Abstract
Gene therapy is proposed as a novel therapeutic strategy for treating glioblastoma multiforme (GBM), a devastating brain cancer. In the clinic, antivector immune responses pose formidable challenges. Herein we demonstrate that high-capacity adenovirus vectors (HC-Ads) carrying the conditional cytotoxic gene herpes simplex virus type 1-thymidine kinase (TK) induce tumor regression and long-term survival in an intracranial glioma model, even in the presence of systemic antiadenovirus immunity, as could be encountered in patients. First-generation Ad-TK failed to elicit tumor regression in this model. These results pave the way for implementing HC-Ad-TK-mediated gene therapy as a powerful adjuvant for treating GBM.
Glioblastoma multiforme (GBM) is a malignant primary brain tumor associated with <2% survival at 5 years postdiagnosis. Advances in neurosurgery, chemotherapy, and radiotherapy have not impacted the dire clinical statistics; therefore, novel therapies are urgently needed. Adenovirus-mediated delivery of the conditionally cytotoxic herpes simplex virus type 1-thymidine kinase (HSV1-TK) gene has been proposed as an adjuvant gene therapy approach for treating GBM (6, 7). HSV1-TK is nontoxic to humans; only actively dividing cells expressing HSV1-TK will convert intravenously administered ganciclovir (GCV) into cytotoxic nucleotides that will kill proliferating GBM cells. This approach has been tested with humans by using first-generation adenovirus vectors (Ads), with the optimal transduction efficiency achieved when the Ads were delivered at multiple injection sites (15). Results from Phase II trials are encouraging, with a 65% increase in the median survival of Ad-TK-treated patients compared to that in control groups, although median life expectancy was increased only to 62.4 weeks (7). Results from a large multicenter Phase III trial are awaited (15). The significant, yet limited, success elicited after Ad-TK was delivered to the tumor mass in situ or to the tumor bed following resection (7) is likely due to the presence of preexisting systemic immune responses against adenoviruses found in many human patients (5). Antiadenoviral immune responses would hamper therapeutic transgene expression from first-generation Ads, resulting in diminished clinical efficacy of the treatment compared to the success attained with preclinical models (1, 6, 7). Along these lines, it has been recently shown that adeno-associated virus serotype 2 (AAV2)-mediated hepatic gene transfer results in transgene product expression, which is stable in preclinical animal models but is short-lived, declining at 4 to 6 weeks after AAV2 delivery to human patients (12). This decline was caused by the elimination of transduced hepatocytes by AAV vectors, mediated by AAV2 capsid-specific CD8+ T cells (12).
The second-generation “gutless,” high-capacity Ads (HC-Ads) have a significantly favorable immunological profile (2, 3, 18). Even in the presence of a preexisting systemic antiadenoviral immune response that eliminates transgene expression from first-generation Ads, the transgene expression from HC-Ads remains stable for up to 1 year (2, 11, 13, 17-19). In this report, using a syngeneic model of intracranial GBM, we demonstrate that the intratumoral delivery of HC-Ad-TK in combination with the peripheral administration of GCV elicited GBM regression and long-term survival, even in the presence of a systemic preexisting immune response against Ads, as is likely to be encountered in the clinic (5). Intratumoral delivery of Ad-TK completely fails to improve long-term survival of tumor-bearing animals that had been preimmunized against Ads. Furthermore, therapeutic efficacy in the presence of systemic anti-Ad immunity ensued without overt neuropathological side effects, following intratumoral administration of HC-Ad-TK. Our data suggest that this gene therapy approach could be a powerful adjuvant for the treatment of GBM, even for patients who would have been preexposed to adenovirus.
