Abstract
Wnt regulation of muscle development is thought to be mediated by the β-catenin-TCF/LEF-dependent canonical pathway. Here we demonstrate that β-catenin, not TCF/LEF, is required for muscle differentiation. We showed that β-catenin interacts directly with MyoD, a basic helix-loop-helix transcription factor essential for muscle differentiation and enhances its binding to E box elements and transcriptional activity. MyoD-mediated transactivation is inhibited in muscle cells when β-catenin is deficient or the interaction between MyoD and β-catenin is disrupted. These results demonstrate that β-catenin is necessary for MyoD function, identifying MyoD as an effector in the Wnt canonical pathway.
Canonical Wnt signaling via β-catenin (β-Cat) has been shown to play a critical role in muscle development (5, 16, 34). Upon Wnt stimulation, activated Dishevelled prevents the destruction complex, including glycogen synthase kinase 3β, axin, and adenomatous poliposis coli protein from targeting ubiquitinated β-Cat by βTrCP. Stabilized β-Cat translocates to nucleus and presumably via forming a complex with TCF/LEF and subsequently activates expression of basic helix-loop-helix (bHLH) myogenic regulatory factors (MRFs) such as Myf5 and MyoD for initiation of myogenesis (10, 47). The Wnt/β-Cat pathway is also necessary for myogenic specification of muscle-derived CD45+ stem cells in response to injury (37), vertebrate limb regeneration (18), and differentiation of multipotent cells into myogenic cells (3, 35, 42, 48). These myogenic programs are thought to require TCF/LEF to activate MRF expression (36). While the canonical Wnt signaling pathway has been studied in earlier steps of muscle development, less is known about its role in muscle differentiation.
bHLH factors bind to E box elements in the promoters of many tissue-specific genes and activate their gene expression (24, 38, 43). Cell fate determination and differentiation in a variety of tissues depend upon the function of different transcription factors, including bHLH factors (12, 17). For instance, MRFs such as MyoD, Myf5, myogenin, and MRF4 are essential for muscle cell fate determination and differentiation (24, 38). When ectopically expressed in other cell types, MRFs including MyoD are able to initiate muscle differentiation (4). They may form complexes with other transcriptional activators (histone acetyltransferase, MEF2, SRF, and retinoblastoma protein) or repressors (mSin3A, NCoR, and histone deacetylase) to coordinate muscle differentiation (40). Deletion of the MyoD gene in mice has no notable defect in skeletal muscle (44), whereas mice deficient in both MyoD and Myf5, a member of the MyoD family, lack myoblasts and differentiated skeletal muscle (45). These observations suggest that MyoD and Myf5 may have functional redundancy in regulation of muscle-specific gene expression.
In the present study, we investigated the role and underlying mechanisms of Wnt signaling during muscle differentiation. We show that β-Cat, but not TCF/LEF, was required for muscle differentiation. Our results demonstrate that β-Cat directly interacts with MyoD and enhances its transcriptional activity necessary for muscle differentiation. These observations implicate the β-Cat/MyoD complex, instead of the β-Cat/TCF complex, in Wnt-mediated regulation of muscle differentiation, identifying MyoD as an effector of the Wnt canonical pathway.
MATERIALS AND METHODS
Cell culture, transfection, luciferase assay, and immunoprecipitation.
C2C12 cells were maintained as described previously (19). Myoblasts (80% confluence) were transiently cotransfected with a muscle-specific tyrosine kinase (MuSK) promoter-luciferase reporter and pRL-TK (as control for transfection efficiency and sample handling) in a ratio of 10:1 using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. In some experiments, cotransfection included wild-type and mutant β-Cat and/or MyoD (in a ratio of 50:10:1 β-Cat/MyoD to reporter to pRL-TK). Twenty-four hours after transfection, myoblasts were switched to the differentiation medium (DM) and luciferase assays were performed after myotubes were fully developed using the dual-luciferase kit (Promega). The firefly luciferase activity was normalized by Renilla luciferase activity. Immunoprecipitation and immunoblotting were performed as described previously (19). Unless otherwise indicated, all experiments were repeated at least three times. 10T1/2 cells were transfected with the indicated vectors and cultured in growth medium (GM) for 24 h. They were then maintained in GM or switched to DM for 4 days. Lysates were examined for expression of myosin heavy chain (MHC) or interaction of myogenic factors and β-catenin.
Plasmid constructs and antibodies.
Antibodies were purchased from Santa Cruz (MyoD, sc-760; Myf5, sc-302; and E47, sc-763), Pharmingen (β-Cat, 610154), and the Developmental Studies Hybidoma Bank (MF20, F5D, and 9E10). β-Catenin and deletion mutants were amplified by PCR with Pfu Turbo (Stratagene) and subcloned between BamHI and EcoRI in pKH3 in frame downstream from the three-hemagglutinin (HA) epitope. Mouse Myf5, MyoD, and deletion mutants were subcloned between EcoRI and XbaI in pCS2+ in frame downstream of the six-Myc epitope. 4RE-Luc contains four E box elements from the MCK enhancer (27, 39), which were generated with the following pair of oligonucleotides (sense, 5′ TCGACCAACACCTGCTGCCCCAACACCTGCTGCCCCAACACCTGCTGCCCCAACACCTGCTGCCGATCTGGGTATATAATGGA; E box elements underlined) subcloned between XhoI and HindIII in pGL2-Basic (Promega). pBS/U6-β-Cat, which encodes 5′ GGAATCCATTCTGGTGCCAC of mouse β-Cat (NM_007614) was constructed as described previously (28, 46). pSuper-E2a encoding common E2a sequence, 5′ GCATGGATCTGAGGTTAATGG (mouse E47, NM_011548) was constructed as described previously (7). His-tagged β-Cat, β-Cat141-519, and MyoD were subcloned in pTrc-His (Invitrogen) and purified.
ChIP assay.
Chromatin immunoprecipitation (ChIP) assays were carried out as described previously (19). Embryonic muscles were chopped into slices and incubated with 1% formaldehyde in phosphate-buffered saline (PBS) at room temperature for 30 min. The cross-linking reaction was stopped by the addition of glycine to a final concentration of 0.125 M. Eluted DNA was used as template in PCR with the following primers: MuSK (335 bp), sense, 5′ TTCCTCATTTGAGTGCAGGA, antisense 5′ GCTCCCGGGTTTACATTTTA; AChRα (237 bp), sense, 5′ GACAAGCCTCTGACTCATGATCTATGT, antisense, 5′ GCTGCCGGTCCTACTCCACCCTGGCT; and MHC (306 bp), sense, 5′ TGAGTAGGGACCTGGCTTTG, antisense, 5′ GCACCCCAGCTTCACTTTTA (26).
EMSA.
