Abstract
Despite their self-sufficient ability to generate capped mRNAs from cytosolic DNA genomes, poxviruses must commandeer the critical eukaryotic translation initiation factor 4F (eIF4F) to recruit ribosomes. While eIF4F integrates signals to control translation, precisely how poxviruses manipulate the multisubunit eIF4F, composed of the cap-binding eIF4E and the RNA helicase eIF4A assembled onto an eIF4G platform, remains obscure. Here, we establish that the poxvirus infection of normal, primary human cells destroys the translational repressor eIF4E binding protein (4E-BP) and promotes eIF4E assembly into an active eIF4F complex bound to the cellular polyadenylate-binding protein (PABP). Stimulation of the eIF4G-associated kinase Mnk1 promotes eIF4E phosphorylation and enhances viral replication and protein synthesis. Remarkably, these eIF4F architectural alterations are accompanied by the concentration of eIF4E and eIF4G within cytosolic viral replication compartments surrounded by PABP. This demonstrates that poxvirus infection redistributes, assembles, and modifies core and associated components of eIF4F and concentrates them within discrete subcellular compartments. Furthermore, it suggests that the subcellular distribution of eIF4F components may potentiate the complex assembly.
A critical event in the life cycle of all viruses involves recruiting host ribosomes to their mRNAs (reviewed in reference 27). For cellular and viral mRNAs that harbor 7-methyl GTP caps on their 5′ ends, this process involves the eukaryotic translation initiation factor 4F (eIF4F), a tripartite complex composed of a cap-binding subunit (eIF4E) and an RNA helicase (eIF4A) anchored to a large molecular scaffold (eIF4G) capable of associating with eIF3-bound 40S ribosome subunits. eIF4G also provides a platform with which to recruit other important translational control proteins to the eIF4F complex. The cap-binding protein eIF4E is phosphorylated by the eIF4G-associated kinase Mnk1 (14, 35, 50). In addition, the cellular poly(A) binding protein (PABP), while itself not a core eIF4F component, physically interacts with eIF4G to bridge the 5′ and 3′ mRNA ends (19, 51) (Fig. 1A). Finally, the assembly of eIF4F is regulated in part by the eIF4E binding protein (4E-BP) family of translational repressors, which sequester eIF4E and prevent its association with eIF4G. Chaperone-like eIF4F assembly factors have also been implicated in binding to eIF4G and facilitating either eIF4F assembly or disassembly (9, 47). Given its pivotal importance in determining the translational fates of numerous mRNAs, eIF4F is the target of numerous cellular signaling pathways that control translation in response to a multitude of environmental conditions (36), including viral infection. Indeed, many viruses utilize a diverse battery of strategies to adroitly manipulate eIF4F through endogenous cellular pathways or by encoding their own regulatory components (27). Whereas viruses whose mRNAs are translated by cap-independent mechanisms often negatively regulate eIF4F to suppress cap-dependent translation, some viruses that utilize a cap-dependent mechanism to translate their mRNAs can actually stimulate eIF4F activity.
FIG. 1.
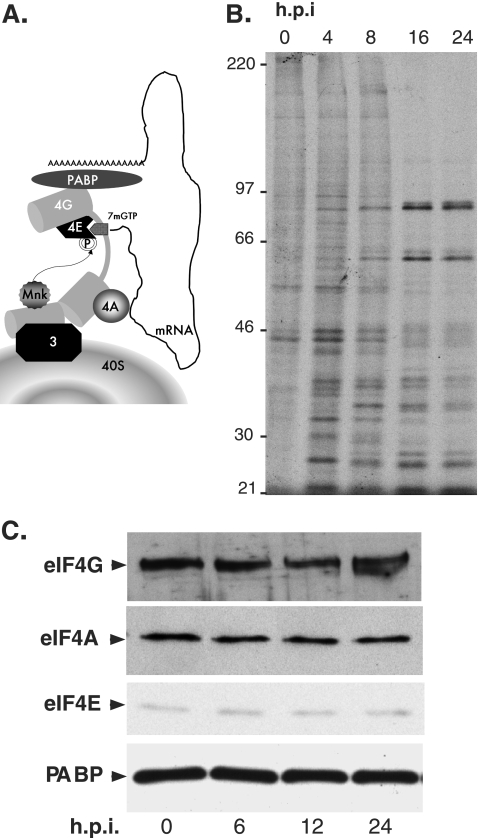
Characterization of eIF4F in poxvirus-infected cells. (A) eIF4F recruits 40S subunits to the mRNA 5′ end. eIF4F is composed of the cap-binding protein (eIF4E), a large protein scaffold (eIF4G), and an RNA helicase (eIF4A). In addition to these core eIF4F components, the PABP and the cellular eIF4E kinase (Mnk1) also associate with eIF4G. The 40S ribosomal subunit is recruited to the 5′ end of the mRNA through an association between eIF4G and eIF3. Once it is localized to the 5′ end, the 40S ribosome commences scanning for the AUG initiator codon. (B) Kinetic profile of newly synthesized proteins in primary human cells infected with VV. NHDFs growth arrested by serum starvation were mock infected (0 h.p.i.) or infected with VV (MOI = 5). At the indicated times (h.p.i.), the cultures were radiolabeled for 1 h with 35S-labeled amino acids. Total protein was subsequently isolated and fractionated by SDS-PAGE, and the fixed, dried gel was exposed to X-ray film. The migration of molecular mass standards (in kDa) appears at the left of the autoradiograph. (C) The abundance of eIF4F core components and ancillary factors remains unchanged following poxvirus infection. As in panel B, except for the following fractionation of total protein by SDS-PAGE, the samples were analyzed by immunoblotting using antisera specific for the indicated proteins.
Poxviruses are large DNA viruses that replicate exclusively within the host cell cytosol (28). Indeed, discrete cytoplasmic regions are dramatically reorganized into replication compartments, termed factories, during the productive growth cycle (8). Despite encoding a varied assortment of functions, including a multisubunit DNA-dependent RNA polymerase, specific transcription factors for different promoter classes, and a type I topoisomerase required for replication of a DNA virus in the cytosol, poxviruses remain, as do all viruses, absolutely dependent upon the cellular translational machinery to produce the viral polypeptides necessary for productive viral growth. Like many viruses, poxviruses can effectively prevent host defenses from inactivating eIF2, the cellular translation initiation factor responsible for chaperoning the initiator tRNA to the 40S ribosome (27). Moreover, poxvirus mRNAs, all of which are capped on their 5′ ends through the action of a viral methyl transferase enzyme complex (6, 26, 45), must effectively commandeer the eIF4F to recruit ribosomes. Although eIF4F is a critical translation initiation factor and a prime target for regulating translation initiation, exactly how poxviruses manipulate eIF4F core and associated proteins has never been fully explored. Earlier studies claimed that translation in poxvirus-infected cells proceeded normally in cells engineered to inducibly express the poliovirus 2A protease (29), while a different study reported that eIF4G cleavage by poliovirus 2A inhibits translation in poxvirus-infected cells (5). In a similar vein, studies of eIF4E modification in poxvirus-infected cells focused exclusively on the lack of apparent dephosphorylation (15, 38). Finally, none of these studies was performed with normal cells; instead, they relied exclusively on transformed, immortalized cell lines now known to contain altered translational regulatory pathways (37).
