Abstract
Parafibromin, a transcription factor associated with the PAF complex, is encoded by the HRPT2 gene, mutations of which cause the hyperparathyroidism-jaw tumor syndrome (OMIM145001). To elucidate the function of parafibromin, we generated conventional and conditional Hrpt2 knockout mice and found that Hrpt2−/− mice were embryonic lethal by embryonic day 6.5 (E6.5). Controlled deletion of Hrpt2 after E8.5 resulted in apoptosis and growth retardation. Deletion of Hrpt2 in adult mice led to severe cachexia and death within 20 days. To explore the mechanism underlying the embryonic lethality and death of adult mice, mouse embryonic fibroblasts (MEFs) were cultured and Hrpt2 was deleted in vitro. Hrpt2−/− MEFs underwent apoptosis, while Hrpt2+/+ and Hrpt2+/− MEFs grew normally. To study the mechanism of this apoptosis, Hrpt2+/+ and Hrpt2−/− MEFs were used in cDNA microarray, semiquantitative reverse transcription-PCR, and chromatin immunoprecipitation assays to identify genes regulated by parafibromin. These revealed that Hrpt2 expression and the parafibromin/PAF complex directly regulate genes involved in cell growth and survival, including H19, Igf1, Igf2, Igfbp4, Hmga1, Hmga2, and Hmgcs2. Thus, our results show that expression of Hrpt2 and parafibromin is pivotal in mammalian development and survival in adults and that these functions are likely mediated by the transcriptional regulation of growth factors.
Loss-of-function HRPT2 mutations are associated with the autosomal dominantly inherited hyperparathyroidism-jaw tumor (HPT-JT) syndrome. Patients with HPT-JT develop parathyroid tumors, ossifying fibromas of the mandible and maxilla, uterine tumors, and renal abnormalities that may include cysts, mixed epithelial and stromal tumors, and Wilms' tumors (3). The HRPT2 gene is located on chromosome 1q31.2, and the observed loss of heterozygosity in HPT-JT-associated tumors and the identification of combined somatic and germ line HRPT2 mutations in some other HPT-JT tumors indicate that HRPT2 is a tumor suppressor gene (2, 7). In addition, HRPT2 mutations are frequently found in sporadic parathyroid carcinomas (18, 43), and expression of parafibromin, which is the 531-amino-acid protein encoded by HRPT2 (7), in parathyroid carcinomas has been reported to be lower than in normal parathyroid tissues (49). Sporadic human renal tumors, from two patients, have also been reported to have frequent allelic imbalances and to harbor HRPT2 mutations (55). These findings are consistent with a tumor suppressor role for HRPT2 and its encoded protein, parafibromin.
Parafibromin shares 32% sequence similarity with the Saccharomyces cerevisiae protein Cdc73, which is a component of the yeast polymerase II-associated factor (PAF) complex, and it also has homologs in Caenorhabditis elegans, Drosophila melanogaster, and mice (7). The yeast PAF complex, which is associated with RNA polymerase II and is composed of Paf1, Ctr9, Leo1, Rtf1, and Cdc73, is involved in RNA polymerase II-mediated transcription initiation and elongation (36), mRNA processing and maturation through maintaining proper poly(A) tail length (28, 34, 44), Rad6-mediated histone H2B-K123 monoubiquitination (30, 52), Set1-mediated histone H3-K4 methylation (21, 31), and Dot1-mediated histone H3-K79 methylation (20). Thus, the PAF complex is involved in gene transcription through the following pathway: PAF complex → histone H2B ubiquitination → histone H3 methylation → chromatin remodeling (33).
Previous studies have shown that the PAF complex is associated with RNA polymerase II on transcriptionally active yeast genes (28, 46). However, only a small subset of genes, related to the cell cycle, protein synthesis, lipid metabolism, and nucleic acid turnover, are reported to be directly regulated by the PAF complex (1, 37, 45). Consistent with the findings in yeast, three separate studies have shown that parafibromin is a member of a human PAF complex that also includes hCtr9, hLeo1, and hPaf1 (40, 53, 57). Moreover, the human PAF complex is necessary for trimethylation of histone H3-K4 and dimethylation of H3-K79, which suggests that the complex may affect gene expression through a mechanism similar to that of the yeast PAF complex (57).
Although Cdc73 is not essential for the growth of S. cerevisiae, a recent study demonstrated that the D. melanogaster homolog of parafibromin, termed Hyrax, is necessary for early development (27). Importantly, human parafibromin, but not yeast Cdc73, can rescue the phenotypic defect caused by a loss of Hyrax, indicating that the biological function of parafibromin may be more complicated than that of Cdc73. In order to further elucidate the function of parafibromin and its target genes, we generated knockout mouse models of Hrpt2. Our study reveals that Hrpt2 heterozygous mice are viable but that Hrpt2 homozygous mice are embryonically lethal. Moreover, deletion of Hrpt2 at various stages of embryonic development demonstrated that Hrpt2 expression and its protein, parafibromin, are necessary for development and that their loss promotes apoptosis. In addition, loss of Hrpt2 expression was found to alter the expression of several target genes that are associated with cell growth and cell death. Thus, our study sheds new light on the functions of Hrpt2 and parafibromin.
MATERIALS AND METHODS
Generation of Hrpt2 conventional knockout mice.
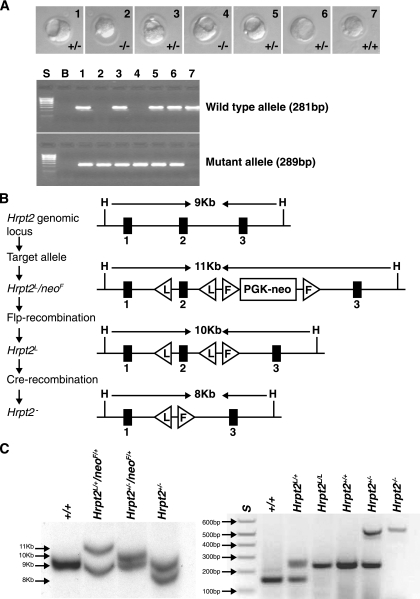
The Hrpt2 conventional knockout mouse was established using the embryonic stem (ES) cell line RRE190, from the BayGenomics Genetrap resource (48). The linearized Genetrap vector had inserted 2,584 bp downstream of the Hrpt2 exon 6, in intron 6, and this was shown to lead to a loss of exons 7 to 17, which would result in a parafibromin/beta-galactosidase fusion protein that lacked the C-terminal 361 amino acids of parafibromin. The RRE190 ES cells were used to generate chimeric mice, which were mated to generate heterozygous (Hrpt2+/−) mice. Hrpt2+/− mice were viable and were intercrossed to obtain Hrpt2−/− mice. Mice were kept in accordance with United Kingdom welfare guidelines and approved licensed restrictions and genotyped using PCR primers F1(5′-GTCACAAAACCAAAGCCTCTGGAACG-3′) and R (5′-GTTACAAGGTCATGGATATTTCCACC-3′) to yield a wild-type band of 281 bp and F2 (5′-CTGCAAGGCGATTAAGTTGGGTAACG-3′) and R (5′-GTTACAAGGTCATGGATATTTCCACC-3′) to yield a mutant band of 289 bp (Fig. 1A).
FIG. 1.