Electrophoretic mobility shift assays (EMSA) were carried out as previously described (19). Oligonucleotides containing the E box element of the MCK enhancer, 5′ GATCCAACACCTGCTGCCTGAG (sense), were labeled with [γ-32P]ATP by polynucleotide kinase. 32P-labeled double-stranded oligonucleotide probes were incubated with His-tagged MyoD and/or β-Cat. The reaction was resolved onto a 6% acrylamide gel in 0.5× Tris-borate-EDTA buffer. Binding complexes were visualized by autoradiogram.
Generation of 35S-labeled proteins and pull-down assays.
To generate the recombinant β-Cat and MyoD, the template DNAs (pCS2-MyoD and pCDNA3-β-Cat) were linearized with the respective gene downstream from the SP6 and T7 promoter, respectively. Linearized DNAs were added to the coupled reticulocyte lysate system (Promega) that first transcribed the gene into mRNAs and subsequently translated the mRNAs into 35S-labeled proteins in the presence of [35S]methionine. The binding partners (i.e., glutathione S-transferase [GST]-MyoD1-318 and GST-β-Cat) were generated in bacteria and purified and conjugated to beads. They were incubated with the respective 35S-labeled proteins in the binding buffer (25 mM HEPES [pH 7.5], 1 mM dithiothreitol, 0.5% Triton X-100, 150 mM NaCl) for 2 h at 4°C on a rotator. After washing with PBS-Tween, bound 35S-labeled proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography.
Reverse transcription-PCR.
Standard reverse transcription-PCR was performed using 5 μg of total RNA isolated from cells or tissues with the following primers: GAPDH (glyceraldehyde-3-phosphate dehydrogenase), sense, 5′ ACCACAGTCCATGCCATCAC, antisense, 5′ TCCACCACCCTGTTGCTGTA; TCF1, sense, 5′ AAACACAGGCAGAACCCAA, antisense, 5′ AGCACTGTCATCGGAAGGAA; TCF3, sense, 5′ TGTTGTTCAGCACCCTCATCA, antisense, 5′ ATTCCTGAGGTGGGCAGA; TCF4, sense, 5′ AACAAAGTACCGGTGGTGCAA, antisense, 5′ TAGATGCGTTGACTGTCAGCG; and LEF, sense, 5′ CACCCTCCAGCTCCTGAAAT, antisense, 5′ TGCCCAGGATCTGGTTGATA.
RESULTS AND DISCUSSION
Essential role of β-Cat in muscle differentiation in vitro.
To investigate the molecular mechanism of β-Cat in muscle differentiation, we first determined whether β-Cat is necessary for muscle differentiation by using a small interferening RNA approach. pBS/U6-β-Cat, which encoded β-Cat short hairpin RNA in pBS/U6 (46), efficiently suppressed expression of coexpressed β-Cat in COS-7 cells and endogenous β-Cat in Wnt3a-stimulated 10T1/2 cells and in C2C12 cells (see Fig. S1 in the supplemental material). Mouse C2C12 cells remain as myoblasts in the GM but differentiate into multinucleated myotubes when cultured in the DM in a manner dependent on MRFs such as MyoD and myogenin (50). C2C12 cells are a well-established model of muscle differentiation and have been used to investigate underlying mechanisms (8, 11, 21, 22, 31, 33).
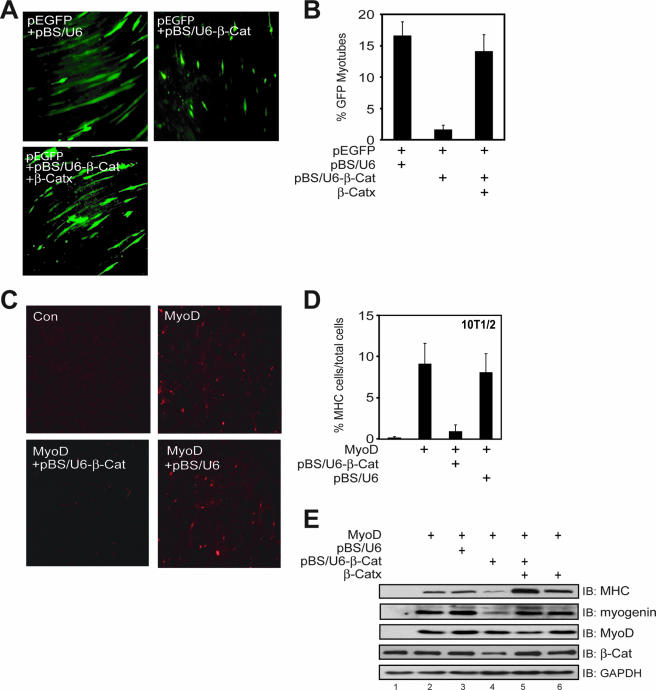
C2C12 cells transfected with control pBS/U6 formed normal myotubes. However, myotube differentiation was inhibited in C2C12 cells transfected with pBS/U6-β-Cat (Fig. 1A and B). This effect could be rescued by the refractory Xenopus β-Cat, β-Catx (Fig. 1A and B; see Fig. S1B in the supplemental material). To further investigate the role of β-Cat in muscle development, we studied multipotent 10T1/2 cells, which undergo myogenic conversion upon expression of exogenous MyoD (4). Four days after being cultured in the DM, MyoD-transfected 10T1/2 cells became positive with MHC, a marker of muscle differentiation (Fig. 1C, upper panel, right). The conversion by MyoD required β-Cat because MHC-positive cells were reduced by pBS/U6-β-Cat. In contrast, the empty pBS/U6 vector had no effect on MyoD-mediated differentiation monitored by MHC expression (Fig. 1D and E).
FIG. 1.
Essential role of β-Cat in muscle differentiation and requirement of β-Cat in MyoD-induced conversion of 10T1/2 fibroblasts into muscle cells. (A) β-Cat is necessary for C2C12 differentiation. C2C12 myoblasts were transfected with pEGFP-C1 and pBS/U6 or pBS/U6-β-Cat (1:20) alone or with β-Catx. Twenty-four hours after transfection, cells were switched to the DM for 4 days. Shown are representative images of GFP-expressing cells under the low-magnification microscope. (B) Quantitative analysis of data in panel A (mean ± standard error of the mean; n = 4). (C) 10T1/2 cells were transfected with pCS2-MyoD with or without pBS/U6 or pBS/U6-β-Cat. Twenty-four hours after transfection, cells were switched to the DM for 4 days, fixed with 4% paraformaldehyde, and stained with monoclonal anti-MHC antibody, which was visualized by rhodamine-conjugated antimouse antibody. Con, control. (D) Quantitative analysis of data in panel C (mean ± standard error of the mean; n = 4). (E) MyoD induction of MHC and myogenin expression in 10T1/2 cells requires β-Cat. 10T1/2 cells were transfected with pcDNA3 (lane 1) or pCS2-MyoD (lanes 2 to 6). pBS/U6 or pBS/U6-β-Cat was cotransfected in the indicated lanes alone or with β-Catx. Four days after growing in the DM, cells were lysed and the resulting lysates subjected to Western immunoblot (IB) analysis using the indicated antibodies. β-Catx was a nondegradable mutant, in which serine 33 was replaced with alanine.
