Abstract
Phosphoinositide 3-kinase (PI3K) is an early signaling molecule that regulates cell growth and cell cycle entry. PI3K is activated immediately after growth factor receptor stimulation (at the G0/G1 transition) and again in late G1. The two ubiquitous PI3K isoforms (p110α and p110β) are essential during embryonic development and are thought to control cell division. Nonetheless, it is presently unknown at which point each is activated during the cell cycle and whether or not they both control S-phase entry. We found that p110α was activated first in G0/G1, followed by a minor p110β activity peak. In late G1, p110α activation preceded that of p110β, which showed the maximum activity at this time. p110β activation required Ras activity, whereas p110α was first activated by tyrosine kinases and then further induced by active Ras. Interference with p110α and -β activity diminished the activation of downstream effectors with different kinetics, with a selective action of p110α in blocking early G1 events. We show that inhibition of either p110α or p110β reduced cell cycle entry. These results reveal that PI3Kα and -β present distinct activation requirements and kinetics in G1 phase, with a selective action of PI3Kα at the G0/G1 phase transition. Nevertheless, PI3Kα and -β both regulate S-phase entry.
The exposure of quiescent cells to growth factors (GF) activates a number of early signaling pathways that trigger cell cycle entry. Class I phosphoinositide 3-kinase (PI3K) represents one of the GF-stimulated pathways that regulate G0/G1 and G1/S transitions. There are four class I PI3K enzymes, composed of a regulatory subunit and a conserved p110 catalytic subunit that triggers phosphatidylinositol (3,4)-biphosphate and phosphatidylinositol (3,4,5)-triphosphate (PIP3) production. Class I PI3K enzymes are further classified as the GF receptor-controlled class IA enzymes and the G protein-coupled receptor-regulated p110γ (class IB PI3K) (12, 42). Three genes encode class IA catalytic subunits (p110α, p110β, and p110δ) (12, 14, 42). Class IA enzymes are activated by tyrosine kinases (TyrK) and Ras and regulate cell growth and DNA synthesis (5, 14, 17). Of the three class IA catalytic subunits, p110δ is expressed mainly in hematopoietic cells and regulates the immune response (30), whereas p110α and -β are ubiquitous and they might control cell division. Mice deficient in p110α or -β isoforms are embryonic lethal, suggesting that at least in development, these two isoforms have nonredundant functions (3, 4).
PI3K activity increases within minutes after GF receptor (GFR) stimulation (first peak) and again in advanced G1 phase (second peak) (18, 19, 24). PI3K has been implicated in the induction of cell growth and regulation of Cdk activity. Pharmacological inhibition of PI3K at the time of GFR stimulation blocks cell division (2). In addition, enhanced PIP3 production after GFR binding accelerates cell cycle entry, whereas PIP3 reduction diminishes this process (1). PI3K regulates cell mass increase by activating p70S6 kinase (p70S6K) and mTOR (9, 10, 23, 34, 35). The upregulation of PI3K activity also enhances Cdk2 activation (21). The mechanisms by which PI3K controls Cdk activity include the induction of cyclin D synthesis and inhibition of cyclin D degradation, an effect mediated by protein kinase B (PKB)-induced glycogen synthase kinase 3β inactivation (31, 33, 41). PI3K also regulates cell cycle entry through PKB-mediated FoxO transcription factor (TF) phosphorylation, which reduces FoxO TF-controlled cyclin G2 and p27INK expression (25, 27). Finally, the late G1 PI3K activity stabilizes c-Myc, an event required for correct cyclin A expression and Cdk2 activation (24).
Although it is well established that PI3K activation regulates progression through early and late G1 phase and cell cycle entry (18, 24), it is unclear which of the two ubiquitous catalytic subunits, p110α or -β, is activated and whether or not they both regulate cell cycle entry. Here we analyzed p110α and -β activation patterns during G1-phase progression, their activation requirements, and their potential contributions to G1-phase progression and cell cycle entry.
MATERIALS AND METHODS
Plasmids.
pSG5-myc-p110α and -p110αCAAX have been described previously (1). pCEF2-hp110βCAAX was a gift from C. Murga (Centro de Biología Molecular/CSIC, Madrid, Spain). The plasmid pAC-CMV encoding Myc-tagged full-length wild-type human p110α (hp110α) was donated by M. White (Howard Hughes Medical Institute, Chevy Chase, MD), and His-tagged wild-type hp110β by B. Vanhaesebroeck (Ludwig Institute for Cancer Research, London, United Kingdom). The mutants myc-K802R-hp110α and myc-K805R-hp110β were generated by using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) with appropriate oligonucleotides and were subcloned into pSG5 and pRV-IRES-GFP (for retroviral infection). Julian Downward donated the cDNAs encoding yellow fluorescent protein-N17-Ras and V12-Ras (London Research Institute, London, United Kingdom). Murine short hairpin RNAs (shRNA) were subcloned in pBluescript/U6 (39). Human shRNA were cloned in the pTER vector as described previously (40). The following target sequences were efficient in reducing target mRNA expression: human/murine p110α1 (h/mp110α1), 5′-GGCATCCACTTGATGCC; h/mp110α2, 5′-GGGAGAACCCAGACATCATGTCA; h/mp110β2, 5′-AAAGCTGGACTACTAAAGTGA; h/mp110β7, 5′-TTGCTCAGCTTCAGGCGCTGC; hp110α7, 5′-CTGTGGGGCATCCACTTGA; and h/mp110β15, 5′-CTGGAATTTGATATTAATAT. The different α shRNA and β shRNA gave similar results. For controls, we used shRNA that did not reduce p110α or -β expression. The following sequences were used for controls: 5′-GGAATGAACCACTGGAATTT (control β) and 5′-CCCAGACATCATGTCAGAG (control α).
Ab and reagents.
Transfections were performed by using Lipofectamine (Invitrogen, Carlsbad, CA). Blots were probed with the following antibodies (Ab): cyclin E (M-20), c-Myc (C-19), p110β (S-19), and p70S6K (C-18) (Santa Cruz Biotechnology, CA). Anti-cyclin D3, anti-phospho-PKB (anti-p-PKB) (Ser-473), anti-Myc (9B11), and anti-p-p70S6K (Thr-389) Ab were from Cell Signaling (Beverly, MA); anti-cyclin A, anti-retinoblastoma protein (anti-RB), and anti-six-His from BD Biosciences (San Jose, CA); and anti-Akt1/PKBα and anti-p-Thr32-FKHRL1 (p-FoxO3a) from Upstate Biotechnology (Millipore, Billerica, MA). Anti-β-actin was from Sigma (St. Louis, MO), anti-Ras was from Oncogene (Merck, Germany), and anti-p110α was donated by A. Klippel (Merck, Boston, MA). [γ-32P]ATP was from Amersham (United Kingdom); lovastatin and herbamycin were from Calbiochem.
Cell lines, cell culture, and retroviral transduction.
