Abstract
Recent studies have demonstrated the importance of insulin or insulin-like growth factor 1 (IGF-1) for regulation of pancreatic β-cell mass. Given the role of tuberous sclerosis complex 2 (TSC2) as an upstream molecule of mTOR (mammalian target of rapamycin), we examined the effect of TSC2 deficiency on β-cell function. Here, we show that mice deficient in TSC2, specifically in pancreatic β cells (βTSC2−/− mice), manifest increased IGF-1-dependent phosphorylation of p70 S6 kinase and 4E-BP1 in islets as well as an initial increased islet mass attributable in large part to increases in the sizes of individual β cells. These mice also exhibit hypoglycemia and hyperinsulinemia at young ages (4 to 28 weeks). After 40 weeks of age, however, the βTSC2−/− mice develop progressive hyperglycemia and hypoinsulinemia accompanied by a reduction in islet mass due predominantly to a decrease in the number of β cells. These results thus indicate that TSC2 regulates pancreatic β-cell mass in a biphasic manner.
The masses of pancreatic islets are decreased in individuals with type 2 diabetes (4), suggesting that this defect may be a cause of this condition. Signaling pathways triggered by insulin or insulin-like growth factor 1 (IGF-1) have been implicated in maintenance of islet mass (8, 17, 19, 28, 31). We have shown that 3-phosphoinositide-dependent protein kinase 1 (PDK1) contributes to signaling by insulin or IGF-1, which is responsible for regulation of both the numbers and the sizes of pancreatic β cells in mice (8). We also found that the signals for regulation of cell number and cell size diverge downstream of PDK1, with the transcription factor Foxo1 contributing to regulation of cell number (8).
Tuberous sclerosis is an autosomal dominant disorder characterized by the formation of hamartomas in the brain, skin, kidneys, and heart, with TSC1 and TSC2 having been identified as causative genes for this condition (13, 30). Tuberous sclerosis complex 2 (TSC2) is a component of the signaling pathway mediated by phosphatidylinositol 3-kinase and Akt, which modulates mTOR (11, 23). TSC2 possesses GTPase-activating protein activity toward Rheb, a member of the Ras family of GTPases (5, 12). Akt-mediated multisite phosphorylation of TSC2 inhibits the ability of TSC2 to act as a GTPase-activating protein toward Rheb, resulting in accumulation of the GTP-bound form of Rheb and consequent activation of mTOR complex 1 (mTORC1) (11). This role of TSC2 in insulin signaling prompted us to examine whether TSC2 contributes to negative regulation of β-cell size. To this end, we generated mice in which Tsc2 is deleted specifically in pancreatic β cells (βTSC2−/− mice).
MATERIALS AND METHODS
Mice.
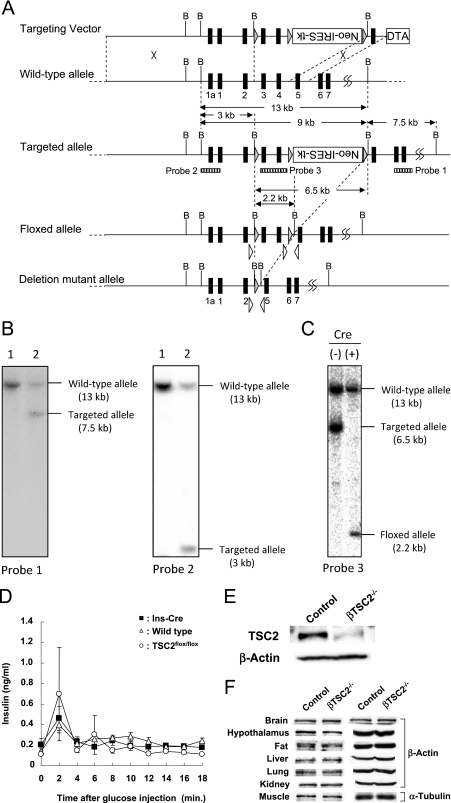
A targeting vector of a floxed Tsc2 allele was constructed using a genomic DNA clone from the 129/Sv mouse strain (Fig. 1A, 17 Briefly, we employed a cassette which expresses both Neo and herpes simplex virus thymidine kinase (HSV-TK) under the control of the internal ribosome entry site (IRES) (a gift from R. Jaenisch) (32). One loxP sequence was introduced in intron 2, and the Neo-IRES-TK cassette was introduced between two loxP sequences in intron 4. The orientation of the Neo-IRES-TK cassette was reverse relative to that of the Tsc2 gene. The diphtheria toxin A chain expression cassette was used as a negative selection marker. The linearized targeting vector was introduced into J1 embryonic stem (ES) cells, and G418-resistant clones were analyzed by Southern blotting to isolate the homologous recombinant (Fig. 1B). Next, Cre recombinase was transiently expressed in homologous recombinant cells by using adenoviral vector (Adex-Cre), and negative selection with medium containing 1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)-5-iodouracil (FIAU; 0.8 μM), an HSV-TK substrate, was performed to ensure deletion of the marker cassette. By Southern blot analysis, ES cell clones carrying a floxed Tsc2 allele were identified (Fig. 1C) and used to generate chimera mice by blastocyst injection. Finally, a mouse strain heterozygous for the floxed Tsc2 allele (TSC2flox/+) was established by crossing chimeras with B6 mice. No apparent abnormality was detected in TSC2flox/flox mice (T. Kobayashi, H. Takano, O. Hino, and T. Noda, unpublished results). By crossing TSC2flox/+ mice with transgenic mice which ubiquitously express Cre, we generated mice heterozygous for a deletion mutant allele and found renal carcinogenesis in those mice, confirming that Tsc2 is inactivated by deletion of exons 3 and 4 (T. Kobayashi et al., unpublished results) (16).
FIG. 1.