This report examines how vaccinia virus (VV), a model poxvirus, effectively manipulates the cellular translation initiation factors required for cap-dependent translation in normal primary human cells. Here, we establish that VV infection results in the destruction of the translational repressor 4E-BP1. Active eIF4F complexes are assembled, and cellular signaling pathways that stimulate the cellular eIF4G-associated kinase Mnk1 to phosphorylate the cap-binding protein eIF4E are triggered. Not only were VV replication and protein production compromised in Mnk1-deficient murine fibroblasts, but preventing eIF4E phosphorylation with the small molecule Mnk1 inhibitor dramatically reduced VV mRNA translation and viral replication in quiescent primary human cells. Remarkably, these changes to eIF4F architecture are accompanied by the redistribution of eIF4E and eIF4G within cytosolic viral replication compartments surrounded by PABP. This establishes that poxvirus infection redistributes, assembles and modifies the core and associated components of eIF4F, concentrating them within discrete subcellular compartments. Furthermore, it suggests that the subcellular distribution of eIF4F components can influence complex assembly. Interfering with this process may provide unique opportunities to develop novel anti-poxvirus compounds.
MATERIALS AND METHODS
Antibodies and chemicals.
Anti-eIF4G polyclonal antiserum was prepared as described previously (46). Polyclonal anti-PABP antiserum was a kind gift from S. Morley (University of Sussex), and polyclonal anti-Dcp1 was generously provided by Jens Lykke-Andersen (University of Colorado, Boulder, CO). The antibodies directed against antigens were purchased from commercial suppliers, as follows: eIF4E (catalog no. E27620; BD Transduction Laboratories, San Diego, CA), 4EBP1 (catalog no. A300-501A; Bethyl Labs, Montgomery, TX), extracellular signal-regulated kinase (ERK) (catalog no. 9102 for total ERK and 9101 for phospho-specific ERK; Cell Signal Technologies, Beverly, MA), p38 mitogen-activated protein kinase (MAPK) (catalog no. 9452 for total p38 and 9216 for phospho-specific MAPK; Cell Signal Technologies, Beverly, MA), anti-VV polyclonal sera (catalog no. 8101; Virostat, Portland, ME), and anti-TIA-1 (catalog no. SC1751; Santa Cruz Biotechnology, Santa Cruz, CA). Phosphonoacetic acid (PAA) and rifampin (catalog nos. 284270 and R3501, respectively) were obtained from Sigma Chemical Co., St. Louis, MO, and cytosine arabinoside (AraC) (catalog no. 251010) was obtained from Calbiochem, San Diego, CA.
Cells and virus.
Primary normal human diploid fibroblasts (NHDFs; Clonetics, Walkersville, MD) were cultured, growth arrested by serum starvation in 0.2% fetal bovine serum (FBS), and infected as described previously (46). Spontaneously immortalized murine fibroblasts from wild-type (WT) or Mnk1-deficient (Mnk1−/−) mice (44) were maintained in Dulbecco's modified Eagle's medium (DMEM) plus 10% FBS and were growth arrested by serum starvation for 72 h in DMEM plus 0.5% FBS. For infections of growth-arrested, serum-starved cells, virus was diluted from concentrated stocks such that a final serum concentration of 0.2% (NHDFs) or 0.5% (murine cells) was maintained at all times during infections. BSC40 cells were maintained in DMEM plus 10% FBS. VV (Western Reserve strain) was propagated in BSC40 cells (at a multiplicity of infection [MOI] of 0.05). Once the cytopathic effect was visible in 100% of the culture, the medium was replaced with serum-free DMEM, and the plates were frozen at −80°C. Under these conditions, the overwhelming majority of virus remained cell associated (D. Walsh and I. Mohr, unpublished observations). Cell-free lysates were prepared by three freeze-thaw cycles, and small aliquots were stored at −80°C. Prior to the infection of cells, the virus stock was treated with 0.125% trypsin for 30 min at 37°C. Samples were adjusted to 1 ml with DMEM, and FBS was added to a final concentration of 0.5%. The virus stock titer was quantified by plaque assay of BSC40 cells.
[35S]methionine labeling, gel electrophoresis, and immunoblotting.
NHDF cells (6 × 105 cells/dish) were incubated for 1 h in 1 ml of methionine-free DMEM containing 77 μCi of a [35S]methionine-cysteine mixture (catalog no. NEG072; Amersham). Total cellular protein was subsequently solubilized in 250 μl of sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate [SDS], 10% glycerol, 0.7 M β-mercaptoethanol), boiled for 3 min, and fractionated by SDS-polyacrylamide gel electrophoresis (PAGE). Labeled proteins were visualized by exposing the fixed, dried gel to X-ray film. Alternatively, where indicated, proteins were transferred to nitrocellulose following SDS-PAGE. Immunoblots were processed, incubated with primary antibody, and developed by using an enhanced chemiluminescence reagent according to the manufacturer's instructions (Amersham). Vertical slab isoelectric focusing (IEF) was performed as described previously (46).
Immunoblotting, IEF, and analysis of eIF4E binding proteins by batch chromatography on 7-methyl GTP Sepharose.
These procedures were performed essentially as described previously (46), with the following minor modifications. NLB buffer containing 50 mM HEPES (pH 7.5), 100 mM NaCl, 1.5 mM MgCl2, 2 mM EDTA, 2 mM Na3VO4, 25 mM glycerophosphate, 0.25% NP-40, and complete miniprotease inhibitor cocktail (Roche) was used to lyse the cells prior to 7-methyl GTP Sepharose chromatography.
Multicycle growth experiments.
NHDF cells (8 ×105 cells/dish) were seeded in 60-mm dishes, serum starved, and treated with either dimethyl sulfoxide (DMSO) or CGP57380, as described previously (46). At the indicated times following infection with either 8,000 PFU (MOI = 10−2), 800 PFU (MOI = 10−3), or 45 PFU (5.6 × 10−5 PFU) of VV, the medium was replaced with serum-free DMEM, and the NHDF cultures were frozen at −80°C. After three freeze-thaw cycles, the amount of infectious virus was quantified by plaque assay of BSC40 cells. WT or Mnk1−/− murine cells were seeded (8 × 105 cells per dish) and serum starved as described above. At the indicated times following infection with 45 PFU of VV, the medium was replaced with serum-free DMEM, and the cultures were frozen at −80°C. After three freeze-thaw cycles, the amount of infectious virus was quantified by plaque assay of BSC40 cells.
Immunofluorescence analysis.
Cells were seeded onto glass coverslips for 24 h and growth arrested by serum deprivation, as described previously (46). Mock-infected or VV-infected cells were fixed with 3.7% formaldehyde for 20 min and permeabilized with 0.1% Triton X-100 plus RNase A. Samples were next incubated with the specified primary antisera for 1 h at 37°C, followed by incubation with the appropriate fluorescently conjugated secondary antibody (anti-mouse fluorescein isothiocyanate [FITC] [Vector Laboratories], anti-rabbit, or anti-goat Alexa flour 633 [Molecular Probes]). Nuclei were counterstained with propidium iodide or 4′,6′-diamidino-2-phenylindole (DAPI). The fluorescent images were collected with a Zeiss LSM510 Meta confocal laser scanning microscope or a Zeiss Axiovert fluorescence microscope, using Metamorph software.