Murine embryonic fibroblasts (MEF) were prepared as reported previously (13). The cells were maintained in Dulbecco's modified Eagle's medium medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 10 mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin. Stable NIH 3T3 p110αCAAX (p110α*) lines were previously described (1). NIH 3T3 p110βCAAX (p110β*) cell lines were prepared by transfecting NIH 3T3 cells with 3 μg pCEF2-hp110βCAAX plus 1 μg p-Pur (Clontech, Mountain View, CA). We failed to obtain stable cell lines expressing K802R-p110α and K805R-p110β mutations; analyses using these mutants were performed by transient transfection or retroviral infection (cultured for 1 week). We expressed pTER-p110α7 or pTER-p110β15 in U2OS cells as described previously (40).
Cell cycle and immunofluorescence analysis.
Cells were synchronized in G0 by serum starvation as reported previously (25). Synchronous cell cycle entry was induced by the addition of serum. Cell cycle distribution was examined by DNA staining using propidium iodide and analyzed by flow cytometry (Beckman-Coulter, Fullerton, CA). U2OS cell cultures were synchronized at the G1/S boundary by double-thymidine block (11) or were synchronized in metaphase with colcemid (13). For retrovirus production, Phoenix cells were transfected by using JetPei-NaCl according to the manufacturer's protocols (Qbiogene, Irvine, CA). Retroviral infection and immunofluorescence analysis were performed as described previously (24).
WB, in vitro transcription and translation, immunoprecipitation, and PI3K assays.
Total cell extracts were prepared in radioimmunoprecipitation buffer (20 mM Tris-HCl, pH 8.0, 137 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 10% glycerol, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) containing protease and phosphatase inhibitors (1 mM Na3VO4, 5 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 nM okadaic acid). Western blotting (WB) and immunoprecipitation were performed as described previously (25). For PI3K assays, cells were transfected with empty vector (pSG5) or with a combination of pSG5-myc-tagged p110α and pSG5-His-tagged p110β and were then synchronized as described above. In some samples, 10 μM lovastatin or 0.3 μg/ml herbamycin was added 1 h before harvest. In vitro transcription and translation and subsequent PI3K activity analysis were performed as reported previously (17). PI3K was immunoprecipitated by using anti-Myc or anti-six-His Ab; the kinase assays were performed as described previously (24).
Quantitation of gel bands and statistical analyses.
Statistical analyses were performed by using StatView 512+ (Calabasas, CA). Gel bands and fluorescence intensities were quantitated with ImageJ software and were normalized according to the fluorescence intensity of the loading control band. Cell cycle profiles were analyzed with multicycle AV for Windows (Phoenix Flow Systems, CA).
RESULTS
p110α and -β contribute differently to downstream signaling.
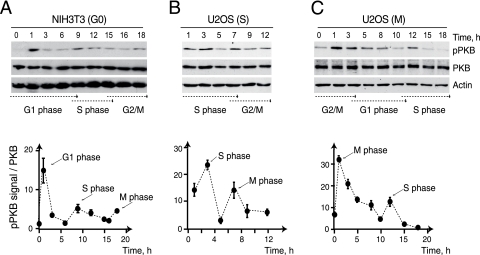
We investigated specific functions of p110α and -β PI3K catalytic subunits in G1 phase by comparing the consequences of interfering with their activation for the induction of different effectors. We examined several PI3K downstream targets, including PKB, FoxO3a, and p70S6K. To synchronize the cells, we arrested immortal nontransformed murine NIH 3T3 cells in G0 phase by serum deprivation and then released them by low-density replating in serum-containing medium for different time periods, as described previously (25). We confirmed that the PI3K effector PKB is activated at G0/G1, in late G1, and at M-phase entry (Fig. 1A), as reported previously (7, 38). We also synchronized human U2OS cells at the G1/S boundary or in metaphase (Fig. 1B and C), as these cells fail to arrest in G0 (7, 38). We confirmed PI3K activation at G1/S transition and M-phase entry in U2OS cells (Fig. 1B and C).
FIG. 1.
PI3K is activated at S-phase entry in different cell lines. (A) NIH 3T3 cells were arrested in G0; (B and C) U2OS cells were synchronized at the G1/S boundary (B) or in metaphase (C) and released for different times. Extracts were examined with WB by using the indicated Ab. Cell cycle distribution was examined in parallel; transits through G1, S, or G2/M are indicated (arrows). Graphs represent the means ± standard deviations of the p-PKB signals in arbitrary units, normalized in comparison to control PKB levels (n = 3).
We confirmed the specificities of the p110 Ab used for this study by transfection of wild-type p110α and p110β under the control of the simian virus 40 promoter (pSG5 vector) in COS cells, which gives rise to high levels of overexpression of recombinant proteins (Fig. 2A). Anti-p110α Ab selectively detected p110α despite the high expression levels of recombinant p110β; similarly, anti-p110β Ab only detected endogenous and recombinant p110β (Fig. 2A). To interfere with p110's cellular activity, we first used the active p110α* (1) and p110β* forms, as well as the kinase-inactive myc-K802R-p110α and myc-K805R-p110β mutants (see Materials and Methods). The interference activities of the mutants were tested by transient transfection of these PI3K forms in asynchronous cultures of NIH 3T3 cells. The expression levels of exogenous p110 were approximately double those of the endogenous proteins (Fig. 2B). Transient transfection of the mutants showed that K802R-p110α and K805R-p110β reduced and p110α* and -β* increased (p110α* had a greater effect) the p-PKB cellular levels (Fig. 2C). Thus, these mutants interfere with endogenous PI3K pathway activation.
FIG. 2.
Interference with p110α or -β activity differentially affects downstream signaling cascades. (A) Extracts (30 μg) from COS cells transfected with pSG5, pefBOS-p110α, pSG5-p110α, or pSG5-p110β were analyzed by WB using anti-p110α, anti-p110β, or actin Ab. (B, C, and F) NIH 3T3 cells were transfected with vectors encoding p110α* or p110β* or the K802R-p110α (KRp110α) or K805R-p110β (KRp110β) mutant, and extracts were examined with WB as described above. (D, E, G, and H) Synchronous p110α*- and p110β*-expressing NIH 3T3 clones (D and G) or NIH 3T3 cells transfected with the K802R-p110α or K805R-p110β mutant (E and H) were lysed, and extracts (30 μg) were examined with WB using the Ab indicated on the left. Graphs (E and H) show the mean percentages ± standard deviations (SD) of the p-Ser-473-PKB (pPKB) or p-Thr-389-p70S6K (pp70S6K) signals normalized in comparison to those of loading controls and compared to the maximum signal (control cells at 1 h, 100%) (n = 3). P values compare results for control cells and those expressing the K802R-p110α mutant at 1 h. (*), P < 0.05; Student's t test. Ctr, control.
