Abstract
Protein phosphatase 1 (PP1), a major protein phosphatase important for a variety of cellular responses, is activated in response to ionizing irradiation (IR)-induced DNA damage. Here, we report that IR induces the rapid dissociation of PP1 from its regulatory subunit inhibitor-2 (I-2) and that the process requires ataxia-telangiectasia mutated (ATM), a protein kinase central to DNA damage responses. In response to IR, ATM phosphorylates I-2 on serine 43, leading to the dissociation of the PP1-I-2 complex and the activation of PP1. Furthermore, ATM-mediated I-2 phosphorylation results in the inhibition of the Aurora-B kinase, the down-regulation of histone H3 serine 10 phosphorylation, and the activation of the G2/M checkpoint. Collectively, the results of these studies demonstrate a novel pathway that links ATM, PP1, and I-2 in the cellular response to DNA damage.
Optimal cellular responses to DNA damage are mediated by protein kinases and phosphatases in order to promote survival and limit genetic instability. The ataxia-telangiectasia mutated kinase, ATM, plays a crucial role in the cellular response to DNA damage. The loss of ATM functions in humans results in progressive neurodegeneration, immunodeficiency, glucose intolerance, sterility, predisposition to lymphoblastoid malignancies, and radiation sensitivity (25). Cellular phenotypes of ATM deficiency include suboptimal activation of radiation-induced cell cycle checkpoints, increased spontaneous and DNA damage-induced chromosomal breakage and gaps, and hypersensitivity to radiation, etc. Human ATM is a 3,056-amino-acid polypeptide which shares several features with other members of the phosphatidylinositol 3-kinase-like kinase family, including a FAT (FRAP, ATR, and TRRAP) domain, a phosphatidylinositol 3-kinase domain, and a FAT carboxyl-terminal domain (14). ATM, like other members of this protein kinase family, phosphorylates its substrates on serines or threonines that are followed by glutamine (the SQ or TQ motif) (12, 22). A number of downstream targets have been identified. These substrates include many tumor suppressors, such as p53, Brca1, and Chk2, and the functional significance of ATM phosphorylation has been studied previously. For example, in response to ionizing radiation (IR), ATM phosphorylates BRCA1 (at Ser 1387) (32), CHK2 (at Thr 68) (7), FANCD2 (at Ser 222) (27), NBS1 (at Ser 278 and Ser 343), and SMC1 (at Ser 957 and Ser 966) (13, 33) to facilitate the S-phase checkpoint. Large-scale proteomic analyses of proteins phosphorylated on the ATM and ATR consensus sites in response to DNA damage have identified more than 900 regulated phosphorylation sites encompassing over 700 proteins. Functional analyses of a subset of this data set have indicated that this list is highly enriched with proteins involved in the DNA damage response (17).
Protein serine/threonine phosphatases, which in humans include protein phosphatase 1 (PP1), PP2A, PP2B, PP4, PP5, PP6, and PP7, function by reversing the phosphorylation of key structural and regulatory proteins (3, 4). In this family, PP2A and PP5 have been reported previously to regulate ATM serine 1981 autophosphorylation after DNA damage (2, 8, 34). It has also been reported previously that PP1 is activated in an ATM-dependent manner in response to DNA damage (9). However, how ATM activates PP1, as well as the physiological function of ATM-mediated PP1 activation in the DNA damage response, remains unknown.
PP1 interacts with its regulatory subunits in controlling the specificity and diversity of the phosphatase function (3). Inhibitor-2 (I-2), a 23-kDa phosphoprotein, is a well-documented PP1 regulatory subunit. I-2 was originally isolated as a heat-stable protein from skeletal muscle extracts that could specifically inhibit PP1 activity (11). PP1 forms a stable and inactive complex with unphosphorylated I-2, and the activation of the complex is accompanied by the phosphorylation of I-2 (21). One established model is that glycogen synthase kinase 3 (GSK-3)-mediated threonine 72 phosphorylation of I-2 promotes a conformational change in the PP1-I-2 complex (1, 16). I-2 can be phosphorylated on other serine sites by casein kinase I (CKI) and CKII, cdc2, and mitogen-activated protein kinases (15, 29). Though phosphorylation by CKII does not alter I-2 activity, it greatly facilitates the subsequent phosphorylation by GSK-3 (3). Previous deletion and mutagenesis studies have demonstrated that the N-terminal domain of I-2 interacts with PP1 (23) and can be dephosphorylated by PP1. Despite these findings, it is not known whether the PP1-I-2 complex is involved in DNA damage responses.
To study the mechanism of ATM-mediated PP1 activation, we investigated the IR-induced dissociation of the PP1-I-2 complex. We report here that the activation of PP1 is governed by ATM phosphorylation of I-2 at serine 43 in response to DNA damage and that ATM-mediated PP1 activation leads to the activation of the G2/M checkpoint through the inhibition of the Aurora-B kinase.
MATERIALS AND METHODS
Cell culture.
Human cells lines 293T and HeLa (from the American Type Culture Collection, Manassas, VA), simian virus 40 (SV40)-transformed human fibroblast cell lines GM0637 and GM9607 (from the NIGMS Human Mutant Cell Repository, Camden, NJ), and SV40-transformed fibroblast cell lines PEB-vector and PEB-YZ5 (32) were grown as monolayers in Dulbecco's modified Eagle medium with high glucose levels. The cell culture medium was supplemented with 10% fetal bovine serum and 1% penicillin and 1% streptomycin. Epstein-Barr virus (EBV)-immortalized lymphoblastoid cell lines from healthy persons (GM0536; NIGMS) and from persons who were homozygous for the ATM mutation (GM1526) were cultured in RPMI 1640 supplemented with 15% fetal bovine serum. All cells were maintained in a humid incubator at 5% CO2 and 37°C.
Irradiation.