(A) Upper panel: embryos at E3.5 obtained from conventional Hrpt2 knockout mouse breedings. The Hrpt2+/+, Hrpt2+/−, and Hrpt2−/− embryos are similar and morphologically normal. Lower panel: genotypes assessed by PCR of mouse embryos 1 to 7 shown in the upper panel; S is the size marker (1-kb ladder), and B is a control water blank. (B) Schematic representation of conditional knockout targeting vectors and strategy for obtaining Hrpt2+/− mice. (C) Left panel: Southern blot analysis of a wild-type mouse (+/+), ES cells (Hrpt2L/+/neoF/+), Hrpt2 heterozygous mouse with neo gene (Hrpt2+/−/neoF/+), and Hrpt2 heterozygous mouse without neo gene (Hrpt2+/−). Right panel: genotyping by PCR of mice with no loxP site (+/+), one loxP site allele (Hrpt2L/+), two loxP site alleles (Hrpt2L/L), wild-type Hrpt2 alleles (Hrpt2+/+), heterozygote Hrpt2 alleles (Hrpt2+/−), and homozygote Hrpt2 null alleles (Hrpt2−/−).
Generation of Hrpt2 conditional knockout mice.
The Hrpt2 targeting vector and Hrpt2L/+/neoF/+ mice were generated, and germ line deletion of the neo gene was achieved by crossing a Hrpt2L/+/neoF/+ mouse with a FLPeR mouse (13) to establish Hrpt2L/+ mice (51). Hrpt2 heterozygotes were generated by mating the Hrpt2L/+ mouse with a cytomegalovirus (CMV)-Cre transgenic mouse (42) (Fig. 1 B). All animals were treated and maintained in accordance with the Institutional Animal Care and Use Committee of Van Andel Research Institute.
Hrpt2L/L mice and Cre-ER transgenic mice (17) were mated to generate three genotypes: Cre-ER/Hrpt2L/L, Cre-ER/Hrpt2L/+, and Cre-ER/Hrpt2+/+. At age 4 to 6 weeks, these animals were injected intraperitoneally (i.p.) daily with 4-OH-tamoxifen (4-OH-TM; 3 mg/40 g of body weight) dissolved in corn oil, for five consecutive days. Timed-pregnant mice were injected i.p. with tamoxifen (4 mg/40 g of body weight) dissolved in corn oil, as previously described (17). Mice were euthanized 2 days later after exposure to tamoxifen and the embryos dissected. For genotyping, primers F (5′-GTATACAGTGGGGTGGAGGATG-3′) and R (5′-ATTCCAACTGGCTTCCAAGCAG-3′) that detected the LoxP site were used to yield a wild-type band of 179 bp and a mutant band of 242 bp; to detect the wild-type and mutant alleles of the Hrpt2 gene, three primers, F1 (5′-ATCTTGTAAGTGCGTCCCTA-3′), F2 (5′-CACCATATCTCACTGTATGT-3′), and R (5′-ATCTGGGGAACCATCTTATT-3′), were used to yield bands of 253 bp and 546 bp, respectively (Fig. 1C).
Murine embryonic fibroblast cultures.
Murine embryonic fibroblasts (MEFs) were isolated from embryonic day 12.5 (E12.5) or E13.5 embryos and maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin. Hrpt2+/+, Hrpt2L/+, and Hrpt2L/L MEFs, as well as Cre-ER/Hrpt2+/+ and Cre-ER/Hrpt2L/L MEFs, were harvested and frozen for later experiments. Hrpt2+/+, Hrpt2L/+, and Hrpt2L/L MEFs were infected with a multiplicity of infection of 25 Ad5CMV-Cre or Ad5CMV-GFP (University of Iowa) for 2 h. In the Cre-ER system, Cre-ER/Hrpt2L/L and Cre-ER/Hrpt2+/+ MEFs were treated with 1 μm 4-OH-TM for 48 h (Sigma).
Embryo culture.
E3.5 blastocysts from natural matings were isolated and placed singly into 96-well plates. Acidic Tryode solution (Sigma) was used to remove the zona pellucida of blastocysts before plating. Embryos were cultured in Dulbecco's modified Eagle's medium and 15% fetal bovine serum for a total of 2 to 5 days and examined by phase-contrast microscopy prior to genotyping by PCR (12).
Histological analysis and immunoblot analysis.
Tissues were fixed overnight in 4% paraformaldehyde and embedded in paraffin. Sections were used for hematoxylin and eosin (H&E) staining and immunostaining using an antiparafibromin antibody as previously reported (49).
Whole-cell lysates were prepared in radioimmunoprecipitation buffer (1% sodium deoxycholine, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 10 mM Tris pH 8.0, 0.14 M NaCl) with protease inhibitor complex (Roche). Western blot analysis was performed using the following antibodies: monoclonal antiparafibromin at 1:5,000 (49), caspase 3 at 1:1,000 (Cell Signaling), cleaved caspase 3 at 1:1,000 (Cell Signaling), LC3 at 1:1,000 (MBL), and HMG1/HMG2 at 1:1,000 (Stressgen).
Cell death and proliferation assays.
Apoptosis terminal deoxynucleotidyltransferase-mediated biotin-dUTP nick end labeling (TUNEL) staining was conducted according to the manufacturer's protocol with the in situ cell death detection kit with POD and the in situ cell death detection kit with fluorescein (Roche). The staining patterns with annexin V and propidium iodide (BD Bioscience) were analyzed by flow cytometry according to the manufacturer's protocol. The bromodeoxyuridine (BrdU; Roche) incorporation assay was carried out following the manufacturer's protocol.
Gene expression profiling and semiquantitative RT-PCR.
Hrpt2+/+ and Hrpt2L/L MEFs were infected with a multiplicity of infection of 25 Ad5CMV-Cre for 2 h, and 4 days later RNA was extracted using TRIzol (Invitrogen), purified, and used for murine cDNA microarray analysis. Experiments were performed on a 15,360-feature cDNA microarray containing full-length cDNA clones that were PCR amplified from the NIA 15k clone set (Bethesda, MD). Arrays were spotted in-house using a custom 204-slide microarrayer (Beta Integrated Concepts, MI) with 32 Point Technologies PT-3000 print pins. All batches of micorarrays passed the quality control and quality assurance criteria. These experiments were also performed using RNA extracted from Cre-ER/Hrpt2+/+ and Cre-ER/Hrpt2L/L MEFs that had been treated with 1 μm 4OH-TM for 4 days. All microarray hybridizations were performed four times, and the signal for each gene was expressed as the signal ratio of the log2(test signal/control signal), where the test signal represented that obtained from the Hrpt2L/L or Cre-ER/Hrpt2L/L MEFs and the control signal represented that obtained from the Hrpt2+/+ or Cre-ER/Hrpt2+/+ MEFs. A gene was designated as being up-regulated if all four of its log2 signal ratios were >0 (i.e., positive) and as being down-regulated if all four of its log2 signal ratios were <0 (i.e., negative). Genes that did not fulfill these criteria were not included in any further analyses. The means ± standard deviations of the log2 signal ratios were calculated for each of the genes that fulfilled the criteria of up-regulation or down-regulation and the anti-log2 value yielded the mean change ± standard deviation (n = 4) in altered expression for each gene, which was designated as being up-regulated if the mean showed a ≥2-fold increase and down-regulated if the mean showed a ≥2-fold decrease. Semiquantitative reverse transcription-PCR (RT-PCR) of selected genes was applied on the same RNA extracted from MEFs.
ChIP assay.
Chromatin immunoprecipitation (ChIP) was undertaken by using the ChIP assay kit (Upstate Biotechnology). Polyclonal antiparafibromin and anti-Paf1 antibodies (Bethyl Laboratories) were used for IP.
RESULTS
Germ line disruption of Hrpt2 leads to embryonic lethality.