We then examined PKB, FoxO, and p70S6K activities during early G1 (until 6 h following serum addition) in synchronized populations of stable p110α* and p110β* transfectants (see Materials and Methods). In these cells, the exogenous p110 expression levels were similar to the levels of endogenous proteins (1) (data not shown). In synchrony, p110α*-expressing cell lines showed sustained p-PKB activation (1) and p110β*-expressing cells showed a minor increase in basal levels of p-PKB and an increase in the p-PKB signal at ∼4 h (Fig. 2D). We failed to stably maintain cells expressing inactive mutants; these mutants were transduced by transient transfection (or infection) of NIH 3T3 cells, which yielded expression levels similar to those of endogenous proteins (Fig. 2B). The expression of the K802R-p110α mutant, but not of the K805R-p110β mutant, reduced p-PKB activation in early G1 (∼1 h) (Fig. 2E), as p110α activity is greater at this point (see below).
We also examined p70S6K activation. In asynchronous cultures, the transient expression of p110-interfering forms decreased and p110-activating mutations enhanced (p110α* had a greater effect than p110β*) p-p70S6K cellular levels (Fig. 2F). In synchronized populations, however, p110α* enhanced p70S6K activation even in G0, whereas p110β* increased p-p70S6K levels most notably at ∼4 h after the addition of serum (Fig. 2G). This suggested a selective action of p110α at the first PI3K activity peak; accordingly, the expression of the K802R-p110α mutant selectively inhibited the initial p-p70S6K peak (∼1 h), whereas the K805R-p110β mutant moderately reduced late p-p70S6K levels (Fig. 2H). The K805R-p110β mutant did not reduce p70S6K activation at 1 h, probably because p110β exhibits a notably lower activity than p110α in early G1 (see below). Quantitation of the gel bands in several assays confirmed the selective effect of the K802R-p110α mutant on the early p-PKB and p-p70S6K activity peaks following the addition of GF (Fig. 2E and H). The reduction of p110α and -β levels with shRNA yielded consistent results (not shown). These results indicate that both p110α and p110β modified PKB and p70S6K activation but that only p110α regulated their early G1 (∼1 h) activity peaks.
p110α regulates FoxO3a phosphorylation.
We also examined FoxO3a (FKHRL1), whose PKB-dependent phosphorylation is required for G0/G1 transition (27). We examined the consequences of reducing the expression of p110α and -β by using interfering mutants or specific shRNA (see Materials and Methods). The expression of p110α shRNA only affected p110α levels; similarly, p110β shRNA reduced only p110β, and not p110α, expression (Fig. 3A). p110β shRNA required longer incubation periods than p110α shRNA (minimum 96 h versus 48 h for p110α shRNA), probably due to the greater stability of the p110β protein (unpublished data). In control cells, the p-FoxO3a signal peaked at 1 to 1.5 h and was reduced by 2 h after GF addition (Fig. 3B). p110α shRNA greatly decreased p-FoxO3a levels at 1 to 1.5 h, whereas p110β shRNA had only a moderate effect on FoxO3a phosphorylation (Fig. 3B). Similar results were obtained by using a different set of shRNA (see Materials and Methods) or the K802R-p110α or K805R-p110β mutant (Fig. 3C). Control cells showed two peaks of increased p-FoxO3a content in cells in G1 phase, an early G1 peak and another peak coincident with the PI3K activity peak in late G1 (Fig. 3C). Whereas the K802R-p110α mutant significantly reduced p-FoxO3a levels throughout G1, the K805R-p110β mutant moderately diminished the duration of the early G1 peak and slightly postponed late G1 FoxO3a phosphorylation. Accordingly, stable p110α*-expressing cell lines exhibited sustained and high p-FoxO3a levels (1) (Fig. 3D), whereas p110β* only moderately and transiently increased FoxO3a phosphorylation (Fig. 3D and data not shown). The more-prominent action of p110α in FoxO TF control in early G1 was confirmed by examining cyclin D (see below). Thus, p110α plays a dominant role in FoxO phosphorylation in early G1. The parallel examination of cell cycle profiles in these assays showed that both the K802R-p110α and the K805R-p110β mutant reduced cell cycle entry (Fig. 3E); the levels of inhibition varied in different assays (see below) but were of similar magnitudes for interference with p110α or p110β. Accordingly, p110α*-expressing cells entered cell cycle earlier (1, 21) (Fig. 3E) and p110β*-expressing cells entered S phase even more efficiently than p110α*-expressing cells (Fig. 3E).
FIG. 3.
Selective action of p110α on FoxO3a phosphorylation. (A and B) NIH 3T3 cells were transfected with pB/U6-α2 (α2) or pB/U6-β2 (β2) shRNA; WB was used to analyze p110α or -β expression at 48 and 96 h posttransfection (A). A cell fraction was arrested in G0 and incubated for different times with serum; p-Thr-32-FoxO3a (pFoxO 3a) levels were analyzed with WB (B). The graph represents the mean percentages ± standard deviations (SD) of the signal normalized with those of the actin loading control and compared to the maximum signal in control cells (100%) (n = 3). (C) Extracts (30 μg) from synchronized NIH 3T3 cells transfected with the K802R-p110α or K805R-p110β mutant were examined with WB as described above. Data were quantitated as described for panel B (n = 3); arrows indicate S-phase progression. (D) Extracts from control or stable p110α*- or p110β*-expressing NIH 3T3 cells synchronized in different phases were examined with WB as described above. (E) Representative cell cycle distributions of the indicated synchronized cells. x axis represents DNA content, and y axis represents cell number. Percentages of cells in G0/G1, S, and G2/M are indicated. (*), P < 0.05 for comparison of results for control cells with results for cells expressing p110α shRNA or the K802R-p110α mutant at 1.5 h. Ctr, control.
p110α and -β control cyclin E and A levels, but only p110α regulates cyclin D.
Early signaling pathways promote cell growth and the expression of G1 cyclins (14). We subsequently examined the consequences for G1 cyclin expression of interfering with p110α and -β activity. Comparison of synchronous stable p110α*- and p110β*-expressing cells showed that enhanced activation of p110α, but not -β, increased cyclin D3 levels (Fig. 4A and B). In contrast, both p110α*- and p110β*-expressing cells upregulated cyclin E levels even before the addition of serum, and p110α*, but not -β*, prolonged cyclin E expression (Fig. 4A and B). Neither p110α* nor -β* expression was sufficient to induce cyclin A expression in G0, but cyclin A appeared earlier in these cells than in controls, and its expression was greater and more prolonged in p110α*-expressing cells (Fig. 4A and B). In p110α*-expressing cells, the higher cyclin D3 levels (Fig. 4A) correlated with their greater p-FoxO3a content (Fig. 3) (1), as p-FoxO3a controls cyclin D synthesis (36).
FIG. 4.
Enhanced p110α and -β activities upregulate G1 cyclins. (A) Levels of cyclins D3, E, and A, as well as actin levels, were examined by WB in synchronous cultures of stable p110α*- or p110β*-expressing NIH 3T3 cells. Transits through S phase are indicated (arrows). Ctr, control. (B) The graphs represent the mean percentages ± standard deviations (SD) of the signals for cyclins normalized with those of loading controls and compared to the maximum signal (100%) in wild-type cells (n = 3). P values (P < 0.05) for the data from some time points are indicated by asterisks. Gray asterisks show comparisons between control and p110α*-expressing cells; black asterisks show comparisons between control and p110β*-expressing cells. Cy, cyclin.