Generation of β-cell-specific TSC2 knockout mice. (A) Structure of targeting vector and Tsc2 alleles. Exons are denoted with filled boxes, some with numbers. Expression cassettes for the diphtheria toxin A chain (DTA) and Neo/HSV-TK (Neo-IRES-TK) are shown as open boxes. Shaded triangles indicate loxP sequences. Dotted lines indicate sites of insertion (targeting vector into wild-type allele) and excision (targeted allele reduced to floxed allele and reduced again to deleted mutant allele). Open triangles indicate primers for genotyping. Positions of probes used for Southern blot analysis are shown as striped boxes below the targeted allele. B; BamHI restriction site. (B) Southern blot analysis of G418-resistant ES clones. BamHI-digested DNAs from two ES clones were analyzed with two probes. Lane 1, nonhomologous recombinant control clone; lane 2, homologous recombinant clone. (C) Southern blot analysis of the floxed Tsc2 allele. BamHI-digested DNA was analyzed. Cre (−), parental homologous recombinant ES clone; Cre (+), FIAU-resistant ES clone with floxed allele. (D) Time course of changes in plasma insulin concentration induced by intraperitoneal administration of glucose (3 mg per gram of body weight) in wild-type (open triangles; n = 3), Ins-Cre (filled squares; n = 3), and TSC2flox/flox (open circles; n = 4) mice at 8 weeks of age. Data are means ± SEM. (E, F) Immunoblot analysis of TSC2 and either β-actin or α-tubulin (loading controls) in pancreatic islets (E) or in the indicated tissues (F) of 9-week-old control (TSC2flox/flox) and βTSC2−/− mice.
We generated pancreatic β-cell-specific TSC2 knockout (βTSC2−/−) mice by breeding TSC2flox/flox mice with mice that express the Cre recombinase gene under the control of the promoter of the rat insulin 2 gene (Ins-Cre mice), which were kindly provided by M. Magnusson (22) and P. Herrera (10). Animals were maintained, and blood glucose, plasma insulin, cholesterol, triglyceride, and free fatty acid concentrations were determined, as described previously (9, 14). Only male mice were used for experiments. This study was performed according to the guidelines of the Animal Ethics Committee of Kobe University Graduate School of Medicine.
Oral glucose tolerance test and acute insulin release.
Mice were deprived of food for 16 h. Blood was collected immediately before as well as 15, 30, 60, and 120 min after the oral administration of glucose (1.5 mg/g of body weight). For measurement of acute insulin release, glucose (3 mg/g) was injected intraperitoneally and blood was collected immediately before and every 2 min for 18 min after injection (19).
Immunoblot analysis.
For determination of the abundance of TSC2, total tissue homogenates were subjected to immunoblot analysis with antibodies to TSC2 (Cell Signaling). Lysates of isolated islets that had been stimulated (or not) with 100 nM recombinant human IGF-1 (Pepro Tech) were prepared as previously described (14, 27). The lysates were probed with antibodies to TSC2, Akt, the phospho-Thr308 form of Akt, the phospho-Ser473 form of Akt, Foxo1, the phospho-Thr24 form of Foxo1, p70 S6 kinase, the phospho-Thr389 form of p70 S6 kinase, 4E-BP1, the phospho-Thr37 and -Thr46 forms of 4E-BP1, S6, the phospho-Ser235 and -Ser236 forms of S6, or the cleaved form of caspase-3 (all from Cell Signaling). Antibodies to insulin receptor substrate 1 (IRS-1) or to IRS-2 (both from Upstate Biotechnology) as well as those to β-actin or to α-tubulin (both from Sigma-Aldrich) were also used.
Immunostaining and morphometric analysis of islets.
Three to five mice of each genotype at 6 or 40 weeks of age were subjected to morphometric analysis. Pancreatic sections were subjected to two-color immunofluorescence staining with antibodies to insulin and to glucagon (both from Dako). For morphometric analysis, images of islets were traced manually and analyzed with the use of WinROOF software (Mitani). The cross-sectional areas of islets with more than five insulin-positive cells were measured for at least three sections separated by 200 μm. Islet density was determined as the number of islets divided by the total area of the pancreas. Total β-cell mass was calculated as the total β-cell area expressed as a percentage of the total area of the pancreas. The sizes of individual β cells were determined as the total β-cell area divided by the total number of β cells, which yielded results similar to those obtained by direct tracing of β cells. The number of β cells was expressed per mm2 of the total area of the pancreas.
Rapamycin treatment.
Control and βTSC2−/− mice were treated from 18 to 40 weeks of age with intraperitoneal injections of 2 mg/kg of body weight of rapamycin (LC Laboratories) every other day. A 10-mg/ml stock solution of rapamycin was made in 100% ethanol, stored at −20°C, diluted to 0.5 mg/ml in vehicle (5% Tween 80 and 5% polyethylene glycol), and then used within 24 h.
Statistical analysis.
Data are presented as means ± standard errors of the means (SEM) and were compared by analysis of variance. A P value of <0.05 was considered statistically significant.
RESULTS
Generation of β-cell-specific TSC2 knockout mice.
We first generated βTSC2−/− mice with the use of mice that overexpress the gene for Cre recombinase under the control of the rat insulin 2 gene promoter (Ins-Cre mice), which were provided by M. Magnuson (22). However, the resulting βTSC2−/− mice died manifesting epileptic seizures around 3 weeks of age, possibly as a result of Cre expression in the brain (18, 21). We therefore generated the βTSC2−/− mice used in the present study with the use of another line of Ins-Cre mice, provided by P. Herrera (10).
We examined whether the insertion of Cre or loxP sequences into the mouse genome affected glucose metabolism or β-cell function. There were no significant differences in blood glucose or plasma insulin concentrations in the fasting or fed state, or in the insulin responses to intraperitoneal glucose administration, among wild-type mice, Ins-Cre mice, or TSC2flox/flox mice at 8 weeks of age (Table 1 and Fig. 1D). We therefore used TSC2flox/flox mice as control animals. Immunoblot analysis showed that the amounts of TSC2 in islets were reduced by ∼90% in βTSC2−/− mice compared with those in control animals (Fig. 1E). The abundances of TSC2 in the brain, hypothalamus, adipose tissue, liver, lung, kidney, and skeletal muscle were similar in βTSC2−/− and control mice (Fig. 1F). These results thus indicated that βTSC2−/− mice indeed are deficient in TSC2, specifically in pancreatic β cells.