Hrpt2+/− mice from the conventional knockout were intercrossed to obtain homozygous (Hrpt2−/−) mice; however, no viable Hrpt2−/− neonates were identified, indicating that Hrpt2−/− mice are embryonically lethal. The time and cause of this embryonic lethality was investigated by obtaining embryos at different stages of gestation. A total of 133 embryos ages E3.5 to E14.5 were obtained. The Hrpt2+/+, Hrpt2+/−, and Hrpt2−/− genotypes of these 133 embryos were identified at a ratio of 1.16:2:0.1, which deviated significantly (P < 0.001) from the expected 1:2:1 Mendelian ratio. Moreover, this genotype ratio (Hrpt2+/+/Hrpt2+/−/Hrpt2−/−) also deviated significantly (P < 0.01) from the expected Mendelian ratio at all the gestational stages above E6.5 to E7.5 (Table 1); the expected Mendelian ratio with Hrpt2−/− embryos was only obtained at gestational stages E3.5 and E5.5. Hrpt2−/− embryos at E3.5 appeared normal and indistinguishable from the Hrpt2+/− and Hrpt2+/+ embryos (Fig. 1A). These results reveal that Hrpt2 null mice are embryonically lethal before E6.5 and indicate that the lethality is likely to occur either at hatching (E4.5) or implantation (E4.5 to 5.5). In order to investigate further these possibilities and the cause of embryonic lethality, we pursued studies in the conditional knockout as it allowed us to temporally control the timing of Hrpt2 deletion during development. For the conditional knockout mouse model, Hrpt2 heterozygous mice were obtained by mating the Hrpt2L/+ mouse with a CMV-Cre transgenic mouse. In order to obtain Hrpt2-null mice, Hrpt2 heterozygous mice were intercrossed. Live Hrpt2-null mice were not obtained, consistent with embryonic lethality. Thus, the conditional and conventional knockout mice yielded similar results.
TABLE 1.
Hrpt2 genotypes of mice and embryos obtained from Hrpt2+/− intercrosses
| Stage | No. of mice with indicated Hrpt2 genotype
|
||
|---|---|---|---|
| +/+ | +/− | −/−a | |
| Neonate | 14 | 16 | 0** |
| E14.5 | 2 | 5 | 0* |
| E13.5 | 1 | 4 | 0* |
| E11.5-E12.5 | 11 | 15 | 0** |
| E8.5-E9.5 | 11 | 15 | 0** |
| E6.5-E7.5 | 13 | 21 | 0** |
| E5.5 | 3 | 14 | 2 |
| E3.5 | 3 | 10 | 3 |
| Total | 58 | 100 | 5*** |
Significantly different from expected: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To gain insight into the etiology of this embryonic lethality, blastocysts at E3.5 were collected and cultured in vitro. Normally, blastocysts are composed of two distinct cell lineages; approximately two-thirds of cells make up the trophectoderm (TE), while the remainder forms the inner cell mass (ICM). When blastocysts are cultured in vitro, these two types of cells have been shown to differentiate normally and to continue growing (12). Under these conditions, the wild-type and heterozygous Hrpt2 blastocysts were observed to attach to the bottom of the plate at E4.5, develop into the ICM and TE at about E5.5, and continue growing. However, the Hrpt2-null blastocysts did not attach to the bottom of the plate, failed to proliferate into the ICM and TE cells, and consequently died within 3 days (Fig. 2A).
FIG. 2.
A) Culture of E3.5 blastocysts in vitro. The wild-type (Hrpt2+/+) and heterozygous (Hrpt2+/−) blastocysts differentiated into an ICM and TE at about E5.5 and continued to grow at E6.5 and E7.5. Hrpt2-null (Hrpt2−/−) blastocysts failed to differentiate into ICM and TE and died. Experiments were repeated three times. B) Temporally controlled deletion of Hrpt2 in conditional knockout mouse embryos. Pregnant mice were treated with tamoxifen at 8.5 dpc, 10.5 dpc, and 12.5 dpc, and embryos were collected 2 days later. Embryos were viewed by microscopy (magnification is indicated in parentheses): E8.5 to 10.5 (4×), E10.5 to 12.5 (2×), and E12.5 to 14.5 (1.6×). Cre-ER/Hrpt2L/L embryos exposed to tamoxifen at E8.5 to 10.5 died and had a developmental defect of the brain (arrow). C) The body sizes of Cre-ER/Hrpt2L/L embryos were smaller than those of control embryos (E8.5 to 10.5, P < 0.01; E10.5 to 12.5, P < 0.01; E12.5 to 14.5, P < 0.05).
To identify whether Hrpt2 expression is essential at other points in development, controlled deletion of Hrpt2 was undertaken by mating Hrpt2L/L mice to Cre-ER transgenic mice. Cre-ER is widely expressed upon administration of tamoxifen in tissues of the Cre-ER transgenic mouse at embryonic and adult stages. For example, in E8.5 embryos, the Cre enzyme recombination can be detected at 6 h and the efficiency of recombination is up to 95% after exposure to tamoxifen for 48 h but declines to about 50 to 60% in E11.5 and E14.5 embryos (17). Tamoxifen was therefore injected i.p. into pregnant mice at 8.5, 10.5, 12.5, and 14.5 days postcoitum (dpc), and the embryos were harvested 2 days later. Exposure of Cre-ER/Hrpt2L/L embryos to tamoxifen at E8.5 to E10.5 resulted in a deletion of the Hrpt2 gene as assessed by PCR, and this was associated with a >50% reduction in parafibromin as assessed by Western blot analysis (data not shown). Cre-ER/Hrpt2L/L embryos at E8.5 to 10.5 were significantly smaller, had a significant delay in the development of the central nervous system, and died rapidly (Fig. 2B and C). The deletion of Hrpt2 in Cre-ER/Hrpt2L/L embryos at later stages, i.e., E10.5 to 12.5 and E12.5 to 14.5, affected growth but not organogenesis or survival.
Widespread deletion of Hrpt2 in adult mice causes damage to multiple tissues and death.
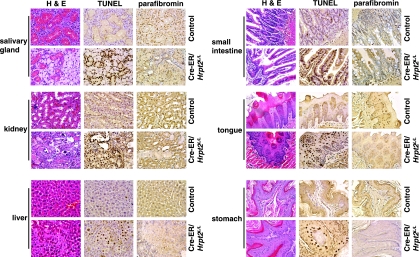
In order to investigate the effects of a loss of Hrpt2 expression in adult tissues, the Hrpt2 gene was deleted in somatic cells using the Cre-ER transgenic mouse. Eleven Cre-ER/Hrpt2L/L mice were generated, and a PCR-based genotype analysis using DNA samples from 15 different organs confirmed deletion of Hrpt2. As expected, low levels of the wild-type band were detected because of incomplete penetration of the Cre enzyme (Fig. 3A). All 13 of the control mice and 7 of the Cre-ER/Hrpt2L/L mice that were injected with corn oil alone were viable and remained healthy until euthanization 3 months later. Null Cre-ER/Hrpt2L/L mice died within 20 days after being exposed to tamoxifen. Prior to this, all of these mice had low activity, labored breathing, slow reaction, weight loss (Fig. 3B), and a reduction in abdominal and subcutaneous adipose tissue (Fig. 3C). In addition, the liver, kidneys, heart, spleen, lungs, stomach, testes, seminal vesicles, and salivary glands were smaller in size than those in the controls (Fig. 3D). Some of the Cre-ER/Hrpt2L/L mice also developed dilation of the gastrointestinal tract and/or developed ascites. The expression of Hrpt2 was detected, by RT-PCR, in multiple adult normal mouse tissues (Fig. 4A), and the presence of parafibromin in these multiple tissues was confirmed by Western blot analysis (Fig. 4B). In addition, the expression and nuclear localization of parafibromin in E12.5 and E16.5 embryos (Fig. 4C) and normal adult tissues (Fig. 4D) were demonstrated by immunohistochemistry. However, in adult Cre-ER/Hrpt2L/L mice parafibromin expression was reduced in the kidney, liver, stomach, and salivary glands (Fig. 5). Moreover, in those adult Cre-ER/Hrpt2L/L mice with a widespread deletion of Hrpt2 there was a rapid onset of cachexia, which suggested the occurrence of multiorgan failure. Indeed, this was consistent with the finding of necrotic cells in the liver and kidneys of the most morbid Cre-ER/Hrpt2L/L mice (Fig. 5). In addition, the morbid Cre-ER/Hrpt2L/L mice had a reduced secretion by the submandibular salivary glands, a decreased number of tubular glands, and diminished secretion in the seminal vesicles. Furthermore, the use of TUNEL staining revealed significant apoptosis in the salivary glands, tongue, stomach, intestine, liver, and kidneys (Fig. 5). The parathyroid glands of the morbid Cre-ER/Hrpt2L/L mice were not found to contain abnormalities (data not shown).
