Tx/91 (34). That study showed that depletion of alveolar macrophages or neutrophils caused uncontrolled virus replication and mortality in mice infected with 1918 HA/NA:Tx/91.
Compared to the lack of mortality of infected pigs not depleted of alveolar macrophages, the high mortality (40%) of infected pigs depleted of alveolar macrophages by MDPCL2 treatment indicates that alveolar macrophages are important in controlling the disease caused by influenza virus infection. Indeed, considerable amounts of virus were detectable in the lungs of infected pigs depleted of alveolar macrophages until 14 days p.i.(4.19 EID50/ml), the last day on which we made observations, while viruses in the lungs of pigs not depleted of alveolar macrophages were detectable only until 6 days p.i. (0.88 log10 EID50/ml). It is possible that the reduced production of the released reactive nitrogen intermediates (nitric oxide) and reactive oxygen species in alveolar macrophages (1) and the diminished phagocytosis of alveolar macrophages and reduced stimulation of dendritic cells for innate and adaptive immunity by TNF-α produced by alveolar macrophages may be responsible for the increased pathogenicity in MDPCL2-treated pigs (20).
Another possible reason for the high mortality of pigs depleted of alveolar macrophages by MDPCL2 treatment may be significantly higher induction of immunosuppressive IL-10 in the lungs compared to that in the lungs of pigs not depleted of alveolar macrophages. IL-10 has been known to inhibit the activation and effector function of T lymphocytes, monocytes, and macrophages (23). The previous studies suggested that IL-10 was associated with lower immune responses to pathogens (6, 22, 25). In a study of influenza virus vaccination, the higher IL-10 production in the elderly was associated with a low antibody response to influenza vaccination (6). A study of Mycobacterium avium infection in BALB/c mice suggested that the increased production of IL-10 was responsible for the increased susceptibility of mice to Mycobacterium avium (25). A study of infection of mice with lymphocytic choriomeningitis virus (clone 13) reported that the increased production of IL-10 in serum caused functional inactivation of cytotoxic T lymphocytes (CTL) (22). Our results showed that the percentage of CD8+ T lymphocytes expressing IFN-γ was lower in infected pigs depleted of alveolar macrophages than in infected pigs not depleted of alveolar macrophages. Other studies showed that CTL is very important in clearing influenza viruses in lungs of animals and humans (4, 11, 21). Respiratory challenge of H1N1-primed mice with H3N2 influenza viruses reduced viral titers in lungs 2 to 3 days earlier than in naïve mice by influenza virus-specific CD8+ CTL (11). In mice infected with A/Japan/305/57 (H2N2), the majority of CD8+ T cells in the lung peaked at days 9 to 11 p.i. and were directed to four epitopes (three dominant and one subdominant) when intracellular cytokine staining and major histocompatibility complex class I tetramer binding were used (21). In humans who have HLA-A2+ major histocompatibility complex class I, CTL responses against influenza virus are directed predominantly to the HLA-A2-restricted epitope of matrix protein (M1 58 to 66) (4).
We also could not rule out the possibility that the reduced induction of antibody for specific influenza viruses was responsible for the increased pathogenicity in MDPCL2-treated infected pigs. Our results showed that HI titers in MDPCL2-treated infected pigs were lower than those in PBS-liposome-treated infected pigs. Another study showed that neutralization of progeny influenza virus played an important role in the process of virus clearance in mice (12).
Our study indicates that alveolar macrophages may be critical for inhibiting influenza viruses in the lungs and reducing mortality and clinical signs in pigs, even though they may contribute to causing some damage to lungs by producing inflammatory cytokines such as IFN-γ and TNF-α.
Acknowledgments
We thank Il Seob Lee and Jin Il Kim for excellent technical support.
Footnotes
Published ahead of print on 20 February 2008.
REFERENCES
- 1.Akaike, T. 2001. Role of free radicals in viral pathogenesis and mutation. Rev. Med. Virol. 1187-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bancroft, J. D., and A. Stevens. 1996. Theory and practice of histological techniques, 4th ed. Churchill Livingstone, New York, NY.