All experimental manipulations with Lewis rats were approved by the Institutional Animal and Care Committee (IACUC) of Cedars-Sinai Medical Center, UCLA. To assess the anti-GBM therapeutic efficacy of HC-Ad-TK and Ad-TK in the presence of systemic anti-Ad immunity, we utilized a first-generation vector with E1 and E3 deleted (Ad-TK) and a helper-dependent HC-Ad-TK, both of which constitutively expressed HSV1-TK under the control of the powerful murine cytomegalovirus (mCMV) promoter (1, 4, 14, 16). Characteristics of Ad-TK delivery were total viral particles (vp), 1.15 × 1013 vp/ml; vector genomes (vg), 1.19 × 1013 vg/ml; and infectious units (iu), 1.46 × 1012 iu/ml. For HC-Ad-TK, total vp were 6.15 × 1012 vp/ml; vg were 4.90 × 1012 vg/ml; and helper virus contamination was 1.0 × 106 iu/ml. Both vectors were free of contaminating replication-competent adenovirus and lipopolysaccharides. The abilities of both vectors to transduce and kill Lewis rat glioma cells (1) in the presence of GCV were confirmed in vitro (data not shown) before in vivo studies were performed. Both HC-Ads and Ads were used at a dose of 1.5 × 108 vg/3 μl delivered into the tumor (from the bregma, +1 mm anterior, +3 mm lateral, and −5 mm from the dura).
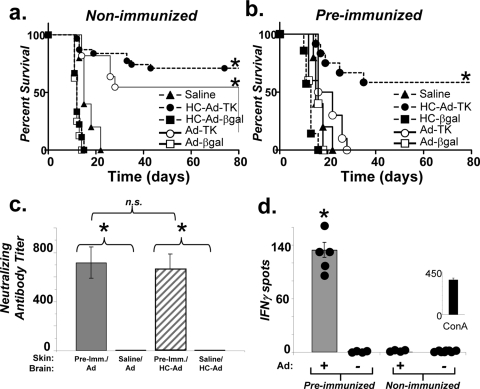
As the majority of patients undergoing gene therapy for glioma are likely to have a preexisting immune response to adenovirus, we wished to test the effectiveness of both of the gene therapy vector platforms with an animal model that more closely mimicked the immunological status that would be encountered in human GBM patients. To do so, we systemically immunized Lewis rats with a first-generation Ad or saline (controls). Two weeks later, animals underwent stereotactic implantation of syngeneic Lewis rat glioma cell line CNS-1 into the striatum (1, 10). One week later, groups of animals received an intratumoral injection of Ad-TK, HC-Ad-TK, Ad-β-galactosidase (Ad-βgal), HC-Ad-βgal, or saline. GCV was administered systemically, and animals were monitored for survival for 80 days. Survival was significantly improved for 75% of the nonimmunized animals treated with HC-Ad-TK and for 60% of the animals treated with Ad-TK (P < 0.0001 versus those treated with saline, log rank test) (Fig. 1a). However, only treatment with HC-Ad-TK significantly extended the survival of preimmunized rats (P < 0.0001 versus that with saline, log rank test). Treatment with Ad-TK failed to improve the survival of preimmunized animals (Fig. 1b). The ability of circulating antiadenovirus antibodies from the sera of immunized mice to neutralize both Ads and HC-Ads was confirmed by a neutralizing antibody assay (Fig. 1c), and the number of gamma interferon-secreting lymphocytes was assessed by enzyme-linked immunospot (ELISPOT) assay (Fig. 1d) (2).
FIG. 1.
Treatment of a syngeneic intracranial glioma model with HC-Ad-TK induces tumor regression and long-term survival, even in the presence of a systemic immune response against adenoviruses. Treatment with Ad-TK failed for preimmunized tumor-bearing rats. (a) A log rank test of Kaplan-Meier survival curves demonstrates that the treatment of nonimmunized animals bearing intracranial gliomas with either HC-Ad-TK or Ad-TK induces tumor regression and long-term survival, while (b) only treatment with HC-Ad-TK is effective at inducing tumor regression and long-term survival for tumor-bearing animals that had been preimmunized with adenoviruses. Treatment with Ad-βgal, HC-Ad-βgal, or saline alone failed (*, P < 0.0001 versus saline, log rank test, n = 5 to 11). (c) Sera from immunized and nonimmunized animals were tested with a neutralizing antibody assay to confirm the ability of circulating antiadenovirus antibodies to neutralize both Ad and HC-Ads (*, P < 0.01, two-way analysis of variance, n = 3 to 5). (d) An ELISPOT assay was performed to confirm the presence of a specific antiadenovirus T-cell response (*, P < 0.01, Student's t test, n = 5). Splenocytes were stimulated with heat-inactivated Ads (+) or without (−). Experiments were repeated at least twice; values represent the means ± standard errors of the means.