We performed a complementary analysis and examined the effects of interfering with p110α and -β expression on G1 cyclin expression. We examined the effect of reducing p110α and -β expression levels by shRNA in murine NIH 3T3 cells (not shown) and human U2OS cells synchronized at the G1/S border (Fig. 5). hp110α shRNA selectively reduced p110α expression, and p110β shRNA acted only on p110β (Fig. 5A). Nonetheless, p110α, but not -β, shRNA reduced cyclin D3 expression (Fig. 5B and C). In contrast, both shRNA (for p110α or -β) delayed the expression of cyclins E and A (Fig. 5B and C). Thus, p110α and p110β regulate the expression of cyclins E and A, but only p110α controls cyclin D levels.
FIG. 5.
p110α and -β control expression of G1 cyclins. (A and B) Expression levels of p110α and p110β in extracts of U2OS cells transfected with pTER-p110α7 (α7) and pTER-p110β15 (β15) shRNA (for 48 and 96 h, respectively) (A), and a fraction of the cells was synchronized at the G1/S boundary, and expression levels of cyclins D3, E, and A were examined by WB at different times after serum addition (B). The percentages of cells in S phase are indicated. (C) Graphs show the mean percentages ± standard deviations (SD) of the signals for each cyclin compared to the maximum signal in wild-type cells (100%), normalized with the signals for loading controls (n = 3). P values (P < 0.05) for data at the 0 time point, prior to release, are shown by asterisks. Gray asterisks show comparisons between control and p110α shRNA-expressing cells; black asterisks show comparisons between cells expressing p110β shRNA and cells expressing control shRNA. Ctr, control; Cy, cyclin; Thy, thymidine.
p110α and -β control late G1 c-Myc levels and RB phosphorylation.
Late G1 PI3K activation stabilizes c-Myc (24); we attempted to determine which of the two ubiquitous isoforms regulated c-Myc levels in late G1. Stable p110α*- and p110β*-expressing NIH 3T3 cell lines, as well as NIH 3T3 cells infected with retroviruses expressing the K802R-p110α or K805R-p110β mutant, were synchronized as described above. The control cells exhibited two peaks of increased c-Myc levels (Fig. 6A and B), as reported previously (24). In p110α*-expressing cells, the c-Myc levels were higher and peaked earlier but the cells still showed the two peaks of c-Myc expression (Fig. 6A and B). p110β* expression also moderately enhanced c-Myc stability, but only in late G1 (Fig. 6A and B). The effect of p110α* at increasing c-Myc levels is consistent with its action on FoxO TF, since FoxO TF represses c-Myc expression (8); it also concurs with the higher cyclin A levels observed in these cells, as c-Myc regulates cyclin A expression (26). Nonetheless, both p110α* and p110β* prolonged c-Myc stability in late G1. Interference with either p110α or -β postponed or reduced, respectively, the c-Myc expression levels in late G1 (Fig. 6C and D), suggesting that both isoforms control c-Myc levels in advanced G1, although they do so differently.
FIG. 6.
p110α and -β regulate c-Myc levels. (A and B) Stable NIH 3T3 cells expressing p110α* or p110β* (A) or NIH 3T3 cells infected with viruses expressing the K802R-p110α or K805R-p110β mutant (B) were arrested in G0 and incubated for different times after serum addition; c-Myc expression levels were analyzed by WB. Graphs show the mean percentages ± standard deviations (SD) of the c-Myc signals compared to the maximum signal in wild-type cells at 12 h after GF addition (100%) and normalized with the signals of loading controls (n = 3). Transits through S phase are indicated (arrows). P values (P < 0.05; Student's t test) for comparisons of data at 9 and 12 h are shown by asterisks. Gray asterisks show comparisons between control and p110α mutant-expressing cells; black asterisks show comparisons between controls and p110β mutant-expressing cells.
We also examined the consequences of interfering with p110α and -β activities for the phosphorylation of RB, a major Cdk2/cyclin substrate (37). In synchronized NIH 3T3 control cells, RB was hyperphosphorylated at ∼12 h after GF addition (Fig. 7A). Both p110α* and -β* expression affected RB phosphorylation, which was observed at low levels even in quiescent cells; in late G1, p110α* and -β* also increased and accelerated (p110β* more so) the appearance of hyperphosphorylated RB (Fig. 7A). Accordingly, interference with p110α or -β activity by the expression of the K802R-p110α or K805R-p110β mutant delayed RB phosphorylation (the K805R-p110β mutant had a greater effect) (Fig. 7B), suggesting that both p110α and -β activities regulate RB phosphorylation.
FIG. 7.
p110α and -β control RB phosphorylation. (A and B) Stable NIH 3T3 cells expressing p110α* or p110β* or infected with viruses encoding the K802R-p110α (p110α) or K805R-p110β (p110β) mutant were treated as described in the Fig. 6 legend; RB expression levels were analyzed by WB. Graphs represent the mean percentages ± standard deviations (SD) of the signals for p-RB (pRB) compared to the maximum p-RB signal in wild-type cells at 15 h (100%) (n = 3). Quantitation was as described in the Fig. 6 legend. (*), P < 0.05.
Distinct activation kinetics of p110α and -β during G1 phase.
The distinct contributions of p110α and -β to early G1 events suggested that p110α and -β might present different activation kinetics. To determine the PI3K isoform(s) activated in early and late G1, we examined NIH 3T3 cells that permit the synchronization of the cultures in G0 phase (25). To isolate p110α and -β, we could not use p85 Ab as it brings down both catalytic subunits, nor we could use anti-p110α and -β Ab, since most of them reduce PI3K activity (unpublished observations). Thus, to evaluate specific isoform activation through G1 phase, we cotransfected NIH 3T3 cells simultaneously with cDNA encoding wild-type p110α and -β fused to two different tags. Recombinant Myc-tagged p110α and His-tagged p110β were expressed at slightly above basal levels (Fig. 8A). p110α and p110β were efficiently immunoprecipitated by using Myc-tagged or His-tagged Ab, as determined by WB using the specific p110α or p110β Ab, respectively (Fig. 8B). Moreover, p110α-p85 and p110β-p85 complexes were at similar levels, as estimated by comparison of the amounts of p85 present in p110α and p110β immunoprecipitates (Fig. 8B, bottom).
FIG. 8.
p110α and -β show distinct activation kinetics. (A) WB analysis of total p110α and p110β levels in NIH 3T3 cells transfected with empty vector or with cDNA encoding Myc-tagged p110α plus His-tagged p110β; expression levels of recombinant proteins are within the range of expression of endogenous p110. (B) NIH 3T3 cell extracts as described for panel A were immunoprecipitated (IP) using anti-Myc-tagged or anti-His-tagged Ab. WB showed p110α or -β expression and the amount of p85 in complex with p110. (C) NIH 3T3 cells transfected with Myc-p110α plus His-p110β were incubated (24 h), arrested in G0, and released in serum alone or with herbimycin or lovastatin at the indicated times. p110α or -β was immunoprecipitated as described for panel B, and kinase activity was assayed in vitro. (D) Immunoprecipitates as described for panel C were resolved by SDS-polyacrylamide gel electrophoresis, and associated p85 was assayed by WB. (A to D) Each assay result shown is representative of five assays with similar results. (E and F) Graphs show the mean percentages ± standard deviations (SD) (n ≥ 4) of p110α and -β activities (as shown in panel C) compared to the activity of p110α at 1 h (100%). The double-ended arrow in panel E indicates the time point for which the P value was calculated. (*), P < 0.05; Student's t test. Ctr, control; +, present; −, absent.