For irradiation treatments, an X-RAD 320 irradiation cabinet (Precision X-Ray, East Haven, CT) was utilized at 320 kV and 160 mA, with a 0.8-mm Sn, 0.25-mm Cu, 1.5-mm Al (half-value layer at 3.7 mm Cu) filter at a target-source distance of 20 cm and a dose rate of 3.4 Gy/min. All irradiation treatments were conducted under normal atmospheric pressure and at room temperatures.
Antibodies.
Rabbit polyclonal antibodies against PP1 and phospho-histone H3 phosphorylated at serine 10 were purchased from Upstate Biotechnology (Temecula, CA). A rabbit polyclonal phospho-histone H1 antibody was obtained from Abcam (Cambridge, MA). A rabbit polyclonal antibody against I-2 was purchased from Calbiochem (San Diego, CA). Mouse anti-Flag antibodies M2 and M5 were obtained from Sigma-Aldrich (St. Louis, MO). The mouse anti-Xpress antibody was purchased from Invitrogen (Carlsbad, CA). The rabbit polyclonal antibody against phospho-Ser 43 of I-2 was generated through Alpha Diagnostic International (San Antonio, TX). Synthetic peptides representing the sequence surrounding serine 43 of I-2 and containing a phosphorylated serine linked with keyhole limpet hemocyanin at the site corresponding to serine 43 were generated. The immunogens were then injected into rabbits, and a polyclonal antibody was generated and purified.
Plasmids.
Vectors that expressed glutathione S-transferase (GST)-conjugated I-2 and PP1 peptides were made by cloning complementary oligonucleotides that encoded the desired peptides (14 amino acids) into the BamHI-SmaI sites of pGEX-2T (Amersham Pharmacia Biotech, Piscataway, NJ). The QuikChange site-directed mutagenesis kit (Stratagene, Cedar, TX) was used to generate the serine-to-alanine mutant peptide. To construct Xpress-tagged I-2 expression vectors, we amplified the entire I-2 coding region by PCR with the following primers: 5′-CTGCGAGTCTCTGCTGTGCC-3′ and 5′-TGTGAAGAACAAGAAGCAACGTAC-3′. The PCR products were cloned into an Xpress-tagged pCDNA6 vector (Invitrogen, Carlsbad, CA) with the EcoRV restriction site. We then utilized the QuikChange site-directed mutagenesis kit to generate the serine-to-alanine mutant form. The oligonucleotides used for mutation were as follows: 5′-GAGCAAAAAAGCCCAGAAGTGG-3′ and 5′-CCACTTCTGGGCTTTTTTGCTC-3′.
Nuclear and cytoplasmic fractionation.
Nuclear and cytoplasmic fractionation was carried out with a nuclear extraction kit (Chemicon, Temecula, CA), which was modified according to our experiments. Cells were collected with trypsinization and rinsed with ice-cold 1× phosphate-buffered saline (PBS) or 1× Tris-buffered saline. Then the sample was centrifuged at 250 × g for 5 min at 4°C. Cell pellets were resuspended with 10 cell pellet volumes of ice-cold 1× cytoplasmic lysis buffer containing 0.5 mM dithiothreitol and diluted protease inhibitor. The cell suspension was then centrifuged, and cell pellets were kept for resuspension with 5 volumes of ice-cold 1× cytoplasmic lysis buffer. The resuspended cells were disrupted using a syringe with a small-gauge needle (27 gauge), and the disrupted cell suspension was centrifuged at 8,000 × g for 20 min at 4°C. The supernatant contained the cytosolic portion of the cell lysates, while the remaining pellet contained the nuclear portion. The nuclear pellet was resuspended in a volume of ice-cold nuclear extraction buffer corresponding to two original cell pellet volumes and containing 0.5 mM dithiothreitol and diluted protease inhibitor. The nuclei were disrupted using a fresh syringe with a 27-gauge needle, and the nuclear suspension was gently agitated with an orbital shaker at 4°C for 1 h. The nuclear suspension was then centrifuged at 8,000 × g for 5 min at 4°C. The supernatant contained the nuclear portion of the cell lysates.
Immunoprecipitation.
Cells were irradiated with 0 or 6 Gy, and the cell lysates were prepared as described in the previous section. The supernatants were incubated with anti-Flag M2, anti-Aurora-B, anti-PP1, or anti-Xpress antibodies. After extensive washing with the lysis buffer, immunoprecipitates were used for in vitro kinase assays or in vitro phosphatase assays or were analyzed by immunoblotting.
In vitro kinase assays.
In vitro kinase assays for Flag-tagged ATM and Aurora-B were performed as described previously (12, 18). The immunoprecipitates were suspended in 50 μl of kinase buffer containing 10 μCi of [λ-32P]ATP, 1 mM unlabeled ATP, and 1 μg of substrates (GST-conjugated peptides, recombinant I-2, or histone H3). The kinase reaction was conducted at 30°C for 30 min and stopped by the addition of sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer. The kinase assay products were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. The phosphorylation signal was analyzed by autoradiography and quantified by a phosphorimager.
In vitro phosphatase assays.
The in vitro phosphatase assays were performed using a Ser/Thr phosphatase assay kit (Upstate). Cytoplasmic, nuclear, or exogenous PP1 was immunoprecipitated, and the PP1 immune complex beads were incubated with a phosphopeptide (KRpTIRR, where p indicates the site of phosphorylation) at room temperature for 30 min. The beads were pelleted, and a 25-μl sample of the supernatant was analyzed for free phosphate in the malachite green assay by dilution with 100 μl of a developing solution (malachite green). After incubation for 15 min, the release of phosphate was quantified by measuring the absorbance at 650 nm in a microtiter plate reader.
Histone H1 and H3 phosphorylation staining.