To assess the efficacy and neuropathological consequences of gene therapy, we analyzed coronal brain sections of moribund tumor-bearing rats or long-term survivors (day 80) by using immunocytochemistry for markers specific for structural integrity and inflammation, i.e., markers for tyrosine hydroxylase (TH), myelin basic protein (MBP), CD8+ T cells (CD8), and macrophages/activated microglia (CD68) (2, 8, 9) (Fig. 2). Coronal brain slices of five animals from each treatment group were examined, and representative images are shown. Immunohistochemistry revealed hypertrophied, activated astrocytes (Fig. 2, GFAP) surrounding the tumor or the injection sites in all animals (Fig. 2a to e). The immunoreactivity of TH (Fig. 2k to o) and MBP (Fig. 2f to j) demonstrated that CNS-1 tumor growth displaces and compresses normal striatal brain tissue (Fig. 2, TH), as well as axon bundles (Fig. 2, MBP) coursing throughout the striatum. TH and MBP staining in long-term survivors’ tissues showed no significant structural disturbances in the striatum compared with that of the untreated, contralateral hemisphere, suggesting that brain tissue compression does not elicit irreversible neuropathological damage. Enlarged ventricles were observed in the hemisphere ipsilateral to tumor implantation in long-term survivors. Striatal CD8+ lymphocytes were observed throughout the tumors of all moribund animals but were absent from the striatum of animals in which tumor regression had ensued in response to the therapy. Macrophages/microglia (CD68) were detected throughout the tumor masses of moribund animals but were confined to the area surrounding the injection site (the site of tumor implantation) in long-term survivors (not shown).
FIG. 2.
The treatment of a syngeneic intracranial glioma with HC-Ad-TK results in minimal neurotoxicity with preimmunized long-term survivors. Immunocytochemical analysis was performed with coronal brain sections from five moribund animals or long-term survivors with markers for (in order from top to bottom) astrocytes (GFAP) (a to e), MBP (f to j), and TH (k to o). Representative images are shown. Immunolabeling reveals the eradication of the tumor (T) in preimmunized animals treated with HC-Ad-TK, with minimal residual neurotoxicity. Scale bars, 1.0 mm.
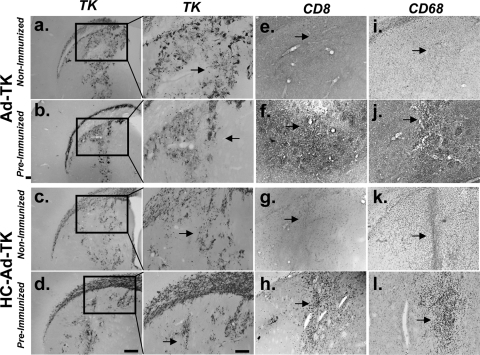
Robust HSV1-TK immunoreactivity in the brains of preimmunized rats at 1 week after intracranial delivery of Ads or HC-Ads (Fig. 3a to d) confirms that neutralizing anti-Ad antibodies are not capable of blocking the vector transduction of brain tissue by either vector (2, 18). Immunocytochemistry revealed that CD8+ T lymphocytes and macrophages/microglia (Fig. 3e to l) are restricted to the injection site, indicating the safety of both vectors in immunized animals. Overt neuropathology was not observed.
FIG. 3.
HC-Ads and Ads mediate high levels of short-term HSV-1 TK expression in preimmunized non-tumor-bearing animals with minimal inflammation at 1 week after vector delivery. Animals were preimmunized with a systemic injection of adenovirus or with saline alone (nonimmunized) and 2 weeks later were injected intracranially with Ad-TK or HC-Ad-TK. Animals were euthanized 1 week after vector delivery, and brains from five animals per group were analyzed by immunocytochemistry (a to d). Representative images are shown. Low-magnification (left panels) and high-magnification (right panels) images of HSV1-TK immunoreactivity demonstrate high levels of HSV1-TK expression in animals treated with HC-Ad-TK or Ad-TK, regardless of their immunization status. Immunocytochemistry reveals minimal CD8+ lymphocytes (e to h) and macrophages/microglia (i to l) in the brains of all animals. Arrows indicate the injection site.