To further investigate the role of β-Cat, we examined expression of MHC and myogenin, another marker of muscle differentiation in MyoD-transfected 10T1/2 cells (Fig. 1E). Cotransfection with pBS/U6-β-Cat reduced levels of both MHC and myogenin without altering MyoD expression (Fig. 1E, see lane 4), suggesting that the effect is not mediated by suppressing MyoD expression. Remarkably, the inhibitory effect can be rescued by coexpression of the refractory β-Catx (Fig. 1E, compare lane 4 to lane 5). Impaired muscle differentiation did not appear to be caused by apoptosis due to β-Cat knockdown. First, the numbers of green fluorescent protein (GFP)-positive myoblasts were similar between control and pBS/U6-β-Cat-transfected cells. Second, the numbers of apoptotic cells after myotube formation (3 days after the switch to the differentiation medium) were also similar between control and pBS/U6-β-Cat-transfected cells (data not shown). These observations indicate a crucial role of β-Cat in MyoD-dependent muscle differentiation.
TCF/LEF and cell adhesion function of β-Cat are dispensable for muscle differentiation.
β-Cat has two well-characterized functions. First, it associates with TCF/LEF to regulate gene expression in the canonical pathway of Wnt signaling (41). Second, it is crucial in forming the cell adhesion complex by interacting with cadherins and α-Cat (32). We therefore investigated which of the two functions may be involved in muscle differentiation.
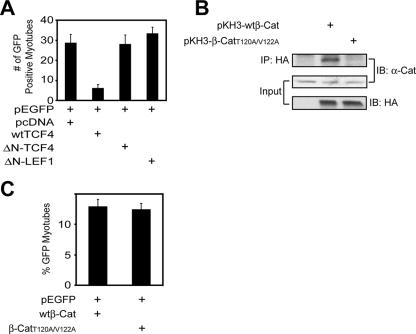
First, we determined whether TCF/LEF is required for β-Cat regulation of muscle differentiation. To test this, we used loss-of-function strategy for involvement of TCF/LEF. N-termini of TCF/LEF proteins bind to β-Cat (23, 30). TCF/LEF proteins without N terminus are unable to associate with β-Cat and thus function as a dominant-negative inhibitor to suppress the canonical pathway of Wnt signaling (30). Accordingly, expression of ΔN-TCF4 has been shown to inhibit β-Cat induction of the TopFlash reporter in SW480 cells that express TCF1, TCF4, and LEF1 (15, 23). Interestingly, ΔN-TCF4 or ΔN-LEF1 had little effect on C2C12 differentiation (Fig. 2A). These results suggest that TCF/LEF is not necessary for muscle differentiation. Consistent with this, TCF/LEF was expressed in myoblasts, but not in myotubes (see Fig. S2 in the supplemental material). Expression of wild-type TCF4 appeared to inhibit muscle differentiation (Fig. 2A), presumably due to neutralizing β-Cat for activating MyoD.
FIG. 2.
TCF/LEF and cell adhesion function of β-Cat are dispensable for muscle differentiation during muscle differentiation. (A) Compromised differentiation in C2C12 cells expressing wild-type TCF4 (wtTCF4) but not ΔN-TCF4 or ΔN-LEF1. C2C12 myoblasts were transfected with pEGFP-C1 and the indicated expression vectors. GFP-expressing cells were counted as in Fig. 1A and B. (B) β-CatT120A/V122A did not interact with α-Cat. HA-tagged wild-type β-Cat (pKH3-wtβ-Cat) or pKH3-β-CatT120A/V122A was transfected in COS-7 cells. Lysates were subjected to immunoprecipitation (IP) with antibody against HA, and the resulting precipitates were immunoblotted (IB) with the indicated antibodies. Lysates were also blotted for α-Cat and HA-tagged proteins to indicate their expression. (C) Expression of β-CatT120A/V122A had little effect on muscle differentiation in C2C12 cells. C2C12 myoblasts were transfected with pEGFP-C1 together with pKH3-wtβ-Cat and pKH3-β-CatT120A/V122A in a ratio of 1:20. Twenty-four hours after transfection, cells were switched to the DM for 4 days. GFP-expressing cells were counted as in Fig. 1A and B.
Next, we determined whether β-Cat deficiency-impaired muscle differentiation results from disruption of cell adhesion. The key amino acid residues in β-Cat to interact with α-Cat have been mapped to Thr-120 and Val-122 (1, 2). The replacement with alanines (hereafter, β-CatT120A/V122A) prevented endogenous β-Cat from binding to α-Cat, and thus β-CatT120A/V122A functions as a dominant-negative inhibitor of cell adhesion (Fig. 2B). However, expression of β-CatT120A/V122A had little effect on muscle differentiation (Fig. 2C), suggesting that the cell adhesion function of β-Cat is dispensable in C2C12 differentiation. Cell adhesion may be maintained by γ-Cat, which has a similar function to β-Cat (52) and is expressed in muscle cells (data not shown). Taken together, these results demonstrate that TCF/LEF and the cell adhesion function of β-Cat are not necessary for muscle differentiation, suggesting that β-Cat functions via a yet-unidentified mechanism.
Dependence of β-Cat regulation of muscle-specific gene expression on E box elements.