The histone H1 and H3 phosphorylation assay results were assessed as described previously (27, 29). Cells were harvested 90 min after IR, washed with PBS, and fixed in a suspension with 2 ml of 70% ethanol. After fixation, cells were washed twice with PBS, suspended in 1 ml of 0.15% Triton X-100 in PBS, and incubated on ice for 5 min. After centrifugation, the cell pellet was suspended in 100 μl of PBS containing 1% bovine serum albumin (BSA) and 0.75 μg of a polyclonal antibody that specifically recognized the phosphorylated form of histone H3 or H1 (Upstate or Abcam, Cambridge, MA, respectively) and the suspension was incubated for 3 h at room temperature. Then the cells were rinsed with PBS containing 1% BSA and incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted at a ratio of 1:30 in PBS containing 1% BSA. After a 30-min incubation at room temperature in the dark, the cells were washed again, resuspended in a mixture of 25 μg of propidium iodide/ml and 0.1 mg of RNase A (Sigma)/ml in PBS, and incubated at room temperature for 30 min before the fluorescence was measured. Cellular fluorescence was measured by using a Becton Dickinson FACSCalibur flow cytometer-cell sorter.
RESULTS
IR induces ATM-dependent dissociation of the PP1-I-2 complex.
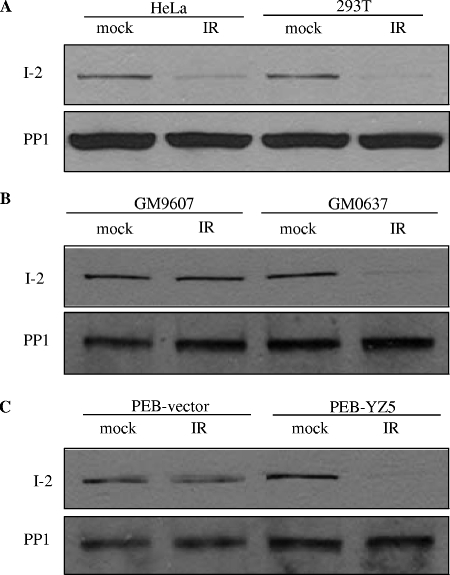
In this study, we first confirmed that PP1 was activated in response to IR-induced DNA damage in an ATM-dependent manner (see Fig. S1A, C, and D in the supplemental material). We also found that only the nuclear fraction of PP1 was activated after IR and that the cytoplasmic fraction did not display IR-induced activation (see Fig. S1B in the supplemental material). Since PP1 is negatively regulated by I-2 in a variety of cellular events, it is possible that PP1 activation involves I-2. PP1 and I-2 form a complex to regulate PP1 activity; therefore, we explored the possibility of an alteration of the PP1-I-2 complex in response to DNA damage. The immunoprecipitation of PP1 from unirradiated cells brought down I-2, but following IR, I-2 was no longer detectable in the PP1 immunoprecipitates (Fig. 1A). We then tested the ATM dependency of this dissociation. While GM0637 cells (with functional ATM) had a noticeable dissociation of PP1-I-2 after IR, no detectable IR-induced PP1-I-2 dissociation occurred in GM9607 (ATM-deficient) cells (Fig. 1B). This phenotype was also observed in isogenic cell lines with an ATM deficiency (PEB-vector cells) and with reconstituted ATM (PEB-YZ5 cells) (Fig. 1C), demonstrating that ATM is required for the IR-induced PP1-I-2 dissociation.
FIG. 1.
The IR-induced dissociation of PP1 and I-2 requires functional ATM. Two hours after IR, immunoprecipitation from nuclear extracts was performed using an anti-PP1 antibody and immunoblot analyses were conducted using antibodies against I-2 or PP1 from HeLa and 293T cells (A), fibroblasts proficient (GM0637) or deficient (GM9607) in ATM (B), and isogenic fibroblasts deficient (PEB-vector) or proficient (PEB-YZ5) in ATM (C).
ATM phosphorylates I-2 at serine 43 in vitro.
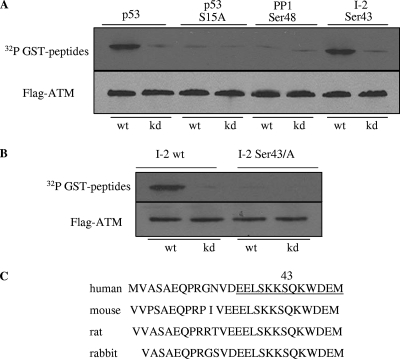
Since the phosphorylation of the inhibitory subunit I-2 can activate PP1 (3), we wondered whether the direct phosphorylation of I-2 by ATM might lead to the activation of the phosphatase after IR. It was also possible that ATM could phosphorylate PP1 to activate it directly. To test these possibilities, we examined the ability of ATM to phosphorylate these substrates in vitro. A sequence search found that there was only one putative ATM target site (SQ or TQ) in PP1 (serine 48 and the adjacent glutamine) and one in I-2 (serine 43 and the adjacent glutamine). Recombinant GST fusion peptides containing amino acid sequences with these SQ sites were used as substrates for ATM in an in vitro kinase assay. GST-p53 (amino acids 1 to 101) and GST-p53 (amino acids 1 to 101 of the S15A mutant form) served as positive and negative substrate controls, respectively. A peptide containing serine 43 of I-2 exhibited a strong phosphorylation signal, while the phosphorylation of the PP1 serine 48 peptide was not above the background level (Fig. 2A). These observations suggested that serine 48 of PP1 was not likely to be a direct target of ATM but that serine 43 of I-2 was promising as a target. Progressing on to full-length I-2 protein as a substrate, we found that ATM phosphorylated wild-type I-2 but not I-2 with a serine 43-to-alanine mutation (Fig. 2B). Thus, serine 43 of I-2 appears to be an ATM target in vitro. It is noted that serine 43 and its surrounding residues are highly conserved among mammalian species (Fig. 2C).
FIG. 2.