- 3.Bender, B. S., and P. A. Small, Jr. 1992. Influenza: pathogenesis and host defense. Semin. Respir. Infect. 738-45. [PubMed] [Google Scholar]
- 4.Boon, A. C., G. de Mutsert, Y. M. Graus, R. A. Fouchier, K. Sintnicolaas, A. D. Osterhaus, and G. F. Rimmelzwaan. 2002. The magnitude and specificity of influenza A virus-specific cytotoxic T-lymphocyte responses in humans is related to HLA-A and -B phenotype.J. Virol. 76582-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung, C. Y., L. L. Poon, A. S. Lau, W. Luk, Y. L. Lau, K. F. Shortridge, S. Gordon, Y. Guan, and J. S. Peiris. 2002. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 3601831-1837. [DOI] [PubMed] [Google Scholar]
- 6.Corsini, E., L. Vismara, L. Lucchi, B. Viviani, S. Govoni, C. L. Galli, M. Marinovich, and M. Racchi. 2006. High interleukin-10 production is associated with low antibody response to influenza vaccination in the elderly. J. Leukoc. Biol. 80376-382. [DOI] [PubMed] [Google Scholar]
- 7.De Haan, A., G. Groen, J. Prop, N. van Rooijen, and J. Wilschut. 1996. Mucosal immunoadjuvant activity of liposomes: role of alveolar macrophages. Immunology 89488-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dingle, J. H., D. G. Badger, and W. S. Jordon, Jr. 1964. Illness in the home: a study of 25,000 illness in a group of Cleveland families, p. 162-187. Western Reserve University Press, Cleveland OH.
- 9.Douglas, R. G., Jr. 1975. Influenza in man, p. 395-447. In E. D. Kilbourne (ed.), The influenza viruses and influenza. Academic Press, Orlando, FL.
- 10.Fleisch, H. 1991. Bisphosphonates. Pharmacology and use in the treatment of tumour-induced hypercalcaemic and metastatic bone disease. Drugs 42919-944. [DOI] [PubMed] [Google Scholar]
- 11.Flynn, K. J., G. T. Belz, J. D. Altman, R. Ahmed, D. L. Woodland, and P. C. Doherty. 1998. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 8683-891. [DOI] [PubMed] [Google Scholar]
- 12.Gerhard, W., K. Mozdzanowska, M. Furchner, G. Washko, and K. Maiese. 1997. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol. Rev. 15995-103. [DOI] [PubMed] [Google Scholar]
- 13.Hinshaw, V. S., R. G. Webster, B. C. Easterday, and W. J. Bean, Jr. 1981. Replication of avian influenza A viruses in mammals. Infect. Immun. 34354-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann, P., H. Sprenger, A. Kaufmann, A. Bender, C. Hasse, M. Nain, and D. Gemsa. 1997. Susceptibility of mononuclear phagocytes to influenza A virus infection and possible role in the antiviral response. J. Leukoc. Biol. 61408-414. [DOI] [PubMed] [Google Scholar]
- 15.Hunninghake, G. W. 1987. Immunoregulatory functions of human alveolar macrophages. Am. Rev. Respir. Dis. 136253-254. [DOI] [PubMed] [Google Scholar]
- 16.Ito, T., J. N. Couceiro, S. Kelm, L. G. Baum, S. Krauss, M. R. Castrucci, I. Donatelli, H. Kida, J. C. Paulson, R. G. Webster, and Y. Kawaoka. 1998. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J. Virol. 727367-7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julkunen, I., K. Melén, M. Nyqvist, J. Pirhonen, T. Sareneva, and S. Matikainen. 2000. Inflammatory responses in influenza A virus infection. Vaccine (Suppl. 1):S32-S37. [DOI] [PubMed]
- 18.Kägi, D., B. Ledermann, K. Bürki, R. M. Zinkernagel, and H. Hengartner. 1996. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu. Rev. Immunol. 14207-232. [DOI] [PubMed] [Google Scholar]
- 19.Kida, H., T. Ito, J. Yasuda, Y. Shimizu, C. Itakura, K. F. Shortridge, Y. Kawaoka, and R. G. Webster. 1994. Potential for transmission of avian influenza viruses to pigs. J. Gen. Virol. 752183-2188. [DOI] [PubMed] [Google Scholar]