RESULTS
Abundances of eIF4F core components and PABP remain constant in VV-infected cells, despite a strong suppression of host protein synthesis.
Following the infection of growth-arrested NHDFs with VV, substantial changes in the overall pattern of the proteins synthesized are noticeable by 3 h postinfection (h.p.i.). As the infection progresses, host mRNA translation is suppressed in part by a mechanism involving mRNA turnover (31-33), and VV proteins accumulate (Fig. 1B). While this is most dramatic between 8 and 16 h.p.i., notable changes in the polypeptides produced, as measured by 35S radiolabeling of newly synthesized proteins, are also evident between 4 and 8 h.p.i. (Fig. 1B). In addition, the overall abundances of the eIF4F core components eIF4E, eIF4G, and eIF4A, along with the eIF4F-associated protein PABP, remain constant in VV-infected NHDFs (Fig. 1C) or murine cells (not shown). Thus, the ability of VV to dominate the host translational machinery and impair host polypeptide production does not appear to involve detectable alterations of the overall steady-state levels of eIF4F core components or of PABP.
Inactivation of the translational repressor 4E-BP1 and eIF4F complex assembly in VV-infected cells.
Depending upon their abundance, a family of small 4E-BPs can sequester eIF4E in a phosphorylation-dependent manner (Fig. 2a). In their hypophosphorylated form, 4E-BPs inhibit cap-dependent translation by preventing eIF4E from associating with eIF4G (reviewed in reference 36). Hyperphosphorylation of the 4E-BPs by the mTOR kinase results in the release of the cap-binding protein, exposing the surface of the eIF4E that interacts with eIF4G, an important step in eIF4F assembly. VV infection results in the appearance of slower-migrating hyperphosphorylated forms of 4E-BP1, visible by 3 h.p.i. and subsequently sustained at later time points, which is clearly resolved by electrophoresis in a high-percentage SDS-polyacrylamide gel (Fig. 2B). The appearance of these hyperphosphorylated forms was blocked by rapamycin, an inhibitor of the cellular kinase mTOR (Fig. 2B). Inactivation of the 4E-BP1 translational repressor was sensitive to the DNA synthesis inhibitor PAA or AraC (Fig. 2C), suggesting that either viral DNA synthesis or an event tightly associated with viral DNA replication such as late viral gene expression is required. Finally, VV infection noticeably reduced the intensity of the 4E-BP1 signal, which could be due to either a reduction in protein abundance or a redistribution of the initial hypophosphorylated population into multiple distinct, phosphorylated species.
FIG. 2.
Inactivation of the 4E-BP1 translational repressor and assembly of eIF4F complexes in poxvirus-infected cells. (A) Inactivation of 4E-BP1 by phosphorylation. In its hypophosphorylated state, the translational repressor 4E-BP1 binds eIF4E and prevents its association with the other eIF4F components. Upon the activation of mTOR, hyperphosphorylation of 4E-BP1 results in the release of eIF4E, exposing a surface on eIF4E that associates with eIF4G. Rapamycin, an mTOR inhibitor, prevents 4E-BP1 hyperphosphorylation. (B) Phosphorylation of 4E-BP1 in VV-infected cells. Growth-arrested NHDFs were mock infected (Mock) or infected (VV) with vaccinia virus (MOI = 5) in the presence (+) or absence (−) of rapamycin (RAPA). At 16 h.p.i, total protein was isolated, fractionated by SDS-PAGE in 17.5% gels, and analyzed by immunoblotting using anti-4E-BP1 serum. The slow-migrating hyperphosphorylated (hyper) and fast-migrating hypophosphorylated (hypo) forms of 4E-BP1 are noted at the right of the panel. (C) Sensitivity of 4E-BP1 phosphorylation to viral DNA synthesis inhibitors in VV-infected cells. As in panel B, except cells were infected in the presence (+) or absence (−) of either PAA (300 μg/ml) or AraC (40 μg/ml). (D) VV infection decreases 4E-BP1 abundance. As in panel B, except the indicated cultures were additionally treated with the proteasome inhibitor MG132. Total protein was fractionated by SDS-PAGE in 7.5% gels, which do not resolve phosphorylated 4E-BP1 isoforms, and analyzed by immunoblotting with the indicated antisera.
To evaluate the overall steady-state level of 4E-BP1 in infected versus uninfected cells, the same samples were fractionated in low-percentage polyacrylamide gels, which do not resolve the phosphorylated isoforms. Whereas the total amount of PABP remains constant, the 4E-BP1 abundance is radically reduced. The treatment of VV-infected cells with either the proteasome inhibitor MG132 or rapamycin eliminated the decline in 4E-BP1 levels (Fig. 2D). Thus, the 4E-BP1 translational repressor is inactivated in VV-infected cells by mTOR-mediated phosphorylation, followed by its destruction within the proteasome. Other DNA viruses use a similar strategy to destroy the 4E-BP1 repressor in infected cells (46).
As the release of the 4E-BP1 repressor exposes a surface on eIF4E that binds to eIF4G, repressor inactivation contributes to eIF4F assembly (16, 23, 25). Collecting eIF4E-containing complexes present in cell lysates on 7-methyl GTP Sepharose beads and analyzing the composition of the bound proteins by immunoblotting can measure the extent of eIF4F assembly. Whereas eIF4E-containing complexes isolated from mock-infected, growth-arrested NHDFs contain an abundance of 4E-BP1 and basal amounts of eIF4G, together with PABP, VV infection or serum stimulation results in the displacement of 4E-BP1 from the complex and the recruitment of greater amounts of PABP and eIF4G (Fig. 3A). Rapamycin or MG132 each blocked the ejection of 4E-BP1 from eIF4E, consistent with our previous observation that mTOR-mediated 4E-BP1 phosphorylation is followed by proteasomal degradation (Fig. 3B). Notably, the amount of PABP recruited into the complex in VV-infected cells exceeds the amount detected in serum-stimulated cells (Fig. 3A). Conceivably, this could reflect a peculiar property of VV mRNAs, which contain polyadenylated 3′ and 5′ ends (2, 18, 39). Nevertheless, VV infection clearly stimulates the assembly of eIF4F core components together with the eIF4F-associated protein PABP. Thus, in addition to suppressing host protein synthesis and inactivating the 4E-BP1 translational repressor, viral functions promote the assembly of the cap-binding protein eIF4E into an eIF4F complex and recruit eIF4F-associated proteins such as PABP.
FIG. 3.