FIG. 3.
Adult conditional Hrpt2 knockout mice of ages 4 to 6 weeks were treated with tamoxifen for 5 days. A) Deletion of the Hrpt2 gene as detected by PCR analysis in DNA obtained from different organs. The asterisk indicates the band following deletion of Hrpt2, and the black arrow indicates the control, wild-type band of Hrpt2. B) Tamoxifen-treated Cre-ER/Hrpt2L/L mice (n = 11) lost 2.28 ± 0.56 g of body weight, while similarly treated control, i.e., Cre-ER/Hrpt2+/+ and Cre-ER/Hrpt2L/+, mice (n = 13) gained 3.50 ± 0.50 g at the time of euthanization (P < 0.01). C) All Cre-ER/Hrpt2L/L mice died within 20 days and had less adipose in the abdominal cavity (upper panel), a smaller body size, less subcutaneous fat, and loss of muscle (lower panel). D) The organs (from top to bottom, heart, lung, spleen, kidney, testis, seminal vesicle, stomach, liver, and salivary gland) of the tamoxifen-treated Cre-ER/Hrpt2L/L mice were also smaller than those of similarly treated Cre-ER/Hrpt2+/+ mice.
FIG. 4.
Expression of Hrpt2 and parafibromin. A) Expression of Hrpt2 in different tissues of normal adult mice was detected by RT-PCR. Lanes: 1, heart; 2, liver; 3, spleen; 4, lung; 5, kidney; 6, brain; 7, eye; 8, tongue; 9, salivary gland; 10, esophagus; 11, bronchus; 12, stomach; 13, duodenum; 14, pancreas; 15, intestine; 16, skin; 17, muscle; 18, bladder; 19, uterus; 20, testis; 21, placenta; 22, pcDNA3.1-HRPT2 (positive control) (54). S is the size marker (1-kb ladder). B) Expression of parafibromin in normal mouse tissues as detected by Western blot analysis. Lanes: a, kidney; b, liver; c, spleen; d, intestine; e, stomach; f, heart; g, lung; h, brain; i, tongue; j, salivary gland; k, muscle; l, eye; m, testis; n, seminal vesicle; o, duodenum; p, ovary; q, uterus. C) Expression of parafibromin in embryos (E12.5 and E16.5) as detected by immunohistochemistry. The inset represents a magnified view of the area denoted by the red square, to show nuclear localization of parafibromin. D) Expression and nuclear localization of parafibromin in adult normal mouse tissues. (I) Brain; (II) liver; (III) kidney; (IV) testis.
FIG. 5.
Pathological changes, detected by H&E, TUNEL, and parafibromin staining, in tamoxifen-treated Cre-ER/Hrpt2L/L mice prior to death. All the tissues from the tamoxifen-treated Cre-ER/Hrpt2L/L mice had more TUNEL staining but less expression of parafibromin compared with control mice. In the tamoxifen-treated Cre-ER/Hrpt2L/L mice, there was reduced glandular tissue in the salivary glands (H&E) but significantly increased cell death in the liver and kidneys (H&E).
Loss of Hrpt2 promotes apoptosis in vitro and in vivo.
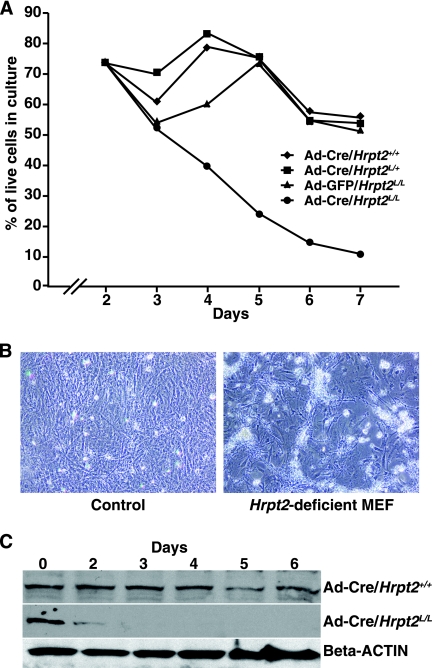
The mechanism of cell death caused by disruption of Hrpt2 was further investigated using Hrpt2-null MEFs. MEFs were obtained from E12.5 embryos of the following genotypes: Hrpt2+/+, Hrpt2L/+, Hrpt2L/L, Cre-ER/Hrpt2+/+, and Cre-ER/Hrpt2L/L. The conditional nature of Hrpt2L allowed for the generation of a null allele of Hrpt2 via the use of either an adenoviral Cre system or exposure of the cultures to tamoxifen in the case of Cre-ER/Hrpt2L/L cells. Loss of Hrpt2 (Hrpt2-deficient MEFs), induced by either method, resulted in reduced cell proliferation (Fig. 6A) and increased cell death (Fig. 6B), and this was associated with decreased amounts of parafibromin (Fig. 6C). This is consistent with the in vivo observations detailed above. Infecting the Hrpt2L/L MEF cells with a control adenovirus did not reveal differences in cellular proliferation or survival relative to Hrpt2-containing cells.
FIG. 6.
Studies with Hrpt2+/+, Hrpt2L/+, and Hrpt2L/L MEFs infected with Ad5CMV-Cre and Hrpt2L/L MEFs infected with Ad5CMV-GFP for 2 days. A) Growth curves; 2 days after infection with adenovirus, all the Hrpt2-containing MEFs (Ad-Cre/Hrpt2+/+, Ad-Cre/Hrpt2L/+, and Ad-GFP/Hrpt2L/L) grew in a similar pattern, while the Hrpt2-deficient MEFs (Ad-Cre/Hrpt2L/L) failed to proliferate. B) Significantly increased cell death was found to occur in the Hrpt2-deficient MEFs. C) Two days after Ad5CMV-Cre infection, parafibromin expression, as detected by Western blot analysis, was found to be decreased in the Hrpt2-deficient MEFs (Ad-Cre/Hrpt2L/L MEFs) but was unchanged in the Hrpt2-containing MEFs (Ad-Cre/Hrpt2+/+ MEFs). All experiments were repeated three times.