ATM phosphorylates I-2 at serine 43 in vitro. (A) Immunoprecipitated Flag-tagged wild-type (wt) or kinase-dead (kd) ATM was incubated with recombinant proteins consisting of fusions between GST and peptides derived from various regions of human PP1 or I-2. The positions of the amino acids corresponding to each peptide are indicated at the top. p53 peptides (amino acids 1 to 101 for either the wild-type or the serine 15-to-alanine mutant form) were used as controls. (B) The full-length wild-type or serine 43-to-alanine mutant form of I-2 was used as the substrate for wild-type or kinase-dead ATM for the in vitro kinase assay. (C) Sequence homology of I-2 around serine 43 in different species. Underlining in the sequence from human I-2 indicates a sequence highly conserved among mammalian species.
ATM phosphorylates I-2 at serine 43 in vivo after IR.
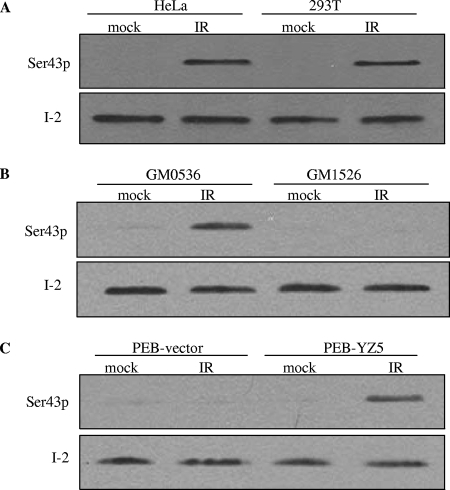
To better study the potential phosphorylation of I-2 in vivo, we generated an antibody that specifically recognizes I-2 serine 43 phosphorylation. The specificity of the phosphospecific antibody was demonstrated by its ability to recognize the phosphorylated, but not the unphosphorylated, peptide sequence (see Fig. S2 in the supplemental material). This antibody was used to probe for I-2 serine 43 phosphorylation in cells before and after IR. Several cell lines were tested, including HeLa and 293T cell lines, EBV-transformed lymphoblast cell lines with (GM 0536) and without (GM1526) ATM, and the isogenic cell lines PEB-vector (ATM null) and PEB-YZ5 (ATM complemented). Though total I-2 protein was easily detectable by Western blot analyses, with no notable changes before and after IR, no reactivity with the phosphoserine-specific antibody in unirradiated cells was seen. However, significant reactivity with the anti-phospho-serine 43 antibody in extracts from irradiated cells with functional ATM was observed (Fig. 3). No reactivity with the phosphoserine-specific antibody in ATM-deficient cells after IR was detected. Thus, ATM directly phosphorylates I-2 at serine 43 in vitro and there is an ATM-dependent phosphorylation of this site in vivo after IR.
FIG. 3.
ATM is required for IR-induced I-2 serine 43 phosphorylation. Shown are Western blot analyses of nuclear extracts from HeLa cells, 293T cells, EBV-transformed lymphoblast cell lines proficient (GM0536) and deficient (GM1526) in ATM, and SV40-transformed human fibroblast isogenic cell lines PEB-vector (ATM deficient) and PEB-YZ5 (expressing reconstituted ATM) with the phospho-serine 43 antibody (Ser43p).
ATM phosphorylation of I-2 at serine 43 is required for IR-induced PP1-I-2 dissociation and PP1 activation.
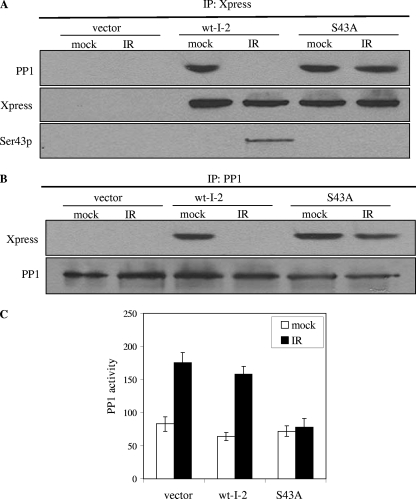
To assess the functional significance of the ATM phosphorylation of I-2, we explored the effects of ATM-dependent I-2 phosphorylation on the PP1-I-2 complex. 293T cells were transiently transfected with Xpress-tagged constructs expressing either a wild-type or a serine 43-to-alanine (S43A) mutant form of I-2, with or without IR. The exogenous I-2 was immunoprecipitated, and the immunoprecipitates were probed with the anti-Xpress or anti-PP1 antibodies. While wild-type I-2 brought down PP1 in the absence of IR, Xpress-tagged wild-type I-2 no longer bound to PP1 after DNA damage. In contrast, the S43A mutant form of I-2 remained bound to endogenous PP1 after IR (Fig. 4A). A reciprocal experiment showed that PP1 still associated with S43A mutant I-2 after IR (Fig. 4B). These observations demonstrate that ATM-mediated I-2 phosphorylation on serine 43 is required for the dissociation process of the PP1-I-2 complex in response to IR-induced DNA damage.
FIG. 4.
ATM phosphorylation of I-2 at serine 43 is required for the dissociation of the PP1-I-2 complex and the activation of PP1. 293T cells were transfected with an empty vector or the Xpress-tagged wild-type or S43A mutant form of I-2 and mock treated (0 Gy) or treated with IR (6 Gy). (A) The exogenous I-2 was immunoprecipitated with an anti-Xpress antibody, and the immunoprecipitates (IP) were probed with an anti-PP1 or anti-Xpress antibody. wt-I-2, wild-type I-2; Ser43p, phospho-serine 43 antibody. (B) Endogenous PP1 was immunoprecipitated with anti-PP1, and the immunoprecipitates were probed with anti-PP1 or anti-Xpress antibodies. (C) Endogenous PP1 was immunoprecipitated and subjected to in vitro phosphatase assays. Error bars represent ±1 standard deviation, and the means of results from three independent experiments are graphed.