TABLE 1.
Blood glucose and plasma insulin concentrations of wild-type, Ins-Cre, and TSC2flox/flox mice at 8 weeks of agea
| Mouse group | Blood glucose concn (mg/dl)
|
Plasma insulin concn (ng/ml)
|
||
|---|---|---|---|---|
| Fasting | Fed | Fasting | Fed | |
| Wild type | 62.0 ± 3.8 | 149.5 ± 11.2 | 0.21 ± 0.03 | 0.72 ± 0.13 |
| Ins-Cre | 63.5 ± 2.3 | 140.3 ± 2.3 | 0.20 ± 0.06 | 0.70 ± 0.09 |
| TSC2flox/flox | 57.7 ± 5.0 | 150.0 ± 5.3 | 0.22 ± 0.03 | 0.82 ± 0.14 |
Data are means ± SEM from four mice of each genotype.
Metabolic characteristics of βTSC2−/− mice.
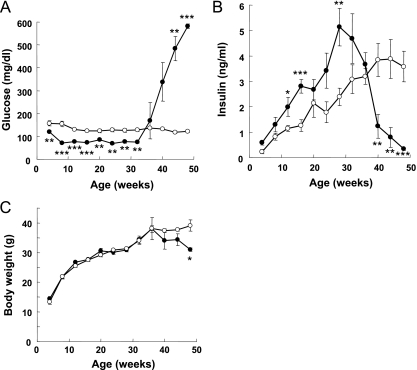
The blood glucose levels of βTSC2−/− mice in the fed state were significantly lower than those in control mice (120 ± 6 versus 158 ± 10 mg/dl) at 4 weeks of age, and this difference was maintained until 32 weeks of age (Fig. 2A). All βTSC2−/− mice survived this hypoglycemic period. The plasma insulin concentrations of βTSC2−/− mice in the fed state tended to be higher at 4 weeks and were significantly higher at 12 weeks than those of control mice (Fig. 2B). The blood glucose and plasma insulin concentrations of βTSC2+/− mice were similar to those of control mice at 8 weeks of age (data not shown). There were no significant differences in the plasma concentrations of free fatty acids, triglyceride, or cholesterol between βTSC2−/− and control mice at 8 or 30 weeks of age (Table 2). The blood glucose levels of βTSC2−/− mice increased markedly after 36 weeks of age, reaching ∼600 mg/dl by 48 weeks (Fig. 2A). Consistent with this observation, the plasma insulin concentrations of βTSC2−/− mice decreased rapidly after 32 weeks and were significantly lower than those of control animals at 40 weeks (Fig. 2B). The body weights of βTSC2−/− mice were also significantly lower than those of control mice after 48 weeks (Fig. 2C).
FIG. 2.
Effect of β-cell TSC2 ablation on glucose metabolism. Blood glucose concentration (A), plasma insulin concentration (B), and body weight (C) are shown for control (open circles; n = 10) and βTSC2−/− (filled circles; n = 12) mice at the indicated ages as measured in the randomly fed state. Data are means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for comparison with the corresponding values for control mice.
TABLE 2.
Metabolic characteristics of control and βTSC2−/− mice at 8 and 30 weeks of agea
| Age and mouse group | Blood glucose concn (mg/dl)
|
Plasma insulin concn (ng/ml)
|
Concn (mg/dl) of:
|
||||
|---|---|---|---|---|---|---|---|
| Fasting | Fed | Fasting | Fed | Plasma FFA | Plasma triglyceride | Plasma cholesterol | |
| 8 wks | |||||||
| Control | 56.9 ± 4.9 | 154.0 ± 12.0 | 0.34 ± 0.08 | 0.82 ± 0.14 | 1.44 ± 0.13 | 59.8 ± 2.7 | 71.7 ± 2.3 |
| βTSC2−/− | 41.0 ± 5.3* | 71.2 ± 2.9*** | 0.84 ± 0.35 | 1.29 ± 0.29 | 1.28 ± 0.18 | 62.5 ± 3.6 | 74.0 ± 3.0 |
| 30 wks | |||||||
| Control | 68.4 ± 5.1 | 126.3 ± 8.4 | 1.14 ± 0.18 | 2.39 ± 0.35 | 1.68 ± 0.06 | 64.3 ± 3.7 | 75.6 ± 3.2 |
| βTSC2−/− | 51.4 ± 4.3* | 77.3 ± 5.3** | 2.02 ± 0.40* | 5.14 ± 0.74** | 1.42 ± 0.19 | 63.2 ± 4.1 | 74.6 ± 3.2 |
Data are means ± SEM (n = 10). *, P < 0.05; **, P < 0.01; ***, P < 0.001 for comparison with the corresponding value for control mice. FFA, free fatty acids.