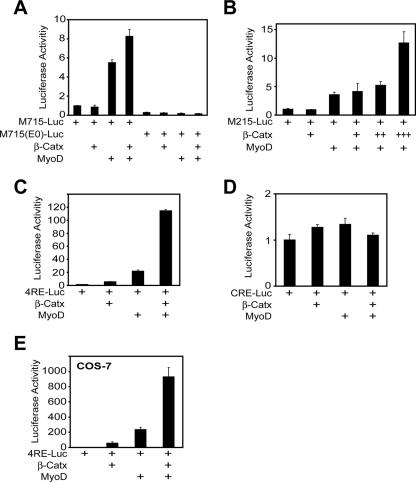
Because suppression of β-Cat expression inhibited MyoD-dependent muscle differentiation, we reasoned that β-Cat may regulate transcriptional activity of MyoD. To test this idea, we examined effects of β-Cat on M715-Luc, a reporter of MuSK, a muscle-specific tyrosine kinase whose expression is regulated by MyoD (20). M715-Luc expression in C2C12 myotubes was enhanced by MyoD, consistent with previous studies (19, 20). Remarkably, the effect was further elevated by coexpression of β-Cat (Fig. 3A), suggesting that β-Cat enhances transcriptional activity of MyoD. To investigate the underlying mechanism, we determined whether the E box element is necessary for β-Cat activation. M715-Luc contains four E box elements (20). Inactivation of the four E box elements by mutation (20) abolished MyoD activation and prevented β-Cat from potentiating MyoD-mediated activity (Fig. 3A). To further demonstrate that β-Cat regulation is via E box elements, we tested the effect of β-Cat on M215-Luc that contains only one E box element without a consensus TCF element (20). As shown in Fig. 3B, β-Cat enhanced MyoD-mediated expression of M215-Luc in a dose-dependent manner. These results suggest that β-Cat acts through E box elements, but not TCF elements. In line with this notion were studies of cyclic AMP-responsive element (CRE)-Luc and 4RE-Luc, a reporter that contained only four repeats of the E box element in the muscle creatine kinase promoter and no other cis-elements (27, 39). As expected, coexpression of β-Cat and/or MyoD increased 4RE-Luc but not CRE-Luc activity (Fig. 3C and D, respectively). Finally, the effect of β-catenin could be reconstituted in nonmuscle COS-7 cells (Fig. 3E), suggesting that MyoD may be a primary target of β-Cat. Taken together, these results demonstrate an essential role of β-Cat, but not TCF/LEF, to promote MyoD activity via the E box element.
FIG. 3.
β-Cat-dependent regulation of muscle-specific gene expression. (A) MyoD-induction of MuSK promoter activity was enhanced by β-Cat in C2C12 myotubes, whereas ablation of E box elements in MuSK promoter led to loss of reporter activity. (B) E box elements were sufficient for β-Cat to potentiate MyoD transcriptional activity in M215-Luc that contains one E box element. +, ++, and +++ indicate relative amounts of DNA used in transfection. (C) E box elements in 4RE-Luc, which contain only four repeats of the E box element in MCK, were sufficient for β-Cat to potentiate MyoD transcriptional activity in C2C12 myotubes. (D) No effect of β-Cat or MyoD on CRE-Luc expression in C2C12 myotubes. (E) The effect of β-Cat was reconstituted in nonmuscle COS-7 cells, which do not express endogenous MyoD. β-Catx was a nondegradable mutant. Twenty-four hours after transfection, luciferase assays were performed as described in Materials and Methods.
β-Cat interaction with MyoD.
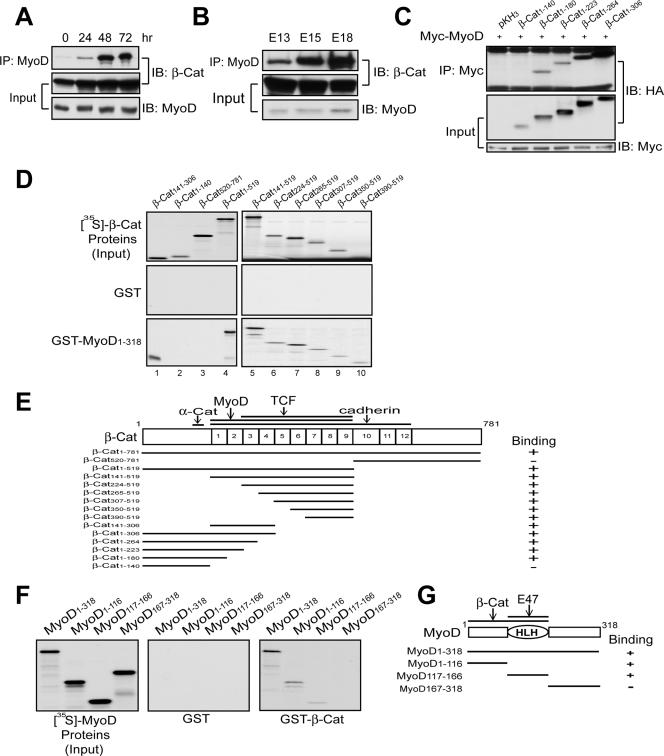
The requirement of E box elements in β-Cat-enhanced MyoD activity suggests that MyoD is a target of β-catenin. To test this hypothesis, we studied the interaction between two proteins in muscle cells. MyoD was precipitated with an anti-MyoD antibody that recognized specifically MyoD, but not other proteins (see Fig. S3A in the supplemental material). This antibody brought down endogenous β-Cat in C2C12 cells and in embryonic muscles (Fig. 4A and B). To map the region in β-Cat necessary for binding to MyoD, HA-tagged β-Cat proteins were coexpressed with Myc-MyoD in COS-7 cells. As shown in Fig. 4C, the N-terminal region of β-Cat was not involved in the binding because β-Cat1-140 did not coprecipitate with MyoD. In contrast, β-Cat-containing Armadillo domains 1 to 4 interacted with MyoD. To determine whether β-Cat interacts directly with MyoD, not via a third component, and to identify the domain in β-Cat necessary for MyoD binding, 35S-labeled proteins were incubated with GST-MyoD immobilized on agarose beads. GST-MyoD, but not GST, bound to full-length β-Cat (Fig. 4D). The C-terminal region (amino acids 520 to 781) including Armadillo domains 10 to 12 was dispensable for the interaction. These results indicate that the binding domain for MyoD is localized in Armadillo domains 1 to 9 (Fig. 4D and E). Reciprocal binding assays were performed with 35S-labeled MyoD proteins and GST-β-Cat immobilized on agarose beads. GST-β-Cat, but not GST, bound to full-length MyoD (Fig. 4F). The β-Cat binding site in MyoD was located in the N-terminal region and HLH domain (Fig. 4F and G). In addition to MyoD, β-Cat also associated with myogenin and Myf5 (see Fig. S3 in the supplemental material). These results indicate a direct interaction between β-Cat and MRFs.
FIG. 4.
β-Cat interaction with MyoD. (A) Characterization of the β-Cat-MyoD interaction in C2C12 cells. C2C12 cells were collected at different times in DM. Lysates were subjected to immunoprecipitation (IP) and immunoblotting (IB) with the indicated antibodies. (B) β-Cat/MyoD complex in embryonic muscles. Rat muscle homogenates were subjected to IP and IB. (C) The N-terminal region of β-Cat does not bind to MyoD. Coexpressed COS-7 cells were subjected to IP with anti-Myc antibody and subsequent IB with anti-HA antibody. (D) Armadillo domains 1 to 9 interact with MyoD. 35S-labeled β-Cat proteins were incubated with GST alone (middle panels) or GST-MyoD1-318 (bottom panels) immobilized on beads. MyoD-associated β-Cat proteins were visualized by autoradiogram. (E) Schematic diagram of β-Cat constructs and their binding activity to MyoD. (F) β-Cat directly binds to the basic and HLH domains of MyoD. 35S-labeled MyoD proteins were incubated with GST alone (middle panel) or GST-β-Cat (right panel) immobilized on beads. (G) Schematic diagram of MyoD constructs and their binding activity to β-Cat.