We then investigated whether serine 43 phosphorylation of I-2 was required for IR-induced PP1 activation. We transfected 293T cells with wild-type or S43A mutant I-2 and assessed potential dominant-inhibitory effects on PP1 activity. 293T cells transiently transfected with either vector only or vectors expressing wild-type I-2 exhibited normal IR-induced PP1 activation. In contrast, cells transfected with vectors expressing the S43A form of I-2 exhibited substantially impaired PP1 activation after IR (Fig. 4C). Thus, S43A mutant I-2 has dominant-inhibitory effects on endogenous PP1 activation after IR, demonstrating that ATM-mediated I-2 phosphorylation is required for IR-induced PP1 activation.
To further study in vivo activation of PP1 toward its substrates, we assessed the dephosphorylation of histone H1, a process mediated by PP1 (24). A flow cytometry-based assay was employed to measure H1 phosphorylation in the absence or presence of DNA damage. We found that H1 phosphorylation was significantly reduced after IR. Expressing vector only or wild-type I-2 did not alter the IR-induced inhibition of H1 phosphorylation, while expressing S43A mutant I-2 abolished the process (see Fig. S3 in the supplemental material). Therefore, our data demonstrate that in vivo PP1 activity is enhanced by ATM-mediated phosphorylation of I-2 after DNA damage.
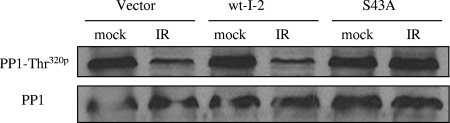
ATM phosphorylation of I-2 is critical for IR-induced PP1 threonine 320 dephosphorylation.
It was shown previously that nuclear PP1 contains the consensus sequence for phosphorylation by Cdk2 (9). PP1 threonine 320 phosphorylation inactivates PP1, and phosphorylation is attenuated after DNA damage in an ATM-dependent manner. Whether ATM-mediated I-2 phosphorylation interacts with the ATM-dependent inhibition of PP1 threonine 320 phosphorylation is not clear. To test this possibility, we performed experiments with 293T cells expressing vector only or wild-type or S43A mutant I-2 to investigate the change in threonine 320 phosphorylation after IR. We found that the S43A mutation can abolish IR-induced threonine 320 dephosphorylation (Fig. 5), suggesting that ATM-mediated I-2 serine 43 phosphorylation may function as an upstream cascade of the signaling pathway.
FIG. 5.
ATM phosphorylation of I-2 at serine 43 is required for the dephosphorylation of PP1 threonine 320 in response to DNA damage. 293T cells were transiently transfected with an empty vector, Xpress-tagged wild-type I-2 (wt-I-2), or S43A mutant I-2 and treated without or with IR (6 Gy). Nuclear extracts were subjected to immunoblotting using anti-phospho-threonine 320 (PP1-Thr320p) or anti-PP1 antibody.
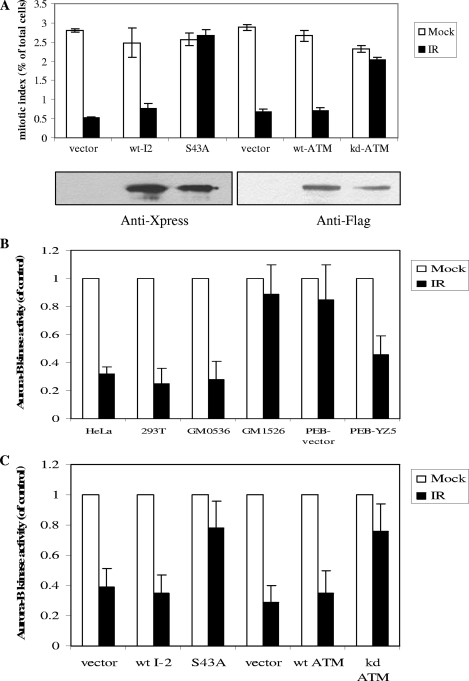
ATM phosphorylation of I-2 at serine 43 is required for the IR-induced G2/M checkpoint.
Previously, we reported that the ATM-dependent G2/M checkpoint is correlated with the down-regulation of histone H3 serine 10 phosphorylation (30, 31). However, how ATM links to H3 serine 10 phosphorylation was not known. Since PP1 is considered to be the major phosphatase required for the down-regulation of serine 10 phosphorylation (10) and the activation of PP1 is associated with the down-regulation of H3 serine 10 phosphorylation (see Fig. S4 in the supplemental material), it seemed likely that ATM-mediated I-2 serine 43 phosphorylation after IR would be important for the inhibition of H3 serine 10 phosphorylation in response to IR. To test this hypothesis, we transfected HeLa cells with the wild-type or the S43A mutant form of I-2 and performed a flow cytometry analysis using an anti-phospho-H3 serine 10 antibody. We found that cells expressing vector only or wild-type I-2 showed robust down-regulation of H3 phosphorylation and activation of the G2/M checkpoint after IR. However, cells expressing the dominant negative form of I-2 (the S43A mutant form) displayed no significant reduction in histone H3 phosphorylation in response to IR (Fig. 6A). These observations demonstrate a role for ATM-dependent I-2 serine 43 phosphorylation in the regulation of histone H3 serine 10 phosphorylation and the G2/M checkpoint in response to DNA damage.
FIG. 6.
ATM phosphorylation of I-2 is required for the activation of the G2/M checkpoint and the inhibition of Aurora-B in response to IR-induced DNA damage. (A) HeLa cells were transfected with an empty vector, Xpress-tagged wild-type I-2 (wt-I2), the S43A mutant form of I-2 (S43A), wild-type ATM (wt-ATM), or kinase-dead ATM (kd-ATM) and treated without IR or with IR (6 Gy). Ninety minutes after IR, cells were harvested and subjected to the flow cytometry-based phospho-histone H3 staining assay. Error bars represent ±1 standard deviation, and the means of results from three independent experiments are graphed. Shown under the bar graph are the Western blot results demonstrating the expression patterns of the exogenous proteins. (B) Cells were treated without or with IR (6 Gy), and Aurora-B was immunoprecipitated and subjected to in vitro kinase assays using histone H3 as the substrate. Phosphorylation signals were quantified by a phosphorimager. (C) HeLa cells were transfected with an empty vector, the Xpress-tagged wild-type or S43A mutant form of I-2, or wild-type or kinase-dead ATM and treated without IR or with IR (6 Gy). The Aurora-B kinase assays were performed 90 min after IR. In panels B and C, values for activity levels are shown relative to the activity level of the control, which was set at 1.