Neutralizing antibody responses to viral capsid proteins are thought to curtail transgene expression upon virus readministration by binding to the virus and inhibiting target cell infection. However, T-cell responses also play an essential role in the elimination of transgene expression from transduced cells. Our results indicate that neutralizing antiadenovirus antibodies do not block transduction of the brain by first-generation vectors or HC-Ads; this finding is supported by the fact that at early times postinfection, there is expression from both vectors (Fig. 3) (2, 17, 18). This is likely due to the fact that antibodies cannot cross the blood-brain barrier and access the brain and, thus, remain unable to block Ad-mediated infection. However, once HC-Ads infect brain cells, they will not express any viral antigenic epitopes, while first-generation Ads will express low levels of adenoviral proteins in infected cells. The slight reduction in transgene expression from HC-Ads is limited by the transient presentation of adenoviral capsid-derived antigenic epitopes with the major histocompatibility complex class I of infected cells. Since those epitopes are derived from input HC-Ad virions, once input virions have been degraded, antiadenovirus immune T cells do not have any adenovirus-derived epitopes that they can recognize, and thus transgene expression from HC-Ads remains stable.
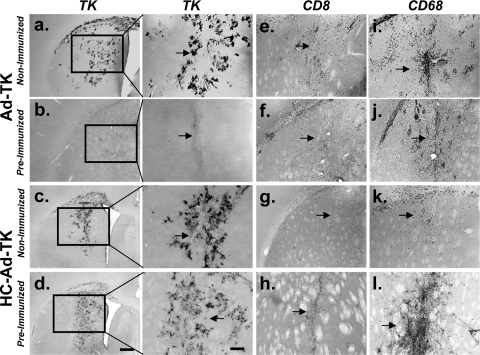
In agreement with the efficacy data (Fig. 1b), HSV1-TK expression in the brain was persistent in preimmunized animals that received injections of HC-Ad-TK in the striatum 30 days earlier (Fig. 4d). The expression of HSV1-TK, however, was greatly reduced in preimmunized animals injected with Ad-TK (Fig. 4b). The infiltration of CD8+ lymphocytes in the brains of preimmunized animals injected with Ad-TK was confirmed by immunocytochemistry (Fig. 4f). CD8+ immunoreactivity was restricted to the area surrounding the injection site in preimmunized animals injected with HC-Ad-TK (Fig. 4h). Overt neuropathology was not observed (not shown). The robust expression of HSV1-TK when encoded within HC-Ads, even in the presence of a preexisting anti-Ad immune response, further emphasizes the usefulness of HC-Ads in clinical trials for GBM therapy.
FIG. 4.
HC-Ad-TK mediates high levels of persistent HSV-1 TK expression in preimmunized non-tumor-bearing animals with minimal inflammation at 1 month after vector delivery. Animals were preimmunized with a systemic injection of adenovirus and 2 weeks later were injected intracranially with HC-Ad-TK or Ad-TK. Animals were euthanized 30 days after vector delivery into the striatum, and brains from five animals were analyzed by immunocytochemistry (a to d). Representative images are shown. Low-magnification (left panels) and high-magnification (right panels) images of HSV1-TK immunoreactivity demonstrate high levels of persistent HSV1-TK expression only in the animals treated with HC-Ad-TK (d). Note the complete abrogation of TK expression in preimmunized animals 30 days after they were injected with Ad-TK (b). Immunocytochemistry indicates high levels of CD8+ lymphocytes in preimmunized animals injected with Ad-TK (f), while CD8+ lymphocytes are confined to the injection site in preimmunized animals injected with HC-Ad-TK. Macrophages/microglia were observed in the brains of all animals (i to l). Arrows indicate the injection site.
In summary, our data indicate that gene therapy using HC-Ads withstands the serious challenge so far imposed by preexisting antiadenoviral immunity. As this issue has been raised as an almost certain stumbling block to the implementation of clinically effective adenovirus-mediated gene therapy, our work indicates that this challenge can be overcome in the context of a clinically relevant experimental GBM model. The implementation of gene therapy clinical trials for GBM by using HC-Ads presents hope for improving the life expectancy and overall prognosis of these patients.