β-Cat associates with E box elements and increases MyoD binding to E box elements.
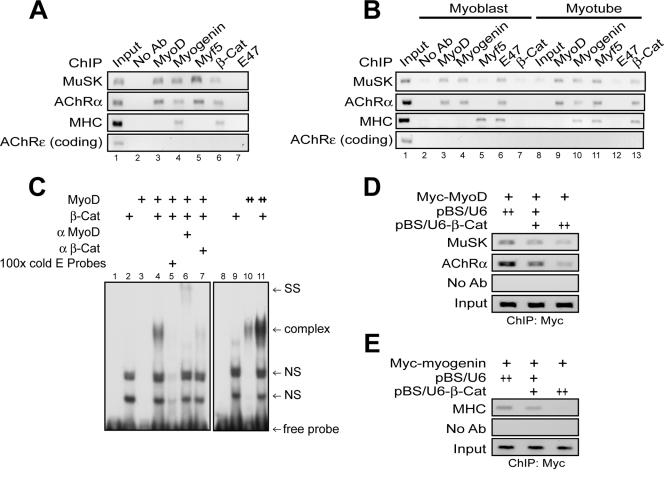
If β-Cat is necessary for muscle differentiation by direct interaction with MyoD, it should be present in the promoters of muscle-specific genes. We tested this hypothesis using ChIP assays. β-Cat was detectable in DNA fragments encompassing the E1 box element in the MuSK gene, the key E box element for MyoD regulation (20) (Fig. 5A, lane 6, and B, lane 13). In contrast, no signal was revealed if antibody was omitted in immunoprecipitation or when primers against the coding region of the AChRɛ gene were used,which does not contain cis-acting elements. β-Cat also bound to the 5′ flanking regions of the AChRα and MHC genes, which have one E box element. In accordance with up-regulation of β-Cat-MRF interaction during differentiation (Fig. 4A and B and see Fig. S3 in the supplemental material), more β-Cat was associated with transcription complexes of MuSK, AChRα, and MHC genes in myotubes (Fig. 5B, compare lanes 7 and 13). These results suggest that β-Cat associates with E box elements in muscle-specific genes via interacting with MRFs.
FIG. 5.
β-Cat associates with E box elements and increases MyoD binding to E box elements. (A) ChIP analysis of β-Cat association with MRF/E box complexes in mouse embryonic muscles. (B) ChIP analysis of β-Cat association with MRF/E box complexes in C2C12 myoblasts and myotubes. ChIP assays were performed as described in Materials and Methods. Precipitates without antibody (No Ab) or with the indicated antibodies (anti-MyoD [α MyoD] and anti-β-Cat [α β-Cat]) were used as template for PCR. PCR products were resolved on 1.5% agarose gel. (C) β-Cat enhanced MyoD binding to E box element in EMSA. 32P-labeled probe containing the MCK E box element was incubated with recombinant His-MyoD (50 ng) and/or His-β-catenin (100 ng), which were generated and purified from bacteria. ++, doubled amounts of indicated proteins. (D and E) Suppression of β-Cat expression attenuated MyoD (D) or myogenin (E) association with E box elements. C2C12 myoblasts were transfected with pCS2-MyoD (D) or pCS2-myogenin (E) with pBS-U6 or pBS-U6-β-Cat. ChIP assays were performed as in panel A with anti-Myc antibody.
We investigated mechanisms by which β-Cat regulates MyoD transactivation. DNA binding activity of MyoD was characterized in the presence or absence of β-Cat. The addition of recombinant MyoD led to weak retardation in the oligonucleotide probes containing E box elements of the MCK enhancer (Fig. 5C, lane 3 versus 10). However, the amount of retarded probes was dramatically increased in the presence of β-Cat (compare lanes 3 and 4 and lanes 10 and 11), suggesting that β-Cat may increase MyoD binding to DNA. Please note that the binding activity was specific because it was abolished by excess of nonlabeled probes (Fig. 5C, lane 5) and there was a supershift by anti-MyoD antibodies (Fig. 5C, lane 6). In addition, anti-β-Cat antibodies appeared to disrupt the complex (Fig. 5C, lane 7). Unlike MyoD, β-Cat alone did not bind to the probes (Fig. 5C, lane 2). Additional ChIP analysis was performed to test whether β-Cat regulates MyoD binding to DNA in vivo. As shown in Fig. 5D and 5E, the amounts of Myc-MyoD and Myc-myogenin associated with the promoters of muscle-specific genes were reduced in C2C12 cells expressing pBS/U6-β-Cat. Together, these results suggest that β-Cat increases MyoD binding to E box elements.
C-terminal domain of β-Cat is required for MyoD transcriptional activity.
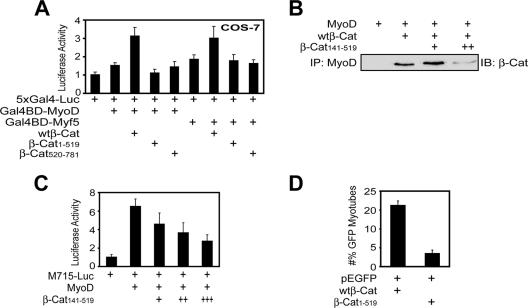
β-Cat has been reported to act as a strong transactivator of Wnt target genes. This action requires the C-terminal domain in β-Cat (49). Thus, we determined whether the activation domain of β-Cat is required for MyoD transcriptional activity using mammalian one-hybrid assays. Expression of Gal4BD-MyoD, a fusion protein of the Gal4 DNA binding (DB) domain and MyoD, increased luciferase activity of 5×Gal4-Luc, a reporter whose expression is controlled by five Gal4 binding elements. MyoD activity was enhanced by β-Cat, but not β-Cat1-519, which binds to MyoD but does not contain the activation domain (Fig. 6A). The potentiation effect was dependent on interaction with MyoD because β-Cat520-781, which does not bind to MyoD but contains the activation domain, was ineffective. Similar results were observed with Gal4BD-Myf5 (Fig. 6A). These results suggest that the transactivation activity in the C-terminal region of β-Cat is necessary for MRFs' activity. To test this notion further, we explored the consequence of inhibiting the MyoD-β-Cat interaction. β-Cat141-519 was able to associate with MyoD and thus prevent endogenous β-Cat from binding to MyoD (Fig. 6B). As shown in Fig. 6C, MyoD induction of M715-Luc activity was reduced in a dose-dependent manner in cells expressing β-Cat141-519. Moreover, C2C12 myoblasts expressing β-Cat1-519 were defective in forming myotubes (Fig. 6D). These results suggest that the activation domain of β-Cat is necessary for MyoD induction on muscle differentiation. Together, these observations indicate that β-Cat association with MRFs enhances their transcriptional activity through the C-terminal domain of β-Cat.