Stimulation of eIF4F assembly in VV-infected cells. (A) Growth-arrested NHDFs were either serum stimulated (serum), mock-infected (Mock), or infected with VV (VV). At 16 h.p.i., detergent lysates were prepared and incubated with 7-methyl GTP Sepharose beads. Material bound to the beads was fractionated by SDS-PAGE and analyzed by immunoblotting with the indicated antisera. (B) Sensitivity of 4E-BP1 release from eIF4E in VV-infected cells to rapamycin and MG132. As in panel A, except cells were infected in the absence (−) or presence (+) of rapamycin (RAPA) or MG132. Material bound to 7-methyl GTP beads was fractionated by SDS-PAGE and analyzed by immunoblotting with the indicated antisera.
Stimulation of eIF4E phosphorylation in VV-infected cells.
Besides PABP, other eIF4F-associated factors fulfill important but not essential regulatory roles. Notable among these factors is the eIF4G-associated kinase Mnk1, which upon activation by ERK or p38 phosphorylates the cap-binding protein eIF4E on Ser 209. Indeed, both ERK and p38 are substantially activated at 5 h.p.i. in VV-infected human (Fig. 4A) or murine cells (not shown). Our observations for primary human cells agree with other reports using established cell lines (4, 11, 24). However, in addition to activating ERK and p38, eIF4E phosphorylation requires eIF4F assembly, illustrating the requirement for proper complex architecture to ensure that the activated kinase Mnk1 is properly oriented in respect to its substrate, eIF4E (35, 43, 47). Phosphorylated eIF4E accumulates between 8 and 16 h.p.i. as VV promotes eIF4F assembly and activates ERK along with p38 (Fig. 4B). While a slight but reproducible reduction in the overall abundance of phospho-eIF4E was observed at 24 h.p.i., the peak of phosphorylated eIF4E accumulation coincides with vigorous viral protein synthesis (Fig. 4B and Fig. 1B). In addition, phosphorylated eIF4E accumulation was sensitive to the viral DNA synthesis inhibitor PAA, suggesting that either viral DNA synthesis or an event tightly linked to viral DNA replication, such as the onset of late gene expression, is required (Fig. 4C). eIF4E phosphorylation in VV-infected cells is entirely dependent upon Mnk1, as phosphorylated eIF4E is not detected in Mnk1-deficient cells infected with VV, even on prolonged exposures of immunoblots (Fig. 4D). Moreover, a small-molecule Mnk1 inhibitor (CGP37580 [22]) effectively blocks eIF4E phosphorylation in VV-infected cells (Fig. 4E).
FIG. 4.
Phosphorylation of eIF4E is stimulated through multiple pathways in poxvirus-infected cells. (A) Activation of both ERK and p38 in VV-infected cells. Growth-arrested NHDFs were either mock-infected (0 h.p.i.) or infected with VV (MOI = 5). At the indicated times (h.p.i.), total protein was isolated, fractionated by SDS-PAGE, and analyzed by immunoblotting using antisera recognizing phospho-ERK [(P) ERK], total ERK [(T) ERK], phospho-p38 [(P) p38], or total p38 [(T) p38]. (B) Accumulation of the phosphorylated eIF4E in poxvirus infected cells. As in panel A, except total protein was fractionated by IEF and analyzed by immunoblotting using anti-eIF4E antisera. The migration of phosphorylated eIF4E [(P)-4E] and unphosphorylated eIF4E (4E) are noted at the left of the panel. A lysate from uninfected, arsenite-treated cells was included in the last lane as a positive control (+) for eIF4E phosphorylation. (C) Inhibition of viral DNA synthesis prevents eIF4E phosphorylation. Cells were infected as shown in panel A in the presence of 300 μg/ml PAA, and total protein was isolated at 16 h.p.i. eIF4E phosphorylation was evaluated by IEF as in panel B. (D) Phosphorylation of eIF4E requires Mnk1 in VV-infected cells. Immortalized murine fibroblasts derived from WT (+/+) or Mnk1-deficient (−/−) embryos were mock infected (M) or infected with VV as shown in panel A. At 8 h.p.i, eIF4E phosphorylation was evaluated by IEF, as shown in panel B. A short and long exposure of the immunoblot from −/− cells is shown. (E) A small-molecule Mnk1 inhibitor prevents eIF4E phosphorylation in VV-infected human cells. Growth-arrested NHDFs were mock infected (M) or infected with VV (MOI = 5) in the presence (+) or absence (−) of CGP57380. eIF4E phosphorylation was evaluated by IEF, as described in the legend to panel B. Both normal (short) exposure and overexposure (long) of the immunoblot are shown.
Enhancement of VV replication by the eIF4E kinase Mnk1.
To determine if Mnk1 contributes to poxvirus replication and the ability of the virus to spread through an infected culture, WT and Mnk1-deficient cells were infected with VV at low multiplicities of infection, and the extent of viral replication and spread was quantified by plaque assay, using permissive monkey kidney (BSC40) cells. Under these conditions, multiple cycles of productive replication are required for the virus to spread through the culture. Remarkably, after 3 days, the cytopathic effect was barely detectable in Mnk1-deficient cultures, whereas it was near 100% in WT cells (Fig. 5A). In addition, the overall yield of infectious virus recovered was reduced by almost 2 orders of magnitude, and the accumulation of VV-encoded polypeptides was dramatically impaired (Fig. 5B). This suggested that VV replication and spread, along with viral protein accumulation, was compromised in Mnk1-deficient cells. As Mnk1 is not required for normal cell growth and development of mice (44), the possibility that Mnk1 inhibitors might suppress VV replication was investigated. Duplicate cultures of growth-arrested NHDFs treated with either DMSO or CGP57380 were again infected with VV at different multiplicities of infection, and the amount of infectious virus produced was quantified by plaque assay. Under these conditions of CGP57380 treatment, phosphorylated eIF4E was undetectable, attesting to the effectiveness of the Mnk inhibitor (Fig. 5C). Taken together, the overall yield of infectious virus measured over a range of time points was reduced by between approximately 40- and 100-fold in each CGP57380-treated culture infected with one of three different quantities of input virus (Fig. 5C and D and data not shown). There was no change in the viability of uninfected cultures treated with CGP57380, and phosphorylated eIF4E was not detected under these conditions (not shown and Fig. 4D). Furthermore, the striking reduction in VV polypeptide abundance in CGP57380-treated normal human cells was similar to that observed with Mnk1-deficient cells. Thus, the eIF4G-associated kinase Mnk1 regulates VV replication, as VV replication is compromised in Mnk1-deficient cells, and a small-molecule Mnk1 inhibitor effectively reduces VV replication and spread in normal human cells.
FIG. 5.