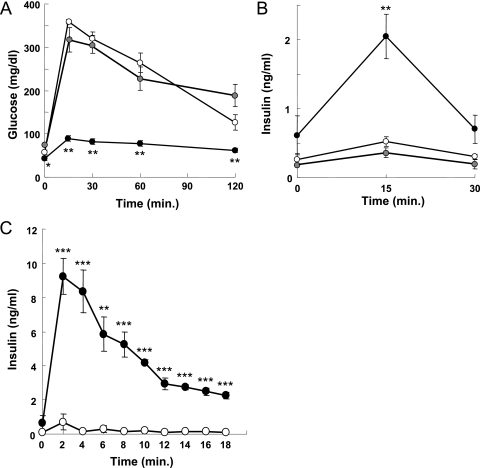
To examine further the hypoglycemia and hyperinsulinemia of young βTSC2−/− mice, we performed oral glucose tolerance tests with 8-week-old animals that had been deprived of food overnight. βTSC2−/− mice exhibited abnormal glucose tolerance, with the increases in blood glucose concentrations after the glucose load being greatly reduced in these mice compared with those in control animals (Fig. 3A). The insulin responses to glucose were also abnormal in βTSC2−/− mice, with the plasma insulin levels 15 min after glucose challenge being significantly higher than those in control mice (Fig. 3B). There were no significant differences in blood glucose or plasma insulin concentrations after oral glucose load between βTSC2+/− and control mice (Fig. 3A and B). We next evaluated acute insulin release in response to intraperitoneal glucose administration. The acute first-phase insulin secretory responses in βTSC2−/− mice were markedly enhanced, with the plasma insulin concentrations in these mice being ∼20 times those in control mice 2 min after glucose challenge and remaining at higher levels thereafter (Fig. 3C).
FIG. 3.
Effect of β-cell-specific ablation of TSC2 on glucose-stimulated insulin secretion in mice at 8 weeks of age. Blood glucose (A) and plasma insulin (B) concentrations during oral glucose tolerance tests are shown for control (open circles; n = 9), βTSC2+/− (gray circles; n = 5), and βTSC2−/− (black circles; n = 10) mice. (C) Acute insulin response to intraperitoneal administration of glucose (3 mg per gram of body weight) in control (open circles; n = 4) and βTSC2−/− (filled circles; n = 4) mice. All data are means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for comparison with the corresponding values for control mice.
Morphology of islets of βTSC2−/− mice.
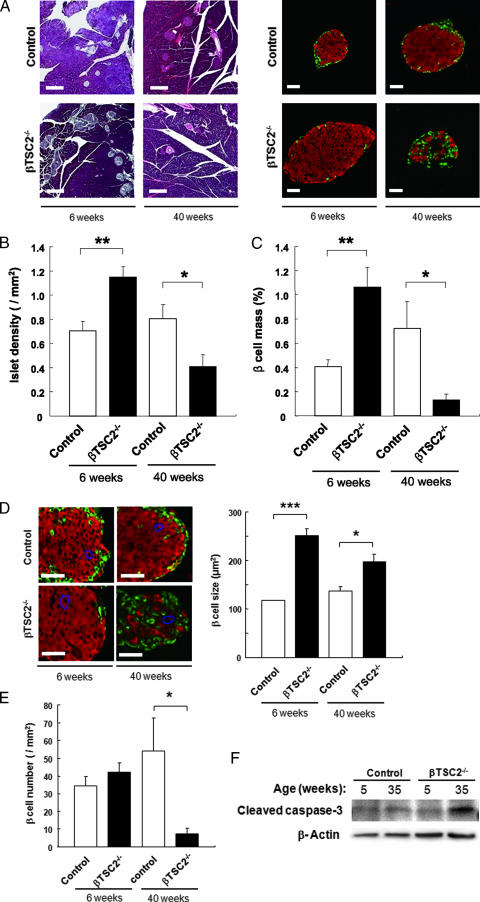
We then examined the effect of TSC2 deficiency on islet morphology. Immunofluorescence analysis revealed that islet densities were increased by ∼50% in βTSC2−/− mice compared with those in control mice at 6 weeks of age (Fig. 4A and B). The total β-cell masses (Fig. 4A and C) and the sizes of individual β cells (Fig. 4D) were also increased by ∼150% and ∼100%, respectively, in βTSC2−/− mice compared with those in control mice at this age. In contrast, β-cell number did not differ between βTSC2−/− and control mice (Fig. 4E). Consistent with the rapid decrease in plasma insulin concentration apparent after 32 weeks of age in βTSC2−/− mice (Fig. 2B), the total β-cell masses were decreased by ∼80% in these animals at 40 weeks of age compared with those in control mice (Fig. 4A and C). Islet density (Fig. 4B) and β-cell number (Fig. 4E) were also decreased in βTSC2−/− mice, by ∼50% and ∼85%, respectively, whereas the sizes of individual β cells remained significantly higher in βTSC2−/− mice than in control mice at this age (Fig. 4D). The abundances of the cleaved form of caspase-3 in islets were similar in control and βTSC2−/− mice at 5 weeks of age but were increased in βTSC2−/− mice compared with those in control animals at 35 weeks (Fig. 4F), suggestive of an increased incidence of β-cell death by apoptosis in βTSC2−/− mice at the latter age.
FIG. 4.
Effects of β-cell TSC2 ablation on pancreatic islet morphology. (A) Pancreatic sections from 6- and 40-week-old control and βTSC2−/− mice were stained with hematoxylin-eosin (left panels) or with antibodies to insulin (red) and to glucagon (green) (right panels). Scale bars, 200 μm (left panels) or 50 μm (right panels). (B) Islet density was determined from the number of islets in pancreatic sections divided by the total area of the pancreas in 6- and 40-week-old control and βTSC2−/− mice. (C) Total β-cell mass was calculated from the area of insulin-positive cells in pancreatic sections divided by the total area of the pancreas in 6- and 40-week-old control and βTSC2−/− mice. (D) Pancreatic sections from 6- and 40-week-old control and βTSC2−/− mice were immunostained as for panel A (left panels). Scale bars, 50 μm. The sizes of individual β cells were determined from the total area of insulin-positive cells in pancreatic sections divided by the number of nuclei in insulin-positive cells. (E) The numbers of β cells in 6- and 40-week-old control and βTSC2−/− mice were determined from the number of β cells in pancreatic sections divided by the total pancreatic area. All quantitative data are means ± SEM (n = 5 and n = 3 for 6- and 40-week-old mice, respectively). *, P < 0.05; **, P < 0.01; ***, P < 0.001. (F) Islets isolated from control and βTSC2−/− mice at 5 or 35 weeks of age were lysed and subjected to immunoblot analysis with antibodies to the cleaved form of caspase-3 and to β-actin.