We immunopurified p110α and -β with the corresponding anti-tag Ab and tested their enzyme activities in vitro. After the addition of serum, p110α activated early, at 5 to 10 min following serum addition; this activity increased at 1 h and then diminished to basal levels, increasing again at ∼7 h (Fig. 8C). p110β exhibited modest activity peaks at 1 and 4 h and a maximum activity at ∼8 h after the addition of serum (Fig. 8C). In NIH 3T3 cells, part of p110β, but not p110α, localizes in the nuclei (our unpublished results); nuclear PI3K activity peaked at ∼8 h after the addition of serum, confirming maximum endogenous p110β activity in late G1 (not shown). We checked that similar amounts of p85 were associated with either p110α or p110β at different time points (Fig. 8D). Therefore, most PI3K activity in early G1 corresponds to that of p110α; p110β exhibits another minor peak by 4 h. In late G1, both p110α and -β are activated and p110β exhibits its maximum activity.
p110α and -β have different activation requirements.
The different activation kinetics of p110α and -β suggested that they exhibit distinct activation requirements. Since TyrK and Ras regulate class IA PI3K activation (17), we tested whether the p110α and -β activities in G1 phase were affected by treatment with inhibitors of TyrK (herbamycin) and Ras (lovastatin). We first checked the selective action of these inhibitors in reducing p-Tyr or active Ras levels (24 and data not shown).
Herbamycin treatment, but not treatment with lovastatin, reduced p110α activity at 7 min. Both herbamycin and lovastatin inhibited p110α activation at 1 and 7 h (Fig. 8C). This suggests that the first increase in the activity of p110α is TyrK dependent, but TyrK and Ras contribute to p110α activation at 1 and 7 h. In contrast, the modest p110β activity at 1 h was sensitive to lovastatin, but not to herbamycin, although both inhibitors blocked later p110β activation peaks (at 4 and 8 h) (Fig. 8C and F). The results of these assays illustrate the distinct activation requirements for p110α and -β activities. The maximum p110α (at 1 h) and p110β (at 8 h) activities, nonetheless, were herbamycin and lovastatin sensitive, suggesting that TyrK and Ras activation contribute to optimal p110α and p110β activities.
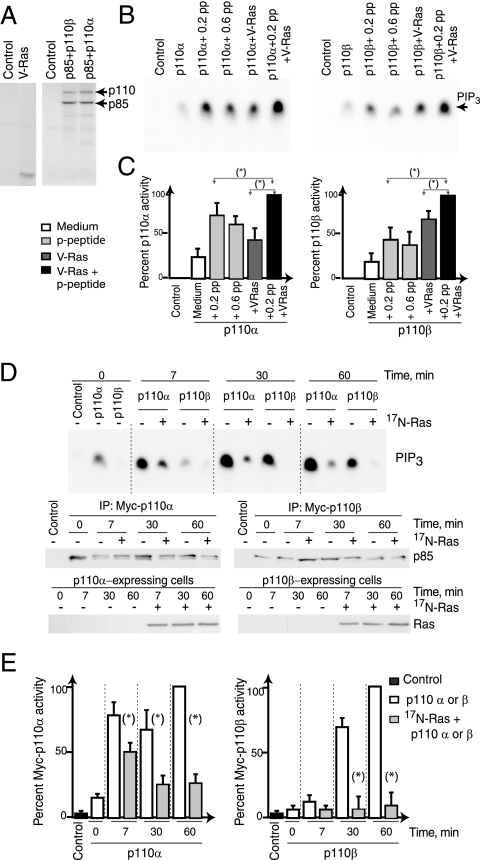
To confirm the distinct activation requirements of p110α and p110β, we examined whether the response of purified p85-p110β complex to activated TyrK and Ras is similar to that of p85-p110α (17). We used Tyr-phosphorylated platelet-derived growth factor receptor (PDGF-R) peptide and purified active Ras in vitro; this analysis confirmed that the Tyr-phosphorylated peptide activates p110α, that active Ras alone exerts a moderate activation effect, and that Ras synergizes with p-Tyr phosphopeptides to enhance p110α activity (Fig. 9B and C) (17). In contrast, although p110β activity also increased with the phosphopeptides and with active Ras and together they induced a greater activation effect (Fig. 9B and C), there was a consistent difference between p110α and p110β activation. Whereas p110α responds better to Tyr phosphopeptides than to V12-Ras alone, Ras consistently induced a greater activating effect than phosphopeptides on p110β (Fig. 9B and C). These assays confirmed the TyrK activation requirement for p110α induction (17) and demonstrated the greater intrinsic Ras dependence for p110β activation.
FIG. 9.
Activation of p110β requires Ras. (A) Control vector or cDNAs encoding V12-Ras or p85 combined with p110α or p110β were transcribed and translated in vitro and then analyzed by SDS-polyacrylamide gel electrophoresis. (B) The activities of purified p85/p110α or p85/p110β complexes were assayed in vitro, alone or in the presence of a PDGF-R phosphopeptide (pp) at the indicated close (μM), V12-Ras, or both. The panels show the results of representative experiments (n = 3). (C) The graphs compare PIP3 spot intensities for three experiments (as in panel B) to maximum p110α or -β activities (pp + VRas [V12-Ras], 100%) (n = 3). Double-ended arrows indicate the two values being compared. (D) NIH 3T3 cells were transfected with empty vector or cotransfected with cDNAs encoding p85 and Myc-p110α or Myc-p110β. p85-p110 cDNAs were transfected alone or with a vector encoding N17-Ras. After 36 h, cells were synchronized in G0 and released by serum addition for different times. p110α or p110β was immunopurified, and their activities assayed in vitro. We examined the amount of p85 in the p85-p110 complexes by WB (middle panels). The different samples expressed similar N17-Ras levels (bottom), as determined by WB. +, present; −, absent; IP, immunoprecipitate. (E) Graphs compare the mean percentages ± standard deviations (SD) of p110α and -β activities of three different assays as described for panel C to the activity of p110α or p110β at 1 h (100%), normalized in comparison with the p85 loading control. (*), P < 0.05. V-Ras/VRas, V12-Ras; N-Ras/17N-Ras, N17-Ras.