FIG. 6.
β-Cat is necessary for MRFs transcriptional activity. (A) The requirement of the C-terminal transactivation domain of β-Cat for MyoD or Myf5 transcriptional activity. Luciferase activities in transfected COS-7 cells were assayed as in Materials and Methods. (B) Disruption of the β-Cat-MyoD interaction by β-Cat141-519. Recombinant His-MyoD, β-Cat141-519, and wild-type β-Cat (wtβ-Cat) were produced and purified from bacteria. His-MyoD was incubated with wtβ-Cat without or with increasing concentrations of β-Cat141-519. Bound β-Cat1-781 was isolated by immunoprecipitation (IP) and visualized by immunoblotting (IB) with the monoclonal antibody that recognizes only the C-terminal region of β-Cat. ++ indicates doubled amounts of β-Cat141-519. (C) β-Cat141-519 inhibited MyoD transcriptional activity. C2C12 myoblasts were transfected with M715-Luc and pRL-TK without or with MyoD and increasing amounts (+, ++, and +++) of β-Cat141-519. Luciferase assays were performed as in Materials and Methods. (D) The transactivation domain of β-Cat was necessary for C2C12 differentiation. C2C12 myoblasts were transfected with pEGFP-C1 together with pKH3-wtβ-Cat orpKH3-β-Cat1-519 in a ratio of 1:20. Twenty-four hours after transfection, myoblasts were switched to the DM for 3 days and examined for myotube formation. GFP-expressing cells were counted as in Fig. 1A and B.
E2a proteins are dispensable for muscle differentiation.
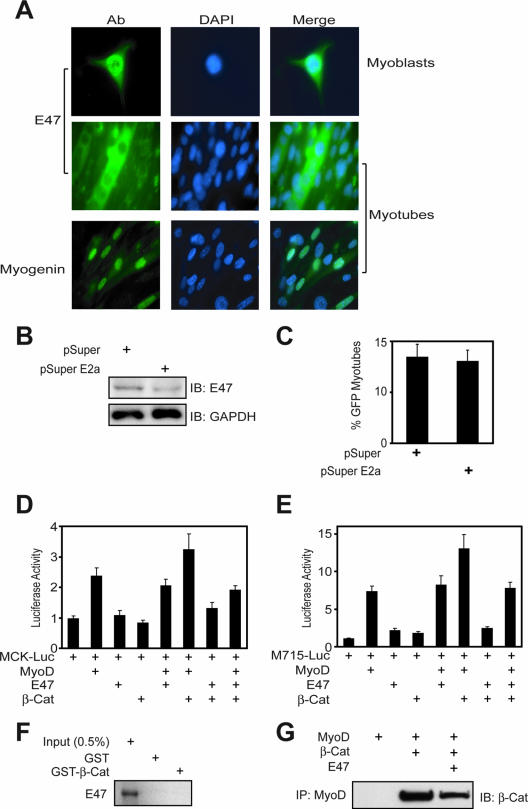
MyoD forms heterodimers with ubiquitously expressed E2a proteins, including E47 and E12, for better binding to E box elements (13, 25). Unlike β-Cat, E47 was only associated with the promoters of muscle-specific genes in C2C12 myoblasts, but not in myotubes (Fig. 5B, compare lanes 6 and 12) and embryonic muscle (Fig. 5A, lane 7). These results were unexpected, because E2a proteins were believed to be necessary for bHLH factors to bind to DNA and act as an activator (13, 25). These observations, however, could suggest that E47 may not be necessary for muscle differentiation. To test this hypothesis, we first characterize E47 distribution in differentiating muscle cells. E47 was present in the nucleus in C2C12 myoblasts (Fig. 7A, top panel), in agreement with the finding that E47 is associated with muscle-specific genes at this stage (Fig. 5B, lane 6). However, E47 was exclusively localized in the cytoplasm in myotubes, suggesting it may not be involved in gene regulation in myotubes (Fig. 7A, middle panel). To test this hypothesis further, short hairpin RNA was used to suppress expression of the E2a gene. Expression of pSuper E2a, which contained common target sequences of the E2a gene and decreased endogenous E47 expression (Fig. 7B), had no effect on C2C12 myotube formation (Fig. 7C), suggesting that E2a proteins are not required for muscle differentiation. Moreover, overexpression of E47 had no apparent effect on MyoD/β-Cat induction of MCK and M715 reporter activity (Fig. 7D and E). Expression of E47 appeared to inhibit muscle differentiation (Fig. 7D and E), presumably due to preventing MyoD from associating with β-Cat (Fig. 7F and G). These results are in agreement with observation that ablation of the E2a gene has no effect on stem cell differentiation into muscles (51) and suggest that E2a proteins are unessential for muscle differentiation.
FIG. 7.
E2a proteins are dispensable for muscle differentiation. (A) E47 proteins distributed differently during C2C12 cell differentiation. Immunostaining was done with anti-E47 or antimyogenin antibody following goat anti-mouse or -rabbit Alexa 488-conjugated secondary antibody. DAPI (4′,6′-diamidino-2-phenylindole) was used to stain the nucleus in C2C12 cells. (B) Reduction of E47 by pSuper E2a was observed using Western blotting. C2C12 myoblasts were transfected with control pSuper parental or pSuper E2a. Twenty-four hours after transfection, cells were collected and subjected to immunoblotting (IB) with the indicated antibodies. (C) No effect of pSuper E2a on C2C12 differentiation. C2C12 myoblasts were transfected with pSuper parental or pSuper E2a. Twenty-four hours after transfection, myoblasts were switched to the DM for 3 days and examined for myotube formation. GFP-expressing cells were counted as in Fig. 1A. (D and E) MyoD-induced MCK and M715 reporter activity was further enhanced by β-Cat and more suppressed by E47. C2C12 cells were transfected with or without MyoD in the presence of the indicated expression vectors. Luciferase assays were performed as described in Materials and Methods. (F) No interaction between E47 and β-Cat. 35S-labeled E47 proteins were generated by in vitro translation as in Materials and Methods and incubated with GST alone or GST-β-Cat immobilized on beads. Bound proteins were visualized by autoradiogram. (G) Inhibition of the β-Cat-MyoD interaction by Ε47. His-MyoD was incubated with His-β-Cat without or with E47. Bound β-Cat was isolated by immunoprecipitation (IP) with anti-MyoD antobody and visualized by IB with anti-β-Cat antibody.