Interfering with Mnk1 suppresses poxvirus replication. (A) Reduction of poxvirus-induced cytopathic effect in Mnk1-deficient cells. Serum-starved murine cells (Mnk1+/+ or Mnk1−/−) were infected with VV at a low MOI, as described in Materials and Methods. At 72 h.p.i., the cultures were photographed. (B) Limited poxvirus replication in the absence of Mnk1. (Left panel) Cultures were infected as shown in panel A and subsequently lysed by repeated freeze-thaw cycles at 72 h.p.i. The amount of infectious virus produced was quantified by plaque assay of permissive BSC40 cells. (Right panel) Following infection with VV, as described in the legend to panel A, total protein was isolated at 72 h.p.i., fractionated by SDS-PAGE, and analyzed by immunoblotting with anti-VV-specific sera. (C) A small-molecule inhibitor of Mnk1 suppresses poxvirus replication. (Left panel) Growth-arrested NHDFs treated with either DMSO or the Mnk1 inhibitor CGP57380 were infected with VV in duplicate at a low MOI. After 4 days, cell-free lysates were prepared by repeated freeze-thaw cycles, and the amount of infectious virus in each independent sample was quantified by plaque assay in BSC40 cells. (Right panel) VV-infected cells were treated with CGP57380 (+) or DMSO (−) as described above. At 4 days postinfection, total protein was isolated and either (i) fractionated by SDS-PAGE and analyzed by immunoblotting with anti-VV specific sera (top panel) or (ii) fractionated by IEF and analyzed by immunoblotting using anti-eIF4E antisera. The migrations of phosphorylated eIF4E [(P)-4E] and unphosphorylated eIF4E (4E) are noted at the left of the panel. (D) Growth-arrested NHDFs treated with either DMSO or the Mnk1 inhibitor CGP57380 were infected with VV in duplicate (MOI = 0.01). At the indicated times (h.p.i.), cell-free lysates were prepared by repeated freeze-thaw cycles, and the amount of infectious virus in each independent sample was quantified by plaque assay of BSC40 cells.
Subcellular redistribution of eIF4F core and associated components accompanies translation initiation factor complex assembly.
Given that VV DNA synthesis and assembly are confined to discrete cytosolic compartments or replication factories (8), the impact of VV infection on the subcellular distribution of eIF4E, eIF4G, and PABP was investigated. In growth-arrested NHDFs, eIF4F core and associated proteins are distributed relatively uniformly throughout the cell (Fig. 6A, B, and C; also see reference 52). However, following the cells' infection with VV, the cap-binding protein eIF4E and eIF4G assembly platform are redistributed between 6 and 12 h.p.i., and a large majority of the proteins appear to concentrate in discrete foci within cytoplasmic viral DNA-containing bodies (Fig. 6A). This coincides with the accumulation of phospho-eIF4E (Fig. 4B) and eIF4F-assembly (Fig. 3) and a period of robust viral protein synthesis (Fig. 1B). Indeed, both eIF4E and eIF4G display substantial colocalization by confocal microscopy (Fig. 6C). VV infection also results in the redistribution of the cellular PABP. Whereas eIF4E and eIF4G colocalize within nucleic acid-containing cytosolic replication compartments or factories, PABP appears to remain concentrated outside, around the periphery of these structures (Fig. 6B). Cytoplasmic DNA-containing bodies were not observed with VV-infected cultures treated with the DNA synthesis inhibitor PAA (Fig. 6B) or AraC (data not shown), demonstrating that these compartments contain replicating viral DNA. Moreover, PAA and AraC each were able to prevent (i) the redistribution of eIF4E, eIF4G, and PABP; (ii) the concentration of eIF4E and eIF4G within these small, cytoplasmic DNA-containing bodies; (iii) the accumulation of PABP in the region that surrounds the replication compartment; and (iv) the phosphorylation of eIF4E by the eIF4G-associated kinase Mnk1 (Fig. 4C), a surrogate marker for eIF4F assembly (43, 47). Treatment of VV-infected cultures with the Mnk inhibitor CGP57380 had no detectable impact on the redistribution of eIF4E, eIF4G, or PABP (data not shown). Thus, either viral DNA synthesis or an event closely linked to viral DNA replication, such as the expression of late viral genes, appears to be required to promote the redistribution of eIF4E, eIF4G, and PABP, together with eIF4E phosphorylation. The greater effective local concentration of eIF4E, eIF4G, and PABP within and around these replication compartments likely contributes to the stimulation observed with eIF4F complex formation seen in infected cells. Finally, both the translation initiation factor redistribution (Fig. 6C) and the eIF4E phosphorylation (data not shown) were observed in the presence of the macrolide antibiotic rifampin, which is believed to interfere with the formation of factory-associated membranes, viral crescents, and immature virions by targeting the VV D13 p65 scaffold protein (7, 40). This suggests that while translation initiation factor redistribution and eIF4E phosphorylation occur late in the viral life cycle, they do so prior to normal crescent formation or do not require normal crescent formation.
FIG. 6.
Redistribution of eIF4E, eIF4G, and PABP in VV-infected cells. (A) Time course of eIF4E-eIF4G redistribution. Growth-arrested NHDFs were infected with VV at high MOI. At the indicated time postinfection (h.p.i.), the cells were fixed with 3.7% formaldehyde, permeabilized with 0.1% Triton X-100, and stained with antisera specific for either eIF4E or eIF4G. Following incubation with a fluorescence-conjugated secondary antibody (eIF4E, FITC, green stain; eIF4G, Alexa Fluor, red stain in merge panel), the samples were viewed with a Zeiss Axiovert fluorescence microscope. Nuclei and cytoplasmic DNA-containing VV replication compartments were visualized by DAPI staining (bodies stained with DAPI alone are shown as white or as blue in the merge panels). White arrowheads (12- and 22-h.p.i. panels) denote representative VV replication compartments (DAPI panels) and the corresponding foci of eIF4E or eIF4G. (B) Growth-arrested cells were mock infected (UN) or infected with vaccinia virus (VV) at a high MOI in the presence or absence of PAA. At 12 to 16 h.p.i., the cells were fixed with 3.7% formaldehyde, permeabilized with 0.1% Triton X-100, and stained with the indicated antibodies (eIF4E, eIF4G, and PABP). DNA was stained red with propidium iodide (eIF4E panel) or blue with DAPI (PABP and eIF4G panels). Following incubation with a fluorescence-conjugated secondary antibody (eIF4E, FITC, green stain; eIF4G or PABP, Alexa Fluor, red stain), the samples were viewed with either a Zeiss LSM510 Meta confocal or a Zeiss Axiovert fluorescence microscope. The bottom center panel shows an enlarged section from the eIF4G-stained VV-infected panel immediately above it (marked by the white *). Note that eIF4G is concentrated in small DNA-containing viral replication factories but is absent from large host nuclei. The accumulation of eIF4G in replication factories is abolished by PAA, an inhibitor of viral DNA synthesis. (C) Cells were infected as shown in panel B in the presence of rifampin (RIF). Fixed and permeabilized cells were incubated with antibodies directed against both eIF4E and eIF4G. Following incubation with the appropriate fluorescence-conjugated secondary antibodies (eIF4E, FITC, green stain; eIF4G, Alexa Fluor, red stain), the samples were viewed with a Zeiss LSM510 Meta confocal microscope, and the extent of colocalization was evaluated. (D) Cells were infected, fixed, and permeabilized as shown in panel B but stained with anti-Dcp1 antiserum. Following incubation with an Alexa Fluor-conjugated secondary antibody (red stain), the samples were viewed with a Zeiss Axiovert fluorescence microscope. DNA was stained with DAPI. For greater contrast, the bodies shown as white were stained with DAPI alone. In the merged image panels, DAPI stained the bodies blue. (E) HeLa cells were infected with VV or treated with arsenite (As), fixed, and permeabilized as shown in panel B but stained with anti-TIA-1 antiserum. Cells were visualized as described in the legend to panel D. Arsenite treatment serves as a positive control known to induce SG formation.