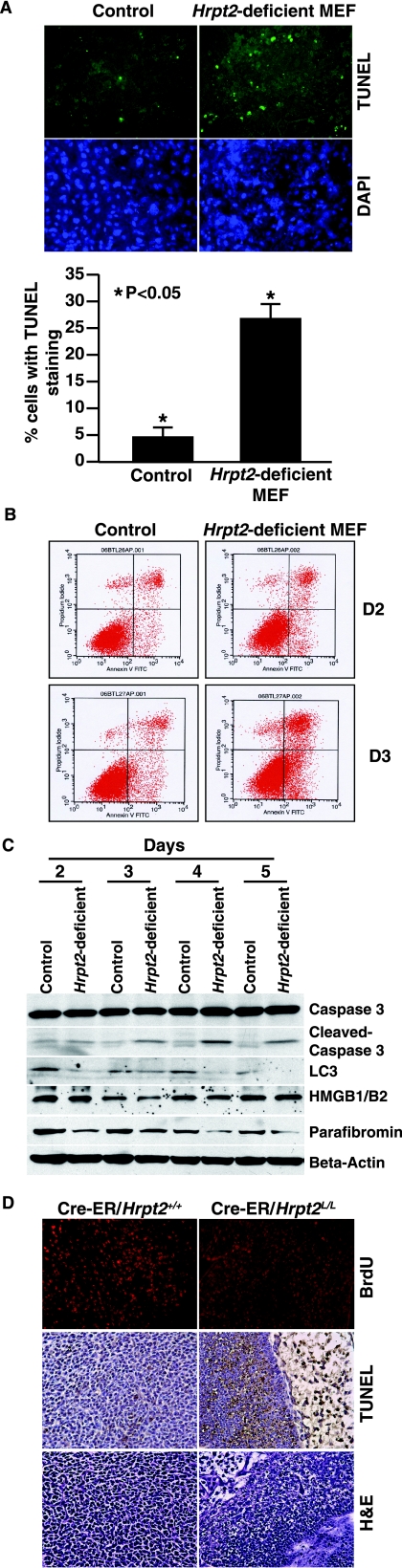
Hrpt2-deficient MEFs also showed increased apoptosis as revealed by the following: increased positive TUNEL staining at day 3 adenovirus infection, relative to the controls (Fig. 7A); significantly increased annexin V staining relative to control cells at 48 h following deletion of Hrpt2 (Fig. 7B); significantly increased levels of cleaved caspase 3 (Fig. 7C). Electron microscopy and investigation for the expression of LC3-II (8, 26) did not reveal any double membrane autophagosomes (data not shown) or the conversion of LC3-I into LC3-II (Fig. 7C), thereby indicating that the Hrpt2-deficient cells were not predisposed to higher levels of autophagic cell death. Moreover, expression of HMGB1/B2 (11) in these Hrpt2-deficient cells was not altered compared to controls (Fig. 7C), thereby indicating that cell death was not associated with an increased level of necrosis. These in vitro findings indicate that there is increased apoptosis following Hrpt2 disruption, and this was further investigated in vivo utilizing Hrpt2-deficient embryos. Hrpt2-deficient embryos at E10.5 had significantly increased TUNEL-positive cells relative to control embryos (Fig. 7D). In addition, cell proliferation, which was assessed in BrdU incorporation assays, was notably decreased in Cre-ER/Hrpt2L/L embryos (Fig. 7D). Thus, these results demonstrate that the loss of Hrpt2 expression is associated with increased apoptosis and reduced cell proliferation.
FIG. 7.
Hrpt2 loss promotes apoptosis in vitro and in vivo. A) Equal numbers of control MEFs and Hrpt2-deficient MEFs were plated and stained (left panel) using TUNEL and DAPI on day 3 after deletion of the Hrpt2 gene. There were more cells with TUNEL staining among the Hrpt2-deficient MEFs (right panel) than among control MEFs (P < 0.05). B) Hrpt2-deficient MEFs had more annexin V-positive cells than did control MEFs at days 2 (D2) and 3 (D3) after deletion of the Hrpt2 gene. C) Whole-cell lysates were isolated from control MEFs and Hrpt2-deficient MEFs at days 2, 3, 4, and 5 after deletion of the Hrpt2 gene. Compared to control MEFs, there was no change of total caspase 3, but the cleaved caspase 3 was increased in Hrpt2-deficient MEFs. There was no conversion of LC-3 I to LC-3 II, but the expression of LC-3 I was decreased in Hrpt2-deficient MEFs. There was no increase of HMGB1/B2 in the supernatant obtained from the Hrpt2-deficient MEFs compared to the control MEFs. All experiments were repeated three times. D) BrdU incorporation and TUNEL and H&E staining of E10.5 Cre-ER/Hrpt2+/+ and Cre-ER/Hrpt2L/L embryos, obtained from tamoxifen-treated pregnant mice at 8.5 dpc. Cre-ER/Hrpt2L/L embryos had increased cell death (H&E), less BrdU incorporation, and significantly more cells with positive TUNEL staining than the control Cre-ER/Hrpt2+/+ embryos.
Hrpt2 and its encoded protein, parafibromin, regulate genes involved in genomic imprinting and cell growth.
Our findings indicate that Hrpt2 and its encoded protein, parafibromin, are involved in regulating mammalian embryonic development and survival in adulthood. In order to identify genes that are regulated in these pathways, differences in gene expression were sought by means of cDNA microarrays between Hrpt2L/L and Hrpt2+/+ MEFs 96 h after exposure to Ad5CMV-Cre and Cre-ER/Hrpt2+/+ and Cre-ER/Hrpt2L/L MEFs 96 h after exposure to 4-OH-TM. This revealed that the expression of 111 genes was altered by the decreased expression of Hrpt2 (threshold, twofold change; see Tables S1, S2, and S3 in the supplemental material). Forty-three of these genes showed up-regulation (see Tables S1 and S3 in the supplemental material) and 68 showed down-regulation (see Tables S1 and S3 in the supplemental material). One group of down-regulated genes was related to cell growth and energy metabolism, and this included H19 fetal liver mRNA (H19), insulin-like growth factor 2 (Igf2), insulin-like growth factor 1 (Igf1), insulin-like growth factor binding protein 4 (Igfbp4), 3-hydroxy-3-methylgutayl-coenzyme A synthase 2 (Hmgcs2), carbonyl reductase 2 (Crb), bone marrow stromal cell antigen 2 (Bst2), high-mobility group AT-hook 1 (Hmga1), high-mobility group AT-hook 2 (Hmga2), and signal transducer and activator of transcription 2 (Stat2) (Fig. 8A). These changes were confirmed for several representative dysregulated genes by the use of semiquantitative RT-PCR on total RNA isolated from the MEFs (Fig. 8A). These findings are consistent with the previous report that the yeast PAF complex is related to protein synthesis and to lipid and nucleic acid metabolism (1).
FIG. 8.
Confirmation of altered gene expression by semiquantitative RT-PCR and CHIP assays. A) Total RNA was extracted from control MEFs and Hrpt2-deficient MEFs at day 4 after deletion of the Hrpt2 gene and semiquantitative RT-PCR was performed. CD24, CK, CXCL7, HSP1, and MnK2 were up-regulated, whereas H19, Igf1, Igf2, Igfbp4, Hmgcs2, Stat2, Hmga2, Hmga1, Crb2, Clu, Bst2, Clac1, and Hrpt2 were down-regulated in Hrpt2-deficient MEFs compared to control MEFs. GAPDH was used as the loading control. Experiments were repeated three times. B) The ChIP assay was performed using normal MEFs and anti-Paf1 and antiparafibromin polyclonal antibodies. Rabbit immunoglobulin G (IgG) was used as the negative control. Parafibromin and Paf1p bound to the promoter and/or open reading frame (ORF) and/or the 3′ untranslated region (3UTR) of several genes, including Igf2, H19, Hmgcs2, Igf1, Hmga1, Hmga2, and Igfbp4. C) The ChIP assay was also performed using Ad-GFP/Hrpt2L/L MEFs and Ad-Cre/Hrpt2L/L MEFs and the antiparafibromin polyclonal antibody. Real-time PCR was performed to assess for quantitative differences in the expression levels of H19, Igf2, and Igf1 in Ad-GFP/Hrpt2L/L MEFs and Ad-Cre/Hrpt2L/L MEFs.