Since p110α activation is greater than that of p110β in early G1 (at 7 min to 1 h), we compared the binding of p110α and -β to PDGF-R at early time points. Both isoforms associated with the PDGF-R at 7 min after the addition of serum (not shown), arguing against a selective binding of p110α as the cause for its selective activation at this point. To gain insight into the mechanisms of p110α and -β activation in early G1, we considered the greater Ras dependence of p110β in vitro and postulated that the activation of p110β in vivo might also rely more on active Ras than that of p110α does. To determine the relative Ras dependence for p110α and -β, we examined the sensitivities of p110α and -β to interference with Ras activation induced by the coexpression of N17-Ras with Myc-tagged versions of p110α and -β. Whereas the first p110α activity peak at 7 min decreased only partially in the presence of N17-Ras (approximately one-third), p110β activation, which was lower than that of p110α, occurred later and was drastically reduced (more than 90%) following the expression of N17-Ras (Fig. 9D and E). These observations show that both in vitro and in vivo, the activation of p110β is more Ras dependent than that of p110α. Considering that Ras activation is moderate at 1 h and maximal in late G1 (24), the greater Ras dependence of p110β explains its activation kinetics in G1 phase.
Interference with p110α or -β expression/activity results in cell cycle entry defects.
During the course of the experiments using synchronized populations, we noticed that cells expressing p110α* and -β* showed an earlier S-phase entry (Fig. 3E, 4, 6A, and 7A). Accordingly, the expression of K802R-p110α and K805R-p110β mutants (Fig. 3E, 6B, and 7B) or the reduction of p110α and -β levels by shRNA in U2OS cells (Fig. 5B) induced a delayed G1/S transition. We also interfered with p110α or -β expression in NIH 3T3 cells by using p110α or -β shRNA, as described above. p110α shRNA selectively reduced p110α expression and p110β shRNA diminished only p110β levels (Fig. 10A). Despite partial reductions in p110α and -β expression, both shRNA delayed S-phase entry (Fig. 10A).
FIG. 10.
Interference with p110α or -β results in cell cycle defects. (A) NIH 3T3 cells were transfected with the indicated shRNA; at 48 or 96 h posttransfection, cells were collected and lysates (30 μg) examined by WB using anti-p110α, anti-p110β, and antiactin Ab (left panels). A fraction of the cells were synchronized, and cell cycle distribution examined at indicated times. The proportion of cells in S phase is represented (mean ± standard deviation) (n = 3) (right panels). P values for comparisons of the data at 12 h are shown. (B) COS cells were transfected with cDNA encoding the K802R-p110α or K805R-p110β mutant; at 24 h posttransfection, the cells were incubated with BrdU (1 h). The percentages of cells incorporating BrdU were examined by immunofluorescence (IF) (means ± standard deviations) (n = 3). (C) Heterozygous (HET/Het) p110α and p110β MEF were screened by specific PCR (left). We show percent BrdU incorporation in exponentially growing heterozygous MEF compared to that in wild-type (WT) MEF from littermate embryos. (*), P < 0.05; Student's t test. Ctr, control; 1 and 2, mice 1 and 2; KO, knockout.
We also examined cell cycle entry by the incorporation of bromodeoxyuridine (BrdU). Interference with endogenous p110α and -β kinase activity in COS cells by the transfection of the inactive K802R-p110α or K805R-p110β mutants reduced BrdU incorporation (Fig. 10B). We also analyzed primary cells. Homozygous deletion of p110α or -β causes embryonic lethality (3, 4). We thus examined MEF from p110α and -β heterozygous mice. Since G0 arrest by serum deprivation or growth to confluence is inefficient in MEF, we analyzed S-phase entry by measuring BrdU incorporation in exponentially growing cultures. Both heterozygous deletions reduced the fraction of BrdU-positive cells compared to the BrdU-positive fraction of wild-type fibroblasts (Fig. 10C). These results demonstrate that both p110α and -β control cell cycle entry.
DISCUSSION
The activation of PI3K is essential for cell division. We examined which one of the two ubiquitous PI3K isoforms (p110α and -β) regulates cell cycle entry. We describe results showing that p110α activated before p110β at the G0/G1 transition exerts a selective action in inducing G1 entry events. In fact, the first activity peak of p110α had already occurred at 5 to 10 min following the addition of GF and it required TyrK activation; p110α further increased its activity at ∼1 h in a TyrK- and Ras-dependent manner and activated again in advanced G1. In contrast, p110β displayed low activity in early G1, with a moderate increase at ∼1 h; Ras induction was essential for p110β activation. p110β displayed another low activity peak in mid-G1 and maximal activation in late G1. p110α and -β activate in a sequential manner in late G1. This concurs with their distinct sensitivities to TyrK and Ras since, also in late G1, the activation of TyrK precedes that of Ras, which is maximal at this point (24). In agreement with the greater activation of p110α in early G1, this isoform regulated early G1 events (such as cyclin D levels and FoxO phosphorylation) more than p110β did. Nonetheless, interference with either p110α or p110β reduced S-phase entry, showing that both isoforms control the G1→S transition. p110α and -β regulated the expression of c-Myc and cyclins E and A, RB phosphorylation, and, in turn, S-phase entry.
The critical role of p110α and -β in the control of cell division was taken into account during the preparation of the cell lines for this study. We used stable cell lines expressing low levels of p110α* and p110β*, since the transient overexpression of high levels of p110α* impairs progression through the G2/M phases (1). p110α*-expressing cells entered cell cycle faster than controls, and p110β*-expressing cells divided at a lower half-life than both p110α*-expressing cells and controls. For the analysis of the consequences of reducing the p110α and p110β activities, we had to use transient transfection or infection, as cell lines of kinase-inactive mutants or shRNA were unstable, showing that p110α and p110β activities control cell survival and/or division.
An open question regarding the select functions of class IA PI3K isoforms is how the specificities of the different isoforms are acquired, as p110 catalytic subunits produce the same lipid products and all class IA p110s associate with p85 molecules, which bring p110 to activated receptors (42, 12). p110δ's specificity seems related to its tissue-specific expression pattern (30). However, in the case of p110α and -β, they are ubiquitous and still they exhibit distinct functions in development (3, 4) and cell division (Fig. 2 and 3). The observations presented here illustrate mechanisms for the p110α and -β functional specificities that are delimited by the different activation requirements determining when these isoforms are activated.
The phenotype of mice expressing a Ras-resistant p110α mutant supports the observation that, physiologically, p110α activity is partially independent of Ras. These mice present a number of defects, including reduced cell proliferation and diminished Ras-dependent tumor formation (15); however, they exhibit a milder phenotype than p110α-deficient mice (3). This shows that despite the fact that K227A p110α is not activated by Ras, it still exerts some of the p110α actions in vivo (15). Interestingly, the expression of wild-type p110β, -δ, and -γ is sufficient to induce chicken embryo fibroblast focus formation; in contrast, p110α requires an activating mutation to trigger transformation (20). The crystal structure analysis of the inter-Src homology 2 domain of p85 in complex with the N-terminal part of p110α suggests that activation by Tyr kinases releases p110α from the inhibition exerted by p85; it is possible that the p85-mediated p110 structural constraint is stronger in the case of p110α (16, 29). The H1047R mutant activates p110α; following the additional K227E mutation, this mutant no longer binds Ras but contributes to cell transformation. In contrast, wild-type p110β loses its transforming activity when Ras binding is impaired (20). It is possible that in the absence of Ras binding, p110β simply exhibits low enzymatic activity, since we show that purified p110β shows a higher Ras dependence for activation than p110α (Fig. 9). In this regard, although late G1 p110β activation was greatly inhibited by the addition of herbamycin at 7 h (Fig. 8C), this treatment reduced late G1 Ras activity (not shown). Future studies will attempt to determine which residues in the p110 Ras-binding site determine the greater Ras dependence of p110β.