The concentration of eIF4E and eIF4G into dense foci within replication compartments raised the possibility that these structures could be antigenically related to characterized aggregate bodies known to contain translation initiation factors in the cytosol of uninfected cells. Translationally inactive mRNAs accumulate in P bodies, subcellular sites of mRNA repression, storage, and degradation (30). To determine if the eIF4E-eIF4G foci observed with VV-infected cells were related to P bodies, infected cells were stained with antisera directed against Dcp1, a host decapping enzyme that is an accepted marker for P bodies (30). While VV infection did not suppress or enhance the relative number of Dcp1-staining P bodies, Dcp1 was not preferentially concentrated within or above DAPI-staining cytoplasmic DNA compartments in VV-infected cells (Fig. 6D). Stress granules (SGs) represent another cytoplasmic subcellular structure reported to contain translation initiation factors. In response to a variety of environmental stressors, including those that activate host eIF2α kinases, stalled translation initiation complexes including 40S ribosome subunits, initiation factors, mRNA, and a collection of RNA binding proteins, one of which is TIA-1, accumulate in foci (3). As TIA-1 is a widely used marker of SG formation, VV-infected cells were stained with antisera specific for TIA-1. While TIA-1 was concentrated within nuclei of uninfected cells and exhibited nucleolar sparing, diffuse TIA-1 staining was also detected in the cytosol. Arsenite treatment reproducibly induced the distinctive accumulation of TIA-1 into dense SGs in virtually every cell in multiple visual fields. In contrast, while TIA-1 nuclear staining was abrogated by VV infection, dense granules were not observed with any infected cells in the population. Instead, TIA-1 remained diffusely distributed in the cytosol and was not observed either to accumulate into aggregates in the cytosol proper or to concentrate within viral DNA replication compartments (Fig. 6E). Thus, while nuclear TIA-1 staining was eliminated, canonical stress granule formation could conceivably be impaired in VV-infected cells, as has been reported for some other viruses (13). This could involve characterized VV functions that prevent the accumulation of phosphorylated eIF2α or perhaps the usurpation of another cellular SG constituent, the rasGAP-associated endoribonuclease G3BP (42), as a transcription factor for viral intermediate-class genes (20). Taken together, the P body marker Dcp1 and the SG marker TIA-1 were not observed to concentrate and accumulate within cytoplasmic DAPI-staining bodies in VV-infected cells, suggesting that the eIF4E-eIF4G-containing foci observed with VV replication compartments are unlikely to be canonical P bodies or SGs. We cannot at this time, however, rule out the possibility that the eIF4E-eIF4G foci are noncanonical P bodies or SGs that lack the well-characterized Dcp1 or TIA-1 subunits thought to contribute to their function.
DISCUSSION
To produce the polypeptide products required to assemble the next generation of virus progeny and complete their replicative cycle, poxviruses, like all viruses, must successfully control the host translational machinery (27). For mRNAs containing a 7-methyl GTP cap on their 5′ end, this process typically requires the multisubunit, cap-binding eIF4F (34). As a first step toward understanding how poxviruses manipulate the host translation machinery, we have established how eIF4F core and associated components respond following infection of normal primary human cells with VV, a model poxvirus. Surprisingly, while host mRNA translation is radically compromised, VV infection inactivates the translational repressor 4E-BP1, promotes assembly of the cap-binding protein eIF4E and PABP into the eIF4F initiation complex, and stimulates the Mnk1-dependent phosphorylation of eIF4E. Not only is VV replication compromised in the absence of the cellular eIF4G-associated kinase Mnk1, but a small-molecule inhibitor of Mnk1 effectively prevents eIF4E phosphorylation, inhibits VV polypeptide accumulation, and suppresses VV replication. Finally, eIF4E, eIF4G, and PABP undergo a remarkable redistribution in infected cells and are concentrated in and around cytosolic VV replication compartments. Thus, VV functions are capable of controlling the assembly, modification, and subcellular distribution of eIF4F-core and associated components.
The numerous profound changes to cellular translation initiation factors and key translational control pathways in infected cells likely result from the actions of discrete VV-encoded or -induced functions. While the identity of these factors and the precise mechanism(s) with which they interface with the host machinery remain to be explained, they are likely to act either by direct binding to cellular translation initiation factors or by triggering critical cellular signaling pathways. For example, functions related to the M-T5 ankyrin repeat containing host range protein encoded by myxoma virus, a rabbit-specific poxvirus that activates Akt, could contribute to mTOR activation (49). Other DNA viruses have successfully utilized a similar strategy of destroying the 4E-BP1 repressor following its phosphorylation by mTOR in infected cells, although the mechanistic details that result in 4E-BP1 destruction remain obscure (46). It is likely, however, to involve the conjugation of ubiquitin to 4E-BP1 (12; D. Walsh and I. Mohr, unpublished observations). Finally, viral functions also regulate eIF4E phosphorylation by producing eIF4G-binding proteins, some of which enhance eIF4F assembly and stimulate eIF4E phosphorylation (47), whereas others displace Mnk1 and effectively reduce the abundance of phospo-eIF4E (10).
Similarly, the dramatic redistribution of translation initiation factors and concentration within and around viral replication structures require viral gene expression. Indeed, eIF4F assembly, eIF4E phosphorylation, and translation factor redistribution all occur with similar late kinetics and are dependent upon viral DNA synthesis. Thus, the act of viral DNA synthesis or, more likely, the expression of one or more viral late-gene products (RNA or protein) plays essential roles in recruiting translation factors to viral factories. Incorporating eIF4E and eIF4G within these subcellular compartments could effectively elevate the local concentration of key initiation factors and thereby promote eIF4F subunit association or assembly without altering overall initiation factor abundance (48) or using a virus-specified chaperone (47). It further raises the possibility that VV factories, besides serving as focal sites of DNA synthesis and virus assembly, may also function as sites of local late-mRNA translation. This would in effect facilitate the accumulation of viral proteins within a subcellular compartment devoted to viral assembly, obviating the need for a specific import mechanism to transport the virion polypeptide components into the factory lumen. The means by which VV redistributes the translation initiation factors may have relevance to other biological systems where localized translation has been observed, such as in neuronal dendritic spines and during development (21, 41).
In addition, the net effect of incorporating eIF4E, eIF4G, and PABP within virus-specific subcellular domains could sequester these important initiation factors into compartments inaccessible to host mRNAs and thereby contribute to the selective translation of VV mRNAs observed with infected cells. Sequestration of eIF4G to perinuclear hsp27-containing structures has been observed with heat-shocked cells and correlates with the inhibition of the capped mRNA translation observed under these conditions (9). Likewise, SGs and P bodies represent discrete cytoplasmic subcellular aggregates associated with translational repression that contain translation initiation factors (3, 30). However, canonical markers of SGs (TIA-1) and P bodies (Dcp1) were not observed to concentrate in cytoplasmic viral replication compartments or colocalize with eIF4E and eIF4G in VV-infected cells. Instead, sequestering translation factors and their bound mRNA ligands within viral replication structures could also protect viral mRNAs from premature degradation and significantly extend their half-life. In particular, initiation factor-bound mRNAs within viral replication compartments might be less susceptible to the action of the VV-decapping enzyme that is unable to distinguish between host and VV mRNAs in vitro (33). At the same time, concentrating translation factors within viral structures would in effect synergize with the VV mRNA decapping enzyme to impair host mRNA translation by limiting their access to host mRNAs.