To further identify the direct target genes whose transcription is controlled by parafibromin or the PAF complex, ChIP assays were used to investigate some growth factors and imprinting genes. This revealed that parafibromin and Paf1 bound to the promoter and/or coding regions of H19, Igf1, Igf2, Igfbp4, Hmgcs2, Hmga2, and Hmga1 (Fig. 8B and C). Thus, these results demonstrate that H19, Igf1, Igf2, Igfbp4, Hmga2, Hmgcs2, and Hmga1 are transcriptional target genes of the murine parafibromin/PAF complex and that these genes are normally regulated by murine parafibromin.
DISCUSSION
Our results have demonstrated that Hrpt2 expression is critical for embryonic development (Table 1 and Fig. 2) and survival in adult mice (Fig. 3) and that disruption of the Hrpt2 gene results in apoptosis (Fig. 5 and 7) by decreasing the expression of several target genes, including H19/Igf2, Igf1, Igfbp4, Hmgcs2, Hmga1, and Hmga2 (Fig. 8). IGF1 and IGF2 are known to provide trophic support for multiple cell types and organ cultures derived from various species, to delay the onset of programmed cell death through the mitochondrial pathway, and to antagonize activation of cytotoxic cytokine signaling (22). Hmga1 and Hmga2, which belong to the HMG family that also includes HMGA1b (6, 39), bind DNA to build a nuclear scaffold that is critical for the assembly of a stereospecific transcriptional complex (41, 50). This complex has roles in promoting DNA repair and in either inducing cellular apoptosis (39) or inhibiting apoptotic activity as revealed by the interaction between HMGA1 and p53 (35). In addition, mitochondrial Hmgcs2 is known to encode a key enzyme in the synthesis of ketones, which have a role in energy metabolism (29). A disruption of these mechanisms may therefore contribute to the observed apoptosis in the Hrpt2-deficient MEFs (Fig. 6 and 7).
These in vitro observations are supported by our in vivo results, which showed that Hrpt2 expression is required for growth of the formed fetus and survival of the adult mouse (Fig. 2 and 3). Moreover, significant apoptosis and developmental defects were observed in the E10.5 fetus with a deletion of the Hrpt2 gene for 48 h (Fig. 2). On the contrary, although deletion of Hrpt2 for 48 h retarded fetal growth, gross developmental defects were not detected at E12.5 or E14.5. Explanations for these differences include the possibility that Cre recombination efficiency may not be as high in the E10.5 to E14.5 embryos as in the E8.5 to E10.5 embryos and that cells at the later embryonic stages may not be as sensitive to loss of Hrpt2 expression as at the earlier stages. Nevertheless, Hrpt2 expression clearly has essential roles in growth and development and in the survival of adult mice, although the organs and cells which are most sensitive to its loss remain to be defined. However, it is important to note that the target genes for Hrpt2 expression and its encoded protein, parafibromin, include Igf1, Igf2, Hmga2, and Hmga1 (Fig. 8), and these are involved in growth of embryos and adult mice. Indeed, Igf1 and Igf2 knockout mice have been reported to have late embryonic lethality and surviving offspring have a reduced body size that is 60% of their wild-type littermates (9, 10, 23, 24, 38). Furthermore, the surviving offspring from Igf1 and Igf2 double knockouts are even smaller, with 30% of the body size of the wild-type littermates (15). Hmga2 knockout mice also have a smaller body size that is associated with a reduction in adipose synthesis (56), while Hmga1 knockout mice develop insulin resistance and diabetes mellitus (14). Thus, it is likely that the observed rapid death of adult Hrpt2-deficient mice (Fig. 3) may be related to a decreased expression of Igf1, Igf2, Hmga2, and Hmga1.
The balance of proapoptotic and antiapoptotic signals is likely to have a major role in contributing to the observed embryonic lethality of Hrpt2−/− embryos before E6.5. This lethality is likely to occur at hatching (E4.5) or implantation (E4.5 to E5.5). Hrpt2−/− blastocysts were normal at E3.5 (Fig. 1A), although they could not proliferate into ICM and TE cells in vitro (Fig. 2A) and died 5.5 to 6.5 dpc. Cell death normally occurs in both the ICM and TE lineages at the blastocyst stage (16), and the cell death index has been reported to be approximately 15% of total cells (19). The balance between anti- and proapoptotic factors is crucial for preimplantation development, and a death-by-default mechanism may force cells into committing suicide upon the withdrawal or nonsynthesis of critical survival factors (32). Indeed, the supplementation of culture medium with growth factors has been shown to increase rates of blastocyst formation that are accompanied by elevated numbers of cells, which likely result from a suppression of cell death (4, 5), whereas the addition of IGF-1 to culture medium results in a significant decrease in the number of apoptotic cells (25, 47). Hence, our results, which have demonstrated that loss of Hrpt2 expression and parafibromin is associated with an induction of apoptosis, provide a likely mechanistic insight into the cause of Hrpt2−/− embryonic lethality at the hatching or implantation stage. Thus, our findings demonstrate an important role of Hrpt2 and its encoded protein, parafibromin, in the transcriptional regulation of growth factors and in mammalian development and survival in adults.
Supplementary Material
Acknowledgments
We thank Eric Kort for editing figures and tables; Ralph Common for confocal microscopy; Pam Swiatek, Bryn Eagleson, and Jamie Bondsfield for mouse husbandry; Pete Haak for cDNA microarray analysis; David Nadziejka for manuscript proofreading; and Sabrina Noyes for preparing and submitting the manuscript.
This work was supported by the Van Andel Research Institute and the Medical Research Council, United Kingdom (M.R.B., A.A., and R.V.T.).