Whereas the results of our studies support the existence of activation specificities for p110α and -β, downstream of p110α and -β we find that they are both capable of regulating the same substrates. In fact, both the mutants interfering with p110α and those interfering with p110β affected PKB and p70S6K activities, illustrating that these p110 isoforms have the potential of regulating the same effectors. The distinct kinetics of p110α activation in early G1 phase explains the specific function for p110α at this point. In fact, in synchronized cells, p110α selectively controlled the first activation wave of PKB and p70S6K and, in turn, FoxO3a phosphorylation and the expression of its effector, cyclin D. Since p110β's activity was low in early G1, interference with its kinase activity affected PKB and p70S6K activities at this point only slightly, although it modulated their activities at later time points (Fig. 2). In contrast, in late G1, both p110α and p110β exhibited remarkable increases in activity and regulated c-Myc and cyclin E and A levels, as well as RB phosphorylation. Therefore, both the p110α and -β isoforms controlled cell cycle regulators at the G1/S boundary, offering a mechanism for the involvement of these isoforms in the control of cell cycle entry.
The expression of p110α shRNA inhibits carcinoma cell growth (28), supporting the role of p110α in cell division. Selective interference with p110α inhibited the early activation of the cell growth regulator p70S6K (Fig. 2). Since cell cycle entry cannot occur without cell growth (35), p110α mutations in human cancer might facilitate G0 exit by upregulating protein synthesis and inhibiting FoxO TF-controlled cell cycle inhibitors. Later in the cell cycle, p110α and -β contribute to enhancing c-Myc stability and Cdk2 activation (24). p110α is thus a potential target for cancer treatment; nonetheless, the inhibition of p110α interferes with glucose metabolism (22). Alternatively, interference with p110β might also be considered a promising approach, since although no activating mutations in p110β in human cancer have been described, the overexpression of the wild-type p110β does promote cell transformation (20). In fact, shRNA for p110β show an antiproliferative effect in tumor cell lines (6, 32) and interference with p110β blocks S-phase entry (Fig. 10).
Altogether, we report that p110α and -β are activated with distinct kinetics during G1 phase, as they respond differently to the activation of TyrK and Ras. p110α primarily controls early G1 events, such as FoxO TF inactivation and cyclin D expression, whereas both p110α and -β regulate later G1 events and G0/G1 transition.
Acknowledgments
M.M. has a predoctoral FPU fellowship from the Spanish Ministry of Education and Science. This work was financed in part by grants from the AICR Foundation, the Fundación Ramón Areces, the AECC, and the Spanish DGCyDT (SAF2004-05955). The Department of Immunology and Oncology was founded and is supported by the Spanish National Research Council (CSIC) and by Pfizer.
We thank M. White for the myc-p110 plasmid, C. Murga for pCEF2-p110β-CAAX, B. Vanhaesebroeck for His-p110β, M. van de Wetering for the pTer vector, and Y. Shi for the pBlue/U6 plasmid. We also thank R. L. Nussbaum for the kind donation of p110α- and p110β-deficient mice, A. Klippel for anti-p110α Ab, and C. Mark for editorial assistance.
Footnotes
Published ahead of print on 19 February 2008.
REFERENCES
- 1.Álvarez, B., C. Martínez-A., B. M. Burgering, and A. C. Carrera. 2001. Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature 413744-747. [DOI] [PubMed] [Google Scholar]
- 2.Álvarez, B., E. Garrido, J. A. Garcia-Sanz, and A. C. Carrera. 2003. Phosphoinositide 3-kinase activation regulates cell division time by coordinated control of cell mass and cell cycle progression rate. J. Biol. Chem. 27826466-26473. [DOI] [PubMed] [Google Scholar]
- 3.Bi, L., I. Okabe, D. J. Bernard, A. Wynshaw-Boris, and R. L. Nussbaum. 1999. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110-alpha subunit of phosphoinositide 3-kinase. J. Biol. Chem. 27410963-10968. [DOI] [PubMed] [Google Scholar]
- 4.Bi, L., I. Okabe, D. J. Bernard, and R. L. Nussbaum. 2002. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI3K. Mamm. Genome 13169-172. [DOI] [PubMed] [Google Scholar]
- 5.Chan, T. O., U. Rodeck, A. M. Chan, A. C. Kimmelman, S. E. Rittenhouse, G. Panayotou, and P. N. Tsichlis. 2002. Small GTPases and tyrosine kinases coregulate a molecular switch in the phosphoinositide 3-kinase regulatory subunit. Cancer Cell 1181-191. [DOI] [PubMed] [Google Scholar]
- 6.Czauderna, F., M. Fechtner, H. Aygun, W. Arnold, A. Klippel, K. Giese, and J. Kaufmann. 2003. Functional studies of the PI(3)-kinase signalling pathway employing synthetic and expressed shRNA. Nucleic Acids Res. 31670-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dangi, S., H. Cha, and P. Shapiro. 2003. Requirement for PI3K activity during progression through S-phase and entry into mitosis. Cell Signal. 15667-675. [DOI] [PubMed] [Google Scholar]
- 8.Domínguez-Cáceres, M. A., J. M. Garcia-Martinez, A. Calcabrini, L. González, P. G. Porque, J. León, and J. Martin-Perez. 2004. Prolactin induces c-Myc expression and cell survival through activation of Src/Akt pathway in lymphoid cells. Oncogene 237378-7390. [DOI] [PubMed] [Google Scholar]
- 9.Fingar, D. C., S. Salama, C. Tsou, E. Harlow, and J. Blenis. 2002. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 161472-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foukas, L. C., M. Claret, W. Pearce, K. Okkenhaug, S. Meek, E. Peskett, S. Sancho, A. J. Smith, D. J. Withers, and B. Vanhaesebroeck. 2006. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature 441366-370. [DOI] [PubMed] [Google Scholar]
- 11.Frouin, I., A. Montecucco, G. Biamonti, U. Hubscher, S. Spadari, and G. Maga. 2002. Cell cycle-dependent dynamic association of cyclin/Cdk complexes with human DNA replication proteins. EMBO J. 212485-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruman, D. A., R. E. Meyers, and L. A. Cantley. 1998. Phosphoinositide kinases. Annu. Rev. Biochem. 67481-507. [DOI] [PubMed] [Google Scholar]