Finally, it is worth mentioning that the virus-induced modifications to eIF4F observed with poxvirus-infected cells could provide new targets for antiviral drug discovery. This assumes even greater importance for biodefense and global health considering the risks associated with smallpox vaccination and the paucity of drugs active against variola virus, the etiological agent responsible for smallpox (1, 17). Along these lines, the inhibition of Mnk1, a nonessential regulatory component associated with the translational machinery, substantially reduces poxvirus protein production and replication without adversely affecting mRNA translation or viability of uninfected cells. Similarly, while cellular translation initiation factors and ancillary proteins are required for the translation of host and viral mRNAs, the redistribution of these factors to viral subcellular structures is uniquely observed with VV-infected cells. Preventing the concentration of eIF4E, eIF4G, and/or PABP within and around replication compartments could interfere with viral mRNA translation and arrest the virus life cycle. The test of this hypothesis awaits the identification of viral gene products required to promote eIF4F assembly, modification, and subcellular redistribution in infected cells.
ADDENDUM
While the present work was in revision, Katsafanas and Moss reported that eIF4E, eIF4G, and G3BP were each individually localized to VV replication compartments in an established transformed cell line (20a).
Acknowledgments
We thank Stewart Shuman (Memorial-Sloan Kettering Cancer Center) for providing the Western Reserve strain of vaccinia, Simon Morley (University of Sussex, United Kingdom) along with Jens Lykke-Andersen (University of Colorado, Boulder, CO) and Bob Schneider (NYU School of Medicine) for antisera, H. Gram (Novartis) for CGP57380, our colleagues in the Mohr laboratory for many insightful discussions, and Diego Acosta-Alvear for critically reading the manuscript.
This work was supported by grants from the NIH to D.W. (2 P30 AI027742; while D.W. was in the United States) and to I.M. (GM056927). I.M. is a scholar of the Irma T. Hirshl Trust. Purchase of the confocal microscope was funded by a shared instrumentation grant from the NIH (S10 RR017970). Work in Ireland was supported by grants from the Science Foundation Ireland (06 IN.1 B80) and the Health Research Board (RP/2007/52) to D.W.
Footnotes
Published ahead of print on 4 February 2008.
REFERENCES
- 1.Alberts, B., and H. V. Fineberg. 2004. Harnessing new science is vital for biodefense and global health. Proc. Natl. Acad. Sci. USA 10111177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, B. Y., and B. Moss. 1989. Capped poly(A) leaders of variable lengths at the 5′ ends of vaccinia virus late mRNAs. J. Virol. 63226-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, P., and N. Kedersha. 2006. RNA granules. J. Cell Biol. 172803-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade, A. A., P. N. G. Silva, A. C. T. C. Pereira, L. P. De Sousa, P. C. P. Ferreira, R. T. Gazzinelli, E. G. Kroon, C. Ropert, and C. A. Bonjardim. 2004. The vaccinia virus-stimulated mitogen-activated protein kinase (MAPK) pathway is required for virus multiplication. Biochem. J. 381437-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barco, A., E. Feduchi, and L. Carrasco. 2000. A stable HeLa cell line that inducibly expresses poliovirus 2A(pro): effects on cellular and viral gene expression. J. Virol. 742383-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boone, R. F., and B. Moss. 1977. Methylated 5′-terminal sequences of vaccinia virus mRNA species made in vivo at early and late times after infection. Virology 7967-80. [DOI] [PubMed] [Google Scholar]
- 7.Charity, J. C., E. Katz, and B. Moss. 2007. Amino acid substitutions at multiple sites within the vaccinia virus D13 scaffold protein confer resistance to rifampicin. Virology 359227-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condit, R. C., N. Moussatche, and P. Traktman. 2006. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 6631-124. [DOI] [PubMed] [Google Scholar]
- 9.Cuesta, R., G. Laroia, and R. J. Schneider. 2000. Chaperone hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes. Genes Dev. 141460-1470. [PMC free article] [PubMed] [Google Scholar]
- 10.Cuesta, R., Q. Xi, and R. J. Schneider. 2001. Adenovirus-specific translation by displacement of kinase Mnk1 from cap-initiation complex eIF4F. EMBO J. 193465-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Magalhaes, J. C., A. A. Andrade, P. N. G. Silva, L. P. De Sousa, C. Ropert, P. C. P. Ferreira, E. G. Kroon, R. T. Gazzinelli, and C. A. Bonjardim. 2001. A mitogenic signal triggered at an early stage of vaccinia virus infection. J. Biol. Chem. 27638353-38360. [DOI] [PubMed] [Google Scholar]
- 12.Elia, A., C. Constantinou, and M. J. Clemens. 2007. Effects of protein phosphorylation on ubiquitination and stability of the translational repressor protein 4E-BP1. Oncogene 27811-822. [DOI] [PubMed] [Google Scholar]
- 13.Emara, M. M., and M. A. Brinton. 2007. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc. Natl. Acad. Sci. USA 1049041-9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukunaga, R., and T. Hunter. 1997. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 161921-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gierman, T. M., R. M. Frederickson, N. Sonenberg, and D. J. Pickup. 1992. The eukaryotic translation initiation factor 4E is not modified during the course of vaccinia virus replication. Virology 188934-937. [DOI] [PubMed] [Google Scholar]
- 16.Haghighat, A., S. Mader, A. Pause, and N. Sonenberg. 1995. Repression of cap-dependent translation by 4E-binding protein 1: competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 145701-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison, S. C., B. Alberts, E. Ehrenfeld, L. Enquist, H. Fineberg, S. L. McKnight, B. Moss, M. O'Donnell, H. Ploegh, S. L. Schmid, K. P. Walter, and J. Theriot. 2004. Discovery of antivirals against smallpox. Proc. Natl. Acad. Sci. USA 10111178-11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ink, B. S., and D. J. Pickup. 1990. Vaccinia virus directs the synthesis of early mRNAs containing 5′ poly(A) sequences. Proc. Natl. Acad. Sci. USA 871536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahvejian, A., G. Roy, and N. Sonenberg. 2001. The mRNA closed-loop model: the function of PABP and PABP-interacting proteins in mRNA translation. Cold Spring Harbor Symp. Quant. Biol. 66293-300. [DOI] [PubMed] [Google Scholar]
- 20.Katsafanas, G. C., and B. Moss. 2004. Vaccinia virus intermediate stage transcription is complemented by Ras-GTPase-activating protein SH3 domain-binding protein (G3BP) and cytoplasmic activation/proliferation-associated protein (p137) individually or as a heterodimer. J. Biol. Chem. 27952210-52217. [DOI] [PubMed] [Google Scholar]
- 20a.Katsafanas, G. C., and B. Moss. 2007. Colocalization of transcription and translation within cytoplasmic poxvirus factories coordinates viral expression and subjugates host functions. Cell Host Microbe 2221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klann, E., and J. D. Richter. 2007. Translational control of synaptic plasticity and learning and memory, p. 485-506. In M. B. Mathews, N. Sonenberg, and J. W. B. Hershey (ed.), Translational control in biology and medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 22.Knauf, U., C. Tschopp, and H. Gram. 2001. Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol. Cell. Biol. 215500-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mader, S., H. Lee, A. Pause, and N. Sonenberg. 1995. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4G and the translational repressors 4E-binding proteins. Mol. Cell. Biol. 154990-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maloney, G., M. Schroder, and A. G. Bowie. 2005. Vaccinia virus protein A52R activates p38 mitogen-activated protein kinase and potentiates liposaccharide-induced interleukin-10. J. Biol. Chem. 28030838-30844. [DOI] [PubMed] [Google Scholar]
- 25.Marcotrigiano, J., A. C. Gingras, N. Sonenberg, and S. K. Burley. 1999. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol. Cell 3707-716. [DOI] [PubMed] [Google Scholar]
- 26.Martin, S. A., and B. Moss. 1975. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J. Biol. Chem. 2509330-9335. [PubMed] [Google Scholar]
- 27.Mohr, I. J., T. Pe'ery, and M. B. Mathews. 2007. Protein synthesis and translational control during viral infection, p. 545-595. In M. B. Mathews, N. Sonenberg, and J. W. B. Hershey (ed.), Translational control in biology and medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 28.Moss, B. 2007. Poxviridae: the viruses and their replication, p. 2849-2883. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA.