We also explored whether PP1 activation regulated the Aurora-B kinase, the kinase essential for H3 serine 10 phosphorylation. It was shown previously that Aurora-B was inhibited after IR-induced DNA damage (18). We found that IR-induced Aurora-B inhibition required functional ATM (Fig. 6B). Further, cells expressing the S43A mutant form of I-2 did not show the inhibition of Aurora-B compared to that in the appropriate controls (Fig. 6C). These observations demonstrate that ATM-mediated I-2 phosphorylation and PP1 activity can inhibit Aurora-B kinase activity, thereby causing reduced H3 phosphorylation and activation of the G2/M checkpoint.
DISCUSSION
Dissecting ATM-mediated signaling pathways in the cellular response to DNA damage can provide important insights into how the loss of ATM function causes such a devastating disease, ataxia-telangiectasia (A-T), in humans. Upon DNA damage, ATM binds strongly to damaged sites and its kinase activity is enhanced. Activated ATM in turn phosphorylates a list of substrates in pathways that together ensure cellular survival and recovery. A number of ATM-mediated signaling pathways have been revealed, and the functional significance of these pathways has been studied extensively. However, due to the complexity of the A-T phenotypes, detailed mechanisms on how the loss of ATM leads to a variety of A-T phenotypes remains to be further explored. In this report, we highlight a novel signaling pathway that involves ATM, PP1, and I-2. We demonstrate that I-2 is a substrate of ATM and that ATM phosphorylation of I-2 at serine 43 is required for the activation of PP1 in response to DNA damage.
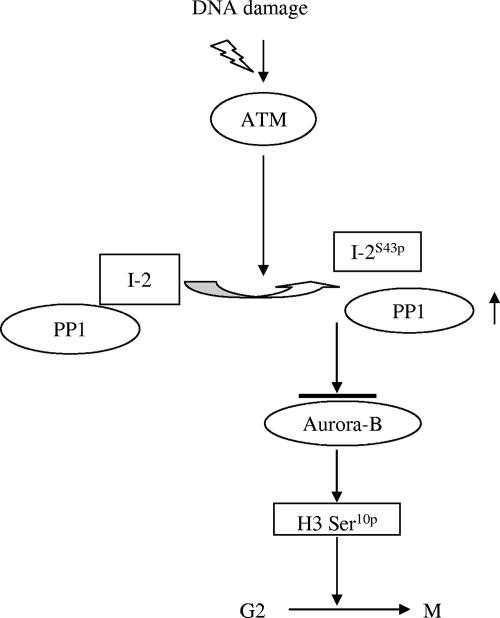
Previously, we reported a rapid and ATM-dependent G2/M checkpoint that correlates with the down-regulation of histone H3 serine 10 phosphorylation in response to IR (30). However, how ATM links to regulators of histone H3 serine 10 phosphorylation was not known. It was reported previously that the enzymatic activities of PP1 are activated in response to IR in an ATM-dependent manner (9) and that Cdk2-mediated PP1 threonine 320 phosphorylation is attenuated after DNA damage. However, a detailed mechanism of ATM-mediated PP1 activation in response to DNA damage remained unknown. Starting with investigations of IR-induced PP1 activity, we found that IR induced an ATM-dependent dissociation of the PP1-I-2 complex. Further studies showed that ATM phosphorylated I-2 on serine 43 and that this phosphorylation led to the dissociation of the complex and the activation of PP1. This effect, in turn, resulted in the inhibition of Aurora-B, the down-regulation of histone H3 serine 10 phosphorylation, and the activation of the G2/M checkpoint (Fig. 7).
FIG. 7.
Proposed role of ATM-mediated I-2 phosphorylation in the activation of PP1 and the signaling cascade in G2/M checkpoint regulation in response to IR-induced DNA damage. I-2S43p, I-2 phosphorylated at serine 43; H3 Ser10p, H3 serine 10 phosphorylation.
Our data also demonstrate that ATM-mediated I-2 phosphorylation is an essential step for the attenuation of IR-induced threonine 320 phosphorylation. One possible explanation is that, after the dissociation of the PP1-I-2 complex, PP1 initiates autodephosphorylation which eventually activates the phosphatase. More-detailed investigations are required to determine whether threonine 320 dephosphorylation may also play a role in ATM-mediated I-2 phosphorylation and PP1-I-2 dissociation.
PP1 activity is also controlled by other regulatory subunits, such as I-1, NIPP1, and DARPP32 (21). Whether these regulators are involved in the DNA damage response and whether they dissociate from PP1 are not known. It is reasonable to suspect that some inhibitors are also involved in regulating PP1 activity in response to DNA damage. For example, I-1 has been shown previously to regulate cell growth and has been linked to PP1 in the G1 cell cycle control (21).
Histone H3 serine 10 phosphorylation is critical for chromosome condensation and segregation, and it has been used previously as a mitotic marker for studying the activation of the G2/M checkpoint. Our data demonstrate that the activation of PP1 governed by ATM phosphorylation of I-2 leads to the down-regulation of H3 serine 10 phosphorylation. We also found that activated PP1 leads to the inhibition of the Aurora-B kinase. Therefore, PP1 may prevent H3 phosphorylation to delay the transition from G2 to M, thereby activating the G2/M checkpoint. However, it is also possible that activated PP1 may directly dephosphorylate the phosphorylated H3 when cells are already in the M phase. Therefore, PP1 and I-2 serine 43 phosphorylation may also have a role to facilitate the mitotic exit. The latter scenario is supported by the evidence that yeast PP1 homolog Dis2 can down-regulate Chk1 activity for a checkpoint release (6). Therefore, the detailed mechanisms of ATM-mediated PP1 activation in the regulation of histone H3 serine 10 phosphorylation remain to be further investigated.