Signaling molecules underlying the phenotypes of βTSC2−/− mice.
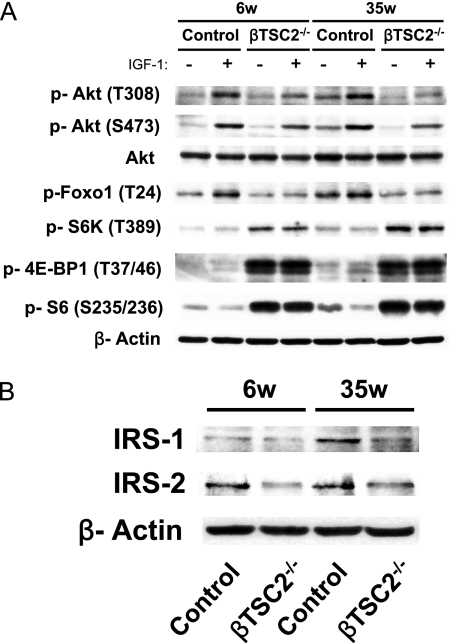
The increase in β-cell mass in βTSC2−/− mice at 6 weeks of age was thus largely attributable to an increase in β-cell size, consistent with a role for TSC2 as a negative regulator of β-cell size. However, at 40 weeks of age, βTSC2−/− mice showed a decreased β-cell mass that was mainly due to a decrease in β-cell number. To clarify the signaling molecules responsible for these phenotypes of βTSC2−/− mice, we isolated islets from control and βTSC2−/− mice at 6 and 35 weeks of age, incubated them with IGF-1 for 1 h, and then subjected them to immunoblot analysis. Ablation of TSC2 increased the amounts of the form of p70 S6 kinase phosphorylated on Thr389, the forms of 4E-BP1 phosphorylated on Thr37 and Thr46, and the forms of S6 phosphorylated on Ser235 and Ser236 at both ages, irrespective of IGF-1 stimulation (Fig. 5A). Given that the phosphorylation of p70 S6 kinase and 4E-BP1 on these residues is mediated by mTORC1 and that of S6 is mediated by p70 S6 kinase, these data suggested that mTORC1 activity in pancreatic β cells was constitutively increased at both 6 and 35 weeks of age as a result of the loss of TSC2.
FIG. 5.
Effects of β-cell TSC2 ablation on insulin signaling in pancreatic islets. Islets isolated from control and βTSC2−/− mice of the indicated ages (in weeks [w]) were deprived of serum for 2 h and then incubated in the presence (+) or absence (−) of 100 nM IGF-1 for 1 h. (A, B) The islets were then lysed and incubated with antibodies for immunoblot analysis of phosphorylated (p) or total forms of the indicated proteins.
Previous studies, including ours, suggesting that Foxo1 is responsible for regulation of β-cell number (8, 15) prompted us to examine IGF-1-induced phosphorylation of Foxo1 in βTSC2−/− mouse islets. The abundances of the Thr24-phosphorylated form of Foxo1 were reduced in the IGF-1-treated islets of βTSC2−/− mice at both 6 and 35 weeks of age (Fig. 5A). We also investigated the effects of TSC2 deletion on the insulin and IGF-1 signaling pathways in pancreatic β cells. PDK1 and TORC2 jointly activate Akt by phosphorylating Akt on Thr308 and Ser473, and Akt phosphorylates TSC2 directly. The abundances of the Thr308- or Ser473-phosphorylated form of Akt were reduced in IGF-1-treated islets of βTSC2−/− mice compared to those in control mice at both 6 and 35 weeks of age (Fig. 5A). Furthermore, the amounts of both IRS-1 and IRS-2 were reduced in βTSC2−/− mice compared to those in controls at both 6 and 35 weeks of age (Fig. 5B). These data suggest that the levels of insulin and IGF-1 signaling in islets were already reduced in βTSC2−/− mice at 6 weeks of age.
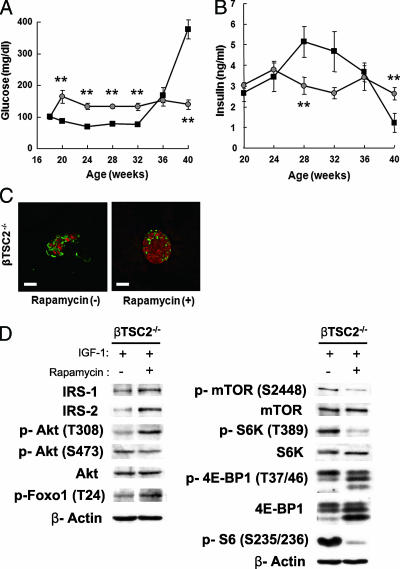
To examine the possibility that chronic activation of mTORC1 might be involved in the reduction of insulin signaling, we delivered an intraperitoneal injection of rapamycin, an inhibitor of mTORC1, into the mice and tested them at 40 weeks of age. Unlike untreated βTSC2−/− mice, rapamycin-treated βTSC2−/− mice did not develop hyperglycemia (Fig. 6A). In addition, rapamycin-treated βTSC2−/− mice did not display a drop in plasma insulin concentrations at 40 weeks of age (Fig. 6B). Moreover, immunohistochemistry revealed that β-cell mass was maintained in rapamycin-treated βTSC2−/− mice compared to that in untreated βTSC2−/− mice at 40 weeks of age (Fig. 6C). Finally, immunoblot analysis of islets isolated from rapamycin-treated and untreated βTSC2−/− mice showed that rapamycin inhibition of mTORC1 led to reduced phosphorylation of p70 S6 kinase and S6 protein (Fig. 6D). In addition, the expressions of IRS-1 and IRS-2 and the phosphorylations of Akt on Thr308 and Foxo1 on Thr24 were increased by rapamycin treatment in islets of βTSC2−/− mice (Fig. 6D). These results suggested that reduced insulin signaling in pancreatic β cells of βTSC2−/− mice is due to the activation of mTORC1.