Τhe canonical pathway of Wnt signaling is thought to regulate muscle differentiation (5, 6, 9, 16). In this pathway, TCF/LEF factors form β-Cat transcriptional complexes necessary for target gene expression and act as Wnt effectors (29). The present study provides evidence that muscle differentiation requires a β-Cat-involved pathway that is independent of TCF/LEF factors. We show that the disruption of TCF/LEF function has no apparent effect on β-Cat-dependent muscle differentiation (Fig. 2), suggesting that β-Cat may regulate gene transcription with a different effector. Interestingly, although both β-Cat and MyoD were detectable at postdifferentiation day 0 (Fig. 4A), no coimmunoprecipitation was detectable (Fig. 4A), suggesting that other cofactors or posttranslational modifications induced by muscle differentiation might be involved in the association of β-Cat and MyoD.
Tissue-specific bHLH factors are able to operate both coordinately and sequentially to regulate consecutive developmental steps (14, 17, 43). For example, myogenic bHLH factors including MyoD, Myf5, myogenin, and MRF4 are known to be essential for muscle development (24, 38). Mice deficient in both MyoD and Myf5, a member of the MyoD family, lack myoblasts and differentiated skeletal muscle (45). Our results suggest that MyoD may be an effector of the Wnt canonical pathway. The following three pieces of evidence suggest that β-Cat interacts directly with MyoD. First, bacterial GST-fusion proteins and respective 35S-labeled MyoD or β-Cat that was generated by reticulocyte lysates coprecipitated in pull-down assays (Fig. 4D and F). Second, we showed in EMSA that further retardation of labeled probes was observed by the addition of bacterial purified β-Cat and MyoD (Fig. 5C). Third, the two proteins, which were purified from bacteria, coimmunoprecipitated (Fig. 6B). Interacting with MyoD and β-Cat enhances its transcription activity necessary for muscle cell differentiation via E box elements (Fig. 3, 4, and 5). In addition, the effect of β-Cat requires the C-terminal region (Fig. 6). This domain contains intrinsic transactivation activity and has been shown to regulate various transcription factors, including TIP49, TIP60, INO80, SWRCAP/SWR1, and p300/CBP (49). Therefore, the β-Cat/MyoD complexes, instead of the β-Cat/TCF complex, may be involved in Wnt-mediated regulation of muscle differentiation. Given the homology among bHLH factors, our findings suggest that β-Cat regulates transcriptional activity of other bHLH factors by a similar mechanism toward tissue differentiation.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the NIH (L.M. and W.C.X.) and Muscular Dystrophy Association (L.M.).
We are grateful to Xi He (Harvard Medical School) and Xian Yu (Institute of Neuroscience, Shanghai, China) for valuable constructs and reagents.
Footnotes
Published ahead of print on 3 March 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aberle, H., H. Schwartz, H. Hoschuetzky, and R. Kemler. 1996. Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to alpha-catenin. J. Biol. Chem. 2711520-1526. [DOI] [PubMed] [Google Scholar]
- 2.Aberle, H., H. Schwartz, and R. Kemler. 1996. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J. Cell Biochem. 61514-523. [DOI] [PubMed] [Google Scholar]
- 3.Akimoto, T., T. Ushida, S. Miyaki, H. Akaogi, K. Tsuchiya, Z. Yan, R. S. Williams, and T. Tateishi. 2005. Mechanical stretch inhibits myoblast-to-adipocyte differentiation through Wnt signaling. Biochem. Biophys. Res. Commun. 329381-385. [DOI] [PubMed] [Google Scholar]
- 4.Aurade, F., C. Pinset, P. Chafey, F. Gros, and D. Montarras. 1994. Myf5, MyoD, myogenin and MRF4 myogenic derivatives of the embryonic mesenchymal cell line C3H10T1/2 exhibit the same adult muscle phenotype. Differentiation 55185-192. [DOI] [PubMed] [Google Scholar]
- 5.Borello, U., M. Coletta, S. Tajbakhsh, L. Leyns, E. M. De Robertis, M. Buckingham, and G. Cossu. 1999. Transplacental delivery of the Wnt antagonist Frzb1 inhibits development of caudal paraxial mesoderm and skeletal myogenesis in mouse embryos. Development 1264247-4255. [DOI] [PubMed] [Google Scholar]
- 6.Borycki, A. G., and C. P. Emerson, Jr. 2000. Multiple tissue interactions and signal transduction pathways control somite myogenesis. Curr. Top. Dev. Biol. 48165-224. [DOI] [PubMed] [Google Scholar]
- 7.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296550-553. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J. F., E. M. Mandel, J. M. Thomson, Q. Wu, T. E. Callis, S. M. Hammond, F. L. Conlon, and D. Z. Wang. 2006. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 38228-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cossu, G., and U. Borello. 1999. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 186867-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cossu, G., R. Kelly, S. Tajbakhsh, S. Di Donna, E. Vivarelli, and M. Buckingham. 1996. Activation of different myogenic pathways: myf-5 is induced by the neural tube and MyoD by the dorsal ectoderm in mouse paraxial mesoderm. Development 122429-437. [DOI] [PubMed] [Google Scholar]
- 11.Dahlqvist, C., A. Blokzijl, G. Chapman, A. Falk, K. Dannaeus, C. F. Ibanez, and U. Lendahl. 2003. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development 1306089-6099. [DOI] [PubMed] [Google Scholar]
- 12.Daniel, V. C., C. D. Peacock, and D. N. Watkins. 2006. Developmental signalling pathways in lung cancer. Respirology 11234-240. [DOI] [PubMed] [Google Scholar]
- 13.French, B. A., K.-L. Chow, E. N. Olson, and R. J. Schwartz. 1991. Heterodimers of myogenic helix-loop-helix regulatory factors and E12 bind a complex element governing myogenic induction of the avian cardiac α-actin promoter. Mol. Cell. Biol. 112439-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatakeyama, J., and R. Kageyama. 2004. Retinal cell fate determination and bHLH factors. Semin. Cell Dev. Biol. 1583-89. [DOI] [PubMed] [Google Scholar]
- 15.Hecht, A., K. Vleminckx, M. P. Stemmler, F. van Roy, and R. Kemler. 2000. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 191839-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoppler, S., J. D. Brown, and R. T. Moon. 1996. Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 102805-2817. [DOI] [PubMed] [Google Scholar]
- 17.Kageyama, R., T. Ohtsuka, J. Hatakeyama, and R. Ohsawa. 2005. Roles of bHLH genes in neural stem cell differentiation. Exp. Cell Res. 306343-348. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami, Y., C. Rodriguez Esteban, M. Raya, H. Kawakami, M. Marti, I. Dubova, and J. C. Izpisua Belmonte. 2006. Wnt/beta-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 203232-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, C.-H., W. C. Xiong, and L. Mei. 2005. Inhibition of MuSK expression by CREB interacting with a CRE-like element and MyoD. Mol. Cell. Biol. 255329-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, C. H., W. C. Xiong, and L. Mei. 2003. Regulation of MuSK expression by a novel signaling pathway. J. Biol. Chem. 27838522-38527. [DOI] [PubMed] [Google Scholar]