The functional significance of ATM-mediated phosphorylation of I-2 and activation of PP1 activity may extend beyond the roles of these processes in histone H3 modification and cell cycle checkpoint regulation. Since PP1 is a major eukaryotic protein serine/threonine phosphatase that regulates a variety of cellular functions, the regulation of PP1 through ATM phosphorylation of I-2 may have a significant impact on many cellular responses to DNA damage. Dephosphorylation by phosphatases can turn signals off or regulate the degradation of phosphorylated substrates, thus balancing the physiological effects of kinases (19).
One of the known physiological roles of I-2 is to control sperm motility (28). A testis-specific isoform of PP1 forms an inactive complex with I-2, and GSK-3-mediated I-2 phosphorylation which activates the PP1-I-2 complex results in an increase in the PP1 activity seen in nonmotile immature sperm. The exposure of the immature sperm to phosphatase inhibitors, such as okadaic acid and calyculin A, induces motility, suggesting that I-2 inhibits PP1 activity in mature mammalian sperm cells to facilitate their motility. The PP1-I-2 complex is also involved in insulin signaling (5, 20). These observations are particularly interesting since both A-T patients and A-T mice are sterile and have glucose intolerance and insulin resistance (26), suggesting a physiologically important link between ATM and PP1-I-2. Indeed, we have observed that ATM phosphorylates I-2 at serine 43 in response to insulin stimulation (unpublished data). The establishment of a serine 43 phosphorylation mutant knock-in mouse model to study the physiological significance of ATM-mediated I-2 phosphorylation is under way.
In summary, our data provide mechanistic insights into the activation process of PP1 in DNA damage response pathways in mammalian cells. The results of these studies also provide a foundation for future studies of the ATM-PP1-I-2 pathway in regulating cellular responses to stress.
Supplementary Material
Acknowledgments
We thank Yosef Shiloh for providing the isogenic fibroblast cell lines deficient or proficient in ATM. We also thank Ratna K. Vadlamudi for providing reagents for constructing the Xpress-tagged I-2. We thank Jaideep Thottassery for helpful discussions.
This work was supported in part by grants from the National Institutes of Health (RR020152-01, CA71387, CA21765, and ES013301) and by the Department of Defense grant W81XWH-05-1-0018.
Footnotes
Published ahead of print on 4 February 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alessi, D. R., A. J. Street, P. Cohen, and P. T. Cohen. 1993. Inhibitor-2 functions like a chaperone to fold three expressed isoforms of mammalian protein phosphatase-1 into a conformation with the specificity and regulatory properties of the native enzyme. Eur. J. Biochem. 2131055-1066. [DOI] [PubMed] [Google Scholar]
- 2.Ali, A., J. Zhang, S. Bao, I. Liu, D. Otterness, N. M. Dean, R. T. Abraham, and X. F. Wang. 2004. Requirement of protein phosphatase 5 in DNA-damage-induced ATM activation. Genes Dev. 18249-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen, P. T. 2002. Protein phosphatase 1—targeted in many directions. J. Cell Sci. 115241-256. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, P. T., N. D. Brewis, V. Hughes, and D. J. Mann. 1990. Protein serine/threonine phosphatases; an expanding family. FEBS Lett. 268355-359. [DOI] [PubMed] [Google Scholar]
- 5.Delibegovic, M., C. G. Armstrong, L. Dobbie, P. W. Watt, A. J. Smith, and P. T. Cohen. 2003. Disruption of the striated muscle glycogen targeting subunit PPP1R3A of protein phosphatase 1 leads to increased weight gain, fat deposition, and development of insulin resistance. Diabetes 52596-604. [DOI] [PubMed] [Google Scholar]
- 6.Den Elzen, N. R., and M. J. O'Connell. 2004. Recovery from DNA damage checkpoint arrest by PP1-mediated inhibition of Chk1. EMBO J. 23908-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falck, J., N. Mailand, R. G. Syljuasen, J. Bartek, and J. Likas. 2001. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410842-847. [DOI] [PubMed] [Google Scholar]
- 8.Goodarzi, A. A., J. C. Jonnalagadda, P. Douglas, D. Young, R. Ye, G. B. Moorhead, S. P. Lees-Miller, and K. K. Khanna. 2004. Autophosphorylation of ataxia-telangiectasia mutated is regulated by protein phosphatase 2A. EMBO J. 234451-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo, C. Y., D. L. Brautigan, and J. M. Larner. 2002. Ionizing radiation activates nuclear protein phosphatase-1 by ATM-dependent dephosphorylation. J. Biol. Chem. 27741756-41761. [DOI] [PubMed] [Google Scholar]
- 10.Hsu, J. Y., Z. W. Sun, X. Li, M. Reuben, K. Tatchell, D. K. Bishop, J. M. Grushcow, C. J. Brame, J. A. Caldwell, D. F. Hunt, R. Lin, M. M. Smith, and C. D. Allis. 2000. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell 102279-291. [DOI] [PubMed] [Google Scholar]
- 11.Huang, F. L., and W. H. Glinsmann. 1976. Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. Eur. J. Biochem. 70419-426. [DOI] [PubMed] [Google Scholar]