FIG. 6.
Effects of rapamycin on insulin signaling in pancreatic islets of βTSC2−/− mice. Intraperitoneal injections of rapamycin were delivered to mice at a concentration of 2 mg/kg every other day from 18 to 40 weeks of age. Blood glucose (A) and plasma insulin (B) concentrations are shown for βTSC2−/− mice treated with (gray circles; n = 6) and without (closed squares; n = 6) rapamycin. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) Pancreatic sections from 40-week-old βTSC2−/− mice untreated (−) or treated (+) with rapamycin were stained with antibodies to insulin (red) and glucagon (green). Scale bars, 50 μm. (D) Islets isolated from 28-week-old βTSC2−/− mice untreated (−) or treated (+) with rapamycin were deprived of serum for 2 h and then incubated in the presence of 100 nM IGF-1 for 1 h. The islets were then lysed and subjected to immunoblot analysis with the indicated antibodies.
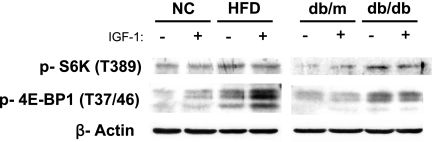
Finally, in order to examine the pathological relevance of mTORC1 activation in pancreatic β cells, we investigated whether the mTORC1 activity was altered in two mouse models of obesity and diabetes: wild-type mice fed a high-fat diet for 3 weeks and 8-week-old mice deficient in the leptin receptor (db/db). As indicated by phosphorylation of p70 S6 kinase on Thr389 and 4E-BP1 on Thr37/46, mTORC1 activity was elevated in the islets of both groups of mice, irrespective of IGF-1 stimulation (Fig. 7).
FIG. 7.
mTORC1 activity in the islets of animal models of obesity and type 2 diabetes. Islets isolated from mice fed with a high-fat diet for 3 weeks (HFD) and 8-week-old db/db mice (db/db) were deprived of serum for 2 h and then incubated in the presence (+) or absence (−) of 100 nM IGF-1 for 1 h. The islets were then lysed and subjected to immunoblot analysis with the indicated antibodies. NC, normal controls; db/m, mice heterozygous for the leptin receptor.
DISCUSSION
With the use of mice with specific gene deletions, either in the whole body or in a specific tissue, several studies have shown that the insulin or IGF-1 signaling pathway plays an important role in maintaining pancreatic islet mass (8, 17, 19, 28, 31). Our previous study with β-cell PDK1-deficient mice prompted us to hypothesize that PDK1 may be responsible for insulin or IGF-1 regulation of both the numbers and the sizes of pancreatic β cells. We found that downstream of PDK1 and Akt the signals diverged: Foxo1 contributed to cell number regulation, and mTOR contributed to cell size regulation (8). To investigate our hypothesis, we generated mice with a pancreatic-specific deletion of TSC2, a negative regulator of mTOR. As expected, these mice manifested increased islet mass largely attributable to increases in the sizes of individual β cells. In addition, the mice displayed hypoglycemia and hyperinsulinemia. Unexpectedly, at 40 weeks of age these mice developed progressive hyperglycemia and hypoinsulinemia accompanied by a reduction in islet mass due predominantly to a reduction in the number of β cells.
Our analysis of β-cell signaling molecules in βTSC2−/− mice revealed an induction of mTORC1 activation and a reduction in IRS/Akt/Foxo1 signaling in mice as young as 6 weeks old. Furthermore, the results with rapamycin, an mTORC1 inhibitor, suggested that the decrease of IRS/Akt/Foxo1 signaling was due to the activation of mTORC1.
Our results are consistent with other studies showing that a TSC2 deficiency in mouse embryonic fibroblasts resulted in the activation of mTORC1; this led to increases in Ser302 phosphorylation of IRS-1 and degradation of the IRS-1 protein through a negative-feedback mechanism mediated by p70 S6 kinase (7). Similarly, chronic activation of mTOR in INS-1 insulinoma cells resulted in increased levels of IRS-2 phosphorylation on Ser and Thr residues, which led to degradation of IRS-2 in the proteasome and increased apoptosis (3). Considering these reports, we speculate that a similar negative-feedback mechanism may operate in vivo in the β cells of βTSC2−/− mice. In support of this notion, mice deficient in p70 S6 kinase remained sensitive to insulin while kept on a high-fat diet as a result of the apparent loss of a negative-feedback loop (29).
Our results suggest that the pancreatic β cells of βTSC2−/− mice likely receive two distinct signals from a young age (6 weeks): one to increase cell size through mTORC1 activation and the other to reduce cell number through nuclear localization of Foxo1, which lacks Thr24 phosphorylation; the latter may possibly be the result of a negative-feedback mechanism induced by mTORC1 activation. It remains unclear why the former signal predominates in younger animals and the latter in older animals. Several other functions of mTORC1 that should be considered in future investigations have been recently reported (2, 24).
Our findings in βTSC2−/− mice at older ages are reminiscent of the decreased islet masses observed in individuals with type 2 diabetes, which also largely appears to be due to a reduction in the number of β cells accompanied by an increase in β-cell size (K. H. Yoon, personal communication). However, several studies have suggested that TSC2 plays a minimal role in the pathogenesis of type 2 diabetes. For example, the ∼70 to 80% depletion of TSC2 in HEK293 cells induced by RNA interference only slightly affected the activities of p70 S6 kinase and Akt (25). Moreover, TSC2 haploinsufficiency in individuals with tuberous sclerosis was not associated with the development of diabetes. Nevertheless, we and others (29) have shown that mTORC1 activity is increased in mice fed a high-fat diet and in db/db mice; thus, mTOR activation in islets may explain the reduced β-cell number and increased β-cell size associated with type 2 diabetes.