Footnotes
Published ahead of print on 22 January 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Betz, J. L., M. Chang, T. M. Washburn, S. E. Porter, C. L. Mueller, and J. A. Jaehning. 2002. Phenotypic analysis of Paf1/RNA polymerase II complex mutations reveals connections to cell cycle regulation, protein synthesis, and lipid and nucleic acid metabolism. Mol. Genet. Genomics 268272-285. [DOI] [PubMed] [Google Scholar]
- 2.Bradley, K. J., B. M. Cavaco, M. R. Bowl, B. Harding, T. Cranston, C. Fratter, G. M. Besser, M. Conceicao Pereira, M. W. Davie, N. Dudley, V. Leite, G. P. Sadler, A. Seller, and R. V. Thakker. 2006. Parafibromin mutations in hereditary hyperparathyroidism syndromes and parathyroid tumours. Clin. Endocrinol. (Oxford) 64299-306. [DOI] [PubMed] [Google Scholar]
- 3.Bradley, K. J., M. R. Hobbs, I. D. Buley, J. D. Carpten, B. M. Cavaco, J. E. Fares, P. Laidler, S. Manek, C. M. Robbins, I. S. Salti, N. W. Thompson, C. E. Jackson, and R. V. Thakker. 2005. Uterine tumours are a phenotypic manifestation of the hyperparathyroidism-jaw tumour syndrome. J. Intern. Med. 25718-26. [DOI] [PubMed] [Google Scholar]
- 4.Brison, D. R. 2000. Apoptosis in mammalian preimplantation embryos: regulation by survival factors. Hum. Fertil. (Cambridge) 336-47. [DOI] [PubMed] [Google Scholar]
- 5.Brison, D. R., and R. M. Schultz. 1997. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor alpha. Biol. Reprod. 561088-1096. [DOI] [PubMed] [Google Scholar]
- 6.Bustin, M., and R. Reeves. 1996. High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog. Nucleic Acid Res. Mol. Biol. 5435-100. [DOI] [PubMed] [Google Scholar]
- 7.Carpten, J. D., C. M. Robbins, A. Villablanca, L. Forsberg, S. Presciuttini, J. Bailey-Wilson, W. F. Simonds, E. M. Gillanders, A. M. Kennedy, J. D. Chen, S. K. Agarwal, R. Sood, M. P. Jones, T. Y. Moses, C. Haven, D. Petillo, P. D. Leotlela, B. Harding, D. Cameron, A. A. Pannett, A. Hoog, H. Heath, 3rd, L. A. James-Newton, B. Robinson, R. J. Zarbo, B. M. Cavaco, W. Wassif, N. D. Perrier, I. B. Rosen, U. Kristoffersson, P. D. Turnpenny, L. O. Farnebo, G. M. Besser, C. E. Jackson, H. Morreau, J. M. Trent, R. V. Thakker, S. J. Marx, B. T. Teh, C. Larsson, and M. R. Hobbs. 2002. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat. Genet. 32676-680. [DOI] [PubMed] [Google Scholar]
- 8.Codogno, P., and A. J. Meijer. 2005. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 12(Suppl. 2)1509-1518. [DOI] [PubMed] [Google Scholar]
- 9.Constancia, M., W. Dean, S. Lopes, T. Moore, G. Kelsey, and W. Reik. 2000. Deletion of a silencer element in Igf2 results in loss of imprinting independent of H19. Nat. Genet. 26203-206. [DOI] [PubMed] [Google Scholar]
- 10.DeChiara, T. M., A. Efstratiadis, and E. J. Robertson. 1990. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature 34578-80. [DOI] [PubMed] [Google Scholar]
- 11.Degryse, B., T. Bonaldi, P. Scaffidi, S. Muller, M. Resnati, F. Sanvito, G. Arrigoni, and M. E. Bianchi. 2001. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J. Cell Biol. 1521197-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobles, M., V. Liberal, M. L. Scott, R. Benezra, and P. K. Sorger. 2000. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell 101635-645. [DOI] [PubMed] [Google Scholar]
- 13.Farley, F. W., P. Soriano, L. S. Steffen, and S. M. Dymecki. 2000. Widespread recombinase expression using FLPeR (Flipper) mice. Genesis 28106-110. [PubMed] [Google Scholar]
- 14.Foti, D., E. Chiefari, M. Fedele, R. Iuliano, L. Brunetti, F. Paonessa, G. Manfioletti, F. Barbetti, A. Brunetti, C. M. Croce, and A. Fusco. 2005. Lack of the architectural factor HMGA1 causes insulin resistance and diabetes in humans and mice. Nat. Med. 11765-773. [DOI] [PubMed] [Google Scholar]
- 15.Gicquel, C., and Y. Le Bouc. 2006. Hormonal regulation of fetal growth. Horm. Res. 65(Suppl. 3)28-33. [DOI] [PubMed] [Google Scholar]
- 16.Hardy, J. F., S. Belisle, A. Couturier, and D. Robitaille. 1997. Randomized, placebo-controlled, double-blind study of an ultra-low-dose aprotinin regimen in reoperative and/or complex cardiac operations. J. Card. Surg. 1215-22. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi, S., P. Lewis, L. Pevny, and A. P. McMahon. 2002. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech. Dev. 119(Suppl. 1)S97-S101. [DOI] [PubMed] [Google Scholar]
- 18.Howell, V. M., C. J. Haven, K. Kahnoski, S. K. Khoo, D. Petillo, J. Chen, G. J. Fleuren, B. G. Robinson, L. W. Delbridge, J. Philips, A. E. Nelson, U. Krause, K. Hammje, H. Dralle, C. Hoang-Vu, O. Gimm, D. J. Marsh, H. Morreau, and B. T. Teh. 2003. HRPT2 mutations are associated with malignancy in sporadic parathyroid tumours. J. Med. Genet. 40657-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jurisicova, A., M. Antenos, K. Kapasi, J. Meriano, and R. F. Casper. 1999. Variability in the expression of trophectodermal markers beta-human chorionic gonadotrophin, human leukocyte antigen-G and pregnancy specific beta-1 glycoprotein by the human blastocyst. Hum. Reprod. 141852-1858. [DOI] [PubMed] [Google Scholar]
- 20.Krogan, N. J., J. Dover, A. Wood, J. Schneider, J. Heidt, M. A. Boateng, K. Dean, O. W. Ryan, A. Golshani, M. Johnston, J. F. Greenblatt, and A. Shilatifard. 2003. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol. Cell 11721-729. [DOI] [PubMed] [Google Scholar]
- 21.Krogan, N. J., M. Kim, A. Tong, A. Golshani, G. Cagney, V. Canadien, D. P. Richards, B. K. Beattie, A. Emili, C. Boone, A. Shilatifard, S. Buratowski, and J. Greenblatt. 2003. Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 234207-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurmasheva, R. T., and P. J. Houghton. 2006. IGF-I mediated survival pathways in normal and malignant cells. Biochim. Biophys. Acta 17661-22. [DOI] [PubMed] [Google Scholar]
- 23.Liu, J. L., A. Grinberg, H. Westphal, B. Sauer, D. Accili, M. Karas, and D. LeRoith. 1998. Insulin-like growth factor-I affects perinatal lethality and postnatal development in a gene dosage-dependent manner: manipulation using the Cre/loxP system in transgenic mice. Mol. Endocrinol. 121452-1462. [DOI] [PubMed] [Google Scholar]
- 24.Liu, J. P., J. Baker, A. S. Perkins, E. J. Robertson, and A. Efstratiadis. 1993. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 7559-72. [PubMed] [Google Scholar]
- 25.Makarevich, A. V., and M. Markkula. 2002. Apoptosis and cell proliferation potential of bovine embryos stimulated with insulin-like growth factor I during in vitro maturation and culture. Biol. Reprod. 66386-392. [DOI] [PubMed] [Google Scholar]
- 26.Melino, G., R. A. Knight, and P. Nicotera. 2005. How many ways to die? How many different models of cell death? Cell Death Differ. 12(Suppl. 2)1457-1462. [DOI] [PubMed] [Google Scholar]
- 27.Mosimann, C., G. Hausmann, and K. Basler. 2006. Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with beta-catenin/Armadillo. Cell 125327-341. [DOI] [PubMed] [Google Scholar]
- 28.Mueller, C. L., S. E. Porter, M. G. Hoffman, and J. A. Jaehning. 2004. The Paf1 complex has functions independent of actively transcribing RNA polymerase II. Mol. Cell 14447-456. [DOI] [PubMed] [Google Scholar]