- 13.García, Z., V. Silió, M. Marqués, I. Cortés, A. Kumar, C. Hernández, A. I. Checa, A. Serrano, and A. C. Carrera. 2006. A PI3K activity-independent function of p85 regulatory subunit in control of mammalian cytokinesis. EMBO J. 254740-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García, Z., A. Kumar, M. Marques, I. I. Cortes, and A. C. Carrera. 2006. PI3K controls early and late events in mammalian cell division. EMBO J. 25655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta, S., A. R. Ramjaun, P. Haiko, Y. Wang, P. H. Warne, B. Nicke, E. Nye, G. Stamp, K. Alitalo, and J. Downward. 2007. Binding of Ras to phosphoinositide 3-kinase p110α is required for Ras-driven tumorigenesis in mice. Cell 129957-968. [DOI] [PubMed] [Google Scholar]
- 16.Jimenez, C., D. R. Jones, P. Rodríguez-Viciana, A. Gonzalez-García, E. Leonardo, S. Wennström, C. von Kobbe, J. L. Toran, L. R.-Borlado, V. Calvo, S. G. Copin, J. P. Albar, M. L. Gaspar, E. Diez, M. A. Marcos, J. Downward, C. Martinez-A., I. Mérida, and A. C. Carrera. 1998. Identification and characterization of a new oncogene derived from the regulatory subunit of phosphoinositide 3-kinase. EMBO J. 17743-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiménez, C., C. Hernández, B. Pimentel, and A. C. Carrera. 2002. The p85 subunit controls activation of PI3K by Tyr kinases and Ras. J. Biol. Chem. 27741556-41562. [DOI] [PubMed] [Google Scholar]
- 18.Jones, S. M., R. Klinghoffer, G. D. Prestwich, A. Toker, and A. Kazlauskas. 1999. PDGF induces an early and a late wave of PI 3-kinase activity, and only the late wave is required for progression through G1. Curr. Biol. 9512-521. [DOI] [PubMed] [Google Scholar]
- 19.Jones, S. M., and A. Kazlauskas. 2001. Growth-factor-dependent mitogenesis requires two distinct phases of signalling. Nat. Cell Biol. 3165-172. [DOI] [PubMed] [Google Scholar]
- 20.Kang, S., A. Denley, B. Vanhaesebroeck, and P. K. Vogt. 2006. Oncogenic transformation induced by the p110β, γ and δ isoforms of class I PI3K. Proc. Natl. Acad. Sci. USA 1031289-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klippel, A., M. A. Escobedo, M. S. Wachowicz, G. Apell, T. W. Brown, M. A. Giedlin, W. M. Kavanaugh, and L. T. Williams. 1998. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Mol. Cell. Biol. 185699-5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight, Z. A., B. Gonzalez, M. E. Feldman, E. R. Zunder, D. D. Goldenberg, O. Williams, R. Loewith, D. Stokoe, A. Balla, B. Toth, T. Balla, A. W. Weiss, R. L. Williams, and K. M. Shokat. 2006. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125733-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozma, S. C., and G. Thomas. 2002. Regulation of cell size in growth, development and human disease: PI3K, PKB and S6K. Bioessays 2465-71. [DOI] [PubMed] [Google Scholar]
- 24.Kumar, A., M. Marqués, and A. C. Carrera. 2006. Phosphoinositide 3-kinase activation in late G1 is required for c-Myc stabilization and S phase entry. Mol. Cell. Biol. 269116-9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martínez-Gac, L., M. Marqués, Z. García, M. R. Campanero, and A. C. Carrera. 2004. Control of cyclin G2 mRNA expression by forkhead transcription factors: novel mechanism for cell cycle control by phosphoinositide 3-kinase and forkhead. Mol. Cell. Biol. 242181-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mateyak, M. K., A. J. Obaya, and J. M. Sedivy. 1999. c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol. Cell. Biol. 194672-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medema, R. H., G. J. Kops, J. L. Bos, and B. M. Burgering. 2000. AFX-like forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404782-787. [DOI] [PubMed] [Google Scholar]
- 28.Meng, Q., C. Xia, J. Fang, Y. Rojanasakul, and B. H. Jiang. 2006. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 182262-2271. [DOI] [PubMed] [Google Scholar]
- 29.Miled, N., Y. Yan, W. C. Hon, O. Perisic, M. Zvelebil, Y. Inbar, D. Schneidman-Duhovny, H. J. Wolfson, J. M. Backer, and R. L. Williams. 2007. Mechanism of two classes of cancer mutations in the PI3K catalytic subunit. Science 317239-242. [DOI] [PubMed] [Google Scholar]
- 30.Okkenhaug, K., A. Bilancio, G. Farjot, H. Priddle, S. Sancho, E. Peskett, W. Pearce, S. E. Meek, A. Salpekar, M. D. Waterfield, A. J. Smith, and B. Vanhaesebroeck. 2002. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science 2971031-1034. [DOI] [PubMed] [Google Scholar]
- 31.Olson, M. F., A. Ashworth, and A. Hall. 1995. An essential role for Rho, Rac and Cdc42 GTPases in cell cycle progression through G1. Science 2691270-1272. [DOI] [PubMed] [Google Scholar]
- 32.Pu, P., C. Kang, Z. Zhang, X. Liu, and H. Jiang. 2006. Downregulation of PIK3CB by siRNA suppresses malignant glioma cell growth in vitro and in vivo. Technol. Cancer Res. Treat. 5271-280. [DOI] [PubMed] [Google Scholar]
- 33.Ryves, W. J., and A. J. Harwood. 2003. The interaction of glycogen synthase kinase-3 (GSK-3) with the cell cycle. Prog. Cell Cycle Res. 5489-495. [PubMed] [Google Scholar]
- 34.Sarbassov, D. D., S. M. Ali, and D. M. Sabatini. 2005. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17596-603. [DOI] [PubMed] [Google Scholar]
- 35.Saucedo, L. J., and B. A. Edgar. 2002. Why size matters: altering cell size. Curr. Opin. Genet. Dev. 12565-571. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt, M., S. Fernandez de Mattos, A. van der Horst, R. Klompmaker, G. J. P. L. Kops, E. W.-F. Lam, B. M. T. Burgering, and R. H. Medema. 2002. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol. Cell. Biol. 227842-7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 131501-1512. [DOI] [PubMed] [Google Scholar]
- 38.Shtivelman, E., J. Sussman, and D. Stokoe. 2002. A role for PI3K and PKB activity in the G2/M phase of the cell cycle. Curr. Biol. 12919-924. [DOI] [PubMed] [Google Scholar]
- 39.Sui, G., C. Soohoo, B. el Affar, F. Gay, Y. Shi, W. C. Forrester, and Y. Shi. 2002. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. USA 995515-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van de Wetering, M., I. Oving, V. Muncan, M. T. Pon Fong, H. H. Brantjes, D. van Leenen, F. C. Holstege, T. R. Brummelkamp, R. Agami, and H. Clevers. 2003. Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep. 4609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Weeren, P. C., K. M. de Bruyn, A. M. de Vries-Smits, J. van Lint, and B. M. Burgering. 1998. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J. Biol. Chem. 27313150-13156. [DOI] [PubMed] [Google Scholar]
- 42.Wymann, M. P., and L. Pirola. 1998. Structure and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta 1436127-150. [DOI] [PubMed] [Google Scholar]