Given that an increase in β-cell size is associated with an increase in the level of glucose-stimulated insulin secretion (6), it may be possible to treat diabetes by inducing an increase in β-cell size. Transgenic mice that overexpress a constitutively active form of Akt1 specifically in β cells manifest both increased β-cell size and hyperinsulinemia (1, 26). However, these mice also exhibit increased numbers of β cells and increased β-cell proliferation, which may potentially cause problems in a diabetes treatment for humans. Our previous data (8) suggested that mTORC1 activation in pancreatic β cells may represent an alternative means to increase β-cell size. However, our present results indicate that this approach would not be feasible, because we observed the activation of a negative-feedback mechanism in vivo. In another study, mice lacking both 4E-BP1 and 4E-BP2 displayed increased insulin resistance associated with increased p70 S6 kinase activity and impairment of Akt signaling; this suggested that 4E-BPs indirectly control p70 S6 kinase activity through competition for binding to raptor and subsequent phosphorylation by mTOR (20). Thus, we speculate that an overexpression of Ser/Thr-phosphorylated 4E-BPs, specifically in pancreatic β cells of mice, may increase β-cell size without activating the negative feedback from insulin or IGF-1 signaling. This hypothesis is currently under investigation in our laboratory.
Acknowledgments
We thank K. H. Yoon (Catholic University of Korea, Seoul, Korea) for helpful discussion and S. Hirahara, M. Nagano, M. Oya, and A. Tanida for technical assistance.
This work was supported by a grant for the 21st Century COE Program “Center of Excellence for Signal Transduction Disease: Diabetes Mellitus as Model” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) to M.K.; a grant for the Cooperative Link of Unique Science and Technology for Economy Revitalization (CLUSTER) from MEXT to M.K.; and a Grant-in-Aid for Creative Scientific Research from MEXT to M.K.
Footnotes
Published ahead of print on 3 March 2008.
REFERENCES
- 1.Bernal-Mizrachi, E., W. Wen, S. Stahlhut, C. M. Welling, and M. A. Permutt. 2001. Islet β cell expression of constitutively active Akt1/PKBα induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J. Clin. Investig. 1081631-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonawitz, N. D., M. Chatenay-Lapointe, Y. Pan, and G. S. Shadel. 2007. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 5265-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briaud, I., L. M. Dickson, M. K. Lingohr, J. F. McCuaig, J. C. Lawrence, and C. J. Rhodes. 2005. Insulin receptor substrate-2 proteasomal degradation mediated by a mammalian target of rapamycin (mTOR)-induced negative feedback down-regulates protein kinase B-mediated signaling pathway in β-cells. J. Biol. Chem. 2802282-2293. [DOI] [PubMed] [Google Scholar]
- 4.Butler, A. E., J. Janson, S. B. Weir, R. Ritzel, R. A. Rizza, and P. C. Butler. 2003. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52102-110. [DOI] [PubMed] [Google Scholar]
- 5.Garami, A., F. J. Zwartkruis, T. Nobukuni, M. Joaquin, M. Roccio, H. Stocker, S. C. Kozma, E. Hafen, J. L. Bos, and G. Thomas. 2003. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol. Cell 111457-1466. [DOI] [PubMed] [Google Scholar]
- 6.Giordano, E., V. Cirulli, D. Bosco, D. Rouiller, P. Halban, and P. Meda. 1993. B-cell size influences glucose-stimulated insulin secretion. Am. J. Physiol. 265C358-C364. [DOI] [PubMed] [Google Scholar]
- 7.Harrington, L. S., G. M. Findlay, A. Gray, T. Tolkacheva, S. Wigfield, H. Rebholz, J. Barnett, N. R. Leslie, S. Cheng, P. R. Shepherd, I. Gout, C. P. Downes, and R. F. Lamb. 2004. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J. Cell Biol. 166213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto, N., Y. Kido, T. Uchida, S. Asahara, Y. Shigeyama, T. Matsuda, A. Takeda, D. Tsuchihashi, A. Nishizawa, W. Ogawa, Y. Fujimoto, H. Okamura, K. C. Arden, P. L. Herrera, T. Noda, and M. Kasuga. 2006. Ablation of PDK1 in pancreatic β cells induces diabetes as a result of loss of β cell mass. Nat. Genet. 38589-593. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto, N., Y. Kido, T. Uchida, T. Matsuda, K. Suzuki, H. Inoue, M. Matsumoto, W. Ogawa, S. Maeda, H. Fujihara, Y. Ueta, Y. Uchiyama, K. Akimoto, S. Ohno, T. Noda, and M. Kasuga. 2005. PKCλ regulates glucose-induced insulin secretion through modulation of gene expression in pancreatic β cells. J. Clin. Investig. 115138-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrera, P. L. 2000. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 1272317-2322. [DOI] [PubMed] [Google Scholar]
- 11.Inoki, K., Y. Li, T. Zhu, J. Wu, and K. L. Guan. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signaling. Nat. Cell Biol. 4648-657. [DOI] [PubMed] [Google Scholar]
- 12.Inoki, K., Y. Li, T. Xu, and K. L. Guan. 2003. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 171829-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kandt, R. S., J. L. Haines, M. Smith, H. Northrup, R. J. M. Gardner, M. P. Short, K. Dumars, E. S. Roach, S. Steingold, S. Wall, S. H. Blanton, P. Flodman, D. J. Kwiatkowski, A. Jewell, J. L. Weber, A. D. Roses, and M. A. Pericak-Vance. 1992. Linkage of an important gene locus for tuberous sclerosis to a chromosome 16 marker for polycystic kidney disease. Nat. Genet. 237-41. [DOI] [PubMed] [Google Scholar]