- 29.Mulder, J., M. E. Robertson, R. A. Seamons, and G. J. Belsham. 1998. Vaccinia virus protein synthesis has a low requirement for the intact translation initiation factor eIF4F, the cap-binding complex, within infected cells. J. Virol. 728813-8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker, R., and U. Sheth. 2007. P bodies and the control of mRNA translation and degradation. Mol. Cell 25635-646. [DOI] [PubMed] [Google Scholar]
- 31.Parrish, S., and B. Moss. 2006. Characterization of a vaccinia virus mutant with a deletion of the D10R gene encoding a putative negative regulator of gene expression. J. Virol. 80553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parrish, S., and B. Moss. 2007. Characterization of a second vaccinia virus mRNA-decapping enzyme conserved in poxviruses. J. Virol. 8112973-12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parrish, S., W. Resch, and B. Moss. 2007. Vaccinia virus D10 protein has mRNA decapping activity, providing a mechanism for control of host and viral gene expression. Proc. Natl. Acad. Sci. USA 1042139-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pestova, T. V., J. R. Lorsch, and C. U. T. Hellen. 2007. The mechanism of translation initiation in eukaryotes, p. 87-128. In M. B. Mathews, N. Sonenberg, and J. W. B. Hershey (ed.), Translational control in biology and medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 35.Pyronnet, S., H. Imataka, A.-C. Gingras, R. Fukunaga, T. Hunter, and N. Sonenberg. 1999. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 18270-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raught, B., and A.-C. Gingras. 2007. Signaling to translation initiation, p. 369-400. In M. B. Mathews, N. Sonenberg, and J. W. B. Hershey (ed.), Translational control in biology and medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 37.Schneider, R. J., and N. Sonenberg. 2007. Translational control in cancer development and progression, p. 401-431. In M. B. Mathews, N. Sonenberg, and J. W. B. Hershey (ed.), Translational control in biology and medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 38.Schnierle, B. S., and B. Moss. 1992. Vaccinia virus-mediated inhibition of host protein synthesis involves neither degradation nor underphosphorylation of components of the cap-binding eukaryotic translation initiation factor complex eIF-4F. Virology 188931-933. [DOI] [PubMed] [Google Scholar]
- 39.Schwer, B., P. Visca, J. C. Vos, and H. G. Stunnenberg. 1987. Discontinuous transcription or RNA processing of vaccinia virus late messengers results in a 5′ poly(A) leader. Cell 50163-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sodeik, B., G. Griffiths, M. Ericsson, B. Moss, and R. W. Doms. 1994. Assembly of vaccinia virus: effects of rifampin on the intracellular distribution of viral protein p65. J. Virol. 681103-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson, B., M. Wickens, and J. Kimble. 2007. Translational control in development, p. 507-544. In M. B. Mathews, N. Sonenberg, and J. W. B. Hershey (ed.), Translational control in biology and medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 42.Tourriere, H., K. Chebli, L. Zekri, B. Courselaud, J. M. Blanchard, E. Bertrand, and J. Tazi. 2003. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 160823-831. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Tuazon, P. T., S. J. Morley, T. E. Dever, W. C. Merrick, R. E. Rhoads, and J. A. Traugh. 1990. Association of initiation factor eIF-4E in a cap binding protein complex (eIF-4F) is critical for and enhances phosphorylation by protein kinase C. J. Biol. Chem. 26510617-10621. [PubMed] [Google Scholar]
- 44.Ueda, T., R. Watanabe-Fukunaga, H. Fukuyama, S. Nagata, and R. Fukunaga. 2004. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol. Cell. Biol. 246539-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkatesan, S., A. Gershowitz, and B. Moss. 1980. Modification of the 5′ end of mRNA: association of RNA triphosphatase with the RNA guanylyltransferase-RNA (guanine-7) methyltransferase complex from vaccinia virus. J. Biol. Chem. 255903-908. [PubMed] [Google Scholar]
- 46.Walsh, D., and I. Mohr. 2004. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 18660-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh, D., and I. Mohr. 2006. Assembly of an active translation initiation factor complex by a viral protein. Genes Dev. 20461-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walsh, D., C. Perez, J. Notary, and I. Mohr. 2005. Regulation of the translation initiation factor eIF4F by multiple mechanisms in human cytomegalovirus-infected cells. J. Virol. 798057-8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, G., J. W. Barrett, M. Stanford, S. J. Werden, J. B. Johnston, X. Gao, M. Sun, J. Q. Cheng, and G. McFadden. 2006. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc. Natl. Acad. Sci. USA 1034640-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waskiewicz, A. J., J. C. Johnson, B. Penn, M. Mahalingam, S. R. Kimball, and J. A. Cooper. 1999. Phosphorylation of the cap-binding protein eukaryotic translation factor 4E by protein kinase Mnk1 in vivo. Mol. Cell. Biol. 191871-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wells, S. E., P. E. Hillner, R. D. Vale, and A. B. Sachs. 1998. Circularization of mRNA by eukaryotic translation initiation factors. Mol. Cell 2135-140. [DOI] [PubMed] [Google Scholar]
- 52.Willet, M., S. A. Flint, S. J. Morley, and V. M. Pain. 2006. Compartmentalisation and localization of the translation initiation factor (eIF) 4F complex in normally growing fibroblasts. Exp. Cell Res. 3122942-2953. [DOI] [PubMed] [Google Scholar]