Acknowledgments
This work is supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) grant 1R01 NS44556.01, minority supplement grants NS445561, 1R21-NSO54143.01, and 1UO1 NS052465.01 to M.G.C.; and by NIH/NINDS grants 1 RO1 NS 054193.01, RO1 NS 42893.01, and 1R21 NS047298-01 to P.R.L. This work also received support from the Bram and Elaine Goldsmith and the Medallions Group endowed chairs in gene therapeutics to P.R.L. and M.G.C., respectively; from the Linda Tallen and David Paul Kane Foundation annual fellowship; and from the Board of Governors at CSMC. G.D.K. is supported by NIH/NINDS grant 1F32N50503034-01. D.L. is supported by a postdoctoral fellowship from the Human Frontier Science Program Organization (HFSPO). P.N. is supported by grants R01DK067324 and R01HL083047.
G.D.K. performed research (in vivo efficacy, Ad neutralizing antibody assays, and gene expression analysis), analyzed data, and contributed to the writing; A.G.M. performed research (in vivo analysis of gene expression in the brain, analysis of immune infiltrates), analyzed data, and contributed to the writing; W.X. performed research (in vivo analysis of HC-Ad-TK-mediated gene expression in the brain); K.M.K. performed research (cloned and characterized the HC-Ad plasmid), analyzed data, and contributed to the writing; M.P. performed research (molecular characterization of HC-Ad-HSV1-TK and NAB assays) and analyzed data; D.L. performed research (ELISPOT assays) and analyzed data; D.P. and P.N. performed research (large scale up and purification of HC-Ads) and analyzed data; P.R.L. and M.G.C. designed the experiments and conceptualized the work described, analyzed the data, and wrote the paper. All authors discussed the results and commented on the manuscript.
The authors declare that they do not have any competing financial interests.
Footnotes
Published ahead of print on 20 February 2008.
REFERENCES
- 1.Ali, S., G. D. King, J. F. Curtin, M. Candolfi, W. Xiong, C. Liu, M. Puntel, Q. Cheng, J. Prieto, A. Ribas, J. Kupiec-Weglinski, N. van Rooijen, H. Lassmann, P. R. Lowenstein, and M. G. Castro. 2005. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 657194-7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barcia, C., M. Jimenez-Dalmaroni, K. M. Kroeger, M. Puntel, A. J. Rapaport, D. Larocque, G. D. King, S. A. Johnson, C. Liu, W. Xiong, M. Candolfi, S. Mondkar, P. Ng, D. Palmer, M. G. Castro, and P. R. Lowenstein. 2007. Sustained, one year expression from high-capacity helper-dependent adenoviral vectors delivered to the brain of animals with a pre-existing systemic anti-adenoviral immune response: implications for clinical trials. Mol. Ther. 152154-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunetti-Pierri, N., T. C. Nichols, S. McCorquodale, E. Merricks, D. J. Palmer, A. L. Beaudet, and P. Ng. 2005. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum. Gene Ther. 16811-820. [DOI] [PubMed] [Google Scholar]
- 4.Candolfi, M., J. F. Curtin, W. D. Xiong, K. M. Kroeger, C. Liu, A. Rentsendorj, H. Agadjanian, L. Medina-Kauwe, D. Palmer, P. Ng, P. R. Lowenstein, and M. G. Castro. 2006. Effective high-capacity gutless adenoviral vectors mediate transgene expression in human glioma cells. Mol. Ther. 14371-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chirmule, N., K. Propert, S. Magosin, Y. Qian, R. Qian, and J. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 61574-1583. [DOI] [PubMed] [Google Scholar]
- 6.Dewey, R. A., G. Morrissey, C. M. Cowsill, D. Stone, F. Bolognani, N. J. Dodd, T. D. Southgate, D. Klatzmann, H. Lassmann, M. G. Castro, and P. R. Lowenstein. 1999. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat. Med. 51256-1263. [DOI] [PubMed] [Google Scholar]