- 14.Kido, Y., D. J. Burks, D. Withers, J. C. Bruning, C. R. Kahn, M. F. White, and D. Accili. 2000. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J. Clin. Investig. 105199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitamura, T., J. Nakae, Y. Kitamura, Y. Kido, W. H. Biggs, C. V. Wright, M. F. White, K. C. Arden, and D. Accili. 2002. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J. Clin. Investig. 1101839-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi, T., O. Minowa, J. Kuno, H. Mitani, O. Hino, and T. Noda. 1999. Renal carcinogenesis, hepatic hematogiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res. 591206-1211. [PubMed] [Google Scholar]
- 17.Kubota, N., K. Tobe, Y. Terauchi, K. Eto, T. Yamauchi, R. Suzuki, Y. Tsubamoto, K. Komeda, R. Nakano, H. Miki, S. Satoh, H. Sekihara, S. Sciacchitano, M. Lesniak, S. Aizawa, R. Nagai, S. Kimura, Y. Akanuma, S. I. Taylor, and T. Kadowaki. 2000. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes 491880-1889. [DOI] [PubMed] [Google Scholar]
- 18.Kubota, N., Y. Terauchi, K. Tobe, W. Yano, R. Suzuki, K. Ueki, I. Takamoto, H. Satoh, T. Maki, T. Kubota, M. Moroi, M. Okada-Iwabu, O. Ezaki, R. Nagai, Y. Ueta, T. Kadowaki, and T. Noda. 2004. Insulin receptor substrate 2 plays a crucial role in β cells and the hypothalamus. J. Clin. Investig. 114917-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni, R. N., J. C. Bruning, J. N. Winnay, C. Postic, M. A. Magnuson, and C. R. Kahn. 1999. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96329-339. [DOI] [PubMed] [Google Scholar]
- 20.Le Bacquer, O., E. Petroulakis, S. Paglialunga, F. Poulin, D. Richard, K. Cianflone, and N. Sonenberg. 2007. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J. Clin. Investig. 117387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, X., A. Taguchi, S. Park, J. A. Kushner, F. Li, Y. Li, and M. F. White. 2004. Dysregulation of insulin receptor substrate 2 in β cells and brain causes obesity and diabetes. J. Clin. Investig. 114908-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postic, C., M. Shiota, K. D. Niswender, T. L. Jetton, Y. Chen, J. M. Moates, K. D. Shelton, J. Lindner, A. D. Cherrington, and M. A. Magnuson. 1999. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274305-315. [DOI] [PubMed] [Google Scholar]
- 23.Potter, C. J., H. Huang, and T. Xu. 2001. Drosophila TSC1 functions with TSC2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell 105357-368. [DOI] [PubMed] [Google Scholar]
- 24.Ravikumar, B., C. Vacher, Z. Berger, J. E. Davies, S. Luo, L. G. Oroz, F. Scaravilli, D. F. Easton, R. Duden, C. J. O'Kane, and D. C. Rubinsztein. 2004. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 36585-595. [DOI] [PubMed] [Google Scholar]
- 25.Shah, O. J., Z. Wang, and T. Hunter. 2004. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 141650-1656. [DOI] [PubMed] [Google Scholar]
- 26.Tuttle, R. L., N. S. Gill, W. Pugh, J. P. Lee, B. Koeberlein, E. E. Furth, K. S. Polonsky, A. Naji, and M. J. Birnbaum. 2001. Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat. Med. 71133-1137. [DOI] [PubMed] [Google Scholar]
- 27.Uchida, T., T. Nakamura, N. Hashimoto, T. Matsuda, K. Kotani, H. Sakaue, Y. Kido, Y. Hayashi, K. I. Nakayama, M. F. White, and M. Kasuga. 2005. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat. Med. 11175-182. [DOI] [PubMed] [Google Scholar]
- 28.Ueki, K., T. Okada, J. Hu, C. W. Liew, A. Assmann, G. M. Dahlgren, J. L. Peters, J. G. Shackman, M. Zhang, I. Artner, L. S. Satin, R. Stein, M. Holzenberger, R. T. Kennedy, C. R. Kahn, and R. N. Kulkarni. 2006. Total insulin and IGF-1 resistance in pancreatic β cells causes overt diabetes. Nat. Genet. 38583-588. [DOI] [PubMed] [Google Scholar]
- 29.Um, S. H., F. Frigerio, M. Watanabe, F. Picard, M. Joaquin, M. Sticker, S. Fumagalli, P. R. Allegrini, S. C. Kozma, J. Auwerx, and G. Thomas. 2004. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431200-205. [DOI] [PubMed] [Google Scholar]
- 30.van Slegtenhorst, M., R. de Hoogt, C. Hermans, M. Nellist, B. Janssen, S. Verhoef, D. Lindhout, A. van den Ouweland, D. Halley, J. Young, M. Burley, S. Jeremian, K. Woodward, J. Nahmias, M. Fox, R. Ekong, J. Osborne, J. Wolfe, S. Povey, R. G. Snell, J. P. Cheadle, A. C. Jones, M. Tachataki, D. Ravine, J. R. Sampson, M. P. Reeve, P. Richardson, F. Wilmer, C. Munro, T. L. Hawkins, T. Sepp, J. B. M. Ali, S. Ward, A. J. Green, J. R. W. Yates, J. Kwiatkowska, E. P. Henske, M. P. Short, E. P. Haines, M. P. Short, J. H. Haines, S. Jozwiak, and D. J. Kwiatkowski. 1997. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277805-808. [DOI] [PubMed] [Google Scholar]
- 31.Withers, D. J., J. S. Gutierrez, H. Towery, D. J. Burks, J. M. Ren, S. Previs, Y. Zhang, D. Bernal, S. Pons, G. I. Shulman, S. B. Weir, and M. F. White. 1998. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391900-904. [DOI] [PubMed] [Google Scholar]
- 32.Wu, H., X. Liu, and R. Jaenisch. 1994. Double replacement: strategy for efficient introduction of subtle mutations into the murine Col1a-1 gene by homologous recombination in embryonic sytem cells. Proc. Natl. Acad. Sci. USA 912819-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]