- 29.Nadal, A., P. F. Marrero, and D. Haro. 2002. Down-regulation of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene by insulin: the role of the forkhead transcription factor FKHRL1. Biochem. J. 366289-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng, H. H., S. Dole, and K. Struhl. 2003. The Rtf1 component of the Paf1 transcriptional elongation complex is required for ubiquitination of histone H2B. J. Biol. Chem. 27833625-33628. [DOI] [PubMed] [Google Scholar]
- 31.Ng, H. H., F. Robert, R. A. Young, and K. Struhl. 2003. Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol. Cell 11709-719. [DOI] [PubMed] [Google Scholar]
- 32.Pampfer, S. 2000. Apoptosis in rodent peri-implantation embryos: differential susceptibility of inner cell mass and trophectoderm cell lineages: a review. Placenta 21(Suppl. A)S3-S10. [DOI] [PubMed] [Google Scholar]
- 33.Pavri, R., B. Zhu, G. Li, P. Trojer, S. Mandal, A. Shilatifard, and D. Reinberg. 2006. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell 125703-717. [DOI] [PubMed] [Google Scholar]
- 34.Penheiter, K. L., T. M. Washburn, S. E. Porter, M. G. Hoffman, and J. A. Jaehning. 2005. A posttranscriptional role for the yeast Paf1-RNA polymerase II complex is revealed by identification of primary targets. Mol. Cell 20213-223. [DOI] [PubMed] [Google Scholar]
- 35.Pierantoni, G. M., C. Rinaldo, F. Esposito, M. Mottolese, S. Soddu, and A. Fusco. 2006. High Mobility Group A1 (HMGA1) proteins interact with p53 and inhibit its apoptotic activity. Cell Death Differ. 131554-1563. [DOI] [PubMed] [Google Scholar]
- 36.Pokholok, D. K., N. M. Hannett, and R. A. Young. 2002. Exchange of RNA polymerase II initiation and elongation factors during gene expression in vivo. Mol. Cell 9799-809. [DOI] [PubMed] [Google Scholar]
- 37.Porter, S. E., T. M. Washburn, M. Chang, and J. A. Jaehning. 2002. The yeast Pafl-RNA polymerase II complex is required for full expression of a subset of cell cycle-regulated genes. Eukaryot. Cell 1830-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powell-Braxton, L., P. Hollingshead, C. Warburton, M. Dowd, S. Pitts-Meek, D. Dalton, N. Gillett, and T. A. Stewart. 1993. IGF-I is required for normal embryonic growth in mice. Genes Dev. 72609-2617. [DOI] [PubMed] [Google Scholar]
- 39.Reeves, R., and J. E. Adair. 2005. Role of high mobility group (HMG) chromatin proteins in DNA repair. DNA Repair (Amsterdam) 4926-938. [DOI] [PubMed] [Google Scholar]
- 40.Rozenblatt-Rosen, O., C. M. Hughes, S. J. Nannepaga, K. S. Shanmugam, T. D. Copeland, T. Guszczynski, J. H. Resau, and M. Meyerson. 2005. The parafibromin tumor suppressor protein is part of a human Paf1 complex. Mol. Cell. Biol. 25612-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saitoh, Y., and U. K. Laemmli. 1994. Metaphase chromosome structure: bands arise from a differential folding path of the highly AT-rich scaffold. Cell 76609-622. [DOI] [PubMed] [Google Scholar]
- 42.Schwenk, F., U. Baron, and K. Rajewsky. 1995. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 235080-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shattuck, T. M., S. Valimaki, T. Obara, R. D. Gaz, O. H. Clark, D. Shoback, M. E. Wierman, K. Tojo, C. M. Robbins, J. D. Carpten, L. O. Farnebo, C. Larsson, and A. Arnold. 2003. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N. Engl. J. Med. 3491722-1729. [DOI] [PubMed] [Google Scholar]
- 44.Sheldon, K. E., D. M. Mauger, and K. M. Arndt. 2005. A Requirement for the Saccharomyces cerevisiae Paf1 complex in snoRNA 3′ end formation. Mol. Cell 20225-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi, X., M. Chang, A. J. Wolf, C. H. Chang, A. A. Frazer-Abel, P. A. Wade, Z. F. Burton, and J. A. Jaehning. 1997. Cdc73p and Paf1p are found in a novel RNA polymerase II-containing complex distinct from the Srbp-containing holoenzyme. Mol. Cell. Biol. 171160-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simic, R., D. L. Lindstrom, H. G. Tran, K. L. Roinick, P. J. Costa, A. D. Johnson, G. A. Hartzog, and K. M. Arndt. 2003. Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J. 221846-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spanos, S., D. L. Becker, R. M. Winston, and K. Hardy. 2000. Anti-apoptotic action of insulin-like growth factor-I during human preimplantation embryo development. Biol. Reprod. 631413-1420. [DOI] [PubMed] [Google Scholar]
- 48.Stryke, D., M. Kawamoto, C. C. Huang, S. J. Johns, L. A. King, C. A. Harper, E. C. Meng, R. E. Lee, A. Yee, L. L'Italien, P. T. Chuang, S. G. Young, W. C. Skarnes, P. C. Babbitt, and T. E. Ferrin. 2003. BayGenomics: a resource of insertional mutations in mouse embryonic stem cells. Nucleic Acids Res. 31278-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan, M. H., C. Morrison, P. Wang, X. Yang, C. J. Haven, C. Zhang, P. Zhao, M. S. Tretiakova, E. Korpi-Hyovalti, J. R. Burgess, K. C. Soo, W. K. Cheah, B. Cao, J. Resau, H. Morreau, and B. T. Teh. 2004. Loss of parafibromin immunoreactivity is a distinguishing feature of parathyroid carcinoma. Clin. Cancer Res. 106629-6637. [DOI] [PubMed] [Google Scholar]
- 50.Tjian, R., and T. Maniatis. 1994. Transcriptional activation: a complex puzzle with few easy pieces. Cell 775-8. [DOI] [PubMed] [Google Scholar]
- 51.Wang, P., D. Kong, M. W. Vanbrocklin, J. Peng, C. Zhang, S. J. Potter, X. Gao, B. T. Teh, N. Zhang, B. O. Williams, and S. L. Holmen. 2006. Simplified method for the construction of gene targeting vectors for conditional gene inactivation in mice. Transgenics 4215-228. [Google Scholar]
- 52.Wood, A., J. Schneider, J. Dover, M. Johnston, and A. Shilatifard. 2003. The Paf1 complex is essential for histone monoubiquitination by the Rad6-Bre1 complex, which signals for histone methylation by COMPASS and Dot1p. J. Biol. Chem. 27834739-34742. [DOI] [PubMed] [Google Scholar]
- 53.Yart, A., M. Gstaiger, C. Wirbelauer, M. Pecnik, D. Anastasiou, D. Hess, and W. Krek. 2005. The HRPT2 tumor suppressor gene product parafibromin associates with human PAF1 and RNA polymerase II. Mol. Cell. Biol. 255052-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, C., D. Kong, M. H. Tan, D. L. Pappas, Jr., P. F. Wang, J. Chen, L. Farber, N. Zhang, H. M. Koo, M. Weinreich, B. O. Williams, and B. T. Teh. 2006. Parafibromin inhibits cancer cell growth and causes G1 phase arrest. Biochem. Biophys. Res. Commun. 35017-24. [DOI] [PubMed] [Google Scholar]
- 55.Zhao, J., A. Yart, S. Frigerio, A. Perren, P. Schraml, C. Weisstanner, T. Stallmach, W. Krek, and H. Moch. 2007. Sporadic human renal tumors display frequent allelic imbalances and novel mutations of the HRPT2 gene. Oncogene 263440-3449. [DOI] [PubMed] [Google Scholar]
- 56.Zhou, X., K. F. Benson, H. R. Ashar, and K. Chada. 1995. Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature 376771-774. [DOI] [PubMed] [Google Scholar]
- 57.Zhu, B., S. S. Mandal, A. D. Pham, Y. Zheng, H. Erdjument-Bromage, S. K. Batra, P. Tempst, and D. Reinberg. 2005. The human PAF complex coordinates transcription with events downstream of RNA synthesis. Genes Dev. 191668-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.