- 7.Immonen, A., M. Vapalahti, K. Tyynela, H. Hurskainen, A. Sandmair, R. Vanninen, G. Langford, N. Murray, and S. Yla-Herttuala. 2004. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol. Ther. 10967-972. [DOI] [PubMed] [Google Scholar]
- 8.King, G. D., K. M. Kroeger, C. J. Bresee, M. Candolfi, C. Liu, C. M. Manalo, A. M. Muhammad, R. J. Pechnick, P. R. Lowenstein, and M. G. Castro. 2008. Flt3L in combination with HSV1-TK mediated gene therapy reverses brain tumor induced behavioral deficits. Mol. Ther. [Epub ahead of print.] doi: 10.1038/mt.2008.18. [DOI] [PMC free article] [PubMed]
- 9.King, G. D., A. K. M. Muhammad, J. F. Curtin, C. Barcia, M. Puntel, C. Liu, S. B. Honig, M. Candolfi, S. Mondkar, P. R. Lowenstein, and M. G. Castro. 2007. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro. Oncol. 1019-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruse, C. A., M. C. Molleston, E. P. Parks, P. M. Schiltz, B. K. Kleinschmidt-DeMasters, and W. F. Hickey. 1994. A rat glioma model, CNS-1, with invasive characteristics similar to those of human gliomas: a comparison to 9L gliosarcoma. J. Neurooncol. 22191-200. [DOI] [PubMed] [Google Scholar]
- 11.Maione, D., C. Della Rocca, P. Giannetti, R. D'Arrigo, L. Liberatoscioli, L. L. Franlin, V. Sandig, G. Ciliberto, N. La Monica, and R. Savino. 2001. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc. Natl. Acad. Sci. USA 985986-5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mingozzi, F., N. C. Hasbrouck, E. Basner-Tschkarajan, S. A. Edmonson, D. J. Hui, D. E. Sabatino, S. Zhou, J. F. Wright, H. Jiang, G. F. Pierce, V. R. Arruda, and K. A. High. 2007. Modulation of tolerance to the transgene product in a non-human primate model of AAV-mediated gene transfer to liver. Blood 1102334-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Neal, W. K., H. Zhou, N. Morral, C. Langston, R. J. Parks, F. L. Graham, S. Kochanek, and A. L. Beaudet. 2000. Toxicity associated with repeated administration of first-generation adenovirus vectors does not occur with a helper-dependent vector. Mol. Med. 6179-195. [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer, D., and P. Ng. 2003. Improved system for helper-dependent adenoviral vector production. Mol. Ther. 8846-852. [DOI] [PubMed] [Google Scholar]
- 15.Pulkkanen, K. J., and S. Yla-Herttuala. 2005. Gene therapy for malignant glioma: current clinical status. Mol. Ther. 12585-598. [DOI] [PubMed] [Google Scholar]
- 16.Southgate, T., P. Kingston, and M. G. Castro. 2000. Gene transfer into neural cells in vivo using adenoviral vectors, p. 4.23.1-4.23.40. In C. R. Gerfen, R. McKay, M. A. Rogawski, D. R. Sibley, and P. Skolnick (ed.), Current protocols in neuroscience, vol. 4.23.1 to 4.23.40. John Wiley and Sons, New York, NY. [Google Scholar]
- 17.Thomas, C. E., G. Schiedner, S. Kochanek, M. G. Castro, and P. R. Lowenstein. 2000. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc. Natl. Acad. Sci. USA 977482-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas, C. E., G. Schiedner, S. Kochanek, M. G. Castro, and P. R. Lowenstein. 2001. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum. Gene Ther. 12839-846. [DOI] [PubMed] [Google Scholar]
- 19.Xiong, W., S. Goverdhana, S. A. Sciascia, M. Candolfi, J. M. Zirger, C. Barcia, J. F. Curtin, G. D. King, G. Jaita, C. Liu, K. Kroeger, H. Agadjanian, L. Medina-Kauwe, D. Palmer, P. Ng, P. R. Lowenstein, and M. G. Castro. 2006. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J. Virol. 8027-37. [DOI] [PMC free article] [PubMed] [Google Scholar]