- 21.Kim, H. K., Y. S. Lee, U. Sivaprasad, A. Malhotra, and A. Dutta. 2006. Muscle-specific microRNA miR-206 promotes muscle differentiation. J. Cell Biol. 174677-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kollias, H. D., R. L. S. Perry, T. Miyake, A. Aziz, and J. C. McDermott. 2006. Smad7 promotes and enhances skeletal muscle differentiation. Mol. Cell. Biol. 266248-6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 2751784-1787. [DOI] [PubMed] [Google Scholar]
- 24.Lassar, A. B., J. N. Buskin, D. Lockshon, R. L. Davis, S. Apone, S. D. Hauschka, and H. Weintraub. 1989. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell 58823-831. [DOI] [PubMed] [Google Scholar]
- 25.Lassar, A. B., R. L. Davis, W. E. Wright, T. Kadesch, C. Murre, A. Voronova, D. Baltimore, and H. Weintraub. 1991. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell 66305-315. [DOI] [PubMed] [Google Scholar]
- 26.Lluis, F., E. Ballestar, M. Suelves, M. Esteller, and P. Munoz-Canoves. 2005. E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 24974-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu, J., R. Webb, J. A. Richardson, and E. N. Olson. 1999. MyoR: a muscle-restricted basic helix-loop-helix transcription factor that antagonizes the actions of MyoD. Proc. Natl. Acad. Sci. USA 96552-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo, Z. G., H. S. Je, Q. Wang, F. Yang, G. C. Dobbins, Z. H. Yang, W. C. Xiong, B. Lu, and L. Mei. 2003. Implication of geranylgeranyltransferase I in synapse formation. Neuron 40703-717. [DOI] [PubMed] [Google Scholar]
- 29.Miyoshi, K., and L. Hennighausen. 2003. Beta-catenin: a transforming actor on many stages. Breast Cancer Res. 563-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molenaar, M., M. van de Wetering, M. Oosterwegel, J. Peterson-Maduro, S. Godsave, V. Korinek, J. Roose, O. Destree, and H. Clevers. 1996. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86391-399. [DOI] [PubMed] [Google Scholar]
- 31.Munz, B., E. Hildt, M. L. Springer, and H. M. Blau. 2002. RIP2, a checkpoint in myogenic differentiation. Mol. Cell. Biol. 225879-5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson, W. J., and R. Nusse. 2004. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 3031483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odelberg, S. J., A. Kollhoff, and M. T. Keating. 2000. Dedifferentiation of mammalian myotubes induced by msx1. Cell 1031099-1109. [DOI] [PubMed] [Google Scholar]
- 34.Pan, W., Y. Jia, T. Huang, J. Wang, D. Tao, X. Gan, and L. Li. 2006. β-Catenin relieves I-mfa-mediated suppression of LEF-1 in mammalian cells. J. Cell Sci. 1194850-4856. [DOI] [PubMed] [Google Scholar]
- 35.Pan, W., Y. Jia, J. Wang, D. Tao, X. Gan, L. Tsiokas, N. Jing, D. Wu, and L. Li. 2005. Beta-catenin regulates myogenesis by relieving I-mfa-mediated suppression of myogenic regulatory factors in P19 cells. Proc. Natl. Acad. Sci. USA 10217378-17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parker, M. H., P. Seale, and M. A. Rudnicki. 2003. Looking back to the embryo: defining transcriptional networks in adult myogenesis. Nat. Rev. Genet. 4497-507. [DOI] [PubMed] [Google Scholar]
- 37.Polesskaya, A., P. Seale, and M. A. Rudnicki. 2003. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell 113841-852. [DOI] [PubMed] [Google Scholar]
- 38.Pownall, M. E., M. K. Gustafsson, and C. P. Emerson, Jr. 2002. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 18747-783. [DOI] [PubMed] [Google Scholar]
- 39.Puri, P. L., K. Bhakta, L. D. Wood, A. Costanzo, J. Zhu, and J. Y. Wang. 2002. A myogenic differentiation checkpoint activated by genotoxic stress. Nat. Genet. 32585-593. [DOI] [PubMed] [Google Scholar]
- 40.Puri, P. L., and V. Sartorelli. 2000. Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J. Cell Physiol. 185155-173. [DOI] [PubMed] [Google Scholar]
- 41.Reya, T., and H. Clevers. 2005. Wnt signalling in stem cells and cancer. Nature 434843-850. [DOI] [PubMed] [Google Scholar]
- 42.Rochat, A., A. Fernandez, M. Vandromme, J. P. Moles, T. Bouschet, G. Carnac, and N. J. Lamb. 2004. Insulin and wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Mol. Biol. Cell 154544-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross, S. E., M. E. Greenberg, and C. D. Stiles. 2003. Basic helix-loop-helix factors in cortical development. Neuron 3913-25. [DOI] [PubMed] [Google Scholar]
- 44.Rudnicki, M. A., T. Braun, S. Hinuma, and R. Jaenisch. 1992. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell 71383-390. [DOI] [PubMed] [Google Scholar]
- 45.Rudnicki, M. A., P. N. Schnegelsberg, R. H. Stead, T. Braun, H. H. Arnold, and R. Jaenisch. 1993. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 751351-1359. [DOI] [PubMed] [Google Scholar]
- 46.Sui, G., C. Soohoo, E. B. Affar, F. Gay, Y. Shi, and W. C. Forrester. 2002. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. USA 995515-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tajbakhsh, S., U. Borello, E. Vivarelli, R. Kelly, J. Papkoff, D. Duprez, M. Buckingham, and G. Cossu. 1998. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development 1254155-4162. [DOI] [PubMed] [Google Scholar]
- 48.Vertino, A. M., J. M. Taylor-Jones, K. A. Longo, E. D. Bearden, T. F. Lane, R. E. McGehee, Jr., O. A. MacDougald, and C. A. Peterson. 2005. Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol. Biol. Cell 162039-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willert, K., and K. A. Jones. 2006. Wnt signaling: is the party in the nucleus? Genes Dev. 201394-1404. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi, A. 1995. Regulation of differentiation pathway of skeletal mesenchymal cells in cell lines by transforming growth factor-beta superfamily. Semin. Cell Biol. 6165-173. [DOI] [PubMed] [Google Scholar]
- 51.Zhuang, Y., C. G. Kim, S. Bartelmez, P. Cheng, M. Groudine, and H. Weintraub. 1992. Helix-loop-helix transcription factors E12 and E47 are not essential for skeletal or cardiac myogenesis, erythropoiesis, chondrogenesis, or neurogenesis. Proc. Natl. Acad. Sci. USA 8912132-12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhurinsky, J., M. Shtutman, and A. Ben-Ze'ev. 2000. Plakoglobin and beta-catenin: protein interactions, regulation and biological roles. J. Cell Sci. 1133127-3139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.