- 12.Kim, S.-T., D.-S. Lim, C. E. Canman, and M. B. Kastan. 1999. Substrate specificities and identification of putative substrates of ATM kinase family members. J. Biol. Chem. 27437538-37543. [DOI] [PubMed] [Google Scholar]
- 13.Kim, S. T., B. Xu, and M. B. Kastan. 2002. Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage. Genes Dev. 16560-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurz, E. U., and S. P. Lees-Miller. 2004. DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair (Amsterdam) 3889-900. [DOI] [PubMed] [Google Scholar]
- 15.Leach, C., S. Shenolikar, and D. L. Brautigan. 2003. Phosphorylation of phosphatase inhibitor-2 at centrosomes during mitosis. J. Biol. Chem. 27826015-26020. [DOI] [PubMed] [Google Scholar]
- 16.MacKintosh, C., A. J. Garton, A. McDonnell, D. Barford, P. T. Cohen, N. K. Tonks, and P. Cohen. 1996. Further evidence that inhibitor-2 acts like a chaperone to fold PP1 into its native conformation. FEBS Lett. 397235-238. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka, S., B. A. Ballif, A. Smogorzewska, E. R. McDonald III, K. E. Hurov, J. Luo, C. E. Bakalarski, Z. Zhao, N. Solimini, Y. Lerenthal, Y. Shiloh, S. P. Gygi, and S. J. Elledge. 2007. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 3161160-1166. [DOI] [PubMed] [Google Scholar]
- 18.Monaco, L., U. Kolthur-Seetharam, R. Loury, J. M. Murcia, M. G. de, and P. Sassone-Corsi. 2005. Inhibition of Aurora-B kinase activity by poly(ADP-ribosyl)ation in response to DNA damage. Proc. Natl. Acad. Sci. USA 10214244-14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moorhead, G. B., L. Trinkle-Mulcahy, and A. Ulke-Lemee. 2007. Emerging roles of nuclear protein phosphatases. Nat. Rev. Mol. Cell Biol. 8234-244. [DOI] [PubMed] [Google Scholar]
- 20.Munro, S., D. J. Cuthbertson, J. Cunningham, M. Sales, and P. T. Cohen. 2002. Human skeletal muscle expresses a glycogen-targeting subunit of PP1 that is identical to the insulin-sensitive glycogen-targeting subunit G(L) of liver. Diabetes 51591-598. [DOI] [PubMed] [Google Scholar]
- 21.Oliver, C. J., and S. Shenolikar. 1998. Physiologic importance of protein phosphatase inhibitors. Front. Biosci. 3D961-D972. [DOI] [PubMed] [Google Scholar]
- 22.O'Neill, T., A. J. Dwyer, Y. Ziv, D. W. Chan, S. P. Lees-Miller, R. H. Abraham, J. H. Lai, D. Hill, Y. Shiloh, L. C. Cantley, and G. A. Rathbun. 2000. Utilization of oriented peptide libraries to identify substrate motifs selected by ATM. J. Biol. Chem. 27522719-22727. [DOI] [PubMed] [Google Scholar]
- 23.Park, I. K., and A. A. Paoli-Roach. 1994. Domains of phosphatase inhibitor-2 involved in the control of the ATP-Mg-dependent protein phosphatase. J. Biol. Chem. 26928919-28928. [PubMed] [Google Scholar]
- 24.Paulson, J. R., J. S. Patzlaff, and A. J. Vallis. 1996. Evidence that the endogenous histone H1 phosphatase in HeLa mitotic chromosomes is protein phosphatase 1, not protein phosphatase 2A. J. Cell Sci. 1091437-1447. [DOI] [PubMed] [Google Scholar]
- 25.Shiloh, Y., and M. B. Kastan. 2001. ATM: genome stability, neuronal development, and cancer cross paths. Adv. Cancer Res. 83209-254. [DOI] [PubMed] [Google Scholar]
- 26.Shiloh, Y. 2006. The ATM-mediated DNA-damage response: taking shape. Trends Biochem. Sci. 31402-410. [DOI] [PubMed] [Google Scholar]
- 27.Taniguchi, T., I. Garcia-Higuera, B. Xu, P. R. Andreassen, R. C. Gregory, S. T. Kim, W. S. Lane, M. B. Kastan, and A. D. D'Andrea. 2002. Convergence of the fanconi anemia and ataxia telangiectasia signaling pathways. Cell 109459-472. [DOI] [PubMed] [Google Scholar]
- 28.Vijayaraghavan, S., D. T. Stephens, K. Trautman, G. D. Smith, B. Khatra, E. F. da Cruz e Silva, and P. Greengard. 1996. Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase 1 activity. Biol. Reprod. 54709-718. [DOI] [PubMed] [Google Scholar]
- 29.Wang, H., and D. L. Brautigan. 2002. A novel transmembrane Ser/Thr kinase complexes with protein phosphatase-1 and inhibitor-2. J. Biol. Chem. 27749605-49612. [DOI] [PubMed] [Google Scholar]
- 30.Xu, B., S.-T. Kim, and M. B. Kastan. 2001. Involvement of Brca1 in S-phase and G2-phase checkpoints after ionizing irradiation. Mol. Cell. Biol. 213445-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu, B., S. T. Kim, D. S. Lim, and M. B. Kastan. 2002. Two molecularly distinct G(2)/M checkpoints are induced by ionizing irradiation. Mol. Cell. Biol. 221049-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu, B., A. H. O'Donnell, S. T. Kim, and M. B. Kastan. 2002. Phosphorylation of serine 1387 in Brca1 is specifically required for the Atm-mediated S-phase checkpoint after ionizing irradiation. Cancer Res. 624588-4591. [PubMed] [Google Scholar]
- 33.Yazdi, P. T., Y. Wang, S. Zhao, N. Patel, E. Y. Lee, and J. Qin. 2002. SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint. Genes Dev. 16571-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang, J., S. Bao, R. Furumai, K. S. Kucera, A. Ali, N. M. Dean, and X. F. Wang. 2005. Protein phosphatase 5 is required for ATR-mediated checkpoint activation. Mol. Cell. Biol. 259910-9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.