- 20.Lambrecht, B. N. 2006. Alveolar macrophage in the driver's seat. Immunity 24366-368. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence, C. W., R. M. Ream, and T. J. Braciale. 2005. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J. Immunol. 1745332-5340. [DOI] [PubMed] [Google Scholar]
- 22.Maris, C. H., C. P. Chappell, and J. Jacob. 2007. Interleukin-10 plays an early role in generating virus-specific T cell anergy. BMC Immunol. 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19683-765. [DOI] [PubMed] [Google Scholar]
- 24.Reed, L. E., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 25.Roque, S., C. Nobrega, R. Appelberg, and M. Correia-Neves. 2007. IL-10 underlies distinct susceptibility of BALB/c and C57BL/6 mice to Mycobacterium avium infection and influences efficacy of antibiotic therapy. J. Immunol. 1788028-8035. [DOI] [PubMed] [Google Scholar]
- 26.Scholtissek, C., W. Rohde, V. Von Hoyningen, and R. Rott. 1978. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 8713-20. [DOI] [PubMed] [Google Scholar]
- 27.Scholtissek, C., H. Burger, O. Kistner, and K. F. Shortridge. 1985. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology 147287-294. [DOI] [PubMed] [Google Scholar]
- 28.Seo, S. H., R. Webby, and R. G. Webster. 2004. No apoptotic deaths and different levels of inductions of inflammatory cytokines in alveolar macrophages infected with influenza viruses. Virology 329270-279. [DOI] [PubMed] [Google Scholar]
- 29.Simonsen, L., M. J. Clarke, G. D. Williamson, D. F. Stroup, N. H. Arden, and L. B. Schonberger. 1997. The impact of influenza epidemics on mortality: introducing a severity index. J. Public Health 871944-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strickland, D. H., T. Thepen, U. R. Kees, G. Kraal, and P. G. Holt. 1993. Regulation of T-cell function in lung tissue by pulmonary alveolar macrophages. Immunology 80266-272. [PMC free article] [PubMed] [Google Scholar]
- 31.Stuart-Harris, C. H. 1961. Twenty years of influenza epidemics. Am. Rev. Respir. Dis. 83S54-S67. [Google Scholar]
- 32.Thepen, T., N. Van Rooijen, and G. Kraal. 1989. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J. Exp. Med. 170499-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thepen, T., C. McMenamin, J. Oliver, G. Kraal, and P. G. Holt. 1991. Regulation of immune response to inhaled antigen by alveolar macrophages: differential effects of in vivo alveolar macrophage elimination on the induction of tolerance vs. immunity. Eur. J. Immunol. 212845-2850. [DOI] [PubMed] [Google Scholar]
- 34.Tumpey, T. M., A. García-Sastre, J. K. Taubenberger, P. Palese, D. E. Swayne, M. J. Pantin-Jackwood, S. Schultz-Cherry, A. Solórzano, N. Van Rooijen, J. M. Katz, and C. F. Basler. 2005. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J. Virol. 7914933-14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Riel, D., V. J. Munster, E. de Wit, G. F. Rimmelzwaan, R. A. Fouchier, A. D. Osterhaus, and T. Kuiken. 2007. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am. J. Pathol. 1711215-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Rooijen, N. 1989. The liposome-mediated macrophage ‘suicide’ technique. J. Immunol. Methods 1241-6. [DOI] [PubMed] [Google Scholar]
- 37.Van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 17483-93. [DOI] [PubMed] [Google Scholar]
- 38.Wijburg, O. L., S. DiNatale, J. Vadolas, N. van Rooijen, and R. A. Strugnell. 1997. Alveolar macrophages regulate the induction of primary cytotoxic T-lymphocyte responses during influenza virus infection. J. Virol. 719450-9457. [DOI] [PMC free article] [PubMed] [Google Scholar]